Abstract
Background
Assisted partner notification (PN) is an effective approach for increasing HIV testing among heterosexual partners. There is sparse evidence on its effect among sexual partners of men who have sex with men (MSM).
Methods
A randomized controlled trial was conducted to compare the effect of assisted PN and passive PN interventions on uptake of HIV testing among male and female sexual partners of newly HIV-diagnosed MSM. In the passive PN group, participants were encouraged to disclose their HIV status and refer and persuade sexual partners to access HIV testing services (HTS). In the assisted PN group, participants were further provided with HIV self-testing kits for sexual partners to take a test at home or allow a community health worker from MSM-serving community-based organization (CBO) to anonymously refer and persuade their sexual partners to access HTS. The primary outcome was the proportion of index cases who had any sexual partner accessing HTS within four months after randomization. This trial is registered with chictr.org.cn, ChiCTR1800017813.
Findings
Between August 2017 and January 2019, 187 MSM newly diagnosed with HIV in a large city Shenyang in northern China were enrolled in the study and randomly assigned to either passive PN (n=90) or assisted PN (n=97) study groups. The proportion of index cases who disclosed their HIV status to any sexual partners within three months of randomization was similar between passive PN (57%, 95% confidence interval [CI]: 46-67%) and assisted PN groups (58%, 95% CI: 48-68%). During four months of follow-up, the number of sexual partners named, referred to HTS, tested and testing positive per index case was 3•2, 0•7, 0•2 and 0•03 in the passive PN group, and 4•0, 1•0, 0•5 and 0•10 in the assisted PN group. Thirty-five percent of index cases in the assisted PN group had any sexual partners accessing HIV testing compared to 17% in the passive PN group (P = 0•004); 49% sexual partners who were disclosed by index cases in the assisted PN group had access HTS compared to 28% in the passive PN group (P = 0•007).
Interpretation
The assisted PN strategy incorporating HIV self-testing and CBO outreach can increase uptake of HIV testing among sexual partners of MSM who were recently diagnosed with HIV.
Funding
National Science and Technology Major Project of China, National Natural Science Foundation of China, and Project for Overseas Visiting Research of Liaoning Province.
Keywords: Assisted partner notification, HIV testing, Men who have sex with men, Community-based organization, Randomized controlled trial
Research in context.
Evidence before this study
We searched peer-reviewed journal articles, as of June 28, 2020, assessing the effect of assisted partner notification (PN) among HIV-infected men who have sex with men (MSM). We used the search terms “partner notification”, “HIV partner service”, “men who have sex with men”, and “male couple” without language restrictions. We found 19 articles about partner notification among MSM living with HIV, mainly from the Americas, Europe, Australia and Taiwan. These studies included one review, two randomized controlled trial (RCT) for syphilis or HIV partner notification and 16 observational studies. We are not aware of any published data on evaluating the efficacy of assisted PN linked with HIV self-testing and community mobilization for improving HIV disclosure and partner testing among HIV-infected MSM.
Added value of this study
To our knowledge, this is the first randomized controlled trial to evaluate the effect of assisted PN on HIV disclosure and sexual partners testing among MSM living with HIV. Though there was no difference in HIV disclosure between assisted PN and passive PN strategies, the assisted PN strategy (incorporating HIV self-testing and community-based organization outreach) significantly increased uptake of HIV testing among sexual partners of MSM who were recently diagnosed with HIV (49% in assisted PN vs. 28% in passive PN group; P = 0•007). More index cases in the assisted PN group had any sexual partners accessing HIV testing (35% in assisted PN vs. 17% in passive PN group; P = 0•004).
Implications of all the available evidence
HIV testing is the gateway to prevention and care, and it is suboptimal among MSM. HIV/AIDS cases are increasing among MSM in China. Passive PN is still widely adopted by HIV public health workers, which results in very low HIV testing coverage among sexual partners of HIV-positive MSM. Our study suggests that the assisted PN strategy is effective and feasible for increasing HIV testing and expanding testing coverage through sexual networks of MSM. Further research is needed to develop more effective PN strategies to improve uptake of HIV testing among MSM sexual partners.
Alt-text: Unlabelled box
Introduction
Although public health officials have focused on reaching the “90-90-90” HIV targets since 2014, these goals have still not been met.1 Globally, 81% of people living with HIV (PLHIV) had been diagnosed in 2019, 67% of those diagnosed received treatment, and 59% of those receiving treatment had achieved HIV suppression. Progress toward achieving these targets, particularly the first one, is even lower in the Asia-Pacific region and China in 2018 (69%-54%-49% and 68%-80%-94%, respectively).2 To achieve the first target of ensuring at least 90% of PLHIV are diagnosed in China, new approaches are needed to increase HIV testing in key populations, such as men who have sex with men (MSM). In China, MSM are disproportionately affected by HIV. The HIV prevalence among MSM in 2018 was 6•9%, which was significantly higher than in the general population (0•09%). Fifty-nine percent of HIV-positive MSM have been diagnosed, but less than half of these patients had received treatment.2,3 In addition, Chinese MSM are discriminated against because their sexual orientation, thus increasing their privacy concerns relating to disclosure of a positive HIV diagnosis to sexual partners.4
Passive HIV partner notification (PN) refers to when PLHIV are encouraged by health care providers to disclose their status to their sexual partners by themselves and suggest their partners to take HIV testing. Assisted PN, i.e., when providers offer assistance to PLHIV to contact and test sexual partners, has been found to be acceptable by those newly diagnosed and highly effective for case-finding among sexual partners.5,6 A meta-analysis of randomized controlled trials (RCTs) from Malawi, Kenya and the United States demonstrated that assisted PN resulted in a 1•5-fold increase in the uptake of HIV testing among sexual partners compared to passive PN.[5], [6], [7], [8] Observational studies conducted in Mozambique and the United States have shown that assisted PN approaches were associated with higher HIV testing uptake among notified partners compared to passive PN.[9], [10] The World Health Organization HIV PN guidelines strongly recommend voluntary assisted PN services be part of a comprehensive package offered to PLHIV.11 However, only 67 countries have policies for HIV PN worldwide in 2016, of which 17% mention provider referral approach.11
In addition, most research on PN focuses on opposite-sex sexual partnerships, and thus may not be generalizable to MSM.12 Several observational studies have investigated the implementation of passive PN and assisted PN among MSM.[13], [14], [15] More data are needed on PN intervention among MSM, but few RCTs have been conducted to evaluate the effectiveness of assisted PN compared with passive PN in this population.
