Abstract
Background and Objective
Peritoneal dialysis (PD) associated peritonitis is the most common cause of morbidity, mortality, and treatment failure in patients undergoing PD. We aimed to identify the incidence, pathogens, antibiotic susceptibility, and the outcome of peritoneal dialysis (PD)-associated peritonitis in children.
Methods
Data from medical records of children who underwent PD between 2007 and 2018 in King Fahad Medical City were retrospectively collected. All children aged <14 years undergoing chronic PD were included. The demographic characteristics of patients, peritonitis rates, and clinical outcomes were collected.
Results
In total, 131 children [boys, 68 (51.9%)] underwent automated PD for 305 years. The most common age group was 6–12 years (61 patients, 46.6%). A total of 74.0% of patients were new to dialysis; 25.2% were transferred from hemodialysis. Peritonitis incidence was 0.6 episodes/patient-year. Gram-positive and -negative organisms were identified in 50.1% and 22% episodes, respectively, whereas cultures remained negative in 20.5% episodes. Coagulase-negative Staphylococcus was the most common isolated organism (22.1%), followed by methicillin-sensitive S. aureus (11.1%). Peritonitis was resolved in 153 (73.6%) episodes, whereas 52 (25.0%) episodes required removal through the catheter. The multivariate logistic regression analysis found the exit site infection to be a risk factor for catheter removal. Three (1.4%) episodes caused death due to peritonitis complicated by septic shock.
Conclusions
Our data showed that the most common organisms causing peritonitis were similar to those reported in the previous international registry. The rate of peritonitis was high, but markedly improved in the past two years.
Keywords: Peritonitis, Peritoneal dialysis, Exit site infection, Outcome
1. Introduction
Chronic kidney disease in childhood, which can be congenital or acquired, is a leading cause to end-stage of several renal or urologic diseases [1]. Peritoneal dialysis (PD) is reported to be the dialysis modality of choice in children with end-stage renal disease (ESRD) worldwide [2]. PD-associated peritonitis is associated with morbidity, mortality, and treatment failure in patients undergoing PD [3]. Peritonitis secondary to bacterial causes is the most frequent cause of morbidity and permanent technical failure in pediatric patients [4]. Therefore, the use of empirical antibiotics according to the most prevalent bacterial organism causing peritonitis has been recommended by the International Society of Peritoneal Dialysis (ISPD) [5]. Early identifications of peritonitis and protocol-guided management may reduce the rate of peritonitis. Proper catheter placement, topical antibiotics for exit-site, frequent training for patients and their families, and contamination treatment are examples for procedures that minimize infection risk in PD patients [6]. This study aims to assess the rate, associated microbiology, antibiotic susceptibility, and outcomes for PD-associated peritonitis in pediatric patients at King Fahad Medical City over 12 years. These data will be beneficial for establishing regional treatment guidelines.
2. Materials and methods
A retrospective study was performed at King Fahad Medical City in Riyadh, Saudi Arabia. Hospitalized pediatric patients aged 0–14 years, diagnosed with PD-associated peritonitis between 2007 and 2018 were retrospectively reviewed and analyzed. Patients above 14 years old were excluded. Both descriptive and inferential statistics were conducted. All the patients underwent surgical catheter insertion and received antibiotic prophylaxis during catheter implantation. Additionally, topical antibiotics of the exit site are used routinely for all of the patients as per ISPD guidelines. Regarding the caregiver training, at least two of the family members (e.g., father and mother) were trained at the time of initiation of dialysis for 1–2 weeks, to reach the required competency of doing dialysis independently.
We used MS Excel version 2010 to analyze 208 episodes of PD-associated peritonitis and the relationships with other study variables. We used IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, N·Y., USA) to analyze the outcomes in 131 patients with peritonitis. Descriptive statistics were presented using counts, proportions (%), and mean ± standard deviation whenever appropriate. The relationship between the sociodemographic data and the outcome of peritonitis had been conducted using Fisher’s Exact test. To adjust for the potential confounders, a multivariate regression analysis was conducted to predict the influence of positive peritonitis from the selected sociodemographic characteristics of the patients. Survival analysis (Kaplan Meier) was also presented to evaluate the differences of the outcome (survival vs. nonsurvival) between male and female subjects measured in months where the log-rank test was also being reported. We considered a P-value of <0.05 as statistically significant. All study variables were carefully assessed and grouped by subject order. Missing values were handled carefully by excluding them from the analyses. This method was applied to maintain the consistency and accuracy of the study results.
