Summary
Glutamate receptor ion channels, including α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, mediate fast excitatory neurotransmission in the CNS. Previous work suggested that AMPA receptors produce a synaptic current with a millisecond duration. However, we find that about two-thirds of principal cells in the hippocampal CA1 region also express AMPA receptors with reduced desensitization that can stay active for half a second after repetitive stimuli. These slow AMPA receptors are expressed at about half of the synapses, with a flat spatial distribution. The increased charge transfer from slow AMPA receptors allows short-term potentiation from a postsynaptic locus and reliable triggering of action potentials. Biophysical and pharmacological observations imply slow AMPA receptors incorporate auxiliary proteins, and their activation lengthens miniature synaptic currents. These data indicate that AMPA receptors are a major source of synaptic diversity. Synapses harboring slow AMPA receptors could have unique roles in hippocampal function.
Graphical abstract

Highlights
-
•
Two-thirds of principal cells in the hippocampal CA1 region express slow AMPA receptors
-
•
Slow AMPA receptors show reduced desensitization and stay active for up to half a second
-
•
Increased charge transfer allows single stimulations to trigger action potentials
-
•
Postsynaptic short-term potentiation has implications for computation and cognition
Pampaloni et al. identify slow AMPA receptors in the hippocampus. Synaptic responses from slow AMPA receptors are distributed in a mosaic fashion across CA1 pyramidal cells. Slow AMPA responses provide massive depolarization that can trigger an action potential from a single stimulation and produce short-term potentiation from a purely postsynaptic locus.
Introduction
Neurons in the vertebrate brain receive excitatory input from their presynaptic partners through the rapid activation of glutamate receptors. The fastest of these glutamate receptor ion channels, the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, activate and deactivate in milliseconds in response to glutamate released from synapses (Hestrin et al., 1990). Other subtypes of glutamate receptors, including NMDA, kainate, and delta receptors, show activation over seconds (Gantz et al., 2020; Kidd and Isaac, 1999; Misra et al., 2000) but are either tonically blocked or expressed in only a handful of neurons. Prior studies suggest that desensitization of native AMPA receptors is nearly complete within tens of milliseconds (Colquhoun et al., 1992; Geiger et al., 1995). These properties allow AMPA receptor activation to follow synaptic input with high temporal accuracy and to participate in short-term depression (Rothman et al., 2009). AMPA channels are retained at synapses by forming complexes with their auxiliary proteins (Bats et al., 2007). These auxiliary subunits act as anchors but also alter receptor responses to glutamate (Tomita et al., 2005). Despite two decades of research, the relevance for brain function of a change in AMPA receptor activity due to auxiliary proteins is lacking.
Auxiliary subunits can slow AMPA receptor kinetics in heterologous expression (Priel et al., 2005) and reduce their tendency to desensitize while boosting activation (Coombs et al., 2017). Evidence for desensitization-resistant AMPA receptors in the brain is so far limited to high-frequency activity at certain connections in the cerebellum (Lu et al., 2017; DiGregorio et al., 2007), where glutamatergic currents with unusual pharmacology were reported (Devi et al., 2016). Recombinant AMPA receptors also show a mode of enhanced gating with high conductance (Zhang et al., 2014) driven by activity (Carbone and Plested, 2016), which led us to hypothesize that certain auxiliary subunits inevitably endow AMPA receptors with slow, desensitization-resistant responses. This effect (which we named superactivation, also known as resensitization) was reported for several transmembrane AMPA receptor regulatory proteins (TARPs) in recombinant expression and should be particularly prevalent for the γ-8 subunit that is strongly expressed in the hippocampus (Rouach et al., 2005; Yamasaki et al., 2016). Repeated application of glutamate to recombinant AMPA receptors in complex with γ-8 at 10–25 Hz produces a substantial, indefatigable superactive current response (Carbone and Plested, 2016). However, work to date failed to identify cognate superactivating/resensitizing responses in neurons and, in particular, deemed them absent from wild-type CA1 pyramidal cells (Kato et al., 2010). However, gradients of gene expression across CA1 (Cembrowski et al., 2016) are consistent with heterogeneity in the pyramidal cell population.
Results
To replicate repetitive activation in the hippocampus while avoiding the potentially confounding effects of presynaptic plasticity, we performed glutamate uncaging at 10 Hz in CA1 pyramidal neurons in organotypic slice cultures (Figure 1A) at visually identified dendritic locations. This stimulation reflects a frequency of synaptic input that hippocampal cells might naturally experience during mu or theta waves (Buzsáki, 2002; Takillah et al., 2017). To limit contamination of the responses to glutamate by other receptors/channels, we performed experiments in a cocktail of inhibitors to block γ-aminobutyric acid (GABA)-A receptors, kainate receptors, metabotropic glutamate receptors (mGluR), and GABA-B receptors. We paid particular attention to blocking all types of NMDA receptors, which represent canonical slow glutamate receptor ion channels. To this end, we worked in normal magnesium (2 mM) and included potent NMDA receptor blockers in both intracellular and extracellular media (MK-801 and APV, respectively).
Figure 1.
Slow pedestal responses in CA1 pyramidal cells
(A) Tiled fluorescence micrograph of a CA1 pyramidal cell in organotypic slice cultures with voltage-clamp responses from 10 Hz uncaging at 10 sites. Dotted white lines are used to divide the hippocampal layers in the CA1 area: o, oriens; p, pyramidale; r, radiatum; l-m, lacunosum moleculare.
(B) Examples of typical fast classical responses (blue, no pedestal) and pedestal responses (orange, with an additional slow inward current and slow decay), with uncaging pulses indicated as purple circles.
(C) Spatial distribution of a normalized pedestal (steady state) current at the end of 20 uncaging pulses at 10 Hz against the distance of the site from the nucleus (dashed line; r2 = 0.003, n = 285 sites, 43 cells). The pie chart shows the fraction of uncaging sites with (orange) and without (blue) pedestal responses.
(D) Incidence and prevalence of pedestal currents with 10 Hz uncaging. Nearly two-thirds of CA1 pyramids present a mosaic distribution of pedestal currents, with one-third apparently lacking pedestal responses.
(E) Pedestal and non-pedestal responses generated from classical 10 Hz Schaffer collateral (SC) electrical stimulation in organotypic slice cultures.
(F) Distribution of pedestal magnitudes in SC stimulation experiments. The pie chart shows the fraction of cells with (orange) or without (blue) pedestal responses from electrical stimulation.
When uncaging glutamate at 10 Hz, we found both classical fast AMPA receptor responses at some sites and fast responses mixed with slow currents at others (Figure 1B). This result was quite unexpected, because many previous studies failed to report slow currents of this nature. We detected these slow currents, which we denote as pedestal responses, in two-thirds of CA1 pyramidal cells, with no relation to distal/proximal dendritic location (Figure 1C) or the peak amplitude of the evoked response (Figure S1). Their distribution appeared to be static on the timescale of our recordings (10–40 min, for a direct comparison with a 15 minute interval; see Figure S1). The incidence and amplitude of the slow responses showed variability within individual neurons (Figure 1A), suggesting variable expression of at least one type of AMPA receptor with atypical properties. Uncaging experiments in the CA3 region of the hippocampus identified similar but less prevalent pedestal responses resistant to the kainate receptor antagonist UBP 310, in addition to the typical slow kainate receptor currents from the mossy fiber-pyramidal cell synapse (Figure S1) (Castillo et al., 1997; Vignes and Collingridge, 1997).
Could these slow currents be artifacts of glutamate uncaging, recording techniques, or variability in our preparation? Several observations suggest this is not the case. First, slow currents were detected in the same cell, and during the same recording, as canonical responses with only a fast component. The slow currents presented without treatment or delay following the start of the recording. Pedestal currents were distributed over the neuron with no obvious spatial pattern and were thus dotted along dendrites, interspersed with classical responses (Figure S2A). In separate experiments, we confirmed that our uncaging spot was restricted to an approximately 1 μm diameter, allowing us to target single identified dendritic spines when they could be discerned (Figures S2B and S2C). However, the amplitude of currents we evoked by uncaging was in general typical of several synaptic sites being targeted. In addition, about one-third of cells from the same slices lacked pedestal responses (Figure 1E), as did dentate gyrus granule cells (described later). We also wondered whether organotypic culture was a factor in generating large pedestal responses. However, recordings from CA1 pyramidal cells in mouse acute hippocampal slices over three age ranges spanning from postnatal day 9 to 80 revealed similar incidence, form, and kinetics of the pedestal current (Figure S3), ruling out this possibility. Finally, we found that the pedestal current was retained (with similar kinetics) as we increased the temperature to 32°C, or as we liberated less glutamate by shortening the duration of the UV uncaging pulses (Figure S4). These observations show that glutamate substantially activating fast, classical responses will also activate slow pedestal currents, if present. Pedestal currents do not require excessive liberation of glutamate and are slow principally because of slow receptor kinetics.
