Abstract
Purpose
To compare the effects of prevention interventions on delirium occurrence in critically ill adults.
Methods
MEDLINE, Embase, PsychINFO, CINAHL, Web of Science, Cochrane Library, Prospero, and WHO international clinical trial registry were searched from inception to April 8, 2021. Randomized controlled trials of pharmacological, sedation, non-pharmacological, and multi-component interventions enrolling adult critically ill patients were included. We performed conventional pairwise meta-analyses, NMA within Bayesian random effects modeling, and determined surface under the cumulative ranking curve values and mean rank. Reviewer pairs independently extracted data, assessed bias using Cochrane Risk of Bias tool and evidence certainty with GRADE. The primary outcome was delirium occurrence; secondary outcomes were durations of delirium and mechanical ventilation, length of stay, mortality, and adverse effects.
Results
Eighty trials met eligibility criteria: 67.5% pharmacological, 31.3% non-pharmacological and 1.2% mixed pharmacological and non-pharmacological interventions. For delirium occurrence, 11 pharmacological interventions (38 trials, N = 11,993) connected to the evidence network. Compared to placebo, only dexmedetomidine (21/22 alpha2 agonist trials were dexmedetomidine) probably reduces delirium occurrence (odds ratio (OR) 0.43, 95% Credible Interval (CrI) 0.21–0.85; moderate certainty). Compared to benzodiazepines, dexmedetomidine (OR 0.21, 95% CrI 0.08–0.51; low certainty), sedation interruption (OR 0.21, 95% CrI 0.06–0.69; very low certainty), opioid plus benzodiazepine (OR 0.27, 95% CrI 0.10–0.76; very low certainty), and protocolized sedation (OR 0.27, 95% CrI 0.09–0.80; very low certainty) may reduce delirium occurrence but the evidence is very uncertain. Dexmedetomidine probably reduces ICU length of stay compared to placebo (Ratio of Means (RoM) 0.78, CrI 0.64–0.95; moderate certainty) and compared to antipsychotics (RoM 0.76, CrI 0.61–0.98; low certainty). Sedative interruption, protocolized sedation and opioids may reduce hospital length of stay compared to placebo, but the evidence is very uncertain. No intervention influenced mechanical ventilation duration, mortality, or arrhythmia. Single and multi-component non-pharmacological interventions did not connect to any evidence networks to allow for ranking and comparisons as planned; pairwise comparisons did not detect differences compared to standard care.
Conclusion
Compared to placebo and benzodiazepines, we found dexmedetomidine likely reduced the occurrence of delirium in critically ill adults. Compared to benzodiazepines, sedation-minimization strategies may also reduce delirium occurrence, but the evidence is uncertain.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00134-021-06490-3.
Keywords: Delirium, Prevention, Pharmacological, Non-pharmacological interventions, Critical care
Take home message
| Compared to placebo and benzodiazepines, dexmedetomidine likely reduces the occurrence of delirium in critically ill adults. Compared to benzodiazepines, sedation minimization strategies may also reduce delirium occurrence, but the evidence is uncertain. |
Introduction
Delirium, a highly prevalent syndrome in critically ill patients, is characterized by acute changes in mental status with inattention, disorganized thinking, and altered level of consciousness not explained by pre-existing conditions [1]. Although delirium is potentially preventable and reversible, it is associated with adverse patient consequences with excess mortality, cognitive impairment, functional decline, and increased healthcare system costs associated with prolonged mechanical ventilation and length of stay [2, 3]. The pathophysiology of delirium is not yet fully understood but is likely multifactorial, although sedatives, especially benzodiazepines, commonly administered for intensive care unit (ICU) sedation, are associated with delirium occurrence [2, 4, 5].
Effective interventions to treat established ICU delirium have not yet been identified [6]. Pharmacological interventions that target known alterations in neurotransmitter pathways, primarily dopaminergic and cholinergic pathways, have failed to demonstrate effect [2, 6]. Antipsychotics are commonly administered to mitigate agitated delirium, but have not yet shown to reduce delirium severity or resolve symptoms in ICU or hospitalized non-ICU patients [6, 7]. Non-pharmacological interventions (e.g., patient orientation, multi-component) shown to be effective in hospitalized non-ICU populations [8] have failed to demonstrate consistent treatment effect in the ICU [9]. In the absence of known effective treatments, it is imperative to identify effective prevention strategies. The current coronavirus disease 2019 (COVID-19) pandemic with the worldwide surge in critical illness has further highlighted the extent of delirium in the ICU and the importance of understanding the best approach to preventing ICU delirium [10, 11].