Data collection obtained from the patient’s medical charts, including demographics, age at initiation of PD, onset of first peritonitis episode after PD initiation, incidence of PD peritonitis, period between PD catheter placement and the first episode of peritonitis, causes of ESRD, comorbid conditions, causing organism, antibiotics susceptibility, and outcomes. The diagnosis of peritonitis was based on ISPD 2016 [5], where the diagnosis of peritonitis requires a minimum of two of the following: (1) clinical manifestations compatible with peritonitis, (2) white cell count >100/μL or >0.1 × 109/L with >50% polymorphonuclear cells of dialysis effluent, and (3) positive culture for dialysis effluent. The removal of PD catheter, transfer to hemodialysis, relapse or recurrent peritonitis, and patient death were the outcomes observed in this study. An episode that happened within four weeks of the therapy completion for a prior episode caused by the same organism or one sterile episode was considered as relapsing peritonitis. If an episode happened within four weeks of the therapy completion for a prior episode, but caused by a different organism was considered as recurrent peritonitis. Peritonitis-related death was recorded if death occurred during the peritonitis treatment course. The standard antimicrobial empirical therapy in our center for PD-associated peritonitis is IP cefazolin and ceftazidime, as per ISPD guidelines then, antibiotics were switched according to the identified organism and its sensitivity. The institutional review board at KFMC provided the ethical approval for this research.
3. Results
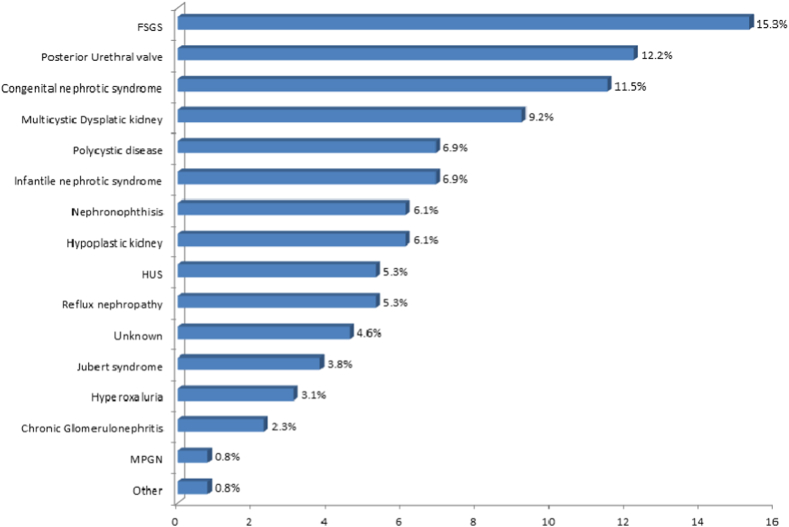
In total, the cases of 146 children undergoing PD were retrospectively reviewed and analyzed. We excluded 15 patients undergoing acute PD. Of the remaining 131 patients, 68 (51.9%) were boys (Table 1). The patients’ age were ranged from 0 to 12 years, with 6–12 years being the most common age (46.6%). In total, 37.4% of patients started PD after 48 months of age and 21.4% of patients started PD between 25 and 48 months of age. Regarding the first peritonitis episode, 27 (20.6%) children developed peritonitis within three months after starting dialysis, and 20 (15.3%) first experienced peritonitis between 3 and 6 months after starting PD. Meanwhile, 50 (38.2%) patients remained peritonitis-free. The mean number of peritonitis episodes was 1.6 (2.5) (Table 1). The most common comorbidities were hypertension (55%), followed by cardiac (29%) and central nervous system dysfunction (22.1%), whereas malignancy was uncommon (0.8%) (Fig. 1). The most common underlying primary diagnosis was focal segmental glomerulosclerosis (FSGS, 15.3%), followed by posterior urethral valves (12.2%) and congenital nephrotic syndrome (11.5%) (Fig. 2). Regarding the presence of peritonitis (negative vs. positive) in relation to the baseline characteristics of participants, only the place of residence (P = 0.020) and total duration of PD (P < 0.001) were significantly related to its presence (Table 2). A multivariate regression analysis had been conducted to predict the influence of positive peritonitis from the selected baseline characteristics of patients. Regression was adjusted in the model, including the place of residence and total duration of PD. It was revealed that the odds of having positive peritonitis for those patients with 6–12 months duration of PD are 17 times higher as compared to those with less than 6 months duration (Adjusted odds ratio (AOR) = 17.425, CI = 4.404–68.942, and P-<0.001); however, the odds increased to 5 times higher in the duration of 13–24 months (AOR = 5.265, CI = 1.362–20.355, P = .016) when compared with less than six months. On the other hand, the place of residence did not reach statistical significance when predicting the effect of positive peritonitis (Table 3).
