Key Points
Question
Among intensive care patients requiring intravenous fluid challenges, does the use of a balanced solution compared with saline solution (0.9% sodium chloride) improve 90-day survival?
Findings
In this randomized clinical that included 10 520 patients in intensive care units, intravenous fluid bolus treatment with a balanced solution vs saline solution resulted in 90-day mortality of 26.4% vs 27.2%, respectively, a difference that was not statistically significant.
Meaning
Among critically ill patients requiring fluid challenges, treatment with a balanced solution compared with saline solution did not significantly reduce 90-day mortality.
Abstract
Importance
Intravenous fluids are used for almost all intensive care unit (ICU) patients. Clinical and laboratory studies have questioned whether specific fluid types result in improved outcomes, including mortality and acute kidney injury.
Objective
To determine the effect of a balanced solution vs saline solution (0.9% sodium chloride) on 90-day survival in critically ill patients.
Design, Setting, and Participants
Double-blind, factorial, randomized clinical trial conducted at 75 ICUs in Brazil. Patients who were admitted to the ICU with at least 1 risk factor for worse outcomes, who required at least 1 fluid expansion, and who were expected to remain in the ICU for more than 24 hours were randomized between May 29, 2017, and March 2, 2020; follow-up concluded on October 29, 2020. Patients were randomized to 2 different fluid types (a balanced solution vs saline solution reported in this article) and 2 different infusion rates (reported separately).
Interventions
Patients were randomly assigned 1:1 to receive either a balanced solution (n = 5522) or 0.9% saline solution (n = 5530) for all intravenous fluids.
Main Outcomes and Measures
The primary outcome was 90-day survival.
Results
Among 11 052 patients who were randomized, 10 520 (95.2%) were available for the analysis (mean age, 61.1 [SD, 17] years; 44.2% were women). There was no significant interaction between the 2 interventions (fluid type and infusion speed; P = .98). Planned surgical admissions represented 48.4% of all patients. Of all the patients, 60.6% had hypotension or vasopressor use and 44.3% required mechanical ventilation at enrollment. Patients in both groups received a median of 1.5 L of fluid during the first day after enrollment. By day 90, 1381 of 5230 patients (26.4%) assigned to a balanced solution died vs 1439 of 5290 patients (27.2%) assigned to saline solution (adjusted hazard ratio, 0.97 [95% CI, 0.90-1.05]; P = .47). There were no unexpected treatment-related severe adverse events in either group.
Conclusion and Relevance
Among critically ill patients requiring fluid challenges, use of a balanced solution compared with 0.9% saline solution did not significantly reduce 90-day mortality. The findings do not support the use of this balanced solution.
Trial Registration
ClinicalTrials.gov Identifier: NCT02875873
This randomized clinical trial compares the effect of a balanced solution vs saline solution on 90-day survival in critically ill patients.
Introduction
Intravenous fluids are routinely used in critically ill patients to sustain or replenish intravascular volume and to deliver drug infusions.1 Although saline solution (0.9% sodium chloride) has remained the primary fluid over time, recent evidence from observational studies2,3,4 and 2 large unblinded cluster-randomized, single-center trials5,6 in the US demonstrated that administration of balanced crystalloids (ie, crystalloids whose sodium and chloride concentrations are similar to plasma) resulted in better outcomes. Furthermore, this effect may be mediated by smaller changes in serum chloride levels. However, these results are not uniform across clinical trials,7 and insufficient evidence is available from large individual randomized multicenter studies.
The Balanced Solutions in Intensive Care Study (BaSICS),8,9 a double-blind, factorial, randomized clinical trial was conducted to assess whether administration of a balanced solution (Plasma-Lyte 148) during intensive care unit (ICU) stay, compared with saline solution, would result in improved 90-day survival in critically ill patients.
Methods
Study Design and Oversight
This was an investigator-initiated randomized clinical trial conducted at 75 ICUs in Brazil. The trial protocol (Supplement 1) and statistical analysis plan (Supplement 2) have been published.8,9 The trial was overseen by an external data and safety monitoring board and approved by each institution’s ethics committee. All patients or their legally authorized representative provided signed informed consent. As per Brazilian law, a posteriori (opt out) consent could be applied when the patient was incapable of providing consent and when no legally authorized representative was available at the time eligibility criteria were met. The patient was then approached once he or she regained capacity and could provide consent or opt out. This study was a factorial trial that assessed both the effects of fluid type (a balanced solution vs saline solution [0.9% sodium chloride] and reported in this article) and compared 2 different infusion speeds to be used during fluid challenges (results are reported separately).
Patients
Patients were randomized if they were admitted to an ICU and needed at least 1 fluid expansion (at the discretion of the attending physician), were not expected to be discharged the next day after enrollment, and met at least 1 of the following criteria for acute kidney injury: (1) older than 65 years; (2) had hypotension (mean arterial pressure <65 mm Hg, systolic blood pressure <90 mm Hg, or vasopressor use at any dose); (3) sepsis (defined as suspected or confirmed infection plus acute organ dysfunction); (4) required mechanical ventilation or noninvasive mechanical ventilation (including high-flow nasal cannula) for at least 12 hours; (5) early signs of kidney dysfunction (oliguria [urine output <0.5 mL/kg/h for ≥3 hours] or serum creatinine level >1.2 mg/d for women or >1.4 mg/dL for men); or (6) had liver cirrhosis or acute liver failure. (To convert creatinine from mg/dL to μmol/L, multiply by 88.4.)
Patients with acute kidney injury who required or who were expected to require kidney replacement therapy within the 6 hours after admission were excluded as were patients with severe electrolyte disturbance (serum sodium level ≤120 mmol/L or ≥160 mmol/L), those whose death was considered imminent within the next 24 hours, those with suspected or confirmed brain death, those receiving palliative or comfort care only, and those previously enrolled in the trial. The only change in eligibility criteria during the study period was the removal of hyperkalemia (serum potassium level >5.5 mEq/L) as an exclusion criterion, which occurred after the second interim analysis.
Randomization
Patients were randomized to receive either saline solution or a balanced solution (Figure 1) via a central, web-based automated randomization system that was available 24 hours per day and maintained by the HCor Research Institute in São Paulo, Brazil. The randomized study group was disclosed only after each patient was registered in the web-based randomization system. The randomization list was generated with online software using random permuted block sizes of 12, stratified by center according to fluid type and infusion speed (2 different speeds). The block size was not disclosed to research personnel.
Figure 1. Flow of Patients in the Trial Comparing a Balanced Solution vs Saline Solution (0.9% Sodium Chloride).
