Abstract
Lynch syndrome is a heritable cancer syndrome caused by a heterozygous germline mutation in DNA mismatch repair (MMR) genes. MMR deficient (dMMR) tumors are particularly sensitive to immune checkpoint inhibitors, an effect attributed to the higher mutation rate in these cancers. However, ~15–30% of patients with dMMR cancers fail to respond to immunotherapy. We now report three patients with Lynch syndrome who each had two primary malignancies: one with dMMR and high tumor mutation burden (TMB) and one with dMMR but, unexpectedly, low TMB. Two of these patients received immunotherapy for their low TMB tumors and did not respond. We have found that not all Lynch-associated dMMR tumors have high TMB and propose that tumors with dMMR and TMB discordance may be resistant to immunotherapy. The possibility of dMMR/TMB discordance should be considered, particularly in less typical Lynch cancers, where TMB evaluation could guide the use of immune checkpoint inhibitors.
Lynch syndrome, also known as hereditary nonpolyposis colorectal cancer (HNPCC), is a heritable cancer syndrome predisposing to colorectal as well as other cancers, including endometrial, gastric, and ovarian.1–3 Lynch syndrome is caused by an autosomal dominant heterozygous germline mutation in the DNA mismatch repair (MMR) genes. MMR deficiency (dMMR) increases the likelihood of acquiring somatic genetic mutations, particularly in short repetitive sequences, leading to varying lengths of these regions, termed microsatellite instability.1,4 However, dMMR is a double-edged sword, because although it predisposes to malignancy, it leads to cancers with more mutations, particularly frameshift alterations, which are associated with “non-self” immunogenic-antigens, high lymphocyte infiltration and immune response.5,6 Due to the high immunogenicity of these tumors, microsatellite instability-high (MSI-H) or dMMR cancers have been shown to respond well to immunotherapy, in contrast to their microsatellite stable (MSS) counterparts.7–10 However, despite the promising response of immunotherapy in dMMR cancers, ~15–30% of patients fail to derive significant benefit with single or combination immunotherapy.7,8
Here, we present three cases of patients with Lynch syndrome who developed a dMMR MSI-H cancer with high TMB (tumor mutation burden), and a second primary malignancy that was dMMR but with low TMB. All tumors were assessed utilizing a next-generation sequencing (NGS) panel (MSK-IMPACT) comprised of >300 cancer-associated genes.11 MSI status was evaluated with the MSIsensor algorithm, where MSIsensor scores ≥10 defines MSI-H status.12 This cutoff for MSI status has been validated against MSI PCR and/or MMR immunohistochemistry (IHC) performed in 138 colorectal cancers (114 MSS and 24 MSI-H) and 40 uterine endometroid cancers (25 MSS, 15 MSI-H) with a concordance of 99.4%.
The first patient is a 43-year-old man who initially presented with multiple primary colon masses. At total colectomy, the most advanced lesion was stage IIB. Pathology was notable for tumor-infiltrating lymphocytes (TILs) and the absence of MLH1 and PMS2 staining on IHC (Figure 1A). Germline genetic testing revealed a deleterious MLH1 likely pathogenic variant confirming Lynch syndrome, and tumor genomic analysis showed an MSI-H phenotype with MSIsensor score of 40.67 and TMB of 54.4 mutations per megabase (mt/Mb). This tumor had driver mutations in APC and TP53, a genomic profile common for colorectal tumors (Figure 1B). Three years later, imaging showed a pancreatic tail mass consistent with a new primary, which was completely resected. Surgical pathology on the resected mass demonstrated a poorly differentiated neuroendocrine carcinoma (Ki-67 60%). IHC confirmed loss of MLH1 and PMS2 in the neuroendocrine carcinoma (Figure 1A). Surprisingly despite his Lynch status, NGS revealed a MSS tumor, with MSIsensor score of 1.8, and low TMB (9.7 mt/Mb) (Figure 1B). Unlike his colon tumor, increased TILs were not seen in the neuroendocrine carcinoma. This second tumor also had a completely different set of somatic mutations, with a truncating RB1 driver mutation consistent with neuroendocrine carcinoma. Unfortunately within 6 months after resection, he was found to have multiple hypervascular metastatic lesions in the liver, consistent with his pancreatic primary. He was started on pembrolizumab given the dMMR status of his primary tumor. His initial scan after 3 cycles showed a mixed response; however, a subsequent scan showed definitive progression of disease with the development of new lesions.