Table 1.
Baseline characteristics of participants.
| Study variable | N (%) (n = 131) |
|---|---|
| Gender | |
| Male | 68 (51.9) |
| Female | 63 (48.1) |
| Nationality | |
| Saudi | 125 (95.4) |
| Non-Saudi | 6 (04.6) |
| Place of residence | |
| Riyadh | 55 (42.0) |
| Outside Riyadh | 76 (58.0) |
| Socioeconomic status | |
| Fair | 57 (43.5) |
| Good | 74 (56.5) |
| Age group (years) | |
| <1 | 7 (05.3) |
| 1–5 | 32 (24.4) |
| 6–12 | 61 (46.6) |
| >12 | 31 (23.7) |
| Age at initiation of PD (months) | |
| <6 | 13 (09.9) |
| 6–12 | 18 (13.7) |
| 13–24 | 23 (17.6) |
| 25–48 | 28 (21.4 |
| >48 | 49 (37.4) |
| The onset of first peritonitis episode after PD initiation (months) | |
| <3 | 27 (20.6) |
| 3–6 | 20 (15.3) |
| 7–12 | 10 (07.6) |
| >12 | 24 (18.3) |
| No peritonitis | 50 (38.2) |
| The number of episodes of peritonitis (mean ± standard deviation) | 1.6 (02.5) |
| Patient source | |
| New to dialysis | 97 (74.0) |
| Failed kidney transplant | 1 (0.80) |
| Transfer from hemodialysis | 33 (25.2) |
PD: peritoneal dialysis.
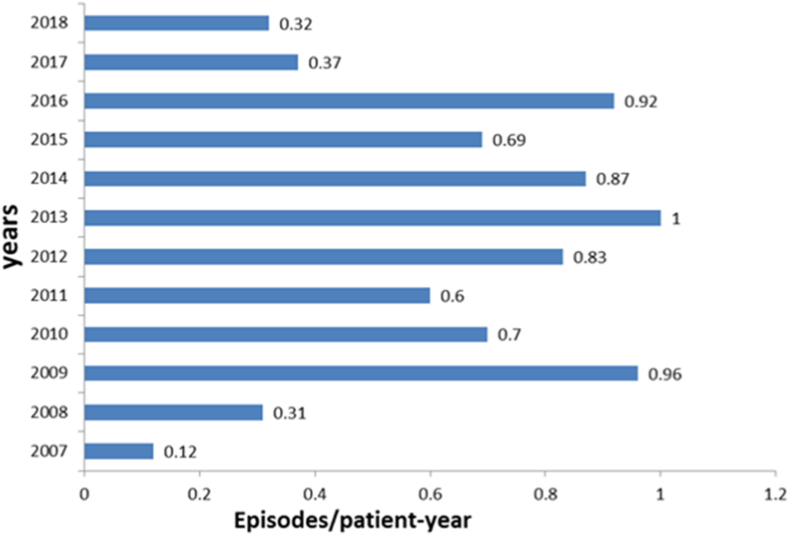
Fig. 1.
The annual rate of peritonitis, revealing the improvement of rate in the last two years.
Fig. 2.
Primary diagnosis of end-stage renal disease. FSGS: Focal segmental glomerulosclerosis; HUS: Hemolytic uremic syndrome; and MPGN: Membranoproliferative glomerulonephritis.
Table 2.
Relationship between baselines characteristics and the presence of peritonitis (n = 131).
| Study variable | Peritonitis |
P-valuea | |
|---|---|---|---|
| Negative N (%) (n = 50) |
Positive N (%) (n = 81) |
||
| Gender | |||
| Male | 26 (52.0) | 42 (51.9) | 1.000 |
| Female | 24 (48.0) | 39 (48.1) | |
| Nationality | |||
| Saudi | 48 (96.0) | 77 (95.1) | 1.000 |
| Non-Saudi | 02 (04.0) | 4 (04.9) | |
| Place of residence | |||
| Riyadh | 15 (30.0) | 41 (50.6) | .029b |
| Outside Riyadh | 35 (70.0) | 40 (49.4) | |
| Socioeconomic status | |||
| Fair | 21 (42.0) | 36 (44.4) | .857 |
| Good | 29 (58.0) | 45 (55.6) | |
| Age group (years) | |||
| ≤5 | 15 (30.0) | 24 (29.6) | 1.000 |
| >5 | 35 (70.0) | 57 (70.4) | |
| The total duration of PD (months) | |||
| <6 | 24 (48.0) | 7 (08.6) | <.001b |
| 6–12 | 12 (24.0) | 12 (14.8) | |
| 13–24 | 6 (12.0) | 19 (23.5) | |
| 25–48 | 4 (08.0) | 21 (25.9) | |
| >48 | 4 (08.0) | 22 (27.2) | |
PD: peritoneal dialysis.
