Abstract
Cells within tissues are routinely subjected to physiological stress and strain, arising from direct interactions with neighboring cells as well as with extracellular matrix components. Accordingly, there is tremendous interest in deciphering how cells sense, and respond to, changes in biomechanical forces. In this study, we explored the effects of mechanostimulation on the differentiation of mouse female germline or oogonial stem cells (OSCs) as a model for adult stem cell function. We report that increasing levels, or repeated application of a subthreshold fixed level, of radial strain to OSCs in culture significantly increased rates of in vitro oocyte formation as a measure of stem cell differentiation. These responses involved changes in F-actin-mediated cytoskeletal tension as well as in activation of intracellular signaling by Rho-associated protein kinase (ROCK) and Yes-associated protein (YAP) phosphorylation. In addition, application of mechanical strain to OSCs enhanced association of YAP with muscle-specific cytidine-adenosine-thymidine (MCAT) response elements in the promoter stimulated by retinoic acid gene 8 (Stra8), the transcriptional activation of which is required for germline meiotic commitment. These data indicate that biomechanical strain directly promotes the differentiation of adult female germline stem cells through a signaling pathway involving F-actin, ROCK, YAP, and Stra8.
Keywords: mechanotransduction, germline stem cell, oogonial stem cell, oogenesis, oocyte, ovary
Introduction
Biomechanical forces have been shown to guide, and alter, cellular function and fate, beginning at the earliest stages of embryogenesis and continuing throughout adult life [1]. Establishment of physical connections between two or more cells, or between a cell and its immediate microenvironment—most often in the form of extracellular matrix (ECM) components [2], creates mechanical forces at points of contact that are both relayed and sensed by cells as tissues develop, remodel, and function. Processing of this information by cells, which is often referred to as mechanotransduction, can have profound effects on many key intracellular decision-making events, ranging from self-renewal and proliferation to differentiation and senescence. Efforts to better understand how such biomechanical forces influence cells are therefore expected to offer important insights into key questions across many disciplines, including developmental biology, stem cell dynamics, biomimetic tissue engineering, and organismal aging [1–3].
Much of what is known about the role of biomechanics in stem cells derives from studies of embryonic patterning and early cell fate decisions during development [1,4–6]. Information obtained from these models, coupled with studies of pluripotent stem cells (embryonic and induced) as well as of endogenous mesenchymal stem cells, have uncovered important, but often cell type specific, roles for the ECM, cytoskeletal reorganization, Hippo kinase activity, Wnt–β-catenin signaling, and Yorki (Yki) orthologs (Yes-associated protein or YAP, and transcriptional coactivator with PDZ binding motif or TAZ) in eliciting and mediating cellular responses to biomechanical forces [1,2,7,8]. Other studies have highlighted the importance of mechanotransduction in stem cell populations that are more limited in potentiality. For example, biomechanical stress has been reported to influence muscle stem cell self-renewal in vitro [9] and regeneration of injured muscles in vivo [10]. Altered biomechanical signaling from the microenvironment has also been linked to epidermal stem cell fate [11], hematopoietic stem and progenitor cell expansion [12], and the malignant transformation of mammary epithelium [13].
Germline stem cells (GSCs) are one of the most important adult stem cell populations in the body since these cells give rise, through meiotic differentiation, to male and female gametes needed for reproduction [14–19]. Despite the fundamental importance of these cells to the successful propagation of essentially all animal species, essentially nothing is known of the significance of mechanobiology to GSC function in either the ovaries or the testes. In adult mammalian females, this issue is particularly interesting to consider, given the dramatic levels of tissue expansion and contraction that occur in the ovaries over the course of each reproductive or menstrual cycle as follicles grow, ovulate, and degenerate [20]. To address this significant gap in knowledge surrounding the role, if any, of mechanobiological forces in modulating GSC function, in this study, we used mice as a model system to begin evaluation of the influence of mechanoactivation on the differentiation capacity of female germline or oogonial stem cells (OSCs). We further assessed potential mechanisms used by OSCs to sense, and respond to, changes in external biomechanical forces that lead to changes in oocyte formation.
Materials and Methods
Animals
Wild-type female C57BL/6 mice were obtained from Charles River Laboratories (Wilmington, MA) at 8 weeks of age for use in the studies described. All experiments reported in this study involving animals were reviewed and approved by the Northeastern University institutional animal care and use committee.
Chemicals and reagents
All chemicals and reagents were obtained from ThermoFisher Scientific (Waltham, MA), unless otherwise indicated.
Ovarian tissue collection and analysis
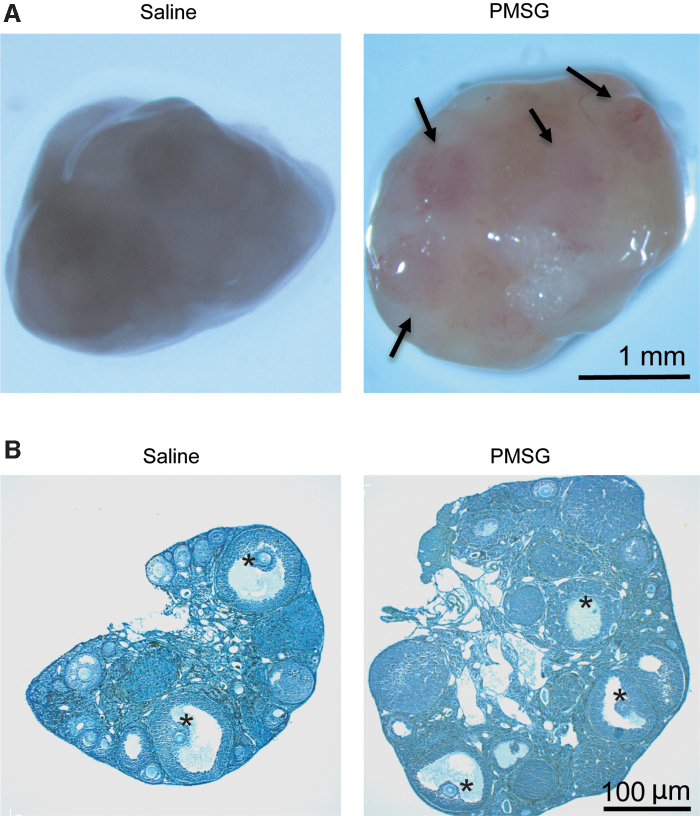
To highlight the extent of volumetric tissue expansion associated with follicular growth during each reproductive cycle, female mice were given 5 IU of pregnant mare serum gonadotropin (MilliporeSigma, Burlington, MA), or sterile saline (vehicle control), by single intraperitoneal injection to promote synchronized development of preovulatory follicles. Forty-six hours following injection, ovaries were collected, washed, and imaged using a Zeiss SteREO Discovery V20 stereomicroscope, and then fixed for paraffin embedding and histological sectioning (8 μm). Cross-sectional areas of gonadotropin-stimulated ovarian tissues were measured using ImageJ software, and normalized to values obtained from parallel analysis of ovaries from unstimulated females.
