Abstract
Background and Purpose
We test the hypothesis that unsatisfactory outcomes after concurrent chemoradiotherapy (RT) for locally advanced non-small cell lung cancer (LA-NSCLC) are due to treatment-related immunosuppression.
Materials and Methods
White blood cells (WBCs) data were retrospectively collected for all stage IIIA/B LA-NSCLC patients before and after (after RT: two weeks, two months, four months) concurrent chemotherapy and intensity-modulated RT in which patients were treated to a median of 63Gy (1.8–2.0 Gy/fractions) in 2004–2014 (N=155). Nine WBC variables were generated from pre-RT normalized absolute number of lymphocytes and neutrophils (L, N) and the N/L thereof. A WBC variable was considered a predictor for overall survival and recurrence (distant/local/nodal/regional) if p≤0.006 (corrected for 9 variables) from Cox regression and competing risk analyses, respectively; both conducted using bootstrap resampling. Finally, a WBC variable predicting any of the outcomes was linearly associated with each of eleven disease/patient/treatment characteristics (p≤0.005; corrected for 11 characteristics).
Results
At the three post-RT time points both L and N significantly decreased (p<0.0003). Overall survival was associated with N and N/L four months post-RT (p=0.00001, 0.0003); regional recurrence was associated with L two months post-RT (p<0.0001). None of the disease/patient/treatment characteristics was significantly associated with any of the three WBC variables that predicted OS or recurrence (lowest p-value: p=0.006 for tumour stage,).
Conclusion
Significantly lower WBC levels after concurrent chemo-RT for LA-NSCLC are associated with worse long-term outcomes. The mechanism behind this treatment-related immunosuppression requires further analysis likely including other characteristics as no statistically significant association was established between any WBC variable and the disease/patient/treatment characteristics.
Keywords: radiotherapy, lung cancer, immune, dose, toxicity, survival
Introduction
Overall survival after radiotherapy (RT) for locally advanced non-small cell lung cancer (LA-NSCLC) continues to be far from optimal despite recent progress with the use of adjuvant immune checkpoint inhibitors, which has resulted in a 10% increase (from 56% to 66%) in the two-year overall survival (OS) rates [1, 2]. Of particular interest, cardio-pulmonary dose has been found to correlate negatively with OS in various LA-NSCLC series, including RTOG 0617 [3–8]. The primary hypothesis addressed to this correlation has been radiation-induced cardiac toxicity, and a number of studies have thereafter established an association between a range of symptomatic cardiac toxicities and cardiac dose [9–14].
Another alternative, potentially related, hypothesis for low survival rates after RT for LA-NSCLC is that concurrent chemo-RT leads to immunosuppression due to decreased number of ‘ immune system cells’, i.e., white blood cells (WBCs), which has been demonstrated following RT for tumour sites ranging from the head and neck to the thorax [15–18]. For LA-NSCLC this hypothesis has, thus far, only been elaborated upon by Contreras et al [15] and Tang et al [16] (note: the cohort in [16] also included 11% stage I and 7% stage II patients). The number of WBCs by the end of chemo-RT [16] or four months after completed chemo-RT [15] were found to predict OS, and these were further correlated with chemotherapy regimen, hyper fractionation, tumour stage and volume [16] or heart dose, lung dose, and tumour volume [15].
In this study, we hypothesized that poor outcomes after concurrent chemo-RT for LA-NSCLC are due to treatment-related immunosuppression. More specifically, we investigated if any of the two WBC components with the highest concentration in the blood, i.e., lymphocytes and neutrophils, are associated with a range of treatment outcomes, and if so we explored potential reasons for this. Unlike in [15] and [16], all WBCs in the current study were normalized with respect to their corresponding pre-treatment values to elucidate the treatment-induced effect on the WBCs.
Materials & Methods
Patient demographics
All stage IIIA/B inoperable LA-NSCLC patients that received definitive intensity-modulated RT (IMRT) combined with chemotherapy between 2004 and 2014 at Memorial Sloan Kettering Cancer Center were considered for inclusion in this Institutional Review Board approved study [19, 20]. From a total of 241 patients, the 155 patients that received concurrent chemotherapy (delivered during RT typically as cisplatin doublets in four cycles) had WBCs collected. Demographics for all included patients are given in Table S1. For the purpose of this study, data were retrospectively collected.
The 155 included patients had been treated to a median of 63Gy (range: 50–80Gy) in 1.8–2.0Gy fractions with IMRT typically using a 5-field beam arrangement [21] (Table S1). All patients had a positron emission tomography (PET) scan available for staging. Dose was prescribed to the 100% isodose line surrounding the primary tumor plus positive lymph nodes and had been re-calculated with the currently used dose-calculation algorithm (Eclipse AAA v.13, Varian Medical Systems, Palo Alto, CA, US).
White blood cell (WBC) variables
White blood cell counts were drawn pre- and post-RT. This study focused on the pre-RT WBCs (within two months pre-RT) as well as WBCs two (range: one to three) months and four (range: three to six) months after RT. The absolute number of lymphocytes and the absolute number of neutrophils (L, N) were extracted. In addition to the aforementioned three time points, L and N were collected at the L nadir, i.e. the lowest L within three months after RT start, which occurred at a median of twelve days after completion of RT (Fig. S1).
From these data, nine WBC variables were defined. For both L and N, the pre-RT normalized ratio of all three post-RT measures were generated, i.e., (post-pre)/pre, which resulted in a total of six WBC variables referred to as LΔ12d, LΔ2mo, and LΔ4mo, as well as NΔ12d, NΔ12mo, and NΔ4mo. In addition, the ratio between N and L was also assessed ( N/L) and, correspondingly for L and N, three variables were generated: N/LΔ12d, N/LΔ2mo, and N/LΔ4mo.