Figure 1.
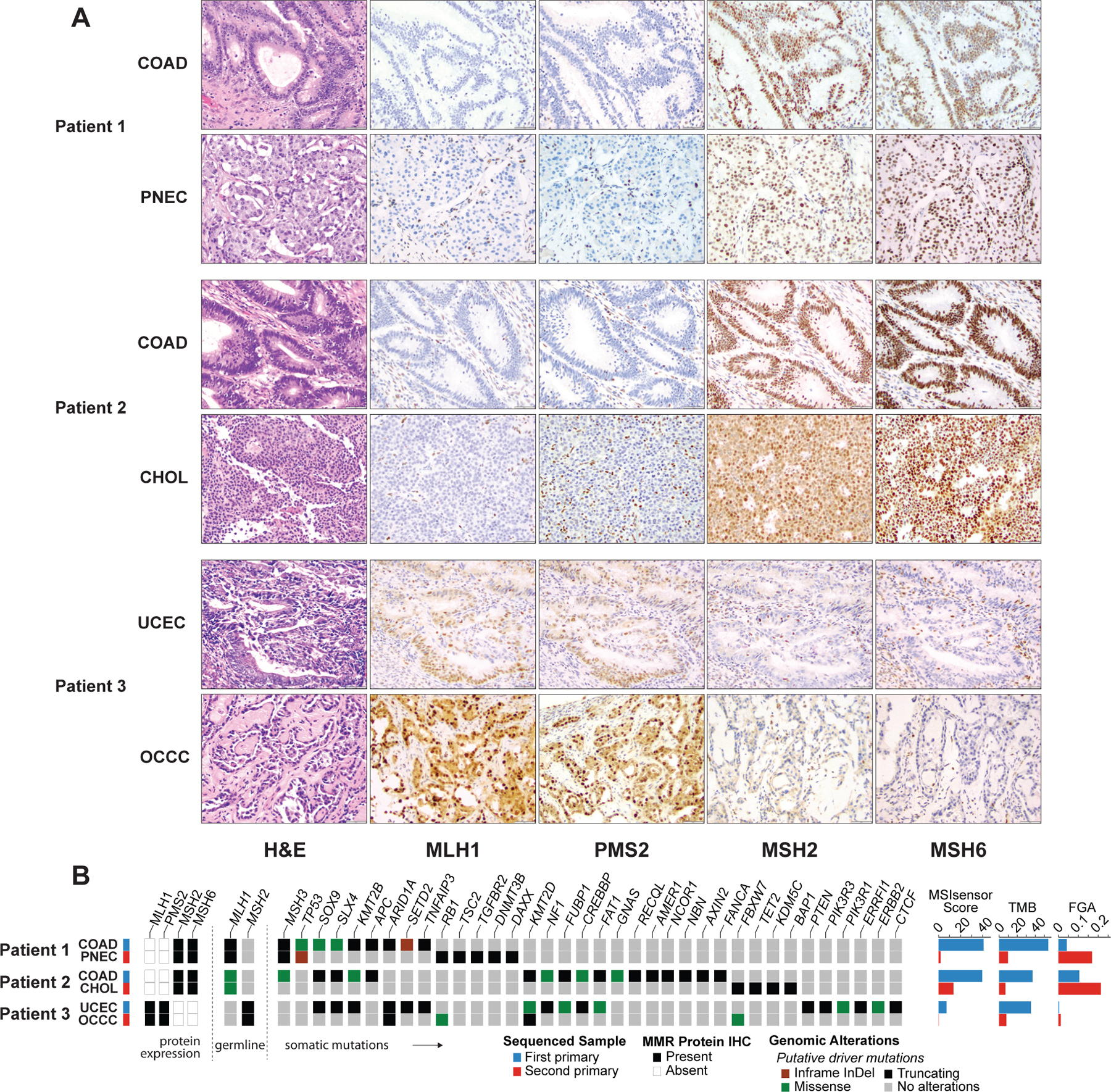
Comparison of histopathological and genomic features across paired primary deficient mismatch repair (dMMR) tumors. (A) Hematoxylin and eosin (H&E) and immunohistochemical (IHC) stains of each tumor pair. In patient 1 and 2, IHC showed normal staining for MSH2 and MSH6 and loss of staining for MLH1and PMS2 in both tumors. In patient 3, IHC showed normal staining for MLH1 and PMS2 and loss of staining for MSH2 and MSH6 in both tumors. (B) Spectrum of oncogenic mutations and differences in MSIsensor scores, tumor mutation burden (TMB), and fraction of genome altered (FGA), as well as depiction of MMR protein status from immunohistochemistry (IHC). The paired tumors from the three patients appear genomically distinct harboring few shared oncogenic mutations. The patients’ secondary primary malignancies all have lower TMBs and MSIsensor scores.
