Abstract
Canine appendicular osteosarcoma is commonly treated with limb amputation; however, limb-sparing options are frequently desired or necessary for a subset of patients. We evaluated 123 patients and 130 sites treated with stereotactic body radiation therapy (SBRT). Eighty-two out of 98 dogs (84%) had maximum lameness improvement at a median of 3 weeks for a median of 6 months duration. Histopathologic evaluation of available samples from amputation or necropsy revealed >80% tumor necrosis in 50% of limbs consistent with local disease control. Of evaluable patients, 41% fractured and 21% pursued an amputation after treatment. Fine needle aspirate (n = 52) and needle core biopsy (n = 28) did not result in increased fracture risk compared to those without tumor sampling (n = 50). Median survival time (MST) was 233 days and time to first event was 143 days. Gross tumor volume and planned target volume were significantly inversely associated with survival and tumor location was significantly associated with survival. Dogs with salvage amputation had a significantly longer MST compared to those without (346 vs 202 days; P = .04). The presence of metastatic disease at the time of treatment in 15 dogs did not significantly impact survival time (200 vs 237 days without metastasis; P = .58). Skin side effects correlated significantly with dose with 33% of patients with acute grade 3 effects developing consequential late grade 3 effects. While SBRT improves lameness in most patients, further investigation is needed to identify candidates with minimal early fracture risk prior to initiating therapy.
Keywords: bone, lameness, limb-spare, radiotherapy, stereotactic
1 |. INTRODUCTION
Osteosarcoma (OSA) is the most common malignant bone tumour in dogs.1 Current standard of care includes amputation for local tumour control followed by adjuvant chemotherapy to delay the onset of metastasis.1 While most clients elect amputation, occasionally canine patients present with arthritis or neurologic disease that makes them poor candidates for amputation. In these cases, a limb-sparing treatment provides an alternative to amputation. Limb sparing surgery allows for resection of the tumour with the goal of maintaining a functional limb; however, complication rates following these surgeries range from 50% to 96%.2–6 Surgical options are limited to the distal radius, ulna, and diaphyseal lesions with poor results reported for limb sparing surgery for the humerus.7 Results of multiple studies have shown the analgesic benefits of palliative radiation therapy for OSA in dogs and a single study demonstrated histologic percent tumour necrosis post radiation as a predictor of local tumour control.8–14
Canine OSA has been established to be a radioresistant tumour with an α/β ratio of 3.47, indicating that a higher dose per fraction is preferential when treating with radiation therapy to achieve a strong biological response.15,16 Intraoperative radiation therapy protocols have shown radiation can achieve local tumour control but is associated with an unacceptable rate of complications.17–22 Stereotactic body radiation therapy (SBRT) involves the precise delivery of high doses of radiation to the tumour over a shortened time period.23 SBRT has been assessed for treatment of canine OSA with prophylactic surgical limb stabilization which ultimately resulted in prohibitively large numbers of infection and surgical implant failure.24,25 In this study, we evaluated patient response to SBRT as a first line treatment using a uniform prescription of 36 Gy in three fractions of appendicular OSA sites by assessing survival time and fracture rate.
The objective of this study is to assess the efficacy of SBRT as a treatment for appendicular OSA and evaluate risk of fracture following the treatment. Additionally, cofactors were assessed to determine their impact on survival and fracture risk.
2 |. MATERIALS AND METHODS
2.1 |. Case selection
Dogs were included in this analysis if they received SBRT at the Colorado State University Veterinary Teaching Hospital for a confirmed or suspected appendicular OSA from May 2009 to February 2019. Only patients prescribed 36 Gy in three fractions were included. Dogs that did not complete the 3 day protocol were excluded as were dogs with scapular lesions. Patients that received prophylactic limb stabilization at the time of radiation therapy were not included in this study. Medical records were reviewed, and follow-up data was collected from referring veterinarians and clients regarding clinical signs, lameness prior to treatment, lameness resolution following treatment, duration of lameness resolution, fracture occurrence, time to fracture, amputation, acute and late adverse radiation effects, time to development of metastatic disease and overall survival time. A fracture was documented if there was newly identified evidence of cortical disruption as seen on radiographs or computed tomography (CT) evaluated by a board-certified veterinary radiologist. Data were collected from staging diagnostic tests including complete blood count, serum biochemical profile, thoracic radiographs, thoracic CT, limb CT, whole body fluorine-18 fluorodeoxyglucose positron emission tomography/computed tomography (F18-FDG PET/CT), and nuclear scintigraphy. Adverse radiation effects were graded according to the VRTOG toxicity grading system.26 Cause of death was classified as local recurrence, metastatic disease, fracture, other, or unknown.
Assumptions included that all dogs were at risk of fracture during the course of the follow up time period, that dogs had 3-view thoracic radiographs to assess for metastatic disease, and that orthogonal view radiographs or CT scans of the affected limb were performed to assess for fracture post SBRT.
2.2 |. CT examination
CT examination was performed using either a Picker PQ2000 CT single slice helical scanner (before November 2009; Picker Medical Systems, Cleveland, Ohio), or a Philips Gemini TF Big Bore 16-slice scanner (after November 2009; Philips Medical Systems, Nederland, B.V.). When obtaining CT images for radiation planning, patients were typically placed in lateral recumbency in a moldable bag (Vac-Lock Cushions; CIVCO Medical Solutions, Coralville, IA). For patients with a lesion in any location of the hind limb or lesion of the proximal humerus, the region of interest was typically immobilized using an AccuForm Cushion (CIVCO Medical Solutions, Coralville, IA) and the affected limb up. For patients with a lesion of the distal radius, the patient was placed in lateral recumbency with the affected limb down. A moldable bag was used with the affected limb stretched away from the body and secured using a thermoplastic net attached to an indexed carbon fibre board (CIVCO Medical Solutions, Coralville, IA). The board and moldable bags were indexed to the CT and radiation therapy couches (Figure 1).
FIGURE 1.

Representative patient with a lesion of the distal radius using the Civco Multifix system including a carbon fibre, indexable baseplate with Posi-cast-Plus 2-point extremity masks placed proximal and distal to the lesion
A non-contrast volumetric (helical) dataset was obtained through the affected limb as well as a segment of the body in the region. Omnipaque 350 (GE Healthcare, Princeton, New Jersey) contrast media then was injected IV (0.7–3.9 mL/kg) before the post-contrast series. When radiation planning CT was done concurrently with F18-FDG PET/CT the contrast media administration was divided into two injections. Images were reconstructed at 2.0 mm contiguous intervals with a 512 matrix, using the smooth algorithm. Additionally, a bone algorithm was reconstructed from the pre-contrast series at 1.0 mm intervals.