Involving an MSM-friendly community-based organization (CBO) in HIV prevention efforts can improve HIV testing and linkage to care among MSM.16,17 HIV self-testing (HIVST) also promotes HIV testing uptake by providing rapid results at home.18,19 Previous research on integrating CBOs or HIVST to a PN strategy is limited; however, one cross-sectional study in Vietnam found that community-led HIV testing services (HTS) such as assisted PN for key populations (i.e., MSM and people who inject drugs) are feasible and effective.20 Most previous studies on PN were carried out in developed countries and evaluated the effectiveness of PN on prevention of sexually transmitted infections.21
The current strategy based on passive PN among MSM in China might be hard to reach sexual partners of PLHIV. With the background of moderate high HIV burden among Chinese MSM,22 evidence on new effective strategies is needed for policy maker to adjust HIV case-finding strategies. We conducted an RCT to assess the feasibility and effectiveness of an assisted PN strategy that incorporated HIV self-testing or MSM-serving CBO outreach on increasing HIV testing among sexual partners of Chinese MSM.
Methods
Study design and participants
This study was designed as an RCT to evaluate the effect of an assisted PN strategy on HIV testing uptake among sexual partners of MSM living with HIV. Participants were recruited in the HIV voluntary counseling and testing (VCT) clinic at a teaching hospital of medical school in Shenyang City in northern China. From August 2017 to January 2019, PLHIV were invited to participate in the study. Men were eligible if they met the following conditions: born male, age 18 years or older, self-report of oral or anal intercourse with male partners in the past six months, a diagnosis with HIV in the past three months, and provision of informed consent for study participation. MSM who had already notified sexual partners of their HIV status were excluded. Following informed consent, an interviewer administered a questionnaire to collect data on demographic and behavioral characteristics, sexual behaviors, and HIV testing history. Then, participants were randomized to either passive PN or assisted PN groups.
Randomization
A randomization sequence was created using SPSS 20•0 statistical software with the number seed of 20170814, and the Visual Binning function in SPSS was then used to divide the sequence into two groups. Participants were enrolled and assigned to either the passive PN or assisted PN group. Enrollment was ongoing for the study period, and participants were followed for four months after randomization.
Study procedures
After randomization, a trained study counselor encouraged index cases of both passive PN and assisted PN groups to disclose their HIV status to their sexual partners during a four-month follow-up period. In addition, index cases were asked by clinical staff to refer and persuade their sexual partners to access HTS at a nearby clinic on themselves.
In the assisted PN group, index cases were additionally offered two options: HIVST kits for at-home testing of their partners, or providing partners’ contact information and providing consent for a trained community health worker (CHW) from an MSM-serving CBO to anonymously notify their sexual partner(s) of their potential exposure to HIV and invite them to take an HIV test at the study clinic. In the HIVST kits option, research staff demonstrated in-person to the index case how to use the HIVST kits and provided enough kits for all sexual partners of the index case. Index cases were instructed to distribute the HIVST kits to their partners and ask their sexual partners to provide online consent. After their sexual partners completed self-testing, the index cases sent the photographs of the test results to research team through website. In the CHW outreach option, index cases provided contact information of their sexual partners (i.e., phone numbers and social media accounts, primarily Blued and WeChat). A full-time employed CHW from an MSM-serving CBO contacted sexual partners using the following message: “Good day. My name is Qiang and I am a counselor from the VCT clinic at the First Affiliated Hospital of China Medical University. Our facility provides free HIV testing services and health education. If you visit our VCT clinic, you will be compensated about seven US dollars and receive free condoms and lubricants. My phone number is ‘***’. Looking forward to seeing you soon.” This invitation did not disclose any information on the index case including their HIV status. The CHW discussed strategies to help overcome any barriers to visiting the VCT clinic, and scheduled appointments for sexual partners to access HTS. The CHW made three phone attempts, followed by three social media attempts to contact each sexual partner provided by the index case. When any sexual partners visited the study clinic, they provided a consent before receiving an HIV testing.
In the passive PN group, the index cases were asked to disclose their HIV status to their sexual partners and suggest their partners to get an HIV test on themselves. Research staff contacted index case in both passive PN and assisted PN groups by phone at one, two, three and four months of follow-ups to ask index cases about whether their sexual partners took a test and what was the testing result. Index cases in both groups who chose not to disclose their HIV status to any partner were asked to provide information on why they decided not to disclose; this data was stratified by type of sexual partner.
Definitions of outcomes and covariates
The primary outcome was the proportion of index cases who successfully had any sexual partner accessing HTS (i.e., documented HIV self-testing or clinic-based testing) within four months after randomization. The secondary outcomes included the proportion of index cases who disclosed their HIV status to any sexual partner within three months after randomization, the proportion of referred sexual partners who accessed HTS during the four months of follow-up, and the proportion of sexual partners who accessed HTS that provided a positive test report for HIV (either HIVST or clinic-based).
Referred sexual partners were defined as individuals who had sex with index case, suggested HTS by the index case or reached out to HTS by the CHW. Accessed HTS was defined as the referred to sexual partner completing an HIV test. Completing an HIV test included providing documentation of the use of an HIVST (i.e., sending a picture of the test results to study staff), receiving a clinic-based HIV test (i.e., providing the official HIV testing report to study staff, with or without HIV result), or going to the clinic and reporting a previous positive test for HIV (i.e., providing a photo of a positive previously taken HIVST result or HIV testing report from health facilities). Tested positive (i.e., newly diagnosed with HIV) was defined as the sexual partner providing a documented positive test result to study staff from a test taken after enrollment of the index case (i.e., a photo of a positive HIVST result or of a positive result from the official HIV testing report).
Regular male sexual partner was defined as a man with whom the index case had a sexual relationship lasting more than three months in past six months, and casual male sexual partner was defined as a man with whom the index case had occasional sex or a sexual relationship lasting three months or fewer in past six months. Female sexual partner (i.e., a wife, girlfriend, or female commercial sex partner) was defined as a woman having any sexual relationship with the index case in past six months.
Statistical analysis
The sexual partner testing index equals to the number of sexual partners who accessed HIV testing divided by the number of index cases. The case-finding index equals the number of newly diagnosed HIV cases among sexual partners divided by the number of index cases. The sample size was calculated based on estimating the difference in the proportion of index cases in two groups who had any sexual partner accessing HTS within four months after randomization. We estimated that a sample size of 192 MSM participants would provide a statistical power of 80% to detect a difference of 20% in the proportions between two study groups at a significance level of 0•05 (two-tailed) (see Supplementary appendix 2).
