Abstract
Objective
Despite conflicting evidence, it is common practice to use continuous antibiotic prophylaxis (CAP) in patients with indwelling double-J (DJ) stents. Cranberry extracts and d-mannose have been shown to prevent colonization of the urinary tract. We evaluated their role in this setting.
Methods
We conducted a prospective randomized study to evaluate patients with indwelling DJ stents following urological procedures. They were randomized into three groups. Group A (n=46) received CAP (nitrofurantoin 100 mg once daily [OD]). Group B (n=48) received cranberry extract 300 mg and d-mannose 600 mg twice daily (BD). Group C (n=40) received no prophylaxis. The stents were removed between 15 days and 45 days after surgery. Three groups were compared in terms of colonization of stent and urine, stent related symptoms and febrile urinary tract infections (UTIs) during the period of indwelling stent and until 1 week after removal.
Results
In Group A, 9 (19.5%) patients had significant bacterial growth on the stents. This was 8 (16.7%) in the Group B and 5 (12.5%) in Group C (p-value: 0.743). However, the culture positivity rate of urine specimens showed a significant difference (p-value: 0.023) with Group B showing least colonization of urine compared to groups A and C. There was no statistically significant difference in the frequency of stent related symptoms (p-value: 0.242) or febrile UTIs (p-value: 0.399) among the groups.
Conclusion
Prophylactic agents have no role in altering bacterial growth on temporary indwelling DJ stent, stent related symptoms or febrile UTIs. Cranberry extract may reduce the colonization of urinary tract, but its clinical significance needs further evaluation.
Keywords: Antibiotic, Prophylaxis, Stent, Cranberry, Infection
1. Introduction
Double‐J (DJ) stents are commonly used in urological practice. They can be associated with various complications such as lower urinary tract symptoms (LUTS), migration, encrustation, urinary tract infection (UTI) and forgotten stents [1]. The incidence of UTIs can vary from 2% to 34% with various risk factors such as chronic kidney disease (CKD), diabetes mellitus and indwelling stent time [[1], [2], [3]]. DJ stents are often colonized within minutes of their placement with bio-film formation. This phenomenon is often associated with stent related UTIs [4].
Despite controversial evidence, it is common practice to use low dose continuous antibiotic prophylaxis (CAP) in patients with indwelling DJ stents with the intention of preventing stent related symptoms and febrile UTIs [5]. While evidence in support of continuous antibiotic prophylaxis is at best controversial, there is ongoing search for new modalities to reduce the incidence of stent related complications. Some authors have shown that anti-adherence agents, such as cranberry juice and d-mannose prevent adherence of bacteria to uroepithelial cells [6]. We conducted a randomized trial to document the role of both antibiotics and anti-adherence agents in patients with indwelling stents and analyzed the rates of colonization, febrile UTI and stent related symptoms.
2. Methods
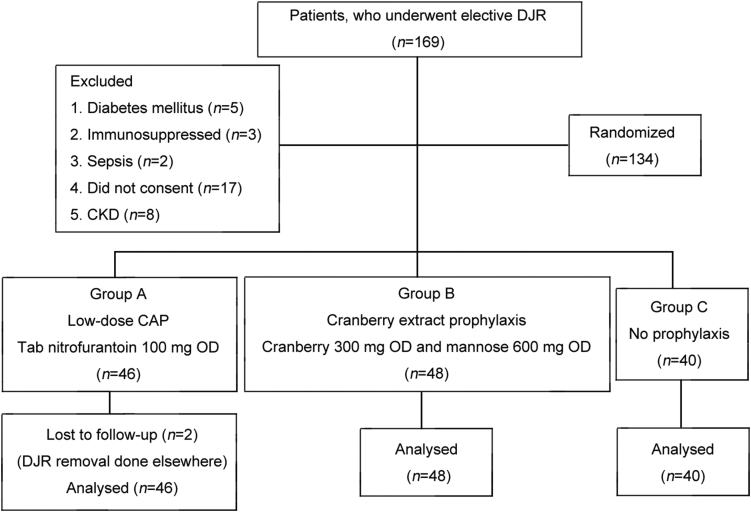
We conducted a randomized controlled trial from 1 February 2017 to 20 July 2017 as per the guidelines of our Institutional Ethics Committee. We included patients, aged 18–65 years, who underwent unilateral elective DJ stenting (polyurethane 6 Fr and 26 cm in length) following various urological procedures. Their enrolments are shown in Fig. 1. The preoperative preparation was done as per our institute's protocol. Procedure was carried out under general or spinal anaesthesia and each patient received a preoperative dose of intravenous cefoperazone 1 g and sulbactam 500 mg. Sterile urine culture was ensured prior to the procedure. Patients with diabetes mellitus, CKD (estimated glomerular filtration rate [eGFR] <30 mL/min), immunosuppressed states or septicemia were excluded. Those who did not consent were also excluded. We evaluated 134 patients who fulfilled our inclusion and exclusion criteria as given in Fig. 1.
