Abstract
Background
Universal screening of congenital cytomegalovirus (cCMV) infection is important for monitoring and intervention during critical stages of speech and language development. This study aimed to explore the optimal detection strategy for cCMV infection screening.
Methods
Serum samples from pregnant women and saliva and urine samples from their newborns were collected for the anti-CMV IgG and CMV DNA PCR tests, respectively. The sensitivity, specificity, and predictive values as well as the likelihood ratios of 12 potential screening strategies for cCMV infection, based on tests for saliva, urine, and their combination, were evaluated.
Findings
A total of 6729 pregnant women were enrolled, and the seroprevalence was 98.1%. Among 6350 newborns that were followed up, 49 were defined as having cCMV infection. In the screening test, the CMV DNA positivity rate remained similar from day 0 to day 5, increased slowly from day 6 to day 13, and became high in newborns beyond 13 days of birth. In the confirmatory testing, the positive rates increased significantly beyond day 21. For the 49 newborns with cCMV infection, the proportion of agreement between saliva and urine testing was poor. Upon evaluating alternative screening strategies, using saliva and urine screening with saliva and urine confirmation as the reference strategy, saliva screening with saliva and urine confirmation showed good diagnostic accuracy and feasibility, with sensitivity, specificity, positive predictive and negative predictive values of 85.7%, 100.0%, 100.0% and 99.9%, respectively.
Interpretation
In populations with high seroprevalence, saliva screening with saliva and urine confirmation might be an alternative strategy for screening cCMV infections. The suggested timeframes for screening and confirmation are within 13 (ideally 5) and 21 (ideally 13) days of birth, respectively.
Funding
National Natural Science Foundation of China, National Science and Technology Major Project of China and Merck & Co., Inc., Kenilworth, New Jersey, U.S.A.
Research in context.
Evidence before this study
We searched PubMed, Embase, Web of science and Cochrane for the studies published between database inception and January 19, 2021, using search term ((“saliva” OR “urine”) AND (“cytomegalovirus” OR “CMV”) AND (“congenital” OR “fetal” OR “vertical transmission” OR “Intrauterine” OR “Prenatal”) AND “infection”), without language restrictions. However, only 9 studies involved saliva and urine in cCMV screening, where inconsistent results reported. In these studies, the collection and detection of saliva and urine was unparallel. For example, saliva was detected for first screening and urine was later collected and detected for confirmation, which limits the comparison of detection strategies using saliva and urine. Meanwhile, the screening population was small. A study from the United States demonstrated the real-time PCR assays of saliva specimens showed high sensitivity and specificity for detecting CMV infection when comparing with rapid culture of saliva and should be considered potential screening tools for CMV in newborns, while how could saliva and urine supplement each other for a better cCMV screening remained inconclusive. Besides, the suggested timeframe for sample collection to diagnose cCMV infection was inconsistent in different consensus (within 14 or 21 days of birth). Hence, as lacking robust evidence, widely accepted agreement on the optimized detection strategy (including the optimal sample types and the acceptable timeframe of sample collection) to identify cCMV infection has not been reached, especially in newborns from population with high seroprevalence, who are responsible for the majority of cCMV cases globally.
Added value of this study
To the best of our knowledge, the present study is the first one that systematically analyze the detection strategy of cCMV screening, by combining the two most accepted specimens (saliva and urine) in the screening and confirmation. The combination of high false-positive rate in the screening, inconsistent results of CMV shedding in saliva and/or urine, and early onset of postnatal infection has complicated the practice of cCMV screening in highly seropositive population. A total of 12 potential detection strategies were evaluated for their performance on the accuracy of cCMV infection diagnosis. Among them, saliva screening with saliva and urine confirmation shows great feasibility and relatively good diagnostic accuracy, which is a rational alternative for cCMV screening program. Besides, the suggested timeframe (screening and confirmation are within 13 (ideally 5) and 21 (ideally 13) days of birth, respectively) is raised based on analysis of the association between screening time and positive rate.
Implications of all the available evidence
Combining saliva with urine, as well as timely confirmatory testing, is necessary for an effective cCMV screening program. Saliva screening with saliva and urine confirmation is a rational alternative for screening program of cCMV infection.
Alt-text: Unlabelled box
Introdction
Human cytomegalovirus (CMV) infection is extremely common, with more than 50% of all adults having been infected worldwide [1]. Maternal-to-foetal transmission of CMV can occur following primary or nonprimary (reactivation or reinfection) maternal CMV infection during pregnancy [2,3]. Congenital CMV (cCMV) infection is one of the leading causes of infant sensorineural hearing loss and developmental delay. Unfortunately, most cCMV infections cannot be identified by pregnancy and neonatal examinations, particularly for newborns of mothers infected with CMV prior to pregnancy, because effective indicators are lacking.
Universal screening of cCMV infection has been frequently discussed for monitoring and intervention purposes during critical stages of speech and language development [4], [5], [6]. In practise, the detection of CMV DNA by polymerase chain reaction (PCR) in saliva and/or urine samples (the two most accepted sample types) is widely adopted for diagnosing cCMV infection [7], [8], [9], [10]. Due to the difficulty of collecting urine from newborns, direct comparisons of the cCMV screening test performances using saliva and urine are very limited and have inconclusive findings [11,12]. The sampling timeframe for cCMV detection is also inconsistent in the literature [13], [14], [15], [16], [17], [18], [19], [20], [21]. A group of multinational experts [5] recommended that repeated positive CMV PCR findings in two samples (either saliva or urine, and preferably saliva) collected within 21 days of birth at two time points be used to confirm the diagnosis of cCMV infection. In contrast, a group of European experts stated that cCMV infection should be diagnosed based on a single CMV-positive PCR result from a urine sample obtained within 21 days (but ideally within 14 days) of birth; saliva PCR testing can be an alternative, but a positive result should be confirmed using urine to avoid false-positive results caused by the contamination of maternal CMV shedding in breastmilk [4]. Hence, a widely accepted agreement on the optimal strategy for detecting cCMV infection, such as the optimal sample type and sampling timeframe, in a large screening population has not been reached.