Associating WBC variables with treatment outcomes
The association between each of the nine WBC variables and five treatment outcomes was assessed. All patients were followed up every 3–4 months the first two years and every six month the following three years. At each visit at least one thoracic CT scan was acquired. These outcomes were distant (non-thorax), local (thorax; in-primary-field), nodal (thorax; in-nodal-field), and regional recurrence (thorax; out-of-field), as well as OS. A competing risk analysis was applied to model the four recurrence outcomes; OS was modelled using Cox proportional hazard regression. For each outcome, significance was denoted at p≤0.0056 to Bonferroni-correct for multiple testing given the nine WBC variables. Further, a WBC variable was considered a predictor only if p≤0.05 between the associated Kaplan-Meier curves or cumulative incidence functions (Cox: log-rank test; Competing risk: Gray’s test) based on tertile splits of the WBC variable. If multiple WBC variables predicted a certain outcome, a multivariate analysis was considered if the Spearman’s rank correlation coefficient (|Rs|) between paired variables did not exceed 0.70; otherwise the variable with the higher p-value was judged redundant. The multivariate analysis was conducted using forward-stepwise variable selection with a retention criterion of p≤0.05 from a likelihood ratio test in the Cox regression, and a Bayesian Information Criterion (BIC) ≤|2| of the minimum BIC [22] for the competing risk analysis. Unless more than one multivariate model presented with comparable model frequency, the most frequently selected model was deemed final.
Investigating relationships between disease/patient/treatment characteristics and identified WBC predictors
To identify a possible link between a statistically significant WBC variable and an outcome, each of the eleven disease/patient/treatment characteristics in Table S1 was linearly regressed against statistically significant WBC variables. The eleven characteristics were age, chemotherapy type (primarily: Carboplatin/taxol vs. Cisplatin doublets), gender, gross tumour volume (GTV), histology, Karnofsky Performance Status (KPS), pericardium mean dose, prescription dose, smoking status, tumor stage, and tumor location. The prescription dose was converted to biologically effective dose (α/β=10Gy) and taking into account repopulation (repopulation time=28 days; potential doubling time=3 days) [23]. The pericardium mean dose was converted into the equivalent dose in 2 Gy fractions (α/β=3Gy). The pericardium was defined using post-treatment contouring that adhered to the RTOG 1106 contouring atlas (https://www.rtog.org/CoreLab/ContouringAtlases/LungAtlas) [24]. The pericardium, thus, encompassed the aorta (mainly ascending), coronary arteries, heart chambers, pulmonary artery and vein, as well as the superior vena cava. In these analyses, linear regression was used. Significance was indicated by p≤0.0045 considering Bonferroni-correction for multiple testing given eleven characteristics per WBC variable. If there was more than one predictor per WBC variable, and |Rs| did not exceed 0.70 between these, a multivariate model was generated. Final predictors (or models in the case of more than one final predictor per WBC variable) were also considered for inclusion in the generated treatment outcome models based on the multivariate criteria outlined above.
All analyses were conducted using bootstrapping with 1000 generated sample populations; the outlined p-value cutoffs were judged as medians across these samples. In the multivariate analyses, the most frequently selected model across the 1000 samples was considered final. The competing risk analyses were conducted using R v.3.5.1 with packages ‘cmprskg’ [25] and ‘crrstep’ [26]; the Cox proportional hazard regression and the linear regression were both performed in MATLAB v. R2016a.
Results
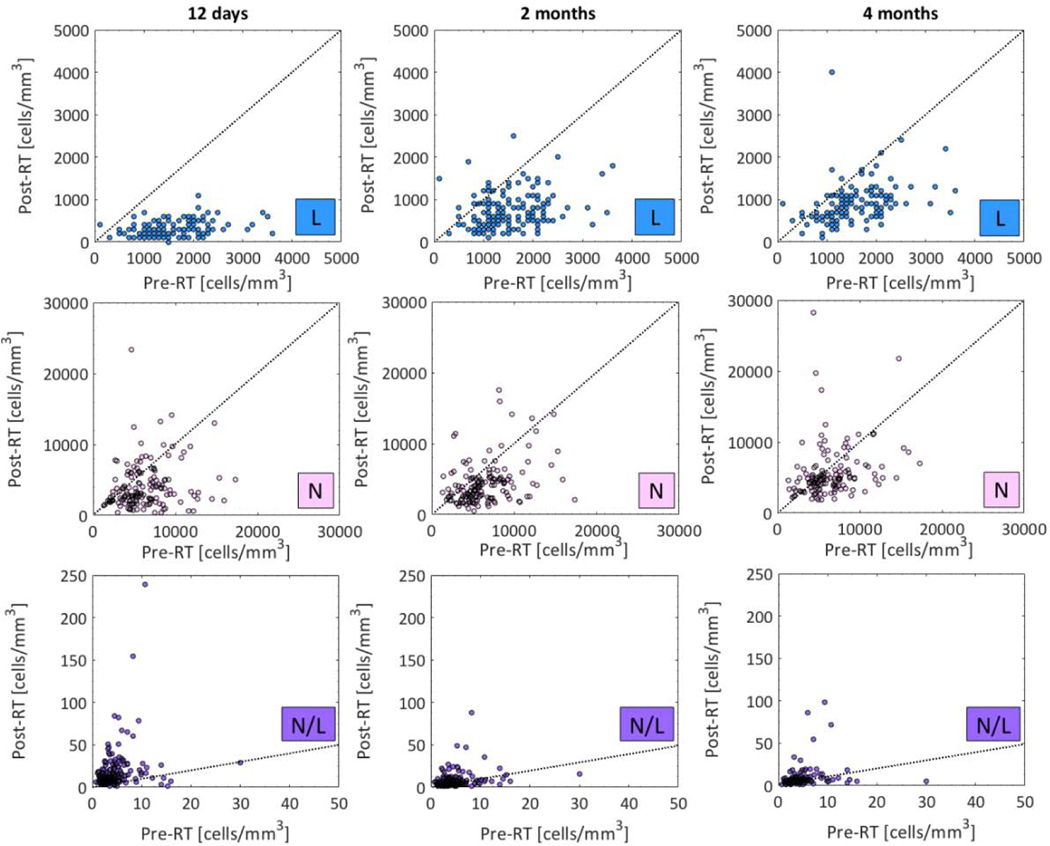
For both L and N, at all three time points, the number of WBCs decreased significantly post-RT (p<0.0003) and this was most pronounced for L (population median: 1500 vs. 300–800 cells/mm3; Fig.1; Table S2). Accordingly, N/L was also significantly different but this ratio was larger post-RT. Thus, concurrent chemo-RT significantly decreases the number of WBCs.