OSC cultures and biomechanical strain application
Ovaries were collected from female mice to isolate OSCs by flow cytometry, which were then established as pure germ cell cultures [21,22]. All experiments utilized OSCs between passages 28 and 35. To monitor differentiation, 1.3 × 104 OSCs per cm2 were plated in triplicate wells of collagen-type 1-coated BioFlex membrane plates (Flexcell® International, Burlington, NC) in OSC culture medium for each experimental replicate. For all experiments, strain was applied 16 h following the initial plating of the cells. Radial strain was applied using the Flexcell system, in which elongation of the flexible membrane at the values indicated for each experiment was performed for 5 s. For repeated strain, a subthreshold level of radial strain was applied for 5 s every 6 h. To monitor differentiation, beginning 8 h after the application of strain (24 h after plating), 20% of culture medium was sampled and evaluated by light microscopy for the number of in vitro-derived (IVD) oocytes formed as an established bioassay for oogenesis [17,21–27]. For time-course evaluation, the remaining medium was removed and replaced with fresh culture medium to evaluate IVD oocyte generation over subsequent 24-h intervals. In some experiments, OSCs were preincubated with 1-μM Y-27632 (Enzo Life Sciences, Farmingdale, NY) for 30 min before the application of strain; in other experiments, OSCs were treated with verteporfin (MilliporeSigma), at the doses indicated, at the time of plating.
Immunofluorescence analysis
For immunofluorescence studies, OSCs were grown to 70% confluence on glass coverslips. At this time, IVD oocytes were manually collected from spent culture medium and fixed in 2% formaldehyde for 30 min at 37°C, and then washed in phosphate-buffered saline (PBS) containing 0.2% polyvinylpyrrolidone (PVP) before immunofluorescence analysis. The remaining adherent cells were washed, fixed in 2% formaldehyde at room temperature for 45 min, and washed again in PBS. All samples were then permeabilized with 0.1% Triton-X in PBS, washed, and incubated in a blocking buffer containing 2% bovine serum albumin and 2% normal goat serum (MilliporeSigma). After washing, the samples were incubated in a 1:100 dilution of anti-YAP primary antibody (14074; Cell Signaling Technology, Danvers, MA), washed, and incubated with a 1:500 dilution of goat anti-rabbit AlexaFluor488-conjugated secondary antibody for 1 h at room temperature. Following additional washing, samples were stained with rhodamine phalloidin as per the manufacturer's protocol. All samples were washed and stained with DAPI before mounting under coverslips with ProLong Gold. For analysis of IVD oocytes, the cells were resuspended in PBS containing 0.2% PVP, and manually pipetted utilizing a 200-μm Flexipet through droplets containing the solutions as described above for OSC analysis. The final wash drops containing the oocytes were overlaid with mineral oil before imaging. All samples were processed in parallel with negative controls, the latter of which entailed omission of primary antibody while holding all other steps constant.
Cell viability assays
Cultured OSCs were treated with increasing concentrations of verteporfin between 25 and 1,000 nM, which represented the full dose range of this inhibitor used in prior studies, or a matched concentration of vehicle (controls; 0.1% dimethyl sulfoxide, v:v) for 24 h. The cells were then harvested, washed, and resuspended in PBS containing 0.1% fetal bovine serum. Untreated cells were used as a positive control for viability, and additional untreated cells were intentionally permeabilized in 0.1% Triton-X for 10 min at room temperature to serve as a positive control for nonviability. Cell suspensions were stained with propidium iodide at a final concentration of 3 μM and analyzed with a BD FACSAria III (Becton Dickinson, Franklin Lakes, NJ) using FACSDiva Software (version 10.2). Data analysis was performed with FlowJo software (version 8.0).
