Abstract
Background
Peritoneal malignancies include primary and metastatic cancer of the peritoneal cavity. The most common origin for peritoneal metastasis is ovarian, gastric, and colorectal cancers. Irrespective of the origin, peritoneal metastases represent the advanced disease and are associated with poor long-term outcomes. The minimally invasive approach of pressurized intraperitoneal aerosol chemotherapy (PIPAC) allows repeated applications and objective assessment of tumor response by comparing histological samples. This study aimed to investigate the initial experience with PIPAC in the Baltic region.
Methods
All patients who underwent PIPAC at Vilnius University Hospital Santaros Klinikos between 2015 and 2020 were included in this retrospective study. The primary outcome of the study was overall survival (OS) in patients with peritoneal carcinomatosis treated by PIPAC. The secondary outcomes included postoperative morbidity; peritoneal carcinomatosis index (PCI) and ascites reduction after treatment by PIPAC.
Results
In total, 15 patients underwent 34 PIPAC procedures. PIPAC-related intraoperative and postoperative morbidity occurred in 3 (8.8%) of 34 procedures. Following PIPAC, the median PCI decreased from 8 (4; 15) to 5 (1; 16) in GC patients, although, the difference failed for significance, p = 0.581. In OC patients, PCI after PIPAC remained stable. Median overall survival after PIPAC procedure was 25 (95% CI 5–44) months. Ovarian cancer patients (22; 95% CI 12–44 months) had significantly higher OS, compared to gastric cancer patients (8; 95% CI 4–16 months), p = 0.018.
Conclusions
PIPAC is safe and feasible for patients with gastric and ovarian cancers peritoneal metastases.
Background
Peritoneal malignancies include primary and metastatic cancer of the peritoneal cavity. The most common origin for peritoneal metastasis is ovarian, gastric, and colorectal cancers [1]. Irrespective of the origin, peritoneal metastases represent the advanced disease and are associated with poor long-term outcomes [2]. Currently, systemic palliative chemotherapy remains the standard treatment for these patients, although the efficacy of such treatment is very limited. One of the limiting factors is the plasma-peritoneal barrier, which restricts the movement of the systemic chemotherapeutic drug to reach the target in the peritoneum [3]. To overcome this issue, the intraperitoneal application of chemotherapy was proposed [4]. Further, intraperitoneal chemotherapy is associated with reduced toxicity because of lower systemic concentrations [4]. Considering these advantages, hyperthermic intraperitoneal chemotherapy (HIPEC), usually combined with cytoreductive surgery, gained attention for peritoneal malignancies. Although, a series of recent studies (PRODIGE7, COLOPEC, CYTO-CHIP, PROFILOCHIP) failed to demonstrate the oncological benefit of the HIPEC [5–8]. Another available strategy for intraperitoneal chemotherapy application is pressurized intraperitoneal aerosol chemotherapy (PIPAC). The rationale behind PIPAC includes (1) optimization of drug distribution by applying an aerosol rather than a liquid solution; (2) applying increased intraperitoneal hydrostatic pressure to increase drug penetration to the target; and (3) limiting blood outflow during drug application [9, 10]. The minimally invasive approach of PIPAC allows repeated applications and objective assessment of tumor response by comparing histological samples [10, 11]. However, PIPAC remains an experimental treatment option for patients with peritoneal malignancy. Thus, this study aimed to investigate the initial experience with PIPAC in the Baltic region.
Materials and methods
Ethics
Vilnius Regional Biomedical Research Ethics Committee approval (No. 2020/11-1279-761) was obtained before this study was conducted. The waiver of informed consent was given by the authority. The study was conducted according to the Declaration of Helsinki.
Patients and data collection
All patients who underwent Pressurized Intraperitoneal Aerosol Chemotherapy (PIPAC) at Vilnius University Hospital Santaros Klinikos between 2015 and 2020 were included in this retrospective study.