2.3 |. Radiation treatment planning
Both the 2 mm pre-contrast and post-contrast CT scans were used for contouring and inverse treatment planning using Varian Eclipse treatment planning system (Varian Medical Systems, Inc Palo Alto, California). Organs at risk (OARs) and gross tumour volume (GTV) were identified and contoured. The skin contour was created using a 2 mm internal expansion from the body contour. A 0 to 3.85 cm clinical target volume (CTV) was extended proximally or distally from the GTV within the bone to include possible microscopic disease that could not be appreciated with the CT images. A 3 to 5 mm isotropic planned target volume (PTV) expansion encompassed the CTV to account for daily set-up positioning error. The OARs included skin in all cases, trachea, oesophagus, and spinal cord when the proximal humerus was treated, and the colon, bladder, and cauda equina when the proximal femur was treated. Target volumes were pulled out of contours that included OARs to meet normal tissue constraints for optimization.
Inverse treatment planning was employed using Eclipse planning software; all plans were designed using coplanar or noncoplanar, isocentrically placed 6 or 10 MV radiation beams or coplanar or noncoplanar 6 MV volumetric arc therapy (VMAT). Radiation beams were modulated using sliding-window technique. The intent for each radiation plan was to deliver 100% of the radiation prescription to 99% of the GTV and CTV and 95% of the PTV.
Quality assurance (QA) was performed by gamma analysis using the Varian portal dosimetry system on individual fields or arcs. A minimum of 95% gamma for a 3 mm distance to agreement and a 3% absolute dose difference were defined as a passing QA score.
When retrospectively reviewing the radiation treatment plans, the following data were collected: GTV, CTV, PTV, dose to 99% of the GTV, dose to 99% of the CTV, dose to 95% of the PTV, maximum, minimum, mean, median, and modal doses to the PTV, conformity index, gradient index, number of beams and energy. The dose to 1.0 cc of skin, maximum skin point dose, and full thickness dose to skin was recorded. Full thickness dose to skin was defined as the dose as seen as the first visible dose on the skin surface based on a single observer (__) evaluation.
2.4 |. Radiation treatment
Patients were anaesthetised for delivery of radiation therapy. Anaesthetic protocols varied but, in most cases, consisted of an opioid pre-medication followed by propofol induction and maintenance with an isoflurane and oxygen admixture. Once anaesthetised, dogs were positioned into the same immobilization devices that were used for their initial CT imaging. Daily patient position verification was performed by online registration of the simulation CT with images of the daily set up obtained using an on-board cone beam CT. Therapy was delivered using a Varian Trilogy linear accelerator.
2.5 |. Histopathology review
When available, pre-radiation histopathological samples were reviewed by a board-certified pathologist to confirm the diagnosis of OSA prior to treatment. Additionally, post-treatment histopathological samples from amputation or necropsy were reviewed to determine if progression of disease was the cause of fracture or pain. Percent tumour necrosis was assessed in pre and post SBRT histopathologic samples when available by a single board-certified veterinary pathologist (DR). Dogs with 79% or less tumour necrosis were classified as recurrent, while dogs with 80% or greater were considered controlled.14
2.6 |. Statistical analysis
Survival time was calculated as the time between the first day of treatment and death. Dogs were censored at the date of analysis if they were still alive or at the date when they were lost to follow-up and were censored at the date of a second SBRT if it was delivered to the same location. Kaplan–Meier survival curves were created and median survival times, median time to fracture, and time to first event determined. Events included death of the patients, fracture of the treated limb, amputation of the treated limb, retreatment with SBRT, and development of metastatic disease. If fracture was reported without a known occurrence date, the date of death was used as the fracture date. A Mantel-Cox Log Rank test was used to evaluate location, the impact of the addition of a CTV, cause of death, and breed with survival. Continuous variables including patient serum ALP concentration, monocyte count, dose to 99% of the GTV, dose to 99% of the CTV, dose to 95% of the PTV, GTV and PTV were evaluated for association to survival time and time to fracture using Cox proportional hazards models with censoring taken into account. Correlation with the development of a fracture was performed for dose to 99% of the GTV, 95% of the PTV, weight, the volumes of GTV, CTV, PTV, ALP serum concentration, administration of bisphosphonate timing, maximum dose, minimum dose, mean dose, median dose, modal dose, conformity index and gradient index were evaluated with Pearson’s correlation. The addition of a CTV to radiation side effects was performed with Pearson’s correlation. Development of acute side effects and the dose to 1 cc of skin, maximum skin dose, and full thickness skin dose was assessed with a Mann-Whitney test. A one-way ANOVA was used to assess acute and late grade of adverse effects to treatment site. Finally, chi-square analysis was used to evaluate the development of fracture with diagnostic technique (fine needle aspirate, biopsy, or neither), metastasis, breed, and age and a Fisher’s exact test was used to evaluate the addition of a CTV to fracture risk. A P-value of .05 was used as a criterion to determine statistical significance. SAS v9.4 (SAS Institute Inc., Cary, NC) or Prism v8.1.0 (GraphPad, San Diego, CA) was used for all statistical analyses.
3 |. RESULTS
Database search revealed 176 treatment sites of appendicular OSA that received SBRT. Twenty-eight patients received alternative SBRT protocols aside from 36 Gy in three fractions and 18 patients had limb stabilization at the time of radiation therapy and were excluded. One patient did not complete the 3 day protocol and was euthanized after the second fraction for acute kidney injury of unknown cause and was excluded. Of the remaining 130 sites, four patients had two sites treated concurrently, two patients had the same site treated at recurrence, and one patient had a metastatic site treated at a later date resulting in a total of 123 patients. The median age was 8.5 years old (range: 2–14 years) and weight was 43.6 kg (20.8–86 kg). Patients included 64 neutered males, 50 spayed females, 8 intact males, and 1 intact female. Twenty-five breeds were represented: Labrador Retrievers (14), Golden Retrievers (12), Great Dane (11), Rottweiler (11), Saint Bernard (9), Great Pyrenees (7), Bernese Mountain Dog (5), Greyhound (5), Mastiff (5), Newfoundland (4), Doberman (3), Malamute (3), German Shepherd (3), Irish Wolfhound (2), Old English Sheepdog (2), one of each of the following Bouvier des Flanders, American Bulldog, Akita, Collie, Saluki, Greater Swiss Mountain Dog, Staffordshire Bull Terrier, Leonberger, German Shorthair Pointer, English Bulldog and 17 mixed breed dogs.
Of the treated sites, 52 (40%) had fine needle aspirates performed for cytologic assessment, 28 (22%) had bone biopsies performed for histopathologic assessment, and 50 (38%) sites had a presumptive diagnoses of OSA based on imaging findings and clinical presentation. Eleven cytology samples were considered non-diagnostic, while only one biopsy was non-diagnostic. Four patients had two separate sites treated concurrently. Two patients received re-irradiation to the original treatment site 2 and 15 months after the first SBRT for presumed recurrence. One patient had two separate sites treated 13 months apart. Eighty-three patients received pamidronate (0.6–2.3 mg/kg), 32 received zoledronate (0.06–0.6 mg/kg), and 8 did not receive a bisphosphonate drug. The two patients that had re-irradiation to the original site received pamidronate prior to treatment each time. The patient that had two separate sites treated 13 months apart received pamidronate prior to the first treatment and zoledronate prior to the second. It was not clear from the medical record for one patient whether they received a bisphosphonate drug.