Chromatin immunoprecipitation–polymerase chain reaction and gene expression analyses
Cultured OSCs were collected 8 h following the application of 10.5% strain. The cells were then processed as per the manufacturer's protocol using an EZ-ChIP kit (MilliporeSigma) with a mouse anti-YAP antibody (2 μg per 100 μL of prepared chromatin solution, SC271134; Santa Cruz Biotechnology, Dallas, TX). In parallel, normal mouse IgG (1 μg per 100 μL of prepared chromatin solution, 12-371B; MilliporeSigma) was used as a negative control, and anti-RNA polymerase II antibody (1 μg per 100 μL of prepared chromatin solution, CTD4H8, 05-623B; MilliporeSigma) was used as a positive control. Primers specific to MCAT elements (5′-CATTCCT-3′) identified in the stimulated by retinoic acid gene 8 (Stra8) promoter by in silico analysis were used to verify the specificity of YAP binding in the immunoprecipitated fraction using GoTaq Green (Promega, Madison, WI). Primer sequences used were as follows:
MCAT1: forward: 5′-AAATTAAAGGCTGAGACCTGTCAGAGG-3′
reverse: 5′-TTAAACCTCTGTACCTGCTGCTGGGG-3′
MCAT2: forward: 5′-AACTTGCCTCCAAGGGGGTAAGGTG-3′
reverse: 5′-ATATGGCTGGCTAAAGGAGCTGGAGC-3′
To quantitate changes in YAP binding, the same primers were employed with SYBR Green (Applied Biosystems, Beverly, MA) using glyceraldehyde 3-phosphate dehydrogenase (Gapdh) as a housekeeping reference gene (forward primer, 5′-GTCCCGTAGACAAAATGGTGA-3′ and reverse primer, 5′-TGCATTGCTGACAATCTTGAG-3′). All data were analyzed using the ΔΔCt method of relative quantitation. For quantitative polymerase chain reaction (PCR), total RNA was extracted from cultured OSCs using RNAzol RT (Molecular Research Center), treated with DNase-I to remove any potential genomic DNA contamination, normalized in concentration across samples to be analyzed, and subjected to first-strand cDNA synthesis using a RevertAid reverse-transcription kit (ThermoFisher Scientific). Quantitative analysis of Stra8 mRNA levels was then conducted using a TaqMan gene expression assay for Stra8 (Applied Biosystems Assay ID: Mm00486473_m1), with values standardized against Gapdh as a housekeeping reference gene (Applied Biosystems Assay ID: Mm99999915_g1).
Protein analyses
To quantitate changes in YAP phosphorylation, OSCs were lysed directly in RIPA buffer [10 mM Tris-HCl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1.0% sodium deoxycholate, 0.1% SDS, and 1.0 mM EDTA] supplemented with a protease inhibitor cocktail (Roche). Lysates were centrifuged at 14,000 g for 10 min at 4°C, and protein concentrations in supernatants were determined by the bicinchoninic acid assay. Protein lysates were resolved by reducing-denaturing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes. Blots were probed with a mouse monoclonal antibody against YAP (SC271134; Santa Cruz Biotechnology) or a rabbit polyclonal antibody against YAP phosphorylated at serine-127 (phospho-YAP) (4911; Cell Signaling Technology) as per the manufacturer's recommendations. Blots were washed and probed with fluorescent secondary antibodies appropriate for each primary antibody (goat anti-mouse AF647; Cell Signaling Technology 4410, goat anti-rabbit AF488; ThermoFisher Scientific A11008). Equality of sample loading was then standardized against GAPDH levels (clone D16H11; Cell Signaling Technology 5174). The membranes were dried, and fluorescence signals were detected and quantified using a ChemiDoc Imaging System (Bio-Rad Laboratories, Hercules, CA).
Data presentation and statistical analysis
All experiments were independently replicated at least three times (refer to text or figure legends for details on each experiment). Quantitative results are presented as the mean ± standard error of the mean of the combined results across all replicate experiments, with statistical significance assessed by ANOVA with post-hoc Tukey honest significant difference (HSD) test. Qualitative data shown for histology and immunofluorescence are representative of results obtained in the independent biological replicates for each experiment.
Results
Mechanical strain promotes the differentiation of OSCs into oocytes
Gonadotropin-driven follicular growth, such as that which occurs during each reproductive cycle [19], results in a significant expansion (∼25%) in ovarian volume in <2 days (Fig. 1). This prompted us to explore how OSCs, which are localized in the outer cortical region of the ovaries [21,24,28] where the highest levels of strain would be sensed by cells as follicles grow to the point of ovulatory rupture, might respond to varying levels of mechanostimulation. Using cultured OSCs as a model, we found that a single application of radial strain failed to induce a detectable change in OSC differentiation within 8 h across any level of applied strain tested (Fig. 2A). However, at 32 h poststrain, a single application of 10.5% elongation, which represented the intermediate level of strain tested in our experiments, significantly increased the rate of IVD oocyte formation over that observed in unstrained control cultures (Fig. 2B). Interestingly, the application of a higher degree of strain (15.8%) did not alter IVD oocyte formation compared with unstrained controls (Fig. 2B), suggesting that the stimulatory effects of mechanoactivation on OSC differentiation are lost if the level of strain sensed by the cells exceeds a given threshold. We then explored the impact of repeated application of a subthreshold level of strain that was set at ∼50% of the maximally effective level identified. The stimulatory effect of 10.5% elongation on OSC differentiation was maintained through 56 h poststrain (Fig. 2C), whereas a single application of 5.5% strain failed to alter IVD oocyte numbers versus controls at this time point (Fig. 2C). However, application of 5.5% elongation every 6 h, 10 times in sequence, significantly increased the rate of IVD oocyte formation over that detected in unstrained control cultures (Fig. 2C).
FIG. 1.
Volumetric expansion of ovaries during gonadotropin-driven follicular growth. Representative appearance (A; arrows indicate large mature follicles) and histology (B; asterisks indicate large mature follicles) of ovaries collected from adult female mice 46 h after injection with saline or PMSG. Across four independent experiments, PMSG injection increased total ovarian volume by 25.14% ± 8.25% compared with ovaries from saline-injected control mice (mean ± SEM, P < 0.05). PMSG, pregnant mare serum gonadotropin; SEM, standard error of the mean.
FIG. 2.