Data on patient characteristics were extracted from the prospectively collected institutional electronic database. They included clinicopathologic characteristics (age; gender; history of previous cancer treatment; origin, number, and size of metastases; peritoneal carcinomatosis index (PCI) score at every PIPAC procedure) and treatment-related characteristics (length of surgery; blood loss; chemotherapeutic drugs; postoperative complications by Clavien-Dindo classification).
Technique of procedure
Indications for the PIPAC procedure were peritoneal carcinomatosis ± refractory ascites. Potentially eligible patients willing to receive experimental treatment by PIPAC were discussed at multidisciplinary team meetings and the decision for such treatment was individual in every case.
The procedures were performed following the protocol adjusted to our infrastructure [12].
All operations were performed under general anesthesia; antibiotic prophylaxis with a single dose of cefazoline 1.0 g IV was administered at the time of induction of anesthesia. A nasogastric tube and urinary drainage were not used unless there was a specific indication for their use.
After insufflation of a 12 mmHg CO2 open access capnoperitoneum was made, two balloon trocars measuring 5 and 10 mm were inserted into the abdominal wall. The preferred sites of insertion were the supraumbilical incision and the left iliac fossa along the same line.
An evaluation of the PCI was done. Biopsies were performed from four different regions of the peritoneal cavity, and ascitic fluid was completely drained and sent for cytological examination.
The 9-mm microinjection pump was connected to an intravenous high-pressure injector and inserted into the abdomen through the 10-mm access port.
A 5-mm camera was inserted through the other port keeping the tip of the Capnopen in view. A safety checklist was performed before the procedure ensuring there is no gas leakage.
One hundred fifty milliliters of NaCl 0.9% containing cisplatin 7.5 mg/m2 body surface and doxorubicin 1.5 mg/m2 body surface area was injected through the Capnopen at a pressure of 200 psi at the rate of 0.5 ml/s to generate the aerosol. The intraabdominal pressure throughout the procedure was maintained at 12 mmHg [12].
The therapeutic capnoperitoneum was then maintained for 30 min. Then, the chemotherapy aerosol was evacuated via a separate hospital air-waste system. Finally, trocars were retracted and laparoscopy was ended.
Patients were allowed oral liquids on the same day and discharged on the following day in the absence of adverse events.
Following procedures were repeated at 6 weeks intervals.
Study outcomes
The primary outcome of the study was overall survival (OS) in patients with peritoneal carcinomatosis treated by PIPAC. OS was defined as the time from the first PIPAC procedure to death. The secondary outcomes included postoperative morbidity; PCI and ascites reduction after treatment by PIPAC. Data on survival and date of death were collected from the Lithuanian National Cancer Registry.
Statistical analysis
All statistical analyses were conducted using the statistical program SPSS 24.0 (SPSS, Chicago, IL, USA). Continuous variables are presented as median with an interquartile range. Categorical variables are shown as proportions. Continuous variables were compared by a Mann-Whitney U test, and categorical variables by the Pearson’s chi-square or Fisher exact test, as appropriate. Related samples were compared by Wilcoxon signed-rank test or McNemar test, as appropriate. Overall rates were analyzed by the Kaplan–Meier method and compared by the log-rank test. Statistical significance was considered when a p value < 0.05 was achieved.
Results
Baseline characteristics
In total, 15 patients underwent 34 PIPAC procedures. The baseline clinicopathologic characteristics are shown in Table 1. All patients received systemic chemotherapy before PIPAC. Different regimens were used for ovarian cancer (OC) and gastric cancer (GC) patients. All OC patients (6/6; 100%) received platinum-based systemic chemotherapy, specifically paclitaxel, and carboplatin. In GC groups, patients received different schemes including XELOX, EOX, FOLFIRI, and FLOT.
Table 1.