Figure 1.
The enrolment criteria and experimental process of the patients who underwent unilateral elective double-J stenting following various urological procedures. CKD, chronic kidney disease; CAP, continuous antibiotic prophylaxis; OD, once daily; DJR, double-J stent.
Patients were randomized into three groups as given in Fig. 1 using simple random sampling. Those in Group A received low dose continuous antibiotic prophylaxis (Tab Nitrofurantoin 100 mg OD [once daily]) throughout the period of indwelling stent. Nitrofurantoin was selected on the basis of the antibiotic sensitivity patterns at our hospital. Patients in Group B received cranberry extract 300 mg and d‐mannose 600 mg BD (twice daily) throughout the period of indwelling stent. Patients in Group C received no prophylaxis. We compared the incidence of febrile UTIs, stent related symptoms and positive cultures in urine specimens and stents among the three groups.
The patients underwent stent removal between 15 days and 45 days of placement of the DJ stents. They were evaluated for any stent related symptoms such as urgency, frequency, dysuria or flank pain and febrile UTI prior to DJ stent removal. Patients were followed up till 7 days after DJ stent removal for any episodes of fever. Febrile UTI was defined as temperature >37.8 °C in the presence of positive urine culture. Patients were said to have stent related symptoms only if they persisted till at least a week after the procedure. We also reviewed the patient after 3 months of the procedure. In this visit, we evaluated our patients for any further episodes of febrile UTIs apart from routine follow-up investigations depending upon the procedure that they had undergone.
Additionally, we also evaluated common adverse effects of the drugs in each group and the rate of multidrug resistant organisms. Multidrug resistance was defined as resistance to more than one drug.
Prior to the removal of DJ stent, we collected urine samples of our patients. Urine was collected using a mid-stream (middle part of the stream) clean catch when patients came for stent removal. In addition, urine was also collected when patients visited the clinic or emergency department with any other lower urinary tract symptoms. The specimen was processed within 1 h. In case the patient visited out of hours, urine sample was stored at 5 °C. Urine was processed on cystine–lactose–electrolyte-deficient (CLED) agar plate using standard loop method. It was incubated aerobically at 37 °C for 24 h. A positive culture was defined as growth of single pathogen of more than 105 colony forming units (CFU) per milliliter of urine. Similarly, stents were collected in a sterile container and processed within 1 h. The surface and the tips were washed with tryptic soy broth agar and incubated aerobically for 24 h at 37 °C. MacConkey, blood and chocolate agar were used for cultures. Positive cultures were defined as representative colonies of single organisms, which numbered more than 10 in a plate. If either culture grew multiple organisms, it was labeled as contaminated. Anaerobic cultures were not performed. Antibiotic sensitivity was tested using the Kirby-Bauer diffusion disk method.
Sample size was calculated using a confidence interval of 95% and 5% margin of error. Estimate of population was based on the number of patients, undergoing elective DJ stenting in our province and estimated colonization rate of 25%. Estimated sample size was 385. Normality of distribution of the variables was checked by Shapiro-Wilk test and Q-Q plots. Normally distributed data were analyzed by comparison of mean. Median was used for variables, which did not show normal distribution. Mean values were compared using student t-test and ANOVA while medians were compared using Kruskal-Wallis test and ANOVA test. In case of categorical data, the comparison was made using Chi‐square test and logistic regression. A predictive model was used to calculate the predicted rates of stent culture positivity among different groups using regression analysis.