Glutamate uncaging bypasses presynaptic release, so we sought to identify pedestal responses according to physiological synaptic function. Recording excitatory postsynaptic currents (EPSCs) in CA1 pyramidal cells following electrical stimulation of the Schaffer collateral (SC) is one of the most commonly performed experiments in cellular neuroscience. We hypothesized that the pedestal currents should appear in such recordings, even though their intensity might be different, because this experiment entails presynaptic plasticity across multiple connections. A similar proportion of neurons developed a pedestal current when SC electrical stimulation was employed (Figure 1F) to the fraction of neurons showing pedestal responses in uncaging experiments. Profound glutamate release was not needed to generate the pedestal response, because minimal stimulation (with a 10%–20% proportion of failures) could still generate a pedestal response when averaging across traces (Figure S4).
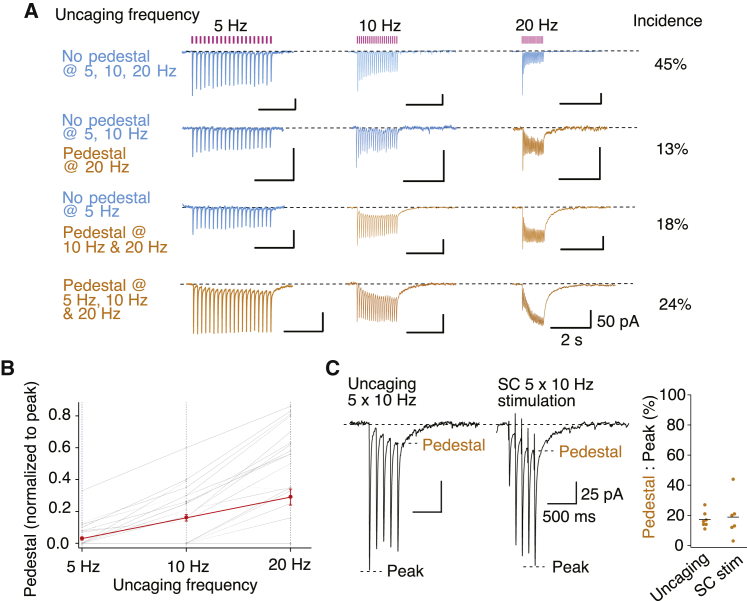
The slow pedestal current becomes apparent with repetitive stimulation, but over what range of frequencies? Uncaging with 5, 10, and 20 Hz trains in CA1 pyramidal cells elicited a spectrum of slow currents (Figure 2A). At 24% of the sites examined, pedestal responses were present at all frequencies, and at 13% of the sites, pedestal responses were only revealed at 20 Hz. There remained 45% of sites for which responses matched canonical AMPA receptors: no pedestal response was observed at any frequency. At sites that showed pedestal currents, the amplitude of the pedestal response increased with stimulation frequency, indicating a general property to report activity in a linear way (Figure 2B). Although we generally used trains of 20 stimuli, the pedestal response usually developed substantially at the start of the train. Consistent with this, 5 pulse trains showed a reliable pedestal current with both uncaging and SC stimulation (Figure 2C).
Figure 2.
Frequency and stimulus dependence of pedestal currents
(A) Uncaging at 5–20 Hz in CA1 pyramidal cells in organotypic slice cultures reveals nearly half of sites have no pedestal response, about a quarter show a prominent pedestal response at all frequencies, and another quarter show an intermediate, frequency-dependent response.
(B) Summary of the pedestal magnitude across 33 uncaging sites from 4 neurons.
(C) In cases of an identified pedestal response, 5 pulse stimulation, either with uncaging (n = 7 sites) or with Schaffer collateral stimulation (n = 6 cells) at 10 Hz, generates a similar pedestal level of about 20% and an anomalous slow decay.
The pedestal current often accumulated during a train, but even more striking was the tail current at the end of stimulation. For amplitude-matched responses from the same cell, the decay of the mixed fast and pedestal responses at the end of the 20 pulse, 10 Hz uncaging train was slower (weighted time constant = 180 ± 30 ms) than for their canonical counterparts (24 ± 4 ms; Figures 3A and 3B). The slow component of the decay for pedestal responses was 440 ± 50 ms, contributing on average 35% ± 4% of the decay. The fast component of the fits to canonical responses (14 ± 1.5 ms, 92% amplitude; Figure S5) corresponded well to previous reports of evoked AMPA receptor EPSCs in CA1 pyramidal cells (11.5 ms) (Tomita et al., 2005). At sites identified to give pedestal responses with 10 Hz uncaging stimulation, the decay of the response to an individual uncaging stimulus was also slower (41 ± 8 ms versus 15 ± 1 ms at sites without pedestal; p of no difference from Student’s t test was 0.006; Figures 3C and 3D), showing that slow AMPA receptors contribute to the decay. The rise time of these responses was the same at each site (Figure 3D).
Figure 3.
The kinetic fingerprint of pedestal responses
(A) Slow tail current follows 10 Hz uncaging at pedestal sites in CA1 pyramidal neurons in organotypic slice cultures, whereas responses at no-pedestal sites have fast decay kinetics like canonical AMPA currents.
(B) Distribution of weighted time constants fitted to the decay after the final pulse following 10 Hz uncaging.
(C) At sites identified to give pedestal currents, the decays of responses to single uncaging events are slower, whereas rise times were indistinguishable.
(D) Distribution of weighted time constants fitted to the current decay after a single uncaging stimulus. The distribution of rise times, which did not change, is also shown.
(E) In neurons showing a pedestal response, a slow tail current followed 10 Hz SC stimulation, whereas responses at no-pedestal cells had fast decay kinetics like canonical AMPA currents.
(F) Distribution of weighted time constants fitted to the decay after the final response following 10 Hz SC stimulation.
(G) Comparison of decays after pedestal responses generated by 5 stimuli at 10 Hz (see Figure 2C).
(H) Dentate gyrus granule cells have no pedestal currents. Tiled fluorescence micrograph of uncaging sites with color-coded recordings of currents driven by 10 Hz uncaging, composited with a differential interference contrast image of the granule cell bodies.
(I) Pedestal responses are absent and decays following uncaging stimuli are universally fast, matching canonical AMPA responses.
(J) Comparison across neuronal types and stimuli, showing the distinction between fast (no pedestal) decays and the slow pedestal response decays, which depend on stimulus intensity.
The kinetics of the decay following Schaffer collateral stimulation (20 × 10 Hz) in organotypic slice cultures were remarkably similar to those obtained with uncaging (Figures 3E and 3F). Cells for which no pedestal current was evoked had responses with a weighted decay constant of 19.6 ± 2.3 ms. In contrast, pedestal responses had on average a longer weighted decay constant of 150 ± 30 ms because of a slow component of 500 ± 80 ms that contributed 73% ± 4% of the decay (Figure S5). Consistent with the receptors underlying the pedestal response being driven into high activity states by activation (Carbone and Plested, 2016), the deactivation decay of pedestal responses after 5 stimuli at 10 Hz (either from uncaging or SC stimulation) (Figure 2C) was intermediate to that from single and 20 stimuli (Figure 3G). The weighted decay time constants were 90 ± 17 ms for 5 uncaging pulses and 93 ± 12 ms for 5 SC stimuli (n = 7 sites and 6 cells, respectively; p of no difference from Student’s t test was 0.9).
To investigate whether the pedestal response depended on neuronal morphology and expression of particular receptor types, we performed 10 Hz uncaging experiments in dentate gyrus granule cells in the same organotypic slice culture system we used for other experiments. To our surprise, the pedestal response was absent (Figure 3H). Across 62 sites in 11 granule cells, we recorded highly stereotypical depressing responses with no buildup of steady-state current. The decay of the last response to 20 stimuli at 10 Hz was uniformly fast (average weighted decay constant = 11 ± 0.5 ms; Figure 3I) and similar to the decay of the response to a single uncaging stimulus at non-pedestal sites in CA1 pyramidal cells (Figure 3J). This result suggests that pedestal currents are regulated by the expression of particular auxiliary proteins in distinct cell types but also provides a benchmark for our system and overall approach.