Baseline clinicopathologic characteristics of patients who received PIPAC
| Malignancy; n (%) | Gastric cancer | 9 (60.0%) |
| Ovarian cancer | 6 (40%) | |
| Median PCI score (Q1; Q3); | Before PIPAC | 8 (4; 15) |
| After PIPAC | 5 (1; 16) | |
| Sex; n (%) | Female (n; %) | 11 (73.3%) |
| Male (n; %) | 4 (26.7%) | |
| Median age (Q1; Q3); years | 58 (51; 68) | |
| Median hospitalization (Q1; Q3); days | 5 (3; 6) | |
| Median BMI (Q1; Q3) | 25 (20; 30) | |
| History of radical surgery for primary tumor; (n; %) | Yes | 8 (53.3%) |
| No | 7 (46.7%) | |
| Median CA125 level (Q1; Q3); kIU/l | 103 (15; 351) | |
| Median CEA level (Q1; Q3); ng/l | 1.4 (0.5; 9.6) | |
| Median CA19.9 level (Q1; Q3); ng/l | 12.3 (6.9; 75.9) | |
| Number of PIPAC procedures | 1 | 5 (33.3%) |
| 2 | 2 (13.3%) | |
| 3–4 | 8 (53.4%) | |
|
Median operation time (Q1; Q3); min minutes |
115 (110; 133) | |
PIPAC procedure characteristics
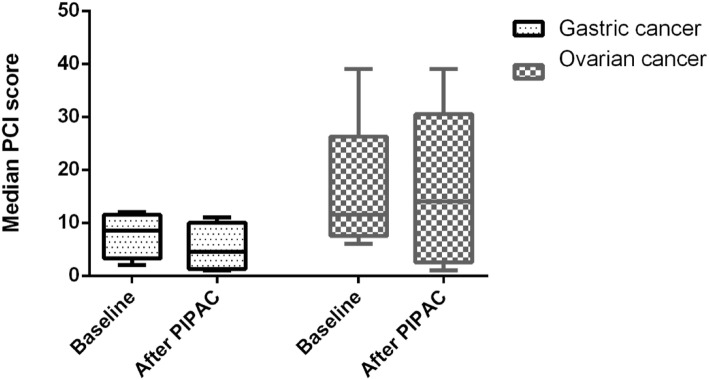
One, two, or three and more PIPAC procedures were performed for 5 (33.3%), 2 (13.3%), and 8 (53.4%) patients, respectively. Following PIPAC, the median PCI decreased from 8 (4; 15) to 5 (1; 16), although, the difference failed for significance, p = 0.999.PIPAC stabilized the PCI score in both—patients with GC and OC (Fig. 1). One of the indications for palliative PIPAC is refractory ascites. Among 10 patients who received at least 2 PIPACs, 7 had ascites at baseline with a median volume of 300 ml (Q1 100; Q3 2200). After PIPAC, 2 (28.6%) of these patients had no ascites and the median volume decreased to 50 ml (Q1 35; Q3 4050); however, the difference was not significant, p = 0.500. PIPAC-related intraoperative and postoperative morbidity occurred in 3 (8.8%) of 34 procedures. One patient developed severe postoperative neutropenia (2.8%) after PIPAC (Clavien-Dindo score 2); one patient (2.8%) developed intraabdominal abscess postoperatively, which was managed with ultrasound drainage (Clavien-Dindo score 3a); and in one case (2.8%) bowel was perforated during initial port placement due to extensive adhesions, it was repaired intraoperatively, and patient’s further recovery was uneventful.
Fig. 1.