A wide-ranging list of prevention strategies evaluated to date include pharmacological, sedation, and non-pharmacological single or multi-component interventions that can be commenced during or immediately prior to (e.g., peri-operative) an ICU admission. Non-pharmacologic multi-component interventions have been studied extensively in hospitalized older non-ICU adults with evidence suggesting these are the most effective method to prevent delirium [12]. Previous systematic reviews investigating the effect of delirium prevention have either focused on direct evidence from head-to-head comparisons for a single intervention (versus placebo or alterative drug class) or have mixed critically ill patients with hospitalized non-ICU patient populations [2, 7, 13]. Given the numerous interventions to choose from, the abundance of trials, and the inconsistent findings reported, we believed a network meta-analysis (NMA) would provide clinicians with additional information to further support bedside decision-making. A NMA is a statistical approach that enables synthesis of both direct and indirect evidence in a multi-treatment comparison analytical framework, allowing assessment and ranking of relative efficacy and safety of multiple interventions that clinicians might consider at the bedside that may or may not have been directly compared in the published trials [14]. Our primary objective was to synthesize data from trials comparing any intervention for preventing delirium in critically ill adults using NMA. Our secondary objectives were to compare the effects of these interventions on the numbers of delirium-free and coma-free days, delirium duration, delirium severity, incidence of sub-syndromal delirium, duration of mechanical ventilation, length of stay, mortality, long-term outcomes (cognitive, discharge disposition, health-related quality of life), and adverse events.
Methods
We registered this review prospectively in PROSPERO (CRD42016036313) and published the protocol [15]. Institutional review board approval was not required as this study did not include individual patient data. Reporting of findings was guided by the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Extension Statement for NMA (eTable 1) [16].
Eligibility criteria, search, and study selection
Using a search strategy developed in consultation with a Medical Information Specialist and peer reviewed by a second using the PRESS framework (search strategy previously published [6]), we searched the following databases from respective inception dates to April 8, 2021: Ovid MEDLINE ALL, Embase Classic + Embase, PsychINFO, CINAHL and Web of Science. We searched the grey literature using sources listed in the Canadian Agency for Drugs and Technologies in Health (CADTH) Grey Matters, the Cochrane Library and Prospero for relevant reviews, and the WHO international clinical trial registry for unpublished and ongoing trials.
We sought randomized and quasi-randomized controlled trials that examined any non-pharmacologic, pharmacologic, or multi-component for prevention of delirium in critically ill adults (≥ 16 years of age in an ICU of any type or high-acuity unit) as well as sedation strategy (e.g., protocolized sedation). We included studies that reported delirium incidence or prevalence and grouped them under the outcome delirium occurrence. We excluded trials using a crossover design, those focused on delirium treatment, and those with interventions applied in the pre- or intra-operative period only. We did not apply restrictions based on publication language, sex, or race. Two authors (LB, LR) independently screened citations against pre-set inclusion–exclusion criteria.
Outcomes
The selection of outcomes was informed by the core outcome sets for effectiveness trials of interventions to prevent and/or treat delirium [17, 18]. The primary outcome was delirium occurrence; secondary outcomes were numbers of delirium-free and coma-free days, delirium duration, delirium severity, incidence of sub-syndromal delirium, duration of mechanical ventilation, length of stay, mortality, long-term outcomes (cognitive, discharge disposition, health-related quality of life), and adverse events. For outcomes reported at multiple time intervals, such as mortality, we used the longest time point available [19].
Data extraction, risk of bias, and GRADE certainty assessment
Working in pairs, two authors independently abstracted data on study characteristics, interventions, outcomes, and risk of bias. Risk of bias was assessed as recommended by the Cochrane Collaboration (version 1), judging the overall risk of bias as the worst score of six domains (random sequence generation, allocation concealment, blinding, attrition, selective reporting, and other biases) [20]. A third author (LB) confirmed extraction, adjudicated inconsistencies, and another (WC) entered data into Review Manager (version 5.3, The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We used the GRADE approach (Grading of Recommendations Assessment, Development and Evaluation, https//gradpro.org) to assess and report the certainty of each NMA estimate as either high, moderate, low, or very low certainty [21, 22]. The authors (WC, LB) assessed the certainty of each direct, indirect, and network meta-analysis estimate using the four-step GRADE approach (i.e., risk of bias, inconsistency, indirectness, and publication bias) with limitations in any of these domains resulting in a downgrade of the certainty. Imprecision was assessed for the NMA estimate. If differences were detected between direct and indirect evidence (i.e., incoherence), we selected the lower certainty of the assessments.
Statistical analysis
For continuous outcomes, we transformed means and standard deviations (SDs) to the log scale due to their skewed nature [23]; medians and interquartile ranges (IQRs) were converted to means and SDs using established methods [24]. We performed DerSimonian–Laird random effects pairwise meta-analyses for all continuous and binary outcomes [25]. We performed NMA for interventions that connected to an evidence network by data available from ≥ 2 studies. For outcomes without adequate network structure, we performed pairwise meta-analyses only. Using established procedures, we assessed validity of assumptions of homogeneity, similarity, and consistency, and performed NMAs using Bayesian fixed and random effect models with normal likelihood and the identify link, accounting for correlations in multi-arm studies [26], with comparisons reported as ratio of means (RoM) with 95% credible intervals (CrI). We addressed transitivity or exchangeability within the network, such that treatment effects in direct comparisons that informed indirect estimates of effect would not be biased by study characteristics. To do so, clinical experts and methodologists reviewed the extracted key clinical and methodological factors (i.e., age, severity of illness, mechanical ventilation, assessment tools for delirium and sedation, and control for analgesia, sedation, agitation, and non-pharmacological interventions) and determined that there was reasonable balance across studies to proceed. For binary outcomes, we fitted both fixed and random effects NMA models with binomial likelihood, with comparisons reported as odds ratios (OR) (95% CrI). If a trial reported multiple mortality outcomes, we prioritized selection of analyzed data as follows: 90-day, hospital, 28/30-day, and ICU mortality. We used a vague prior distribution for the common between-study variance parameter in random effects NMAs [specifically, Uniform (0, 3)], and vague prior distribution for log RoM for each intervention compared with placebo [specifically, Normal (0, 100)].
