Abstract
Background:
Sacubitril-valsartan reduced risks of death and hospitalization for heart failure (HF) versus enalapril in ambulatory patients with HF and reduced ejection fraction in the PARADIGM-HF trial. However, the comparative effectiveness of sacubitril-valsartan and ACE inhibitors / angiotensin receptor blockers (ACE/ARB) in patients treated in routine clinical practice is unclear.
Objectives:
To compare the effectiveness of sacubitril-valsartan and ACE/ARB in systolic HF.
Methods:
We identified patients with systolic HF in a U.S. administrative claims database treated with sacubitril-valsartan or ACE/ARB from 07/01/15–02/01/18. One-to-one propensity score matching was used to balance patients on 29 clinical variables. Cox models were used to compare outcomes between treatment groups.
Results:
A total of 7893 matched pairs were included; mean (SD) follow-up was 6.3 (5.4) months. Sacubitril-valsartan was associated with lower risks of all-cause mortality or all-cause hospitalization (HR 0.86, 95% CI 0.81–0.91, p<0.001), all-cause mortality (HR 0.80, 95% CI 0.66–0.97, p=0.027), and all-cause hospitalization (HR 0.86, 95% CI 0.80–0.91, p<0.001), but not HF hospitalization (HR 1.07, 95% CI 0.96–1.19, p=0.26). A lower risk of the primary outcome with sacubitril-valsartan was observed in whites (HR 0.83, 95% CI 0.76–0.90) but not blacks (21% of population, HR 1.00, 95% CI 0.88–1.15, interaction p=0.032). No statistically significant differences in treatment response by sex or age were observed.
Conclusion:
Sacubitril-valsartan was associated with lower risks of death and hospitalization compared with ACE/ARB in a heterogeneous cohort of patients with systolic HF. However, our finding that outcomes with sacubitril-valsartan and ACE/ARBs were similar in black patients warrants further evaluation.
Keywords: heart failure, medication, mortality, hospitalization, sacubitril-valsartan
Introduction
Medical therapy for heart failure with reduced ejection fraction (HFrEF) has evolved over the last several decades due to the identification of effective pharmacotherapies that have significantly reduced morbidity and mortality. In 2014, the first-in-class small molecule LCZ696, which combined the neprilysin inhibitor sacubitril with the angiotensin II receptor blocker (ARB) valsartan, was shown to decrease all-cause mortality by 16% and HF hospitalization by 21% compared with enalapril in patients with symptomatic HFrEF enrolled in the Prospective Comparison of Angiotensin II Receptor Blocker Neprilysin Inhibitor with Angiotensin Converting Enzyme Inhibitor to Determine Impact on Global Mortality and Morbidity in HF (PARADIGM-HF) trial.(1) In July 2015, sacubitril-valsartan was approved by the U.S. FDA for use in chronic symptomatic HFrEF. Despite receiving a Class I recommendation in HF guidelines,(2,3) use of sacubitril-valsartan in clinical practice has been lower than expected.(4,5)
While numerous factors can impact the adoption of novel pharmacotherapies, uncertainty about the effectiveness of sacubitril-valsartan outside of clinical trial populations may have contributed to slow uptake. This uncertainty is especially pertinent for patient groups historically underrepresented in clinical trials, such as women, older individuals, and racial and ethnic minorities.(6) In PARADIGM-HF, only 5% of participants were black, and 21% were women, and as such, confidence in the effectiveness of sacubitril-valsartan in these populations is less robust. Furthermore, observational data suggest that clinicians are prescribing sacubitril-valsartan in ways that vary from the PARADIGM-HF treatment protocol.(5) Many patients initiated on sacubitril-valsartan have not been taking an angiotensin converting enzyme inhibitor (ACE) or ARB, and the effectiveness of sacubitril-valsartan in ambulatory ACE/ARB naïve patients is unknown.
To address potential uncertainties about the effectiveness of sacubitril-valsartan in real-world clinical practice, we compared differences in mortality and hospitalization in patients with HFrEF taking sacubitril-valsartan and ACE/ARB therapy represented in a large commercial insurance claims database.
Methods
Data Source.
This study was a retrospective cohort study from the OptumLabs® Data Warehouse (OLDW), which includes claims data for privately insured and Medicare Advantage enrollees in a large, private, U.S. health plan.(7,8) We included individuals with both medical and pharmacy insurance coverage. The study was exempt from institutional board review as it used pre-existing de-identified data.
Study Population.
We identified all individuals at least 18 years of age that filled a prescription for sacubitril-valsartan or ACE/ARB between 07/01/2015 and 02/01/2018. We restricted to those with a prior diagnosis of systolic HF using International Classification of Diseases [ICD] billing codes (9th Edition, 428.2X; 10th Edition, I50.2X). This approach is 97.7% specific for individuals with HF and an ejection fraction (EF)<45%/(5,9) Patients were required to have ≥180 days of continuous enrollment in a medical health plan with prescription coverage prior to their index medication fill date to ensure adequate capture of baseline characteristics. The index date of the sacubitril-valsartan cohort was a patient’s first prescription of sacubitril-valsartan, whereas the index date of the ACE/ARB cohort was the first fill of an ACE/ARB in the study period after patients met the 180 day enrollment requirement. Patients in the sacubitril-valsartan cohort could have ACE/ARB prescriptions prior to their index date.
Baseline Characteristics.
Clinical variables were defined by the presence of a claim with corresponding diagnosis codes, procedure codes, or prescription fills. Race in OLDW is classified based on self-report or derived rule sets (10,11), and is classified here as non-Hispanic white (white), non-Hispanic black (black) or other. Household income is estimated based on a model using both public and private consumer data. Comorbidities were captured by ICD-9 or ICD-10 codes in any position on claims in the 6 months prior to index prescription fill.(12) Prior hospitalizations,cardiologist, and primary care office visits were captured using medical claims. Prior medication use was defined as having a prescription fill within 120 days prior to the index date. The total daily dose of the ACE/ARB was categorized as low, intermediate or high (Supplementary Table S1).