Median peritoneal carcinomatosis index in patients who received PIPAC for gastric and ovarian cancer peritoneal metastases
Long-term outcomes
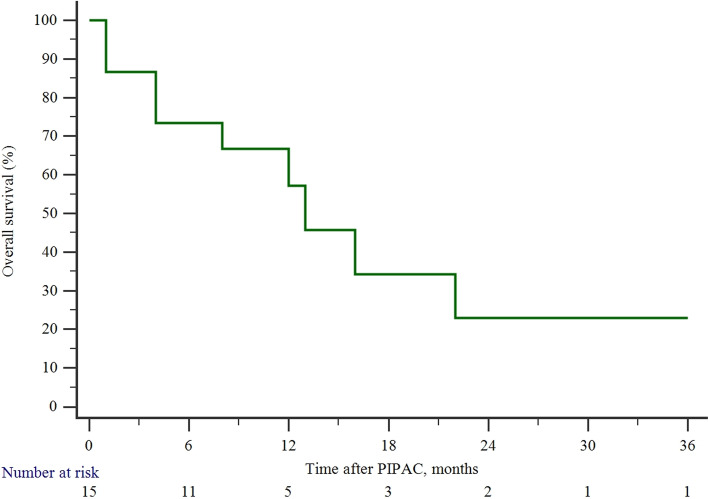
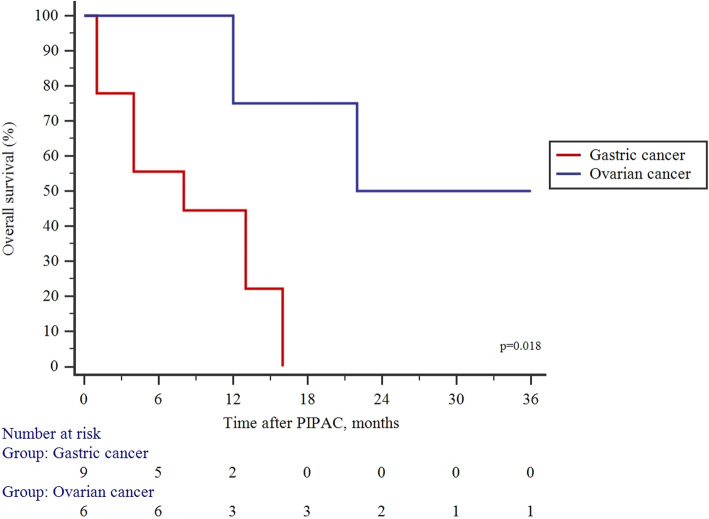
The median time to follow-up after PIPAC was 10 (Q1:4; Q3: 16) months and the median survival by Kaplan–Meier analysis was 25 (95% CI 5–44) months (Fig. 2). OC patients (22; 95% CI 12–44 months) had significantly higher OScompared to GC patients (8; 95% CI 4–16 months), p = 0.018 (Fig. 3).
Fig. 2.
Overall survival in the entire cohort of study patients who received PIPAC for peritoneal metastases
Fig. 3.
Comparison of overall survival between gastric and ovarian cancer patients treated by PIPAC
Discussion
The present study demonstrated the initial results of the first PIPAC program in the Baltic region country—Lithuania. PIPAC was safe and feasible for patients with gastric and ovarian cancer peritoneal metastases. The repeated PIPAC procedures were performed for 66.7% of patients, and postoperative complications occurred after 8.8% of procedures, with no postoperative mortality. PIPAC reduced the mean PCI in gastric cancer patients and stabilized the disease for ovarian cancer patients.
PIPAC is a new and emerging technique for peritoneal metastases of various cancers. Some evidence shows that it is one of the best methods to manage the burden of advanced intraperitoneal cancer by reducing or halting disease progression and improving quality of life [13]. Further, the minimally invasive approach is one of the major advantages of the PIPAC procedure, as it is associated with a low intraoperative and postoperative morbidity ranging between 0 and 11% in previous and our study [1]. A typical candidate for PIPAC suffers from miliary peritoneal carcinomatosis, which is considered an incurable disease. Although, PIPAC can stabilize the progression of peritoneal carcinomatosis and sometimes even downgrade the disease to the level, where potentially curative cytoreductive surgery with or without HIPEC is feasible [14]. In the present study, we found stabilization of the disease in ovarian cancer patients and regression of the PCI in gastric cancer patients, although the difference failed for significance. GC patients with a limited PCI may benefit from curative cytoreductive surgery + HIPEC as shown by a recent meta-analysis [15]. Thus, because of PCI score reduction after PIPAC in GC patients, it may be considered as a conversion therapy from unresectable to potentially resectable disease.
A second most common indication for PIPAC is a refractory accumulation of ascites, which impairs quality of life [16]. It has been reported that PIPAC is an excellent method to control ascites, thus it improves the quality of life at the final stages of the disease [17]. In our study, we have found that 28.6% of patients suffering ascites resolved after PIPAC. Further, the median volume of ascites decreased substantially, although the difference failed for significance.