P-value was calculated by using the Fisher’s exact test.
Significant at p < 0.05 level.
Table 3.
Multivariate regression analysis to predict the effect of positive peritonitis from the selected baseline characteristics of the patients (n = 131).
| Factor | AOR | 95% CI | P value |
|---|---|---|---|
| Place of residence | |||
|
Ref | ||
|
.433 | 0.182–1.029 | .058 |
| The total duration of PD (months) | |||
|
Ref | ||
|
17.425 | 4.404–68.942 | < .001a |
|
5.265 | 1.362–20.355 | .016a |
|
1.736 | 0.418–7.215 | .448 |
|
0.909 | 0.197–4.200 | .903 |
AOR: adjusted odds ratio and CI: Confidence Interval.
Significant at P < .05 level.
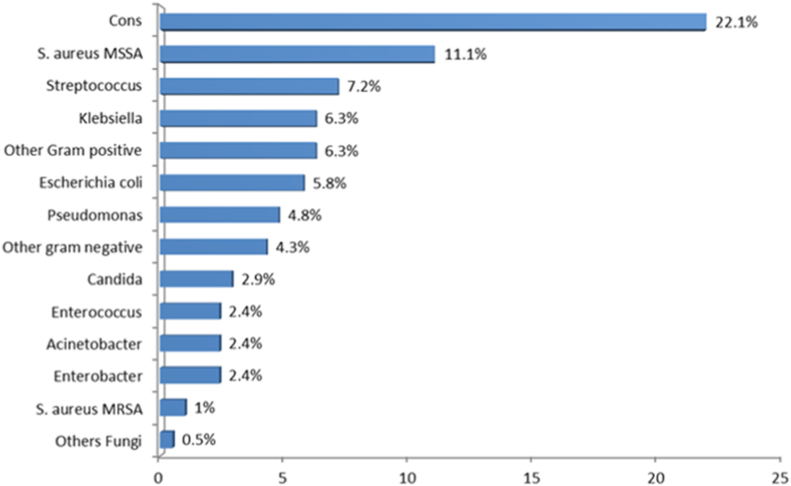
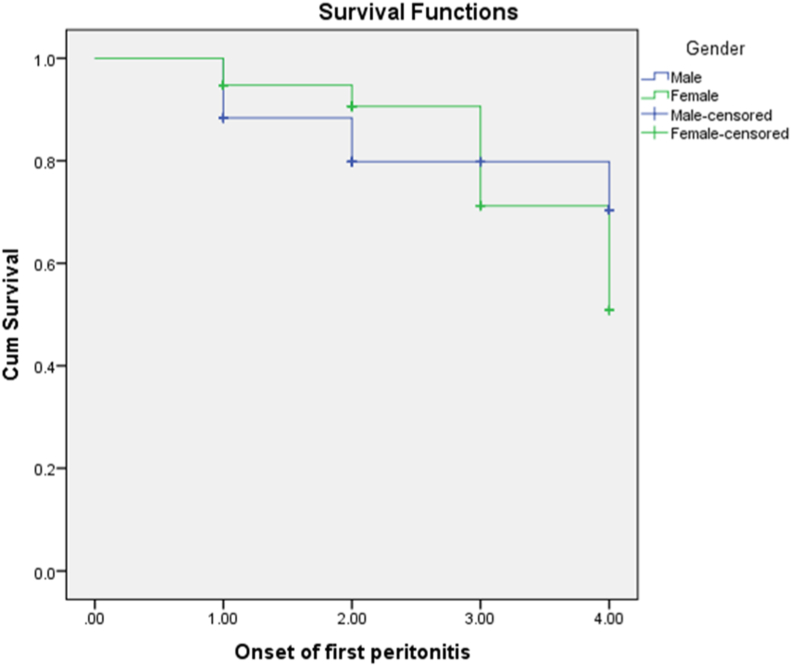
Most of the patients had a single episode of peritonitis (25.2%), followed by two (19.1%) and three episodes (6.9%). Gram-positive bacteria were most common causative organisms (50.1%), then gram-negative microbes (26%) and fungi (3.4%). Meanwhile, 20.5% of cases were culture-negative (Fig. 3). Among gram-positive organisms, coagulase-negative Staphylococcus was the most common (22.1%), whereas Klebsiella (6.3%) was the most common gram-negative microbe. Most of the gram-positive microorganisms were resistant to penicillin, whereas first-generation cephalosporins were effective against most streptococci and methicillin-sensitive Staphylococcus aureus. However, methicillin-resistant S. aureus was resistant to most antibacterial agents tested excluding vancomycin (Table 4). In general, aminoglycosides had the highest sensitivity for gram-negative pathogens, whereas gram-negative microbes were sensitive to ceftazidime and cefepime (Table 5). Among the presenting symptoms, 56.7%, 50%, and 49% of patients presented with abdominal pain, fever, and cloudy effluent, respectively. In total, 18.9% of episodes were associated with exit-site infection. The vast majority of episodes (95.9%) were not associated with bloodstream infections. Peritonitis was resolved in 153 episodes (73.6%), whereas 52 (25.0%) episodes required catheter removal. Three episodes (1.4%) were fatal. In the multivariate regression model, to predict catheter removal from the selected organisms, it was found that the odds of catheter removal for those who had exit site infection was 5 times higher as compared to those without it (AOR = 5.64, CI = 0.52–61.32, and P = .049). On the other hand, gram positive, gram negative, polymicrobial, and fungi did not reach statistical significance after adjustment for confounders (Table 6). The survival plot of male and female subjects regarding the onset of first peritonitis revealed that the mean survival of male was 3.48 months while for female it was 3.57 months, whereas the overall mean survival rate was 3.53. (Fig. 4). However, this result did not reach statistical significance based on log-rank (Mantel-Cox) (X2 = 0.095, P = .758).