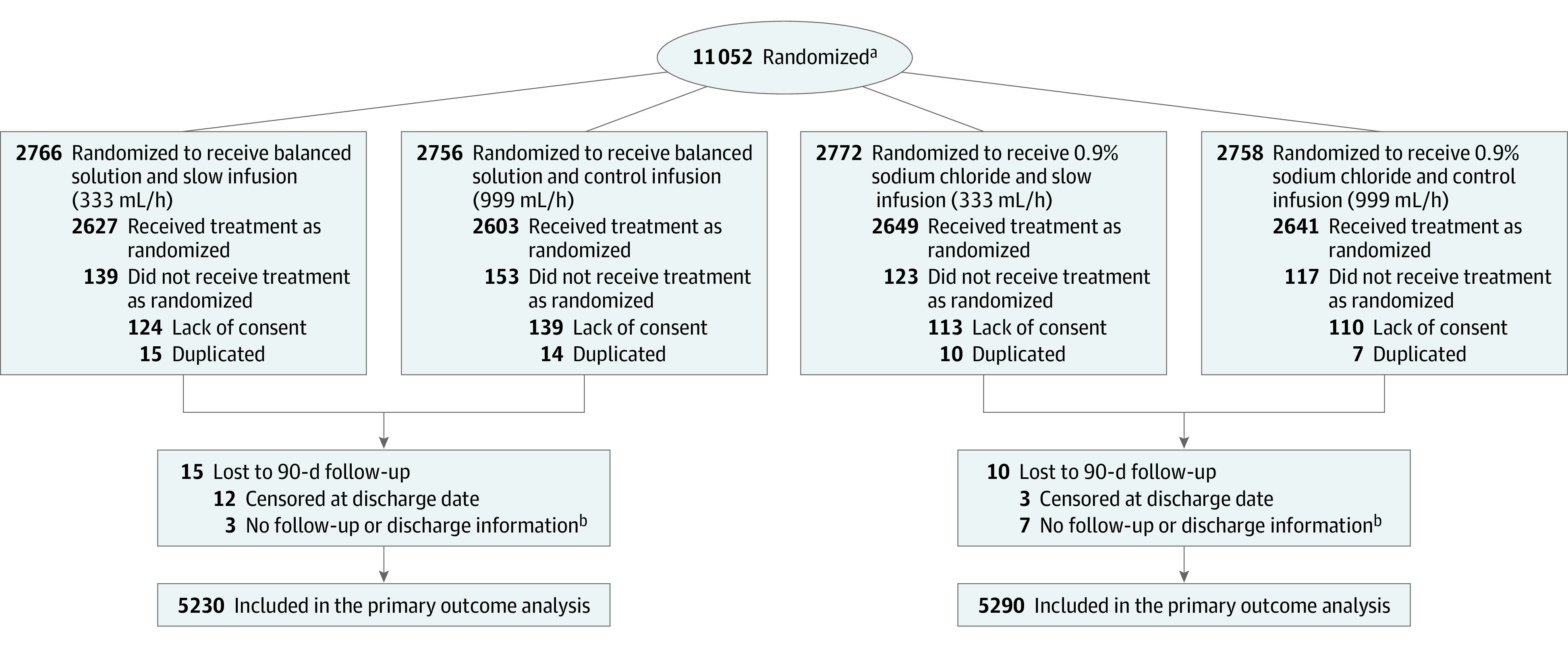
aThere was no screening log; therefore, the number of patients assessed for eligibility cannot be presented.
bData for the primary outcome were imputed.
Interventions
The fluids were supplied to enrolling sites in identical 500 mL bags labeled only by a code corresponding to the fluid type. The fluids were supplied by Baxter Hospitalar. All fluid challenges, maintenance fluids, and drug infusions greater than 100 mL were requested to be performed using the trial fluids during the ICU stay up to 90 days after enrollment (eMethods and eFigures 1-2 in Supplement 3). Physicians, patients, and individuals who assessed the outcomes were blinded to the assigned treatment. Overall patient management, including the decision to perform fluid challenges, was left to the discretion of the attending physician. Protocol adherence was checked at specific time points (days 1, 2, 3, and 7 after enrollment).
Clinical and Laboratory Data
The following patient data were collected: baseline demographic information, illness severity scores (Acute Physiology and Chronic Health Evaluation II [APACHE II] and Sequential Organ Failure Assessment [SOFA]),10,11 presence of acute kidney injury (defined as Kidney Disease: Improving Global Outcomes [KDIGO] stage),12 the need for organ support (mechanical ventilation, vasopressors, kidney replacement therapy), admission type, and administration of fluids within the 24 hours before ICU admission. In addition, the following data were collected in a dedicated case report form on days 1, 2, 3, and 7 after randomization: organ dysfunction (measured by SOFA score), laboratory values (creatinine level, and, if available, chloride level), and volume of fluids administered (including adherence to study fluid).
Data on hospital outcomes, including ICU and hospital length of stay and days not requiring mechanical ventilation, also were recorded in the case report form. A follow-up contact was performed (centrally at the HCor Research Institute) by telephone at 90 days after enrollment to assess vital status and need for kidney replacement therapy after discharge. In cases when phone contact failed, several methods were used to obtain follow-up, including telegrams, hospital records for further visits after discharge, personal visits at the patient’s provided address and, as a last resource, national information on vital status obtained from the Brazilian government.
Data on unexpected treatment-related serious adverse events also were collected and reported in the case report form. On-site or remote monitoring during the trial (risk-based monitoring) was performed for a random sample of fast recruiting sites (those that enrolled >10 patients per week for >4 consecutive weeks).
Outcomes
Primary Outcome
The primary outcome was 90-day survival.
Secondary Outcomes
Several secondary outcomes were evaluated and included (1) the need for kidney replacement therapy up to 90 days after enrollment; (2) the occurrence of acute kidney injury defined as KDIGO12 stage 2 or 3 evaluated at days 3 and 7 (measured at those specified days, not cumulatively, and only for patients without acute kidney injury [ie, KDIGO stage 0 or 1] at enrollment); (3) SOFA score assessed both as a continuous total value and as its individual components (cardiovascular, neurological, coagulation, hepatic, and respiratory dichotomized as ≤2 or >2) at days 3 and 7; and (4) the number of days not requiring mechanical ventilation within 28 days.
Tertiary Outcomes
The tertiary outcomes were ICU and hospital mortality and ICU and hospital length of stay. Details on the definitions used appear in the eMethods in Supplement 3. Because both medications were already approved for use, we did not collect safety outcomes other than unexpected treatment-related serious adverse events.