The highest effect of PIPAC is achieved when procedures can be repeated. Alyami et al. reported a clinical response rate of 50–90%, in cases where 3 PIPACs were utilized [1]. Our results show that repeated PIPAC procedures are feasible in approximately two-thirds of patients. Although, the utilization of repeated PIPACs depends on the origin of peritoneal metastases, as three cycles were feasible for 83.3% with OC and only one-third of patients with GC. Such differences may be explained by the different severity of the disease by different origin peritoneal metastases [1]. The different origins of metastases are also, associated with different prognoses. Grass et al. reported that median survival following PIPAC ranges between 11–14.1 and 13.4–15.4 months, for OC and GC patients, respectively [11]. In contrast, our study demonstrated a longer survival for OC patients. The unclarities on the subgroup of patients who benefit the most from PIPAC have to be elucidated in future clinical studies.
A minimally invasive approach associated with low morbidity and potential therapeutic effect for incurable disease makes PIPAC an attractive novel treatment strategy for peritoneal metastases. Thus, there is a growing number of clinical studies investigating PIPAC for various types of cancers and various combinations with systemic therapy or even PIPAC as neoadjuvant therapy [17–21]. Furthermore, some novel anti-tumorigenic agents, such as taurolidine are under investigation for PIPAC [22]. These novel agents may increase the effectiveness and thus the attractiveness of PIPAC. Although to date, there is a lack of robust evidence from prospective randomized studies on the efficacy of PIPAC, thus it still has to be considered as an experimental treatment option.
Our study has some limitations. The retrospective design and small sample size are the major limitations that could lead to the selection bias and underestimation of the positive and negative effects of PIPAC for gastric and ovarian cancer patients with peritoneal metastases. Therefore, the findings of the current study must be validated with larger cohorts.
Conclusions
The present study demonstrated the initial results of the first PIPAC program in the Baltic region country—Lithuania. PIPAC was safe and feasible for patients with gastric and ovarian cancer peritoneal metastases.
Acknowledgements
Not applicable
Abbreviations
- OC
Ovarian cancer
- GC
Gastric cancer
- PIPAC
Pressurized intraperitoneal aerosol chemotherapy
- PCI
Peritoneal carcinomatosis index
- OS
Overall survival
- HIPEC
Hyperthermic intraperitoneal chemotherapy
Authors’ contributions
RR contributed to data collection and analysis and was a major contributor in writing the manuscript. AB contributed to analyzing the data and writing the manuscript. ML, JJ, and MP contributed to writing and reviewing the manuscript. KS contributed to data analysis, manuscript writing, and review of the manuscript. All authors read and approved the final manuscript.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Vilnius Regional Biomedical Research Ethics Committee approval (No. 2020/11-1279-761) was obtained before this study was conducted. The study was conducted according to the Declaration of Helsinki. Informed consent was waived by the Vilnius Regional Biomedical Research Ethics Committee that approved the study.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alyami M, Hübner M, Grass F, Bakrin N, Villeneuve L, Laplace N, Passot G, Glehen O, Kepenekian V. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol. 2019;20(7):e368–e377. doi: 10.1016/S1470-2045(19)30318-3. [DOI] [PubMed] [Google Scholar]