From 2015 to 2018, we conducted a prospective cohort study to understand the disease burden of cCMV in China, a country with high CMV seroprevalence. Through the detection of CMV using paired saliva and urine samples collected from newborns on different days after birth for screening and confirmation, different detection strategies were herein analysed.
Methods
Study cohort
The study was a multicentre, mother-child cohort study. From June 2015 to September 2017, volunteers were recruited among pregnant women attending their first pregnancy check at three county-level maternal and child health hospitals located in Xinmi, Zhongmu and Jiaxian, Henan Province of China. The participants donated their serum samples at enrolment for anti-CMV IgG testing to define their history of exposure to CMV. The saliva and urine samples were typically collected from their newborns within 3 days of birth for the CMV DNA screening test. Following positive saliva and/or urine results, a subsequent saliva and urine sample was collected soon after diagnosis (typically within 21 days of birth) for a confirmatory test.
The study was approved by the Ethical Committee of the School of Public Health, Xiamen University (ClinicalTrials.gov, NCT02645396). Informed consent was obtained from each participant at enrolment.
Specimen collection
All specimens were collected under a predefined strict standard operation procedure to minimize contamination during specimen collection, processing, and testing. For pregnant woman, 5 ml of blood was collected. Saliva specimens were collected from newborns at least one hour after feeding by swabbing the inside of the mouth with a sterile cotton swab softly and sufficiently until it was soaked with saliva. The saliva swabs were placed in transport medium (DMEM) immediately after collection. Urine specimens were collected by infant urine drainage bags (GuanKe Bio, NingBo, China), and faecal contamination was carefully avoided. All the specimens were stored at -20°C and transported on dry ice to the central laboratory at Xiamen University at ten-day intervals for DNA PCR testing. Additional collection was scheduled as soon as a positive PCR result was obtained.
Laboratory measurement
Serum samples were tested for a CMV IgG antibody by a well-validated enzyme-linked immunosorbent assay as previously reported [22]. Briefly, 8 mM ethylenediaminetetraacetic acid (EDTA) was added to the saliva or urine samples to inhibit DNA degradation, and DNA was extracted using a Total Nucleic Acid Isolation Kit (GenMag, Beijing, China) according to the manufacturer's instructions. Then, the CMV DNA was quantitated by real-time PCR using one of three pairs of primers and probes targeting UL123 and UL54, respectively (eMethod and eTable 1, supplementary material). The viral DNA load was quantitated using the World Health Organization (WHO) standard for human CMV nucleic acid amplification technique (09/162, NIBSC, UK). The viral loads of the specimens were calculated from the standard curves with a cycle threshold (CT) value of the standard samples. This method of quantitative real-time PCR was used for both screening and confirmatory testing.
Case definition of congenital CMV infection
The positive criterion for the screening and confirmatory tests was a positive PCR test result in saliva and/or urine. A cCMV infection was diagnosed if a newborn with a CMV-positive screening result in saliva and/or urine was further tested positive in saliva and/or urine in a confirmatory test of subsequent samples collected within 21 days of birth, which was recommended by the International Congenital Cytomegalovirus Recommendations Group [5].
Statistical analysis
Pearson chi-square or Fisher's exact test was used to statistically analyse the categorical variables. The kappa value and proportion of agreement were used to evaluate the agreement of the results in the two groups. The kappa coefficient is a measure of interrater agreement, and its magnitude reflects the strength of agreement. When there is perfect agreement between the two ratings, the kappa coefficient equals +1; when the observed agreement exceeds chance agreement, the value of kappa is positive; when agreement is worse than chance, the value of kappa is negative. The exact 95% confidence intervals (CIs) for each rate were calculated.
We evaluated the performances of 12 strategies (eTable 2, supplementary material) for detecting cCMV infection, which were formed by different combinations of saliva and urine in the screening and confirmatory tests. Using saliva and urine screening with saliva and urine confirmation as the reference standard strategy, the sensitivity, specificity and predictive indices of each strategy were calculated by standard methods to determine the proportions and exact 95% confidence limits. It should be noticed that predictive indices are influenced by disease prevalence, and the predicative indices calculated here were based on population with extremely high seroprevalence where the cCMV prevalence is about 1.3%. The Youden index and likelihood ratios are composite index to evaluate diagnostic tests, which were calculated based on the ratio of sensitivity and specificity. Youden index is seen to be the sum of the two fractions showing the proportions correctly diagnosed for newborns with cCMV infection and uninfected newborns, which was measured by the formulation of “sensitivity + specificity-1” [23]. The formula to calculate the positive likelihood ratio was the sensitivity divided by (1 - specificity), and the negative likelihood ratio was as (1 - sensitivity) divided by the specificity [24]. The positive likelihood ratio expresses change in odds favouring cCMV infection given a positive test result, whereas the negative likelihood ratio expresses the change in odds favouring infection given a negative test result [24].
Table 2.
CMV-positive rates of newborns fed via different methods in the screening and confirmatory testing rounds
| N | Positive rates among sample types |
|||
|---|---|---|---|---|
| Saliva and/or urine | Saliva | Urine | ||
| Screening rounda | ||||
| Breastfeeding No. (%) | 5357 | 240 (4.5) | 205 (3.8) | 55 (1.0) |
| Mixed feeding No. (%) | 421 | 23 (5.5) | 20 (4.8) | 5 (1.2) |
| Formula feeding No. (%) | 505 | 23 (4.6) | 23 (4.6) | 3 (0.6) |
| P value | 0.6 | 0.5 | 0.6 | |
| Confirmatory round (within 21 days of birth) b | ||||
| Breastfeeding No. (%) | 141 | 40 (28.4) | 37 (26.2) | 17 (12.1) |
| Mixed feeding No. (%) | 18 | 6 (33.3) | 6 (33.3) | 0 (0.0) |
| Formula feeding No. (%) | 11 | 3 (27.3) | 3 (27.3) | 0 (0.0) |
| P value | 0.9 | 0.8 | 0.2 | |
Note.
The feeding method was missing for 67 newborns.