Power Analysis and Sample Size Calculation
The study was designed to enroll 11 000 patients.8 The sample size was calculated by estimating 35% mortality within 90 days in the control group (saline solution), with an estimated 89% power to detect a hazard ratio (HR) for mortality of 0.90 or less with an α level of .05. The sample size was calculated for this factorial trial assuming the absence of interaction between the 2 interventions (fluid type and infusion speed). Additional details appear in Supplement 2.7,8
Statistical Analysis
Three interim analyses were conducted at 25%, 50%, and 75% of the study cohort. The stopping rule for safety was P < .001 (Haybittle-Peto boundary13) for 90-day mortality. The primary outcome, 90-day survival, was tested using mixed-effects Cox proportional hazards models, considering enrolling sites as the random variable (frailty models) and adjusting for age, baseline SOFA score, and the type of admission (planned, unplanned with baseline sepsis, or unplanned without baseline sepsis). The proportionality of the HR was assessed using the Grambsch and Therneau method.14 For patients with missing data for the primary outcome, 5 sets of multiple imputation using multiple imputation chains were used considering age, sex, enrolling site, creatinine level at randomization, SOFA score, admission type, use of fluids within the 24 hours before enrollment, presence of heart failure or cirrhosis, traumatic brain injury at enrollment, hypotension at enrollment, mechanical ventilation status at enrollment, and outcome. The medians of the imputed results (or the most frequent category) were used for the analysis.
Kidney replacement therapy up to 90 days was estimated using a mixed Poisson model adjusted for age, baseline SOFA score, and admission type and is reported as the incidence per 1000 patient-days. Alternatively, kidney replacement therapy at 90 days was reported using a competing risk model that considered death as a competitor for the need for kidney replacement therapy. Occurrence of acute kidney injury at days 3 and 7 was tested with mixed generalized linear models with a binomial distribution and the logit link function. SOFA score was assessed using a mixed generalized linear model. Individual SOFA components, dichotomized as less than or equal to 2 or greater than 2, were analyzed using mixed logistic regression models. The proportion of days not requiring mechanical ventilation during the 28-day time frame was tested using β-binomial regression.
Subgroup analyses for the primary outcome were performed for the following prespecified subgroups: (1) patients with sepsis vs those without sepsis; (2) patients with KDIGO stage of less than 2 vs those with KDIGO stage of 2 or greater (ie, KDIGO stage of 0-1 vs 2-3) at enrollment; (3) surgical vs nonsurgical patients; (4) patients with traumatic brain injury vs those without traumatic brain injury; (5) patients with an APACHE II score of 25 points or greater vs those with an APACHE II score of less than 25 points; and (6) patients who received greater than 1 mL of saline solution vs those who received 1 mL or less of saline solution within the 24 hours before randomization.
Additional prespecified exploratory analyses were conducted. The first analysis assessed treatment effect on the primary outcome according to baseline chloride levels (stratified as ≥110 mEq/L or <110 mEq/L). The second was an exploratory analysis using bayesian networks to evaluate the potential association between fluid type and key SOFA components at days 3 and 7 (especially the neurological and hemodynamic components) while accounting for multiple competing events (discharge and death).
Other post hoc sensitivity analyses were performed for the primary outcome, the first including only patients with known results for the primary outcome; the second including only patients who did not receive fluids before enrollment. Post hoc exploratory analyses were conducted to assess treatment effect on (1) the composite outcome of mortality or use of kidney replacement therapy during hospital stay both in patients who did and in those who did not receive fluids within the 24 hours prior to enrollment; (2) the composite outcome of death and the need for kidney replacement therapy in the hospital or the doubling of creatinine level; and (3) the different definitions of acute kidney injury. In addition, a post hoc analysis compared trends for chloride levels between groups among the patients with available serum chloride levels measured at days 1, 2, 3, and 7. Post hoc sensitivity analyses for the primary, secondary, and tertiary outcomes also were performed after excluding patients admitted due to a traumatic brain injury.
P values are reported for the primary outcome and for the subgroup analyses; the remaining outcomes are reported with the mean effect and 95% CI. Because of the potential for type I error due to multiple comparisons, the findings for the analyses of the secondary outcomes should be interpreted as exploratory. All tests were 2-sided with an α level of .05. All analyses were performed using R software version 4.03 (R Foundation for Statistical Computing).15
Results
Patients
A total of 11 052 patients were randomized between May 29, 2017, and March 2, 2020, at 75 ICUs. There were 532 patients who were excluded from the analysis (486 patients subsequently refused to provide consent and 46 were duplicate patients [the first enrollment for those patients was kept in the analysis]), leaving 10 520 patients for the analysis (5230 patients randomized to a balanced solution and 5290 randomized to saline solution; Figure 1). Follow-up concluded on October 29, 2020.
The patient characteristics were well-balanced between the groups (mean age, 61.1 [SD, 17] years; 44.2% were women) (Table 1; the 4 groups of the original trial design appear in eTable 1 in Supplement 3). Of all the patients, 48.4% were admitted to the ICU after elective surgery and the majority were randomized during their first day in the ICU. Approximately 68% of all patients received a crystalloid fluid bolus before ICU admission (45% received >1 L). Of all patients, 60.6% had hypotension or vasopressor use and 44.3% required mechanical ventilation at enrollment.
Table 1. Baseline Characteristics of Patients in the Intensive Care Unit (ICU).