Cox proportional hazards regression was used to determine the adjusted hazard ratio (aHR) for factors associated with the index case who had any sexual partner access HTS within four months of randomization, including intervention group. Cox regression analysis was also performed to calculate aHR for the same factors plus the type of sexual partner, which are associated with more partners accessing to HTS. Covariates included age, residence permit in the past six months, marital status, occupation, education, monthly income, main venue for seeking male sexual partners in the past six months (i.e., internet and mobile applications or other), self-reported sexual orientation (i.e., homosexual or other), and the number of regular male sexual partners, casual male sexual partners, and female sexual partners in the past six months, and the condom use with each type of sexual partner in the past six months (i.e., always, mostly, occasionally, never).
Time to having any sexual partner access HTS among index cases (both HIV self-testing or clinic-based) and time to test HIV among sexual partners were calculated using the Kaplan-Meier estimator. In addition, we compared our findings to the HIV testing cascade stratified by each PN group and type of sexual partner: 1) number of sexual partners identified; 2) proportion of identified sexual partners who were disclosed/referred to HTS (by index case or by CHW); 3) proportion of those referred who were persuaded to access HIV testing (both HIV self-testing and clinic-based testing); and 4) proportion of those persuaded to access HIV testing who provided a documented HIV positive test report (i.e., were diagnosed with HIV). Above findings were compared using a Chi-square test. An α level of 0•05 was defined as statistically significant. All data analysis was conducted using SPSS 20•0 (IBM Corp., Armonk, NY, USA). This trial is registered with chictr.org.cn, ChiCTR1800017813.
Role of the funding source
The funders had no role in study design, data collection, data analysis, interpretation, or writing of the report.
Results
Demographic and behavioral characteristics of index cases
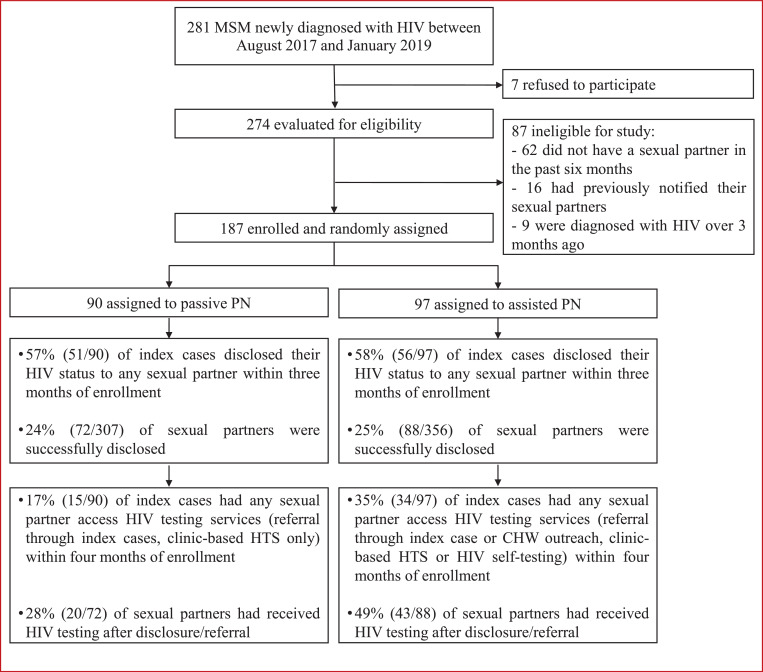
A total of 281 MSM were newly diagnosed with HIV at the study clinic between August 2017 to January 2019. Seven refused to participate, and of the remaining 274 potential participants, 187 (68%) were eligible for the study and randomly assigned to either passive PN (n=90) or assisted PN (n=97) study groups. The main reason for ineligibility was not having any sexual partners in the past six months (Figure 1).
Figure 1.
Study design of randomized controlled trial
Of 187 men enrolled in this study, the median age was 29•2 years (interquartile range (IQR): 24•6–36•1 years), 40% were single, and 61% self-reported their sexual orientation as homosexual. Approximately 65% of participants met sexual partners in the past six months mainly through the Internet or mobile applications, and about 58% were employed full-time. Participant characteristics were similar in both the passive PN and assisted PN groups (Table 1).
Table 1.
Baseline sociodemographic characteristics and sexual behaviors of 187 participants
| Variable | Total | Passive PN group | Assisted PN group |
| N=187 (%) | n=90 (%) | n=97 (%) | |
| Age, years | |||
| ≤24 | 50 (26•7) | 25 (27•8) | 25 (25•8) |
| >24 | 137 (73•3) | 65 (72•2) | 72 (74•2) |
| Median (IQR) | 29•2 (24•6-36•1) | 29•1 (24•2-35•1) | 29•8 (24•7-36•9) |
| Residence permit in the past six months | |||
| Shenyang | 61 (32•6) | 34 (37•8) | 27 (27•8) |
| Other cities of Liaoning Province | 67 (35•8) | 29 (32•2) | 38 (39•2) |
| Other provinces | 59 (31•6) | 27 (30•0) | 32 (33•0) |
| Education | |||
| High school and below | 63 (33•7) | 28 (31•1) | 35 (36•1) |
| College and above | 124 (66•3) | 62 (68•9) | 62 (63•9) |
| Marital status | |||
| Single | 75 (40•1) | 34 (37•8) | 41 (42•3) |
| Cohabitated with man | 62 (33•2) | 33 (36•7) | 29 (29•9) |
| Ever married to woman | 50 (26•7) | 23 (25•6) | 27 (27•8) |
| Occupation | |||
