Abstract
Intracranial hemorrhage after traumatic brain injury (TBI) can be life threatening and requires prompt diagnosis. Computed tomography (CT) scans are a rapid and accurate way to evaluate for hemorrhage. In patients with mild and moderate TBI, however, in whom the incidence of intracranial pathology is low, scanning every patient with CT can be costly. The Food and Drug Administration recently approved a novel biomarker screen, the Banyan Trauma Indicator (BTI), to help streamline the decision for CT scanning in mild to moderate TBI. The BTI screen diagnoses intracranial lesions with a sensitivity and specificity of 97.5% and 99.6%, respectively. We performed cost analyses of the BTI screen to determine the threshold of cost-effectiveness, compared with application of clinical decision rules or routine CT scans, for cases of mild or moderate TBI. With a 0.104 probability of an intracranial lesion in mild TBI, the biomarker screen is cost-effective if the cost is $308.96 or below per test. In moderate TBI, because of the greater prevalence of intracranial lesions at 0.663, there is a lower need for screening, and BTI becomes cost-effective up to $73.41 per test.
Keywords: biomarker, cost-effectiveness, traumatic brain injury
Introduction
Traumatic brain injury (TBI) is cited to be the cause of 2.8 million emergency department visits in 2013 and another 1 million cases seen in the community health clinics in the United States.1,2 Of these injuries, more than 75% are categorized as mild TBIs, which may include a brief change in mental status or consciousness, post-traumatic amnesia, and/or focal neurologic deficits that may or may not be transient. Proper diagnosis of TBI is of utmost importance with an impetus to capture all cases of mild or moderate TBI with an occult intracranial lesion that may require close observation or operative intervention.
Head computed tomography (CT) scans are a fast and accurate imaging modality to identify TBIs that may require surgical intervention. Only 10% of mild TBI cases, however, consist of any CT-visible intracranial pathology with approximately 1% necessitating neurosurgical intervention.3 Hence, it is unreasonable to scan every at-risk patient with a head CT. Recent estimates cite more than 62 million annual CT scans.4 To reduce the number of unnecessary CT scans for patients with TBI, clinicians often rely on clinical decision rules, such as the Canadian CT Head Rule,5 which is the best validated and most widely used rule.
Recently, the U.S. Food and Drug Administration (FDA) approved the use of the first brain-specific, biomarker blood screen to evaluate for TBI, the Banyan Brain Trauma Indicator (BTI).6 The Banyan screen aims to reduce the number of CT scans by stratifying the likelihood of an intracranial lesion in patients with TBI by measuring levels of ubiquitin C-terminal hydrolase (UCH-L1) and glial fibrillary acidic protein (GFAP), two proteins that are released into the bloodstream and detected within hours after head injury.
Numerous research groups, including TRACK-TBI, have reported the utility of testing for UCH-L1 and GFAP as surrogates for brain injury.7–10 Most notably, TRACK-TBI found that the combination of UCH-L1 and GFAP levels has higher sensitivity and specificity for distinguishing TBI compared with each individual biomarker.10 Measuring the predictive ability of BTI with results from CT scans, the FDA found that the blood screen could predict the presence of an intracranial lesion 97.5% of the time (sensitivity) and the absence of a lesion 99.6% of the time (specificity).6
In this study, we analyze cost utility models for mild and moderate TBI to determine the price at which the Banyan Brain Trauma Indicator, compared with clinical decision rules or routine CT scans, becomes cost-effective.
Methods
Definitions and assumptions
We elected to analyze moderate and mild TBI separately, because most authorities treat them differently. Standard treatment for patients with moderate TBI is hospital admission, usually to a specialized nursing unit and accompanied by routine head CT. In contrast, hospital admission for mild TBI is usually reserved for patients with risk factors identified on examination and/or a positive CT scan. We define moderate TBI as a Glasgow Coma Scale (GCS) score of 9–13. Although traditionally defined as scores of 9–12,11 there is evidence that a score of 13 is more properly included in the moderate category,12 and most recent studies of moderate TBI13–20 use the latter definition. By exclusion, mild TBI is limited to GCS scores 14–15. We took as our base case a 20-year-old male, but also investigated 40-, 60-, and 80-year old males and females.
Literature search
PubMed, Embase, and the Cochrane database were searched in February 2018 for the subject heading “head injury,” associated with “mild” or “moderate” or “minor” in the title published since 1980. Abstracts were screened, and inappropriate articles were excluded. The rest were downloaded as full text and reviewed by at least two authors. We excluded studies involving animals, those not published in English, those with too few cases (100 for moderate TBI, 250 for mild), those in which fewer than 80% of qualifying patients received CT scans, and those lacking original data (reviews, editorials, duplicate publications, intracranial lesions not reported, etc.). The search returned 3240 abstracts, 22 of which were used in various analyses of the study. The review process is summarized in Figure 1. Because all data come from non-analytic studies, by definition they all represent Level III evidence.21
FIG. 1.
Summary of literature search.
Data management and analysis
We abstracted estimates of the probability of intracranial lesions, the cost of a CT scan, and the operating characteristics (sensitivity and specificity) of clinical screening tests and the BTI.6 The reported point estimates of pooled data represent variance-weighted means (random-effects meta-analytic model) of observational data22 and were tested for heterogeneity. Models and additional data required for analysis are discussed below. Uncertainties of the point estimates were addressed using one- to three-way sensitivity analyses, in which values of all parameters were varied within their 95% confidence intervals. All costs are calculated from a societal perspective, and costs are expressed in 2018 dollars. Point estimates were generated using Stata 12 (StataCorp, College Station, TX), and analyses of the model employed TreeAge Pro 2017 (Tree Age Software, Inc., Williamstown, MA).