Models were evaluated for adequacy of fit by comparing posterior total residual deviance to the number of unconstrained data points (i.e., total number of study arms); fit was considered adequate if these quantities were of similar magnitude. We compared models using the deviance information criterion (DIC), with lower values indicating better model fit [27]. We also fitted unrelated means models to the data and compared DIC values and posterior mean deviance contributions with those from consistency models to detect violations of the consistency assumption. We assessed model convergence with established methods including inspection of the Gelman–Rubin–Brooks diagnostics plots and the potential scale reduction factor (with threshold 1.01) [28].
For each outcome, we estimated secondary measures of effect, including surface under the cumulative ranking curve (SUCRA) values [29]. Methodological heterogeneity was assessed using similarity of point estimates, overlap of confidence intervals (CIs), and statistical tests (χ2 test for homogeneity and I2 measure for heterogeneity) [30]. All NMAs were performed using Open Bayesian inference Using Gibbs Sampling (BUGS) software version 3.2.3 and the R2WinBUGS package version 3.2–3.2 in R [31–33].
Results
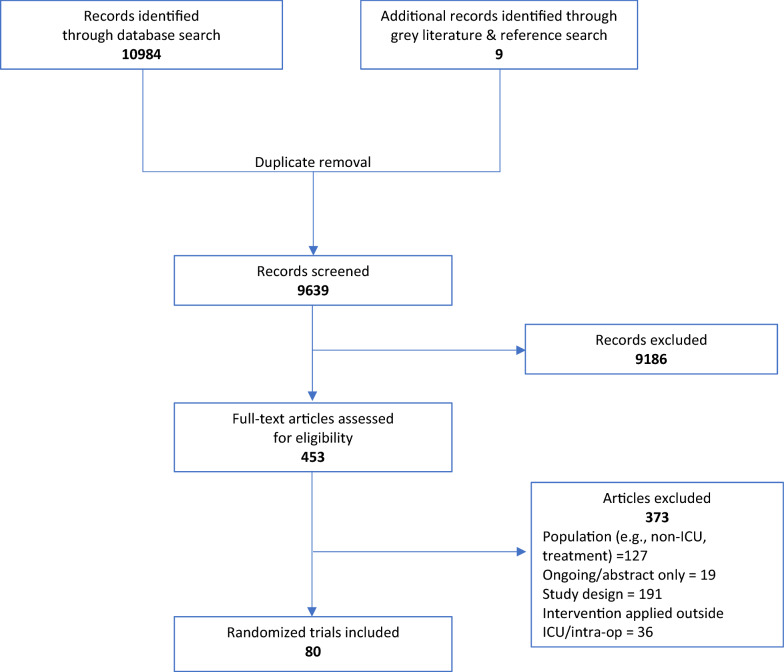
The search strategy resulted in 80 trials that met inclusion criteria (Fig. 1), with a total of 17,140 participants [34–113]. Included trials were comprised of 54 (67.5%) pharmacological or sedation intervention studies [34–36, 38–40, 42–45, 47, 49–51, 55–57, 59–63, 67–69, 71, 73–76, 78, 79, 81, 82, 85–87, 89, 90, 92–94, 97–100, 102, 103, 105–108, 110, 112, 113] with 14,224 participants, 25 (31.3%) studies of non-pharmacological single or multi-component interventions with 2904 participants [37, 41, 46, 48, 52, 53, 57, 58, 64–66, 70, 72, 77, 80, 83, 84, 88, 91, 95, 96, 101, 104, 109, 111], and 1 study (1.2%) included a combination non-pharmacological with a pharmacological intervention with 12 participants [54]. Key features of all included trials are presented in detail in eTable 2. Trials were geographically dispersed but primarily conducted in North America (22.5%), Europe (25.0%) and Asia (26.3%). All trials were published between 2006 and 2021 and 43 (53.8%) were conducted in mixed ICUs. Trials allocated participants to two to four study arms and enrolled between 11 and 4000 ICU participants. The mean or median age at randomization ranged from 34.6 to 77.4 years, and 56 (70%) of trials reported a mean or median age of 60 or greater. Nearly all trials (78 trials, 97.5%) used a validated delirium assessment tools; 72 trials (90.0%) used either the Confusion Assessment Method for the ICU (CAM-ICU) or Intensive Care Delirium Screening Checklist (ICDSC). From the perspective of the primary outcome, 51% (41) trials had high risk of bias, primarily due to lack of blinding and risk of differential co-interventions (eTable 3).