One hundred nineteen patients received single agent carboplatin (150–300 mg/m2) and one received single agent doxorubicin after radiation therapy. One patient with same site re-irradiation received carboplatin at first treatment and no chemotherapy at second treatment. The patient that received SBRT for a secondary location received carboplatin for the first SBRT and doxorubicin for the second. Two patients received alternating carboplatin and doxorubicin and one patient received palladia and cyclophosphamide. Chemotherapy was delivered a median of 1 day post the first day of SBRT.
The mean ALP serum concentration was 134.0 IU/L (range: 12–549 IU/L, N: 20–170 U/L). The mean monocyte count was 510/μL (range: 0–2900/μL).
3.1 |. Imaging and radiation treatment
Thirty-two patients had nuclear scintigraphy and 32 were staged with an F18-FDG PET/CT prior to radiation therapy. The patient that had a secondary lesion treated 13 months after the initial SBRT had an F18-FDG PET/CT prior to both radiation treatments. All patients had a CT scan for radiation planning.
One hundred twenty-two patients had treatment on consecutive operating days (Monday–Friday) and one patient had treatment every other day due to compromised skin prior to treatment. Nineteen radiation treatments utilized volumetric modulated arc therapy and 110 received treatment with static beams. The median number of beams was 7 (mean = 7.7) and median number of arcs was 2 (mean 2.6). Eighteen plans used half arcs and full arcs were used in one plan. The median volumes of the GTV, CTV, and PTV were 74.1, 75.5, and 126.0 cc, respectively (mean 90.9, 87.6 and 145.3 cc). Eighty-three plans included a CTV expansion for microscopic disease while 46 did not. Various dose statistics can be found in Table 1. The median dose to 1 cc of skin, the median maximum dose to skin, and the median full thickness skin dose were 25.5, 32.3 and 23.2 Gy, respectively (mean 25.8, 31.7, and 22.8 Gy).
TABLE 1.
Median, mean and full range of doses (in Gy) for the variables listed from all radiation treatments performed in the study
| Dose at isocenter | 99% GTV | 99% CTV | 95% PTV | Max dose | Minimum dose | Mean Dose | Median Dose | Modal Dose | CI | GI | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 37.6 | 33.9 | 33.5 | 31.4 | 41.0 | 18.2 | 36.8 | 37.6 | 37.9 | 0.9 | 1.3 |
| Mean | 37.7 | 35.9 | 35.4 | 33.9 | 40.8 | 18.4 | 37.1 | 37.6 | 37.9 | 0.9 | 1.1 |
| Range | 21.2–45.1 | 20.2–37.4 | 15.5–37.4 | 15.5–36.9 | 36.6–46.8 | 0.2–32.4 | 33.2–40.4 | 34.2–40.1 | 34.1–45.4 | 0.09–1.69 | 0.48–3.2 |
3.2 |. Fracture development
Information regarding fracture was available for 125 sites. Of those, 51 patients (41%) went on to fracture 106 days after SBRT. When censoring dogs who died prior to fracture, the median time to fracture was 333 days. No significance was found for dose to 99% of the GTV, 99% of CTV, 95% of PTV, weight, age, GTV, CTV, PTV, serum ALP concentration, the administration of bisphosphonates prior, maximum dose, minimum dose, mean dose, median dose, modal dose, conformity index, or gradient index to fracture risk. Sampling method of primary tumour site was not significantly associated with fracture risk (P = .08; Figure 2). Additionally, the development of metastasis, the addition of a CTV, breed, and age were not associated with fracture occurrence.
FIGURE 2.

Bar graph showing the distribution of lesions that were sampled with needle aspirate, core biopsy, or were not sampled and the number of patients in each that resulted in a pathologic fracture (P = .08)
Amputation information was available for 124 treated limbs. Of those, 26 limbs (21%) were amputated during the follow up period. Nineteen limbs were amputated due to fracture, 4 due to uncontrollable pain, 2 due to recurrence of disease, and 1 due to infection.
Three patients had fracture fixation with two patients having trans-carpal plating and one treated with internal fixation and carpal arthrodesis. One of these patients had surgery 4 months after SBRT and did not develop any surgical complications. Two bone metastatic lesions in this same patient were noted 19 months after the initial SBRT and these lesions were treated with an SBRT protocol. This patient lived for 23 months after SBRT. Another of these patients had surgery 2 months after SBRT and developed pyogranulomatous inflammation 5 months after the plating procedure and 7 months after SBRT. This patient went on to develop an open non-healing wound 6 months post-surgical fixation, had an amputation 11 months post-surgery, and was euthanized for declining quality of life 17 months after SBRT. The final patient had plating 2 months after SBRT, developed a chronic ongoing infection 2 weeks after surgery treated with long courses of antibiotics, and was eventually euthanized for progressive disease 23 months after SBRT.
3.3 |. Histopathology review
Eighteen formalin-fixed paraffin embedded tumour samples were available for retrospective histopathological review. These included eight pre-radiation treatment samples, diagnosed as OSA (1), chondroblastic OSA (1), osteoblastic OSA (4), osteoblastic/chondroblastic OSA (1), and reactive woven bone/fibroplasia (1).
Three samples were evaluated from amputation specimens and diagnosed as OSA (1), osteoblastic OSA (1) and diffuse tumour necrosis preventing adequate tumour interpretation (1). Percent tumour necrosis was 0%, 20% and 100%, respectively, in these samples with two patients being considered recurrent. In the patient with 0% tumour necrosis, the first SBRT was delivered 38 months prior, the second SBRT was delivered 57 days prior in combination with antebrachial transarticular stabilization for a pathologic fracture and was amputated due to a deep methicillin-resistant Staphylococcus pseudintermedius infection. The patients with 100% and 20% necrosis received SBRT 56 days and 101 days prior respectively.
Seven samples were collected at time of necropsy. Of those, four were likely OSA; however, diffuse tumour necrosis made interpretation difficult. The remaining three cases were osteoblastic OSA. Percent tumour necrosis ranged from 0% to 100% (median 98%) in these samples with three patients considered recurrent. One patient with 99% necrosis was euthanized due to a fracture and another patient with 98% necrosis was euthanized because of pain and lethargy without evidence of a fracture. Two patients were euthanized because of metastatic lesions in the ribs and vertebrae, one patient had cranial abdominal pain and was unable to walk, one patient arrested with a colonic volvulus, and one presented deceased on arrival.
Dose to the GTV, PTV, and maximum, minimum, mean, median, and modal dose was evaluated between the patients classified as having greater or less than 80% tumour necrosis and no significant difference was found for any of the parameters.