Fig.1.
Distributions of L, N, and N/L (upper, middle, and lower) pre- RT (x-axis) and at the three post-RT time points (y-axis; 12 days, 2 months, and 4 months post-RT: left, middle, and right panel). Note: See Table 2 for population medians (range) and p-values for post-RT and pre-RT comparisons. Dotted line: Identity line.
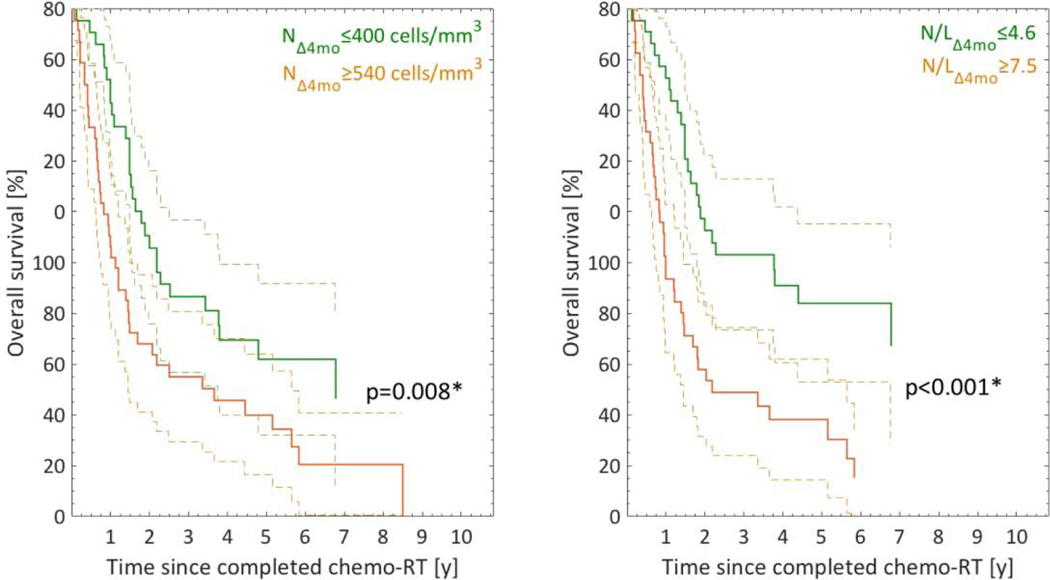
Of the nine studied WBC variables, NΔ4mo and N/LΔ4mo significantly predicted OS (p=0.00001, 0.0003; Fig.2; Table 1). Based on tertile splits, a significant separation was observed in the Kaplan-Meier curves for both (p=0.008, p<0.001; Fig.2). None of the remaining WBC variables predicted OS (p=0.009–0.66); NΔ2mo presented with the lowest p-value). The correlation between NΔ4mo and N/LΔ4mo was moderate to strong, but did not exceed 0.70 (|Rs|=0.70). Therefore, a multivariate analysis was undertaken. The most frequently selected model with a frequency of 83% included NΔ4mo and N/LΔ4mo and both these WBC variables were deemed final OS predictors.
Fig.2.
Kaplan-Meier (KM) curves for NΔ4mo and N/LΔ4mo that significantly predicted OS stratified into tertiles (riskiest: orange; least riskiest: green). Note: Dashed lines: 95% CIs; *significantly different KM curves (log-rank test).
Table 1.
Specifics for the established significant associations (p≤0.0056) between the investigated WBC variables and the treatment outcomes.
| Outcome | Modeling | WBC variable | p | β | HR (95%CI) |
|---|---|---|---|---|---|
|
| |||||
| Overall survival | Univariate | N Δ4mo | 0.00001 | 0.61 | 1.8 (1.4–2.4) |
| N/L Δ4mo | 0.0003 | 0.16 | 1.2 (1.1–1.3) | ||
| Multivariate | N Δ4mo | 0.005 | 0.14 | 1.1 (1.0–1.3) | |
| N/L Δ4mo | 0.09 | 0.02 | 1.0 (1.0–1.0) | ||
|
| |||||
| Regional recurrence | Univariate | L Δ12d | 0.002 | 0.39 | - |
| L Δ2mo | 1E-10 | 0.12 | - | ||
Note: None of the WBCs predicted distant, local, or nodal recurrence (p-value range: 0.18–0.49, 0.13–0.49, 0.11–0.51); the p-values for the WBCs that did not predict OS and regional recurrence ranged between 0.02–0.67 and 0.008–0.47, respectively.
In a sub analysis, both NΔ4mo and N/LΔ4mo were found to also significantly predict progression-free survival (progression: distant, nodal, primary, or regional; p=0.002,0.003) while none of the remaining seven WBC variables did (data not shown).