Fig. 1.
Summary of study retrieval and identification. Figure describes the flow of selection of included trials. Inclusion criteria applied included: randomized controlled trials, examined any pharmacological, sedation, non-pharmacological or multi-component intervention for prevention of delirium in critically ill adults
Neither single nor multi-component non-pharmacological intervention trials connected to evidence networks for any outcomes of interest; pairwise comparisons are presented in eFigure 1. In the presentation of results below, we focus on the NMA estimates from random effects models for interventions (pharmacological and sedation strategies) that connected to the network; random effects models were superior to fixed effects. Model fit details including posterior mean deviance contribution plots, DIC, between-study SD and funnel plots are presented in eTable 4 and eFigures 2 and 3.
Delirium occurrence
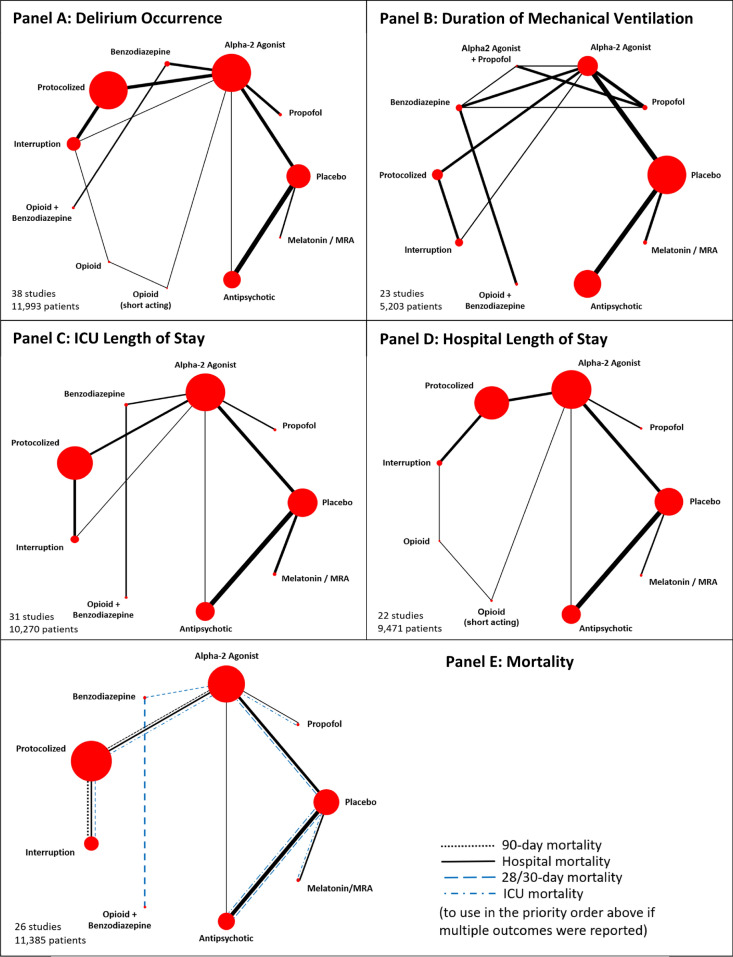
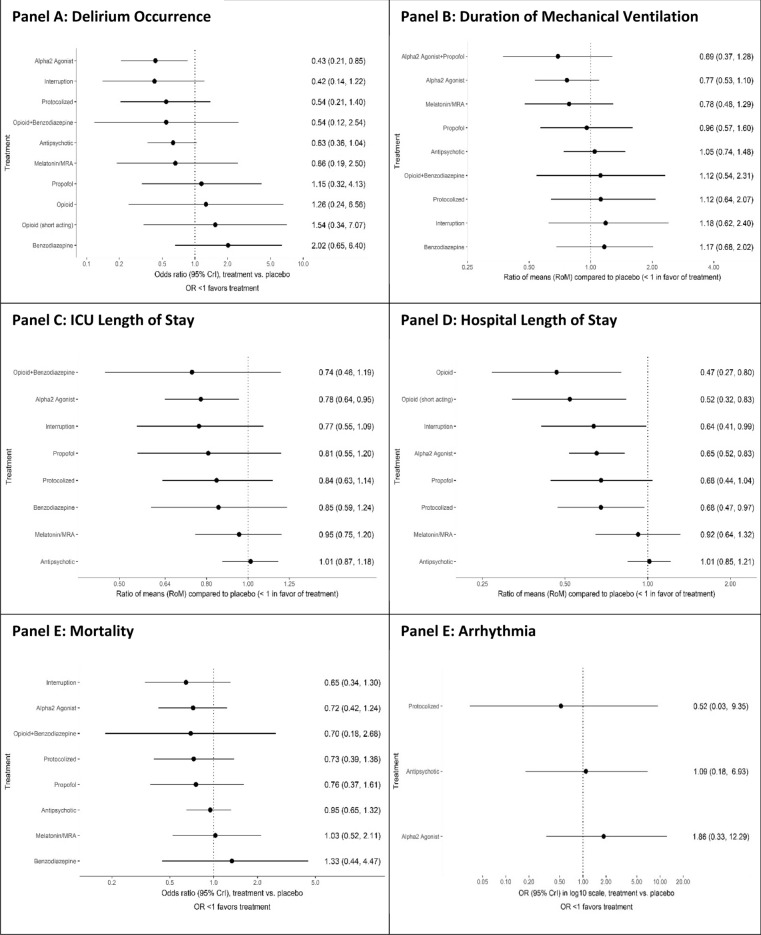
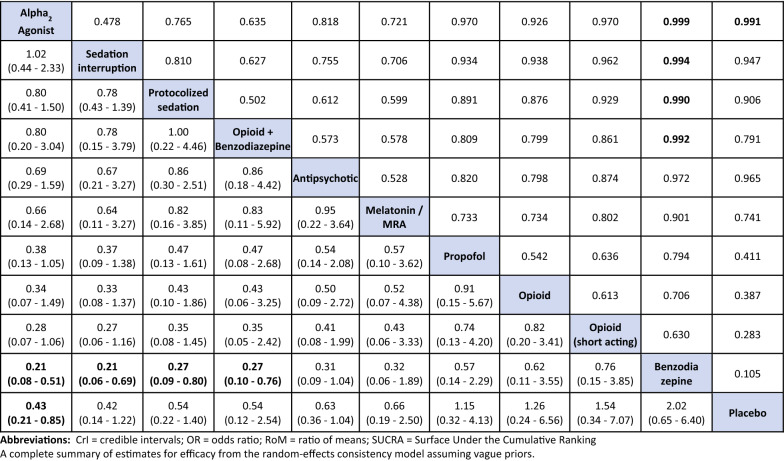
Eleven pharmacological interventions studied in 38 trials [34–36, 38, 40, 43, 44, 49–51, 56, 59, 61, 67, 69, 71, 73, 74, 76, 79, 81, 82, 85, 86, 89, 90, 93, 94, 97–100, 103, 105–107, 112, 113] (N = 11,993) connected to the evidence network (Table 1, Fig. 2A, eTable 5 summarizes node references); 24% (13/55) of the pairwise comparisons included direct evidence. Compared to placebo, only alpha2 agonists (all trials but one examined dexmedetomidine) probably reduce delirium occurrence (OR 0.43, 95% CrI 0.21–0.85; moderate certainty) (Fig. 3A, Table 2, eTable 6). Compared to benzodiazepines, dexmedetomidine (OR 0.21, 95% CrI 0.08–0.51; low certainty), sedation interruption (OR 0.21, 95% CrI 0.06–0.69; very low certainty), opioid plus benzodiazepine (OR 0.27, 95% CrI 0.10–0.76; very low certainty), and protocolized sedation (OR 0.27, 95% CrI 0.09–0.80; very low certainty) may reduce delirium occurrence, but the evidence is uncertain. The Bayesian NMA Summary of Findings with GRADE is presented in Table 3. Pairwise comparisons for environmental or multi-component interventions found no differences compared to standard care, with wide CIs (0.83, 95% CI 0.49–1.41 and 0.65, 95% CI 0.40–1.05, respectively) (eFigure 1).
Table 1.
Summary of randomized trials and interventions included in the network meta-analysis
| Study, year [reference] | N | Intervention | Control | Delirium occurrence | Duration of mechanical ventilation | Length of stay—ICU | Length of stay—hospital | Mortality | Arrhythmia | Risk of bias* |