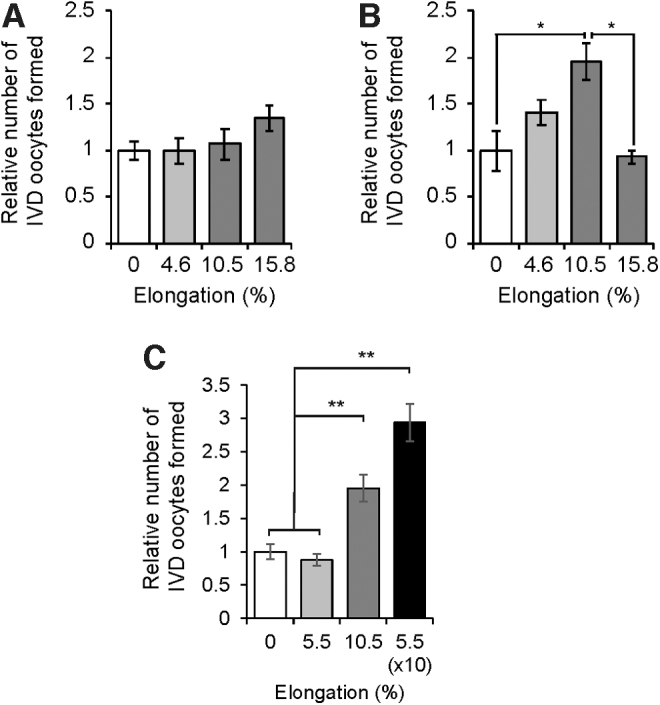
Mechanical strain enhances OSC differentiation. Rate of IVD oocyte formation by OSCs at 8 (A), 32 (B), or 56 (C) h following the application of radial strain at the indicated levels. While 10.5% elongation increased in vitro oogenesis at both 32 and 56 h poststrain, lower levels of strain (4.6%–5.5%) were without effect at any time point tested. However, repeated application of a subthreshold level of strain (5.5%) 10 times in succession increased rates of IVD oocyte formation (C). Results are the mean ± SEM from three independent experiments. *P < 0.05 compared with the 0% elongation group, **P < 0.01 compared with the 0% elongation group. IVD, in vitro derived; OSC, oogonial stem cell.
Mechanoactivation of OSCs involves Rho-associated protein kinase signaling
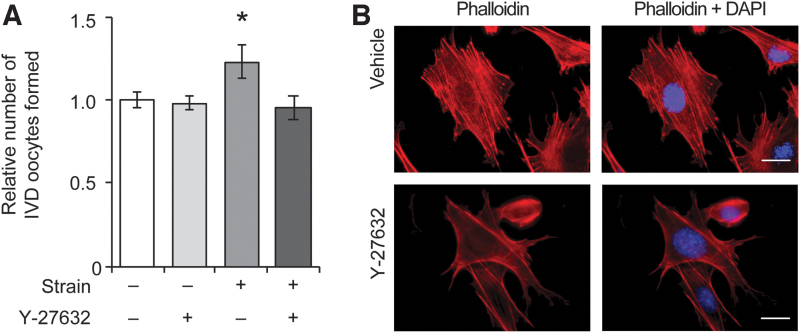
To explore potential mechanisms underlying the transduction of mechanical signals in OSCs that lead to enhanced differentiation into oocytes, the cells were exposed to Y-27632, a small molecule inhibitor of the Rho-associated protein kinase (ROCK) pathway [29]. Activation of ROCK is a critical early signal that conveys biomechanical information from outside the cell to the cytoskeleton [30,31]. Treatment of OSCs with Y-27632 had no significant impact on basal rates of IVD oocyte formation (Fig. 3A); however, Y-27632 suppressed the enhancement of OSC differentiation induced by the application of 10.5% elongation at 32 h poststrain (Fig. 3A). Immunofluorescence-based analysis of F-actin in control and Y-27632-treated OSCs revealed a reduction in highly organized stress fibers in cells exposed to the ROCK inhibitor (Fig. 3B), in agreement with past studies highlighting the importance of cytoskeletal changes and ROCK signaling in mechanotransduction in other cell lineages [30,31].
FIG. 3.
Differentiation of OSCs in response to mechanical strain involves ROCK signaling and cytoskeletal tension. The ability of 10.5% elongation to increase IVD oocyte formation at 32 h poststrain was abolished by treatment with the ROCK inhibitor, Y-27632 (A). Results are the mean ± SEM from three independent experiments. *P < 0.05 compared with unstrained cells. Cells exposed to the ROCK inhibitor also exhibited reduced and disorganized F-actin stress fibers compared with vehicle-treated OSCs (B). Scale bars = 10 μm. ROCK, Rho-associated protein kinase.