- 2.Solass W, Kerb R, Mürdter T, Giger-Pabst U, Strumberg D, Tempfer C, Zieren J, Schwab M, Reymond MA. Intraperitoneal chemotherapy of peritoneal carcinomatosis using pressurized aerosol as an alternative to liquid solution: first evidence for efficacy. Ann Surg Oncol. 2014;21(2):553–559. doi: 10.1245/s10434-013-3213-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg PK, Brandl A, Rau B. Hyperthermic intraperitoneal chemotherapy - fading perspective in the light of modern systemic chemotherapy? Visc Med. 2018;34(6):412–416. doi: 10.1159/000493493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Solass W, Giger-Pabst U, Zieren J, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC): occupational health and safety aspects. Ann Surg Oncol. 2013;20(11):3504–3511. doi: 10.1245/s10434-013-3039-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quénet F, Elias D, Roca L, Goéré D, Ghouti L, Pocard M, Facy O, Arvieux C, Lorimier G, Pezet D, Marchal F, Loi V, Meeus P, Juzyna B, de Forges H, Paineau J, Glehen O, MARIANI P, BRIGAND C, BEREDER JM, MSIKA S, PORTIER G, RAT P. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256–266. doi: 10.1016/S1470-2045(20)30599-4. [DOI] [PubMed] [Google Scholar]
- 6.Klaver CEL, Wisselink DD, Punt CJA, Snaebjornsson P, Crezee J, Aalbers AGJ, Brandt A, Bremers AJA, Burger JWA, Fabry HFJ, Ferenschild F, Festen S, van Grevenstein WMU, Hemmer PHJ, de Hingh IHJT, Kok NFM, Musters GD, Schoonderwoerd L, Tuynman JB, van de Ven AWH, van Westreenen HL, Wiezer MJ, Zimmerman DDE, van Zweeden AA, Dijkgraaf MGW, Tanis PJ, Andeweg CS, Bastiaenen VP, Bemelman WA, van der Bilt JDW, Bloemen J, den Boer FC, Boerma D, ten Bokkel Huinink D, Brokelman WJA, Cense HA, Consten ECJ, Creemers GJ, Crolla RMPH, Dekker JWT, Demelinne J, van Det MJ, van Diepen KK, Diepeveen M, van Duyn EB, van den Ende ED, Evers P, van Geloven AAW, van der Harst E, Heemskerk J, Heikens JT, Hess DA, Inberg B, Jansen J, Kloppenberg FWH, Kootstra TJM, Kortekaas RTJ, Los M, Madsen EVE, van der Mijle HCJ, Mol L, Neijenhuis PA, Nienhuijs SW, van den Nieuwenhof L, Peeters KCMJ, Polle SW, Pon J, Poortman P, Radema SA, van Ramshorst B, de Reuver PR, Rovers KP, Schmitz RF, Sluiter N, Sommeijer DW, Sonneveld DJA, van Sprundel TC, Veltkamp SC, Vermaas M, Verwaal VJ, Wassenaar E, Wegdam JA, de Wilt JHW, Westerterp M, Wit F, Witkamp AJ, van Woensdregt K, van der Zaag ES, Zournas M. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019;4(10):761–770. doi: 10.1016/S2468-1253(19)30239-0. [DOI] [PubMed] [Google Scholar]
- 7.Bonnot P-E, Piessen G, Kepenekian V, Decullier E, Pocard M, Meunier B, Bereder JM, Abboud K, Marchal F, Quenet F, Goere D, Msika S, Arvieux C, Pirro N, Wernert R, Rat P, Gagnière J, Lefevre JH, Courvoisier T, Kianmanesh R, Vaudoyer D, Rivoire M, Meeus P, Passot G, Glehen O, on behalf of the FREGAT and BIG-RENAPE Networks Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (CYTO-CHIP study): a propensity score analysis. J Clin Oncol Off J Am Soc Clin Oncol. 2019;37(23):2028–2040. doi: 10.1200/JCO.18.01688. [DOI] [PubMed] [Google Scholar]
- 8.Moran BJ. PROPHYLOCHIP: no benefit of second-look surgery plus HIPEC for colorectal peritoneal metastases. Lancet Oncol. 2020;21(9):1124–1125. doi: 10.1016/S1470-2045(20)30338-7. [DOI] [PubMed] [Google Scholar]
- 9.Graversen M, Detlefsen S, Bjerregaard JK, Fristrup CW, Pfeiffer P, Mortensen MB. Prospective, single-center implementation and response evaluation of pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis. Ther Adv Med Oncol. 2018;10:1758835918777036. doi: 10.1177/1758835918777036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nadiradze G, Horvath P, Sautkin Y, Archid R, Weinreich F-J, Königsrainer A, et al. Overcoming drug resistance by taking advantage of physical principles: pressurized intraperitoneal aerosol chemotherapy (PIPAC) Cancers. 2019;12(1):34. doi: 10.3390/cancers12010034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grass F, Vuagniaux A, Teixeira-Farinha H, Lehmann K, Demartines N, Hübner M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. BJS Br J Surg. 2017;104(6):669–678. doi: 10.1002/bjs.10521. [DOI] [PubMed] [Google Scholar]