| Full-time | 108 (57•8) | 49 (54•4) | 59 (60•8) |
| Part-time | 30 (16•0) | 16 (17•8) | 14 (14•4) |
| Unemployed | 28 (15•0) | 16 (17•8) | 12 (12•4) |
| Student | 21 (11•2) | 9 (10•0) | 12 (12•4) |
| Monthly income, US dollars | |||
| ≤ 430 | 60 (32•1) | 30 (33•3) | 30 (30•9) |
| 431-720 | 75 (40•1) | 32 (35•6) | 43 (44•3) |
| > 720 | 52 (27•8) | 28 (31•1) | 24 (24•7) |
| Main venue for seeking male sexual partners in the past six months | |||
| Internet / mobile applications | 121 (64•7) | 56 (62•2) | 65 (67•0) |
| Othera | 66 (35•3) | 34 (37•8) | 32 (33•0) |
| Self-reported sexual orientation | |||
| Homosexual | 114 (61•0) | 57 (63•3) | 57 (58•8) |
| Otherb | 73 (39•0) | 33 (36•7) | 40 (41•2) |
| Total number of sexual partners per index case in the past six monthsc | |||
| Median (IQR) | 3 (1-4) | 3 (1-4) | 3 (2-5) |
| Number of regular male sexual partners in the past six months | |||
| 0 | 65 (34•8) | 27 (30•0) | 38 (39•2) |
| 1 | 97 (51•9) | 49 (54•4) | 48 (49•5) |
| ≥ 2 | 25 (13•4) | 14 (15•6) | 11 (11•3) |
| Condom use with regular male sexual partners in the past six months (N=122) | |||
| Always | 38 (31•1) | 22 (34•9) | 16 (27•1) |
| Mostly | 26 (21•3) | 11 (17•5) | 15 (25•4) |
| Occasionally | 21 (17•2) | 9 (14•3) | 12 (20•3) |
| Never | 37 (30•3) | 21 (33•3) | 16 (27•1) |
| Number of casual male sexual partners in the past six months * | |||
| 0 | 55 (29•4) | 35 (38•9) | 20 (20•6) |
| 1−2 | 73 (39•0) | 33 (36•7) | 40 (41•2) |
| > 2 | 59 (31•6) | 22 (24•4) | 37 (38•1) |
| Condom use with casual male sexual partners in the past six months (N=132) | |||
| Always | 54 (40•9) | 29 (52•7) | 25 (32•5) |
| Mostly | 35 (26•5) | 13 (23•6) | 22 (28•6) |
| Occasionally | 15 (11•4) | 5 (9•1) | 10 (13•0) |
| Never | 28 (21•2) | 8 (14•5) | 20 (26•0) |
| Female sexual partners in the past six months | |||
| No | 160 (85•6) | 76 (84•4) | 84 (86•6) |
| Yes | 27 (14•4) | 14 (15•6) | 13 (13•4) |
| Condom use with female sexual partners in the past six months (N=27) | |||
| Always | 13 (48•1) | 8 (57•1) | 5 (38•5) |
| Mostly | 4 (14•8) | 2 (14•3) | 2 (15•4) |
| Occasionally | 2 (7•4) | 1 (7•1) | 1 (7•7) |
| Never | 8 (29•6) | 3 (21•4) | 5 (38•5) |
Used Fisher exact chi-square test.
Other venues of seeking male partners in the past six months: park 2 cases, bar 3 cases, public bath 5 cases, have regular partner and do not need to seek male partners 56 cases.
Other self-reported sexual orientation: heterosexual 3 cases, bisexual 49 cases, uncertain 21 cases.
Total sexual partners number: including regular male sexual partners, casual male sexual partners, and female sexual partners.
PN = partner notification; IQR = interquartile range
Comparison of HIV disclosure and partner testing between assisted and passive PN groups
The proportion of index cases in the assisted PN group who disclosed their HIV status to any sexual partners within three months following randomization was 58% (56/97; 95% confidence interval [CI]: 48-68%); a similar proportion was found in the passive PN group as 57% (51/90, 95% CI: 46-67%; Figure 1).
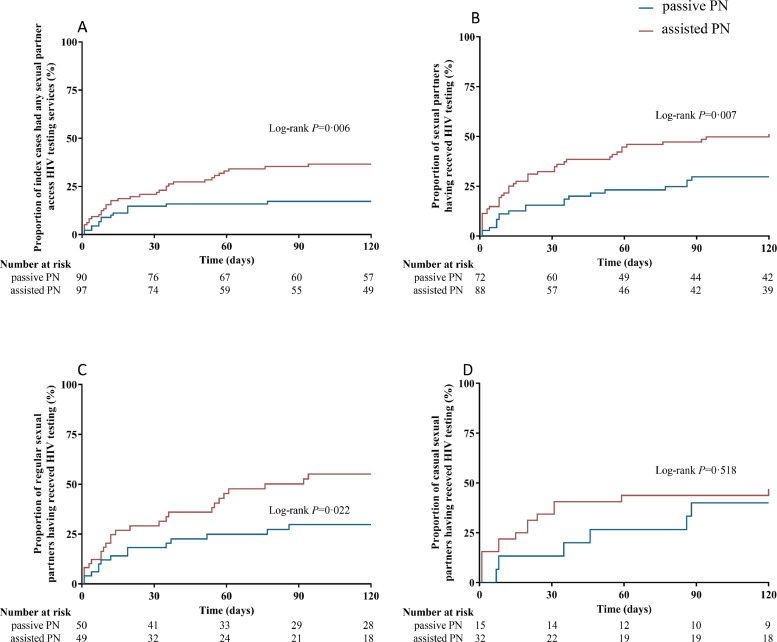
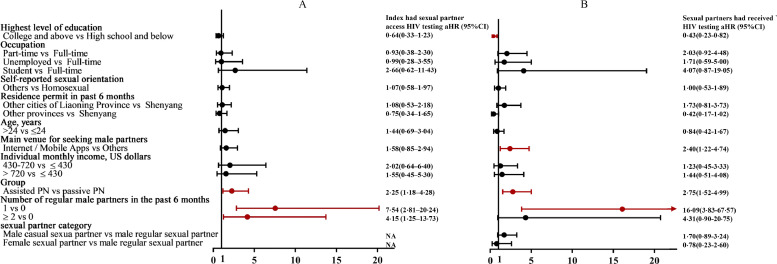
Over one third (35%, 34/97; 95% CI: 26-45%) of index cases in the assisted PN group had any sexual partner accessing HIV testing compared to 17% (15/90; 95% CI: 10-26%) in passive PN group (risk difference [RD]: 18%; 95% CI: 6-30%; P = 0•004); 49% (43/88; 95% CI: 39-59%) sexual partners in the assisted PN group who were disclosed by index cases received HTS, which was higher than 28% (20/72; 95% CI: 19-39%) in the passive PN group (RD: 21%; 95% CI: 6-35%; P = 0•006) (Figure 1 and 2). After adjusting for sociodemographic characteristics and number of sexual partners, participants in the assisted PN groups had more than two times faster to refer their sexual partners accessing HTS compared to those in the passive PN group (aHR = 2•25, 95% CI: 1•18–4•28). For partners who were disclosed by index cases, those in the assisted PN group were more than two times likely to have access to HTS (aHR = 2•78, 95% CI: 1•54–5•02) (Figure 3).
Figure 2.