Role of YAP in OSC differentiation induced by mechanical strain
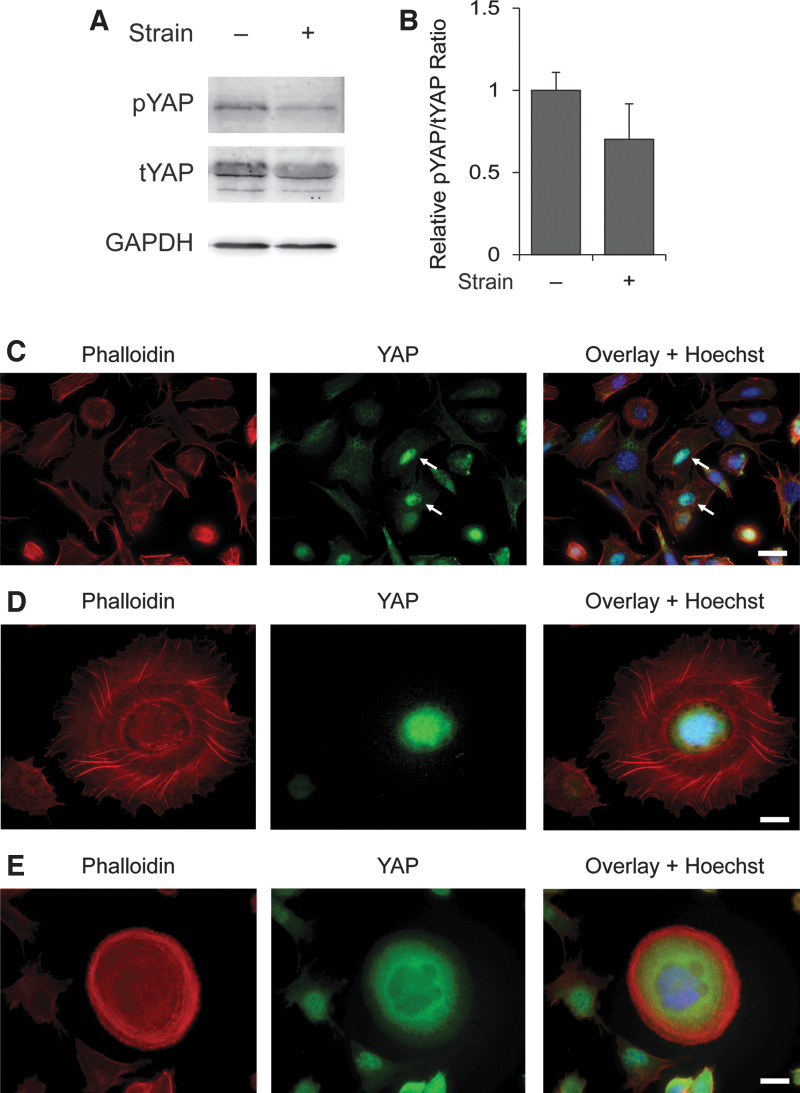
In assessing the presence of YAP protein in OSCs (Fig. 4A), we observed that at 12 h following the application of 10.5% strain, the ratio of phosphorylated YAP, which is retained within the cytoplasm and thus unable to act as a transcriptional regulator [8], to total YAP in OSCs was reduced by ∼30% compared with unstrained control cells (Fig. 4B). Across the total cell population, we identified a small subset of cells in which YAP was exclusively localized to the nucleus (Fig. 4C). As those cells with nuclear YAP underwent dynamic cytoskeletal organization associated with the transition of individual OSCs from adherence in monolayer culture (Fig. 4C) to three-dimensional IVD oocytes (Fig. 4D), YAP became progressively localized only to the cytoplasm of newly formed IVD oocytes released into the medium (Fig. 4E). These observations with in vitro oogenesis from OSCs mirror YAP localization patterns during oogenesis in vivo [32].
FIG. 4.
Analysis of YAP expression in OSCs and IVD oocytes. Western blot analysis was used to confirm the presence of YAP protein in OSCs, with an apparent shift in the ratio of pYAP to tYAP observed at 12 h following the application of 10.5% strain (A). Densitometric analysis showed that the ratio of pYAP to tYAP, normalized against GAPDH levels as a control for sample loading, was decreased by ∼30% at 12 h after the application of 10.5% strain (B). Results are the mean ± SEM from three independent experiments (P = 0.1). Immunofluorescence-based analysis of YAP in cultured OSCs identified a small number of cells with nuclear YAP localization (C; white arrows). As these cells differentiated into IVD oocytes, there occurred a dramatic reorganization of F-actin stress fibers (D) followed by a progressive relocalization of YAP from the nucleus to the cytoplasm, at which time F-actin fiber reorganization to a subcortical location was also completed (E). Scale bars = 20 μm. GAPDH, glyceraldehyde 3-phosphate dehydrogenase; pYAP, phosphorylated YAP; tYAP, total YAP; YAP, Yes-associated protein.
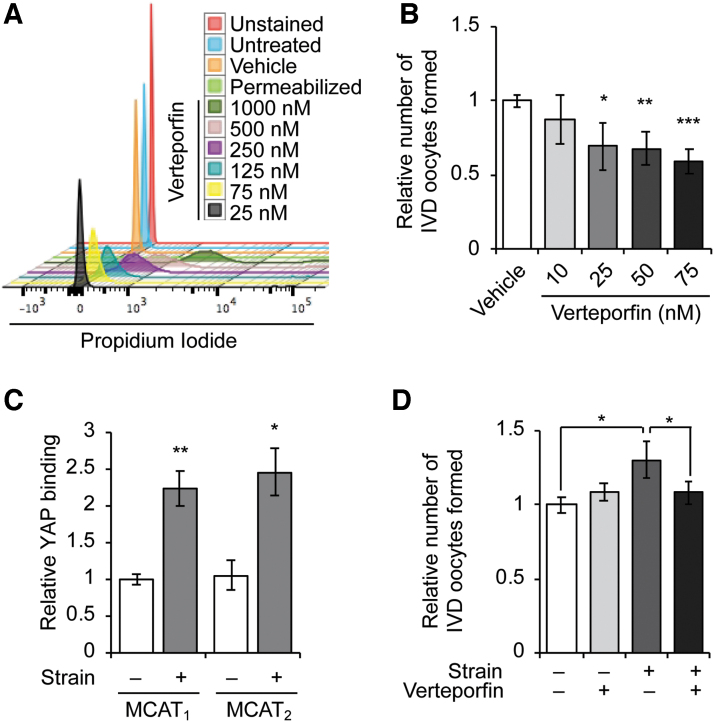
Prior genetic studies in mice have established that the commitment of primitive germ cells to meiotic differentiation in males (spermatogenesis) and females (oogenesis) requires transcriptional activation and expression of Stra8 [33–35]. Recent experiments have also shown that the generation of IVD oocytes by OSCs in culture similarly works through Stra8 activation [17,23,27]. We therefore next tested if YAP directly signals for meiotic commitment in OSCs under basal growth conditions or following the application of mechanical strain using verteporfin, a small molecule inhibitor that blocks YAP-mediated transcriptional activation of its target genes through retention of YAP in the cytoplasm [36]. After empirically establishing a maximal concentration of the drug that was without toxicity in OSCs (Fig. 5A), we found that exposure of OSCs to verteporfin in the absence of mechanical strain significantly decreased basal rates of IVD oocyte formation at doses between 25 and 75 nM (Fig. 5B).