| Characteristic | Balanced solution, No./total (%)a | Saline solution, No./total (%)a,b |
|---|---|---|
| No. of patients | 5230 | 5290 |
| Age, mean (SD), y | 60.9 (17.0) | 61.2 (16.9) |
| Sex, No. (%) | ||
| Female | 2321 (44.4) | 2334 (44.1) |
| Male | 2909 (55.6) | 2956 (55.9) |
| Admission type | ||
| No. of missing patients | 18 | 9 |
| Planned (elective surgery) | 2491/5212 (47.8) | 2588/5281 (49.0) |
| Unplannedc | 2721/5212 (52.2) | 2693/5281 (51.0) |
| Emergency department | 1194/5212 (22.9) | 1188/5281 (22.5) |
| Nonelective surgery | 653/5212 (12.5) | 652/5281 (12.3) |
| Ward | 549/5212 (10.5) | 507/5281 (9.6) |
| Transfer from another hospital | 288/5212 (5.5) | 306/5281 (5.8) |
| Transfer from another ICU | 37/5212 (0.7) | 40/5281 (0.8) |
| Illness severity at enrollment | ||
| APACHE II scored | ||
| No. of patients | 5195 | 5271 |
| No. of missing patients | 35 | 29 |
| Median (IQR) | 12 (8-17) | 12 (8-17) |
| SOFA scoree | ||
| No. of patients | 5195 | 5271 |
| No. of missing patients | 35 | 29 |
| Median (IQR) | 4 (2-7) | 4 (2-7) |
| KDIGO stagef | 1683/5198 (32.4) | 1765/5265 (33.5) |
| No. of missing patients | 32 | 25 |
| Sepsis | 966/5212 (18.5) | 1015/5280 (19.2) |
| No. of missing patients | 18 | 10 |
| Traumatic brain injury | 247/5212 (4.7) | 236/5281 (4.5) |
| No. of missing patients | 18 | 9 |
| Hypotensiong | 3161/5211 (60.7) | 3195/5280 (60.5) |
| No. of missing patients | 19 | 10 |
| Type of mechanical ventilation | ||
| No. of missing patients | 18 | 9 |
| Noninvasive for >12 h | 332/5212 (6.4) | 341/5281 (6.5) |
| Invasive | 2304/5212 (44.2) | 2340/5281 (44.3) |
| Serum creatinine level, mean (SD), mg/dL | ||
| No. of patients | 5187 | 5247 |
| No. of missing patients | 43 | 43 |
| Mean (SD) | 1.2 (0.9) | 1.2 (0.9) |
| Creatinine level, mg/dL | ||
| <1.5 | 4139/5187 (79.8) | 4162/5247 (79.3) |
| 1.6-2.5 | 719/5187 (13.9) | 720/5247 (13.7) |
| >2.5 | 329/5187 (6.3) | 365/5247 (7.0) |
| Cirrhosis or acute liver failure | 132/5212 (2.5) | 135/5281 (2.5) |
| No. of missing patients | 18 | 9 |
| Heart failure | 593/5212 (11.4) | 543/5281 (10.3) |
| No. of missing patients | 18 | 9 |
| Time from ICU admission to randomization, d | ||
| No. of patients | 5212 | 5281 |
| No. of missing patients | 18 | 9 |
| Median (2.5%-97.5%) | 0 (0-1) | 0 (0-1) |
| Administration of balanced solution within the 24 h before enrollmenth | ||
| No. of missing patients | 18 | 10 |
| Received any | 2503/5212 (48.0) | 2561/5280 (48.5) |
| Received >1000 mL | 1626/5212 (31.2) | 1692/5280 (32.0) |
| Volume of balanced solutions adminstered within the 24 h before enrollment | ||
| No. of patients | 5212 | 5280 |
| No. of missing patients | 18 | 10 |
| Median (IQR), mL | 0 (0-1500) | 0 (0-1500) |
| Administration of saline solution within the 24 h before enrollment | ||
| No. of missing patients | 18 | 10 |
| Received any | 1987/5212 (38.1) | 1971/5280 (37.3) |
| Received >1000 mL | 935/5212 (17.9) | 994/5280 (18.8) |
| Volume of saline solution adminstered within the 24 h before enrollment | ||
| No. of patients | 5212 | 5280 |
| No. of missing patients | 18 | 10 |
| Median (IQR), mL | 0 (0-1000) | 0 (0-1000) |
| Administration of any fluid within the 24 h before enrollment | ||
| No. of missing patients | 18 | 10 |
| Received any | 3551/5212 (68.1) | 3609/5280 (68.4) |
| Received >1000 mL | 2327/5212 (44.6) | 2427/5280 (46) |
| Volume of any fluids adminstered within the 24 h before enrollment | ||
| No. of patients | 5212 | 5280 |
| No. of missing patients | 18 | 10 |
| Median (IQR), mL | 1000 (0-2500) | 1000 (0-2500) |
Abbreviations: APACHE II, Acute Physiology and Chronic Health Evaluation II; IQR, interquartile range; KDIGO, Kidney Disease: Improving Global Outcomes; SOFA, Sequential Organ Failure Assessment.
SI conversion factor: To convert creatinine to μmol/L, multiply by 88.4.
Unless otherwise indicated. eTable 1 in Supplement 3 compares all 4 treatment groups.
Saline solution is 0.9% sodium chloride.
Total sum does not include patients whose admission type was imputed; additional details appear in Supplement 3.
Measures illness severity. Scores range from 0 to 71; a higher score indicates more severe disease. A score of 12 predicts an in-hospital mortality rate of 15%.
Measures illness severity. Scores range from 0 to 24; a higher score indicates more severe disease. A score of 4 predicts an in-hospital mortality rate of 20%.
Classifies acute kidney injury. Stages range from 0 (no acute kidney injury) to 3 (severe kidney injury).
Defined as mean arterial pressure of less than 65 mm Hg, systolic blood pressure of less than 90 mm Hg, or vasopressor use.
Plasma-Lyte 148 and lactated ringer.
Interventions
Patients in both groups received a median of 1.5 L of fluid during the first day after enrollment. The accumulated median fluid administered (including study fluid and nonstudy fluid) during the first 3 days after enrollment was 4.1 L (SD, 2.9 L) and the median study fluid administered during the same period was 2.9 L (SD, 2.4 L). Adherence to study fluid on the measured days appears in Figure 2A and in eTable 2 in Supplement 3.
Figure 2. Volume of Infused Fluids at Days 1, 2, 3, and 7 and Boxplot of Serum Chloride Levels.
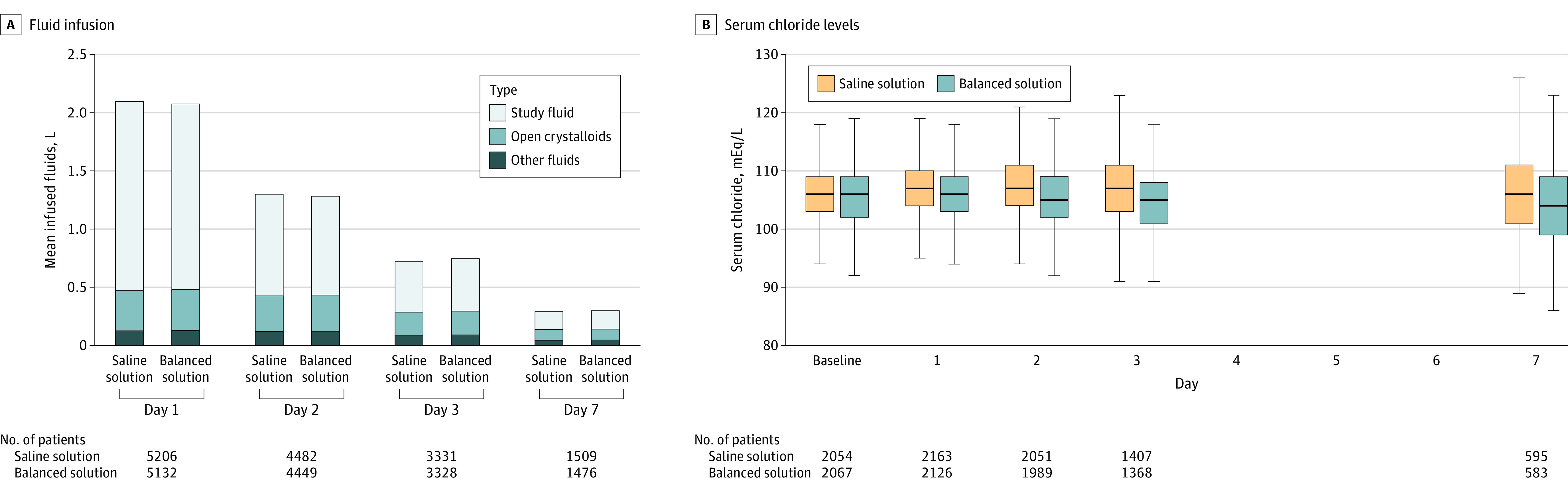
A, The y-axis represents the total volume of infused fluids given on each day. Open crystalloids refer to open label (nonstudy fluid) use. The proportion of each fluid for each day is shown inside the bars. B, Data are expressed as the median serum chloride levels, first and third quartiles (boxes), and range (lines) at baseline and days 1, 2, 3, and 7 for both groups. Saline solution is 0.9% sodium chloride.