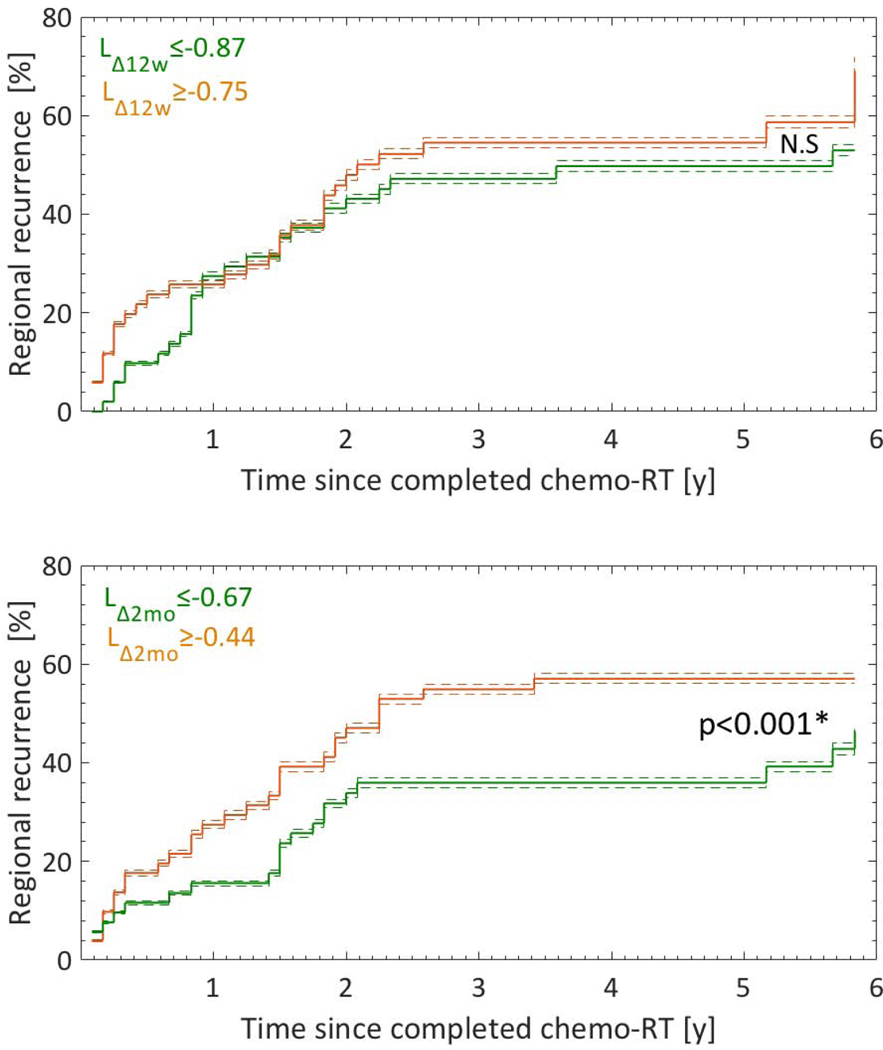
An association was established between regional recurrence and LΔ12d as well as LΔ2mo (p=0.02, 1.2E-10; Fig.3; Table 1). A separation was observed between the cumulative incidence functions of risk groups for LΔ2mo (p<0.001; Fig.3), but not for LΔ12d. Therefore, LΔ2mo alone was considered a final predictor for regional recurrence. None of the nine WBC variables was associated with any of the other four recurrence outcomes (p-values ranged from 0.11 to 0.51).
Fig.3.
Cumulative incidence functions (CIFs) for the two WBC variables LΔ12w and LΔ2mo that significantly predicted regional recurrence stratified into tertiles (riskiest: orange; least riskiest: green). Note: Dashed lines: 95% CIs; *significantly different CIFs (Gray’s test).
None of the eleven characteristics in Table S1 was linearly associated with NΔ4mo or N/LΔ4mo (Table 2). Similarly, neither LΔ12d nor LΔ2mo was linearly associated with any of these characteristics (Table 3). A Spearman’ s rank test, assuming a non-structural relationship, confirmed this lack of association (data not shown). Regardless, among all comparisons, the overall lowest p-value was observed between tumour stage and N/LΔ4mo (p=0.0059; Table 2), which was still not significant given the a priori defined significance level of p≤0.0045. For NΔ4mo and LΔ12d, the lowest p-values were observed for the pericardium mean dose (p=0.02; Fig.S2), whereas for LΔ2mo KPS presented with the lowest p-value (p=0.02).
Table 2.
Linear regression results for NΔ4mo and N/LΔ4mo, which were final predictors for OS, based on the characteristics in Table 1.