The feeding method was missing for one newborn.
The two-tailed P values < 0.05 were considered to be indicative of statistical significance. All statistical analyses were performed using SAS software (version 9.4; SAS Institute, Cary, NC).
Role of the funding source
The sponsor of the study has involved in study design, but hasn't involved in data collection, data analysis, data interpretation, and writing of the report. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Study cohort
A total of 6729 pregnant women were enrolled and underwent CMV serological testing at their first antenatal visit (<25.4 gestational weeks [gw], with a median value of 13.6 gw), and the seroprevalence rate was 98.1% (6602/6729) (Figure 1). Among 6350 live newborns, 287 were positive in either their saliva or urine specimens at the screening visit, and 49 were finally defined as having cCMV infection by confirmatory tests conducted within 21 days of birth; 48 (98.0%) of these newborns were from CMV-seropositive mothers. The characteristics of all the newborns are shown in Table 1.
Figure 1.
Flow diagram of enrolment and cCMV screening
Table 1.
Baseline characteristics of the women and their newborns
| Total newborns (n=6350) | Newborns with congenital CMV infection (n=49) | |
|---|---|---|
| Maternal age (years) | ||
| Mean (SD) | 27.3 (4.2) | 25.2 (3.1) |
| Median (min, max) | 27 (16, 46) | 25 (18, 34) |
| Maternal serostatus at the first antenatal visit (within 25.4 gw), No. (%) | ||
| Seronegative | 120 (1.9) | 1 (2.0) |
| Seropositve | 6185 (98.1) | 48 (98.0) |
| Sex of newbornsa, No. (%) | ||
| Male | 2983 (50.9) | 26 (53.1) |
| Female | 2873 (49.1) | 23 (46.9) |
| Newborn feedinga, No. (%) | ||
| Breast only | 5357 (85.3) | 40 (81.6) |
| Formula | 505 (8.0) | 3 (6.1) |
| Mixed | 421 (6.7) | 6 (12.3) |
Note.
Information regarding sex was missing from 494 newborns, and information regarding the feeding of 67 newborns was missing. SD: standard deviation.
Impacts of sample collection age and feeding method on the positive rates of congenital cytomegalovirus infection screening and confirmatory tests
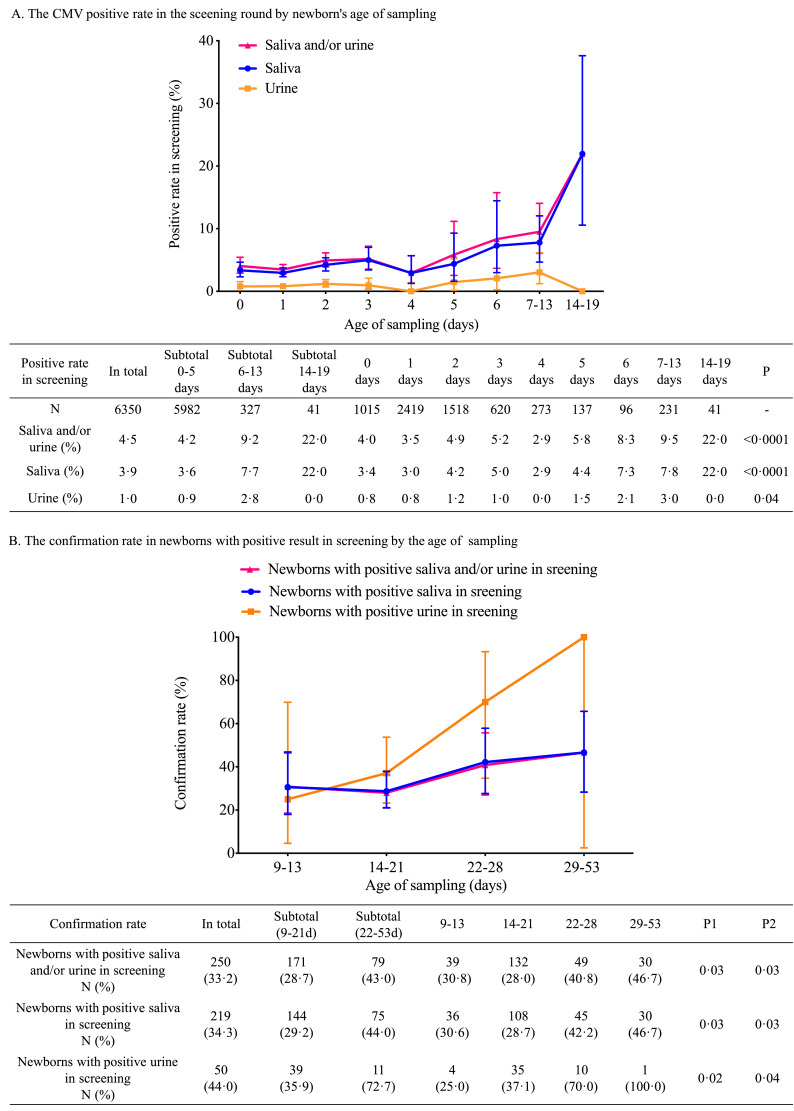
In the screening test, the CMV DNA-positive rates were 3.9% (249/6350) in saliva, 1.0% (63/6350) in urine and 4.5% (287/6350) in saliva and/or urine. The age at sample collection significantly impacted the positive rate in urine (P=0.04), saliva (P<0.0001) and in the urine and saliva combination (P<0.0001) (Figure 2). The positive rates remained similar from day 0 to day 5 (4.2%), increased slightly from day 6 to day 13 (9.2%), and jumped to a high level beyond 13 days of age (22.0%) (Figure 2A).
Figure 2.
