Abstract
Takotsubo cardiomyopathy (TCM) secondary to an infusion reaction is extremely rare in the literature. Here, we present an unusual case of TCM in a patient with cervical squamous cell carcinoma who presented with acute hypoxic respiratory failure following the initiation of the first-cycle paclitaxel infusion therapy.
Keywords: cardiovascular medicine, arrhythmias, cancer - see oncology, interventional cardiology, ischaemic heart disease
Background
Takotsubo cardiomyopathy (TCM) or broken heart syndrome is described as sudden, transient and dramatic left ventricular (LV) akinesis that mimics acute coronary syndrome (ACS) in terms of symptoms, ECG changes and elevated troponin due to myocardial injury without significant coronary artery stenosis. One possible aetiology of TCM is impaired coronary microcirculation.1 Prognosis of TCM is excellent, owing to improvement of LV ejection fraction (LVEF) from 20%–49.9% to 59%–76% within a mean time of 7–37 days and an in-hospital mortality rate of 1.7%.2 It is more prevalent in postmenopausal women with a recent history of emotional or physical stress.3
Case presentation
A 79-year-old women with a history of stage IIb squamous cell carcinoma of the cervix, hypertension and non-ischaemic cardiomyopathy with recovered LV function, was sent to the emergency department (ED) from the oncology clinic after becoming unresponsive for 15–20 s following 1.3 mL infusion of paclitaxel as the initial agent for her scheduled chemotherapeutic course. The patient reported dyspnoea at rest and was found to be tachycardic with heart rates in the 150s–160s, hypoxaemic with oxygen saturations of 72%, requiring a 100% non-rebreather mask and hypertensive with blood pressure 158/100 mm Hg. The paclitaxel infusion was stopped immediately, and intravenous crystalloid fluid, Benadryl 50 mg intravenous and hydrocortisone 100 mg intravenous were administered as initial treatment. The patient was transferred to the ED for further evaluation.
Investigations
The initial ECG showed sinus tachycardia without any ST or T-wave abnormalities (figure 1). Subsequent ECGs had shown new-onset T-wave inversions in the lateral precordial leads, specifically in V5–V6 (figure 2). Cardiac enzymes showed a high sensitivity initial troponin of 16 ng/mL and up trended to a peak of 1100 ng/mL. A transthoracic echocardiogram (TTE) showed an EF of 15%–20% with severely hypokinetic to akinetic mid to apical segments with preserved contractility of basal segments. The global longitudinal strain was significantly abnormal with a severe reduction in a segmental strain more at the apical segments. The regional dysfunction raised suspicion for stress induced cardiomyopathy or TCM (figure 3 and video 1). A coronary angiogram was done and showed normal coronary arteries (figure 4). Chest X-ray was unremarkable for cardiomegaly, pneumothorax, pulmonary vascular congestion or infiltrates. CT angiogram of the thorax was negative for pulmonary embolism. Complete blood count, liver function tests, renal function tests and electrolytes were within normal limits.
Figure 1.
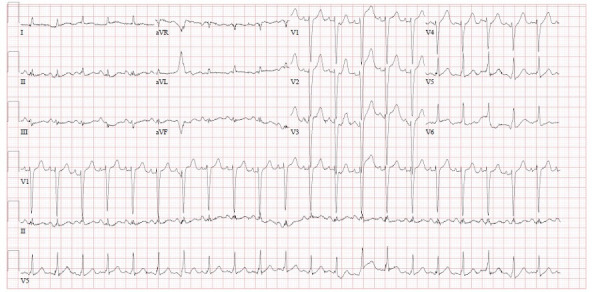
ECG during the initial presentation showing sinus tachycardia. aVF, Augmented vector foot; aVL, Augmented vector left.
Figure 2.
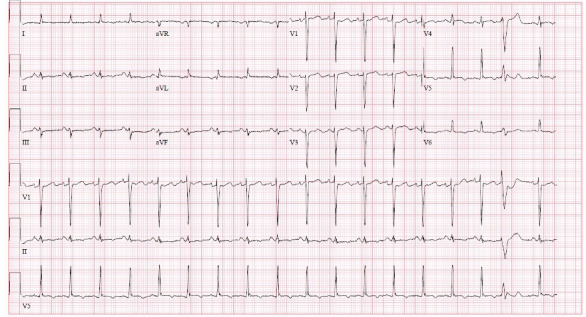
ECG after 6 hours from the presentation showing new T-wave inversions the in lateral precordial leads V5–V6. aVF, Augmented vector foot; aVL, Augmented vector left.