Primary Outcome
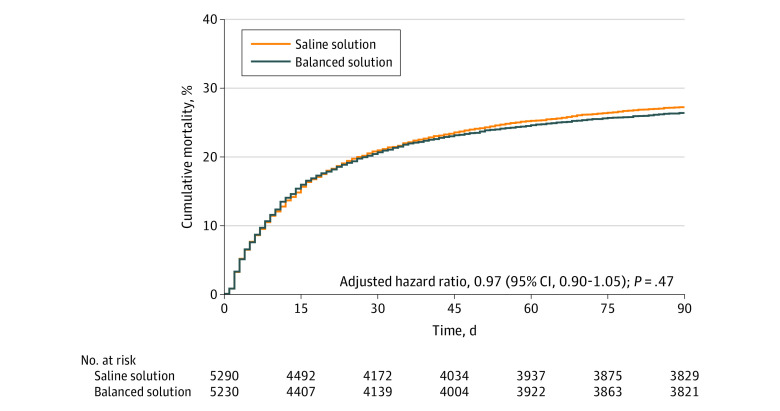
The primary outcome information was available for all but 25 patients. Fifteen of these 25 patients had hospital outcome data available and were censored at the discharge date. The primary outcome was imputed for the remaining 10 patients. The proportionality of the hazards assumption was met (P = .23). Within 90 days, 1381 of 5230 patients (26.4%) assigned to a balanced solution died vs 1439 of 5290 patients (27.2%) assigned to saline solution (adjusted HR, 0.97 [95% CI, 0.90-1.05]; P = .47). The cumulative incidence plot for the primary outcome appears in Figure 3 and the full model results are reported in eTable 3 in Supplement 3. There was no significant interaction between the 2 interventions (fluid type and infusion speed; P = .98) or between groups for the primary outcome (Figure 3 and eFigure 3 in Supplement 3).
Figure 3. Cumulative Incidence of the Primary Outcome of 90-Day Survival for a Balanced Solution vs Saline Solution (0.9% Sodium Chloride).
The median follow-up was 90 days (interquartile range, 59.2-90.0 days) for the balanced solution group and 90 days (interquartile range, 54-90 days) for the saline solution group.
Secondary Outcomes
The secondary outcomes appear in Table 2. A total of 19 secondary outcomes were evaluated, of which 2 were statistically different between the groups. SOFA score at day 7 was significantly different for the balanced solution group (median difference, 0.27 [95% CI, 0.08-0.45]), mostly due to a higher neurological SOFA score (>2) at day 7 (32.1% vs 26.0% for the saline solution group; odds ratio, 1.40 [95% CI, 1.18-1.66]).
Table 2. Primary, Secondary, and Tertiary Outcomes Comparing a Balanced Solution With Saline Solution (0.9% Sodium Chloride).
| Outcomes | No./total (%) | Absolute difference (95% CI) | Effect measure (95% CI) | |
|---|---|---|---|---|
| Balanced solution | Saline solution | |||
| Primary outcome | ||||
| 90-d Mortalitya | 1381/5230 (26.4) | 1439/5290 (27.2) | −1.0 (−2.9 to 0.8) | HR, 0.97 (0.90 to 1.05) |
| P value | .47 | |||
| Secondary outcomes | ||||
| Incidence of acute kidney failure with need for kidney replacement therapy within 90 d per 1000 patient-days | 414/471 (0.88) | 445/476 (0.93) | −0.05 (−0.15 to 0.06) | RR, 0.95 (0.83 to 1.08) |
| At day 1 | 28/5218 (0.5) | 30/5287 (0.6) | ||
| At day 2 | 115/5174 (2.2) | 137/5242 (2.6) | ||
| At day 3 | 181/5052 (3.6) | 213/5123 (4.2) | ||
| At day 7 | 267/4808 (5.6) | 314/4884 (6.4) | ||
| In the hospital (≥1 episode during stay) | 393/5218 (7.5) | 427/5287 (8.1) | −0.5 (−1.5 to 0.4) | OR, 0.93 (0.81 to 1.06) |
| Acute kidney injury assessed as KDIGO stage ≥2b | ||||
| At day 3 | 850/3128 (27.2) | 859/3094 (27.8) | −1.9 (−4.0 to 0.2) | OR, 0.99 (0.88 to 1.11) |
| At day 7 | 276/1180 (23.4) | 273/1170 (23.3) | −1.5 (−3.6 to 0.6) | OR, 1.07 (0.88 to 1.30) |
| KDIGO stage ≥2 or death | ||||
| At day 3 | 851/3128 (27.2) | 865/3094 (28.0) | −1.9 (−4.0 to 0.2) | OR, 0.98 (0.87 to 1.10) |
| At day 7 | 278/1180 (23.6) | 275/1170 (23.5) | −1.5 (−3.6 to 0.6) | OR, 1.07 (0.88 to 1.30) |
| Total SOFA score at day 3c | ||||
| No. of patients | 3789 | 3846 | ||
| Median (IQR) | 4 (2 to 6) | 4 (2 to 6) | 0.09 (−0.02 to 0.16) | |
| SOFA score >2 at day 3 | ||||
| Cardiovasculard | 1319/3789 (34.8) | 1271/3846 (33.0) | 1.7 (−0.4 to 3.9) | OR, 1.11 (1.00 to 1.22) |
| Neurologicale | 654/3789 (17.3) | 636/3846 (16.5) | 0.6 (−0.8 to 1.9) | OR, 1.06 (0.93 to 1.22) |