CMV-positive rates in the screening and confirmatory testing rounds sorted by newborn age at sampling. A: CMV-positive rates in the screening round sorted by newborn age at sampling. The detailed positive rates are listed in the table below the figure. The P statistic represents the significance of the positive rate by age at sampling as determined by the chi-square test. B: Confirmation rates in newborns with positive screening results sorted by age at sampling. The detailed confirmation positive rates are shown in the table supporting the figure. P1= P value for the positive rate tendency in confirmatory testing sorted by the sampling day. P2= P value for the significance of positive rates between newborns tested within 21 days of birth and beyond 22 days of age. Among 287 newborns with a positive screening result, the median age (range) at subsequent sample collection was 19 (9-53) days, and the median interval between the first and second samples was 16 (6-52) days.
For the confirmatory test, the impacts of age of sample collection on the positive rate were also observed in urine (P=0.02), saliva (P=0.03) and their combination (P=0.03). In contrast to the minor differences in the positive rates between newborns tested within 13 days and newborns tested from 14 to 21 days of age, the positive rate increased significantly after day 21 (Figure 2B). The confirmed positive rate of newborns tested within 21 days of age was similar to those of newborns with a positive saliva screening test (29.2%, 42/144) and newborns with a positive urine screening test (35.9%, 14/39) (P=0.5).
For newborns that tested positive beyond 13 days of age in the screening, all of the confirmatory samples were collected beyond 21 days of age, and the positivity rate was 42.9%. In newborns that tested positive within 13 days of birth in screening, the positive rates in confirmatory test were similar between those of newborns confirmed within 13 days of birth and newborns confirmed from 14 to 21 days of age (27.5% vs 29.1%) and lower than that of newborns confirmed beyond 21 days of age (43.1%).
When comparing the CMV-positive rates in saliva obtained from breastfeeding newborns, mixed feeding newborns and formula-feeding newborns, no significant differences in the positive screening (3.8%, 4.8% and 4.6%, P=0.5) and confirmatory (26.2%, 33.3% and 27.3%, P=0.8) testing rates were found (Table 2).
Cytomegalovirus shedding in saliva and urine of congenital infected newborns
Among 6350 newborns tested for CMV in their paired saliva and urine specimens at the screening visit, the kappa values for saliva and urine testing were as low as 0.15 (95% CI: 0.13-0.17). Among 171 newborns who underwent confirmatory testing within 21 days of birth, the kappa value was also unsatisfactory (0.35, 95% CI: 0.22-0.48) (eTable 3, supplementary material).
Table 3.
Performances of the detection strategies for diagnosing congenital CMV infection in newborns
| cCMV-positive (n=49) | cCMV- negative (n=6185) | Total (n=6234) | Sensitivity (95% CI)-% | Specificity (95% CI)-% | Youden index | Positive predictive value* (95% CI)-% | Negative predictive value* (95% CI)-% | Positive likelihood ratio (95% CI) | Negative likelihood ratio (95% CI) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Saliva and urine screening with saliva and urine confirmation (Strategy 1, the reference) | ||||||||||
| Positive | 49 | 0 | 49 | 100.0 | 100.0 | 1.00 | 100.0 | 100.0 | - | - |
| Negative | 0 | 6185 | 6185 | |||||||
| Saliva only screening (Strategy 2) | ||||||||||
| Positive | 42 | 102 | 144 | 85.7 (73.3, 92.8) | 98.4 (98.0, 98.6) | 0.84 | 29.2 (22.4, 37.1) | 99.9 (99.8, 99.9) | 52.0 (50.6, 53.4) | 0.15 (0.11, 0.19) |
| Negative | 7 | 6083 | 6090 | |||||||
| Urine only screening (Strategy 3) | ||||||||||
| Positive | 14 | 25 | 39 | 28.6 (17.9, 42.4) | 99.6 (99.4, 99.7) | 0.28 | 35.9 (22.7, 51.6) | 99.4 (99.2, 99.6) | 70.7 (46.1, 108.5) | 0.72 (0.68, 0.76) |
| Negative | 35 | 6160 | 6195 | |||||||
| Saliva and urine screening (Strategy 4) | ||||||||||
| Positive | 49 | 122 | 171 | 100.0 (92.7, 100) | 98.0 (97.7, 98.4) | 0.98 | 28.7 (22.4, 35.8) | 100.0 (99.9, 100.0) | 50.7 (49.9 – 51.5) | 0.0 (0.0, -) |
| Negative | 0 | 6063 | 6063 | |||||||
| Saliva screening with saliva confirmation (Strategy 5) | ||||||||||
| Positive | 39 | 0 | 39 | 79.6 (66.4, 88.5) | 100.0 (99.9, 100.0) | 0.80 | 100.0 (91.0, 100.0) | 99.8 (99.7, 99.9) | - | 0.20 (0.17, 0.25) |
| Negative | 10 | 6185 | 6195 | |||||||
| Saliva screening with urine confirmation (Strategy 6) | ||||||||||
| Positive | 12 | 0 | 12 | 24.5 (14.6, 38.1) | 100.0 (99.9, 100.0) | 0.24 | 100.0 (75.7, 100.0) | 99.4 (99.2, 99.6) | - | 0.76 (0.72, 0.80) |
| Negative | 37 | 6185 | 6222 | |||||||
| Saliva screening with saliva and urine confirmation (Strategy 7) | ||||||||||
| Positive | 42 | 0 | 42 | 85.7 (73.3, 92.9) | 100.0 (99.9, 100.0) | 0.86 | 100.0 (91.6, 100.0) | 99.9 (99.8, 100.0) | - | 0.14 (0.11, 0.19) |
| Negative | 7 | 6185 | 6192 | |||||||
| Urine screening with urine confirmation (Strategy 8) | ||||||||||
| Positive | 12 | 0 | 12 | 24.5 (14.6, 38.1) | 100.0 (99.9, 100.0) | 0.24 | 100.0 (75.7, 100.0) | 99.4 (99.2, 99.6) | - | 0.76 (0.72, 0.80) |
| Negative | 37 | 6185 | 6222 | |||||||
| Urine screening with saliva confirmation (Strategy 9) | ||||||||||
| Positive | 14 | 0 | 14 | 28.6 (17.9, 42.4) | 100.0 (99.9, 100.0) | 0.29 | 100.0 (78.5, 100.0) | 99.4 (99.2, 99.6) | - | 0.71 (0.68, 0.76) |
| Negative | 35 | 6185 | 6220 | |||||||
| Urine screening with saliva and urine confirmation (Strategy 10) | ||||||||||
| Positive | 14 | 0 | 14 | 28.6 (17.9, 42.4) | 100.0 (99.9, 100.0) | 0.29 | 100.0 (78.5, 100.0) | 99.4 (99.2, 99.6) | - | 0.71 (0.68, 0.76) |
| Negative | 35 | 6185 | 6220 | |||||||
| Saliva and urine screening with saliva confirmation (Strategy 11) | ||||||||||
| Positive | 46 | 0 | 46 | 93.9 (83.5, 97.9) | 100.0 (99.9, 100.0) | 0.94 | 100.0 (92.3, 100.0) | 100.0 (99.9, 100.0) | - | 0.06 (0.03, 0.12) |
| Negative | 3 | 6185 | 6188 | |||||||
| Saliva and urine screening with urine confirmation (Strategy 12) | ||||||||||
| Positive | 17 | 0 | 17 | 34.7 (22.9, 48.7) | 100.0 (99.9, 100.0) | 0.35 | 100.0 (81.6, 100.0) | 99.5 (99.3, 99.6) | - | 0.65 (0.61 – 0.69) |
| Negative | 32 | 6185 | 6217 | |||||||
Note: *Predictive indices would be influenced by disease prevalence, and the predicative indices calculated here were based on the cCMV prevalence of about 1.3%
For the 49 newborns with cCMV, the detection of CMV in both saliva and urine was uncommon in the screening and confirmation rounds (Figure 3). However, the confirmatory tests of both saliva and urine were positive for all 7 of the newborns infected with cCMV who had a viral load higher than 105 IU/mL in their saliva or urine at the screening test (6 of the 7 had CMV-positive saliva and urine samples at the screening).