Mild TBI
Biomarker screening test
The model assumes that positive screening tests result in the performance of a CT scan and prompt treatment of positive findings; negative screening will result in hospital discharge. Possible outcomes of biomarker or clinical screening are discussed in the Supplementary Appendix; see online supplementary material at http://www.liebertpub.com.
Clinical decision rules
A number of clinical decision rules have been introduced to assist in the decision about CT scans for patients with mild TBI.5,23–27 Each rule uses slightly different clinical indicators for the risk of intracranial lesions; thus, all have different operating characteristics. For our model, we employed the Canadian CT Head rule.5
The model
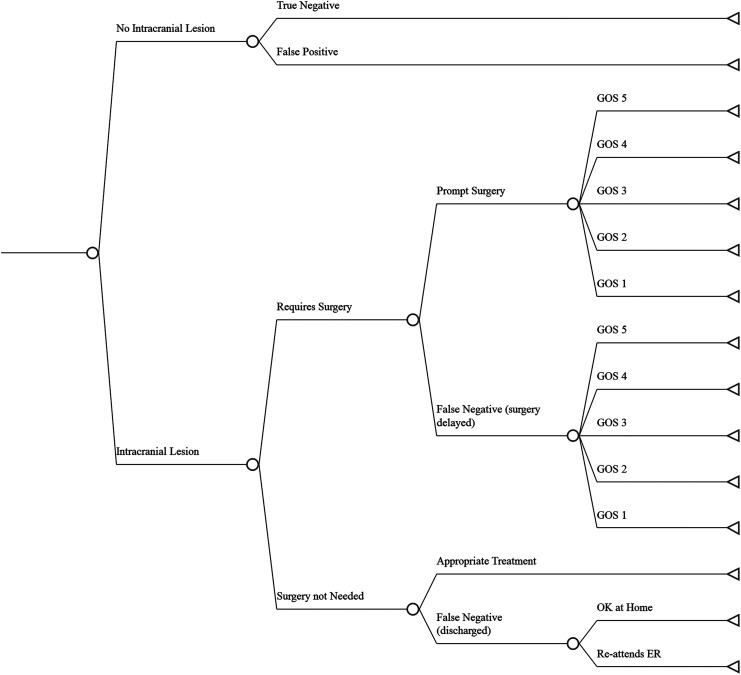
Illustrated in Figure 2, the model compares four different management strategies for deciding whether to perform a CT scan on a patient presenting after mild TBI. These strategies are outlined in the Supplementary Appendix; see online supplementary material at www.liebertpub.com. The various pathways and outcomes following implementation of a treatment strategy are illustrated in Figure 3. These are discussed in more detail in the Supplementary Appendix. We assume all patients found to have surgical lesions are treated aggressively. Outcomes reflect the negative effects of delayed surgery, including increased death and morbidity (and increased associated costs). A proportion of patients with non-surgical lesions will recover at home. The rest will return to the hospital and incur additional testing, observation, and treatment.
FIG. 2.
Decision tree for initial testing in mild traumatic brain injury: summarizes four management strategies and decision-making. CT, computed tomography.
FIG. 3.
Decision tree of possible outcomes for a management strategy in mild TBI. GOS (Glasgow Outcome Scale) score (5 good outcome; 4 moderate disability; 3 severe disability; 2 vegetative state; 1 dead).
Analysis
We abstracted the probabilities of surgical and non-surgical lesions, the other probabilities listed in Figure 4, as the operating characteristics (sensitivity and specificity) of the four management strategies from our literature search. We obtained the 2017 life expectancy of a 20-year-old male from the Social Security Administration28 for patients who had no lesion, non-surgical lesions, and Glasgow Outcome Scale (GOS) scores of 4 or 5 after operation. We adjusted life expectancies downward for patients with GOS of 2 or 3 after surgical procedure to reflect GOS-specific mortality rates.29–32 Long-term quality-adjusted life years (QALYs)33 were calculated based on the six-month GOS scores, which were assumed to last for the remainder of life expectancy. A description of QALYs and how they are calculated can be found in the Supplementary Appendix; see online supplementary material at www.liebertpub.com. Calculations follow those used in our previous study of diagnostic screening for mild TBI.34
FIG. 4.
Decision tree for initial testing in moderate traumatic brain injury (TBI): all pathways and outcomes are shown. CT, computed tomography.
An additional sensitivity analysis for effectiveness involved Monte Carlo simulation,35 in which all parameters follow beta distributions. We simulated 100 trials, each with 100 subjects in each group. We compared the results using one-way analysis of variance. Lacking the cost of the Banyan Brain Trauma Indicator, we were unable to perform Monte Carlo simulations of cost or cost-effectiveness.