|---|---|---|---|---|---|---|---|---|---|---|
| Abbasi 2018 [34] | 137 | Melatonin PO | Placebo PO | + | + | + | + | + | Low | |
| Abdelgalel 2016 [35] | 90 | Dexmedetomidine IV infusion with optional LD | Placebo IV intermittent | + | + | + | + | + | Low | |
| Al-Qadheeb 2016 [36] | 68 | Haloperidol IV intermittent | Placebo IV intermittent | + | + | + | Low | |||
| Azeem 2018 [38] | 60 | Dexmedetomidine IV LD + infusion | Morphine IV + midazolam IV | + | + | + | Low | |||
| vanden Boogaard 2018 [40] | 1789 | Haloperidol IV intermittent | Placebo IV intermittent | + | + | + | + | + | Low | |
| Chang 2018 [43] | 60 | Dexmedetomidine IV infusion | Propofol IV infusion | + | + | High | ||||
| Chanques 2017 [44] | 137 | IV sedation interruption | Usual sedation | + | + | + | + | + | High | |
| DeJonghe 2018 [49] | 1174 | IV sedation protocol | Usual sedation | + | + | High | ||||
| Devlin 2014 [50] | 33 | Dexmedetomidine IV infusion | Placebo IV infusion | + | + | + | + | Low | ||
| Djaiani 2016 [51] | 183 | Dexmedetomidine IV LD + infusion | Propofol IV infusion | + | + | + | + | + | Low | |
| Gandolfi 2020 [55] | 203 | Melatonin PO | Placebo PO | + | + | + | + | Low | ||
| Girard 2008 56] | 336 | IV sedation interruption | Usual sedation | + | + | + | High | |||
| Hakim 2012 [59] | 101 | Risperidone PO | Placebo PO | + | + | + | + | Low | ||
| Hu 2015 [60] | 76 |
Intervention 1: dexmedetomidine IV infusion + propofol IV infusion Intervention 2: propofol IV infusion |
Midazolam IV infusion | + | High | |||||
| Huang 2014 [61] | 108 | Dexmedetomidine IV infusion | Propofol IV infusion | + | + | High | ||||
| Hughes 2021 [62] | 432 | Dexmedetomidine IV infusion | Propofol IV infusion | + | Low | |||||
| Javaherforooh Zadeh 2021 [63] | 60 | Melatonin PO | Placebo PO | + | + | High | ||||
| Kawazoe 2017 [67] | 201 | Dexmedetomidine IV infusion | Sedation IV infusion without dexmedetomidine | + | + | + | + | + | + | High |
| Khan 2018 [69] | 135 | Haloperidol IV intermittent dose | Placebo IV intermittent dose | + | + | + | + | + | Low | |
| Li 2016 [71] | 70 | 3 groups of dexmedetomidine, propofol or combination IV infusion to circadian clock | Sedation IV infusion without regulation to circadian clock | + | + | + | High | |||
| Liu 2017 [73] | 105 |
Intervention 1: remifentanil IV + midazolam IV infusions Intervention 2: fentanyl + midazolam IV infusions |
Placebo + midazolam IV infusion | + | + | + | + | Low | ||
| Lyu 2015 [74] | 140 | Remifentanil + midazolam IV infusion | Midazolam IV infusion | + | + | + | + | High | ||