Quinoxaline dione antagonists act as partial agonists on AMPA receptors endowed with auxiliary proteins (Menuz et al., 2007). NBQX, likely the most commonly employed AMPA receptor antagonist (Sheardown et al., 1990), is less effective on receptors in complex with γ-2 (MacLean et al., 2014; Devi et al., 2016) than on AMPA receptors not complexed with TARPs. In uncaging experiments, we first confirmed that classical responses were fully blocked by NBQX (data not shown; n = 5). However, at pedestal sites, NBQX (10 μM) only inhibited the pedestal current by 40% while almost abolishing the fast peak component (Figure 4A). To confirm that the pedestal currents derive from AMPA receptors, we added the AMPA receptor selective non-competitive antagonist GYKI 52466 (Donevan and Rogawski, 1993) at the end of the recording, which blocked the pedestal response. We repeated these experiments with electrical stimulation and found largely similar results (Figure 4B). We noticed during wash-in of 10 μM NBQX that the fast (canonical) response was inhibited first and the pedestal current was inhibited subsequently. Consistent with less glutamate acting in competition following axonal stimulation than in uncaging, only a lower concentration of NBQX (1 μM) could spare the pedestal current generated by electrical stimulation while still inhibiting the early, fast component. In separate uncaging experiments, application of GYKI 52466 (100 μM) blocked both fast and pedestal responses (Figure S6). The slow current at sites showing a pedestal response was also resistant to N-acetyl-spermine (Figure S6). The fast component at pedestal sites and canonical fast AMPA responses showed variable sensitivity to N-(3-((4-((3-aminopropyl)amino)butyl)amino)propyl)-2-(naphthalen-1-yl)acetamide (NASPM), ranging from no inhibition to 40% reduction at some sites. This result is consistent with previous work reporting occasional rectifying responses to 2-photon uncaging in CA1 pyramidal cells (Soares et al., 2013). Nonetheless, these results suggest the AMPA receptor complexes that generate slow pedestal currents contain GluA2 and thus are unlikely to flux substantial calcium.
Figure 4.
Pedestal currents have aberrant AMPA receptor pharmacology
(A) In CA1 pyramidal cells in organotypic slice cultures, steady-state (pedestal) currents that develop during a 20 × 10 Hz uncaging stimulation are selectively spared by NBQX (10 μM), compared with the peak response to the first stimulation, but abolished following addition of GYKI 52466 (100 μM).
(B) Equivalent experiment to (A) but with stimulation of SC gave similar results with NBQX (1 μM).
(C) Peak and steady-state currents from heteromeric GluA1:A2 receptors are inhibited by GYKI (100 μM) and NBQX (3 μM) in a voltage-independent manner. Auxiliary proteins γ-2 and γ-8 each endow heteromeric AMPA receptors expressed in HEK293 cells with steady-state pedestal responses that are spared by NBQX (3 μM) but abolished by GYKI (100 μM). Note the slow superactivation in the γ-8 example. Recordings were made at +50 mV to exclude GluA1 homomeric receptors not complexed by TARPs, which are blocked at this voltage by 50 μM spermine.
What could the composition of an AMPA receptor with this unexpected pharmacology be? To answer this question, we performed fast perfusion electrophysiology experiments on defined combinations of AMPA receptor subunits in heterologous expression. In these experiments, we recorded at +50 mV in the presence of 50 μM spermine, a condition designed to exclude homomeric GluA1 receptors lacking TARPs (Carbone and Plested, 2016). A1A2R heteromers lacking TARPs showed robust inhibition by NBQX and GYKI at both +50 and −60 mV. Recordings from patches containing A1A2R plus γ-2 or γ-8 gave a similar mix of a canonical fast response and a slow steady-state current, reminiscent of the pedestal current in neurons. NBQX (3 μM) selectively inhibited the fast peak current while sparing or even boosting the slow pedestal response to the extended glutamate application, whereas mirroring our results in pyramidal cells (Figure S6), a separate application of GYKI 52466 (100 μM) inhibited both components readily (Figure 4C). These results suggest that auxiliary proteins, including but perhaps not limited to γ-2 and γ-8, can endow AMPA receptors with slow pedestal activity and NBQX resistance.
To understand the influence of the pedestal expression on CA1 pyramidal cell activity, we made current-clamp recordings (Figure 5). Following a 10 Hz train stimulation to determine whether a putative synaptic connection had a pedestal response, we switched to current-clamp mode and repeated the stimulation. Comparison between non-pedestal and pedestal sites with matched response amplitudes revealed a massive increase in charge transfer (+146% ± 10%) (Figure 5). Accordingly, uncaging at these sites in current-clamp mode had a high probability of triggering an action potential (Figure 5) that was correlated to the magnitude of the pedestal response (r2 = 0.64). The mean frequency of spiking during a 10 Hz train was 32% (n = 12 pedestal sites), suggesting strong coupling that should be robust in the face of low release probability. In contrast, uncaging at single sites lacking a pedestal response almost never evoked an action potential (Figure 5), as expected. We confirmed the specificity of this coupling by addition of GYKI 52466 at the end of the experiment, which blocked plateau depolarization and evocation of action potentials (Figure 5E).
Figure 5.
Pedestal connections are instructive, providing a large depolarizing drive
(A) Pedestal responses produced by 10 Hz uncaging in CA1 pyramidal cells in organotypic slice cultures evoke charge transfer of increased amplitude and duration (shaded region).
(B) At amplitude-matched sites in the same cell, pedestal responses produce about 3 times more charge transfer in response to 10 Hz train stimulation. Values in picoampere milliseconds are equivalent to nanocoulombs (nC).
(C) Current-clamp recordings (upper traces) show that uncaging at pedestal sites reliably triggers action potentials, whereas similar amplitude canonical responses almost never do. Lower traces are the responses of the same sites in voltage clamp.
(D) Relation between pedestal magnitude and reliability of action potential firing during a train. The large blue circle indicates 17 classical sites where zero action potentials were fired from 10 Hz stimulation. The coefficient of determination (r2) for the line fitted to pedestal responses was 0.64.
(E) Application of GYKI 52466 abolished both uncaging excitatory postsynaptic potentials (EPSPs) and consequent firing of spikes.
A concern from these uncaging experiments might be that excessive glutamate is liberated over a wide volume. We expect that AMPA receptors are concentrated at synapses. However, we could conceivably have obtained pedestal currents by activating a special class of receptors that are either excluded systematically from synapses or distributed widely, including substantial populations distant from glutamate release sites. These receptors might therefore avoid native synaptic release. Unlikely as this scenario is, we sought to confirm directly that slow AMPA receptors were located at synapses and activated even by spontaneous release. Although two-photon uncaging is the gold standard for releasing glutamate in the most accurate way, the volume liberated is still large compared with vesicular release, and such experiments would not confirm that synaptically released glutamate activates slow AMPA receptors.
To address synaptic localization and activation of slow AMPA receptors directly, we recorded miniature EPSCs (mEPSCs) in naive CA1 pyramidal neurons for 5 min before performing our normal scan of 10–20 putative synaptic sites with glutamate uncaging at 10 Hz. This scan allowed us to characterize the prevalence of pedestal responses into three categories: no pedestal, weak pedestal, and strong pedestal (see the legend to Figure 6 for criteria). Individual miniature currents (minis) showed a range of 90%–10% decay times (from around 2–50 ms), but the distribution of decay times was different across the different categories. In cells classified as non-pedestal, miniature ESPCs had a fast decay typical of classical AMPA receptors (mean weighted tau = 4.7 ± 0.5 ms, n = 12 cells; Figure 6B), whereas in cells where we could identify strong pedestal currents, decays were markedly slower (mean weighted tau = 9.5 ± 1 ms, n = 10 cells; p of no difference from no pedestal by Dunnett’s multiple comparison test was 0.01). In cells classified as weak pedestal, the decay time constant was 6.3 ± 0.7 ms (n = 6 cells; p of no difference from no pedestal was 0.7). These decays are faster than those following uncaging and Schaffer collateral stimulation, probably because such stimulation activates multiple sites asynchronously, entailing more free glutamate overall compared with spontaneous release. Overexpression of γ-8, the most abundant TARP in the hippocampus (Tomita et al., 2003), by single-cell electroporation further lengthened mEPSC decays (weighted decay tau = 18 ± 3 ms, n = 4 cells; p of no difference from no pedestal was 5 × 10−6; Figure 6B).
Figure 6.
Pedestal currents correspond to slow individual miniature synaptic currents
(A) Experimental design. Miniature currents were recorded for >5 min in a naive cell in organotypic slice cultures, preceding an uncaging survey to classify the pedestal prevalence.