On subgroup analysis, we categorized our patients into two groups depending upon when their stents were removed between 15 days and 29 days and between 30 days and 45 days. We used this categorization to determine if duration of indwelling stent had any effect on the stent cultures. We also used this categorization to create six groups and then build a predictive model giving us the expected rates of bacterial growth on the indwelling DJ stents in different scenarios.
3. Results
We analyzed 134 patients in our study. The demographic data of our patients are given in Table 1. After stenting, we followed our patients for any stent related symptoms or drug related adverse effects, which is also given in Table 1. There was no significant difference in the reporting of febrile UTI or other stent related symptoms among the three groups as in Table 1.
Table 1.
Baseline parameters of our patients and categorisation into groups.
| Characteristic | Antibiotic group (Group A) | Cranberry group (Group B) | No prophylaxis (Group C) | p-Value |
|---|---|---|---|---|
| Patient, n | 46 | 48 | 40 | |
| Median age (range), year | 36 (19–64) | 39 (21–59) | 33 (18–55) | 0.811 |
| Male/female, n | 35/11 | 40/8 | 31/9 | 0.917 |
| Procedure, n | ||||
| Percutaneous nephrolithotomy | 19 | 17 | 20 | 0.837 |
| Ureteroscopy and lithotripsy | 18 | 20 | 14 | 0.975 |
| Laparoscopic pyeloplasty | 9 | 11 | 6 | |
| Staghorn calculi, n | 7 | 6 | 7 | |
| Location of calculi, n | ||||
| Kidney | 15 | 13 | 15 | 0.705 |
| Upper ureter | 10 | 10 | 6 | |
| Mid ureter | 5 | 10 | 6 | |
| Lower ureter | 7 | 4 | 5 | |
| Median indwelling stent time, mean (range), day | 30 (18–41) | 29 (21–45) | 26 (19–36) | 0.842 |
| Febrile urinary tract infection (temperature >37.8 °C with positive urine culture), n | 6 | 7 | 3 | 0.399 |
| Dysuria, n | 15 | 13 | 16 | 0.425 |
| Macroscopic hematuria, n | 3 | 1 | 4 | 0.242 |
| Flank pain, n | 25 | 22 | 19 | 0.754 |
| Drug related adverse effects, n | ||||
| Nausea | 14 | 17 | 11 | 0.684 |
| Vomiting | 5 | 3 | 0 | 0.174 |
| Upper gastrointestinal discomfort/heartburn | 11 | 13 | 7 | 0.562 |