Figure 3.
Viral loads in 49 cCMV newborns at the screening round. The red dots indicate the viral loads of newborns with positive saliva and urine samples at the screening round. The blue dots indicate the viral loads of newborns with only positive saliva samples at the screening round. The green dots indicate the viral loads of newborns with only positive urine samples at the screening round.
Performances of the detection strategies for cCMV diagnosis
Considering the different sample type choices and the necessity of a confirmatory test, 12 potential detection strategies (strategies 1 to 12) were formulated, and their diagnostic accuracies for detecting cCMV infection were compared (Table 3). Strategy 1 (saliva and urine screening with saliva and urine confirmation) was defined as the reference standard in this analysis. All strategies showed negative predictive values (NPVs) not less than 99.4% owing to the low incidence of cCMV in the study cohort and the high specificity of the assays. Strategies 2, 4, 5, 7 and 11 showed relatively high Youden index values (> 0.8).
Four strategies (strategies 2–4) without confirmatory testing showed low positive predictive values (PPVs), ranging from 28.7% to 35.9% (Table 3). Strategy 3 (using urine only) showed a low sensitivity value (28.6%) and a poor negative likelihood ratio (0.72).
The performances of eight strategies (strategy 5 to 12) with confirmatory testing were determined to be 100% specific, and the PPVs were 100%. Due to the low sensitivity of urine-based testing (28.6%, strategy 3), the strategies using urine as the sole screening or confirmatory sample (strategy 8, 9, 10) showed poor sensitivities (24.5%–28.6%). Strategy 11 (saliva and urine screening with saliva confirmation) showed high sensitivity (93.9%), followed by strategy 7 (saliva screening with saliva and urine confirmation, 85.7%) and strategy 5 (saliva screening and confirmation, 79.6%).
To improve the diagnostic accuracy, we explored an optimal cut-off value for defining positive screening results with saliva and urine samples using receiver operating characteristic (ROC) analysis. At cut-off values of 657.4 IU/mL and 1206.0 IU/mL, the Youden index peaked (0.20 and 0.77, respectively) for saliva and urine screening, respectively. Upon applying the two cut-off values in the screening of saliva and urine, the PPV unfortunately remained low (58.3%) even when the sensitivity was dropped to 42.9%.
In the strategies using saliva as the only screening sample, the highest viral load in the urine of newborns with false-negative cCMV detection was 2.0 ×105 IU/mL (strategies 2, 5, 6 and 7). In the strategies using urine as the only screening sample, the highest viral load in the saliva of newborns with false-negative cCMV detection was 6.8 ×104 IU/mL (strategies 3, 8, 9 and 10).
Discussion
In this large-scale cohort study, 49 of 6350 newborns were diagnosed with cCMV infection based on positive CMV DNA results in saliva and/or urine at both screening and subsequent confirmatory testing within 21 days of birth. To the best of our knowledge, this study is the first to systematically analyse the detection strategies for cCMV screening by combining the two most accepted specimens (saliva and urine) in the screening, and the results provided robust evidence for the optimal cCMV screening programme. In the evaluated strategies, using saliva and urine screening with saliva and urine confirmation as reference strategies, saliva screening with saliva and urine confirmation was thought to be feasible and reliable for screening for cCMV infection in settings with limited medical resources. To avoid confounding factors, the suggested timeframes for screening and confirmation are within 13 (ideally 5) and 21 (ideally 13) days of birth, respectively.
The estimated global CMV seroprevalence is 83% [1], and nonprimary infection is responsible for the majority of cCMV cases in developing and developed countries [25]. This study was based on a population with extremely high seroprevalence, as nearly all of the cCMV infections (approximately 98.0%) were caused by maternal nonprimary infection during pregnancy. This study provides important additional evidence for selecting a cCMV screening programme in newborns of highly seroprevalent populations.