3.4 |. Survival
The median survival time for all patients was 233 days (Figure 3) and the time to first event was 143 days (Figure 4). No significance was found for the addition of a CTV (P = .70), the cause of death (0.2), and breed (0.51) with survival. Cause of death was due to presumed or histologically confirmed local recurrence in 17 (14%), metastatic disease in 35 (29%), fracture in 14 (11%), unknown in 11 (9%), other causes in 38 (30%), and a combination of local recurrence and metastatic disease in 1 (0.8%). When dogs that did not die due to OSA were censored, the median survival time was 322 days (Figure 5). Two patients were euthanized for presumed recurrence based on physical exam or imaging findings consistent with a fracture; however, on histopathological evaluation there was 98% and 99% tumour necrosis. Seven patients were censored due to loss of follow up or were still alive at the time of data collection. Three patients were censored at the time of retreatment with SBRT alone or in combination with surgical limb stabilization.
FIGURE 3.
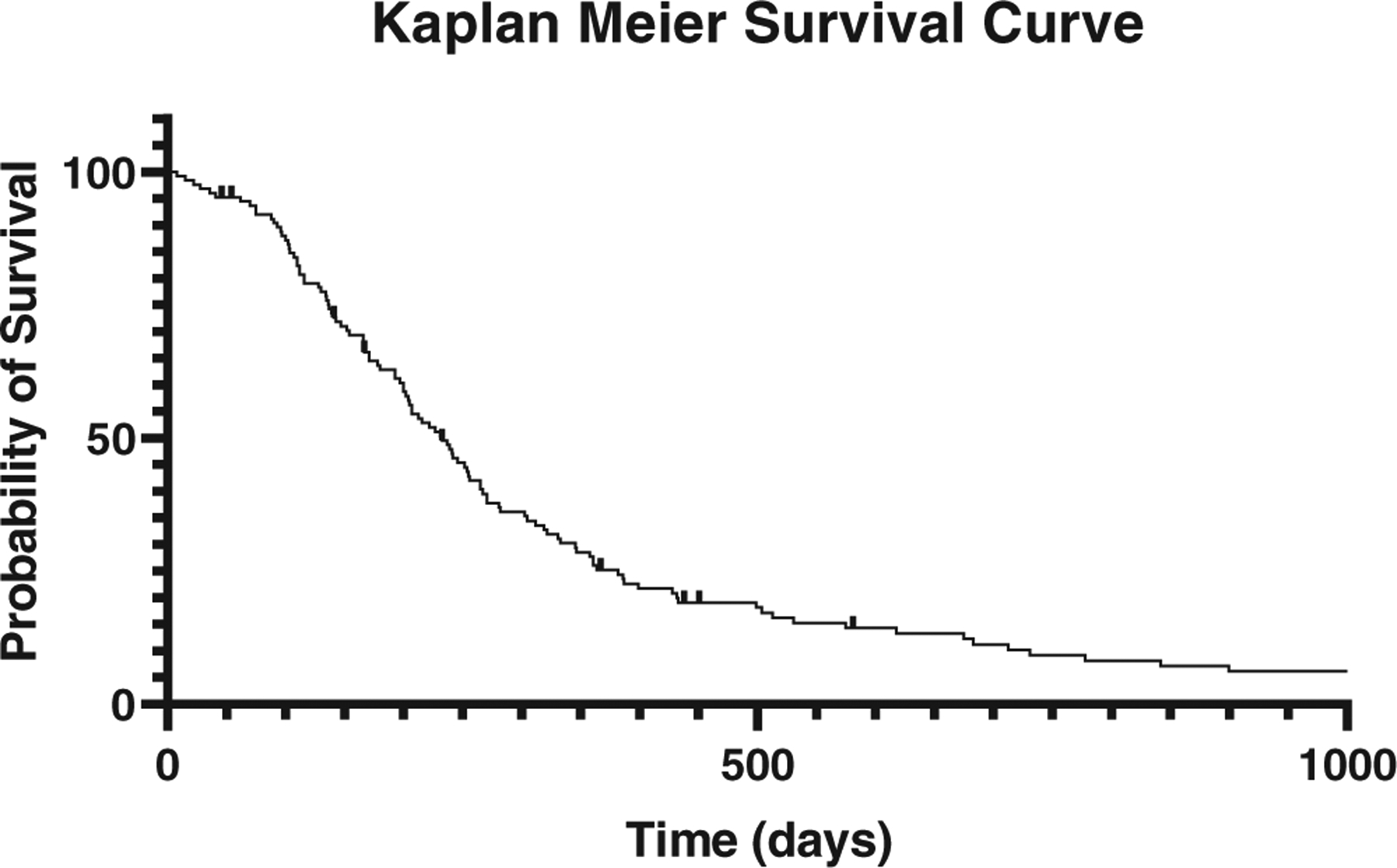
Kaplan–Meier curve depicting survival of dogs that were treated with stereotactic body radiation therapy for appendicular osteosarcoma. Dogs censored from the analysis are depicted with a crosshair. Median survival time shown here is 233 days
FIGURE 4.

Kaplan–Meier survival curve showing the time to first event. Events included death of the patient, fracture of the treated limb, amputation of the treated limb, retreatment with SBRT, and development of metastatic disease. Dogs censored from the analysis are depicted with a crosshair. Median time to first event shown here was 143 days
FIGURE 5.

Kaplan–Meier survival curve with dogs censored if they died due to a cause other than osteosarcoma. Dogs censored from the analysis are depicted with a crosshair. Median survival time shown here is 322 days
Dose to 99% of GTV, 99% of CTV, 95% of PTV, serum ALP concentration, and monocyte count were not significantly associated with survival. Tumour volumes GTV (P = .008) and PTV (P = .003) were significantly inversely associated with survival. Location was found to significantly impact survival (P < .0001) with proximal femur, distal radius, and distal tibia living the longest and patients with multiple lesions treated concurrently living the shortest period of time (Table 2).
TABLE 2.
List of the different sites treated and the median survival time and range for each site
| Location | Number of treated sites | MST (days) | Survival range (days) |
|---|---|---|---|
| Bilateral proximal humeri | 2 | 148.5 | 143–154 |
| Distal femur | 10 | 219 | 19–675 |
| Distal fibula | 1 | 252 | – |
| Distal radius | 45 | 303 | 8–1308 |
| Distal radius and distal ulna | 1 | 97 | – |
| Distal tibia | 7 | 274.5 | 28–842 |
| Distal ulna | 3 | 227 | 116–382 |
| Middiaphyseal radius | 1 | 271 | – |
| Middiaphyseal ulna | 1 | 200 | – |
| Proximal femur | 3 | 331 | 305–346 |
| Proximal humerus | 43 | 202 | 15–1048 |
| Proximal tibia | 7 | 180 | 137–731 |
| Distal radius and distal tibia | 1 | 28 | – |
| Proximal humerus and distal radius | 1 | Undefined | – |
No significant difference in survival was evident between dogs that developed a fracture (median survival 281 days) and those that did not (median survival 207 days; P = .06). There was a significant difference between dogs that had an amputation vs those that did not (P = 0.04) with a median survival time of 346 vs 202 days, respectively (Figure 6).