| Skin rashes | 3 | 1 | 0 | 0.385 |
| Diarrhea | 7 | 3 | 2 | 0.310 |
| Multidrug resistance (resistance to more than 1 drug) | 4 | 1 | 1 | 0.574 |
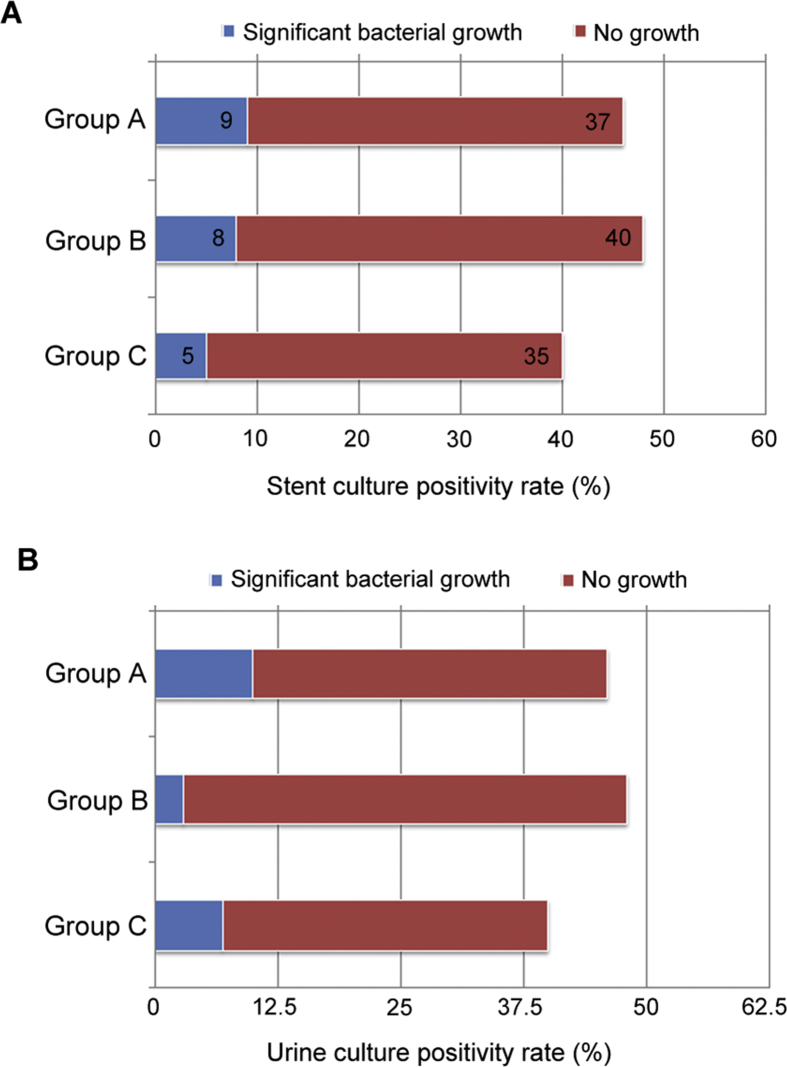
Out of 134 patients, 22 showed significant bacterial growth upon culture of DJ stent, which shows a colonization rate of 16.4%. In Group A, 9 (19.6%) out of 46 patients had positive cultures. In Group B, 8 (16.7%) out of 48 had significant bacterial growth in the culture of their DJ stent. In Group C, only 5 (12.5%) out of 40 had significant bacterial growth. Thus lowest culture positivity rate was seen in the no prophylaxis group, but this was not statistically significant (p=0.743) as shown in Fig. 2. In case of urine cultures, the cranberry extract group or Group B showed a significantly lower culture positivity rate as seen in Fig. 2 (p=0.023). Overall, 20 out of 134 patients (14.93%) had a positive urine culture. In the antibiotic group, 7 out of 40 patients (15.6%) had a positive culture. In the cranberry group, 3 out of 48 patients (6.7%) and in the no prophylaxis group, 10 out of 46 (27.8%) patients had a positive culture. However this did not translate into any clinical advantage, as there is no significant difference among the three groups in terms of fever, dysuria or any other stent related symptoms. Additionally, the rates of adverse effects were least in the no prophylaxis group, but this did not reach statistical significance. Similarly, the maximum number of multidrug resistant organisms was isolated among patients on CAP but again statistical significance could not be achieved (Table 1). The details of bacterial growth in the three groups are shown in Table 2. Lastly, during the next follow-up visit at 3 months post stent removal, only one patient had developed any further episode of febrile UTI (CAP group). However due to small number of patients, no statistical analysis was performed at 3 months.
Figure 2.
The culture positivity rate of the stent cultures and urine cultures among the three groups. (A) Stent culture positivity rate (p‐value: 0.743); (B) Urine culture positivity rate (p‐value: 0.023).
Table 2.