Fig. 3.
Most common microorganisms causing peritonitis. CONS: coagulase-negative Staphylococcus aureus; MSSA: Methicillin sensitive Staphylococcus aureus; and MRSA: Methicillin-resistant Staphylococcus aureus.
Table 4.
Gram-positive organisms’ susceptibility.
| Gram-positive organisms | Penicillin | Vancomycin | Cephalothin | |||
|---|---|---|---|---|---|---|
| S | R | S | R | S | R | |
| CONS % | 4.4 | 95.6 | 100 | 0 | 28.2 | 71.8 |
| MSSA % | 7.5 | 92.5 | 100 | 0 | 96.3 | 3.7 |
| Streptococcus % | 77 | 33 | 100 | 0 | 80 | 20 |
| Enterococcus % | 28.5 | 71.5 | 83.4 | 16.6 | 50 | 50 |
| MRSA % | 0 | 100 | 100 | 0 | 0 | 100 |
| Others % | 100 | 0 | 100 | 0 | 100 | 0 |
S: Sensitive; R: Resistant; CONS: Coagulase negative Staphylococcus aureus; MSSA: Methicillin-sensitive Staphylococcus aureus; MRSA: Methicillin-resistant Staphylococcus aureus.
Table 5.
Gram-negative organisms’ susceptibility.
| Gram-negative organisms | Ceftazidime |
Cefepime |
Aminoglycoside |
Tazocin |
Ciprofloxacin |
Meropenem |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S | R | S | R | S | R | S | R | S | R | S | R | |
| Pseudomonas% | 92.8 | 7.2 | 91.6 | 8.4 | 100 | 0 | 92.8 | 7.2 | 100 | 0 | 100 | 0 |
| Klebsiella including ESBL % | 60 | 40 | 63.6 | 36.4 | 100 | 0 | 90 | 10 | 81.8 | 18.2 | 91 | 9 |
| Escherichia coli including ESBL % | 66.6 | 33.4 | 66.6 | 33.4 | 88.8 | 11.2 | 78.5 | 12.5 | 85.7 | 14.3 | 100 | 0 |
| Acinetobacter baumanni% | 87.5 | 12.5 | 100 | 0 | 100 | 0 | 87.5 | 12.5 | 100 | 0 | 100 | 0 |
| Others % | 55.5 | 44.5 | 83.3 | 16.7 | 100 | 0 | 75 | 25 | 83.3 | 16.7 | 83.3 | 16.7 |
S: Sensitive; R: Resistant; and ESBL: Extended-spectrum beta-lactamases.
Table 6.
Association between the outcome of peritonitis among the selected baseline characteristics and selective organisms of children with peritonitis (n = 208).
| Factor | Resolved N (%) (n = 153) |
Catheter Removal N (%) (n = 52) |
Death N (%) (n = 3) |
AOR (95% CI) | P-valuea |
|---|---|---|---|---|---|
| Gram Positive | 67 (43.8) | 29 (55.8) | 02 (66.7) | 0.47 (0.16–1.41) | .275 |
| Gram Negative | 35 (22.9) | 11 (21.2) | 01 (33.3) | 0.32 (0.10–1.00) | .739 |
| Polymicrobial | 09 (05.9) | 02 (03.8) | 0 | 0.96 (0.22–4.14) | .774 |
| Fungi | 08 (05.2) | 03 (05.8) | 0 | 0.10 (0.02–0.55) | 1.000 |
| Exit Site Infection | 28 (18.3) | 04 (7.7) | 0 | 5.64 (0.52–61.32) | .049b |
AOR – Adjusted Odds Ratio; CI – Confidence Interval.