(A) Kaplan-Meier curve of the proportions of index cases in two study groups who had any sexual partners accessing HIV testing services within four months of follow-up; (B) Kaplan-Meier curve of the proportions of total sexual partners who were disclosed by index cases had received HIV testing within four months of follow-up; (C) Kaplan-Meier curve of the proportions of regular sexual partners who were disclosed by index cases had received HIV testing within four months of follow-up; (D) Kaplan-Meier curve of the proportions of casual sexual partners who were disclosed by index cases had received HIV testing within four months of follow-up.
Number at risk is the number of index cases having not had partners’ access to HTS (A), or the number of sexual partners having not received HTS (B, C and D).
PN = partner notification
Figure 3.
(A) Factors associated with index cases had any sexual partner access HIV testing within four months of follow-up; (B) Factors associated with sexual partners had received HIV testing within four months of follow-up. The Cox multiple regression model adjusted for the following factors: age, residence permit in the past six months, highest level of education, occupation, monthly income, main venue for seeking male sexual partners in the past six months, and self-reported sexual orientation.
HTS = HIV testing services; CI = Confidence interval; PN = partner notification
Reasons for HIV non-disclosure to sexual partners
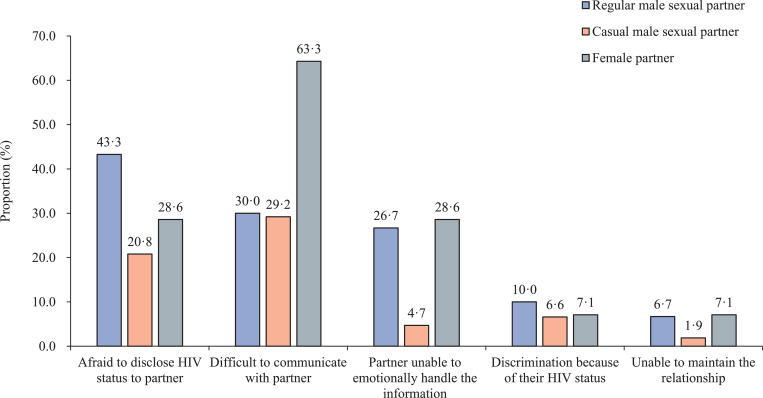
Figure 4 depicted the challenges, stratified by type of sexual partner, to implementing both passive and assisted PN groups. Of those who refused to disclose their HIV status to sexual partners, 30 index cases were willing to explain their reasoning, and they had a total of 30 regular male sexual partners, 106 casual male sexual partners, and 14 female sexual partners. The main reasons for non-disclosure included: 1) fear of disclosing their HIV status to their regular (43%, 13/30) and to casual male sexual partners (21%, 22/106); 2) difficulty with how to communicate a positive HIV diagnosis to their regular (30%, 9/30) and casual male sexual partners (29%, 31/106); and 3) concern that their regular (27%, 8/30) or casual male sexual partners (5%, 5/106) would be unable to emotionally handle the information of their HIV diagnosis (Figure 4).
Figure 4.
Self-reported barriers to implementing passive or assisted PN by type of sexual partner among MSM participants
HIV testing cascades by type of sexual partner
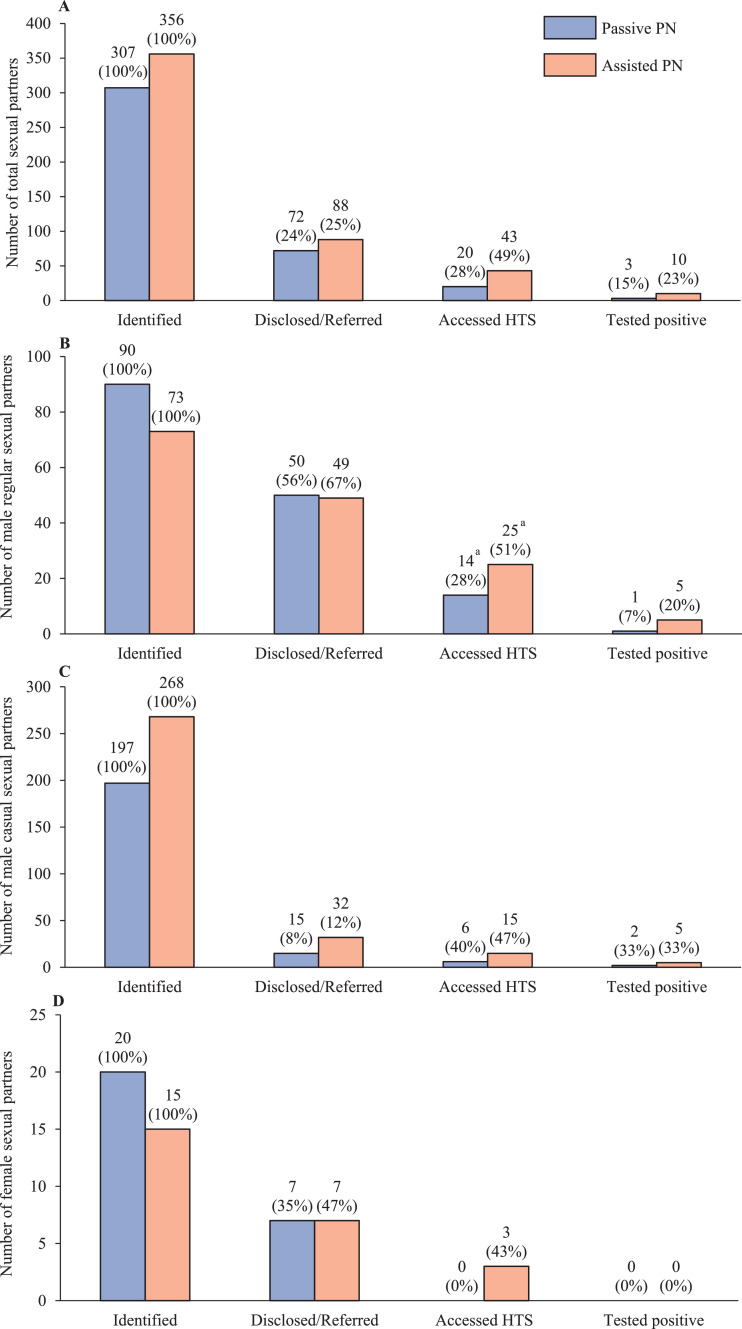
Sixty-five percent of index cases reported having regular male partners, 71% reported casual male partners and 15% reported female partners. The total number of identified sexual partners of any type (i.e., regular male, casual male, or female) was 307 in the passive PN group and 356 in the assisted PN group. Among sexual partners who accessed HTS, the percentage who provided a documented positive HIV test report (either HIVST or clinic-based) was higher but did not differ significantly between the assisted PN group (10/43, 23%) and passive PN group (3/20, 15%, Chi-square P = 0•451). Among identified regular male sexual partners, the percentage who were referred to HTS was similar between the assisted PN (49/73, 67%) and passive PN groups (50/90, 56%; chi-square P = 0•133). Among referred regular male sexual partners, the percentage who accessed HTS was significantly higher in the assisted PN group (25/49, 51%) than passive PN group (14/50, 28%; chi-square P = 0•019). There were no significant differences for casual male sexual partners or female sexual partners between the passive and assisted PN groups in the HIV testing cascades (Figure 5).