| Coagulationf | 163/3789 (4.3) | 163/3846 (4.2) | 0 (−0.8 to 0.9) | OR, 1.02 (0.82 to 1.27) |
| Respiratoryg | 266/3789 (7.0) | 258/3846 (6.7) | 0.2 (−0.7 to 1.1) | OR, 1.04 (0.87 to 1.24) |
| Hepatich | 44/3789 (1.2) | 49/3846 (1.3) | −0.1 (−0.5 to 0.3) | OR, 0.94 (0.65 to 1.36) |
| Total SOFA score at day 7c | ||||
| No. of patients | 1531 | 1594 | ||
| Median (IQR) | 4 (2 to 7) | 4 (2 to 7) | 0.27 (0.08 to 0.45) | |
| SOFA score >2 at day 7 | ||||
| Cardiovasculard | 420/1531 (27.4) | 409/1594 (25.7) | 2.0 (−1.0 to 5.1) | OR, 1.14 (0.97 to 1.34) |
| Neurologicale | 492/1531 (32.1) | 415/1594 (26.0) | 5.0 (2.3 to 7.8) | OR, 1.40 (1.18 to 1.66) |
| Coagulationf | 62/1531 (4.0) | 70/1594 (4.4) | −0.1 (−1.3 to 1.0) | OR, 1.01 (0.73 to 1.39) |
| Respiratoryg | 171/1531 (11.2) | 154/1594 (9.7) | 1.3 (−0.4 to 2.9) | OR, 1.21 (0.96 to 1.52) |
| Hepatich | 25/1531 (1.6) | 27/1594 (1.7) | 0 (−0.6 to 0.6) | OR, 1.04 (0.67 to 1.62) |
| Days not requiring mechanical ventilation within 28 d | 0.14 (−0.35 to 0.64) | |||
| No. of patients | 5217 | 5287 | ||
| Median (IQR) | 27 (13 to 28) | 27 (10 to 28) | ||
| Tertiary outcomes | ||||
| Died | ||||
| In the ICU | 907/5218 (17.4) | 922/5287 (17.4) | 0 (−0.6 to 0.6) | OR, 1.01 (0.90 to 1.13) |
| In the hospital | 1177/5218 (22.6) | 1217/5287 (23) | −0.6 (−2.4 to 1.1) | OR, 0.98 (0.88 to 1.09) |
| ICU length of stay, d | ||||
| No. of patients | 5218 | 5287 | ||
| Median (IQR) | 3 (2 to 7) | 3 (2 to 7) | 0 (−0.4 to 0.4) | MR, 0.99 (0.94 to 1.04) |
| Hospital length of stay, d | ||||
| No. of patients | 5218 | 5287 | ||
| Median (IQR) | 8 (5 to 18) | 9 (5 to 18) | −0.3 (−1.1 to 0.5) | MR, 0.98 (0.93 to 1.03) |
Abbreviations: HR, hazard ratio; ICU, intensive care unit; IQR, interquartile range; KDIGO, Kidney Disease: Improving Global Outcomes; MR, mean ratio; OR, odds ratio; RR, risk ratio; SOFA, Sequential Organ Failure Assessment.
Fifteen patients had missing data and their information was imputed.
Measured on specific day and not cumulative.
Measures illness severity. Six organs or systems are assessed and each receives 0 points (no dysfunction) to 4 points (more severe dysfunction). The sum of the scores for all systems ranges from 0 to 24; a higher score indicates more severe illness.
Patient received 5 µg/kg/min or greater dose of dopamine, any dose of epinephrine, or any dose of norepinephrine.
Glasgow Coma Scale score was 9 or less.
Platelet count was 49 000/μL or less.
Ratio of Pao2 to fraction of inspired oxygen was less than 200 mm Hg.
Serum bilirubin level was 6 mg/dL (102.6 μmol/L) or greater.
Subgroup Analyses
The prespecified subgroup analyses for the primary outcome appear in Figure 4. There was a statistically significant interaction between presence of traumatic brain injury, fluid type, and 90-day mortality (31.3% for the balanced solution group vs 21.1% for the saline solution group [HR, 1.48; 95% CI, 1.03-2.12]; 26.2% vs 27.5%, respectively, for patients without a traumatic brain injury [HR, 0.96; 95% CI, 0.89-1.03]; P = .02 for interaction). There were no other significant interactions for the predefined subgroups.
Figure 4. Forest Plot for the Primary Outcome of 90-Day Survival in the Prespecified Subgroup Analyses.
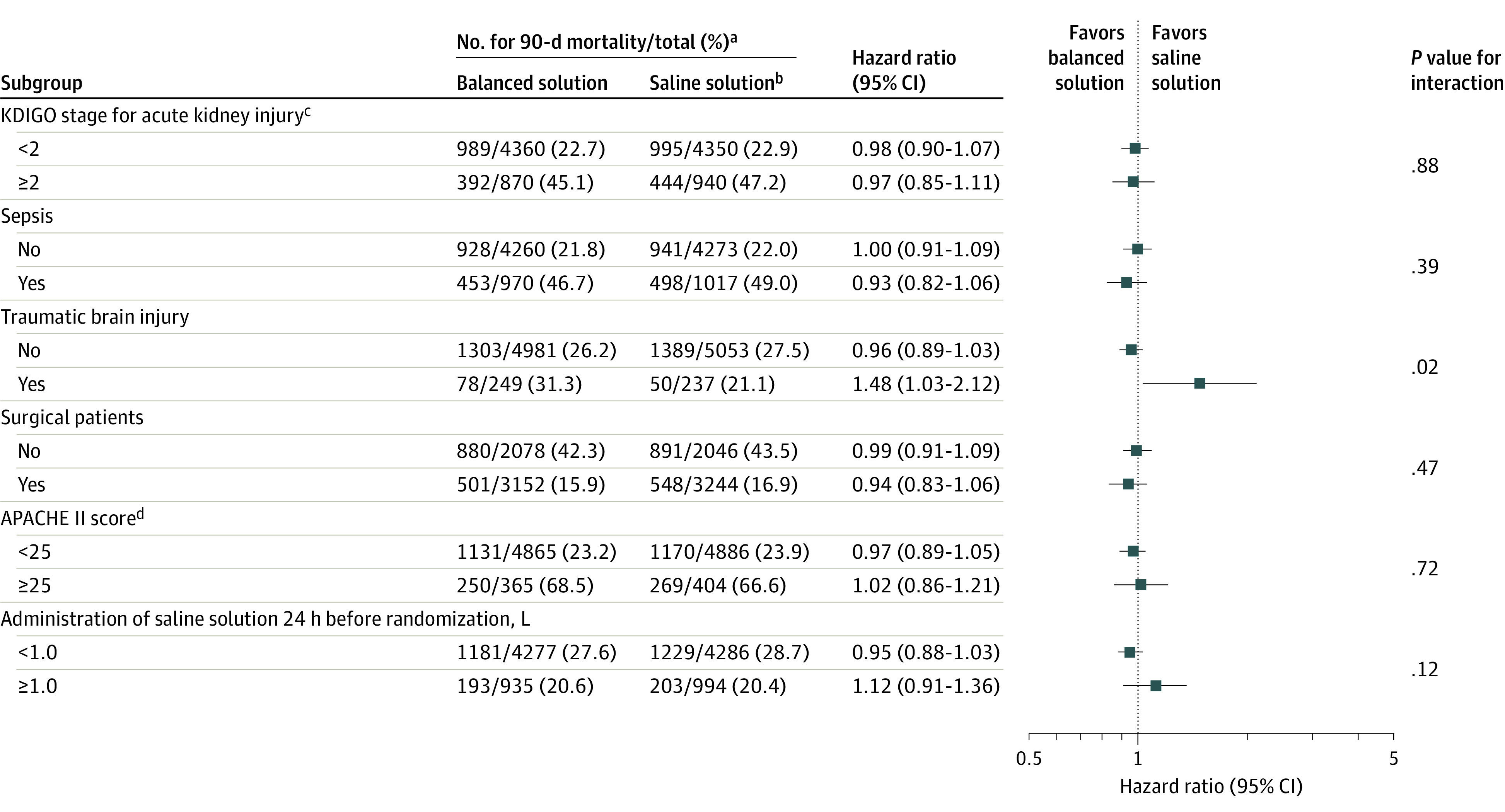
aThe denominators do not match the data presented in Table 1 because the data in this figure were imputed. Additional details appear in the statistical analysis plan in Supplement 2.