FIG. 5.
Role of YAP in mechanotransduction-induced OSC differentiation. Empirical testing of verteporfin concentrations between 25 and 1,000 nM was used to determine noncytotoxic doses for further analysis (A). Verteporfin treatment resulted in a dose-dependent suppression of basal oogenesis in OSC cultures at concentrations between 25 and 75 nM (B). ChIP-PCR analysis of YAP binding to MCAT response elements in the Stra8 promoter identified significantly increased YAP binding at both sites 8 h after the application of 10.5% strain (C). While 10-nM verteporfin had no effect on basal oogenesis, this same low concentration of YAP inhibitor completely abolished the increase in IVD oocyte formation 32 h after the application of 10.5% strain (D). Results are the mean ± SEM from three independent experiments. *P < 0.05 compared with the respective control group, **P < 0.01 compared with the respective control group, ***P < 0.001 compared with the respective control group. ChIP, chromatin immunoprecipitation; MCAT, muscle-specific cytidine-adenosine-thymidine; PCR, polymerase chain reaction; Stra8, stimulated by retinoic acid gene 8.
Consistent with these observations and the established role of Stra8 in signaling premeiotic germ cell differentiation, we identified two consensus MCAT elements (5′-CATTCCT-3′) in the promoter region of the mouse Stra8 gene. These elements serve as genomic DNA binding sites for YAP, when complexed with transcription enhancer factor-1 and abaA (TEA) domain transcription factors, to modulate gene transcription [37]. Using chromatin immunoprecipitation (ChIP)-PCR analysis, YAP was found to directly associate with both Stra8 MCAT elements in OSCs, and this association was significantly increased at both MCAT sites 8 h following the application of 10.5% strain (Fig. 5C). This was paralleled by a near 50% (1.43 ± 0.3-fold) increase in Stra8 mRNA levels in OSC cultures poststrain compared with unstrained control OSCs (n = 4, P = 0.19). While a low dose of verteporfin (10 nM) did not inhibit basal rates of in vitro oogenesis, it effectively abolished the increase in IVD oocyte formation resulting from the application of 10.5% elongation to OSCs at 32 h poststrain (Fig. 5D).
Discussion
There has been growing interest in deciphering the role of mechanotransduction in ovarian follicle development and oocyte meiotic maturation [38]. For example, in mammalian ovaries, Hippo signaling has been implicated in growth activation of quiescent primordial follicles, possibly offering a new avenue for the clinical management of fertility issues in women [38,39]. In addition, aging in mice is associated with altered ovarian expression of genes that comprise the Hippo signaling pathway [40], although the functional relevance of these observations to aging-related ovarian failure remains unknown. In studies of Drosophila, antagonism of Hippo signaling in niche somatic cells promotes the differentiation of adjacent GSCs [41]. However, we are not aware of any prior studies that have assessed the direct impact of mechanoactivation in the context of GSC differentiation in either males or females. Our results collectively demonstrate that biomechanical strain, applied either as a one-time stimulus or as a sequential subthreshold stimulus, directly activates the meiotic differentiation of OSCs into new oocytes, and this likely involves ROCK activation, redistribution of F-actin stress fibers, and YAP signaling. Of interest, it appears that YAP activation and nuclear translocation enable the association of YAP with MCAT sites in the Stra8 promoter, consistent with a central role for this gene in oogenesis as reported in prior studies of female germ cell meiotic commitment during prenatal and postnatal life [17,23,27,33–35].
It has been shown previously that the numbers of immature (primordial) oocyte-containing follicles in adult mouse ovaries fluctuate relative to different stages of the female reproductive cycle [42,43]. The largest numbers of immature oocytes are detected in each cycle at the point of final follicle maturation in preparation for ovulation, when the greatest level of biomechanical strain would be sensed by cells in the tissue proximal to the maturing follicle pool (refer to Fig. 1, which depicts the significant expansion of ovarian volume in response to gonadotropin stimulation that generates the ovulatory cohort of follicles). Accordingly, changes in biomechanical forces within the ovaries during each reproductive cycle associated with follicular growth, which causes an acute expansion of the tissue by more than one-fifth of its starting volume before gonadotropin stimulation, could directly influence the rate of de novo oogenesis by resident OSCs [17,27]. In this regard, physical contact between cells and their surrounding ECM has been identified as a principal route through which these changes in external forces are initially transduced to cells for sensing [44,45]. In turn, recent studies have shown that ECM proteins directly modulate the differentiation of both mouse and human OSCs into oocytes in a species-specific manner, potentially through integrin-mediated signaling [26].
We believe our findings reported herein may also be of value in further assessing the role of OSC dysfunction as a potential mechanism that contributes to exhaustion of the oocyte-containing follicle pool as females age. A number of studies have shown that OSCs persist in ovaries past the time of functional failure in both mice and women [25,46,47], and yet these cells somehow lose the capacity to support adult ovarian function through de novo oogenesis with advancing age [17]. Notably, ovarian aging is associated with dramatic changes in the architecture of the tissue leading to increased fibrosis, which would be indicative of the gonads becoming more rigid as females grow older [26,48]. The ensuing changes in the structural capacity of ovarian tissue to generate cyclic biomechanical signals with age, along with alterations in ECM proteins [26] as well as declining availability of local hormones that support OSC differentiation (ie, estrogen; [27]), may all combine to produce suboptimal conditions for OSCs to continue maintenance of an adequate oocyte reserve during female reproductive life. While more work is needed to test these hypotheses in vivo, our findings provide a foundation for such studies and add to a now large body of knowledge on OSC biology [19] accumulated since the initial reports of the existence of these cells and their support of postnatal oogenesis in mammalian ovaries almost two decades ago [16]. In the shorter term, the results reported herein may prove useful in the design and optimization of new technology platforms aimed at the reconstitution of functional ovarian tissue from autologous stem cells for the ultimate purpose of generating oocytes entirely ex vivo [18].