Costs
In the case of mild TBI, we analyzed lifetime direct and indirect societal costs for a 20-year-old male. Medicare reimbursements were used as proxies for direct (medical) costs.36 Rehabilitation and nursing care costs for survivors were calculated,37 as were short- (first six months) and long-term indirect costs (lost productivity).38 We employed an overall discount rate of 3% per year and converted all costs to 2018 values.39 Details of the cost calculations can be found in Whitmore and associates.40
Moderate TBI
Calculations for moderate TBI are much simpler. For the purposes of this analysis, we assume all moderately injured patients are admitted to a special care unit. This allows for frequent nursing checks and rapid access to CT scanning, neurosurgical consultation, and treatment, if necessary. We further assume that no patient has a delay in diagnosis of an intracranial lesion. Accordingly, there is no difference in effectiveness of various management strategies; the only differences relate to costs, which are limited to the diagnostic tests. Hence, this part of the analysis is more appropriately called a cost-minimization rather than a cost-effectiveness analysis.33
The model
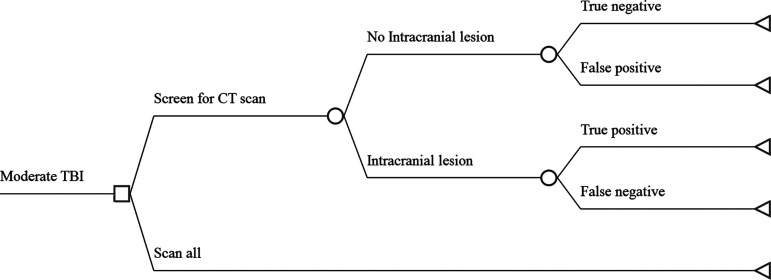
Figure 4 illustrates the possible pathways and outcomes in moderate TBI. Patients found to have moderate TBI on hospital admission receive either a head CT scan or a biomarker test to screen for the need for a scan. In the latter case, there may or may not be an intracranial lesion. Those who do not have lesions may have a correct diagnosis (true negative) and save the expense of an unnecessary CT scan or they may have an incorrect diagnosis (false positive) and receive the scan. Patients who do have intracranial lesions will either have a correct diagnosis (true positive) by the screening test, or the lesion will be missed (false negative). Because we assume the lesion will at some point present clinically, all patients with lesions will receive both the screening test and an intracranial CT scan. Thus, the result of the model is the cost an average patient can expect if each management strategy is followed.
Results
Mild TBI
Data
Our meta-analyses revealed the pooled mean probability that a patient with mild TBI has an intracranial lesion is 0.104 (95% confidence interval [CI] = 0.085 to 0.123) (Fig. S1). The probability that a lesion will require surgery is 0.115 (95% CI = 0.087 to 0.135). Thus, the combined probability of a mild TBI patient requiring operation for an intracranial lesion is 0.104 × 0.115, or 0.01196 (Fig. S2). Point estimates of probabilities, utilities, and costs used in the model are shown in Table 1. The table also reports the 95% CIs for these estimates, along with the sources for each estimate. The average life expectancy for a 20-year-old male is 62.3 years.28 At a 3% annual discount rate, this translates to 28.3353 QALYs, the maximum expected outcome with perfect management.
Table 1.
Probabilities, Utilities, and Costs in Mild Traumatic Brain Injury
| Baseline | 95% CI | References | |
|---|---|---|---|
| Probabilities | |||
| Intracranial lesion | 0.104 | 0.085 to 0.123 | 5,34,45–56 |
| Lesion requires surgery | 0.115 | 0.087 to 0.135 | 5,22,34,45–47, 49–54, 57 |
| Canadian CT Head Rule false positive | 0.568 | 0.530 to 0.607 | 53 |
| Canadian CT Head Rule false negative | 0.052 | 0.025 to 0.078 | |
| Biomarker screen false positive | 0.004 | 0–0.01 | 6 |
| Biomarker screen false negative | 0.025 | 0–0.05 | |
| Prolonged symptoms from non-surgical lesion | 0.43 | 0.33 to 0.52 | 34 |
| Prompt surgery results in: | |||
| GOS score = 5 | 0.988 | 0.977 to 1.0 | |
| GOS score = 4 | 0.006 | 0 to 0.1 | |
| GOS score = 3 | 0.005 | 0 to 0.1 | |
| GOS score = 2 | 0.001 | 0 to 0.001 | |
| GOS score = 1 | 0.009 | 0.004 to 0.013 | |
| Delayed surgery results in: | |||
| GOS score = 5 | 0.67 | 0.61 to 0.73 | |
| GOS score = 4 | 0.096 | 0.06 to 0.13 | |
| GOS score = 3 | 0.083 | 0.05 to 0.12 | |
| GOS score = 2 | 0.010 | 0.003 to 0.011 | |
| GOS score = 1 | 0.142 | 0.10 to 0.18 | |
| Utilities | |||
| GOS score = 5 | 1 | Definition | 58 |
| GOS score = 4 | 0.755 | 0.726 to 0.784 | 59 |
| GOS score = 3 | 0.445 | 0.405 to 0.485 | |
| GOS score = 2 | 0.11 | 0.071 to 0.149 | |
| GOS score = 1 | 0 | Definition | 58 |
| Costs | |||
| Cranial CT scan – no contrast | 218.48 | 36 | |
| Surgery, resulting in: | |||
| GOS score = 5 | 137,728.49 | Not reported | 40 |
| GOS score = 4 | 486,999.06 | ||
| GOS score = 3 | 2,333,901.07 | ||
| GOS score = 2 | 3,184,896.16 | ||
| GOS score = 1 | 2,315,579.95 | ||
| Hospitalization, concussion with complications | 15,178.77 | 36 | |
| Hospitalization, concussion without complications | 9473.10 | ||
CI, confidence interval; CT, computed tomography; GOS, Glasgow Outcome Scale.