| Characteristics | NΔ4mo | N/LΔ4mo | |||||||
|---|---|---|---|---|---|---|---|---|---|
| R2 | Intercept | β | p | R2 | p | Intercept | β | p | |
|
| |||||||||
| Age at RT [y] | −4.2E-3 | 0.08 | −1.0E-3 | 0.51 | −2.0E-3 | 0.39 | 0.87 | 4.3E-3 | 0.39 |
|
| |||||||||
| Chemotherapy type | |||||||||
| (ref: Carboplatin/taxol) | 1.9E-3 | −0.07 | 0.15 | 0.27 | −2.8E-3 | 0.43 | 1.3 | −0.18 | 0.43 |
| (ref: Cisplatin doublets) | 0.01 | 0.07 | −0.28 | 0.12 | 1.3E-3 | 0.28 | 1.3 | −0.49 | 0.28 |
| (ref: Other) | −5.1E-3 | 7.0E-3 | 0.03 | 0.57 | 0.01 | 0.13 | 1.0 | 0.64 | 0.13 |
|
| |||||||||
| Gender (ref: Female) | −4.0E-3 | −0.02 | 0.02 | 0.49 | 4.0E-3 | 0.49 | 0.99 | 0.10 | 0.49 |
|
| |||||||||
| GTV [cm3] | 8.8E-4 | 0.10 | −4.1E-4 | 0.29 | −5.7E-3 | 0.61 | 1.1 | 2.7E-4 | 0.61 |
|
| |||||||||
| Histology | |||||||||
| (ref: Adeno ca) | −3.2E-3 | 0.04 | −0.07 | 0.45 | −3.6E-3 | 0.47 | 1.2 | −0.20 | 0.47 |
| (ref: SCC) | −3.3E-3 | −0.02 | 0.05 | 0.46 | 2.0E-4 | 0.31 | 0.89 | 0.38 | 0.31 |
|
| |||||||||
| KPS | −5.1E-3 | 0.20 | −2.2E-3 | 0.57 | −4.3E-3 | 0.51 | 0.24 | 0.01 | 0.51 |
|
| |||||||||
| PMD [Gy] | 0.03 | −0.39 | 0.02 | 0.02 | −6.8E-3 | 0.74 | 60 | 0.03 | 0.74 |
|
| |||||||||
| Prescription dose [Gy] | 0.01 | 1.2 | −0.02 | 0.13 | −2.4E-3 | 0.41 | 1.1 | 1.1E-3 | 0.41 |
|
| |||||||||
| Smoking status | |||||||||
| (ref: Current) | 4.8E-3 | 0.11 | −0.17 | 0.20 | −3.3E-3 | 0.45 | 1.3 | −0.2 | 0.45 |
| (ref: Former) | 6.1E-3 | −0.05 | 0.19 | 0.18 | −2.6E-3 | 0.42 | 1.1 | 0.2 | 0.42 |
|
| |||||||||
| Tumor stage (ref: IIIA) | 0.03 | −0.44 | 0.29 | 0.03 | 0.05 | 5.9E-3 | −0.45 | 1.0 | 0.0059* |
|
| |||||||||
| Tumor location | |||||||||
| (ref: inferior) | 0.03 | −0.05 | 0.38 | 0.03 | −1.8E-3 | 0.38 | 1.1 | 0.28 | 0.38 |
| (ref: left) | 4.9E-3 | −0.05 | 0.17 | 0.20 | 0.01 | 0.10 | 0.92 | 0.60 | 0.10 |
| (ref: right) | 2.2E-3 | 0.10 | −0.14 | 0.26 | 0.01 | 0.10 | 1.53 | −0.60 | 0.10 |
| (ref: superior) | 0.01 | 0.18 | −0.22 | 0.13 | −4.2E-3 | 0.50 | 1.1 | 0.11 | 0.50 |
Note: Significance is denoted by p≤0.0045 given eleven comparisons; R2 is the coefficient of determination. Abbreviations as in Table 1;
Characteristics with the lowest p-value.
Table 3.
Linear regression results for the WBC variables LΔ12d and LΔ2mo, which were both candidate predictors for regional recurrence (only LΔ2mo was a final predictor), based on the characteristics in Table 1.
| Characteristics | LΔ12d | LΔ2mo | ||||||
|---|---|---|---|---|---|---|---|---|
| R2 | Intercept | β | p | R2 | Intercept | β | p | |
|
| ||||||||
| Age at RT [y] | −2.2E-3 | −0.85 | 1.3E-3 | 0.41 | 8.8E-3 | −1.1 | 0.01 | 0.13 |
|
| ||||||||
| Chemotherapy type | ||||||||
| (ref: Carboplatin/taxol) | 2.2E-3 | −0.74 | −0.05 | 0.25 | 2.9E-3 | −0.27 | −0.21 | 0.23 |
| (ref: Cisplatin doublets) | 1.3E-3 | −0.76 | −0.06 | 0.28 | 3.8E-3 | −0.38 | −0.12 | 0.51 |
| (ref: Other) | 0.03 | −0.8 | 0.1 | 0.03 | 0.01 | −0.49 | 0.39 | 0.08 |
|
| ||||||||
| Gender (ref: Female) | 6.2E-3 | −0.86 | 0.07 | 0.17 | 4.7E-3 | −0.72 | 0.22 | 0.19 |
|
| ||||||||
| GTV [cm3] | 0.02 | −0.71 | −2.8E-4 | 0.06 | −1.3E-4 | −0.31 | 4.2E-4 | 0.32 |
|
| ||||||||
| Histology | ||||||||
| (ref: Adeno ca) | −4.3E-3 | −0.76 | −0.01 | 0.54 | −4.3E-3 | −0.38 | −0.07 | 0.52 |
| (ref: SCC) | 0.02 | −0.70 | −0.10 | 0.04 | 0.01 | −0.18 | −0.33 | 0.10 |
|
| ||||||||
| KPS | 0.02 | −0.13 | −7.5E-3 | 0.04 | 0.03 | 2.0 | −0.03 | 0.02 |
|
| ||||||||
| PMD [Gy] | 0.03 | −0.46 | −0.02 | 0.02 | 4.4E-4 | −0.20 | −0.01 | 0.30 |
|
| ||||||||
| Prescription dose [Gy] | 2.6E-3 | −1.1 | 5.8E-3 | 0.24 | −6.0E-3 | −0.23 | 2.9E-3 | 0.74 |
|
| ||||||||
| Smoking status | ||||||||
| (ref: Current) | 8.2E-3 | −0.72 | −0.07 | 0.14 | 3.9–3 | −0.26 | −0.23 | 0.21 |
| (ref: Former) | 0.02 | −0.80 | 0.10 | 0.05 | 9.4E-3 | −0.49 | 0.30 | 0.12 |
|
| ||||||||
| Tumor stage (ref: IIIA) | 0.03 | −0.60 | −0.11 | 0.03 | 2.0E-3 | −0.09 | −0.19 | 0.26 |
|
| ||||||||
| Tumor location | ||||||||
| (ref: inferior) | −3.2E-3 | −0.76 | −0.04 | 0.47 | −3.4E-3 | −0.38 | −0.10 | 0.48 |
| (ref: left) | 4.9E-3 | −0.75 | −0.05 | 0.30 | −2.2E-3 | −0.35 | −0.11 | 0.41 |
| (ref: right) | −4.5E-4 | −0.79 | 0.04 | 0.34 | −2.0E-3 | −0.48 | 0.12 | 0.40 |
| (ref: superior) | 4.6E-3 | −0.73 | −0.06 | 0.20 | 7.2E-3 | −0.21 | 0.28 | 0.15 |
Note: Significance is denoted by p≤0.0045 given eleven comparisons; R2 is the coefficient of determination. Abbreviations as in Table S1.