Follow-Up.
Follow-up started from the index date and continued until end of treatment. End of treatment was defined as the earliest date of: discontinuation of index medication, end of enrollment in health plan, death, or end of the study period (February 1, 2018). Discontinuation of index medication was defined as not refilling a prescription within 30 days of end of supply.
We calculated adherence using the medical possession ratio (MPR).(13) Specifically, we used the prescription fill dates and days supply for each medication group (sacubitril-valsartan or ACE/ARB) to calculate total days supply and divided by the number of days in the follow-up period.
Study Outcomes.
The primary outcome was a composite of all-cause mortality or all-cause hospitalization. Mortality was identified using the Social Security Death Master File and discharge status of expired after a hospitalization.(14) All-cause hospitalization was captured using medical claims. Secondary outcomes included all-cause mortality, all-cause hospitalization, and HF hospitalization. HF hospitalization was defined as a hospitalization with primary ICD-9 codes 428.X, 402.X1, 404.X1, 404.X3 or ICD-10 codes I11.0, I13.0, I13.2, or I50. (15–17) To assess differences in safety, we compared the risks of angioedema (ICD-9 995.1 or ICD-10 T78.3XXA), hypotension (ICD-9 458 or ICD-10 I95), and hyperkalemia (ICD-9 276.7 or ICD-10 E87.5) between treatment groups during follow-up. We included hospitalizations or outpatient visits where the ICD codes were listed as the primary diagnosis.
Statistical Analysis.
We used propensity score matching to identify patients treated with ACE/ARB who were similar to those treated with sacubitril-valsartan. Logistic regression was used to estimate the probability of being treated with sacubitril-valsartan. Covariates included in the logistic model were age, sex, race/ethnicity, census region, depression, renal failure, cardiac arrhythmia, peripheral vascular disease, valvular heart disease, anemia, hypertension, diabetes, prior myocardial infarction, dementia, cerebrovascular disease, chronic obstructive pulmonary disease, implantable cardioverter defibrillator (ICD), prior use of HF medications (beta blockers, loop diuretics, aldosterone antagonists, digoxin), strength of ACE/ARB dose (among prior/ current users), Charlson comorbidity index, office visit with a cardiologist and primary care provider, and hospitalization (all-cause, prior HF) in the last 6 months. One-to-one nearest-neighbor caliper matching was used to match patients based on the logit of the propensity score using a caliper equal to 0.2 of the standard deviation of the logit of the propensity score (18). To account for potential effects of initiating renin-angiotensin therapy, we exact matched new users of sacubitril-valsartan that had not used an ACE/ARB in the last 6 months to new users of ACE/ARB. For those switching to sacubitril-valsartan from ACE/ARB (had filled a prescription for ACE/ARB in prior 6 months), we matched to prevalent ACE/ARB users. Standardized difference was used to assess the balance of covariates after matching, with a difference of no more than 10% considered acceptable.(19)
Cox proportional hazards regression was used to compare the risk of outcomes between treatment groups in the propensity-matched cohort. Robust sandwich estimates were included to account for clustering within matched sets.(20) The proportional hazards assumption was tested on the basis of Schoenfeld residuals and found to be valid.(21) Differences in hazard ratios (HR) by subgroups of interest were tested using interaction terms. All analyses were conducted using SAS 9.4 (SAS Institute Inc., Cary, NC) and Stata version 14.1 (StataCorp).
Sensitivity Analyses.
First, we required at least 14 days of follow-up after cohort entry; results were similar (data not shown). Second, to ensure that the two treatment groups were well balanced following propensity matching, we compared laboratory values pre- and post-matching where available, including serum creatinine, calcium, albumin, hemoglobin and sodium (Supplementary Table S2). The values and the proportion of missing values were balanced after matching. Finally, analysis using a falsification endpoint was performed to test for residual confounding.(22) Risk of outpatient urinary tract infection was selected as was unlikely to be impacted by treatment with sacubitril-valsartan versus ACE/ARB.
Results
Patient Characteristics.
A total of 8291 and 83318 adults filling a prescription for sacubitril-valsartan or ACE/ARB, respectively, were identified. Prior to matching, patients initiating sacubitril-valsartan were more often men, more often taking evidence-based HF medications, and more likely to have seen a cardiologist recently (Table 1). The final propensity-matched cohort included 7893 pairs taking sacubitril-valsartan or ACE/ARB. Overall, demographic and clinical characteristics were well-balanced between the two treatment groups (standardized differences <10%, Table 1). One-third of patients were women. There was excellent representation of racial and ethnic minority groups (20% black, 11% Hispanic). Over one-third of patients initiating sacubitril-valsartan had not filled a prescription for an ACE or ARB in the last 6 months. Adherence in both treatment groups was high, with mean MPRs of 0.94 (SD 0.096) and 0.98 (SD 0.05) and in the sacubitril-valsartan and ACE/ARB groups, respectively. Adherence to sacubitril-valsartan (mean MPR 0.93 vs. 0.95) and ACE/ARBs (mean MPR 0.97 vs. 0.98) was numerically slightly lower in black compared with white patients.