FIGURE 6.

Kaplan–Meier survival curve for dogs with amputation and no amputation. Dogs censored from the analysis are depicted with a cross hair. Median survival time for dogs with amputation was 346 days and dogs without an amputation was 202 days (P = .02)
3.5 |. Metastasis
At the time of treatment, pulmonary metastasis was seen on CT or thoracic radiographs in two patients and boney metastasis was presumed in five based on regional radiographs or CT imaging. Two patients had bilateral proximal humeral lesions which may represent metastatic lesions or bilateral synchronous primary lesions.27 Lymph node metastasis was suspected based on F18-FDG PET/CT in two patients; however, microscopic evaluation was not performed. One patient had a cytologically confirmed bone metastasis and suspected lymph node metastasis based on imaging findings of an F18-FDG PET/CT. Additionally, in one patient the metastatic lesion was treated with SBRT as amputation was performed on the primary site 18 months prior. In one patient the primary lesion was treated with SBRT and 13 months later the metastatic lesion was treated with a second SBRT protocol. And, finally, in one patient two bone lesions were noted at the time of treatment and SBRT was pursued for one lesion and amputation in the other.
In total, 15 patients had metastatic disease (2 pulmonary, 10 boney, 3 lymph node) at the time of treatment. Of these patients, five had only the primary lesion treated with SBRT, eight had both the primary and metastatic lesion treated with SBRT, and two had the primary lesion treated with surgery and the metastatic lesion treated with SBRT. Median survival for dogs that had metastatic disease was 200 vs 237 days for those that did not (P = .58). Median survival time was not different between dogs that had the metastatic lesion treated compared to those that did not (P = .17). There was no significance found for location of the metastasis at the time of treatment with MST for bone, lung, and suspected lymph node metastasis resulting in 154, 190.5 and 428 days, respectively (P = .66).
3.6 |. Lameness improvement
Ninety-eight patients had follow-up information regarding improvement in pain and lameness. Of those dogs, 82 (84%) had reported maximum improvement within a median of 3 weeks (range: 0.4–20 weeks) post SBRT. Ninety-nine patients had medical records that included the duration of lameness improvement. Of those, the median duration of lameness improvement was 6 months (range: 1–43 months) and 36/99 (36%) died free from pain in the treated limb and another 24/99 (24%) became acutely lame after the development of a fracture.
3.7 |. Adverse effects
Acute radiation effects were assessed in 120 treatment sites. Of those, 82 (68%) had VRTOG grade 0 effects, 22 (18%) had grade 1, 4 (3%) had grade 2, and 12 (10%) had grade 3. Late radiation effects were assessed in 95 treatment sites. Of those, 75 (79%) had grade 0, 11 (12%) had grade 1 and 9 (9%) had grade 3. Four dogs that had acute grade 3 effects also had late grade 3 effects and one dog that had acute grade 2 progressed to a late grade 3. There was no significant difference in treatment location and the development of adverse effects for acute (P = .88) or late effects (P = .64).
A significant difference was found for the development of acute adverse radiation effects resulting in any grade of VRTOG side effects to the skin and the dose to 1 cc of skin (P = .0005), maximum skin dose (P = .007) and full thickness skin dose (P = .0002). The median dose to 1 cc of skin and the development of no acute side effects was 25.0 Gy and the median dose for a development of grades 1 to 3 was 28.4 Gy. Dogs that did not develop any side effects had a median maximum skin dose of 31.9 Gy and dogs that developed a grade 1 skin side effect or higher had a median maximum skin dose of 33.7 Gy. The median full thickness skin dose for no side effects was 21.9 Gy and for grades 1 to 3 was 26.2 Gy. Additionally, a significant difference was found between dogs that developed VRTOG grades 0 to 1 effects and those that developed VRTOG grades 2 to 3 effects and full thickness skin dose (P = .02). The median full thickness skin dose for dogs that had no effects or grade 1 was 22.6 and 26.6 Gy for those that developed VRTOG grades 2 to 3 acute effects (Table 3).
TABLE 3.
VRTOG acute skin side effects and the associated median dose and range received for each of the parameters evaluated.
| Acute VRTOG skin side effects | |||
|---|---|---|---|
| Parameter assessed | N | Median (range) | Sig. |
| Dose to 1 cc skin | |||
| Grade 0 | 82 | 25.0 Gy (16.6–30.8 Gy) | .0005 * |
| Grades 1 to 3 | 38 | 28.4 Gy (18.9–31.6 Gy) | |
| Grades 0 to 1 | 104 | 25.4 Gy (16.6–31.4 Gy) | .066 |
| Grades 2 to 3 | 16 | 28.2 Gy (22.4–31.6 Gy) | |
| Maximum skin dose | |||
| Grade 0 | 82 | 31.9 Gy (21.7–36.6 Gy) | .007 * |
| Grades 1 to 3 | 38 | 33.7 Gy (21.9–37.6 Gy) | |
| Grades 0 to 1 | 104 | 32.2 Gy (21.7–37.1 Gy) | .208 |
| Grades 2 to 3 | 16 | 33.5 Gy (27.4–37.6 Gy) | |
| Full thickness skin dose | |||
| Grade 0 | 82 | 21.9 Gy (10.9–32.4 Gy) | .0002 * |
| Grades 1 to 3 | 38 | 26.2 Gy (12.8–35.5 Gy) | |
| Grades 0 to 1 | 104 | 22.6 Gy (10.9–32.4 Gy) | .014 * |
| Grades 2 to 3 | 16 | 26.6 Gy (15.2–35.5 Gy) | |
Significant differences are identified with an asterisk.
A significant difference was also found for the development of late VRTOG grades 0 to 1 and grade 3 skin side effects and dose to 1 cc of skin (P = .02) and full thickness skin dose (P = .003). The median dose to 1 cc of skin for dogs that developed grades 0 to 1 late effects was 25.4 and 30.2 Gy for those that developed grade 3. The median full thickness skin dose for dogs that developed grades 0 to 1 late skin effects was 22.1 Gy and 27.3 for those that developed grade 3 late effects. Additionally, there was a significant difference between dogs that developed any grade of late side effects and full thickness skin dose (P = .04) with the median dose for no side effects being 21.8 and 24.2 Gy for any degree of side effects (Table 4).
TABLE 4.