Figure 3.
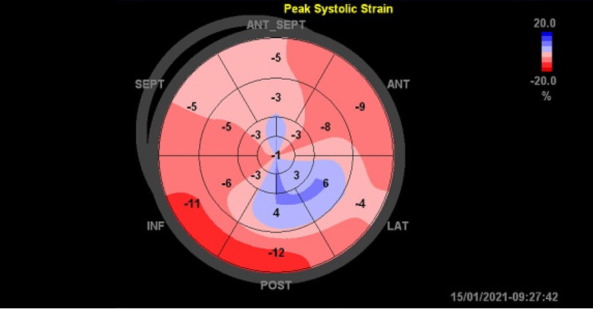
Bull’s eye map of the longitudinal strain pattern on echocardiography. ANT, Anterior; ANT_SEPT, antero-septal; INF, Inferior; LAT, lateral; SEPT, Septal.
Video 1.
Figure 4.
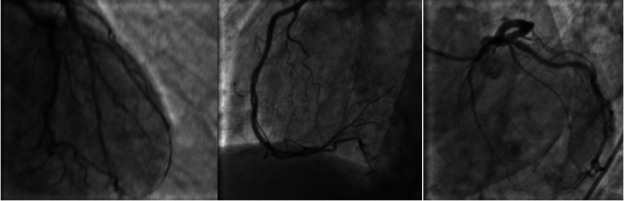
Coronary angiogram with normal coronary arteries.
Differential diagnosis
1-Anaphylaxis to chemotherapy given sudden onset of dyspnea and hypotension following starting the infusion. 2-Acute coronary syndrome patient is elderly and will have a variable degree of coronary atherosclerosis disease presented with sudden onset dyspnea which equivalent to angina. 3-Pulmonary embolism given patient at high risk of venous thromboembolism due to malignancy present with sudden onset of dyspnea. 4-Acute systolic Heart failure is possible, the patient has a remote history of Heart failure with reduced ejection fraction (HFrEF) with recovered EF (ejection fraction) can be exacerbated by malignancy or stress. 5-pneumothorax sudden onset of dyspnea, might have metastasis to the lung and causing necrosis and subsequent pneumothorax.
Treatment
The patient was loaded with aspirin and started on a heparin drip per ACS protocol. The patient was hypotensive before left heart catheterisation (LHC) and was started on a dobutamine drip for suspected cardiogenic shock. Heparin drip was stopped after the catheterisation revealed normal coronaries. When the patient’s blood pressure improved, she was transitioned from dobutamine to digoxin and started on captopril. A TTE was repeated on day 5 showed recovered function to 55%–60% and no regional wall motion abnormalities (figure 5 and video 2). Her maps improved to 60s–70s. Subsequently, digoxin was stopped, and carvedilol and losartan were initiated. The patient’s clinical symptoms improved gradually during the admission and were discharged home on day 6 with heart failure guideline-directed medical therapy with an anticipated plan to restart chemotherapy with desensitisation protocol outpatient.
Figure 5.
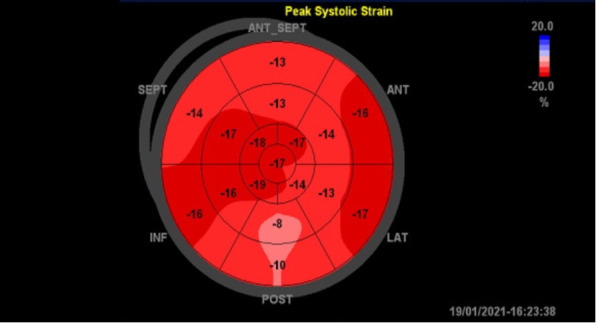
Bull’s eye map of strain pattern on repeated echocardiography 5 days after showing recovered contractility of apex and free wall of left ventricle. ANT, Anterior; ANT_SEPT, antero-septal; INF, Inferior; LAT, lateral; SEPT, Septal.
Video 2.
Outcome and follow-up
The patient presented to the cardiology clinic 2 weeks after discharge, she remains asymptomatic and tolerates losartan and carvedilol. Medical management was continued with no change.