Analyses
Monte Carlo simulations of effectiveness are shown in Table 2. Although the strategy of employing the biomarker screen first, followed by the Canadian Rule for negative results, is the most effective, differences are extremely small and not significant (F = 1.360, p = 0.255). One-way sensitivity analysis demonstrated that the parameters with the greatest influence on cost-effectiveness are the probability of an intracranial lesion, the probability that the lesion necessitates surgery, and the cost of the biomarker screen (Supplementary Fig. S3). A two-way sensitivity analysis, in which biomarker cost is plotted against the probability of intracranial lesion, is shown in Figure 5.
Table 2.
Differences in Effectiveness of Four Strategies for Mild Traumatic Brain Injury
| Management strategy | Expected QALYs | SD |
|---|---|---|
| Canadian Head Rule only | 28.2915 | 0.0170 |
| Biomarker Screen only | 28.2935 | 0.0068 |
| Canadian Head Rule first | 28.2898 | 0.0359 |
| Biomarker Screen first | 28.2952 | 0.0011 |
QALYs, quality-adjusted life years; SD, standard deviation.
FIG. 5.
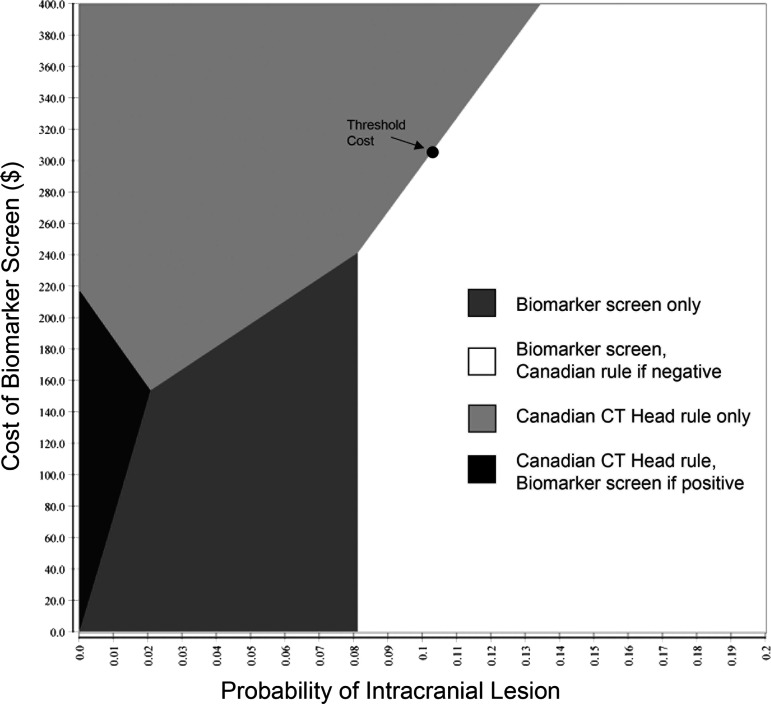
Two-way sensitivity analysis in mild traumatic brain injury. For a given value of probability of intracranial lesion and cost of the biomarker screen, the intersection lands in the area of the best strategy. For example, for the pooled mean prevalence of intracranial lesion of 0.104, the strategy of biomarker screen first, Canadian rule if negative is best, as long as the biomarker screen costs no more than $308.96. If the cost is greater, using the Canadian CT Head rule is the most cost-effective strategy. CT, computed tomography.
The ideal management strategy varies with the two parameters. Under baseline estimates, the biomarker first strategy is cost-effective if the societal cost is $308.96 or less. There are modest changes if the proportion of intracranial lesions necessitating a surgical procedure is lower or higher than estimated (Supplementary Figs. S4–S6). This calculation refers to 20-year-old males; however, neither age nor sex was associated with a significant change in the threshold. Cost-effectiveness also varies with the willingness of a society to pay (WTP) for additional QALYs. Traditionally, this has been considered $50,000 per QALY; however, there are calls to perform cost-effectiveness studies at multiple WTP values.41 Our original analysis was performed at a WTP of $50,000 per QALY. Raising the WTP threshold to $100,000 per QALY has only a minimal effect on the threshold cost of the biomarker screen ($312.70).
Moderate TBI
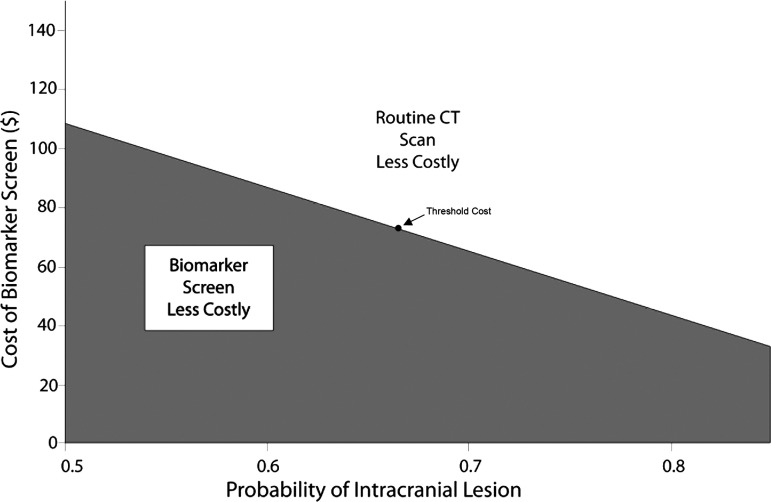
The pooled mean probability that a patient with moderate TBI has an intracranial lesion is 0.663 (95% CI = 0.492 to 0.834), demonstrated in Supplementary Figure S7. Using the same values for the operating characteristics of the BTI screen and the cost of a CT scan as above, one-way sensitivity analysis identifies the main drivers of cost-effectiveness to be the probability of intracranial lesions and the cost of the biomarker screen (Supplementary Fig. S8). The cost threshold for the biomarker screen is $73.41. Above this cost, performing CT scans on all patients is the more cost-effective alternative. At a lower cost, a biomarker screen (CT scan only if positive) is more cost-effective (Fig. 6).