Figure 5.
HIV testing cascades by type of sexual partners in passive and assisted PN groups. (A). HIV testing cascade for all sexual partners; (B). HIV testing cascade for regular male sexual partners; (C). HIV testing cascade for casual male sexual partners; (D). HIV testing cascade for female sexual partners. The percentage in the parenthesis for each bar was obtained through comparing with the number in the left adjacent bar in the cascade.
a Among sexual partners who were referred, the proportion who accessed HIV testing services were significantly higher in the assisted PN group than in the passive PN group (Chi-square test P<0•05).
During four months of follow-up, the number of sexual partners named and disclosed/referred to HTS per index case was 3•2 and 0•7 in the passive PN group and 4•0 and 1•0 in the assisted PN group. The sexual partner testing index was 0•44 (43/97) in the assisted PN group and 0•22 (20/90) in the passive PN group. The case-finding index was 0•10 (10/97) in the assisted PN group and 0•03 (3/90) in the passive PN group. In both study groups, the use of HIVST was low; approximately 10% of referred sexual partners who accessed HTS provided a documented test report through HIVST (10% (2/20) in passive PN, 12% (5/43) in assisted PN). About half of referred sexual partners who accessed HTS did not provide documentation of their test results (50% (10/20) in passive PN, and 47% (20/43) in assisted PN).
Discussion
Assisted partner notification that incorporated HIVST and outreach by a CHW from an MSM-serving CBO resulted in a 56% increase in the proportion of referred sexual partners who accessed HTS compared to passive partner notification. Among any disclosed sexual partners, the percentage of accessing HTS was also higher in the assisted PN group (49%) than in the passive PN group (28%). While the percentage of sexual partners disclosed/referred to HTS was similar in both two groups, the percentage of disclosed/referred sexual partners received HIV testing was significantly higher if the index case was assigned to assisted PN that included HIVST and CHW outreach compared to passive PN. For both PN groups, regular male sexual partners were more likely to be disclosed/referred to HTS compared to casual male sexual partners. Through a randomized controlled trial, we determined the effectiveness of assisted PN on increasing HIV testing among sexual partners of Chinese MSM recently diagnosed with HIV.
To the best of our knowledge, this study is the first randomized assessment of assisted partner notification (HIVST plus CHW outreach) among HIV-positive MSM in China, including HIV disclosure and accessing HTS among sexual partners. The proportion of index cases who had any sexual partner access HTS was significantly higher in the assisted PN group compared to the passive PN group; sexual partners in the assisted PN group, in addition to referral by the index case, could have been referred to HTS through CHW outreach and this outreach could have increased sexual partner accessing HTS. These results are consistent with findings from an observational study examining feasibility of PN in collaboration with Chinese MSM-serving CBOs.15 Another cross-sectional survey in Yunnan showed that 60% of MSM in chronic HIV infected stage successfully referred their sexual partners for HIV testing.23 Index cases also refer a higher percentage of regular male sexual partners to HTS compared to casual male sexual partners, possibly because the index case knows the contact information of their regular male sexual partner and has a sense of ethical responsibility for their partners’ health.24 In contrast, casual male sexual partners may be difficult to reach as the index case may not have established the relationship needed discuss HIV serostatus and testing. Innovative strategies such as structural intervention of social networks25,26 and procedures for health providers to offer assisted PN services10 could increase the percentage of casual male sexual partners who are referred to HTS.
The percentage of any sexual partner who accessed HTS and were diagnosed with HIV (i.e., provided a documented positive HIV test report dated after index case enrollment) in both passive PN and assisted PN groups (15% and 23%, respectively) was much higher than previously reported in studies conducted among MSM in China. For example, the HIV prevalence among sexual partners recruited from MSM living with HIV in Chinese cities was 11%.15,23,27 Unfortunately, only 1% of newly diagnosed PLHIV from 2008 to 2017 were identified by PN testing services in Shenyang, China.28 As 39% of referred sexual partners from both PN groups accessed HTS, PN can potentially increase uptake of HIV testing in this key population. Assisted PN strategy can target potentially high-risk populations, increasing the number of PLHIV who know their HIV status, and thus help to achieve the first goal of ensuring at least 90% of PLHIV are diagnosed in China.
MSM engage in bisexual behaviors and their female partners may be at high risk of HIV infection and have low prevalence of HIV disclosure. 29,30 Our study showed the low rate of accessed HTS among female sexual partners, which suggested services in HIV disclosure and HTS should be enhanced. More attention should be paid to HIV testing among female partners among Chinese MSM. Due to social and family pressure, many MSM in China choose to be married with a woman. They tend to hide both sexual orientation and HIV status from their female partner. Our study showed that the main reasons for the index cases not to disclose their HIV status to female partners were communication and emotional barriers. MSM with female partners may serve as a bridging role in HIV transmission from high-risk MSM community to their low-risk wives; therefore, MSM's female partners are at risk and should take HIV testing.
This study has implications for policy and future research. Our assisted PN strategy included an option for a CHW from an MSM-serving CBO to reach out and refer sexual partners to HTS. As the CHW did not disclose the name of index cases to sexual partners, this anonymous message gave index cases the option to not disclose their HIV status and still refer sexual partners to HTS through the CHW outreach. In addition, the CHW was hired from an MSM-serving CBO, and thus trained in communicating and developing rapport with the MSM community.31 Both the anonymous aspect and the sensitivity of the CHW with the MSM community likely improved acceptability and ultimately uptake of the option of CHW outreach option. The Chinese government recently issued an implementation plan to mitigate the spread of HIV/AIDS in China (2019-2022) and recommended that health facilities work together with CBOs to improve HIV testing and prevention services.32 With further training in assisted PN, CBOs can reach out to sexual partners to increase uptake of HTS in key populations.