bSaline solution is 0.9% sodium chloride.
cKDIGO indicates Kidney Disease: Improving Global Outcomes. Stage 1 is defined by an increase in serum creatinine level by 0.3 mg/dL or greater within 48 hours or an increase in serum creatinine level to 1.5 times or greater than baseline, which is known or presumed to have occurred within the prior 7 days, or a urine output of less than 0.5 mL/kg/h for 6 hours. Stage 2 is defined by an increase in serum creatinine level of 2.0 to 2.9 times baseline or a urine output of less than 0.5 mL/kg/h for 12 hours or longer. Stage 3 is defined by an increase in serum creatinine level 3.0 times or greater than baseline or to 4.0 mg/dL or greater, initiation of kidney replacement therapy, or a urine output of less than 0.3 mL/kg/h for 24 hours or longer or anuria for 12 hours or longer. To convert creatinine to μmol/L, multiply by 88.4.
dAPACHE II indicates the Acute Physiology and Chronic Health Evaluation II. APACHE II scores can range from 0 to 71; a higher value indicates greater illness severity.
Exploratory Analyses
There were no significant between-group differences for the tertiary outcomes (Table 2). There was no statistically significant interaction between fluid type and the primary outcome according to baseline chloride levels (P = .37; eTable 4 in Supplement 3). The bayesian network analysis accounting for the competing events of death or hospital discharge was consistent with a high probability that use of the balanced solution is associated with a lower Glasgow Coma Scale score (≤12) for patients that required mechanical ventilation at day 7 (eFigure 4 and eTable 5 in Supplement 3).
Post Hoc Analyses
The results of the post hoc sensitivity analyses appear in Supplement 3. The sensitivity analyses considering only patients with known data for the primary outcome or only patients who did not receive any fluid before randomization showed no significant difference for the primary outcome. There was no significant difference for the composite outcome of mortality and use of kidney replacement therapy during hospital stay both in patients who did and those who did not receive fluids within the 24 hours prior to enrollment (eTable 6 in Supplement 3) or for the subgroups stratified according to KDIGO stage (0, 1, 2, or 3) at enrollment (eTables 7-8 in Supplement 3).
There was no statistically significant difference in the incidence of the composite outcome of death and need for kidney replacement therapy in the hospital or doubling of creatinine level (1452/5218 [27.8%] in the balanced solution group and 1527/5287 [28.9%] in the saline solution group; odds ratio, 0.95 [95% CI, 0.86-1.04]). Other post hoc sensitivity analyses exploring different definitions of acute kidney injury also rendered results that were not statistically significant (eTable 8 and eFigures 5-6 in Supplement 3). Patients randomized to the balanced solution group had lower chloride levels than patients randomized to the saline solution group (Figure 2B and eFigure 7 in Supplement 3; P < .001). After excluding patients with traumatic brain injury, the results for the primary, secondary, and tertiary outcomes were mostly unchanged (eTable 9 in Supplement 3).
Adverse Events
There were no unexpected treatment-related severe adverse events in either group.
Discussion
In this randomized clinical trial of critically ill patients requiring fluid therapy, use of a balanced solution did not change 90-day survival compared with saline solution.
Intravenous fluid composition represents an interesting target for clinical trials aiming to improve outcomes in critically ill patients because virtually all patients will receive fluids during their ICU stay. Therefore, even small benefits in the reduction of mortality and organ failure could have important population effects. Balanced solutions are designed to have a composition closer to blood plasma through use of organic anions as sodium buffers instead of equimolar chloride.1 Chloride load (and serum chloride levels) have been suggested to be associated with organ failure and mortality in critically ill patients; solutions with lower chloride concentrations were therefore hypothesized to be related to improved outcomes through reducing chloride load, although the exact mechanism is unclear.2,4,16
A large cluster-randomized clinical trial (Isotonic Solutions and Major Adverse Renal Events Trial [SMART])5 conducted at a large center reported that balanced solutions were associated with improvement in a composite outcome of death, new receipt of kidney replacement therapy, persistent kidney dysfunction censored at 30 days, and hospital discharge. Another study on patients in the emergency department who were not critically ill reported a similar reduction for this outcome.6 However, in another smaller randomized trial,7 there was no significant between-group difference for survival or other outcomes. Despite differences in design, a similar gradient to the SMART trial5 regarding serum chloride levels was obtained in the current study (ie, saline solution resulted in significantly more cases of hyperchloremia).
None of the secondary or subgroup analyses demonstrated benefit with use of the balanced solution. There was a signal of possible harm for patients in the balanced solution group with a traumatic brain injury and a worse neurological SOFA component score at day 7 (measured using the Glasgow Coma Scale, which is prone to measurement error and may be suboptimal in sedated patients). Hypotonic balanced solutions have been suggested to be potentially harmful for patients with traumatic brain injury,17 although the results are inconsistent.18 In the current study, all of the subgroup and secondary outcome analyses should be considered as only hypothesis-generating, particularly given the large number of statistical comparisons.
Limitations
This study has several limitations. First, although adherence to the study fluids was good, with close to 80% of all fluids received during the first days being the study drug, allowing the use of nonstudy fluids for small dilutions led to some degree of contamination. Second, patients frequently received fluids in the emergency department or in the operating room prior to enrollment. Fluid use before the ICU stay may affect the effect of fluid type on outcomes for critically ill patients.19 Third, due to its design, postrandomization exclusions occurred, mostly due to lack of consent for the trial. Fourth, there was a high number of planned surgical admissions that may have reduced the overall trial mortality.