COAD, colorectal adenocarcinoma; PNEC, pancreatic neuroendocrine carcinoma; UCEC, uterine corpus endometrial carcinoma; OCCC, ovarian clear cell carcinoma
The second patient is a 54-year-old man with two resected colon primaries that were dMMR with loss of MLH1/PMS2 on IHC (Figure 1A), and germline NGS confirming MLH1 likely pathogenic variant. He later presented with a new liver lesion on surveillance imaging. Before it was clear that he had a second primary, the patient received ipilimumab and nivolumab for presumed liver metastasis from colon cancer, with clear progression (the mass grew from 5.7 × 5.4 cm to 9.0 × 8.4 cm) after 3 cycles of therapy. Biopsy of the mass showed a morphologically different tumor from the prior colon cancer that was poorly differentiated, suggestive of a new GI primary. Subsequent resection of the mass showed a cholangiocarcinoma with positive staining for albumin in situ hybridization. The cholangiocarcinoma was MMR deficient (absent MLH1/PMS2) and MSI-H with MSIsensor score of 13.51 but low TMB of 7 mt/Mb (Figure 1B). The colorectal tumor had driver mutations in APC, SOX9, and NF1. No somatic mutations were shared between the two tumors from this patient. Despite treatment with ipilimumab and nivolumab, no lymphocytic infiltrate was seen in the resected tumor.
The third patient is a 40-year-old woman with MSH2 pathologic germline mutation who had synchronous primary endometrial and ovarian cancers found on screening. Both tumors demonstrated loss of MSH2/MSH6 by IHC (Figure 1A). The endometrial adenocarcinoma was MSI-Intermediate (MSIsensor 7.16) with high TMB of 35.1 mt/Mb. However, sequencing of her ovarian clear cell carcinoma showed an MSS phenotype (MSIsensor 0.12) and low TMB (7.9 mt/Mb) (Figure 1B). Hematoxylin and eosin (H&E) stains identified increased TILs in the primary endometrial adenocarcinoma, as compared to the ovarian clear cell carcinoma without TILs. Consistent with what is expected in these cancer types, both tumors from this patient harbored an ARID1A mutation, however these mutations were at different positions and private to each of their respective tumors.
We are reporting 3 cases of Lynch patients who each developed two primary tumors with confirmed dMMR status. Unexpectedly, despite confirmed dMMR within each tumor pair, one tumor had an expected high TMB, but one had a lower TMB. In agreement with their TMB status, the two patients treated with immunotherapy for their low TMB tumors did not respond. Using FACETs, an allele-specific copy number algorithm, we assessed ploidy and loss of heterozygosity of MLH1 in the paired cancers from the first two patients.13 Both paired cases demonstrated loss of the normal MMR allele. However, the germline MLH1 mutant allele was enriched in the first TMB-high cancer compared to the second cancer.
While it has been shown that Lynch patients can develop sporadic cancers with retained MMR machinery, Lynch-associated tumors with confirmed dMMR but with discordantly low TMB have not been well described. It is known that some MMR variants are associated with dMMR on IHC but not MSI-H – but in this case the same germline MMR variant in each tumor pair lead to a MSI-H and TMB-high phenotype in one tumor, but a TMB-low phenotype in the other tumor14. Consistent with our data, Georgiadis et al reported that while TMB and MSI status were highly correlated, TMB and MSI discordance did occur, and 6 of 7 MSI-H tumors with progressive disease on immunotherapy were TMB-low.15 Similarly, Schrock et al found that within MSI-H colorectal cancer, TMB was predictive of response to immunotherapy.16
We acknowledge that the immunogenic response in Lynch tumors is complicated, as Lynch syndrome polyps with low mutational rate still illicit an immune response.17 In the three patients analyzed here, high levels of tumor infiltrating lymphocytes were not detected in the TMB low tumors, in contrast to the high TMB tumors. Additionally, in patient 2, no immune activation was seen after dual immune checkpoint blockade.