Table 1:
Baseline Characteristics Before and After Propensity Score Matching
| Characteristic | Pre-Match | Post-Match | ||||
|---|---|---|---|---|---|---|
| ACE/ARB (n=83318) | Sacubitril-Valsartan (n=8291) | Std Diff | ACE/ARB (n=7893) | Sacubitril-Valsartan (n=7893) | Std Diff | |
| Age | ||||||
| Mean (SD) | 69.9 (12.2) | 68.2 (12.0) | 0.14 | 68.1 (11.9) | 68.2 (12.0) | −0.01 |
| Median | 71 | 70 | 68 | 70 | ||
| Q1, Q3 | 62.0, 80.0 | 61.0, 77.0 | 61.0, 77.0 | 61.0, 77.0 | ||
| Sex | ||||||
| Female | 34318 (41.2%) | 2719 (32.8%) | −0.17 | 2588 (32.8%) | 2625 (33.3%) | 0.01 |
| Male | 49000 (58.8%) | 5572 (67.2%) | 0.17 | 5305 (67.2%) | 5268 (66.7%) | −0.01 |
| Race | ||||||
| Asian | 1638 (2.0%) | 168 (2.0%) | 0.00 | 164 (2.1%) | 160 (2.0%) | −0.01 |
| Black | 16246 (19.5%) | 1741 (21.0%) | 0.04 | 1608 (20.4%) | 1649 (20.9%) | 0.01 |
| Hispanic | 7062 (8.5%) | 920 (11.1%) | 0.09 | 900 (11.4%) | 866 (11.0%) | −0.01 |
| Unknown | 5294 (6.4%) | 531 (6.4%) | 0.00 | 569 (7.2%) | 528 (6.7%) | −0.02 |
| White | 53078 (63.7%) | 4931 (59.5%) | −0.09 | 4652 (58.9%) | 4690 (59.4%) | 0.01 |
| Income | ||||||
| <$40,000 | 27263 (32.7) | 2351 (28.4) | −0.09 | 2493 (31.6) | 2242 (28.4) | −0.07 |
| $40,000–$74,999 | 20394 (24.5) | 2154 (26) | 0.03 | 1865 (23.6) | 2041 (25.9) | 0.05 |
| $75,000–$124,999 | 13826 (16.6) | 1600 (19.3) | 0.07 | 1278 (16.2) | 1520 (19.3) | 0.08 |
| $125,000–$199,999 | 4349 (5.2) | 570 (6.9) | 0.07 | 374 (4.7) | 536 (6.8) | 0.09 |
| $200,000+ | 1735 (2.1) | 239 (2.9) | 0.05 | 150 (1.9) | 228 (2.9) | 0.06 |
| Missing | 15751 (18.9) | 1377 (16.6) | −0.06 | 1733 (22.0) | 1326 (16.8) | −0.13 |
| Patient Census Region | ||||||
| Midwest | 23865 (28.6%) | 1671 (20.2%) | −0.20 | 1622 (20.5%) | 1596 (20.2%) | −0.01 |
| Northeast | 12550 (15.1%) | 1148 (13.8%) | −0.04 | 1047 (13.3%) | 1054 (13.4%) | 0.00 |
| South | 39389 (47.3%) | 4973 (60.0%) | 0.26 | 4715 (59.7%) | 4759 (60.3%) | 0.01 |
| West | 7514 (9.0%) | 499 (6.0%) | −0.11 | 509 (6.4%) | 484 (6.1%) | −0.01 |
| Medical Comorbidities | ||||||
| Depression | 18362 (22.0%) | 1670 (20.1%) | −0.05 | 1423 (18.0%) | 1523 (19.3%) | 0.03 |
| Renal Failure | 28024 (33.6%) | 2791 (33.7%) | 0.00 | 2533 (32.1%) | 2613 (33.1%) | 0.02 |
| Cardiac Arrhythmia | 60174 (72.2%) | 6461 (77.9%) | 0.13 | 6056 (76.7%) | 6077 (77.0%) | 0.01 |
| Peripheral Vascular Disease | 35640 (42.8%) | 4799 (57.9%) | 0.31 | 4342 (55.0%) | 4469 (56.6%) | 0.03 |
| Valvular Disease | 41951 (50.4%) | 4928 (59.4%) | 0.18 | 4442 (56.3%) | 4568 (57.9%) | 0.03 |
| Anemia | 16628 (20.0%) | 1536 (18.5%) | −0.04 | 1333 (16.9%) | 1408 (17.8%) | 0.02 |
| Hypertension | 76899 (92.3%) | 7710 (93.0%) | 0.03 | 7250 (91.9%) | 7321 (92.8%) | 0.03 |
| Diabetes | 44095 (52.9%) | 4532 (54.7%) | 0.04 | 4188 (53.1%) | 4273 (54.1%) | 0.02 |
| History of Myocardial Infarction | 26853 (32.2%) | 3021 (36.4%) | 0.09 | 2579 (32.7%) | 2734 (34.6%) | 0.04 |
| Dementia | 9485 (11.4%) | 453 (5.5%) | −0.21 | 385 (4.9%) | 427 (5.4%) | 0.02 |
| Cerebrovascular Disease | 26086 (31.3%) | 2377 (28.7%) | −0.06 | 2024 (25.6%) | 2170 (27.5%) | 0.04 |
| COPD | 40802 (49.0%) | 4008 (48.3%) | −0.01 | 3554 (45.0%) | 3716 (47.1%) | 0.04 |
| Pacemaker/ ICD | 24851 (29.8%) | 4058 (48.9%) | 0.40 | 3739 (47.4%) | 3781 (47.9%) | 0.01 |
| Medications | ||||||
| β-blockers | 59875 (71.9%) | 7283 (87.8%) | 0.41 | 6894 (87.3%) | 6894 (87.3%) | 0.00 |
| Loop diuretics | 43347 (52.0%) | 5500 (66.3%) | 0.29 | 5216 (66.1%) | 5177 (65.6%) | −0.01 |
| Aldosterone antagonist | 14167 (17.0%) | 3129 (37.7%) | 0.48 | 2887 (36.6%) | 2856 (36.2%) | −0.01 |
| Digoxin | 7830 (9.4%) | 1202 (14.5%) | 0.16 | 1094 (13.9%) | 1107 (14.0%) | 0.00 |
| Prior ACE/ARB (120 Days Prior) | 50449 (60.5%) | 5309 (64.0%) | 0.07 | 4911 (62.2%) | 4911 (62.2%) | 0.00 |