Fifth, the trial tested a specific balanced solution; however, it is unclear if other solutions with different buffers (such as lactate) would provide similar results.4 Sixth, the trial was designed with a higher expected mortality at 90 days than the observed mortality that could be related to low illness severity and the large number of surgical patients. This may have resulted in a lower power to observe a clinically important difference. Seventh, patients received relatively small amounts of fluid during the trial that may have contributed to the neutral results of this study.
Conclusions
Among critically patients requiring fluid challenges, use of a balanced solution compared with 0.9% saline solution did not significantly reduce 90-day mortality. The findings do not support the use of this balanced solution.
Trial protocol
Statistical analysis plan
eMethods
eFigure 1. Study scheme
eFigure 2. Fluid management in BaSICS
eFigure 3. Primary outcome results according to both interventions in BaSICS (infusion speed and fluid type)
eFigure 4. Bayesian Network
eFigure 5. Creatinine values at the measured days according to group
eFigure 6. Density plot of creatinine (in log values, x-axis) and diuresis (y-axis) values at the measured days (panels) according to group
eFigure 7. Chloride levels over time displayed at mean and standard deviation
eTable 1. Baseline characteristics of the included patients on the four groups of the trial
eTable 2. Adhesion to allocated fluid use
eTable 3. Primary outcome model
eTable 4. Results for primary endpoint stratified by baseline chloride values (complete case analysis)
eTable 5. Results for queries in the Bayesian Network
eTable 6. Composite endpoint of mortality and use of renal replacement therapy during hospital stay
eTable 7. Primary endpoint analysis according to KDIGO at enrollment
eTable 8. Sensitivity creatinine analyses
eTable 9. Results for primary, secondary, and tertiary endpoints excluding patients with traumatic brain injury
Nonauthor collaborators. BaSICS investigators and the BRICNet members
Data sharing statement
References
- 1.Finfer S, Myburgh J, Bellomo R. Intravenous fluid therapy in critically ill adults. Published correction appears in Nat Rev Nephrol. 2018;14(11):717. Nat Rev Nephrol. 2018;14(9):541-557. [DOI] [PubMed] [Google Scholar]
- 2.Yunos NM, Bellomo R, Hegarty C, et al. Association between a chloride-liberal vs chloride-restrictive intravenous fluid administration strategy and kidney injury in critically ill adults. JAMA. 2012;308(15):1566-1572. [DOI] [PubMed] [Google Scholar]
- 3.Raghunathan K, Shaw A, Nathanson B, et al. Association between the choice of IV crystalloid and in-hospital mortality among critically ill adults with sepsis. Crit Care Med. 2014;42(7):1585-1591. [DOI] [PubMed] [Google Scholar]
- 4.Zampieri FG, Ranzani OT, Azevedo LC, et al. Lactated ringer is associated with reduced mortality and less acute kidney injury in critically ill patients: a retrospective cohort analysis. Crit Care Med. 2016;44(12):2163-2170. [DOI] [PubMed] [Google Scholar]
- 5.Semler MW, Self WH, Wanderer JP, et al. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378(9):829-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Self WH, Semler MW, Wanderer JP, et al. Balanced crystalloids versus saline in noncritically ill adults. N Engl J Med. 2018;378(9):819-828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young P, Bailey M, Beasley R, et al. ; SPLIT Investigators; ANZICS CTG . Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. Published correction appears in JAMA. 2015;314(23):2570. JAMA. 2015;314(16):1701-1710. [DOI] [PubMed] [Google Scholar]
- 8.Zampieri FG, Azevedo LCP, Corrêa TD, et al. ; BaSICS Investigators and the BRICNet . Study protocol for the Balanced Solution versus Saline in Intensive Care Study (BaSICS): a factorial randomised trial. Crit Care Resusc. 2017;19(2):175-182. [PubMed] [Google Scholar]
- 9.Damiani LP, Cavalcanti AB, Biondi RS, et al. Statistical analysis plan for the Balanced Solution versus Saline in Intensive Care Study (BaSICS). Rev Bras Ter Intensiva. 2020;32(4):493-505. doi: 10.5935/0103-507X.20200081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818-829. [PubMed] [Google Scholar]
- 11.Vincent JL, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. [DOI] [PubMed] [Google Scholar]
- 12.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group . KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2012;2:1-138. [Google Scholar]
- 13.Haybittle JL. Repeated assessment of results in clinical trials of cancer treatment. Br J Radiol. 1971;44(526):793-797. [DOI] [PubMed] [Google Scholar]
- 14.Grambsch PA, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;81:515-526. doi: 10.1093/biomet/81.3.515 [DOI] [Google Scholar]
- 15.R Core Team . R: a language and environment for statistical computing. Accessed April 1, 2021. http://www.R-project.org/
- 16.Semler MW, Kellum JA. Balanced crystalloid solutions. Am J Respir Crit Care Med. 2019;199(8):952-960. doi: 10.1164/rccm.201809-1677CI [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rowell SE, Fair KA, Barbosa RR, et al. The impact of pre-hospital administration of lactated ringer’s solution versus normal saline in patients with traumatic brain injury. J Neurotrauma. 2016;33(11):1054-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roquilly A, Loutrel O, Cinotti R, et al. Balanced versus chloride-rich solutions for fluid resuscitation in brain-injured patients. Crit Care. 2013;17(2):R77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jackson KE, Wang L, Casey JD, et al. Effect of early balanced crystalloids before ICU admission on sepsis outcomes. Chest. 2021;159(2):585-595. doi: 10.1016/j.chest.2020.08.2068 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
Statistical analysis plan
eMethods
eFigure 1. Study scheme
eFigure 2. Fluid management in BaSICS
eFigure 3. Primary outcome results according to both interventions in BaSICS (infusion speed and fluid type)
eFigure 4. Bayesian Network
eFigure 5. Creatinine values at the measured days according to group
eFigure 6. Density plot of creatinine (in log values, x-axis) and diuresis (y-axis) values at the measured days (panels) according to group
eFigure 7. Chloride levels over time displayed at mean and standard deviation
eTable 1. Baseline characteristics of the included patients on the four groups of the trial
eTable 2. Adhesion to allocated fluid use
eTable 3. Primary outcome model
eTable 4. Results for primary endpoint stratified by baseline chloride values (complete case analysis)
eTable 5. Results for queries in the Bayesian Network
eTable 6. Composite endpoint of mortality and use of renal replacement therapy during hospital stay
eTable 7. Primary endpoint analysis according to KDIGO at enrollment
eTable 8. Sensitivity creatinine analyses
eTable 9. Results for primary, secondary, and tertiary endpoints excluding patients with traumatic brain injury
Nonauthor collaborators. BaSICS investigators and the BRICNet members
Data sharing statement