Discussion
In this study, we postulated that chemotherapy delivered concurrently with IMRT for LA-NSCLC suppresses the immune system and decrease the number of WBCs, which would be related to treatment outcomes, and explained by disease/patient/treatment characteristics. In particular, the number of WBCs four months after RT significantly decreased. Also, this neutrophil decrease, and the neutrophil/lymphocyte ratio was significantly associated with mortality. For lymphocytes, in contrast, the corresponding reduction was related to regional (out-of-field) recurrences. Tested disease/patient/treatments characteristics did not explain the observed treatment-induced WBC decrease.
All included patients had stage III disease and received concurrent chemo-IMRT. The distribution of disease/patient/treatment characteristics was similar between our cohort and the cohort studied by Contreras et al [15], although that cohort included patients with stage II-III disease that received concurrent, sequential, or no chemotherapy (e.g., the population median age was 64 years vs. 62 years; the population median prescribed dose was 63Gy vs. 66Gy; the rate of SCC was ca 30% in both cohorts; every other patient was a woman). However, while all our patients were planned using IMRT, this was only the case for 47% of the patients in [15]. The latter may have resulted in lower heart doses in favour of the current study [27] (the population median fractionation-corrected pericardium mean dose in the current study was 17Gy; no corresponding information was given in [15]). Another key difference is that, unlike this study, the WBCs in [15] were not normalized to their corresponding pre-treatment values. In the following, comparisons to the results in [15] will be made against their concurrent chemo-RT subgroup that all had stage III disease (N=127; cf. Table 2 in [15]) but with a smaller fraction of stage IIIB compared to the current cohort (35% vs. 58%). Similar to [15], our WBCs approached the corresponding pre-treatment values around four month post-RT, but our L and N values were overall slightly higher and lower, respectively. The multivariate OS model in [15] included N/L4mo (p<0.001) while both N/LΔ4mo and NΔ4mo predicted OS in this study (p=0.0003, 0.00001). The N/L4mo in [15] was significantly correlated with heart dose and the strongest relationships were observed with the mean dose or with the relative heart volume irradiated to 50 Gy (both: p<0.001). A similar association with N/L4mo did not manifest in the current study (p=0.74); the lowest p-value with the pericardium mean dose (p=0.02) was instead seen with NΔ4mo, but this was inconclusive accounting for multiple testing (significance level: p≤0.0045).
The only association for which significance was approached between the eleven investigated characteristics and any of the WBC predictors, i.e. that were candidate predictors for either OS or regional recurrence (NΔ4mo, N/LΔ4mo or LΔ12d, LΔ2mo), was between tumour stage and N/LΔ4mo (p=0.0059; again the significance level was p≤0.0045). Per AJCC’s staging definition [28], stage IIIB implicates a larger extent of nodal involvement than stage IIIA. Our population median nodal GTV was 77 cm3 and 39 cm3 for stage IIIB and IIIA patients, respectively (Wilcoxon rank-sum test: p=0.004), and also stage IIIB patients trended towards higher pericardium mean doses (population median: 17Gy vs. 16Gy; p=0.07). However, neither of these two characteristics was associated with N/LΔ4mo or the other three WBC variables. While the association between stage and WBC was not investigated in [15], Tang et al [16] found that both stage (N=341; III vs. I+II) and GTV were linearly associated with LNadir (Nadir: close to the end of RT; p=0.05, p<0.0001) in their mixed stage I-III NSCLC subset that received concurrent chemo-RT. It is, however, unclear if the GTVs in [16] refer to the primary and/or nodes, but given their GTV distribution of 1.5–960 cm3 and ours being 12–1040 cm3 for the primary and nodal envelope in stage III patients only, it is likely that their GTVs refer to the primary plus nodes. We did not establish a similar association between the GTV envelope and any of our four WBC predictors (lowest p-value: p=0.06 with LΔ12d). Again, differences in WBC definition (ours: pre-treatment normalized; [16]: absolute post-treatment) along with stage differences could have influenced these differences. In addition, separate primary and nodes analyses were inconclusive (GTV primary: p=0.12–0.46; GTV nodes: p=0.01– 0.37). A more fine-grained analysis including nodal extent and specific lymph node location is likely to shed further light on these findings.
Implications have previously been made regarding the platinum chemotherapy agents carboplatin and cisplatin as immunosuppressive [29], partially, as recently exploited, since they down-regulate the programmed death ligand PD-L2 (which inhibits T cell activation [30]). All patients included in this study received concurrent chemotherapy administered primarily as carboplatin/taxol, or cisplatin doublets, but chemotherapy type was not associated with any of the WBC variables that predicted treatment outcomes. Tang et al [31] also could not establish a difference between the post-treatment decreases in WBCs of the same two platinum agents as used in this study (carboplatin and cisplatin). In the larger cohort in [15], sequential chemotherapy and RT alone were OS predictors (p=0.002, 0.03), but these two characteristics were not included in their OS model derived for the concurrent chemo-RT and stage III subgroup. For LNadir Tang et al [16] noticed a similar effect between induction and concurrent chemotherapy with the latter resulting in significantly higher LNadir. This may suggest that chemotherapy timing/type (chemotherapy type not specified in [15]) plays a role in immunosuppression. Unfortunately, consistently collected WBCs for the 86 patients treated with sequentially chemotherapy in our original cohort were not available, and we could not quantitatively assess this hypothesis further.