| ACE | 33651 (40.4%) | 3138 (37.8%) | −0.05 | 2961 (37.5%) | 2961 (37.5%) | 0.00 |
| ARB | 16798 (20.1%) | 2171 (26.2%) | 0.14 | 1950 (24.7%) | 1950 (24.7%) | 0.00 |
| Prior Dose (ACE/ARB) | ||||||
| Low dose | 16369 (32.4%) | 1739 (32.8%) | 0.01 | 1604 (32.7%) | 1614 (32.9%) | 0.00 |
| Medium dose | 20712 (41.1%) | 2234 (42.1%) | 0.02 | 2046 (41.7%) | 2060 (41.9%) | 0.00 |
| High dose | 13368 (26.5%) | 1336 (25.2%) | −0.03 | 1261 (25.7%) | 1237 (25.2%) | −0.01 |
| Charlson Index Score | ||||||
| Mean (SD) | 5.2 (3.2) | 5.3 (3.1) | −0.03 | 5.0 (2.9) | 5.2 (3.1) | −0.07 |
| Median (IQR) | 5.0 | 5.0 | 5.0 | 5.0 | ||
| Office Visit | ||||||
| Cardiologist | 45641 (54.8) | 6641 (80.1) | 0.56 | 6278 (79.5) | 6269 (79.4) | 0.00 |
| Primary care | 35281 (42.3) | 3730 (45.0) | 0.05 | 3500 (44.3) | 3559 (45.1) | 0.02 |
| Prior Hospitalization | ||||||
| “0” | 49116 (59.0%) | 5016 (60.5%) | 0.03 | 4827 (61.2%) | 4780 (60.6%) | −0.01 |
| “1” | 24021 (28.8%) | 2214 (26.7%) | −0.05 | 2103 (26.6%) | 2117 (26.8%) | 0.00 |
| “2+” | 10181 (12.2%) | 1061 (12.8%) | 0.02 | 963 (12.2%) | 996 (12.6%) | 0.01 |
| Prior HF Hospitalization | ||||||
| “0” | 69129 (83.0%) | 6525 (78.7%) | −0.11 | 6283 (79.6%) | 6245 (79.1%) | −0.01 |
| “1” | 12004 (14.4%) | 1421 (17.1%) | 0.07 | 1303 (16.5%) | 1330 (16.9%) | 0.01 |
| “2+” | 2185 (2.6%) | 345 (4.2%) | 0.09 | 307 (3.9%) | 318 (4.0%) | 0.01 |
| Year of Index | ||||||
| 2015 | 39135 (47.0%) | 162 (2.0%) | −1.23 | 191 (2.4%) | 162 (2.1%) | −0.02 |
| 2016 | 22356 (26.8%) | 2213 (26.7%) | 0.00 | 2234 (28.3%) | 2191 (27.8%) | −0.01 |
| 2017 | 20569 (24.7%) | 5383 (64.9%) | 0.88 | 5021 (63.6%) | 5075 (64.3%) | 0.01 |
| 2018 | 1258 (1.5%) | 533 (6.4%) | 0.25 | 447 (5.7%) | 465 (5.9%) | 0.01 |
ACE/ARB= angiotensin converting enzyme inhibitor/ angiotensin receptor blocker, COPD= chronic obstructive pulmonary disease; HF= heart failure
Outcomes.
The mean (SD) and median (IQR) follow-up times were 6.3(5.4) and 4.8(2.1–8.4) months, respectively. The primary outcome occurred in 1764(22.3%) individuals treated with sacubitril-valsartan and 2110(26.7%) individuals taking ACE/ARBs. Compared to ACE/ARB, sacubitril-valsartan was associated with a lower risk of all-cause mortality or all-cause hospitalization during follow-up (HR 0.86; 95% confidence interval [CI] 0.81 to 0.91, p<0.001, Central Illustration A).
CENTRAL ILLUSTRATION: Cumulative Risk of Outcomes in Patients Treated with Sacubitril-Valsartan or ACE/ARB.
Cumulative risk for the primary outcome (all-cause mortality or hospitalization; Panel A), all-cause mortality (Panel B), all-cause hospitalization (Panel C), and HF hospitalization (Panel D) for patients on sacubitril-valsartan or ACE/ARB are shown.
Sacubitril-valsartan was also associated with lower risks of both components of the primary endpoint. During follow-up, 2.2% (n=170) of patients treated with sacubitril-valsartan died, compared with 2.9% (n=229) treated with ACE/ARB (HR 0.80, 95% CI 0.66 to 0.97, p=0.027, Central Illustration B). All-cause hospitalization occurred in 1716(21.8%) patients taking sacubitril-valsartan versus 2060(26.1%) taking ACE/ARBs (HR 0.86, 95% CI 0.80 to 0.91, p<0.001, Central Illustration C). The proportion of all-cause hospitalizations that included a code for HF in any position was 84.7% in the overall cohort (86.9% sacubitril-valsartan and 80.9% ACE/ARB group).During follow-up, 646(17.2%) patients treated with sacubitril-valsartan were admitted with a primary diagnosis of HF, compared with 648(15.9%) patients taking ACE/ARBs. Risk of HF hospitalization did not differ in patients taking sacubitril-valsartan and ACE/ARB (HR 1.07, 95% CI 0.96 to 1.19, p=0.26, Central Illustration D).Kaplan-Meier curves extended to two years of follow-up are included in the Supplementary Material (Figure S1).