(B) Aligned miniature currents from 4 exemplary cells. Each average miniature current was constructed from (left to right) 63, 88, 129, and 85 minis, respectively.
(C) Uncaging surveys from 12 sites for each cell in (B). Cells that had 2 or more sites with <20% normalized pedestal amplitude were classified as weak pedestal (incidence range = 13%–33%), and cells with 2 or more sites with >20% pedestal amplitude were classified as strong pedestal (incidence range = 17%–50%).
(D) Distributions of fitted 90%–10% decay times of individual miniature currents. Each column represents a cell classified according to the post hoc uncaging results, and each point is the decay time of an individual current. Black bars are the mean values. Numbers of miniature currents averaged in each group are as follows (left to right): no pedestal, 63, 91, 141, 208, 315, 454, 116, 31, 73, 33, 135, 49; weak pedestal, 26, 361, 36, 88, 37, 32; pedestal, 148, 37, 10, 129, 49, 24, 107, 35; γ-8 overexpression, 101, 26, 85, 14.
(E) Summary of miniature currents with decays longer than 20 ms. Increasing intensity of pedestal currents correlates with detection of more miniature currents with long decays (r2 = 0.67).
(F) Miniature current amplitudes according to the same classification. Each point represents the amplitude of an individual miniature current, bars are averages (no pedestal, 13 ± 2 pA; weak pedestal, 9.8 ± 0.5 pA, p versus no pedestal = 0.75; strong pedestal, 17 ± 2 pA, p versus no pedestal = 0.49; γ-8 overexpression, 17 ± 3 pA, p versus no pedestal = 0.7), and the number of cells in each group is indicated in brackets.
Pedestal cells have a mixture of fast and slow sites, but a key expectation if slow AMPA receptors are involved in synaptic transmission is that in cells with more pedestal sites, the proportion of miniature currents with a long decay should increase. We plotted the fraction of miniature currents with a 90%–10% decay time > 20 ms (on average, 85% ± 4% of minis were faster than this cutoff under the no-pedestal condition), which correlated well with the prevalence of pedestal currents from our survey (r2 = 0.67; Figure 6E). This experiment indicates that receptors with slower kinetics participate in individual synaptic currents, suggesting they have a similar synaptic localization to classical AMPA receptors. We observed large miniature EPSCs in cells that had strong pedestal prevalence (Figure 6F), but comparing mean amplitudes across cells revealed no effect. The mean amplitude of minis in strong pedestal cells was 17 ± 2 pA, compared with 13 ± 2 pA for cells without the pedestal condition (p of no difference was 0.49).
To understand which TARPs determine pedestal responses, we performed further single-cell electroporation experiments in CA1 pyramidal neurons in organotypic slice cultures. The principal advantage of this method is that it allowed us to use both wild-type and gating-deficient TARPs that associate normally into receptor complexes (Riva et al., 2017) to perturb synaptic transmission, without the confounding effects of affecting trafficking of receptors to synaptic sites. This approach also avoids large-scale perturbations and subsequent homeostasis from deleting TARPs in knockout mice. Strikingly, overexpression of γ-8 increased the prevalence of pedestal currents in transfected neurons to ∼100%, which inevitably increased the mean normalized pedestal response (Figure 7B; p of no difference to control was 2 × 10−6).
Figure 7.
Overexpression of γ-8 converts almost all synaptic responses to pedestal responses
(A) Glutamate uncaging at 10 Hz at 9 sites on a neuron electroporated with the auxiliary subunit γ-8. In the example shown, all sites tested exhibited pedestal responses, although the magnitude of the pedestal response at site 9 was small. Tiled micrograph of Alexa Fluor 594 fluorescence.
(B) Overexpression of γ-8 increased the fraction of pedestal responses to 95% (n = 4 cells) and significantly increased the mean pedestal level (Dunnett’s test for multiple comparisons). Overexpression of dominant-negative forms of gamma-8 and gamma-2 eliminated large pedestal responses but did not change the mean pedestal response, because the fraction of sites giving no pedestal response was reduced.
(C) Gamma-8 overexpression slowed the pedestal rise time, whereas dominant-negative TARP expression sped it up. Each point represents a single response to uncaging.
(D) Pedestal current off-kinetics were almost insensitive to TARP overexpression.
Overexpression of γ-8 increased the amplitudes of peak currents evoked by uncaging (Figure S7; p of no difference to control was 0.004), consistent with receptors not being saturated by γ-8 in control conditions. These observations confirm, from the specificity of γ-8, that pedestal currents and slow mEPSC decays result from AMPA receptors. In contrast, dominant-negative mutants of γ-2 and γ-8 (Riva et al., 2017) that show reduced modulation of receptor gating did not change the distribution of the pedestal overall (Figure 7B; p of no difference to control > 0.9 in both cases), even though pedestal currents became somewhat more prevalent.
Pedestal responses did not have uniform kinetics. At 84% of sites in control conditions, the pedestal response developed slowly with repetitive 10 Hz uncaging, but at 16% of sites, the slow current was instant and did not augment during the train (Figure S7). Across 79 sites, the onset of the pedestal during 10 Hz uncaging was well fit by a single exponential function with a mean time constant of 550 ± 60 ms. Overexpression of γ-8 by single-cell electroporation in organotypic slice cultures slowed this onset time constant (800 ± 100 ms) (Figure 7C; p of no difference to control was 0.026). In contrast, overexpression of null γ-2 and γ-8 mutants made pedestal onset faster (140 ± 30 ms at 24 sites and 280 ± 50 ms at 37 sites for null γ-2 and γ-8, respectively) while reducing its amplitude (from 61 ± 6 pA in control conditions to 31 ± 6 pA for null γ-2 and to 18 ± 2 pA for null γ-8; p of no difference to control was 0.02 and 0.00003, respectively).
Expression of the dominant-negative γ-8 construct reduced the peak amplitude of the entire response evoked by uncaging (from 111 ± 5 pA, n = 281 sites, in control conditions to 54 ± 5 pA, n = 80 sites; p of no difference from control was 4 × 10−6; Figure S7), similar to experiments from γ-8 knockout mice (Rouach et al., 2005), and suggested that modulation of receptor gating by γ-8 may play a role in synaptic current amplitudes. Similar electroporation experiments for overexpression of wild-type γ-2 and wild-type CNIH2 yielded inconclusive results. A low success rate of these electroporation experiments suggested these auxiliary proteins might be cytotoxic to CA1 pyramidal neurons when overexpressed. Overall, these overexpression experiments revealed a striking role for γ-8 in specifying pedestal responses and show that the pedestal is perturbed, but not abolished, by dominant-negative TARPs, strongly suggesting the involvement of multiple types of auxiliary proteins in slow AMPA responses in CA1 pyramidal cells.
Discussion
Our isolation of desensitization-resistant slow AMPA receptors, likely associated with γ-2, γ-8, or other auxiliary subunits, suggests that a subset of extremely powerful excitatory inputs is present in hippocampal CA1 and CA3 pyramidal cells. Previous work suggested that TARPs are ubiquitously expressed in CNS neurons (Tomita et al., 2003), and that TARP modulation of gating is widespread (Menuz et al., 2007). Therefore, slow AMPA currents are likely found elsewhere in the hippocampus and cortex. However, TARP expression is not a simple determinant of pedestal responses. Dentate granule cells, which transiently express AMPA receptors with reduced desensitization (Schmidt-Salzmann et al., 2014) and have abundant expression of γ-8, appear to lack these slow currents (Figure 3H). With higher-frequency stimulation than used here, slow AMPA currents were also reported in cerebellum (Devi et al., 2016; Lu et al., 2017) and calyx of Held (Taschenberger et al., 2002). An obvious question is, if pedestal currents are so prominent in hippocampal pyramidal cells, why were they not previously reported? To some extent, the right experiments (involving repetitive stimulation) were not done. More importantly, we fear that the literature is biased by reports of synaptic responses that fit best the canonical view of the AMPA receptor as a purely fast ion channel receptor. It is possible that experiments in which slow responses were observed were systematically discarded (for example, CA1 pyramidal cell responses from SC stimulation), presuming errors in space clamp. Selectively omitting these cells (Figure 1F; one-third of cells have a pedestal response with 10 Hz stimulation) would lead to a general underestimate of the relation between synaptic input and pyramidal CA1 cell excitability.