Various microorganisms isolated from our patients.
| Organism | Urine culture, n |
Stent culture, n |
||||
|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group A | Group B | Group C | |
| Escherichia coli | 7 | 2 | 6 | 6 | 5 | 3 |
| Enterococcus faecalis | 1 | 1 | 0 | 2 | 1 | 1 |
| Klebsiella | 0 | 0 | 0 | 1 | 0 | 1 |
| Streptococcus | 0 | 0 | 1 | 0 | 0 | 0 |
| Pseudomonas aeruginosa | 1 | 0 | 0 | 0 | 1 | 0 |
| Staphylococcus | 1 | 0 | 0 | 0 | 1 | 0 |
| Total | 10 | 3 | 7 | 9 | 8 | 5 |
Out of 134 patients, 60 patients had their stents removed in 15–29 days. Out of these 60, 8 (13.33%) had a positive stent culture. Seventy-four patients had their stents removed between days 30–45 after surgery. Out of these 74, 14 (19.18%) had positive stent culture. The stent culture positivity rate did not vary significantly between the two groups (p=0.385). Using available data, we calculated prediction tables using regression analysis for a patient having a colonized stent, which is given in Table 3.
Table 3.
Prediction of positive cultures depending on duration of stent.
| Duration of indwelling stent (day) | Prophylaxis group | Predictive value of a positive stent culture (%) |
|---|---|---|
| 15–29 | -No prophylaxis | 3.4–25.0 |
| -Cranberry prophylaxis | 5.0–29.0 | |
| -Antibiotic prophylaxis | 7.4–32.1 | |
| 30–45 | -No prophylaxis | 5.9–32.0 |
| -Cranberry prophylaxis | 9.3–34.2 | |
| -Antibiotic prophylaxis | 11.5–42.1 |
4. Discussion
Despite controversial evidence and in the absence of specific guidelines, it is common practice to start patients with indwelling DJ stents on low dose antibiotics to reduce the probability of developing febrile UTIs and stent related symptoms. However, there is no definite evidence to support this school of thought although few authors have supported the use of antibiotics and other prophylactic agents [[5], [6], [7], [8]]. Abuse of antibiotics can lead to rising antibiotic resistance and increased cost of care.
While the American Urological Association (AUA) guidelines on urologic procedures and antimicrobial prophylaxis states that no prophylaxis is needed for the period of indwelling double‐J stents, it concedes that this recommendation is based on low quality of evidence [9]. Another review by Beysens and Tailly [10] recommended no advantage of low dose continuous antibiotic prophylaxis in patients with indwelling stents. European Association of Urology (EAU) guidelines also recommend avoiding giving antibiotics during the period of indwelling stents [11]. While most guidelines concur on avoidance of antibiotic prophylaxis for patient with indwelling stents, the evidence that this is based on is not high level. There is only one randomized controlled trial by Moltzahn et al. [12], which evaluated this question and has formed the basis of most guidelines. They randomized patients into two groups: Continuous antibiotic prophylaxis and no prophylaxis (all patients received a single preoperative dose of antibiotics at least). They did not demonstrate any significant difference in rate of UTI, fever, bacteriuria or stent related symptoms among the two groups [12]. Similar to our study, the two groups had 44 and 51 patients each. They used amoxicillin/clavulanic acid (625 mg) once daily in contrast to nitrofurantoin 100 mg used by us. Moltzahn et al. [12] demonstrated maximum number of multidrug resistant organisms in the antibiotic group, which is similar to our findings.
We demonstrated lower incidence of bacteriuria in the cranberry group. This is expected as cranberry extract is well known to prevent adherence of bacteria to the urothelial tract [6,13].
There is some evidence in favour of use of continuous antibiotic prophylaxis in patients with indwelling DJ stents. Alsaywid et al. [14] reported a significant difference in rates of symptomatic UTIs when patients were on continuous antibiotic prophylaxis (7% vs. 25%). Similarly, Wilson et al. [15] reported that antibiotic prophylaxis with cotrimoxazole 480 mg daily led to a reduced risk of stent related UTIs. The Canadian Agency for Drugs and Technologies in Health issued a guideline in 2017 and recommended the use of antibiotics in this setting [16]. Similarly, Ramaswamy and Shah [5] recommended the use of perioperative antibiotics in patients after ureteroscopy, but they gave antibiotics to their patients for 1 week only.
Thus, it is clear that there is a paucity of evidence that is required to answer the question about the need of continuous antibiotic prophylaxis in patients with indwelling stents. In our study, we have documented that antibiotics make no difference to rates of colonization as well as febrile UTIs. In addition, they do not bring any changes to stent related symptoms. Further, no studies have evaluated the role of cranberry extract and d-mannose in this setting.