Sacubitril-valsartan was associated with a higher risk of hypotension (3.4 vs. 2.5 events per 100 person-years, HR 1.35, 95% CI 1.05 to 1.75, p=0.022) compared with ACE/ARB (Table S3). No difference in risk of hyperkalemia was observed (0.89 vs. 0.84 events per 100 person-years, HR 1.05, 95% CI 0.66 to 1.67, p=0.84). Angioedema risk was very low in both groups (<11 events total).
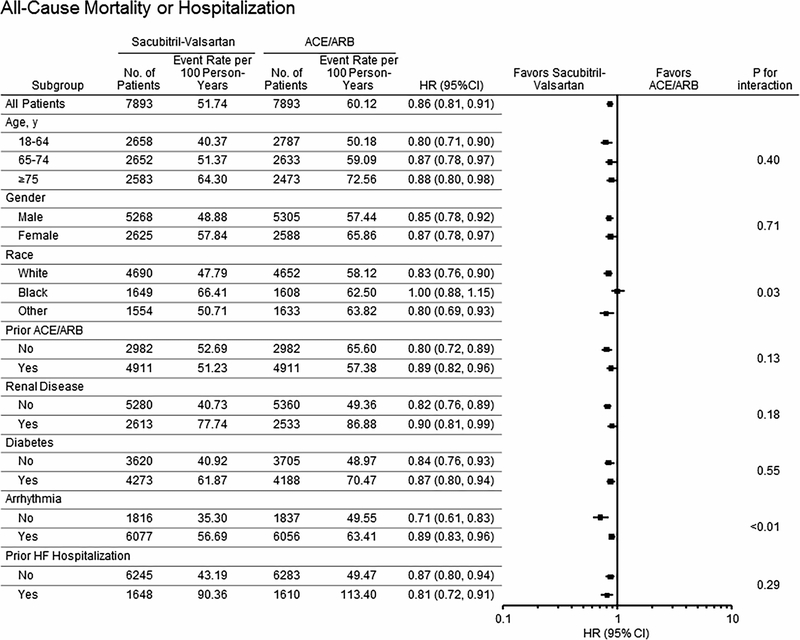
Subgroup Analyses.
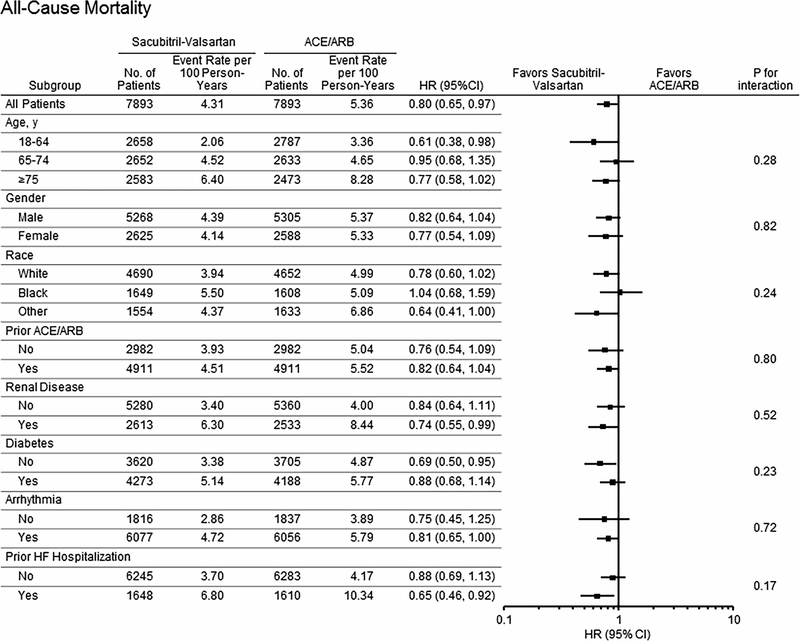
Subgroup analyses for the combined endpoint of death or all-cause hospitalization are shown in Figure 1 and Tables S4-S7. Sacubitril-valsartan was associated with a lower risk of the combined endpoint compared with ACE/ARB in white patients (HR 0.83, 95% CI 0.76–0.90) and in non-black patients of other races/ ethnicities (HR 0.80, 95% CI 0.69–0.93), but not black patients (HR 1.00, 95% CI 0.88–1.15; interaction race* treatment p=0.032). A difference in the comparative risk of all-cause hospitalization in patients taking sacubitril-valsartan versus ACE/ARB by race was also observed (HR 0.83, 95% CI 0.76–0.90 in whites vs. HR 1.00, 95% CI 0.87–1.14 in blacks, interaction race*treatment p=0.045, Figure 2). There was a trend toward greater survival associated with sacubitril-valsartan use versus ACE/ARBs in white (HR 0.78, 95% CI 0.60–1.02) compared with black (HR 1.04, 95% CI 0.68–1.59) patients (Figure 3). To investigate if adherence impacted results observed, we excluded patients with poor adherence (MPR<0.80), and findings were similar. The HR (95% CI) associated with use of sacubitril-valsartan vs. ACE/ARB was 1.00 (0.87–1.12) for black patients, 0.82 (0.75–0.90) for whites, 0.81 (0.69–0.95) for other racial and ethnic groups (p value for interaction=0.040).
Figure 1: Differences in Risk of Mortality or All-Cause Hospitalization in Patients Taking Sacubitril-Valsartan Compared With ACE/ARB by Patient Characteristics.
Differences in risk of all-cause hospitalization or mortality (HR, 95% CI and p value for interaction) according to patient baseline characteristics are shown.
Figure 2: Differences in Risk of All-Cause Hospitalization in Patients Taking Sacubitril-Valsartan Compared With ACE/ARB by Patient Characteristics.
Differences in risk of all-cause hospitalization (HR, 95% CI and p value for interaction) according to patient baseline characteristics are shown.
Figure 3: Differences in Risk of Mortality in Patients Taking Sacubitril-Valsartan Compared With ACE/ARB by Patient Characteristics.