Slow receptors not only resist desensitization but also produce responses with slow decays, about 100-fold slower than canonical AMPA responses. The fast component of the decay for a single uncaging stimulus (80% ± 6% on average) at a no-pedestal site was about 5 ms, and the slow component after a 10 Hz train (on average 35% ± 4% for uncaging and 74% ± 6% for SC simulation) was on average about 500 ms. This slow decay is not due to an accumulation of glutamate associated with an overload of glutamate transporter capacity, because it was similar at room temperature and at 32°C when transporters should be more effective (Figure S4). Therefore, it appears that slow pedestal responses come from receptors with a higher affinity for glutamate than a classical fast AMPA receptor. This observation may be related to the apparently higher affinity for glutamate of receptors in complex with TARPs (Coombs et al., 2017). However, the previously measured increases in apparent affinity did not isolate slow decay components and are therefore likely underestimates. The decay kinetics following trains of stimuli are congruent between uncaging and SC stimulation (Figure 3J), and these decay kinetics were robust to overexpression of different TARPs (Figure 7). However, several observations support the idea that CA1 pyramidal neurons express more than one type of slow AMPA receptor complex. The onset of the slow pedestal component had a range of kinetics, and overexpression of γ-8 altered this kinetic profile differently from null TARP mutants. Experiments with different stimulation frequencies (Figure 2A) revealed sites that gave pedestal responses already at 5 Hz stimulation, whereas others only produced a pedestal response at 20 Hz. The slow current response in NBQX developed rapidly for γ-2 (Figure 4C); for A1A2R γ-8 complexes, the slow current developed slower than in CA1 pyramidal cells. When using repetitive 1 ms pulses of 10 mM glutamate in fast perfusion experiments, the slow response developed even more slowly. This observation suggests that complexes that produce pedestal currents are formed with more different mixtures of GluA and TARP subunits than we have so far examined. In turn, we speculate that subcellular expression of these complexes permits distinct mixtures of superactivation and desensitization (Carbone and Plested, 2016) and frequency-dependent sensitivity of the post-synaptic response. Although several factors, including synapse geometry and glutamate clearance, may contribute to the slow decay, the slow AMPA receptors we describe here are essential to produce this variability and a slow synaptic response.
The involvement in synaptic transmission of pedestal responses because of AMPA receptors with high affinity for glutamate challenges some fundamental ideas about glutamatergic synapses. Receptors with higher glutamate affinity that do not desensitize need not be directly opposed to vesicle release sites (Raghavachari and Lisman, 2004), making synaptic architecture more flexible. Synaptic currents generated by such complexes thus have diluted dependence on receptor diffusion (Heine et al., 2008) and clustering (Savtchenko and Rusakov, 2013). At the same time, pedestal currents are more potent at depolarizing target neurons than classical fast responses, because they derive from channels with larger mean conductance and slow deactivation. These slow kinetics match the observations of modal gating for AMPA receptor-TARP tandem complexes in heterologous expression, in which long-lived bursts of single-channel openings up to 700 ms long are reported that often outlive the agonist application (Zhang et al., 2014). Our results urge further care in the interpretation of results in which quinoxaline dione antagonists are employed to block AMPA receptors (Menuz et al., 2007; Cossart et al., 2002). In particular, some experiments involving the long-term application of quinoxaline dione antagonists to block AMPA receptors might require re-evaluation.
Considering mechanisms of short-term synaptic plasticity, the progressive augmentation of slow AMPA receptor currents is a distinct postsynaptic complement to classical descriptions of potentiation with repetitive activity that occurs due to presynaptic calcium accumulation (Katz and Miledi, 1968). Pedestal currents represent a long-sought short-term potentiation mechanism from a purely postsynaptic locus, with a similar wide dynamic range to presynaptic adaptation (Zucker and Regehr, 2002). In particular, postsynaptic neurons can trivially implement diversity in the synaptic response in an autonomous manner. We speculate that such diversity can be regulated by the relative expression of pedestal currents on a per-synapse basis (Figure 1). Instant, long-lasting depolarizations from a single input have substantial implications for long-term plasticity induction and synaptic integration (Remy and Spruston, 2007). Likewise, the slow AMPA currents offer a complement to NMDA receptors as temporal integrators, with some favorable properties. Our results indicate that pedestal responses should not necessarily be directly associated with a high calcium influx but might allow NMDA-receptor-independent plasticity to occur.
Inhibition by GYKI 52466 indicates that slow pedestal currents are generated from AMPA receptor subunits. Therefore, slow pedestal currents are likely regulated by long-term plasticity (Nicoll, 2017; Diering and Huganir, 2018) and homeostatic plasticity mechanisms (Turrigiano, 2008), but the biochemical and biophysical interplay may be distinct from that of canonical fast AMPA currents. The consensus view that many or all synaptic AMPA receptors are decorated by auxiliary proteins may require revision, because neither the pharmacology of the fast responses we observed nor the effect of γ-8 overexpression fit well to the idea that all receptors are fully clad with TARPs. Evidence for synaptic receptors lacking TARPs was shown under knockout conditions in cerebellar cells (Bats et al., 2012), and further work will be needed to assess the situation in other brain regions. The detonator function of connections in which slow AMPA receptors are found is likely more general than the previous example of the mossy terminal (Henze et al., 2002). The strong depolarizing drive has knock-on effects for the excitability of cells where pedestal responses are found, with downstream effects for network function. Finally, it is intriguing to speculate as to whether pedestal currents participate in feature recognition (Bittner et al., 2015) or enable particular inputs to rapidly instruct cellular responses to stimuli or environments, such as conversion into place cells (Epsztein et al., 2011).
Limitations of the study
Although with GYKI 52466 we could abolish the pedestal response, dominant-negative TARP constructs did not abolish slow currents and instead only speed up their onset kinetics. Therefore, a role for other auxiliary proteins in slow responses appears likely, but we did not address this point. Our pharmacological experiments in HEK293 cells demonstrating that γ-2 and γ-8 confer selective resistance to NBQX, but not GYKI, used a simpler stimulation compared with the one we employed in slice cultures and acute slices. Our data showing that γ-8 overexpression converts almost all uncaging sites to pedestal sites do not guarantee that γ-8 is responsible for slow responses in native conditions. In dentate gyrus granule cells, γ-8 expression is high, and another auxiliary subunit could be blocking expression of slow AMPA receptor responses, but this study did not address this point. Finally, our data do not provide information about the subunit composition of the AMPA receptors involved in slow pedestal currents beyond the likely involvement of GluA2. Preferential incorporation of subunits GluA1, GluA3, or GluA4 might preclude or promote slow activation, which will require further investigation.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Chemicals, peptides, and recombinant proteins | ||
| Tetrodotoxin (TTX) | Tocris | Cat#1078 |
| D-AP5 | Tocris | Cat#0106 |
| Gabazine | Tocris | Cat#1262 |
| UBP-310 | Tocris | Cat# 3621 |
| NBQX disodium salt | HelloBio | Cat#HB0443 |
| GYKI 52466 | AbCam | Cat#ab146716 |
| NASPM trihydrochloride | Bio-techne | Cat# 2766 |
| Alexa Fluor-594 Hydrazide | ThermoFisher | Cat#A10438 |
| (+)-MK 801 maleate | AbCam | Cat#ab120027 |
| CGP 55845 Hydrochloride | Sigma Aldrich | Cat#SML0594 |
| (RS)-MCGP | Tocris | Cat#0336 |
| MNI-caged-L-Glutamate | HelloBio | Cat#HB0423 |
| Experimental models: Cell lines | ||
| HEK293 cells | Leibniz Institute German Collection of Microorganisms and Cell Cultures | CVCL_0045 |
| Experimental models: Organisms/strains | ||
| Mouse C57BL/6J | Jackson Laboratory | N/A |
| Recombinant DNA | ||
| γ-8-DsRed-Max-N1 pRK5 | Roger Nicoll, Carbone and Plested (2016) | N/A |
| Null γ-8 (γ2-in-γ8-chim1-short-ECS2) DsRed Max N-1 pRK5 | Riva et al. (2017) | N/A |
| Null γ-2 (Stg-ECS1-del-ECS2-mut) DsRed Max-N1 pRK5 | Riva et al. (2017) | N/A |
| Software and algorithms | ||
| Axograph | Axograph | SCR_014284 |
| pClamp | Molecular Devices | SCR_011323 |
| IGOR Pro 8 | WaveMetrics | RRID: SCR_000325 |
| Fiji | ImageJ | RRID: SCR_003070 |
| Excel | Microsoft | RRID: SCR_016137 |
| SysCon | Rapp Optoelectronic | - |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Andrew Plested (andrew.plested@hu-berlin.de).
Materials availability
This study did not generate new unique reagents.
Experimental model and subject details
Animals
Animals were maintained in compliance with the EU Legislation on the protection of animals used for scientific purposes and were approved by Landesamt für Gesundheit und Sociales Berlin (LaGeSo).