P-value has been calculated by using the Fisher’s Exact Test.
Significant at P < .05 level.
Fig. 4.
The survival plot of male and female subjects during the onset of first peritonitis. Kaplan Meier.
4. Discussion
PD continues to be an effective and safe dialysis for children with ESRD [6]. However, according to several epidemiological studies, peritonitis is a serious complication that is commonly associated with PD [7]. This study is the largest of this type conducted in Saudi Arabia. In our 12-year study, the peritonitis rate exceeded those recorded in the USA (0.26 episodes/patient-year), but the rate was markedly reduced in the last two years [8]. Furthermore, our rate is lower as compared to the Annual Dialysis Report of the North American Pediatric Renal Trials and Collaborative Studies (NAPRTCS) (0.64 episodes/patient-year) and reports in Australia and New Zealand (0.71 episodes/patient-year) [8]. [9]. Our results were similar to those reported from the King Saud University Hospital in Riyadh (1 per 4.7 patient-months) [10]. In contrast, better results were reported by Mirza et al. (one per nine patient-months) from the Riyadh Medical Complex and King Fahd National Guard Hospital [11]. Our findings were also in line with those reported by Alharthi [12]. Per ISPD guidelines, the peritonitis rate should not exceed 0.5 episodes per year [5]. This probably reflects the improvement in our PD program, which included retraining and strict protocols to prevent infections. The importance of preserving the peritoneal membrane and its function in children, who will require a lifetime of ESRD care, dictates an effective approach to prevent peritonitis. The ISPD pediatric guidelines have been developed by taking this approach into consideration. However, it is reflecting an opinion-based rather than evidence-based approach, as there are limited studies in both the pediatric nephrology and infectious disease literature.
It has been reported that gram-positive bacteria account for more than 50% of peritonitis episodes, whereas 20–30% of these episodes are related to gram-negative bacteria [12]. Our findings were in line with previous results. Among gram-positive bacteria, Staphylococcus epidermidis and S. aureus are commonly the causative organisms. S. epidermidis can form biofilms on silastic material more quickly than other microorganisms [13]. Meanwhile, the rate of fungal infections was in line with the findings of international studies [14].
Peritonitis with culture-negative is rare in North America (11%) and Argentina (15%), whereas it was causative of about 59% of all episodes in a multicenter Brazilian prospective study [13,15]. However, the rate of culture-negative peritonitis was 20.5% in our study. The culture-negative peritonitis rate should not exceed 20% of all peritonitis episodes, with a goal to be lower than 10% in any center [5]. The empiric therapy for peritonitis episodes consist of intraperitoneal cefepime monotherapy or can be a first-generation cephalosporin, such as cefazolin or a glycopeptide (vancomycin or teicoplanin) combined with a third-generation cephalosporin such as ceftazidime according to the current pediatric ISPD guidelines. The guidelines also recommended the use of vancomycin with a positive history of methicillin-resistant Staphylococcus aureus (MRSA) colonization/infection, is seriously sick, or has a positive history of allergy to penicillins and cephalosporins [5,6]. Nevertheless, there is no consensus concerning the best empirical antimicrobial therapy of PD-related peritonitis. An empirical treatment with glycopeptide and ceftazidime was superior to cefazolin and ceftazidime as demonstrated by a meta-analysis study [16,17]. However, our study results showed that the use of vancomycin is essential in cases with coagulase-negative Staphylococcus aureus (CONS) and MRSA peritonitis; however, the use of cefazolin plus ceftazidime as empirical therapy for peritonitis is still effective, when there is no concern for CONS and MRSA. Most of the gram-negative organisms were sensitive to aminoglycoside; however, it was used only in cases resistant to ceftazidime, secondary to its effect on residual renal function. Regarding monotherapy cefepime, additional prospective research is needed to assess its effectiveness in our population.
The International Pediatric Peritonitis Registry from 44 pediatric dialysis centers reported the outcomes of 548 episodes of peritonitis in 392 pediatric patients, 89% of patients achieved full functional recovery in line with exit-site infection in 18.9%, which makes exit-site infection an important risk factor [18]. Another risk factor is prolonged PD; patients undergoing PD for more than 84 months developed more peritonitis episodes (28.8%), when compared with patients undergoing PD for less than six months (6.2%). P value was <.001. In the current study, 82% of full functional recovery was the outcomes of the peritonitis episodes comparable to the rate of 89%, which was reported in large multicenter studies with data extracted from the IPPR [18].