Differences in risk all-cause mortality (HR, 95% CI and p value for interaction) according to patient baseline characteristics are shown.
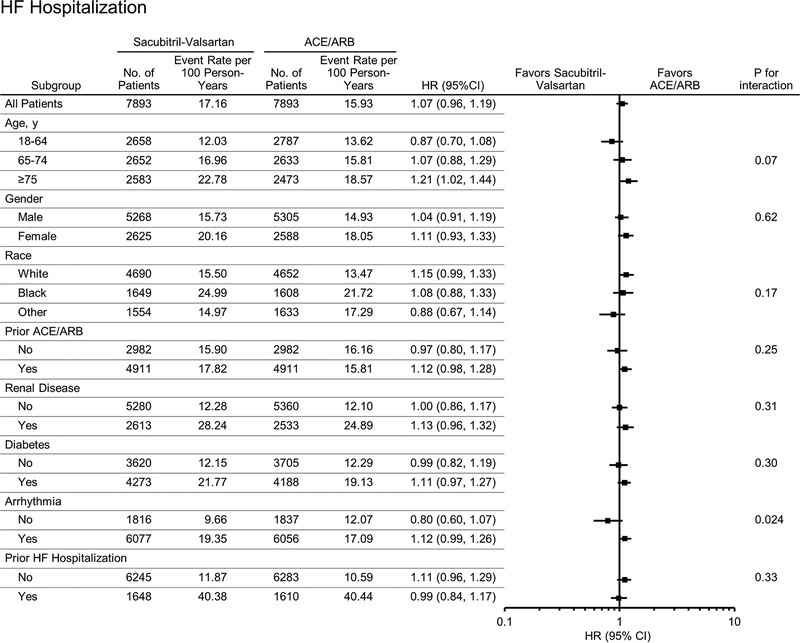
The magnitude of decreased risk of the primary outcome associated with sacubitril-valsartan was also more pronounced in patients without prior arrhythmia (HR 0.71, 95% CI 0.61–0.83) compared to patients with arrhythmias (HR 0.89, 95% CI 0.83–0.96; interaction arrhythmia*treatment p=0.006). This interaction of arrhythmia history and treatment was also observed for all-cause hospitalizations (p value for interaction<0.005, Figure 2) and HF hospitalizations (p value for interaction 0.024, Figure 4) Otherwise, treatment effects were similar by subgroup (p values for interaction>0.05, Figures 1–4). There were no differences in treatment response in men and women for all outcomes examined. There were no significant differences in treatment response by age (interaction >0.05 for all outcomes examined). For the combined endpoint of all-cause mortality or all-cause hospitalization, point estimates were similar in all three age groups examined (Figure 2). Conversely, sacubitril-valsartan was associated with a lower risk of HF hospitalization compared with ACE/ARB in younger patients, but the risk of HF hospitalization was higher with sacubitril-valsartan in the elderly (Central Illustration). However, differences in treatment response by age were not statistically significant (p value for interaction 0.07). Treatment effects were similar in patients who were and were not recently taking an ACE/ARB. While only a small percentage of patients (0.6%) initiated sacubitril-valsartan within one week of a HF hospitalization, the lower risk of the combined endpoint observed with sacubitril-valsartan compared with ACE/ARBs was similar in patients with and without a HF hospitalization in the last 6 months.
Figure 4: Differences in Risk of Heart Failure Hospitalization in Patients Taking Sacubitril-Valsartan Compared With ACE/ARB by Patient Characteristics.
Differences in risk of hospitalization for heart failure (HR, 95% CI and p value for interaction) according to patient baseline characteristics are shown.
Sensitivity Analysis.
No difference in risk of urinary tract infection was observed in patients treated with sacubitril-valsartan versus ACE/ARB (HR 0.91, 95% CI 0.72–1.15, p=0.43).
Discussion
In this study of nearly 16,000 patients with HFrEF, those taking sacubitril-valsartan were significantly less likely to experience death or hospitalization from any cause compared with those on ACE/ARB therapy. The benefits observed with sacubitril-valsartan were similar in men and women and among those who were and were not taking an ACE/ARB previously. However, in contrast to patients of other races and ethnicities, outcomes with sacubitril-valsartan and ACE/ARBs were similar in black patients.
This study provides real-world effectiveness data comparing sacubitril-valsartan with ACE/ARB outside of a clinical trial. Our data suggest that patients prescribed sacubitril-valsartan in clinical practice are older (mean 68 vs. 64 years), more often women (33% vs. 22%), and more racially and ethnically diverse compared with participants in the PARADIGM-HF trial.(1) Despite these dissimilarities, the treatment benefits of sacubitril-valsartan were observed in both sexes and in patients with a variety of comorbidities. Sacubitril-valsartan was associated with better outcomes compared with ACE/ARB even in patients who had not been taking an ACE/ARB previously. The lower risk of all-cause mortality or all-cause hospitalization with sacubitril-valsartan was observed across the age spectrum. Hence, our data indicate that the benefits seen with sacubitril-valsartan in the PARADIGM-HF study are translatable to a representative population of patients with HFrEF in the U.S.
However, our observation that black patients had no better outcomes with sacubitril-valsartan compared with ACE/ARB suggests that further data are needed to fully understand the optimal treatment strategy for this population. Black patients were known to be underrepresented in PARADIGM-HF, with a total of 428 (5%) black patients included in both arms.(1) The recent PIONEER-HF trial enrolled a higher proportion of black patients (36%), but was a small study (n=881 total patients), so the absolute number of black patients enrolled was small.(23) Our study included more than 7 times the number of black patients in PARADIGM-HF and 10 times the number enrolled in PIONEER-HF. There is a growing body of literature demonstrating that the level of natriuretic peptides, which mediate a number of cardiorenal protective properties, vary across ethnic groups. African-Americans have lower natriuretic peptide levels, on average, than Caucasians.(24,25) This relative deficiency in natriuretic peptides has been hypothesized to contribute to an increased tendency for salt retention, hypertension, cardiac remodeling, and adverse cardiovascular outcomes.(26) Although the neprilysin inhibition from sacubitril may increase existing natriuretic peptide availability, this effect may be blunted in individuals who synthesize lower amounts of natriuretic peptides, providing a possible mechanism underlying the lack of benefit over ACE/ARBs we observed among black patients. More thorough investigation into potential racial differences in treatment effect and biological mechanisms mediating these differences are warranted.