Author Disclosure Statement
J.A.M. declares no competing financial interests. D.C.W. declares interest in intellectual property described in U.S. Patent 8,642,329, U.S. Patent 8,647,869, U.S. Patent 9,150,830, and U.S. Patent 10,525,086. J.L.T. declares interest in intellectual property described in U.S. Patent 7,195,775, U.S. Patent 7,850,984, U.S. Patent 7,955,846, U.S. Patent 8,642,329, U.S. Patent 8,647,869, U.S. Patent 8,652,840, European Patent Specification No. EP1765085, U.S. Patent 9,150,830, U.S. Patent 9,267,111, U.S. Patent 9,845,482, U.S. Patent 9,962,411, and U.S. Patent 10,525,086.
Funding Information
This work was supported by a grant from the National Institutes of Health to Jonathan L. Tilly (R01-AG012279).
References
- 1.Vining KH and Mooney DJ. (2017). Mechanical forces direct stem cell behavior in development and regeneration. Nat Rev Mol Cell Biol 18:728–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith LR, Cho S and Discher DE. (2017). Stem cell differentiation is regulated by extracellular matrix mechanics. Physiology 33:16–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillip JM, Aifuwa I, Walston J and Wirtz D. (2015). The mechanobiology of aging. Annu Rev Biomed Eng 17:113–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steinberg MS. (1963). Reconstruction of tissues by dissociated cells. Science 141:401–408 [DOI] [PubMed] [Google Scholar]
- 5.Beloussov LV, Dorfman JG and Cherdantzev VG. (1975). Mechanical stresses and morphological patterns in amphibian embryos. J Embryol Exp Morphol 34: 559–574 [PubMed] [Google Scholar]
- 6.Rauzi M, Verant P, Lecuit T and Lenne PF PF. (2008). Nature and anisotropy of cortical forces orienting Drosophila tissue morphogenesis. Nat Cell Biol 10:1401–1410 [DOI] [PubMed] [Google Scholar]
- 7.Hong W and Guan K-L. (2012). The YAP and TAZ transcription co-activators: key downstream effectors of the mammalian Hippo pathway. Semin Cell Dev Biol 23:785–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Totaro A, Panciera T and Piccolo S S. (2018). YAP/TAZ upstream signals and downstream responses. Nat Cell Biol 20:888–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, Nguyen NK, Thrun S, Lutolf MP and Blau HM. (2010). Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science 329:1078–1081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cezar CA, Roche ET, Vandenburgh HH, Duda GN, Walsh CJ and Mooney DJ. (2016). Biologic-free mechanically induced muscle regeneration. Proc Natl Acad Sci U S A 113:1534–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Connelly JT, Gautrot JE, Trappmann B, Tan DW, Donati G, Huck WT and Watt FM. (2010). Actin and serum response factor transduce physical cues from the microenvironment to regulate epidermal stem cell fate decisions. Nat Cell Biol 12:711–718 [DOI] [PubMed] [Google Scholar]
- 12.Holst J, Watson S, Lord MS, Eamegdool SS, Bax DV, Nivison-Smith LB, Kondyurin A, Ma L, Oberhauser AF, Weiss AS and Rasko JE. (2010). Substrate elasticity provides mechanical signals for the expansion of hemopoietic stem and progenitor cells. Nat Biotechnol 28:1123–1128 [DOI] [PubMed] [Google Scholar]
- 13.Stowers RS, Shcherbina A, Israeli J, Gruber JJ, Chang J, Nam S, Rabiee A, Teruel MN, Snyder MP, Kundaje A and Chaudhuri O. (2019). Matrix stiffness induces a tumorigenic phenotype in mammary epithelium through changes in chromatin accessibility. Nat Biomed Eng 3:1009–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Rooij DG. (2017). The nature and dynamics of spermatogonial stem cells. Development 144:3022–3030 [DOI] [PubMed] [Google Scholar]
- 15.Kubota H and Brinster R. (2018). Spermatogonial stem cells. Biol Reprod 99:52–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnson J, Canning J, Kaneko T, Pru JK and Tilly JL. (2004). Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature 428:145–150 [DOI] [PubMed] [Google Scholar]
- 17.Wang N, Satirapod C, Ohguchi Y, Park E-S, Woods DC and Tilly JL. (2017). Genetic studies in mice directly link oocytes produced during adulthood to ovarian function and natural fertility. Sci Rep 7:10011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akahori T, Woods DC and Tilly JL. (2019). Female fertility preservation through stem cell-based ovarian tissue reconstitution in vitro and ovarian regeneration in vivo. Clin Med Insights Reprod Health 13:1179558119848007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin JJ, Woods DC and Tilly JL. (2019). Implications and current limitations of oogenesis from female germline or oogonial stem cells in adult mammalian ovaries. Cells 8:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baerwald AR, Adams GP and Pierson RA. (2012). Ovarian antral folliculogenesis during the human menstrual cycle: a review. Hum Reprod Update 18:73–91 [DOI] [PubMed] [Google Scholar]
- 21.White YAR, Woods DC, Takai Y, Ishihara O, Seki H and Tilly JL. (2012). Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med 18:413–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woods DC and Tilly JL. (2013). Isolation, characterization and propagation of mitotically active germ cells from adult mouse and human ovaries. Nat Protoc 8:966–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park ES, Woods DC and Tilly JL. (2013). Bone morphogenetic protein 4 (BMP4) promotes mammalian oogonial stem cell differentiation via SMAD1/5/8 signaling. Fertil Steril 100:1468–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding X, Liu G, Xu B, Wu C, Hui N, Ni X, Wang J, Du M, Teng X and Wu J. (2016). Human GV oocytes generated by mitotically active germ cells obtained from follicular aspirates. Sci Rep 6:28218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silvestris E, Cafforio P, D'Oronzo S, Felici C, Silvestris F and Loverro G. (2018). In vitro differentiation of human oocyte-like cells from oogonial stem cells: single-cell isolation and molecular characterization. Hum Reprod 33:464–473 [DOI] [PubMed] [Google Scholar]