Moreover, concerning the long-term outcomes of children on PD, our findings regarding the shift to hemodialysis were similar to those of international and European registries (8.1% and 21%, respectively) [19]. Besides, another study found that 18% of episodes of peritonitis in the US and 16% of those in Canada required catheter removal [12]. There is an appreciable variation in the reported prevalence of peritonitis-associated mortality in the literature [20]. In our study, peritonitis-related mortality was considered for any death during the peritonitis course. However, the rate of deaths due to peritonitis complicated by septic shock was lower than that reported in Italy (9.5%) [21].
The main limitations of our study include the retrospective design and sample size and subsequent bias in results and conclusions.
5. Conclusion
The results of our study showed that the prevalence of peritonitis was comparable with those of other large pediatric studies and previous international registries related to common organisms. The rate of peritonitis was noticeably high in our study, although the rate was markedly reduced in the last two years, most likely related to the prolonged duration of PD and exit site infection. Further studies are required to identify other risk factors in our populations, including the assessment of environmental factors. Our data suggest that ceftazidime and cefazoline are still considered as effective options in the empirical therapy. Vancomycin instead of cefazoline should be considered when there is a concern for CONS and MRSA. Renal transplants within the first two years after the initiation of PD will decrease the overall rate of peritonitis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgment
The authors would like to thank the Research Center at King Fahd Medical City, Riyadh, for their valuable support in the preparation of this manuscript.
Footnotes
Peer review under responsibility of King Faisal Specialist Hospital & Research Centre (General Organization), Saudi Arabia.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpam.2020.09.001.
Contributor Information
Saeed M. AlZabli, Email: alzablis@ymail.com.
Mohammed A. Alsuhaibani, Email: msuhaibani@qumed.edu.sa.
Meshail A. BinThunian, Email: mbinthunian@kfmc.med.sa.
Dayel A. Alshahrani, Email: daalshahrani@kfmc.med.sa.
Abdulkarim Al anazi, Email: aalanazi@kfmc.med.sa.
Sibi Varghese, Email: svarghese@kfmc.med.sa.
Vernice Rose, Email: vfernando@kfmc.med.
Khawla A. Rahim, Email: krahim@kfmc.med.sa.
Visual abstract
The following are the Supplementary data to this article:
Fig S1.
References
- 1.Prasad N., Rangaswamy D., Patel M., Gulati S., Bhadauria D., Kaul A. Long-term outcomes in children on chronic continuous ambulatory peritoneal dialysis: a retrospective cohort study from a developing country. Pediatr Nephrol. 2019;34(11):2389–2397. doi: 10.1007/s00467-019-04311-w. [DOI] [PubMed] [Google Scholar]
- 2.Melek E., Baskın E., Gülleroğlu K.S., Kırnap M., Moray G., Haberal M. Timing for removal of peritoneal dialysis catheters in pediatric renal transplant patients. Exp Clin Transplant. 2016;14(Suppl 3):74–77. [PubMed] [Google Scholar]
- 3.Ye H., Zhou Q., Fan L., Guo Q., Mao H., Huang F. The impact of peritoneal dialysis-related peritonitis on mortality in peritoneal dialysis patients. BMC Nephrol. 2017;18(1):186. doi: 10.1186/s12882-017-0588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethna C.B., Bryant K., Munshi R., Warady B.A., Richardson T., Lawlor J. Risk factors for and outcomes of catheter-associated peritonitis in children: the SCOPE collaborative. Clin J Am Soc Nephrol. 2016;11(9):1590–1596. doi: 10.2215/CJN.02540316. CJN-02540316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li P.K., Szeto C.-C., Piraino B., de Arteaga J., Fan S., Figueiredo A.E. Peritoneal Dialysis International; 2016. ISPD peritonitis recommendations: 2016 update on prevention and treatment. pdi-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Szeto C.-C., Li P.K.-T., Johnson D.W., Bernardini J., Dong J., Figueiredo A.E. ISPD catheter-related infection recommendations: 2017 update. Perit Dial Int. 2017;37(2):141–154. doi: 10.3747/pdi.2016.00120. [DOI] [PubMed] [Google Scholar]