Both patients with and without a history of arrhythmias experienced lower risk of the primary outcome with sacubitril-valsartan, but this effect was more pronounced among patients without a history of arrhythmias. Sacubitril-valsartan may be associated with decreased ventricular arrhythmia burden and ICD shocks, possibly due to reverse remodeling (27,28). However, patients with arrhythmias may have also had more underlying comorbidities, which may have contributed to results observed. This finding is worthy of future study.
While patients treated with sacubitril-valsartan had lower risks of all-cause hospitalization compared with those treated with ACE/ARBs, the risk of hospitalization for HF was similar in both treatment groups. We were surprised by this given the large reduction in HF hospitalization risk with sacubitril-valsartan in PARADIGM-HF.(1) However, while we relied upon use of validated codes for HF hospitalization, the potential that differences in coding practices may have influenced the analysis of cause-specific hospitalization still exists. The fact that, despite matching on history of HF hospitalization in the baseline period, patients on sacubitril-valsartan still had a higher proportion of total hospitalizations where HF was coded in any position during follow-up, underscores that this may be the case. By selecting a measure that is not influenced by coding practices (all-cause hospitalization), we avoided this potential source of bias. Furthermore, while we found no statistically significant difference in treatment effects by age for outcomes examined in our study, there was some variation in point estimates of risk for HF hospitalization by age, suggesting that older patients on sacubitril-valsartan may have higher risk of HF hospitalization than those taking ACE/ARBs. Previously noted limitations to using codes to identify HF hospitalization may have contributed to these findings, which are in contrast to those observed in post-hoc analyses of PARADIGM-HF, where the benefits of sacubitril-valsartan were observed even in those 75 years and older.(29)
In-hospital initiation of sacubitril-valsartan was recently shown in an 881 patient randomized controlled trial to lead to a greater reduction in NT-proBNP compared with enalapril among patients hospitalized with acute decompensated HF.(24) In exploratory analyses, sacubitril-valsartan was also associated with greater reductions in risks of death and rehospitalization for HF at 8 weeks compared with enalapril. Our findings indicate that, to date, patients are rarely initiated on sacubitril-valsartan in the hospital. We suspect that practice patterns may change following publication of PIONEER-HF,(24) and the effectiveness of sacubitril-valsartan with ACE/ARB when initiated in hospitalized patients should be compared in future clinical practice studies.
Limitations and Strengths.
The OLDW includes patients enrolled in private and Medicare Advantage health plans; as such, our findings may not be generalizable to patients with other types of health insurance. The observational nature of the study precluded our ability to make conclusions regarding causality. The possibility of residual confounding between treatment groups cannot be ruled out despite the use of robust propensity matching techniques. The follow-up period was relatively short and it will be important to delineate long-term outcomes for sacubitril-valsartan in future investigations. Finally, there is the potential for misclassification when relying on billing codes for identifying comorbidities. However, to the best of our knowledge, this is the first study to compare risks of death and hospitalization in large numbers of patients with HFrEF prescribed sacubitril-valsartan and ACE/ARB therapies outside of a clinical trial. While randomized controlled trials are the gold standard way to compare the effectiveness of two interventions, observational data can be incredibly helpful, as patients with HF treated in clinical practice are often different than those in clinical trials. Minorities, the elderly, women, and those with comorbidities have historically been underrepresented in clinical trials, but are treated with new therapies once available in clinical practice. Our study population is direct evidence of this phenomenon, as our population was older, had a higher proportion of women, blacks, and diabetics than patients enrolled in PARADIGM-HF. Furthermore, our study included nearly twice as many patients on sacubitril-valsartan as were enrolled in PARADIGM- HF. These data thereby add to our knowledge of the effectiveness of this novel HFrEF therapy in populations often underrepresented in clinical trials.
Conclusions.
In a large cohort of patients with HFrEF, sacubitril-valsartan was associated with lower risks of mortality and hospitalization when compared with ACE/ARB therapy. Our findings suggest that, unlike other racial and ethnic groups, outcomes with sacubitril-valsartan and ACE/ARBs were similar in black patients. More research is needed to determine if there are racial differences in treatment response to sacubitril-valsartan.
Supplementary Material
Perspectives.
Competency in Medical Knowledge:
In a large cohort of patients with systolic heart failure, use of sacubitril-valsartan was associated with lower risks of mortality or hospitalization compared with use ACE inhibitor / angiotensin receptor blocker (ACE/ARB) therapy.
Competency in Patient Care:
Sacubitril-valsartan is associated with lower risks of mortality and hospitalization compared with ACE/ARBs in patients with systolic heart failure.
Translational Outlook 1:
It will be essential to compare long term mortality and cardiovascular outcomes of sacubitril-valsartan against that of ACE/ARB therapy.
Translational Outlook 2:
More research is needed to determine if there are racial differences in treatment response to sacubitril-valsartan.
Funding:
S.M. Dunlay’s time to do this work was supported by the NIH (K23 HL116643). Disclosures: Authors have received research funding including S.J. Sangaralingham (RO1 HL132854), N.D. Shah (FDA U01FD005938, AHRQ 01HS025164; R01HS025402; 1U19HS024075; R03HS025517, NIH R56HL130496; R01HL131535, and from the Patient Centered Outcomes Research Institute to develop a Clinical Data Research Network), and S.M. Dunlay (R03 HL135225).