Discussion
TCM is a rare but transient form of LV systolic dysfunction that mimics ACS in the absence of clinically significant obstructive coronary artery disease with most of the cases being attributed to a physiological or emotional stressor.4 The pathogenesis of TCM is elusive. Several pathophysiological mechanisms involving myocardial ischaemia (multivessel coronary artery spasm, microvascular dysfunction, aborted myocardial infarction), LV outlet tract obstruction, bloodborne catecholamine myocardial toxicity, epinephrine-induced switch in signal trafficking, and autonomic nervous system dysfunction have been proposed.5 The most common form of TCM is the apical ballooning variant. ECG findings that distinguish ACS from TCM are mainly T-wave inversion appeared when ST elevation had subsided; when T-wave inversion appeared, QT prolongation occurred; there was no reciprocal change in limb leads or atrioventricular block; Q-waves were reversible, and there was no significant arrhythmia even.6
Patients with malignancy have been shown to have a higher incidence of TCM, with as high as 10% among patients with malignancy as opposed to the 1%–2% estimated in the general population.7 Several mechanisms to explain the pathophysiology of TCM and the role of malignancy in its incidence have been suggested including emotional response, the chronic inflammatory state of cancer, the physical/surgical stress of cancer treatments, radiotherapy, chemotherapy and paraneoplastic mediators.8 Chemotherapy can cause coronary endothelial dysfunction and increased coronary spasticity that may lead to diffuse, transient spastic obliteration of coronary arteries and critical ischaemia contributing to the development of TCM.7–9
Numerous chemotherapeutic agents are well known to be associated with TCM. The antimetabolite class of drugs (5-fluorouracil, capecitabine and cytarabine) are the most reported agents as TCM triggers, followed by tyrosine kinase inhibitors (sunitinib, axitinib and pazopanib), HER2 monoclonal antibodies (trastuzumab and pertuzumab), angiogenesis inhibitors (bevacizumab), CD20 monoclonal antibodies (rituximab), microtubule-targeting drugs (paclitaxel and combretastatin) and anthracyclines (doxorubicin and daunorubicin).10 To the best of our knowledge, only a limited number of cases of paclitaxel-induced TCM have been reported.11–14
In our case report, we are presenting a very uncommon and under-reported case of TCM that happened minutes after initiating paclitaxel infusion and was completely resolved with discontinuation of the drug. With the acute onset in symptoms relative to initiation of paclitaxel, the TCM was likely related to the stress of the infusion reaction, rather than the cardiotoxic effects of paclitaxel. Paclitaxel is known to cause a type 1 hypersensitivity-like response that is mediated via histamine release, typically taxanes provoke reactions in the beginning portion of the first dose, as in our patient.15 There are only two cases of infusion reactions precipitating TCM reported, both were monoclonal antibodies given as chemotherapeutic agents including trastuzumab and daratumumab.16 17 To the best of our knowledge, there is no infusion mediated TCM secondary to paclitaxel reported previously in the literature.
Conclusions
Rapid-onset TCM is a rare and under-reported complication of paclitaxel infusion. Treatment and recovery of LV dysfunction are expected after discontinuation of the medication with heart failure guideline-directed medical therapy.
Patient’s perspective.
‘I felt like I am going to die when the infusion started, you guys did a great job, today I feel like I am back to my normal life’
Learning points.
An oncologist should be aware of the possibility of rapid takotsubo syndrome development even minutes following paclitaxel infusion. A complication that can be so significant leading to cardiogenic shock with increased mortality, morbidity and in-hospital length of stay.
We must take into our consideration the predisposing factors that increase the risk for takotsubo cardiomyopathy (TCM) before starting paclitaxel including but not limited to the female gender, postmenopausal, malignancy, history of neurological disease (seizure, epilepsy) and history of psychiatric illness. To predict the incidence of TCM and take action to prevent it. These steps need to be studied further.
The list of chemotherapy induced TCM is growing despite under-reported cases. Thus, we must keep this syndrome in mind as a side effect of all chemotherapy regimens including new monoclonal antibodies.