Organotypic Slice culture preparation
350 μm-thick organotypic hippocampal slice cultures were prepared from P6 to P9 WT C57 mice of either sex. Slices were prepared on filter paper according to the interface method (Stoppini et al., 1991; De Simoni and Yu, 2006) and cultured in a MEM-based mouse slice culture medium, with the addition of 15% Horse Serum; 1x B27; 25 mM HEPES; 3mM L-Glutamine; 2.8 mM CaCl2; 1.8 mM MgSO4; 0.25 mM Ascorbic Acid; 6.5 g/L D-Glucose. 3 days after plating, the medium was replaced and then exchanged every 4 days. Cultures were grown in an incubator with 5% CO2 at 34°C.
Preparation of acute hippocampal slices
Hippocampal acute slices were obtained from postnatal day P9–P80 mice of both sexes, using a standard protocol (Papouin and Haydon, 2018). Briefly, after cervical dislocation, the brain was quickly removed from the skull and placed in ice-cold slicing solution (aCSF) containing (in mM): 10 Glucose, 125 NaCl, 1.25 NaH2PO4, 2.5 KCl, 26 NaHCO3, 2 MgCl2, 1 CaCl2, saturated with 95% O2 and 5% CO2, pH 7.3–7.4. Transverse hippocampal slices (300 μm thick) were cut with a Leica VT 1200S vibratome and stored at room temperature in a holding bath containing the same solution as above. After incubation for at least 1 h, an individual slice was submerged in the recording chamber and continuously superfused at a rate of 5 ml/min with oxygenated experimental ACSF containing (in mM): 10 Glucose, 125 NaCl, 1.25 NaH2PO4, 2.5 KCl, 26 NaHCO3, 1 MgCl2, 2 CaCl2, saturated with 95% O2 and 5% CO2, pH 7.3–7.4.
HEK293 cell cultures
HEK293 cells were obtained from the German Collection of Cell cultures and micro-organisms and tested negative for mycoplasma. They were cultivated with Minimum Essential Media (PAN Biotech GmbH) supplemented with 5%–10% FBS, and were maintained at 37°C, 95% air and 5% CO2 in a humidified incubator.
Method details
Single cell electroporation
Single cell electroporation (SCE) was performed at 15-16 days of slice culture (Wiegert et al., 2017). Visually identified CA1 pyramidal neurons were transfected by single cell electroporation. Slices were placed in the microscope chamber in the presence of 3-4 mL sterile pre-warmed (34°C) HEPES-based artificial cerebrospinal fluid (aCSF) containing 145 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES and 10 mM glucose, adjusted to 310 mOsm /l and pH 7.3 with NaOH at room temperature. Borosilicate glass pipettes were filled with intracellular solution containing 135 mM K·CH3SO3, 4 mM NaCl, 2 mM MgCl2, 2 mM Na2ATP, 0.3 mM Na2GTP, 0.06 mM EGTA, 0.01 mM CaCl2, 10 mM HEPES, adjusted to 300 mOsm/l and pH 7.2-3.
For SCE experiments, cDNA was added to the intracellular solution the day of the experiment at a concentration of 4 ng/μl. A pipette with resistance in saline of 8-11 MΩ was brought to the cell body and held in loose-cell attached configuration. DNA was delivered into the target neuron through stimulation of 500 ms at 50 Hz with a pulse amplitude of −10 to −12 V, using an Axoporator 800A (Axon Instruments). After transfection, slices were placed back into the incubator, and the culture medium was enriched with 10 μg/ml Gentamicin. Transfected cells were recorded 24 to 48 hours after electroporation.
Electrophysiology and glutamate uncaging in organotypic hippocampal cultures and acute slices
Somatic whole cell patch clamp recordings of visually-identified CA1 and CA3 principal cells, and dentate gyrus granule cells, were performed after 16-23 days of slice culture. Microelectrodes (3-8 MΩ tip resistance) were prepared from borosilicate glass capillaries (1B150F-4 World Precision Instruments) using a Sutter P-1000 puller. Slices were superfused with recirculating aCSF (5 mL/min at room temperature) containing 4-methoxy-7-nitroindolinyl-glutamate (MNI-caged-L-Glutamate; HelloBio HB0423) at a concentration of 0.5 mM and used for the whole day of recordings.
Glutamate was uncaged by a 405 nm laser diode (one-photon excitation) mounted on a custom-built upright microscope (Scientifica SliceScope). The collimated beam was directed through a UGA-42 Firefly laser scanner (Rapp Optoelectronic GmbH), and directed through a Zeiss epifluorescence reflector (Examiner A1) into the water dipping 60x objective (Olympus LUMPlanFL N; N.A. 1). The uncaging laser and Jenoptik ProgRes MF camera were controlled SysCon software (Rapp) and ImageJ (https://imagej.nih.gov/ij/) respectively. After reaching the whole cell modality, we waited several minutes to allow the diffusion of AlexaFluor-594 dye (ThermoFisher; 20 μM, dissolved into the intracellular solution) into the processes of the neuron. Afterward, dendritic regions were selected by illuminating the sample with a 595 nm LED (Thorlabs). UV light pulses (usually 1 ms) were delivered by triggering the laser (usually at 50% power). Simultaneous passage of red emission (600 nm) and 405 nm light for uncaging was achieved by using a 405/488/594nm Laser Triple Band filter set (TRF 69902; Chroma) mounted in a Zeiss TIRF cube. At the end of each experiment, we documented the uncaging sites and their distances from the cell nucleus using a tiled, multifocal plane fluorescence micrograph of the filled neuron, neglecting the z-displacements which were typically small.
For voltage clamp recordings neurons were held at –60 mV (not corrected for the liquid junction potential which was calculated to be –6.6 mV). To monitor the uncompensated series resistance (< 20 MΩ), a hyperpolarizing voltage step (–10 mV, 100 ms) was delivered at the beginning of each recording. Recordings with series resistance changes > 20% were discarded. For the current clamp experiment, the bridge balance compensation was adjusted before the beginning of each recording. Most recordings were done at room temperature (23°C)(Herring et al., 2013), but for some recordings we heated the bath inflow with a TC02 inline heater (Multichannel Systems, Germany), adjusting as needed to produce a final temperature of 32°C in the bath.
To evoke whole cell EPSCs in CA1 principal cells from stimulating the Schaffer Collateral, we used an electrode that had resistance of 1-3 MΩ when filled with aCSF. The stimulating electrode was placed in CA3/CA1 interface at the stratum radiatum to activate Schaffer collateral/commissural afferents (300-500 μm away from the recording electrode). Monopolar stimulation was applied with an Iso-Flex constant-current stimulator (API Instruments, Jerusalem, Israel), and the stimulation trigger (5 or 20 x 1 ms pulses at 10 Hz) was controlled by Axograph software.
For most experiments in slices, drugs were added to the aCSF at the following concentrations: AP-5 (HelloBio; 20 μM), SR-99531 (HelloBio; 10 μM), CGP-55845 (Tocris; 10 μM), (RS)-MCGP (Tocris; 200 μM) and UBP-310 (HelloBio; 10 μM), while MK-801-maleate (HelloBio; 1mM) was added to the intracellular solution. For current clamp recordings the same pharmacological cocktail was used, but we omitted TTX. For the experiments involving stimulation of the Schaffer Collateral, besides the exclusion of TTX from the ACSF, QX-314 (5 mM, HelloBio) was added to the pipette solution.
Data were acquired with a Multiclamp 700B amplifier (Molecular Devices) and digitized at 20 kHz under the control of Axograph (Axograph Scientific).