VRTOG late skin side effects and the associated median dose received for each of the parameters evaluated.
| Late VRTOG skin side effects | |||
|---|---|---|---|
| Parameter assessed | N | Median (range) | Sig. |
| Dose to 1 cc skin | |||
| Grade 0 | 75 | 25.4 Gy (16.6–31.6 Gy) | .115 |
| Grades 1 to 3 | 21 | 27.8 Gy (18.9–30.7 Gy) | |
| Grades 0 to 1 | 87 | 25.4 Gy (16.6–31.6 Gy) | .024 * |
| Grade 3 | 9 | 30.2 (24.2–30.7 Gy) | |
| Maximum skin dose | |||
| Grade 0 | 75 | 32.2 Gy (21.7–37.6 Gy) | .159 |
| Grades 1 to 3 | 21 | 32.6 Gy (21.9–37.1 Gy) | |
| Grades 0 to 1 | 87 | 32.2 Gy (21.7–37.6 Gy) | .08 |
| Grade 3 | 9 | 34.3 Gy (29.5–35.8 Gy) | |
| Full thickness skin dose | |||
| Grade 0 | 75 | 21.8 Gy (10.9–35.5 Gy) | .044 * |
| Grades 1 to 3 | 21 | 24.2 Gy (15.2–30.7 Gy) | |
| Grades 0 to 1 | 87 | 22.1 Gy (10.9–35.5 Gy) | .003 * |
| Grade 3 | 9 | 27.3 Gy (22.4–30.7 Gy) | |
Significant differences are identified with an asterisk.
4 |. DISCUSSION
The median survival time for this population of dogs with appendicular OSA treated with SBRT was 233 days for all dogs and 322 days for dogs whose deaths were related to OSA or treatment. This is shorter than other reports of the use of SBRT for the treatment of OSA which range from 295 to 897.13,25,28,29 The current study evaluated a larger patient cohort than the previously published cases suggesting a more accurate expected survival time. There was no significant survival difference between dogs that developed a fracture and those that did not; however, if dogs underwent an amputation following treatment, they lived a significantly longer time than those that did not. Less than half of patients that had a fracture pursued amputation (19/51). The remaining 32 patients elected for euthanasia after being diagnosed with a pathologic fracture, leading to the shorter survival time. Improved survival following amputation is a similar finding to a recent publication using a limb salvage surgical procedure as the initial primary treatment.2
The cause of death was not significantly associated with survival in this cohort of patients. Percent tumour necrosis has been shown to impact tumour control and is histopathologically described as necrotic debris with ghost outlines of cells and remnants of tumour bone or osteoid devoid of cells with possible haemorrhage.14 It has been previously established that the hypofractionated non-modulated radiation dose required to cause at least 80% tumour necrosis consistent with higher chances of local tumour control is 42.2 Gy with radiation alone or 28.1 Gy when radiation is combined with intraarterial cisplatin.14 While 73% of plans had 28.1 Gy or higher to 95% of the PTV, only 50% of the samples evaluated had greater than 80% necrosis; however, the patients that did well generally did not have histopathologic evaluation post SBRT and, therefore, the actual patients with local control is likely higher. Additionally, if 80% of the tissue is devoid of viable cells, a concomitant lack in associated structural matrix necessary to endure daily impact and sustain an intact bone is also likely, thus leading to an increase in fracture rate.
While breed did not significantly impact survival, it is interesting to note that MST for Bernese Mountain Dogs was 713 days, Newfoundland was 475 days, and Old English Sheepdogs was 468 days compared to the shortest MST breeds including St. Bernard at 152 days, Doberman at 167 days and Greyhound at 171 days.
No significant difference in survival was apparent for dogs with evidence of metastasis at the time of treatment (median 200 days) vs those that did not. Dogs that present with stage III OSA have previously been shown to have a survival time of 76 days.30 Within that study, dogs that received radiation therapy and chemotherapy lived significantly longer than dogs in other groups with a MST of 130 days.30 One theory for the longer survival time is due to concomitant tumour resistance. This is a phenomenon in which a tumour-bearing patient is resistant to the development or progression of metastasis.31 With radiation therapy, the primary lesion remains and cells die gradually due to mitotic cell death potentially allowing for the continued release of metastatic suppressing cytokines and may participate in suppressing metastasis. In dogs treated with amputation followed by chemotherapy, 75% to 90% succumb to metastatic disease within 1 year, supporting the concomitant tumour resistance hypothesis.
Two patients had bilateral proximal humeral lesions diagnosed and treated with SBRT concurrently. It is impossible to determine if these lesions represent the occurrence of bilateral primary lesions or a primary lesion and metastasis. Bilateral synchronous OSA is rare in both dogs and humans and expected to occur in about 0.4% of primary bone tumours.27 A previous case series suggested a similar outcome and prognosis to dogs with a singular lesion; however, the two patients described here had a significantly shorter survival period compared to other locations living for only 143 and 154 days.27 Both patients represented here developed a fracture in one of their limbs and one developed suspected vertebral metastasis as well.
Cytology was used commonly to confirm a diagnosis of OSA. Recent data suggests a comparable accuracy for diagnosis of neoplasia between cytology and histopathology in canine bone lesions, with the advantage that cytology lends to fewer complications.32–34 It should be noted, however, that biopsy for histopathology was not associated with an increase in fracture risk for this population. Cytology samples resulted in 19% non-diagnostic samples while only 3% of biopsy samples were non-diagnostic. There were no reported adverse events that occurred from sampling of the tumour. Based on this information, either diagnostic approach could be considered with the anticipation that cytology may not provide a definitive result.
Bisphosphonates are known to inhibit osteoclast function to reduce bone resorption and have direct anti-tumour effects, including inhibiting tumour cell proliferation, adhesion and invasion.11,35–37 The pain palliation effects of bisphosphonates as well as their impact on survival are controversial.11,12,37 One hundred eighteen patients in the current cohort received a bisphosphonate while only eight did not. Given the small number of patients that did not receive bisphosphonates, it is not possible to draw further conclusions regarding bisphosphonate pain palliation effects or its impact on survival. Further studies on SBRT for OSA without the use of a bisphosphonate is warranted to compare the two groups.
Over one-third of irradiated sites in this study resulted in a fracture. This is similar to a previously reported publication utilizing SBRT for OSA.13 Rabbit models showed that bone changes post high dose irradiation included a loss of bending strength, increase in porosity, reduced new bone formation and reduced cellularity of the bone marrow all leading to an increased fracture risk.38 Additionally, murine models suggested that reduced cortical mineral/matrix ratios could also contribute to post radiation fragility fractures that are reported clinically.39 While these studies describe post radiation changes to normal bone, the current population suffered from a defective bone prior to irradiation in addition to the previously described changes leading to an increased risk for fragility fractures. Additional methods of bone evaluation should be identified to determine which patients are at higher risk for developing a fracture post SBRT.