Footnotes
Twitter: @n/a
Contributors: TMU is the first author. MMU is the corresponding author.TMU, AS and MMU made the conception, design and abstract for this paper. TMU, AS and MMU have helped with writing case presentation, discussion section, data collection from the EMR including lab findings and medical histories, echocardiography image collection from cardiology echocardiography lab, collecting CT scan images in addition to talking to Radiologist for deeper interpretation of the images in the study. TMU, MMU, AS, KB, AE have collected all the citations, interpreting the summary of each citation article, and worked on in-text citations that are relevant to the article. SC was the supervising or senior author who finalised the paper for submission and finished data interpretation.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Ito K, Sugihara H, Katoh S, et al. Assessment of Takotsubo (ampulla) cardiomyopathy using 99mTc-tetrofosmin myocardial SPECT--comparison with acute coronary syndrome. Ann Nucl Med 2003;17:115–22. 10.1007/BF02988449 [DOI] [PubMed] [Google Scholar]
- 2.Pilgrim TM, Wyss TR. Takotsubo cardiomyopathy or transient left ventricular apical ballooning syndrome: a systematic review. Int J Cardiol 2008;124:283–92. 10.1016/j.ijcard.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 3.Gianni M, Dentali F, Grandi AM, et al. Apical ballooning syndrome or takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523–9. 10.1093/eurheartj/ehl032 [DOI] [PubMed] [Google Scholar]
- 4.Khan NAJ, Pacioles T, Alsharedi M. Atypical takotsubo cardiomyopathy secondary to combination of chemo-immunotherapy in a patient with non-small cell lung cancer. Cureus 2020;58. 10.7759/cureus.9429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Y-Hassan S, Tornvall P, Epidemiology TP. Epidemiology, pathogenesis, and management of takotsubo syndrome. Clin Auton Res 2018;28:53–65. 10.1007/s10286-017-0465-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Namgung J. Electrocardiographic findings in takotsubo cardiomyopathy: ECG evolution and its difference from the ECG of acute coronary syndrome. Clin Med Insights Cardiol 2014;8:CMC.S14086–34. 10.4137/CMC.S14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cammann VL, Sarcon A, Ding KJ, et al. Clinical features and outcomes of patients with malignancy and takotsubo syndrome: observations from the International takotsubo registry. J Am Heart Assoc 2019;8:e010881. 10.1161/JAHA.118.010881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai A, Noor A, Joshi S, et al. Takotsubo cardiomyopathy in cancer patients. Cardiooncology 2019;5:1–6. 10.1186/s40959-019-0042-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Angelini P, Uribe C. Is transient takotsubo syndrome associated with cancer? why, and with what implications for Oncocardiology? J Am Heart Assoc 2019;8:e013201. 10.1161/JAHA.119.013201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storey K, Sharkey SW. Clinical features and outcomes of patients with chemotherapy-induced takotsubo syndrome. US Cardiology Review 2019;13:74–82. 10.15420/usc.2019.10.1 [DOI] [Google Scholar]
- 11.Vejpongsa P, Banchs J, Reyes M, et al. Takotsubo cardiomyopathy in cancer patients: triggers, recovery, and resumption of therapy. J Am Coll Cardiol 2015;65:A927. 10.1016/S0735-1097(15)60927-5 [DOI] [Google Scholar]
- 12.Giza DE, Lopez-Mattei J, Vejpongsa P, et al. Stress-Induced cardiomyopathy in cancer patients. Am J Cardiol 2017;120:2284–8. 10.1016/j.amjcard.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 13.Ionescu D, Stone D, Stone J. Takotsubo syndrome and use of paclitaxel. J Clin Oncol 2017;35 (suppl 15):e14012. 10.1200/JCO.2017.35.15_suppl.e14012 [DOI] [Google Scholar]
- 14.Balanescu DV, Liu VY, Donisan T. Clinical features and outcomes of patients with chemotherapy-induced takotsubo stress cardiomyopathy. Eur Heart J 2018;39 (suppl 1):ehy565–1253. 10.1093/eurheartj/ehy565.P1253 [DOI] [Google Scholar]
- 15.Picard M, Castells MC. Re-visiting hypersensitivity reactions to taxanes: a comprehensive review. Clin Rev Allergy Immunol 2015;49:177–91. 10.1007/s12016-014-8416-0 [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto T, Oda T, Yoshida Y, et al. Takotsubo cardiomyopathy caused by infusion reaction to trastuzumab. Int Cancer Conf J 2020;9:28–31. 10.1007/s13691-019-00396-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustehsan MH, Jahufar F, Arora S. A diagnostically challenging infusion Reaction-Kounis, takotsubo, or the ATAK! JAMA Intern Med 2019;179:99–100. 10.1001/jamainternmed.2018.6155 [DOI] [PubMed] [Google Scholar]