| Mehta 2012 [76] | 423 | Protocolized sedation + daily interruption | Protocolized sedation | + | + | + | + | + | High | |
| Mokhtari 2020 [79] | Aripiprazole PO | Placebo PO | + | + | High | |||||
| Nassar 2014 [81] | Daily IV sedation interruption | Intermittent IV sedation | + | + | + | + | High | |||
| Nishikimi 2018 [82] | 88 | Ramelteon PO | Placebo PO | + | + | + | Low | |||
| Pandharipande 2007 [85] | 103 | Dexmedetomidine IV LD + infusion | Lorazepam IV LD + infusion | + | + | + | Low | |||
| Park 2014 [86] | 142 | Dexmedetomidine IV LD + infusion | Remifentanil IV infusion | + | + | High | ||||
| Prakanrattana 2007[89] | 126 | Risperidone PO | Placebo PO | + | + | + | + | Low | ||
| Priye 2015 [90] | 64 | Dexmedetomidine IV infusion | Placebo IV infusion | + | Low | |||||
| Rubino 2010 [93] | 30 | Clonidine IV LD + infusion | Placebo IV infusion | + | + | + | Moderate | |||
| Ruokonen 2009 [94] | 85 | Dexmedetomidine IV infusion | Propofol or midazolam IV infusion | + | Low | |||||
| Shehabi 2009 [97] | 299 | Dexmedetomidine IV infusion | Morphine IV infusion | + | + | + | + | + | + | Low |
| Shehabi 2013 [98] | 37 | Early goal directed sedation Dexmedetomidine IV infusion | Propofol or midazolam IV infusion | + | + | + | + | High | ||
| Shehabi 2019 [99] | 4000 | Dexmedetomidine IV infusion | Propofol or midazolam IV infusion | + | + | + | + | + | High | |
| Shu 2019 [100] | 80 | Dexmedetomidine IV LD + infusion | Midazolam IV infusion | + | High | |||||
| Skrobik 2018 [102] | 100 | Dexmedetomidine IV infusion | Placebo IV infusion | + | + | + | + | Low | ||
| Song 2015 [103] | 90 | Dexmedetomidine IV infusion | Midazolam IV infusion | + | + | + | High | |||
| Spies 2011 [105] | 60 | Remifentanil IV infusion | Fentanyl IV infusion | + | + | Low | ||||
| Strom 2010 [106] | 113 | No sedation; analgesia with opioid | Analgesia with opioid + propofol IV infusion | + | + | High | ||||
| Su 2016 [107] | 700 | Dexmedetomidine IV infusion | Placebo IV infusion | + | + | + | + | + | + | Low |
| Wan 2011 [112] | 200 | Dexmedetomidine IV infusion | Midazolam IV infusion | + | + | High | ||||
| Wang 2012 [113] | 457 | Haloperidol IV infusion | Placebo IV infusion | + | + | + | + | + | + | Low |
See supplementary eTables 2 and 3 for detailed description of included studies and risk of bias assessment
IV intravenous, LD loading dose, PO per os
*The overall risk of bias was the lowest for any domain in the risk of bias tool (i.e., sequence generation, allocation concealment, incomplete outcome data, selective reporting, or other bias)
Fig. 2.