One dog had an amputation due to chronic infection, and ultimately septic shock, 138 days after SBRT. This patient had ulceration of the skin in the treatment field prior to SBRT and radiation was delivered every other day in an attempt to spare the skin. Four patients had an amputation for uncontrollable pain without evidence of a fracture. One patient had a lesion of the distal femur and three patients had lesions of the proximal humerus. An additional patient with a lesion of the proximal humerus was euthanized due to uncontrollable pain without evidence of a fracture and 98% tumour necrosis was noted on necropsy, resulting in 9% of patients with proximal humeral lesions developing uncontrollable pain. While challenging to prove with imaging, a possible mechanism may be radiation induced (RI) plexopathy. While uncommonly seen after subclavian irradiation for breast cancer, clinical manifestations are sensorimotor symptoms by direct RI nerve injury, and severity depends on the underlying fibrotic process including axonal loss and arterial stenosis.40–42 A recent study in people did not find benefit to treatment with pentoxyfilline-vitamin E combination requiring further investigation to this quality of life debilitating sequela to radiation; however, careful contouring and constraints on the area of the brachial plexus are important to minimize this development.40
Of the treatment sites with reported adverse events, 10% developed acute and 8% developed late grade 3 VRTOG effects consisting of confluent moist desquamation with edema, ulceration, and necrosis in the acute setting and severe fibrosis and necrosis in the late setting. One-third of the patients with acute grade 3 effects developed late grade 3 effects consistent with a consequential late effect. The skin dose constraints have adjusted over time as follow-up has been accrued for treated sites with the initial allowance of no more than 1 cc of skin receiving 30 Gy to, more recently, 1 cc of skin at 24 Gy. Based on the results of this study, new recommendations include allowing for 1 cc of skin at 25.4 Gy, a maximum skin point dose of 32.2 Gy, and full thickness skin dose at 22.1 Gy in three fractions to reduce the number of patients affected by severe late radiation effects while continuing to get a maximal dose to the tumour.
The primary limitations of the study include its retrospective nature and lack of mature normal tissue constraints. There are also limitations in tissue sampling and confirmatory diagnosis of OSA with 47% of treated sites having a presumptive diagnosis. The follow-up schedule and limb radiographs were inconsistent leading to local progression assumptions and possible early euthanasia.
SBRT offers dogs with appendicular OSA a non-surgical limb spare option that can be combined with chemotherapy. MST is less than historical amputation and chemotherapy protocols and longer than purely palliative radiation therapy protocols. Complication rates can be high, with post SBRT fracture the most common complication followed by late radiation effects. Smaller tumour volumes correlate with smaller volumes of irradiated normal tissues which may impact fracture and adverse radiation effects in larger sample sizes. Recommended skin dose constraints for 1 cc of skin at 25.4 Gy, maximum skin point dose of 32.2 Gy and full thickness skin dose at 22.1 Gy should be applied when using this therapy. While certain patients may not be limb amputation candidates, a more reliable method for bone integrity evaluation should be created to help identify patients who are more likely to develop a pathologic fracture.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1.Ehrhart NP, Christensen NI, Fan TM. Tumors of the skeletal system. In: Vail DM, Thamm DH, Liptak J, Page RL, eds. Small Animal Clinical Oncology. 6th ed.St Louis: Elsevier Saunders; 2020:524–564. [Google Scholar]
- 2.Wustefeld-Janssens BG, Séguin B, Ehrhart NP, Worley DR. Analysis of outcome in dogs that undergo secondary amputation as an end-point for managing complications related to limb salvage surgery for treatment of appendicular osteosarcoma. Vet Comp Oncol. 2020;18(1):84–91. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell KE, Boston SE, Kung M, et al. Outcomes of limb-sparing surgery using two generations of metal endoprosthesis in 45 dogs with distal radial osteosarcoma. A veterinary Society of Surgical Oncology retrospective study. Vet Surg. 2016;45(1):36–43. [DOI] [PubMed] [Google Scholar]
- 4.Liptak JM, Dernell WS, Ehrhart N, Lafferty MH, Monteith GJ, Withrow SJ. Cortical allograft and endoprosthesis for limb-sparing surgery in dogs with distal radial osteosarcoma: a prospective clinical comparison of two different limb-sparing techniques. Vet Surg. 2006; 35(6):518–533. [DOI] [PubMed] [Google Scholar]
- 5.Séguin B, O’Donnell MD, Walsh PJ, Selmic LE. Long-term outcome of dogs treated with ulnar rollover transposition for limb-sparing of distal radial osteosarcoma: 27 limbs in 26 dogs. Vet Surg. 2017;46(7): 1017–1024. [DOI] [PubMed] [Google Scholar]
- 6.Withrow SJ, Liptak JM, Straw RC, et al. Biodegradable cisplatin polymer in limb-sparing surgery for canine osteosarcoma. Ann Surg Oncol. 2004;11(7):705–713. [DOI] [PubMed] [Google Scholar]
- 7.Kuntz CA, Asselin TL, Dernell WS, Powers BE, Straw RC. Limb salvage surgery for osteosarcoma of the proximal humerus: outcome in 17 dogs. Vet Surg. 1998;27(5):417–422. [DOI] [PubMed] [Google Scholar]
- 8.Green EM, Adams WM, Forrest LJ. Four fraction palliative radiotherapy for osteosarcoma in 24 dogs. J Am Anim Hosp Assoc. 2002;38(5): 445–451. [DOI] [PubMed] [Google Scholar]
- 9.Knapp-Hoch HM, Fidel JL, Sellon RK, Gavin PR. An expedited palliative radiation protocol for lytic or proliferative lesions of appendicular bone in dogs. J Am Anim Hosp Assoc. 2009;45(1):24–32. [DOI] [PubMed] [Google Scholar]
- 10.Pagano C, Boudreaux B, Shiomitsu K. Safety and toxicity of an accelerated coarsely fractionated radiation protocol for treatment of appendicular osteosarcoma in 14 dogs: 10 Gy × 2 fractions. Vet Radiol Ultrasound. 2016;57(5):551–556. [DOI] [PubMed] [Google Scholar]
- 11.Oblak ML, Boston SE, Higginson G, Patten SG, Monteith GJ, Woods JP. The impact of pamidronate and chemotherapy on survival times in dogs with appendicular primary bone tumors treated with palliative radiation therapy. Vet Surg. 2012;41(3):430–435. [DOI] [PubMed] [Google Scholar]
- 12.Fan TM, Charney SC, de Lorimier LP, et al. Double-blind placebo-controlled trial of adjuvant pamidronate with palliative radiotherapy and intravenous doxorubicin for canine appendicular osteosarcoma bone pain. J Vet Intern Med. 2009;23(1):152–160. [DOI] [PubMed] [Google Scholar]