FIG. 6.
Two-way sensitivity analysis in moderate traumatic brain injury. For a given value of probability of intracranial lesion and cost of the biomarker screen, the intersection lands in the area of the best strategy. For example, for the pooled mean prevalence of intracranial lesion of 0.663, the biomarker screen is less costly only if it costs $73.41 or less. If the cost is greater, routine computed tomography (CT) scanning is then the less costly alternative.
The cost-effectiveness threshold changes in the opposite direction of lesion prevalence. For example, if lesion prevalence were only 50%, approximately $108 for the biomarker screen would still be cost-effective. At a lesion prevalence of 85%, the cost-effectiveness threshold is roughly $32.
Discussion
Clinical decision rules have been utilized as strategies to selectively scan patients with mild TBI with CT. Among them, the Canadian CT Head Rule is best validated, with 100% sensitivity for detecting an intracranial lesion in patients with high-risk factors and 98.4% sensitivity for patients with medium-risk factors.5,42 The BTI is a new biomarker test with a sensitivity of 97.5% that the FDA approved of in February 2018 as part of a selective CT strategy and to help streamline management practices for TBI across providers.
Our study calculated a pooled mean probability of 10.4% for an intracranial lesion in patients with mild TBI. We found that the most effective strategy to detect an intracranial lesion is to screen first with the BTI and then utilize the Canadian CT Head rule. With such a strategy, the biomarker screen is cost-effective up to $308.96. In contrast, for moderate TBI, the cost-effective threshold for the BTI screen is lowered to $73.41. Above such prices, it is more cost-effective to directly scan with CT every patient who has mild or moderate TBI, respectively. The difference in cost thresholds between mild and moderate TBI reflects the increased likelihood of an intracranial lesion in patients with moderate TBI (mean probability of 66.3%). Our calculations are for the base case of a 20-year-old male. There are no significant changes in the cost-effectiveness thresholds for older patients (≤ age 80) or for females, however.
While the focus of this article is not intended to support or refute the implementation of the BTI screen, there are several factors that must be addressed because they may affect the cost-effectiveness of the screen. As with all models, we made several simplifying assumptions, any of which may detract from the reliability of our conclusions. Among them is the assumption that all patients sent home with non-surgical lesions will return to the hospital for further care. Some of these patients will not but will still have brief periods of lost productivity; however, the effect on overall outcome and costs is very small. We also assume that no missed intracranial hematomas result in death outside the hospital (this proportion is unknown and would influence direct costs). In addition, we used 6-month GOS as a proxy for utility. While not an ideal representation, there is no better measure of outcome in the literature. From a cost standpoint, Medicare and Medicaid reimbursements were used as proxies for direct healthcare costs. While commonly accepted,33 this practice may not represent true societal costs.
Further, heterogeneity among populations used to calculate the pooled prevalence of intracranial lesions is unavoidable. Our model also assumes that our patients maintain a stable neurologic examination during the time clinicians must wait for the result of the BTI, which the FDA predicts will take an average of three to four hours.6 During this time, if a patient were to deteriorate or is found to have abnormal laboratory findings (ie., coagulopathy, positive drug screen), then the threshold for a clinician to obtain a head CT is different. Our findings may be particularly relevant for a specific subcategory of patients who present to the emergency department with both TBI and intoxication. Given that commonly used clinical metrics and neurologic examinations would be unreliable in intoxicated patients, the BTI would be an objective metric to help guide diagnostics.
Biomarker screening has also been promoted to reduce the radiation risk associated with unnecessary CT scans. It should be noted, however, that adjusting for the extremely small, published cancer risks of a head CT scan in a 20-year-old (or older) patient43,44 did not alter the calculated life expectancy or QALYs for the average patient. Last, conservative estimates were made regarding length of hospital stay, although the exact figures may vary with local practices.
Conclusion
There is considerable controversy over imaging policies for mild TBI. To curb the number of unnecessary head CTs performed, the FDA approved the BTI as a new blood screen to evaluate for the presence of an intracranial lesion. The BTI, when combined with the Canadian CT Head rule, is cost-effective for screening for mild TBI at a cost of approximately $309 compared with a cost of $73 for moderate TBI. We hope this study can provide guidance on not only the implementation of the screen, but also the societal cost.
Supplementary Material
Acknowledgments
We would like to thank Dr. Ramon Diaz-Arrastia for commentary and edits.
Author Disclosure Statement
No competing financial interests exist.