Abbreviations:
- ACE/ARB
Angiotensin-converting enzyme inhibitor, angiotensin receptor blocker
- CI
confidence interval
- COPD
chronic obstructive pulmonary disease
- FDA
Food and Drug Administration
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- HR
hazard ratio
- ICD
implantable cardioverter defibrillator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McMurray JJ, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, Jessup M, Bozkurt B et al. 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2016;68:1476–1488. [DOI] [PubMed] [Google Scholar]
- 4.Luo N, Fonarow GC, Lippmann SJ et al. Early Adoption of Sacubitril/Valsartan for Patients With Heart Failure With Reduced Ejection Fraction: Insights From Get With the Guidelines-Heart Failure (GWTG-HF). JACC Heart Fail 2017;5:305–309. [DOI] [PubMed] [Google Scholar]
- 5.Sangaralingham LR, Sangaralingham SJ, Shah ND, Yao X, Dunlay SM. Adoption of Sacubitril/Valsartan for the Management of Patients With Heart Failure. Circ Heart Fail 2018;11:e004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahhan AS, Vaduganathan M, Greene SJ et al. Enrollment of Older Patients, Women, and Racial and Ethnic Minorities in Contemporary Heart Failure Clinical Trials: A Systematic Review. JAMA Cardiol 2018;3:1011–1019. [DOI] [PubMed] [Google Scholar]
- 7.Labs O Real World Healthcare Experiences. [Google Scholar]
- 8.Wallace PJ, Shah ND, Dennen T, Bleicher PA, Crown WH. Optum Labs: building a novel node in the learning health care system. Health Aff (Millwood) 2014;33:1187–94. [DOI] [PubMed] [Google Scholar]
- 9.Li Q, Glynn RJ, Dreyer NA, Liu J, Mogun H, Setoguchi S. Validity of claims-based definitions of left ventricular systolic dysfunction in Medicare patients. Pharmacoepidemiol Drug Saf 2011;20:700–8. [DOI] [PubMed] [Google Scholar]
- 10.Hershman DL, Tsui J, Wright JD, Coromilas EJ, Tsai WY, Neugut AI. Household net worth, racial disparities, and hormonal therapy adherence among women with early-stage breast cancer. J Clin Oncol 2015;33:1053–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sangaralingham LR, Shah ND, Yao X, Roger VL, Dunlay SM. Incidence and Early Outcomes of Heart Failure in Commercially Insured and Medicare Advantage Patients, 2006 to 2014. Circ Cardiovasc Qual Outcomes 2016;9:332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Quan H, Sundararajan V, Halfon P et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130–9. [DOI] [PubMed] [Google Scholar]
- 13.Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health 2007;10:3–12. [DOI] [PubMed] [Google Scholar]
- 14.Yao X, Gersh BJ, Holmes DR Jr. et al. Association of Surgical Left Atrial Appendage Occlusion With Subsequent Stroke and Mortality Among Patients Undergoing Cardiac Surgery. JAMA 2018;319:2116–2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol 2013;61:1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA 2011;306:1669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bonow RO, Bennett S, Casey DE Jr. et al. ACC/AHA clinical performance measures for adults with chronic heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures (Writing Committee to Develop Heart Failure Clinical Performance Measures) endorsed by the Heart Failure Society of America. J Am Coll Cardiol 2005;46:1144–78. [DOI] [PubMed] [Google Scholar]
- 18.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat 2011;10:150–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med 2009;28:3083–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gayat E, Resche-Rigon M, Mary JY, Porcher R. Propensity score applied to survival data analysis through proportional hazards models: a Monte Carlo study. Pharm Stat 2012;11:222–9. [DOI] [PubMed] [Google Scholar]
- 21.Grambsch PM, Therneau TM. Proportional Hazards Tests and Diagnostics Based on Weighted Residuals. Biometrika 1994;81:515–526. [Google Scholar]
- 22.Prasad V, Jena AB. Prespecified falsification end points: can they validate true observational associations? JAMA 2013;309:241–2. [DOI] [PubMed] [Google Scholar]
- 23.Velazquez EJ, Morrow DA, DeVore AD et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2019;380:539–548. [DOI] [PubMed] [Google Scholar]
- 24.Gupta DK, Claggett B, Wells Q et al. Racial differences in circulating natriuretic peptide levels: the atherosclerosis risk in communities study. J Am Heart Assoc 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta DK, de Lemos JA, Ayers CR, Berry JD, Wang TJ. Racial Differences in Natriuretic Peptide Levels: The Dallas Heart Study. JACC Heart Fail 2015;3:513–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dries DL, Victor RG, Rame JE et al. Corin gene minor allele defined by 2 missense mutations is common in blacks and associated with high blood pressure and hypertension. Circulation 2005;112:2403–10. [DOI] [PubMed] [Google Scholar]
- 27.Martens P, Nuyens D, Rivero-Ayerza M et al. Sacubitril/valsartan reduces ventricular arrhythmias in parallel with left ventricular reverse remodeling in heart failure with reduced ejection fraction. Clin Res Cardiol 2019. [DOI] [PubMed] [Google Scholar]
- 28.de Diego C, Gonzalez-Torres L, Nunez JM et al. Effects of angiotensin-neprilysin inhibition compared to angiotensin inhibition on ventricular arrhythmias in reduced ejection fraction patients under continuous remote monitoring of implantable defibrillator devices. Heart Rhythm 2018;15:395–402. [DOI] [PubMed] [Google Scholar]
- 29.Jhund PS, Fu M, Bayram E et al. Efficacy and safety of LCZ696 (sacubitril-valsartan) according to age: insights from PARADIGM-HF. Eur Heart J 2015;36:2576–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.