The impact of the sampling time on the positive CMV testing rate was assessed in this study. In the screening round, the CMV-positive rate was stable within 5 days of birth and tended to increase beyond 5 days of birth, especially beyond 13 days of birth, particularly for saliva. A multicentre cohort study in China showed that the CMV-positive rate in infants without congenital infection was 20.3% at 12 weeks of age and 66.9% at one year old as determined by urine testing [26]. Hence, a plausible explanation is that perinatal or postnatal CMV infection occurs quickly after birth due to close contact with people who shed the virus by breastmilk feeding, kissing, sharing food and utensils, etc. The positive rate in newborns screened beyond 13 days of birth was substantially higher, but potential contamination was excluded as the reason after analysing the data related with processes of sample collection and detection. For newborns screened positive beyond 13 days of age, all the confirmatory samples were collected beyond 21 days of age, and the positive rate was 42.9%, which was similar as that in newborns screened within 13 days and confirmed beyond 21 days of age (43.1%), but higher than that in newborns screened within 13 days of birth and confirmed within 21 days of birth (28.7%). The higher positive rate in both screening and confirmational testing likely indicated the confounding of perinatal or postnatal infection. Hence, infection by viral particles shed during the perinatal or postnatal period might be established in newborns beyond 13 days of age. In addition, based on a positive screening result within 13 days of birth, the positive rates of confirmatory testing in newborns within 13 days of birth and between 14 and 21 days of age were similar (27.5% and 29.1%), while they were lower than that in newborns tested beyond 21 days of age (43.1%). This result indicated that as long as the screening was conducted within 13 days of birth, the influence of perinatal or postnatal infection was small when the confirmatory samples were collected beyond 13 days but within 21 days of birth. Taking the above information into consideration, we preliminarily suggest that the cCMV infection screening test should be performed within 13 days (ideally 5 days) of birth and that the confirmation test should be performed within 21 days (ideally 13 days) of birth.
Even among newborns screened within 5 days of birth, a large proportion (72.5%, 121/167) were negative at the confirmatory testing. Newborns with a high viral load (higher than 1000 IU/mL in saliva or urine) had a higher positive rate at the confirmatory testing, but there was still a considerable proportion of newborns (53.6%, 15/28) who were negative at the confirmatory testing. To improve the diagnostic accuracy, we explored an optimal cut-off value for defining positive results at the screening stage with saliva and urine; unfortunately, even when the sensitivity was dropped to 41.7%, the PPV remained low (58.8%).
The high false-positive screening rate, for which the reasons are unclear, highlights the necessity of confirmatory testing for cCMV. Contamination from breastmilk is a possible explanation; however, this was not the main reason in our study since (1) the CMV-positive rates were not significantly different among newborns fed breastmilk only, breastmilk and formula, or formula only in either the screening or confirmation rounds; (2) the proportions of positive result in confirmation tests were similar between newborns with positive urine and positive saliva screening results; and (3) the tendencies of positive rates in the screening and confirmatory testing rounds were similar between saliva and urine. Hence, the collection of saliva at least 1 hour after breastfeeding potentially limits the influence of breastmilk contamination.
The combinatory testing of saliva and urine greatly improved the sensitivity. In total, 14.3% and 71.4% of cCMV diagnoses were missed by screening only saliva or only urine, respectively; for confirmatory testing, these proportions were 6.1% and 65.3%, respectively. Previous studies have reported that the viral load in blood of newborns with cCMV after birth might be closely related to adverse clinical outcomes [8,27,28]. Screening using only saliva or only urine would have resulted in the false-negative diagnosis of 31.3% of the 16 cCMV newborns with a high viral load (>1000 IU/mL) in our study. The data on CMV shedding in saliva and urine among the 49 newborns with cCMV infection were in poor agreement. For cCMV newborns with viral loads higher than 1000 IU/mL, the agreement was improved but still unsatisfactory. High agreement exists only in newborns with high viral loads (>105 IU/mL in saliva or urine). Large-scale studies comparing CMV DNA testing between saliva and urine are lacking. In several studies from America and Europe, highly consistent testing results from saliva and urine were shown in newborns mainly born to mothers with primary infection [8,11,29]. However, an increasing number of studies have reported inconsistent testing results between saliva and urine, especially in studies from Asia, where a substantial majority of newborns with cCMV are born to seropositive mothers with nonprimary infection during pregnancy. A study in Israel [12] showed that the urine confirmatory positive rate was 54.4% in newborns with a positive saliva screening result. The kappa value between saliva and urine testing was low (0.31) in a study from Indonesia [30]. Inconsistent saliva and urine results were also reported in studies from Spain and Germany [31,32]. The discrepant results were obtained from populations in different regions, where the dominant infection types (primary infection, reinfection or reactivation) in pregnant women are potentially very different. cCMV infections in newborns caused by maternal primary infection, reinfection and reactivation might have different virus shedding patterns in saliva and urine due to the differential characteristics of immunity and viruses in the microenvironment at the maternal–foetal interface. This study revealed a clue regarding this hypothesis, as among newborns from seropositive pregnant women who exhibited inconsistent viral shedding in different body fluids, the single newborn with cCMV infection from a mother with primary infection in this study exhibited shedding in both their saliva and urine at both the screening and confirmatory rounds.
Testing for CMV in saliva and/or urine with confirmation of positive results by testing a subsequent sample (either saliva or urine) was recommended by the International Congenital Cytomegalovirus Recommendations Group for the diagnosis of CMV in newborns [5]. To analyse the optimal strategy for a large screening programme, twelve detection strategies formulated by different combinations of saliva and urine testing were evaluated in this study. Saliva and urine screening with saliva and urine confirmation was deemed the most comprehensive and accurate diagnostic strategy and was therefore set as the reference strategy for evaluation. Saliva and urine screening with saliva confirmation showed a relatively good diagnostic accuracy, with sensitivity, specificity, positive predictive and negative predictive values of 93.9%, 100.0%, 100.0% and 100.0%, respectively. However, the widely perceived challenges of urine collection, such as the uncertainty regarding the time of newborn urination, possible faecal contamination and easily droppable urine bags, makes the application of large-scale screening strategies difficult. The strategy of saliva screening with saliva and urine confirmation significantly increased the feasibility of conduction while maintaining good diagnostic accuracy (Se=85.7%, Sp=100.0%, PPV=100%, NPV=99.9%) and thus might be an alternative for large-scale screenings with limited medical resources.