Supplementary Material
References
- 1.Taylor C.A., Bell J.M., Breiding M.J., and Xu L. (2017). Traumatic brain injury-related emergency department visits, hospitalizations, and deaths - United States, 2007 and 2013. MMWR Surveill. Summ. 66, 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coronado V.G., McGuire L.C., Sarmiento K., Bell J., Lionbarger M.R., Jones C.D., Geller A.I., Khoury N., and Xu L. (2012). Trends in Traumatic Brain Injury in the U.S. and the public health response: 1995–2009. J. Safety Res. 43, 299–307 [DOI] [PubMed] [Google Scholar]
- 3.Jeret J.S., Mandell M., Anziska B., Lipitz M., Vilceus A.P., Ware J.A., and Zesiewicz T.A. (1993). Clinical predictors of abnormality disclosed by computed tomography after mild head trauma. Neurosurgery 32, 9–15 [DOI] [PubMed] [Google Scholar]
- 4.Brenner D.J. and Hall E.J. (2007). Computed tomography—an increasing source of radiation exposure. N. Eng. J. Med. 357, 2277–2284 [DOI] [PubMed] [Google Scholar]
- 5.Stiell I.G., Wells G.A., Vandemheen K., Clement C., Lesiuk H., Laupacis A., McKnight R.D., Verbeek R., Brison R., Cass D., Eisenhauer M.E., Greenberg G., and Worthington J. (2001). The Canadian CT Head Rule for patients with minor head injury. Lancet 357, 1391–1396 [DOI] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration (2018). FDA authorizes marketing of first blood test to aid in the evaluation of concussion in adults. www.fda.gov/newsevents/newsroom/pressannouncements/ucm596531.htm (Last accessed January27, 2019)
- 7.Papa L., Mittal M.K., Ramirez J., Silvestri S., Giordano P., Braga C.F., Tan C.N., Ameli N.J., Lopez M.A., Haeussler C.A., Mendez Giordano D., and Zonfrillo M.R. (2017). Neuronal biomarker ubiquitin C-terminal hydrolase detects traumatic intracranial lesions on computed tomography in children and youth with mild traumatic brain injury. J. Neurotrauma 34, 2132–2140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papa L., Brophy G.M., Welch R.D., Lewis L.M., Braga C.F., Tan C.N., Ameli N.J., Lopez M.A., Haeussler C.A., Mendez Giordano D.I., Silvestri S., Giordano P., Weber K.D., Hill-Pryor C., and Hack D.C. (2016). Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol. 73, 551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis L.M., Schloemann D.T., Papa L., Fucetola R.P., Bazarian J., Lindburg M., and Welch R.D. (2017). Utility of serum biomarkers in the diagnosis and stratification of mild traumatic brain injury. Acad. Emerg. Med. 24, 710–720 [DOI] [PubMed] [Google Scholar]
- 10.Diaz-Arrastia R., Wang K.K., Papa L., Sorani M.D., Yue J.K., Puccio A.M., McMahon P.J., Inoue T., Yuh E.L., Lingsma H.F., Maas A.I., Valadka A.B., Okonkwo D.O., Manley G.T. and TRACK-TBI Investigators. (2014). Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J. Neurotrauma 31, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rimel R.W., Giordani B., Barth J.T., Boll T.J. and Jane J.A. (1981). Disability caused by minor head injury. Neurosurgery 9, 221–228 [PubMed] [Google Scholar]
- 12.Stein S.C. (2001). Minor head injury: 13 is an unlucky number. J. Trauma 50, 759–760 [DOI] [PubMed] [Google Scholar]
- 13.Andriessen T.M., Horn J., Franschman G., van der Naalt J., Haitsma I., Jacobs B., Steyerberg E.W., and Vos P.E. (2011). Epidemiology, severity classification, and outcome of moderate and severe traumatic brain injury: a prospective multicenter study. J. Neurotrauma 28, 2019–2031 [DOI] [PubMed] [Google Scholar]
- 14.Compagnone C., d'Avella D., Servadei F., Angileri F.F., Brambilla G., Conti C., Cristofori L., Delfini R., Denaro L., Ducati A., Gaini S.M., Stefini R., Tomei G., Tagliaferri F., Trincia G., and Tomasello F. (2009). Patients with moderate head injury: a prospective multicenter study of 315 patients. Neurosurgery 64, 690–696 [DOI] [PubMed] [Google Scholar]
- 15.Fabbri A., Servadei F., Marchesini G., Stein S.C., and Vandelli A. (2008). Early predictors of unfavourable outcome in subjects with moderate head injury in the emergency department. J. Neurol. Neurosurg. Psychiatry 79, 567–573 [DOI] [PubMed] [Google Scholar]
- 16.Fearnside M. and McDougall P. (1998). Moderate head injury: a system of neurotrauma care. Aust. N. Z. J. Surg. 68, 58–64 [DOI] [PubMed] [Google Scholar]
- 17.Godoy D.A., Rubiano A., Rabinstein A.A., Bullock R., and Sahuquillo J. (2016). Moderate traumatic brain injury: the grey zone of neurotrauma. Neurocrit. Care 25, 306–319 [DOI] [PubMed] [Google Scholar]
- 18.Lund S.B., Gjeilo K.H., Moen K.G., Schirmer-Mikalsen K., Skandsen T., and Vik A. (2016). Moderate traumatic brain injury, acute phase course and deviations in physiological variables: an observational study. Scand. J. Trauma Resusc. Emerg. Med. 24, 77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall S.A. and Riechers R.G., 2nd (2012). Diagnosis and management of moderate and severe traumatic brain injury sustained in combat. Mil. Med. 177, Suppl 8, 76–85 [DOI] [PubMed] [Google Scholar]