- 12.Blanco A, Giger-Pabst U, Solass W, Zieren J, Reymond MA. Renal and hepatic toxicities after pressurized intraperitoneal aerosol chemotherapy (PIPAC) Ann Surg Oncol. 2013;20(7):2311–2316. doi: 10.1245/s10434-012-2840-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alyami M, Bonnot P-E, Mercier F, Laplace N, Villeneuve L, Passot G, et al. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable peritoneal metastasis from gastric cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2020;47(1):123-7. [DOI] [PubMed]
- 14.Graversen M, Detlefsen S, Asmussen J, Mahdi B, Fristrup C, Pfeiffer P, et al. Treatment of peritoneal carcinomatosis with pressurized intraperitoneal aerosol chemotherapy – PIPAC-OPC2. Pleura Peritoneum. 2018;3:20180108. doi: 10.1515/pp-2018-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Granieri S, Bonomi A, Frassini S, Chierici AP, Bruno F, Paleino S, et al. Prognostic impact of cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients: a meta-analysis of randomized controlled trials. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol. 2021;S0748-7983(21):00492–00493. doi: 10.1016/j.ejso.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 16.Gockel I, Jansen-Winkeln B, Haase L, Niebisch S, Moulla Y, Lyros O, Lordick F, Schierle K, Wittekind C, Thieme R. Pressurized IntraPeritoneal Aerosol Chemotherapy (PIPAC) in patients with peritoneal metastasized colorectal, appendiceal and small bowel cancer. Tumori. 2020;106(1):70–78. doi: 10.1177/0300891619868013. [DOI] [PubMed] [Google Scholar]
- 17.Tempfer CB, Celik I, Solass W, Buerkle B, Pabst UG, Zieren J, Strumberg D, Reymond MA. Activity of pressurized intraperitoneal aerosol chemotherapy (PIPAC) with cisplatin and doxorubicin in women with recurrent, platinum-resistant ovarian cancer: preliminary clinical experience. Gynecol Oncol. 2014;132(2):307–311. doi: 10.1016/j.ygyno.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 18.Ploug M, Graversen M, Pfeiffer P, Mortensen MB. Bidirectional treatment of peritoneal metastasis with pressurized intraperitoneal aerosol chemotherapy (PIPAC) and systemic chemotherapy: a systematic review. BMC Cancer. 2020;20(1):105. doi: 10.1186/s12885-020-6572-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Girshally R, Demtröder C, Albayrak N, Zieren J, Tempfer C, Reymond MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol. 2016;14(1):253. doi: 10.1186/s12957-016-1008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siebert M, Alyami M, Mercier F, Gallice C, Villeneuve L, Laplace N, Passot G, Bakrin N, Glehen O, Kepenekian V. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) in association with systemic chemotherapy and bevacizumab, evaluation of safety and feasibility. A single center comparative study. Eur J Surg Oncol. 2021;47(1):139–142. doi: 10.1016/j.ejso.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 21.Robella M, Vaira M, De Simone M. Safety and feasibility of pressurized intraperitoneal aerosol chemotherapy (PIPAC) associated with systemic chemotherapy: an innovative approach to treat peritoneal carcinomatosis. World J Surg Oncol. 2016;14(1):128. doi: 10.1186/s12957-016-0892-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schubert J, Khosrawipour V, Chaudhry H, Arafkas M, Knoefel WT, Pigazzi A, Khosrawipour T. Comparing the cytotoxicity of taurolidine, mitomycin C, and oxaliplatin on the proliferation of in vitro colon carcinoma cells following pressurized intra-peritoneal aerosol chemotherapy (PIPAC) World J Surg Oncol. 2019;17(1):93. doi: 10.1186/s12957-019-1633-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.