Our urine culture positivity rate was comparable to other studies, which have evaluated post procedural UTI rates. Most studies demonstrated this to be between 6% and 38% [12,[17], [18], [19], [20]]. While some studies have documented a stent colonization rate of 42.00%, ours was lower at 16.42% [19]. This can be explained by the fact that our indwelling DJ stent duration was less than 45 days. We demonstrated that giving patients continuous prophylaxis throughout the duration of indwelling stent either with antibiotics or cranberry led to no significant difference in the colonization of the stents of these patients. With regard to the colonization of stents, no such previous randomized trials exist to the best of our knowledge.
Since we have also classified patients depending upon the duration of stents, we had a total of six categories. We have calculated the predictive rates of colonization, which one can expect depending upon which category the patients fall into. After taking these into account, we conclude that prophylaxis by either cranberry or antibiotics does not prevent colonization of the stents. Anti-adherence agent may however have a role in preventing colonization of the urinary tract in patients with indwelling stents.
5. Limitations
Our study has a few limitations, which include small number of patients, heterogeneous study population and a short follow-up. The heterogeneity was due to the multiple elective procedures and range of period of indwelling stent. The study was underpowered and recruitment was withheld as preliminary analysis showed no benefit in groups A and B. In addition, we did not perform cultures on the stones and this might have added a limited amount of bias to the results despite urine cultures being clear. However, since all the procedures were evenly matched among groups, this bias is likely to have had a minimal effect on the results.
6. Conclusion
Prophylactic antibiotics and antiadhesion agents have no role in altering colonization on temporary indwelling DJ stent, stent related symptoms or symptomatic UTI. Cranberry extract may reduce the colonization of urinary tract. This may not necessarily transform into any clinical benefit in terms of stent related symptoms or fever. However, these results need to be validated externally in a larger setting.
Author contributions
Study concept and design: Uday Pratap Singh, Sanjoy Kumar Sureka, Rakesh Kapoor, Anessh Srivastava, M S Ansari.
Data acquisition: Sanchit Rustagi, Rahul Jena, Sanjoy Kumar Sureka.
Data analysis: Kumar Madhavan.
Drafting of manuscript: Kumar Madhavan.
Critical revision of the manuscript: Sanjoy Kumar Sureka, Rakesh Kapoor, Anessh Srivastava, M S Ansari.
Conflicts of interest
The authors declare no conflict of interest.
Footnotes
Peer review under responsibility of Second Military Medical University.
References
- 1.Klis R., Korczak-Kozakiewicz E., Denys A., Sosnowski M., Rozanski W. Relationship between urinary tract infection and self-retaining double-J catheter colonization. J Endourol. 2009;23:1015–1019. doi: 10.1089/end.2008.0518. [DOI] [PubMed] [Google Scholar]
- 2.Akay A.F., Aflay U., Gedik A., Sahin H., Bircan M.K. Risk factors for lower urinary tract infection and bacterial stent colonization in patients with a double‐J ureteral stent. Int Urol Nephrol. 2007;39:95–98. doi: 10.1007/s11255-006-9150-1. [DOI] [PubMed] [Google Scholar]
- 3.Paick S.H., Park H.K., Oh S.J., Kim H.H. Characteristics of bacterial colonization and urinary tract infection after indwelling of double‐J ureteral stent. Urology. 2003;62:214–217. doi: 10.1016/s0090-4295(03)00325-x. [DOI] [PubMed] [Google Scholar]
- 4.Ando E., Monden K., Mitsuhata R., Kariyama R., Kumon H. Biofilm formation among methicillin resistant Staphylococcus aureus isolates from patients with urinary tract infection. Acta Med Okayama. 2004;58:207–214. doi: 10.18926/AMO/32090. [DOI] [PubMed] [Google Scholar]