- 13.Farese JP, Milner R, Thompson MS, et al. Stereotactic radiosurgery for treatment of osteosarcomas involving the distal portions of the limbs in dogs. J Am Vet Med Assoc. 2004;225(10):1567–1572. [DOI] [PubMed] [Google Scholar]
- 14.Powers BE, Withrow SJ, Thrall DE, et al. Percent tumor necrosis as a predictor of treatment response in canine osteosarcoma. Cancer. 1991;67(1):126–134. [DOI] [PubMed] [Google Scholar]
- 15.Fitzpatrick CL, Farese JP, Milner RJ, et al. Intrinsic radiosensitivity and repair of sublethal radiation-induced damage in canine osteosarcoma cell lines. Am J Vet Res. 2008;69(9):1197–1202. [DOI] [PubMed] [Google Scholar]
- 16.Walter CU, Dernell WS, LaRue SM, et al. Curative-intent radiation therapy as a treatment modality for appendicular and axial osteosarcoma: a preliminary retrospective evaluation of 14 dogs with the disease. Vet Comp Oncol. 2005;3(1):1–7. [DOI] [PubMed] [Google Scholar]
- 17.Liptak JM, Dernell WS, Lascelles BD, et al. Intraoperative extracorporeal irradiation for limb sparing in 13 dogs. Vet Surg. 2004;33(5): 446–456. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi S, Sugimoto M, Kotoura Y, et al. Incorporation of cortical bone autografts following intraoperative extracorporeal irradiation in rabbits. Int J Radiat Oncol Biol Phys. 1991;21(5):1221–1230. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto T, Akisue T, Marui T, Nagira K, Kurosaka M. Osteosarcoma of the distal radius treated by intraoperative extracorporeal irradiation. J Hand Surg Am. 2002;27(1):160–164. [DOI] [PubMed] [Google Scholar]
- 20.Oike N, Kawashima H, Ogose A, et al. Long-term outcomes of an extracorporeal irradiated autograft for limb salvage operations in musculoskeletal tumours: over ten years’ observation. Bone Joint J. 2019;101-b(9):1151–1159. [DOI] [PubMed] [Google Scholar]
- 21.Chauhan S, Khan SA, Prasad A. Irradiation-induced compositional effects on human bone after extracorporeal therapy for bone sarcoma. Calcif Tissue Int. 2018;103(2):175–188. [DOI] [PubMed] [Google Scholar]
- 22.Sharma DN, Rastogi S, Bakhshi S, et al. Role of extracorporeal irradiation in malignant bone tumors. Indian J Cancer. 2013;50(4):306–309. [DOI] [PubMed] [Google Scholar]
- 23.Timmerman RD, Herman J, Cho LC. Emergence of stereotactic body radiation therapy and its impact on current and future clinical practice. J Clin Oncol. 2014;32(26):2847–2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boston SE, Vinayak A, Lu X, et al. Outcome and complications in dogs with appendicular primary bone tumors treated with stereotactic radiotherapy and concurrent surgical stabilization. Vet Surg. 2017;46:829–837. [DOI] [PubMed] [Google Scholar]
- 25.Covey JL, Farese JP, Bacon NJ, et al. Stereotactic radiosurgery and fracture fixation in 6 dogs with appendicular osteosarcoma. Vet Surg. 2014;43(2):174–181. [DOI] [PubMed] [Google Scholar]
- 26.Ladue T, Klein MK. Toxicity criteria of the veterinary radiation therapy oncology group. Vet Radiol Ultrasound. 2001;42(5):475–476. [DOI] [PubMed] [Google Scholar]
- 27.Selmic LE, Ryan SD, Ehrhart NP, Withrow SJ. Bilateral appendicular bone tumors in four dogs. J Am Anim Hosp Assoc. 2013;49(2):135–141. [DOI] [PubMed] [Google Scholar]
- 28.Nolan MW, Green NA, DiVito EM, Lascelles BDX, Haney SM. Impact of radiation dose and pre-treatment pain levels on survival in dogs undergoing radiotherapy with or without chemotherapy for presumed extremity osteosarcoma. Vet Comp Oncol. 2020;18:538–547. [DOI] [PubMed] [Google Scholar]
- 29.Kubicek L, Vanderhart D, Wirth K, et al. Association between computed tomographic characteristics and fractures following stereotactic radiosurgery in dogs with appendicular osteosarcoma. Vet Radiol Ultrasound. 2016;57(3):321–330. [DOI] [PubMed] [Google Scholar]
- 30.Boston SE, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. Evaluation of survival time in dogs with stage III osteosarcoma that undergo treatment: 90 cases (1985–2004). J Am Vet Med Assoc. 2006;228(12):1905–1908. [DOI] [PubMed] [Google Scholar]
- 31.Chiarella P, Bruzzo J, Meiss RP, Ruggiero RA. Concomitant tumor resistance. Cancer Lett. 2012;324(2):133–141. [DOI] [PubMed] [Google Scholar]
- 32.Berzina I, Sharkey LC, Matise I, Kramek B. Correlation between cytologic and histopathologic diagnoses of bone lesions in dogs: a study of the diagnostic accuracy of bone cytology. Vet Clin Pathol. 2008;37(3):332–338. [DOI] [PubMed] [Google Scholar]
- 33.Sabattini S, Renzi A, Buracco P, et al. Comparative assessment of the accuracy of cytological and histologic biopsies in the diagnosis of canine bone lesions. J Vet Intern Med. 2017;31(3):864–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Britt T, Clifford C, Barger A, et al. Diagnosing appendicular osteosarcoma with ultrasound-guided fine-needle aspiration: 36 cases. J Small Anim Pract. 2007;48(3):145–150. [DOI] [PubMed] [Google Scholar]
- 35.Hoddinott K, Oblak ML, Wood GA, Boston S, Mutsaers AJ. Evaluation of effects of radiation therapy combined with either pamidronate or zoledronate on canine osteosarcoma cells. Can J Vet Res. 2019;83(1): 3–10. [PMC free article] [PubMed] [Google Scholar]
- 36.Curtis RC, Custis JT, Ehrhart NP, et al. Combination therapy with zoledronic acid and parathyroid hormone improves bone architecture and strength following a clinically-relevant dose of stereotactic radiation therapy for the local treatment of canine osteosarcoma in athymic rats. PLoS One. 2016;11(6):e0158005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fan TM, de Lorimier LP, O’Dell-Anderson K, Lacoste HI, Charney SC. Single-agent pamidronate for palliative therapy of canine appendicular osteosarcoma bone pain. J Vet Intern Med. 2007;21(3):431–439. [DOI] [PubMed] [Google Scholar]
- 38.Sugimoto M, Takahashi S, Toguchida J, et al. Changes in bone after high-dose irradiation. Biomechanics and histomorphology. J Bone Joint Surg Br. 1991;73(3):492–497. [DOI] [PubMed] [Google Scholar]
- 39.Mandair GS, Oest ME, Mann KA, Morris MD, Damron TA, Kohn DH. Radiation-induced changes to bone composition extend beyond periosteal bone. Bone Rep. 2020;12:100262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delanian SE, Lenglet T, Maisonobe T, Resche-Rigon M, Pradat PF. Randomized, placebo-controlled clinical trial combining pentoxifylline-tocopherol and clodronate in the treatment of radiation-induced plexopathy. Int J Radiat Oncol Biol Phys. 2020;107(1):154–162. [DOI] [PubMed] [Google Scholar]
- 41.Delanian S, Lefaix JL, Pradat PF. Radiation-induced neuropathy in cancer survivors. Radiother Oncol. 2012;105(3):273–282. [DOI] [PubMed] [Google Scholar]
- 42.Delanian S, Lefaix JL. The radiation-induced fibroatrophic process: therapeutic perspective via the antioxidant pathway. Radiother Oncol. 2004;73(2):119–131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