Network plots for delirium prevention strategies for outcomes. Network geometry displays nodes as interventions and head-to-head direct comparisons as lines connecting these nodes. The width of the edges each representing a pairwise comparison was weighted by the corresponding number of studies, while the size of treatment nodes was weighted by the number of patients
Fig. 3.
Forest plots with interventions ordered in descending order of SUCRA values for each network. All outcomes are reported as network odds or ratio of means with 95% credible intervals (Crl)
Table 2.
Delirium occurrence league table of pairwise ORs with 95% CrI (lower triangle) and pairwise probabilities of superiority (upper triangle)
Treatments other than placebo are ranked in order (upper left–lower right) of decreasing SUCRA value. For pairwise probabilities of superiority for each comparison (i.e., a treatment is better than another), the lower/right-most treatment is the reference treatment. Thus, values < 1 favor the upper/left-most intervention. Differences where the 95% CrI excludes the null value of 1 are shown in bold font
Table 3.
Bayesian NMA Summary of Findings—delirium occurrence.
| Patient or population: critically ill adults, includes both non-ventilated and mechanically ventilated patients. | |||||||
| Interventions: any interventions and strategies for sedation titration (e.g., protocolized and interruption). | |||||||
| Comparator (reference): placebo. | |||||||
| Outcome: delirium occurrence. | |||||||
| Setting(s): mixed intensive care unit settings |
| Total studies: 38 Total participants: 11,993 |
Relative effect * (95% CrI) | Anticipated absolute effect (95% CrI) | Certainty of the evidence | Number of participants (trials) | Ranking*** (95% CrI) | ||
|---|---|---|---|---|---|---|---|
| Placebo | Intervention | Risk difference** | |||||
| Alpha2 agonist vs placebo |
OR 0.43 (0.21–0.85) NMA estimate |
278 per 1000 (147/528 based on 5 trials) | 163 per 1000 (86/527 based on 5 trials) | 136 fewer per 1000 (from 204 to 30 fewer) |
⊕ ⊕ ⊕ ◯ Moderate Due to inconsistency2 |
Direct evidence: 1055 (5 trials) | 2.73 (1–5) |
| Antipsychotics vs placebo |
OR 0.63 (0.36–1.04) NMA estimate |
309 per 1000 (375/1199 based on 8 trials) | 301 per 1000 (473/1577 based on 8 trials) | 91 fewer per 1000 (from 170 fewer to 9 more) |
⊕ ⊕ ◯◯ Low Due to imprecision3, and inconsistency2 |
Direct evidence: 2776 (8 trials) | 4.80 (1–9) |
| Melatonin/MRA vs placebo |
OR 0.66 (0.19–2.50) NMA estimate |
186 per 1000 (21/113 based on 2 trials) |
125 per 1000 (14/112 based on 2 trials) |
55 fewer per 1000 (from 144 fewer to 178 more) |
⊕ ⊕ ◯◯ Low Due to imprecision3 and inconsistency2 |
Direct evidence: 225 (2 trials) | 5.22 (1–11) |
| Sedation interruption vs placebo |
OR 0.42 (0.14–1.22) NMA estimate |
330 per 1000 1 | No head-to-head comparison with placebo | 157 fewer per 1000 (from 265 fewer to 46 more) |
⊕ ◯◯◯ Very low Due to imprecision3, indirectness4, inconsistency5 and risk of bias |
No direct evidence. Indirect evidence only | 2.81 (1–7) |
| Protocolized sedation vs placebo |
OR 0.54 (0.21–1.40) NMA estimate |
330 per 1000 1 | No head-to-head comparison with placebo | 119 fewer per 1000 (from 238 fewer to 77 more) |
⊕ ◯◯◯ Very low Due to imprecision3, indirectness4, inconsistency6 and risk of bias |
No direct evidence. Indirect evidence only | 4.27 (1–8) |
| Opioid + benzodiazepine vs placebo |
OR 0.54 (0.12–2.54) NMA estimate |
330 per 1000 1 | No head-to-head comparison with placebo | 119 fewer per 1000 (from 275 fewer to 225 more) |
⊕ ◯◯◯ Very low Due to imprecision3, Serious indirectness7, inconsistency8 and risk of bias |
No direct evidence. Indirect evidence only | 4.36 (1–10) |
| Propofol vs placebo |
OR 1.15 (0.32–4.13) NMA estimate |
330 per 10001 | No head-to-head comparison with placebo | 31 more per 1000 (from 192 fewer to 341 more) |
⊕ ◯◯◯ Very low Due to imprecision3, indirectness4, and inconsistency5 |
No direct evidence. Indirect evidence only | 7.77 (2–11) |
| Opioid vs placebo |
OR 1.26 (0.24–6.56) NMA estimate |
330 per 1000 1 | No head-to-head comparison with placebo | 53 more per 1000 (from 222 fewer to 434 more) |
⊕ ◯◯◯ Very low Due to imprecision3, serious indirectness9, inconsistency6 |
No direct evidence. Indirect evidence only | 7.91 (2–11) |
| Opioid (short acting) vs placebo |
OR 1.54 (0.34 to 7.07) NMA estimate |
330 per 1000 1 | No head-to-head comparison with placebo |
102 more per 1000 (from 188 fewer to 447 more) |