Additionally, we provided HIVST kits to index cases, and encouraged them to distribute these HIVST kits to their sexual partners. HIVST can increase HIV testing uptake by providing results at-home. While the first HIVST guidelines in China were released in 2019 and provided technical guidance for medical and health personnel, CBO volunteers, and self-testers, these guidelines did not provide information on how to incorporate HIVST in PN strategies.33 In addition, documented uptake of HIVST was low in this study, and thus future studies on PN should evaluate potential strategies to expand uptake of HIVST among MSM in China.
There were several limitations in our study. First, in comparison to passive PN, assisted PN interventions increased uptake of HIV testing among sexual partners of HIV-infected MSM. However, these increases resulted in only seven more new HIV cases (ten in assisted PN and three in passive PN). The main reasons might include barriers to disclosing their HIV status to their sexual partners among MSM and reluctance to disclose their sexual partners to CBO staff for the purpose of assisted PN intervention. Therefore, more innovative interventions should be developed to increase HIV testing in this population. Second, the participants in our study were followed for only four months, thus our understanding of the effectiveness of assisted PN is limited to a short time period. It is possible that more partners would test for HIV after four months of follow up, for individuals who may have been exposed to HIV, testing at the earliest is important and encouraged. The sample size has sufficient power for primary outcome - proportion of index cases having sexual partners accessing to HTS, but might not for other study outcomes such as proportion of sexual partners accessing to HTS. Future studies should explore potential long-term effects and larger scale of assisted PN on HIV testing uptake among the sexual partners of MSM. Third, this study was conducted one clinic in one city, and the study population may differ from MSM in other Chinese cities in terms of demographics and sexual behaviors and HIV testing service may also be heterogenous across China. These factors may limit the generalizability of the study findings. However, the homosexuality culture, HIV testing policies and CBO's role in HIV testing are consistent in China, so our study findings may apply to other parts of China. Forth, this study may suffer from a Hawthorn effect. Participants in the assisted group may be more active in promoting their partners for testing because they know what the study was trying to do. This bias may lead to overestimation of the intervention effects. In addition, the two study arms ascertained partner testing outcomes in different ways, which might hard to avoid ascertainment bias. A single-blind design should be performed in further research to avoid potential bias during data collection and assessment.
In conclusion, assisted PN strategy that incorporated HIVST and outreach by a CHW from an MSM-serving CBO was effective at increasing HIV testing uptake among referred sexual partners of Chinese MSM newly diagnosed with HIV. The evidence from this study suggests that assisted PN should be adopted by HIV public health workers to expand HIV testing among HIV-positive MSM in China. Further real-world research is required to establish the practical effect of assisted PN strategy.
Contributors
QHH, JML, JJX, and HS conceived and designed the research study. QHH, JML, ZXC, and HBD collected the data for the study. QHH, HZQ and JJX analyzed the data and interpreted the results. QHH and HZQ wrote the first draft of the manuscript. JJX, HZQ, SIL, SHV, DT, YJJ, and HS revised the manuscript. SIL and DT helped in editing the manuscript. All authors reviewed and approved the final version of the manuscript.
Data sharing statement
An anonymized dataset and all statistical codes are available upon approved request from the corresponding author.
Declaration of Competing Interest
The authors have no competing interests to declare.
Acknowledgements
The authors extend their sincere gratitude to all participants in this study. We sincerely appreciate the support of implementing the assisted PN strategy by Qiang Kang (Sunshine Voluntary Services Center, Shenyang, China). The study was funded by the Mega-Projects of National Science Research for the 13th Five-Year Plan (2017ZX10201101); the National Science and Technology Major Project (2018ZX10101-001-001-003); Fund of National Natural Science Foundation of China (81703277, 81872674, 82073620); and the Project for Overseas Visiting Research of Liaoning Province (2018LNGXGJWPY-ZD004).
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100171.
Contributor Information
Qing-Hai Hu, Email: qinghaihucn@163.com.
Han-Zhu Qian, Email: han-zhu.qian@yale.edu.
Sequoia I. Leuba, Email: sequoia.leuba@unc.edu.
DeAnne Turner, Email: deanne.turner@yale.edu.
Sten H. Vermund, Email: sten.vermund@yale.edu.
Jun-Jie Xu, Email: xjjcmu@163.com.
Hong Shang, Email: hongshang100@hotmail.com.
Appendix. Supplementary materials
References
- 1.Joint United Nations Programme on HIV/AIDS. Prevailing against pandemics by putting people at the centre - World AIDS Day report 2020. 2020. https://www.unaids.org/en/resources/documents/2020/prevailing-against-pandemics (accessed December 12, 2020).
- 2.Joint United Nations Programme on HIV/AIDS. UNAIDS data 2019. 2019. https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf (accessed August 21, 2020).