- 7.Sahlawi M Al, Wilson G., Stallard B., Manera K.E., Tong A., Pisoni R.L. Peritoneal Dialysis International; 2019. Peritoneal dialysis-associated peritonitis outcomes reported in trials and observational studies: a systematic review. 0896860819893810. [DOI] [PubMed] [Google Scholar]
- 8.Perl J., Fuller D.S., Bieber B.A., Boudville N., Kanjanabuch T., Ito Y. American Journal of Kidney Diseases; 2020. Peritoneal dialysis–related infection rates and outcomes: results from the peritoneal dialysis outcomes and practice patterns study (PDOPPS) [DOI] [PubMed] [Google Scholar]
- 9.Chang H.J., Han K.H., Cho M.H., Park Y.S., Kang H.G., Cheong H Il. Outcomes of chronic dialysis in Korean children with respect to survival rates and causes of death. Korean journal of pediatrics. 2014;57(3):135. doi: 10.3345/kjp.2014.57.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al Salloum A.A., Al Mugeiren M.M., Al Rasheed S., Al Mazrou A., Al Zamil F. CAPD in Saudi Arabian children: ten years experience from a single center. Saudi Journal of Kidney Diseases and Transplantation. 1997;8(3):298. [PubMed] [Google Scholar]
- 11.Mirza K., Elzouki A.Y. Peritonitis in continuous ambulatory peritoneal dialysis in children living in Saudi Arabia. Pediatr Nephrol. 1997;11(3):325–327. doi: 10.1007/s004670050286. [DOI] [PubMed] [Google Scholar]
- 12.Alharthi A.A. Peritonitis in children with automated peritoneal dialysis. Clin Nephrol. 2015;84(5):289–294. doi: 10.5414/CN108631. [DOI] [PubMed] [Google Scholar]
- 13.Ponce D., De Moraes T.P., Pecoits-Filho R., Figueiredo A.E., Barretti P. Peritonitis in children on chronic peritoneal dialysis: the experience of a large national pediatric cohort. Blood Purif. 2018;45(1–3):118–125. doi: 10.1159/000484344. [DOI] [PubMed] [Google Scholar]
- 14.Levallois J., Nadeau-Fredette A.-C., Labbe A.-C., Laverdiere M., Ouimet D., Vallée M. Ten-year experience with fungal peritonitis in peritoneal dialysis patients: antifungal susceptibility patterns in a North-American center. Int J Infect Dis. 2012;16(1):e41–e43. doi: 10.1016/j.ijid.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 15.Chadha V., Schaefer F.S., Warady B.A. Dialysis-associated peritonitis in children. Pediatr Nephrol. 2010;25(3):425–440. doi: 10.1007/s00467-008-1113-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barretti P., Doles J.V.P., Pinotti D.G., El Dib R. Efficacy of antibiotic therapy for peritoneal dialysis-associated peritonitis: a proportional meta-analysis. BMC Infect Dis. 2014;14(1):445. doi: 10.1186/1471-2334-14-445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kussmann M., Schuster L., Zeitlinger M., Pichler P., Reznicek G., Wiesholzer M. The influence of different peritoneal dialysis fluids on the in vitro activity of ampicillin, daptomycin, and linezolid against Enterococcus faecalis. Eur J Clin Microbiol Infect Dis. 2015;34(11):2257–2263. doi: 10.1007/s10096-015-2477-8. [DOI] [PubMed] [Google Scholar]
- 18.Warady B.A., Feneberg R., Verrina E., Flynn J.T., Müller-Wiefel D.E., Besbas N. Peritonitis in children who receive long-term peritoneal dialysis: a prospective evaluation of therapeutic guidelines. J Am Soc Nephrol. 2007;18(7):2172–2179. doi: 10.1681/ASN.2006101158. [DOI] [PubMed] [Google Scholar]
- 19.Vidal E., van Stralen K.J., Chesnaye N.C., Bonthuis M., Holmberg C., Zurowska A. Infants requiring maintenance dialysis: outcomes of hemodialysis and peritoneal dialysis. Am J Kidney Dis. 2017;69(5):617–625. doi: 10.1053/j.ajkd.2016.09.024. [DOI] [PubMed] [Google Scholar]
- 20.Mehrotra R., Devuyst O., Davies S.J., Johnson D.W. The current state of peritoneal dialysis. J Am Soc Nephrol. 2016;27(11):3238–3252. doi: 10.1681/ASN.2016010112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vidal E., Edefonti A., Murer L., Gianoglio B., Maringhini S., Pecoraro C. Peritoneal dialysis in infants: the experience of the Italian Registry of paediatric chronic dialysis. Nephrol Dial Transplant. 2012;27(1):388–395. doi: 10.1093/ndt/gfr322. [DOI] [PubMed] [Google Scholar]