Our pre-treatment N values are higher than those of the general population (in 27000 civilians aged 66 years and above in the NHANES III survey [32]): 5600 cells/mm3 at a median vs. 4850 cells/mm3, probably reflecting cancer burden, while the pre-treatment L are considerably lower (1500 vs. 2400 cells/mm3), probably reflecting the immunosuppressive impacts of cancer. However, in the current study both L and N values dropped considerably below their pre-treatment values after RT (L: 1500 vs. 300–800 cells/mm3; N: 5600 vs. 3000–4800 cells/mm3). Consequently, the pre-treatment N/L also differed from those of the general population and was 4.1 compared to 1.7; the latter assessed from 413 healthy volunteers [33]. Even though the pre-treatment L, N and N/L were considerably different compared to the general population, they were worse predictors for OS or regional recurrence compared to LΔ, NΔ, and N/LΔ (p=0.001–0.47 vs. 1.2E-10 to 0.66). This motivates the hypothesis that the treatment itself negatively impacts long-term outcomes. Also, the absolute post-treatment WBC values were poorer predictors of treatment outcomes compared to the baseline-normalized WBCs. This could also explain differences with both [15] and [16] given that their WBC characteristics were not normalized to the corresponding pre-treatment values.
For regional recurrence, LΔ2mo was a final predictor (p=1E-10). None of the other three recurrence outcomes was associated with any of the nine WBC variables (p=0.11–0.51). Regional recurrence was not investigated in [15] or [16]. In [15] an association was found between N/L4mo and freedom from distant metastasis, but not with local recurrence or progression-free survival. Even though Tang et al [16] found that LNadir predicted event-free survival, overall survival, and loco-regional failure (p=0.03, 0.005, 0.05), other characteristics were more strongly associated with these outcomes (multivariate analysis: stage and chemotherapy; p<0.0001–0.001 and p<0.0001–0.0002). Also, worth noting is that the associated p-values for LNadir in [16] are considerably higher than those for the regional recurrence predictor LΔ2mo in the current study (p=0.005–0.05 vs. p=1E-10). However, direct comparisons between our results for regional recurrence and the recurrence results in [15] and [16] are challenging since no competing risk analyses were undertaken in the latter studies, thereby leading to considerably different treatment outcomes studied.
Several studies have established relationships between cardio-pulmonary dose and OS after RT for LA-NSCLC [3–9]. In an additional analysis, we found that the pericardium mean dose was associated with OS but not regional recurrence (p=0.004, 0.47). However, the magnitude of association between the pericardium mean dose and OS was weaker than for both WBC variables that predicted OS (NΔ4mo: p=0.00001; N/LΔ4mo: p=0.0003). Given that no investigated characteristics was found to be associated with any of the four WBC predictors leaves both causality and related mechanism as open questions. Further, we also assumed that the pericardium mean dose was a surrogate for WBCs and thereby immunosuppression, but with this assumption we cannot discriminate whether the treatment-induced decrease in the number of WBCs is mostly due to irradiation of circulating WBCs in the lymphatic system, blood system, or other organs, and/or if this is due to irradiation of the red bone marrow that in turn produces WBCs.
In summary, for LA-NSCLC patients treated with concurrent chemo-IMRT, this study has demonstrated a significant treatment-related WBC decrease within the first four months after completed treatment. This decrease was further associated with worse survival and regional recurrence. Even though that no association was established between this decrease and disease/patient/treatment characteristics, our findings still support RT delivery respecting regions key to immune function, e.g., blood-carrying structures and the bone marrow. Lastly, Pike et al [34] found that (metastatic) patients are more likely to develop severe lymphopenia after the initiation of treatment with immune checkpoint inhibitors. This further motivates active monitoring of WBCs in order to identify optimal patient-specific time points for which to administer immunotherapy in future trial designs that combine concurrent chemo-RT and immunotherapy.
Supplementary Material
Highlights.
The number of white blood cells (WBCs) significantly decreases within four months after concurrent chemo-RT of LA-NSCLC
The treatment-induced neutrophil decrease and the ratio between the decreased neutrophil and lymphocyte decreases is associated with worse overall survival
No investigated disease/patient/treatment characteristics was significantly associated with the WBC drop
Acknowledgements
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748 (AH, AJ, AR, AW, JOD, LL, MM, MT), as well as through NIH/NCI R01 CA198121 (JOD, MT)
AR reports relevant grants outside the submitted work from Varian Medical Systems, Boehringer Ingelheim, Pfizer, and AztraZeneca, personal fees from AztraZeneca, Merck, Research to Practice, and Cybrexa, as well as non-financial support from Philips/Elekta
Footnotes
Conflict of interest
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Antonia SJ, Villegas A, Daniel D, et al. Durvalumab after chemoradiotherapy in stage III non-small cell lung cancer. N Eng J Med 2017;377:1919–29 [DOI] [PubMed] [Google Scholar]
- [2].Antonia SJ, Villegas A, Daniel D, et al. Overall survival with Durvalumab after chemoradiotherapy in stage III NSCLC. N Eng J Med 2018;379:2342–50 [DOI] [PubMed] [Google Scholar]
- [3].Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non- small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Thor M, Deasy JO, Hu C, et al. The role of heart-related dose-volume metrics on overall survival in the RTOG 0617 clinical trial. Int J radiat Oncol Biol Phys 2018;102:S96 [Google Scholar]