- 3.Zhang L, Peng P, Wu Y. Modelling the Epidemiological Impact and Cost-Effectiveness of PrEP for HIV Transmission in MSM in China. AIDS Behav. 2019;23(2):523–533. doi: 10.1007/s10461-018-2205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shang H, Xu J, Han X, Spero Li J, Arledge KC, Zhang L. HIV prevention: Bring safe sex to China. Nature. 2012;485(7400):576–577. doi: 10.1038/485576a. [DOI] [PubMed] [Google Scholar]
- 5.Dalal S, Johnson C, Fonner V. Improving HIV test uptake and case finding with assisted partner notification services. AIDS. 2017;31(13):1867–1876. doi: 10.1097/QAD.0000000000001555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenberg NE, Mtande TK, Saidi F. Recruiting male partners for couple HIV testing and counselling in Malawi's option B+ programme: an unblinded randomised controlled trial. Lancet HIV. 2015;2(11):e483–e491. doi: 10.1016/S2352-3018(15)00182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown LB, Miller WC, Kamanga G. HIV partner notification is effective and feasible in sub-Saharan Africa: opportunities for HIV treatment and prevention. J Acquir Immune Defic Syndr. 2011;56(5):437–442. doi: 10.1097/qai.0b013e318202bf7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landis SE, Schoenbach VJ, Weber DJ. Results of a randomized trial of partner notification in cases of HIV infection in North Carolina. N Engl J Med. 1992;326(2):101–106. doi: 10.1056/NEJM199201093260205. [DOI] [PubMed] [Google Scholar]
- 9.Myers RS, Feldacker C, Cesár F. Acceptability and Effectiveness of Assisted Human Immunodeficiency Virus Partner Services in Mozambique: Results From a Pilot Program in a Public, Urban Clinic. Sex Transm Dis. 2016;43(11):690–695. doi: 10.1097/OLQ.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 10.Golden MR, Gift TL, Brewer DD. Peer referral for HIV case-finding among men who have sex with men. AIDS. 2006;20(15):1961–1968. doi: 10.1097/01.aids.0000247118.74208.6a. [DOI] [PubMed] [Google Scholar]
- 11.WHO. Guidelines on HIV self-testing and partner notification: Supplement to consolidated guidelines on HIV testing services. 2016. https://www.who.int/hiv/pub/vct/hiv-self-testing-guidelines/en/ (accessed August 21, 2020). [PubMed]
- 12.Beyrer C, Baral SD, Collins C. The global response to HIV in men who have sex with men. Lancet. 2016;388(10040):198–206. doi: 10.1016/S0140-6736(16)30781-4. [DOI] [PubMed] [Google Scholar]
- 13.Cheng W, Jin W, Gu Y. HIV Partner Notification Across Different Sexual Partner Types Among Men Who Have Sex with Men in Guangzhou, China. AIDS Patient Care STDS. 2019;33(7):295–298. doi: 10.1089/apc.2019.0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semple SJ, Pines HA, Strathdee SA. Uptake of a Partner Notification Model for HIV Among Men Who Have Sex With Men and Transgender Women in Tijuana, Mexico. AIDS Behav. 2018;22(7):2042–2055. doi: 10.1007/s10461-017-1984-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fu X, Qi J, Hu Y. Partner notification in cooperation with community-based organizations among HIV-positive men who have sex with men in two Chinese cities. Int J STD AIDS. 2016;27(10):821–831. doi: 10.1177/0956462416648827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapatava E, Rios A, Shelley G, Milan J, Smith S, Uhl G. Community-Based Organization Adaptations to the Changing HIV Prevention and Care Landscape in the Southern United States. AIDS Educ Prev. 2018;30(6):516–527. doi: 10.1521/aeap.2018.30.6.516. [DOI] [PubMed] [Google Scholar]
- 17.National Center for AIDS/STD Control and Prevention China CDC. Performance-Based Funding Management Framework for CBOs in China's Response to HIV. 2013. ncaids.chinacdc.cn/fzyw_10256/xgzl/201401/W020140107513637717847.pdf (accessed August 21, 2020).
- 18.Tang W, Wu D. Opportunities and challenges for HIV self-testing in China. Lancet HIV. 2018;5(11) doi: 10.1016/S2352-3018(18)30244-3. e611-e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dovel K, Shaba F, Offorjebe OA. Effect of facility-based HIV self-testing on uptake of testing among outpatients in Malawi: a cluster-randomised trial. Lancet Glob health. 2020;8(2) doi: 10.1016/S2214-109X(19)30534-0. e276-e87. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen VTT, Phan HT, Kato M. Community-led HIV testing services including HIV self-testing and assisted partner notification services in Vietnam: lessons from a pilot study in a concentrated epidemic setting. J Int AIDS Soc. 2019;22(Suppl 3):e25301. doi: 10.1002/jia2.25301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferreira A, Young T, Mathews C, Zunza M, Low N. Strategies for partner notification for sexually transmitted infections, including HIV. Cochrane Database Syst Rev. 2013;(10) doi: 10.1002/14651858.CD002843.pub2. Cd002843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang X, Wang N, Vermund SH. Interventions to improve the HIV continuum of care in China. Curr HIV/AIDS Rep. 2019;16(6):448–457. doi: 10.1007/s11904-019-00469-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Zhang R, Wang J. Feasibility of their sex partners accepting HIV antibody tests mobilized by MSM infected with HIV. Chin J AIDS STD. 2016;22(11):883–886. [Google Scholar]
- 24.Tomnay JE, Hulme-Chambers A, Bilardi J, Fairley CK, Huffam S, Chen MY. A Qualitative Study of Means to Improve Partner Notification After an HIV Diagnosis Among Men Who Have Sex with Men in Australia. AIDS Patient Care STDS. 2017;31(6):269–274. doi: 10.1089/apc.2017.0080. [DOI] [PubMed] [Google Scholar]
- 25.Zhang C, Koniak-Griffin D, Qian HZ. Impact of providing free HIV self-testing kits on frequency of testing among men who have sex with men and their sexual partners in China: A randomized controlled trial. PLoS Med. 2020;17(10) doi: 10.1371/journal.pmed.1003365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luo M, Hann K, Zhang G. HIV testing uptake and yield among sexual partners of HIV-positive men who have sex with men in Zhejiang Province, China, 2014-2016: A cross-sectional pilot study of a choice-based partner tracing and testing package. PloS One. 2020;15(6) doi: 10.1371/journal.pone.0232268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lian Y, Zhao Y, Wang J. A health communication intervention to integrate partner testing with antiretroviral therapy service among men who have sex with men in China: an observational cohort study. BMC Public Health. 2018;18(1):1235. doi: 10.1186/s12889-018-6147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mu JP, Song W, Wang L. Analysis of epidemiological characteristics of HIV/AIDS in Shenyang from 2008 to 2017 (in Chinese) Modern Preventive Medicine. 2018;45(16):2894–2897. [Google Scholar]
- 29.Chow EP, Wilson DP, Zhang L. Estimating HIV incidence among female partners of bisexual men in China. Int J Infect Dis. 2012;16(5):e312–e320. doi: 10.1016/j.ijid.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Yan H, Cao W, Mo P. Prevalence and associated factors of HIV serostatus disclosure to regular female sex partners among HIV-positive men who have sex with both men and women in China. AIDS Care. 2019;31(8):1026–1034. doi: 10.1080/09540121.2019.1612002. [DOI] [PubMed] [Google Scholar]
- 31.Tucker JD, Muessig KE, Cui R. Organizational characteristics of HIV/syphilis testing services for men who have sex with men in South China: a social entrepreneurship analysis and implications for creating sustainable service models. BMC Infect Dis. 2014;14:601. doi: 10.1186/s12879-014-0601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.National Health Commission of the People's Republic of China. The Implementation Plan to Stop HIV Transmission (2019-2022). 2019. http://www.nhc.gov.cn/jkj/s7925/201910/adc374d0613144b2b7bb5d6c58a60223.shtml (accessed August 21, 2020).
- 33.National Center for AIDS/STD Control and Prevention China CDC. Seminar of expanded HIV testing and self-testing was held in Hangzhou. 2019. http://www.chinacdc.cn/zxdt/201910/t20191024_206463.html (accessed August 21, 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.