- 26.MacDonald JA, Takai Y, Ishihara O, Seki H, Woods DC and Tilly JL. (2019). Extracellular matrix signaling activates differentiation of adult ovary-derived oogonial stem cells in a species-specific manner. Fertil Steril 111:794–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Satirapod C, Wang N, MacDonald JA, Sun M, Woods DC and Tilly JL. (2020). Estrogen regulation of female germline stem cell differentiation as a mechanism contributing to female reproductive aging. Aging (Albany, NY) 12:7313–7333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo K, C-h Li, X-y Wang, D-j He and Zheng P. (2016). Germ stem cells are active in postnatal mouse ovary under physiological conditions. Mol Hum Reprod 22:316–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M and Narumiya S. (2000). Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol 57:976–983 [PubMed] [Google Scholar]
- 30.Burridge K and Wittchen ES. (2013). The tension mounts: stress fibers as force-generating mechanotransducers. J Cell Biol 200:9–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohashi K, Fujiwara S and Mizuno K. (2017). Roles of the cytoskeleton, cell adhesion and rho signaling in mechanosensing and mechanotransduction. J Biochem 161:245–254 [DOI] [PubMed] [Google Scholar]
- 32.Abbassi L, Malki S, Cockburn K, Macaulay A, Robert C, Rossant J and Clarke HJ. (2016). Multiple mechanisms cooperate to constitutively exclude the transcriptional co-activator YAP from the nucleus during murine oogenesis. Biol Reprod 94:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baltus AE, Menke DB, Hu YC, Goodheart ML, Carpenter AE, de Rooij DG and Page DC. (2006). In germ cells of mouse embryonic ovaries, the decision to enter meiosis precedes premeiotic DNA replication. Nat Genet 38:1430–1434 [DOI] [PubMed] [Google Scholar]
- 34.Anderson EL, Baltus AE, Roepers-Gajadien HL, Hassold TJ, de Rooij DG, van Pelt AM and Page DC. (2008). Stra8 and its inducer, retinoic acid, regulate meiotic initiation in both spermatogenesis and oogenesis in mice. Proc Natl Acad Sci U S A 105:14976–14980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng CW, Bowles J and Koopman P. (2014). Control of mammalian germ cell entry into meiosis. Mol Cell Endocrinol 382:488–497 [DOI] [PubMed] [Google Scholar]
- 36.Wang C, Zhu X, Feng W, Yu Y, Jeong K, Guo W, Lu Y and Mills GB. (2016). Verteporfin inhibits YAP function through up-regulating 14-3-3σ sequestering YAP in the cytoplasm. Am J Cancer Res 6:27–37 [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao B, Ye X, Yu J, Li L, Li W, Li S, Yu J, Lin JD, Wang CY, Chinnaiyan AM, Lai ZC and Guan KL. (2008). TEAD mediates YAP-dependent gene induction and growth control. Genes Dev 22:1962–1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shah JS, Sabouni R, Cayton Vaught KC, Owen CM, Albertini DF and Segars JH. (2018). Biomechanics and mechanical signaling in the ovary: a systematic review. J Assist Reprod Genet 35:1135–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cordeiro CN, Christianson MS, Selter JH and Segars Jr JH. (2016). In vitro activation. A possible new frontier for treatment of primary ovarian insufficiency. Reprod Sci 23:429–438 [DOI] [PubMed] [Google Scholar]
- 40.Li J, Zhou F, Zheng T, Pan Z, Liang X, Huang J, Zheng L and Zheng Y. (2015). Ovarian germline stem cells (OGSCs) and the Hippo signaling pathway association with physiological and pathological ovarian aging in mice. Cell Physiol Biochem 36:1712–1724 [DOI] [PubMed] [Google Scholar]
- 41.Li C, Kan L, Chen Y, Zheng X, Li W, Zhang W, Cao L, Lin X, Ji S, et al. (2015). Ci antagonizes Hippo signaling in the somatic cells of the ovary to drive germline stem cell differentiation. Cell Res 25:1152–1170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Allen E. (1923). Ovogenesis during sexual maturity. Am J Anat 31:439–482 [Google Scholar]
- 43.Johnson J, Bagley J, Skaznik-Wikiel M, Lee H-J, Adams GB, Niikura Y, Tschudy KS, Tilly JC, Cortes ML, et al. (2005). Oocyte generation in adult mammalian ovaries by putative germ cells derived from bone marrow and peripheral blood. Cell 122:303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen CS, Tan J and Tien J. (2014). Mechanotransduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng 6:275–302 [DOI] [PubMed] [Google Scholar]
- 45.Humphrey JD, Dufresne ER and Schwartz MA. (2014). Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol 15:802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Niikura Y, Niikura T and Tilly JL. (2009). Aged mouse ovaries possess rare premeiotic germ cells that can generate oocytes following transplantation into a young host environment. Aging (Albany, NY) 1:971–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woods DC and Tilly JL. (2015). Autologous germline mitochondrial energy transfer (AUGMENT) in human assisted reproduction. Semin Reprod Med 33:410–421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laszczynska M, Brodowska A, Starczewski A, Masiuk M and Brodowski J. (2008). Human postmenopausal ovary—hormonally inactive fibrous connective tissue or more? Histol Histopathol 23:219–226 [DOI] [PubMed] [Google Scholar]