This study does have some limitations. First, we did not conduct hearing tests in infants infected with cCMV. Clinical data are needed, integrating with laboratory finding, to better understand the true burden of cCMV in infants with discordant results between saliva and urine. We hope to perform follow-up examinations to determine the clinical outcomes of all newborns potentially infected with cCMV and will update the results in future reports. Second, we excluded newborns who screened positively but were sampled beyond 21 days of age from the confirmatory testing, which may have affected the diagnostic accuracy analysis. Nonetheless, as a sensitivity analysis, we re-evaluated all the strategies without the requirement of confirming the time window. Similar results and conclusions were obtained (eTable 4, supplementary material). Taking the above into consideration, the main results and conclusions of this study are still reliable despite these limitations.
The epidemiology of CMV infection differs among regions due to not only the varied sociodemographic and environmental factors underlying CMV infection but also the differential virological and immune characteristics among regions and races, which may lead to differential patterns of cCMV infection in newborns. Studies from Asia are limited, and most of the current knowledge about cCMV infection is based on studies from Europe and America. Our study could provide helpful information for future screening programmes in Asia.
In conclusion, the combination of high false-positive screening rates, inconsistent CMV shedding in saliva and/or urine, and early-onset postnatal infection complicates the practise of cCMV screening in highly seropositive populations. Our data showed that combining saliva with urine, as well as timely confirmatory testing, are necessary for effective cCMV screening in populations with high seroprevalence. Saliva screening with saliva and urine confirmation is an alternative cCMV screening strategy in settings with limited medical resources. In addition, the suggested timeframes of screening and confirmation are within 13 (ideally 5) and 21 (ideally 13) days of birth.
Contributors
YH, HY, QS, FW, CL (Caihong Li), JW, CL (Caihong Liang), FZ, HL, HL, HW and ZL conducted the study. HW JT XG performed the sample detection. JZ, SG, TF, NX, TL, TW and YS provided critical guidance during the conduction of the study. YH performed the data analysis and drafted the manuscript. YH, HW and TL verified the underlying data. JZ, SG, TF, NX, TL, TW and YS commented on and revised the manuscript. The corresponding authors attest that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. JZ, SG and TF are the guarantors.
Data sharing
The data of this study are available from inquiring the corresponding author (Jun Zhang, zhangj@xmu.edu.cn) upon reasonable request for academic purpose.
Declaration of Competing Interest
Jun Zhang received grant from Merck & Co., Inc. during the conduct of the study. All other authors declare no competing interests.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grants 81973058 and 81672111), the National Science and Technology Major Project of China (2017ZX10302201-002-003) and Merck & Co., Inc., Kenilworth, New Jersey, U.S.A.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanwpc.2021.100182.
Contributor Information
Shengxiang Ge, Email: sxge@xmu.edu.cn.
Tong-Ming Fu, Email: tong-ming.fu@uth.tmc.edu.
Jun Zhang, Email: zhangj@xmu.edu.cn.
Appendix. Supplementary materials
References
- 1.Zuhair M., Smit G.S.A., Wallis G. Estimation of the worldwide seroprevalence of cytomegalovirus: A systematic review and meta-analysis. Rev Med Virol[J] 2019:e2034. doi: 10.1002/rmv.2034. [DOI] [PubMed] [Google Scholar]
- 2.Boppana S.B., Rivera L.B., Fowler K.B. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med[J] 2001;344(18) doi: 10.1056/nejm200105033441804. 1366-71. [DOI] [PubMed] [Google Scholar]
- 3.Britt W. Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy. Medical microbiology and immunology[J] 2015;204(3):263–271. doi: 10.1007/s00430-015-0399-9. [DOI] [PubMed] [Google Scholar]
- 4.Luck S.E., Wieringa J.W., Blazquez-Gamero D. Congenital Cytomegalovirus: A European Expert Consensus Statement on Diagnosis and Management. Pediatr Infect Dis J[J] 2017;36(12):1205–1213. doi: 10.1097/INF.0000000000001763. [DOI] [PubMed] [Google Scholar]
- 5.Rawlinson W.D., Boppana S.B., Fowler K.B. Congenital cytomegalovirus infection in pregnancy and the neonate: consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect Dis[J] 2017 doi: 10.1016/S1473-3099(17)30143-3. [DOI] [PubMed] [Google Scholar]
- 6.Gantt S., Dionne F., Kozak F.K. Cost-effectiveness of Universal and Targeted Newborn Screening for Congenital Cytomegalovirus Infection. JAMA Pediatr[J] 2016;170(12) doi: 10.1001/jamapediatrics.2016.2016. 1173-80. [DOI] [PubMed] [Google Scholar]
- 7.Cardoso E.S., Jesus B.L., Gomes L.G. The use of saliva as a practical and feasible alternative to urine in large-scale screening for congenital cytomegalovirus infection increases inclusion and detection rates. Rev Soc Bras Med Trop[J] 2015;48(2):206–207. doi: 10.1590/0037-8682-0200-2014. [DOI] [PubMed] [Google Scholar]
- 8.Ross S.A., Ahmed A., Palmer A.L. Detection of Congenital Cytomegalovirus Infection by Real-Time Polymerase Chain Reaction Analysis of Saliva or Urine Specimens. Journal of Infectious Diseases[J] 2014;210(9):1415–1418. doi: 10.1093/infdis/jiu263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Vries J.J.C., van der Eijk A.A., Wolthers K.C. Real-time PCR versus viral culture on urine as a gold standard in the diagnosis of congenital cytomegalovirus infection. Journal of Clinical Virology[J] 2012;53(2) doi: 10.1016/j.jcv.2011.11.006. 167-70. [DOI] [PubMed] [Google Scholar]