- 20.Stein S.C. and Ross S.E. (1992). Moderate head injury: a guide to initial management. J. Neurosurg. 77, 562–564 [DOI] [PubMed] [Google Scholar]
- 21.Harbour R. and Miller J. (2001). A new system for grading recommendations in evidence based guidelines. BMJ 323, 334–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Einarson T.R. (1997). Pharmacoeconomic applications of meta-analysis for single groups using antifungal onychomycosis lacquers as an example. Clin. Ther. 19, 559–569 [DOI] [PubMed] [Google Scholar]
- 23.Haydel M.J., Preston C.A., Mills T.J., Luber S., Blaudeau E., and DeBlieux P.M. (2000). Indications for computed tomography in patients with minor head injury. N. Engl. J. Med. 343, 100–105 [DOI] [PubMed] [Google Scholar]
- 24.Ingebrigtsen T., Romner B., and Kock-Jensen C. (2000). Scandinavian guidelines for initial management of minimal, mild, and moderate head injuries. The Scandinavian Neurotrauma Committee. J. Trauma 48, 760–766 [DOI] [PubMed] [Google Scholar]
- 25.Mower W.R., Hoffman J.R., Herbert M., Wolfson A.B., Pollack C.V., Jr., and Zucker M.I.; NEXUS II Investigators. National Emergency X-Radiography Utilization Study. (2002). Developing a clinical decision instrument to rule out intracranial injuries in patients with minor head trauma: methodology of the NEXUS II investigation. Ann. Emerg. Med. 40, 505–514 [DOI] [PubMed] [Google Scholar]
- 26.Servadei F., Teasdale G., and Merry G.; Neurotraumatology Committee of the World Federation of Neurosurgical Societies. (2001). Defining acute mild head injury in adults: a proposal based on prognostic factors, diagnosis, and management. J. Neurotrauma 18, 657–664 [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Clinical Excellence (2007). Head Injury Triage, Assessment, Investigation and Early Management of Head Injury in Infants, Children and Adults. Vol Clinical Guideline 56. National Collaborating Centre for Acute Care at the Royal College of Surgeons of England: London: [PubMed] [Google Scholar]
- 28.Social Security Administration (2017). Retirement & Survivors Benefits: Life Expectancy Calculator [Google Scholar]
- 29.Brown A.W., Leibson C.L., Malec J.F., Perkins P.K., Diehl N.N., and Larson D.R. (2004). Long-term survival after traumatic brain injury: a population-based analysis. NeuroRehabilitation 19, 37–43 [PubMed] [Google Scholar]
- 30.Harrison-Felix C., Whiteneck G., DeVivo M., Hammond F.M., and Jha A. (2004). Mortality following rehabilitation in the Traumatic Brain Injury Model Systems of Care. NeuroRehabilitation 19, 45–54 [PubMed] [Google Scholar]
- 31.Shavelle R.M., Strauss D., Whyte J., Day S.M., and Yu Y.L. (2001). Long-term causes of death after traumatic brain injury. Am. J. Phys. Med. Rehabil. 80, 510–516 [DOI] [PubMed] [Google Scholar]
- 32.Strauss D.J., Shavelle R.M., and Ashwal S. (1999). Life expectancy and median survival time in the permanent vegetative state. Pediatr. Neurol. 21, 626–631 [DOI] [PubMed] [Google Scholar]
- 33.Gold M.R., Siegel J.E., Russell L.B., and Weinstein M.C. (1996). Cost-Effectiveness in Health and Medicine. Oxford University Press: New York [Google Scholar]
- 34.Stein S.C., Burnett M.G., and Glick H.A. (2006). Indications for CT scanning in mild traumatic brain injury: a cost-effectiveness study. J. Trauma 61, 558–566 [DOI] [PubMed] [Google Scholar]
- 35.Concato J. and Feinstein A.R. (1997). Monte Carlo methods in clinical research: applications in multivariable analysis. J. Investig. Med. 45, 394–400 [PubMed] [Google Scholar]
- 36.Centers for Medicare and Medicaid Services (2017). FY 2017 IPPS Final Rule Homepage [Google Scholar]
- 37.Faul M., Wald M.M., Rutland-Brown W., Sullivent E.E., and Sattin R.W. (2007). Using a cost-benefit analysis to estimate outcomes of a clinical treatment guideline: testing the Brain Trauma Foundation guidelines for the treatment of severe traumatic brain injury. J. Trauma 63, 1271–1278 [DOI] [PubMed] [Google Scholar]
- 38.Finkelstein E.A., Corso P.S., and Miller T.R. (2006). The Incidence and Economic Burden of Injuries in the United States. Oxford University Press: New York [Google Scholar]
- 39.US Department of Labor Bureau of Labor Statistics (2018). CPI Inflation Calculator [Google Scholar]
- 40.Whitmore R.G., Thawani J.P., Grady M.S., Levine J.M., Sanborn M.R., and Stein S.C. (2012). Is aggressive treatment of traumatic brain injury cost-effective? J. Neurosurg. 116, 1106–1113 [DOI] [PubMed] [Google Scholar]
- 41.Neumann P.J., Cohen J.T., and Weinstein M.C. (2014). Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Engl. J. Med. 371, 796–797 [DOI] [PubMed] [Google Scholar]
- 42.Stiell I.G., Lesiuk H., Wells G.A., Coyle D., McKnight R.D., Brison R., Clement C., Eisenhauer M.A., Greenberg G.H., Macphail I., Reardon M., Worthington J., Verbeek R., Rowe B., Cass D., Dreyer J., Holroyd B., Morrison L., Schull M., Laupacis A., Canadian C.T.Head and C-S pine Study Group. (2001). Canadian CT head rule study for patients with minor head injury: methodology for phase II (validation and economic analysis). Ann. Emerg. Med. 38, 317–322 [DOI] [PubMed] [Google Scholar]