- 5.Ramaswamy K., Shah O. Antibiotic prophylaxis after uncomplicated ureteroscopic stone treatment: is there a difference? J Endourol. 2012;26:122–125. doi: 10.1089/end.2011.0360. [DOI] [PubMed] [Google Scholar]
- 6.Foo L.Y., Lu Y., Howell A.B., Vorsa N. A-Type proanthocyanidin trimers from cranberry that inhibit adherence of uropathogenic P-fimbriated Escherichia coli. J Nat Prod. 2000;63:1225–1228. doi: 10.1021/np000128u. [DOI] [PubMed] [Google Scholar]
- 7.Grabe M. Antibiotic prophylaxis in urological surgery, a European viewpoint. Int J Antimicrob Agents. 2011;38:58–63. doi: 10.1016/j.ijantimicag.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Grabe M. Controversies in antibiotic prophylaxis in urology. Int J Antimicrob Agents. 2004;23:17–23. doi: 10.1016/j.ijantimicag.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 9.Urologic procedures and antimicrobial prophylaxis. 2019. https://www.auanet.org/guidelines/urologic-procedures-and-antimicrobial-prophylaxis-(2019)
- 10.Beysens M., Tailly T.O. Ureteral stents in urolithiasis. AsianJ Urol. 2018;5:274–286. doi: 10.1016/j.ajur.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bonkat B, Bartoletti R, Bruyère F, Cai T, Geerlings SE, Köves B, et al. EAU guidleines on urological infections. https://uroweb.org/guideline/urological-infections/. [Access 20 Mar 2020].
- 12.Moltzahn F., Haeni K., Birkhäuser F.D., Roth B., Thalmann G.N., Zehnder P. Peri-interventional antibiotic prophylaxis only vs. continuous low-dose antibiotic treatment in patients with JJ stents: a prospective randomised trial analysing the effect on urinary tract infections and stent-related symptoms. BJU Int. 2013;111:289–295. doi: 10.1111/j.1464-410X.2012.11592.x. [DOI] [PubMed] [Google Scholar]
- 13.Jensen H.D., Struve C., Christensen S.B., Krogfelt K.A. Cranberry juice and combinations of its organic acids are effective against experimental urinary tract infection. Front Microbiol. 2017;8:542. doi: 10.3389/fmicb.2017.00542.eCollection.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alsaywid B.S., Mesawa A.A., Mohammedkhalil A.K., Almarghoub M., Barnawi Z., Abuznadah W.T. Antibiotic prophylaxis in children with ureteric stents: bliss or misery? Urol Ann. 2019;11:421–425. doi: 10.4103/UA.UA_116_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson C.H., Bhatti A.B., Rix D.A., Manas D.M. Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst Rev. 2005:CD004925. doi: 10.1002/14651858.CD004925.pub2. [DOI] [PubMed] [Google Scholar]
- 16.Reynen E., Picheca L. Canadian Agency for Drugs and Technologies in Health; Ottawa (ON): 2017. Ureteral stents: a review of clinical effectiveness and guidelines. [internet] PMID: 29266911. [PubMed] [Google Scholar]
- 17.Kliś R., Szymkowiak S., Madej A., Blewniewski M., Krześlak A., Forma E. Rate of positive urine culture and double‐J catheters colonization on the basis of microorganism DNA analysis. Cent European J Urol. 2014;67:81–85. doi: 10.5173/ceju.2014.01.art18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi R., Singh D.R., Sharma S. Lower urinary tract infection and bacterial colonization in patient with double‐J ureteral stent. J Nepal Health Res Counc. 2011;9:165–168. [PubMed] [Google Scholar]
- 19.Yeniyol C.Ö., Tuna A., Yener H., Zeyrek N., Tilki A., Coskuner A. Bacterial colonization of double‐J stents and bacteriuria frequency. Int Urol Nephrol. 2002;34:199–202. doi: 10.1023/a:1023285422278. [DOI] [PubMed] [Google Scholar]
- 20.Kehinde E.O., Rotimi V.O., Al-Hunayan A., Abdul-Halim H., Boland F., Al-Awadi K.A. Bacteriology of urinary tract infection associated with indwelling J ureteral stents. J Endourol. 2004;18:891–896. doi: 10.1089/end.2004.18.891. [DOI] [PubMed] [Google Scholar]