HEK293 transfection and electrophysiology
Human embryonic kidney (HEK) 293 cells were co-transfected with the AMPAR subunits GluA1 and GluA2 (edited at the Q/R site) plus the auxiliary protein TARP γ-2 or γ-8. γ-2 was transfected using a DNA mass ratio of 1:1:2 GluA1:A2:γ-2, whereas for γ-8 the ratio was 1:1:5 GluA1:A2:γ-8. Patch-clamp experiments in the outside-out configuration were performed 24 hours after transfection. Cells were selected based on simultaneous EGFP and DsRed fluorescence signals indicating co-expression of GluA2 and the TARP respectively. In most experiments, the presence of GluA1 subunits was assessed based on the current-voltage (I-V) relationships showing partial voltage-dependent block by intracellular spermine. The intracellular solution contained (in mM): 120 NaCl, 10 NaF, 0.5 CaCl2, 5 Na4BAPTA, 5 HEPES and 0.05 spermine. The extracellular solution contained (in mM): 150 NaCl, 0.1 MgCl2, 0.1 CaCl2 and 5 HEPES. Both solutions were titrated to pH 7.3. Using a glass tool (Plested and Poulsen, 2021) mounted on a piezo stack (Physik Instrument, Germany) for fast solution application, outside-out patches were held in extracellular control solution and exposed to brief pulses (400-700 ms) of 10 mM glutamate. To test inhibition by antagonists, either 3 μM NBQX or 100 μM GYKI-52466 were added to both control and glutamate barrels; in other words, patches were pre-equilibrated with antagonist before the jump into glutamate. Currents from AMPAR-TARP complexes were acquired at the holding potential of +50 mV. All recordings were filtered with a 5 kHz low-pass filter using an Axopatch 200B amplifier (Molecular Devices, U.S.A.) and sampled at 10 kHz with AxoGraph. Because both NBQX and GYKI abolished the fast peak response to glutamate, we normalized the effects of NBQX or GYKI-52466 on the peak and steady state currents as the ratio of the peak (Ipk, antagonist) or steady-state (Iss, antagonist) response to the mean peak current amplitude (Ipk, control) evoked by glutamate in the absence of antagonist.
Data analysis
Data were analyzed offline with Axograph, Clampfit (Molecular Devices) and IgorPRO 8 (WaveMetrics). All recorded traces were low-pass filtered at 1 kHz. For each uncaging site or whole-cell evoked current, the pedestal value was calculated as the steady-state current (Iss) at the end of the train stimulus, in ratio to the peak current at the 5th or 20th pulse. The Iss was taken to be 0 when smaller than 5 pA. Analysis of spontaneous miniature AMPA-mediated synaptic excitatory currents (mEPSCs) was performed for each recording by generating a template based on the events recorded in the same trace in Axograph. This template was then used to collect the mEPSCs from which we measured amplitudes and decay time (90 to 10% of the peak). Events with an amplitude lower than three times the root mean square (RMS) noise were discarded to reduce false-positive detections. We compared miniature current decays using a bi-exponential fit to the average of aligned miniature currents from each cell, to account fairly for biological variability. For the analysis of the charge transfer, responses with a similar Ipeak at the 20th pulse (113 ± 4 pA for non-pedestal and 112 ± 3 pA for pedestal, n = 6 for both groups) were selected. Similarly, for the firing probability of pedestal versus non-pedestal inputs, inputs were selected so that the evoked amplitude at the 20th pulse was 100 ± 20 pA. For this analysis, only inputs within 70 μm from the cell nucleus were analyzed, in order to minimize spatial filtering effects. Pedestal current on-kinetics were fitted with single exponential functions by masking the fast AMPA component. Weighted time constants were obtained from bi-exponential fits to decays by averaging individual time constants according to their amplitudes.
Quantification and statistical analysis
Graphs are displayed as dot plots with the mean, and/or as individual points with standard deviation of the mean. We did not employ a cut-off for determining statistical significance, rather we provide exact P-values. Two-sided multiple comparisons testing the null hypothesis (either against “no-pedestal” condition or against control conditions) were done with Dunnett’s Multiple Comparison test in IgorPRO. All statistical analyses were carried out using the Igor PRO software. Further statistical details are indicated in the figure legends.
Acknowledgments
We thank Andrew Penn, Mario Carta, and Mauro Pulin for advice on slice culture and electroporation; Marcus Wietstruk for plasmid cloning and helpful discussions; Sabine Fievre and Christoph Mulle for help with initial experiments in CA3 cells; Francesca Logiacco for advice on acute slice preparation; Paul Kammermeier for advice on pharmacology; Mark Mayer, Estelle Toulmé, and Christoph Schmidt-Hieber for comments on the manuscript; and Gert Rapp and for assistance with SysCon software and microscope optimization. N.P.P. was recipient of an EMBO Long Term Fellowship (ALTF 873-2018), and A.L.C. was recipient of a NeuroCure Female Fellowship (EXC-257). This work was funded by the Deutsche Forschungsgemeinschaft (DFG) Heisenberg Professorship (PL619/3-1), DFG RU2518 DynIon (P3; PL619/5-1), DFG under Germany’s Excellence Strategy (EXC-2049-390688087-NeuroCure), and ERC (647895 “GluActive”) (all to A.J.R.P.). This manuscript is dedicated to the memory of James R. Howe, whose generous sharing of data before publication was critical at early stages of this work.
Author contributions
Conceptualization, N.P.P., A.L.C., and A.J.R.P.; methodology, N.P.P., I.R., A.L.C., and A.J.R.P.; investigation, N.P.P., I.R., A.L.C., and A.J.R.P.; writing – original draft, N.P.P. and A.J.R.P.; writing – review & editing, N.P.P., I.R., A.L.C., and A.J.R.P.; funding acquisition, N.P.P., A.L.C., and A.J.R.P.; supervision, A.L.C. and A.J.R.P.
Declaration of interests
The authors declare no competing interests.
Published: August 3, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.celrep.2021.109496.
Supplemental information
Data and code availability
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This study did not generate any code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- Bats C., Groc L., Choquet D. The interaction between Stargazin and PSD-95 regulates AMPA receptor surface trafficking. Neuron. 2007;53:719–734. doi: 10.1016/j.neuron.2007.01.030. [DOI] [PubMed] [Google Scholar]
- Bats C., Soto D., Studniarczyk D., Farrant M., Cull-Candy S.G. Channel properties reveal differential expression of TARPed and TARPless AMPARs in stargazer neurons. Nat. Neurosci. 2012;15:853–861. doi: 10.1038/nn.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittner K.C., Grienberger C., Vaidya S.P., Milstein A.D., Macklin J.J., Suh J., Tonegawa S., Magee J.C. Conjunctive input processing drives feature selectivity in hippocampal CA1 neurons. Nat. Neurosci. 2015;18:1133–1142. doi: 10.1038/nn.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- Carbone A.L., Plested A.J.R. Superactivation of AMPA receptors by auxiliary proteins. Nat. Commun. 2016;7:10178. doi: 10.1038/ncomms10178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo P.E., Malenka R.C., Nicoll R.A. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Cembrowski M.S., Bachman J.L., Wang L., Sugino K., Shields B.C., Spruston N. Spatial Gene-Expression Gradients Underlie Prominent Heterogeneity of CA1 Pyramidal Neurons. Neuron. 2016;89:351–368. doi: 10.1016/j.neuron.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Colquhoun D., Jonas P., Sakmann B. Action of brief pulses of glutamate on AMPA/kainate receptors in patches from different neurones of rat hippocampal slices. J. Physiol. 1992;458:261–287. doi: 10.1113/jphysiol.1992.sp019417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs I.D., MacLean D.M., Jayaraman V., Farrant M., Cull-Candy S.G. Dual Effects of TARP γ-2 on Glutamate Efficacy Can Account for AMPA Receptor Autoinactivation. Cell Rep. 2017;20:1123–1135. doi: 10.1016/j.celrep.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart R., Epsztein J., Tyzio R., Becq H., Hirsch J., Ben-Ari Y., Crépel V. Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron. 2002;35:147–159. doi: 10.1016/s0896-6273(02)00753-5. [DOI] [PubMed] [Google Scholar]
- De Simoni A., Yu L.M. Preparation of organotypic hippocampal slice cultures: interface method. Nat. Protoc. 2006;1:1439–1445. doi: 10.1038/nprot.2006.228. [DOI] [PubMed] [Google Scholar]