- 43.Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation NRC. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. (2005). National Academies Press: Washington, D.C: [PubMed] [Google Scholar]
- 44.Brenner D., Elliston C., Hall E., and Berdon W. (2001). Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am. J. Roentgenol. 176, 289–296 [DOI] [PubMed] [Google Scholar]
- 45.Arab A.F., Ahmed M.E., Ahmed A.E., Hussein M.A., Khankan A.A., and Alokaili R.N. (2015). Accuracy of Canadian CT head rule in predicting positive findings on CT of the head of patients after mild head injury in a large trauma centre in Saudi Arabia. Neuroradiol. J. 28, 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouida W., Marghli S., Souissi S., Ksibi H., Methammem M., Haguiga H., Khedher S., Boubaker H., Beltaief K., Grissa M.H., Trimech M.N., Kerkeni W., Chebili N., Halila I., Rejeb I., Boukef R., Rekik N., Bouhaja B., Letaief M., and Nouira S. (2013). Prediction value of the Canadian CT head rule and the New Orleans criteria for positive head CT scan and acute neurosurgical procedures in minor head trauma: a multicenter external validation study. Ann. Emerg. Med. 61, 521–527 [DOI] [PubMed] [Google Scholar]
- 47.Easter J.S., Haukoos J.S., Meehan W.P., Novack V., and Edlow J.A. (2015). Will neuroimaging reveal a severe intracranial injury in this adult with minor head trauma? The rational clinical examination systematic review. JAMA 314, 2672–2681 [DOI] [PubMed] [Google Scholar]
- 48.Haydel M. (2012). Management of mild traumatic brain injury in the emergency department. Emerg. Med. Pract. 14, 1–24 [PubMed] [Google Scholar]
- 49.Ibanez J., Arikan F., Pedraza S., Sanchez E., Poca M.A., Rodriguez D., and Rubio E. (2004). Reliability of clinical guidelines in the detection of patients at risk following mild head injury: results of a prospective study. J. Neurosurg. 100, 825–834 [DOI] [PubMed] [Google Scholar]
- 50.Papa L., Stiell I.G., Clement C.M., Pawlowicz A., Wolfram A., Braga C., Draviam S., and Wells G.A. (2012). Performance of the Canadian CT Head Rule and the New Orleans Criteria for predicting any traumatic intracranial injury on computed tomography in a United States Level I trauma center. Acad. Emerg. Med. 19, 2–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ro Y.S., Shin S.D., Holmes J.F., Song K.J., Park J.O., Cho J.S., Lee S.C., Kim S.C., Hong K.J., Park C.B., Cha W.C., Lee E.J., Kim Y.J., Ahn K.O., and Ong M.E. (2011). Comparison of clinical performance of cranial computed tomography rules in patients with minor head injury: a multicenter prospective study. Acad. Emerg. Med. 18, 597–604 [DOI] [PubMed] [Google Scholar]
- 52.Smits M., Dippel D.W., de Haan G.G., Dekker H.M., Vos P.E., Kool D.R., Nederkoorn P.J., Hofman P.A., Twijnstra A., Tanghe H.L., and Hunink M.G. (2005). External validation of the Canadian CT Head Rule and the New Orleans Criteria for CT scanning in patients with minor head injury. JAMA 294, 1519–1525 [DOI] [PubMed] [Google Scholar]
- 53.Stein S.C., Fabbri A., Servadei F., and Glick H.A. (2009). A critical comparison of clinical decision instruments for computed tomographic scanning in mild closed traumatic brain injury in adolescents and adults. Ann. Emerg. Med. 53, 180–188 [DOI] [PubMed] [Google Scholar]
- 54.Stiell I.G., Clement C.M., Rowe B.H., Schull M.J., Brison R., Cass D., Eisenhauer M.A., McKnight R.D., Bandiera G., Holroyd B., Lee J.S., Dreyer J., Worthington J.R., Reardon M., Greenberg G., Lesiuk H., MacPhail I., and Wells G.A. (2005). Comparison of the Canadian CT Head Rule and the New Orleans Criteria in patients with minor head injury. JAMA 294, 1511–1518 [DOI] [PubMed] [Google Scholar]
- 55.Tan D.W., Lim A.M., Ong D.Y., Peng L.L., Chan Y.H., Ibrahim I., and Kuan W.S. (2017). Computed tomography of the head for adult patients with minor head injury: are clinical decision rules a necessary evil? Singapore Med. J. 59, 199–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Unden L., Calcagnile O., Unden J., Reinstrup P., and Bazarian J. (2015). Validation of the Scandinavian guidelines for initial management of minimal, mild and moderate traumatic brain injury in adults. BMC Med. 13, 292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sharp A.L., Nagaraj G., Rippberger E.J., Shen E., Swap C.J., Silver M.A., McCormick T., Vinson D.R., and Hoffman J.R. (2017). Computed tomography use for adults with head injury: describing likely avoidable emergency department imaging based on the Canadian CT Head Rule. Acad. Emerg. Med. 24, 22–30 [DOI] [PubMed] [Google Scholar]
- 58.Sox H.C., Blatt M.A., Higgins M.C., and Marton K.I. (2007). Medical Decision Making. Vol 2. American College of Physicians: Philadelphia [Google Scholar]
- 59.Kosty J., Macyszyn L., Lai K., McCroskery J., Park H.R., and Stein S.C. (2012). Relating quality of life to Glasgow outcome scale health states. J. Neurotrauma 29, 1322–1327 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.