- [5].Tucker SL, Liu A, Gomez D, et al. Impact of heart and lung dose on early survival in patients with non-small cell lung cancer treated with chemoradiation. Radiother Oncol 2016;119:495–500 [DOI] [PubMed] [Google Scholar]
- [6].McWilliam A, Kennedy J, Hodgson C, Vasquez Osario E, Faivre-Finn C, and van Herk M. Radiation dose to heart base linked with poorer survival in lung cancer patients. Eur J Cancer 2017;85:106–13 [DOI] [PubMed] [Google Scholar]
- [7].Speirs CK, DeWees TA, Rehman S, et al. Heart dose is an independent dosimetric predictor of overall survival in locally advanced non-small cell lung cancer. J Thorac Oncol 2017;12:293–301 [DOI] [PubMed] [Google Scholar]
- [8].Stam B, van der Bijl E, van Diessen J, et al. Heart dose associated with overall survival in locally advanced NSCLC patients treated with hypofractionated chemoradiotherapy. Radiother Oncol 2017;125:63–5 [DOI] [PubMed] [Google Scholar]
- [9].Vivekanandan S, Landau DB, Counsell N, Warren DR, Khwanda A, Rosen SD, et al. The Impact of Cardiac Radiation Dosimetry on Survival After Radiation Therapy for Non-Small Cell Lung Cancer. Int J Radiat Oncol 2017;99:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dess RT, Sun Y, Matuszak MM, et al. Cardiac events after radiation therapy: combined analysis of prospective multicenter trials for locally advanced non-small-cell lung cancer. J Clin Oncol 2017;35:1395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ning MS, Tang L, Gomez DR, et al. Incidence and Predictors of Pericardial Effusion After Chemoradiation Therapy for Locally Advanced Non-Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys 2017;99:70–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35:1387–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wang K, Pearlstein KA, Patchett ND, et al. Heart dosimetric analysis of three types of cardiac toxicity in patients treated on dose-escalation trials for Stage III non-small-cell lung cancer. Radiother Oncol 2017; 125:293–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Yegya-Raman N, Wang K, Kim S, et al. Dosimetric Predictors of Symptomatic Cardiac Events after Conventional-Dose Chemoradiation Therapy for Inoperable Non-Small Cell Lung Cancer. J Thorac Oncol 2018;13:1508–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Contreras JA, Lin AJ, Weiner A, et al. Cardiac dose is associated with immunosuppression and poor survival in locally advanced non-small cell lung cancer. Radiother Oncol 2017;128:498–504 [DOI] [PubMed] [Google Scholar]
- [16].Tang C, Liao D, Gomez D, et al. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys 2014;89:1084–91 [DOI] [PubMed] [Google Scholar]
- [17].Davaluri R, Jiang W, Fang P, et al. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J radiat Oncol Biol Phys 2017;99:128–35 [DOI] [PubMed] [Google Scholar]
- [18].Lin AJ, Rao YJ, Chin RI, et al. Post-operative radiation effects on lymphopenia, neutrophil to lymphocyte ratio, and clinical outcomes in palatine tonsil cancers. Oral Oncol 2018;86:1–7 [DOI] [PubMed] [Google Scholar]
- [19].Oberije C, De Ruysscher D, Houben R, et al. A validated prediction model for overall survival from stage III non-small cell lung cancer: Toward survival prediction for individual patients. Int J Radiat Oncol Biol Phys 2015;92:935–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Sonnick MA, Oro F, Yan B, et al. Identifying the optimal radiation dose in locally advanced non-small-cell lung cancer treated with definitive radiotherapy without concurrent chemotherapy. Clin Lung Cancer 2018;19:131–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chang JY, Kestin KK, Barriger RB, et al. ACR Appropriateness Criteria: nonsurgical treatment for non-small-cell lung cancer: good performance status/definitive intent. Oncology 2014;28:706–10 [PubMed] [Google Scholar]
- [22].Kass RE, and Raftery AE. Bayes factors. J Am Stat Assoc 1995;90:773–95 [Google Scholar]
- [23].Fowler JF. 21 years of biologically effective dose. Br J Radiol 2010;83:554–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wheatley MD, Gore EM, Bar Ad V, Robinson CG, and Bradley JD. Defining a novel cardiac contouring atlas for NSCLC using cadaveric anatomy. Int J Radiat Oncol Biol Phys 2014;90(Suppl):658 [Google Scholar]
- [25].Fine JP, and Gray RJ. A proportional hazards model for the sub distribution of a competing risk. J Am Stat Assoc 1999;94:496–509 [Google Scholar]
- [26].Kuk D, and Varadhan R. Model selection in competing risk regression. Stat Med 2013; 32:3077–88 [DOI] [PubMed] [Google Scholar]
- [27].Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: A secondary analysis of the NRG oncology RTOG 0617 randomized controlled trial. J Clin Oncol 2017;35:56–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. http://cancerstaging.org/references-tools/quickreferences/documents/lungmedium.pdf. [Google Scholar]
- [29].Lesterhuis WJ, Punt CJA, Hato SV, et al. Platinum-based drugs disrupt STAT6-mediated suppression of immune responses against cancer in humans and mice. J Clin Invest 2011;121:3100–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Latchman Y, Wodd CR, Chernova T, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immun 2001;2:261–8 [DOI] [PubMed] [Google Scholar]
- [31].Tang C, Lee MS, Gomez D, et al. Effects of chemotherapy regimen and radiation modality on hematologic toxicities in patients receiving definitive platinum-based doublet chemoradiation for non-small cell lung cancer. Am J Clin Oncol 2017;40:625–30 [DOI] [PubMed] [Google Scholar]
- [32].Hollowell JG, van Assendelft OW, Gunter EW, et al. Hematological and iron-related analytes – reference data for persons aged 1 year and over: United States, 1988–94. Vital Health Stat 2005;247:1–156 [PubMed] [Google Scholar]
- [33].Forget P, Khalifa C, Defour JP, Latinne D, Van Pel MC, and De Kock M. What is the normal value of the neutrophil-to-lymphocyte ratio? BMC Res Notes 2017;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.