- 10.Boppana S.B., Ross S.A., Shimamura M. Saliva polymerase-chain-reaction assay for cytomegalovirus screening in newborns. N Engl J Med[J] 2011;364(22):2111–2118. doi: 10.1056/NEJMoa1006561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto A.Y., Mussi-Pinhata M.M., Marin L.J. Is saliva as reliable as urine for detection of cytomegalovirus DNA for neonatal screening of congenital CMV infection? J Clin Virol[J] 2006;36(3) doi: 10.1016/j.jcv.2006.03.011. 228-30. [DOI] [PubMed] [Google Scholar]
- 12.Eventov-Friedman S., Manor H., Bar-Oz B. Saliva Real-Time Polymerase Chain Reaction for Targeted Screening of Congenital Cytomegalovirus Infection. J Infect Dis[J] 2019;220(11):1790–1796. doi: 10.1093/infdis/jiz373. [DOI] [PubMed] [Google Scholar]
- 13.Tanimura K., Yamada H. Maternal and neonatal screening methods for congenital cytomegalovirus infection. Journal of Obstetrics and Gynaecology Research[J] 2019;45(3):514–521. doi: 10.1111/jog.13889. [DOI] [PubMed] [Google Scholar]
- 14.Dietrich M.L., Schieffelin J.S. Congenital Cytomegalovirus Infection. Ochsner J[J] 2019;19(2) doi: 10.31486/toj.18.0095. 123-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fowler K.B., Boppana S.B. Congenital cytomegalovirus infection. Semin Perinatol[J] 2018;42(3):149–154. doi: 10.1053/j.semperi.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Marsico C., Kimberlin D.W. Congenital Cytomegalovirus infection: advances and challenges in diagnosis, prevention and treatment. Ital J Pediatr[J] 2017;43(1):38. doi: 10.1186/s13052-017-0358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naing Z.W., Scott G.M., Shand A. Congenital cytomegalovirus infection in pregnancy: a review of prevalence, clinical features, diagnosis and prevention. Aust N Z J Obstet Gynaecol[J] 2016;56(1):9–18. doi: 10.1111/ajo.12408. [DOI] [PubMed] [Google Scholar]
- 18.van Zuylen W.J., Hamilton S.T., Naing Z. Congenital cytomegalovirus infection: Clinical presentation, epidemiology, diagnosis and prevention. Obstetric medicine[J] 2014;7(4):140–146. doi: 10.1177/1753495X14552719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bale J.F., Jr. Congenital cytomegalovirus infection. Handb Clin Neurol[J] 2014;123:319–326. doi: 10.1016/B978-0-444-53488-0.00015-8. [DOI] [PubMed] [Google Scholar]
- 20.Soper D.E. Congenital cytomegalovirus infection: an obstetrician's point of view. Clin Infect Dis[J] 2013;57(Suppl 4):S171–S173. doi: 10.1093/cid/cit611. [DOI] [PubMed] [Google Scholar]
- 21.Buonsenso D., Serranti D., Gargiullo L. Congenital cytomegalovirus infection: current strategies and future perspectives. Eur Rev Med Pharmacol Sci[J] 2012;16(7):919–935. [PubMed] [Google Scholar]
- 22.Huang X., Li J.J., Ge S.X. Establishment and validation of an enzyme-linked immunosorbent assay for IgG antibody against cytomegalovirus based on pp150 antigen. J Virol Methods[J] 2017;240:21–25. doi: 10.1016/j.jviromet.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Wallace S.J., Swann R., Donnelly M. Mortality and morbidity of locally acquired hepatitis E in the national Scottish cohort: a multicentre retrospective study. Aliment Pharmacol Ther[J] 2020;51(10):974–986. doi: 10.1111/apt.15704. [DOI] [PubMed] [Google Scholar]
- 24.Simel D.L., Samsa G.P., Matchar D.B. Likelihood ratios with confidence: sample size estimation for diagnostic test studies. J Clin Epidemiol[J] 1991;44(8):763–770. doi: 10.1016/0895-4356(91)90128-v. [DOI] [PubMed] [Google Scholar]
- 25.de Vries J.J., van Zwet E.W., Dekker F.W. The apparent paradox of maternal seropositivity as a risk factor for congenital cytomegalovirus infection: a population-based prediction model. Rev Med Virol[J] 2013;23(4):241–249. doi: 10.1002/rmv.1744. [DOI] [PubMed] [Google Scholar]
- 26.Group for the Study of Maternal and Infantile Cytomegalovirus Infection in Beijing Prospective study on the impact of infantile cytomegalovirus infection on growth and development of infants. Chinese Journal of Pediatrics[J] 2010;48(5):385–389. [PubMed] [Google Scholar]
- 27.Forner G., Abate D., Mengoli C. High Cytomegalovirus (CMV) DNAemia Predicts CMV Sequelae in Asymptomatic Congenitally Infected Newborns Born to Women With Primary Infection During Pregnancy. J Infect Dis[J] 2015;212(1):67–71. doi: 10.1093/infdis/jiu627. [DOI] [PubMed] [Google Scholar]
- 28.Marsico C., Aban I., Kuo H. Blood Viral Load in Symptomatic Congenital Cytomegalovirus Infection. J Infect Dis[J] 2019;219(9):1398–1406. doi: 10.1093/infdis/jiy695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams E.J., Kadambari S., Berrington J.E. Feasibility and acceptability of targeted screening for congenital CMV-related hearing loss. Arch Dis Child Fetal Neonatal Ed[J] 2014;99(3):F230–F236. doi: 10.1136/archdischild-2013-305276. [DOI] [PubMed] [Google Scholar]
- 30.Putri N.D., Wiyatno A., Dhenni R. Birth prevalence and characteristics of congenital cytomegalovirus infection in an urban birth cohort, Jakarta, Indonesia. Int J Infect Dis[J] 2019;86:31–39. doi: 10.1016/j.ijid.2019.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Nagel A., Dimitrakopoulou E., Teig N. Characterization of a universal screening approach for congenital CMV infection based on a highly-sensitive, quantitative, multiplex real-time PCR assay. PLoS One[J] 2020;15(1) doi: 10.1371/journal.pone.0227143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blázquez-Gamero D., Soriano-Ramos M., Vicente M. Prevalence and Clinical Manifestations of Congenital Cytomegalovirus Infection in a Screening Program in Madrid (PICCSA Study) Pediatr Infect Dis J[J] 2020 doi: 10.1097/inf.0000000000002808. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.