- Devi S.P.S., Howe J.R., Auger C. Train stimulation of parallel fibre to Purkinje cell inputs reveals two populations of synaptic responses with different receptor signatures. J. Physiol. 2016;594:3705–3727. doi: 10.1113/JP272415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering G.H., Huganir R.L. The AMPA Receptor Code of Synaptic Plasticity. Neuron. 2018;100:314–329. doi: 10.1016/j.neuron.2018.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGregorio D.A., Rothman J.S., Nielsen T.A., Silver R.A. Desensitization properties of AMPA receptors at the cerebellar mossy fiber granule cell synapse. J. Neurosci. 2007;27:8344–8357. doi: 10.1523/JNEUROSCI.2399-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donevan S.D., Rogawski M.A. GYKI 52466, a 2,3-benzodiazepine, is a highly selective, noncompetitive antagonist of AMPA/kainate receptor responses. Neuron. 1993;10:51–59. doi: 10.1016/0896-6273(93)90241-i. [DOI] [PubMed] [Google Scholar]
- Epsztein J., Brecht M., Lee A.K. Intracellular determinants of hippocampal CA1 place and silent cell activity in a novel environment. Neuron. 2011;70:109–120. doi: 10.1016/j.neuron.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gantz S.C., Moussawi K., Hake H.S. Delta glutamate receptor conductance drives excitation of mouse dorsal raphe neurons. eLife. 2020;9:e56054. doi: 10.7554/eLife.56054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger J.R., Melcher T., Koh D.S., Sakmann B., Seeburg P.H., Jonas P., Monyer H. Relative abundance of subunit mRNAs determines gating and Ca2+ permeability of AMPA receptors in principal neurons and interneurons in rat CNS. Neuron. 1995;15:193–204. doi: 10.1016/0896-6273(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Heine M., Groc L., Frischknecht R., Béïque J.-C., Lounis B., Rumbaugh G., Huganir R.L., Cognet L., Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henze D.A., Wittner L., Buzsáki G. Single granule cells reliably discharge targets in the hippocampal CA3 network in vivo. Nat. Neurosci. 2002;5:790–795. doi: 10.1038/nn887. [DOI] [PubMed] [Google Scholar]
- Herring B.E., Shi Y., Suh Y.H., Zheng C.-Y., Blankenship S.M., Roche K.W., Nicoll R.A. Cornichon proteins determine the subunit composition of synaptic AMPA receptors. Neuron. 2013;77:1083–1096. doi: 10.1016/j.neuron.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S., Sah P., Nicoll R.A. Mechanisms generating the time course of dual component excitatory synaptic currents recorded in hippocampal slices. Neuron. 1990;5:247–253. doi: 10.1016/0896-6273(90)90162-9. [DOI] [PubMed] [Google Scholar]
- Kato A.S., Gill M.B., Ho M.T., Yu H., Tu Y., Siuda E.R., Wang H., Qian Y.-W., Nisenbaum E.S., Tomita S., Bredt D.S. Hippocampal AMPA receptor gating controlled by both TARP and cornichon proteins. Neuron. 2010;68:1082–1096. doi: 10.1016/j.neuron.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The role of calcium in neuromuscular facilitation. J. Physiol. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd F.L., Isaac J.T. Developmental and activity-dependent regulation of kainate receptors at thalamocortical synapses. Nature. 1999;400:569–573. doi: 10.1038/23040. [DOI] [PubMed] [Google Scholar]
- Lu H.W., Balmer T.S., Romero G.E., Trussell L.O. Slow AMPAR Synaptic Transmission Is Determined by Stargazin and Glutamate Transporters. Neuron. 2017;96:73–80.e4. doi: 10.1016/j.neuron.2017.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean D.M., Ramaswamy S.S., Du M., Howe J.R., Jayaraman V. Stargazin promotes closure of the AMPA receptor ligand-binding domain. J. Gen. Physiol. 2014;144:503–512. doi: 10.1085/jgp.201411287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuz K., Stroud R.M., Nicoll R.A., Hays F.A. TARP auxiliary subunits switch AMPA receptor antagonists into partial agonists. Science. 2007;318:815–817. doi: 10.1126/science.1146317. [DOI] [PubMed] [Google Scholar]
- Misra C., Brickley S.G., Wyllie D.J., Cull-Candy S.G. Slow deactivation kinetics of NMDA receptors containing NR1 and NR2D subunits in rat cerebellar Purkinje cells. J. Physiol. 2000;525:299–305. doi: 10.1111/j.1469-7793.2000.t01-1-00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll R.A. A Brief History of Long-Term Potentiation. Neuron. 2017;93:281–290. doi: 10.1016/j.neuron.2016.12.015. [DOI] [PubMed] [Google Scholar]
- Papouin T., Haydon P.G. Obtaining Acute Brain Slices. Bio. Protoc. 2018;8:e2699. doi: 10.21769/BioProtoc.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plested A.J.R., Poulsen M.H. Crosslinking glutamate receptor ion channels. Methods Enzymol. 2021;652:161–192. doi: 10.1016/bs.mie.2021.03.005. [DOI] [PubMed] [Google Scholar]
- Priel A., Kolleker A., Ayalon G., Gillor M., Osten P., Stern-Bach Y. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J. Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavachari S., Lisman J.E. Properties of quantal transmission at CA1 synapses. J. Neurophysiol. 2004;92:2456–2467. doi: 10.1152/jn.00258.2004. [DOI] [PubMed] [Google Scholar]
- Remy S., Spruston N. Dendritic spikes induce single-burst long-term potentiation. Proc. Natl. Acad. Sci. USA. 2007;104:17192–17197. doi: 10.1073/pnas.0707919104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva I., Eibl C., Volkmer R., Carbone A.L., Plested A.J. Control of AMPA receptor activity by the extracellular loops of auxiliary proteins. eLife. 2017;6:e28680. doi: 10.7554/eLife.28680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J.S., Cathala L., Steuber V., Silver R.A. Synaptic depression enables neuronal gain control. Nature. 2009;457:1015–1018. doi: 10.1038/nature07604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N., Byrd K., Petralia R.S., Elias G.M., Adesnik H., Tomita S., Karimzadegan S., Kealey C., Bredt D.S., Nicoll R.A. TARP gamma-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nat. Neurosci. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- Savtchenko L.P., Rusakov D.A. Moderate AMPA receptor clustering on the nanoscale can efficiently potentiate synaptic current. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2013;369:20130167. doi: 10.1098/rstb.2013.0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Salzmann C., Li L., Bischofberger J. Functional properties of extrasynaptic AMPA and NMDA receptors during postnatal hippocampal neurogenesis. J. Physiol. 2014;592:125–140. doi: 10.1113/jphysiol.2013.267203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheardown M.J., Nielsen E.O., Hansen A.J., Jacobsen P., Honoré T. 2,3-Dihydroxy-6-nitro-7-sulfamoyl-benzo(F)quinoxaline: a neuroprotectant for cerebral ischemia. Science. 1990;247:571–574. doi: 10.1126/science.2154034. [DOI] [PubMed] [Google Scholar]
- Soares C., Lee K.F., Nassrallah W., Béïque J.C. Differential subcellular targeting of glutamate receptor subtypes during homeostatic synaptic plasticity. J. Neurosci. 2013;33:13547–13559. doi: 10.1523/JNEUROSCI.1873-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppini L., Buchs P.A., Muller D. A simple method for organotypic cultures of nervous tissue. J. Neurosci. Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Takillah S., Naudé J., Didienne S., Sebban C., Decros B., Schenker E., Spedding M., Mourot A., Mariani J., Faure P. Acute Stress Affects the Expression of Hippocampal Mu Oscillations in an Age-Dependent Manner. Front. Aging Neurosci. 2017;9:295. doi: 10.3389/fnagi.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taschenberger H., Leão R.M., Rowland K.C., Spirou G.A., von Gersdorff H. Optimizing synaptic architecture and efficiency for high-frequency transmission. Neuron. 2002;36:1127–1143. doi: 10.1016/s0896-6273(02)01137-6. [DOI] [PubMed] [Google Scholar]
- Tomita S., Chen L., Kawasaki Y., Petralia R.S., Wenthold R.J., Nicoll R.A., Bredt D.S. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J. Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S., Adesnik H., Sekiguchi M., Zhang W., Wada K., Howe J.R., Nicoll R.A., Bredt D.S. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- Turrigiano G. Homeostatic Synaptic Plasticity. In: Hell J.W., Ehlers M.D., editors. Springer; 2008. pp. 535–552. (Structural And Functional Organization Of The Synapse). [Google Scholar]
- Vignes M., Collingridge G.L. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Wiegert J.S., Gee C.E., Oertner T.G. Single-Cell Electroporation of Neurons. Cold Spring Harb. Protoc. 2017;2017:prot094904. doi: 10.1101/pdb.prot094904. [DOI] [PubMed] [Google Scholar]
- Yamasaki M., Fukaya M., Yamazaki M., Azechi H., Natsume R., Abe M., Sakimura K., Watanabe M. TARP γ-2 and γ-8 Differentially Control AMPAR Density Across Schaffer Collateral/Commissural Synapses in the Hippocampal CA1 Area. J. Neurosci. 2016;36:4296–4312. doi: 10.1523/JNEUROSCI.4178-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., Devi S.P.S., Tomita S., Howe J.R. Auxiliary proteins promote modal gating of AMPA- and kainate-type glutamate receptors. Eur. J. Neurosci. 2014;39:1138–1147. doi: 10.1111/ejn.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker R.S., Regehr W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this paper will be shared by the lead contact upon request.
-
•
This study did not generate any code.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.