These cases reveal an important practical clinical point; patients with Lynch syndrome can develop dMMR tumors with low TMB. While we cannot draw general conclusions from a three-person case series, these results, taken with previous evidence showing that TMB associates with response to immunotherapy within MSI-H tumors, suggest that dMMR tumors with low TMB would not respond to immunotherapy.15,16 We propose that dMMR/TMB discordance should be considered in patients with Lynch syndrome, particularly in those who develop secondary, less typical Lynch cancers, and TMB should be evaluated in these tumors to inform the use of immune checkpoint inhibitors.
Acknowledgments
Supported by the National Institutes of Health Cancer Center Core Grant P30 CA 008748 and ACS Postdoctoral Fellowship 134065-PF-19-125-01-CSM (to A.A.B.).
Footnotes
The authors declare no conflicts of interest.
References:
- 1.Sinicrope FA. Lynch Syndrome–Associated Colorectal Cancer. Solomon CG, ed. N Engl J Med. 2018;379(8):764–773. doi: 10.1056/NEJMcp1714533 [DOI] [PubMed] [Google Scholar]
- 2.Watson P, Vasen HFA, Mecklin J-P, et al. The risk of extra-colonic, extra-endometrial cancer in the Lynch syndrome. Int J Cancer. 2008;123(2):444–449. doi: 10.1002/ijc.23508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Latham A, Srinivasan P, Kemel Y, et al. Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer. J Clin Oncol. 2019;37(4):286–295. doi: 10.1200/JCO.18.00283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lynch HT, Snyder CL, Shaw TG, Heinen CD, Hitchins MP. Milestones of Lynch syndrome: 1895–2015. Nat Rev Cancer. 2015;15(3):181–194. doi: 10.1038/nrc3878 [DOI] [PubMed] [Google Scholar]
- 5.Buckowitz A, Knaebel HP, Benner A, et al. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer. 2005;92(9):1746–1753. doi: 10.1038/sj.bjc.6602534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schwitalle Y, Kloor M, Eiermann S, et al. Immune Response Against Frameshift-Induced Neopeptides in HNPCC Patients and Healthy HNPCC Mutation Carriers. Gastroenterology. 2008;134(4):988–997. doi: 10.1053/j.gastro.2008.01.015 [DOI] [PubMed] [Google Scholar]
- 7.Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med. 2015;372(26):2509–2520. doi: 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773–779. doi: 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 9.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair–deficient cancer: Results from the phase II KEYNOTE-158 study. J Clin Oncol. 2020;38(1):1–10. doi: 10.1200/JCO.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (80- ). 2017;357(6349):409–413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng DT, Mitchell TN, Zehir A, et al. Memorial sloan kettering-integrated mutation profiling of actionable cancer targets (MSK-IMPACT): A hybridization capture-based next-generation sequencing clinical assay for solid tumor molecular oncology. J Mol Diagnostics. 2015;17(3):251–264. doi: 10.1016/j.jmoldx.2014.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Middha S, Zhang L, Nafa K, et al. Reliable Pan-Cancer Microsatellite Instability Assessment by Using Targeted Next-Generation Sequencing Data. JCO Precis Oncol. 2017;2017(1):1–17. doi: 10.1200/po.17.00084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen R, Seshan VE. FACETS vignettes. Nucleic Acids Res. 2016;44(16):1–6. doi: 10.1093/nar/gkw520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerretelli G, Ager A, Arends MJ, Frayling IM. Molecular pathology of Lynch syndrome. J Pathol. 2020;250(5):518–531. doi: 10.1002/path.5422 [DOI] [PubMed] [Google Scholar]
- 15.Georgiadis A, Durham JN, Keefer LA, et al. Noninvasive detection of microsatellite instabilit and high tumor mutation burden in cancer patients treated with PD-1 blockade. Clin Cancer Res. 2019;25(23):7024–7034. doi: 10.1158/1078-0432.CCR-19-1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schrock AB, Ouyang C, Sandhu J, et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol. 2019;30(7):1096–1103. doi: 10.1093/annonc/mdz134 [DOI] [PubMed] [Google Scholar]
- 17.Chang K, Taggart MW, Reyes-Uribe L, et al. Immune profiling of premalignant lesions in patients with Lynch syndrome. In: JAMA Oncology. Vol 4. American Medical Association; 2018:1085–1092. doi: 10.1001/jamaoncol.2018.1482 [DOI] [PMC free article] [PubMed] [Google Scholar]