⊕ ◯◯◯ Very low Due to Imprecision3, Indirectness4, and Inconsistency5 |
No direct evidence. Indirect evidence only |
8.73 (3–11) |
| Benzodiazepine vs placebo |
OR 2.02 (0.65–6.40) NMA estimate |
330 per 1000 1 | No head-to-head comparison with placebo | 169 more per 1000 (from 86 fewer to 429 more) |
⊕ ◯◯◯ Very low Due to imprecision3, indirectness4, inconsistency5 |
No direct evidence. Indirect evidence only | 9.87 (6–11) |
| Placebo | Reference comparator | – | – | – | – | 7.53 (4–10) | |
GRADE Working Group grades of evidence (or certainty in the evidence)
High certainty: We are very confident that the true effect lies close to that of the estimate of the effect
Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different
Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect
Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect
Abbreviations
CrI credible interval, OR odds ratio
NMA-SoF table definitions
*Network meta-analysis estimates are reported as odds ratio. CrI: credible interval (rather than confidence interval), since a Bayesian network meta-analysis has been conducted
**Anticipated absolute effect: risk difference is calculated based on the control group risk and the estimated odds ratio
***Median and credible intervals are presented. Rank statistics is defined as the probabilities that a treatment out of n treatments in a network meta-analysis is the best, the second, the third and so on until the least effective treatment
Explanatory footnotes
1Given that there were no head-to-head trials for these comparisons, the control group rate is based on the placebo arm of a large, randomized control trial (Boogaard et al. 2018, antipsychotic vs placebo)
2Inconsistency: due to heterogeneity in the direct comparison
3Imprecision: due to wide credible intervals in the OR estimate
4Indirectness: only indirect evidence available (through one degree of intermediary, alpha2 agonist)
5Inconsistency: due to heterogeneity in the direct comparison of alpha2 agonist vs placebo
6Inconsistency: due to heterogeneity in the direct comparison of alpha2 agonist vs placebo and the direct comparison of protocolized vs alpha2 agonist
7Serious indirectness: only indirect evidence available (through two degrees of intermediaries, alpha2 agonist and benzodiazepine)
8Inconsistency: due to heterogeneity in the direct comparison of alpha2 agonist vs placebo and the direct comparison of benzodiazepine vs alpha2 agonist
9Serious indirectness: only indirect evidence available (through three degrees of intermediaries, interruption / opioid (short acting), alpha2 agonist, and benzodiazepine)
Duration of mechanical ventilation
Ten interventions studied in 23 trials (N = 5203) [36, 38, 40, 44, 50, 51, 55, 60, 61, 63, 67, 69, 71, 73, 74, 76, 93, 97, 102, 103, 107, 112, 113] connected the evidence network (Table 1, Fig. 2B, eTable 5); 29% (13/45) of the pairwise comparisons included direct evidence. No intervention reduced the duration of mechanical ventilation compared to placebo or each other (Fig. 3B, eTables 7, 8 and 9). Compared to benzodiazepines, duration of mechanical ventilation may be reduced by dexmedetomidine (OR 0.66, 95% CrI 0.44–0.98; low certainty). Pairwise comparisons for neither environmental nor multi-component interventions found differences compared to standard care (eFigure 1).
Length of stay
Nine interventions studied in 31 trials (N = 10,270) [34–36, 38, 40, 44, 50, 51, 55, 56, 59, 63, 67, 69, 71, 73, 74, 76, 79, 81, 82, 85, 89, 93, 97, 98, 102, 103, 107, 113] connected to the evidence network for ICU length of stay (Table 1, Fig. 2C, eTable 5); 28% (10/36) of the pairwise comparisons included direct evidence. Compared to placebo, only alpha2 agonists (all trials but one examined dexmedetomidine) probably reduce ICU length of stay (RoM 0.78, 95% CrI 0.64–0.95; moderate certainty) (Fig. 3C; eTables 10, 11 and 12). Alpha2 agonists may reduce ICU length of stay compared to antipsychotics (RoM 0.76, 95% CrI 0.61–0.98; low certainty). Pairwise comparisons for single or multi-component non-pharmacological interventions found no differences compared to standard care (eFigure 1).
For the outcome of hospital length of stay, 9 interventions studied in 22 trials (N = 9471) [34, 35, 40, 43, 44, 51, 55, 59, 67, 69, 76, 81, 86, 89, 97–99, 102, 105–107, 113] connected the evidence network (Table 1, Fig. 2D, eTable 5); 28% (10/36) of the pairwise comparisons included direct evidence. Compared to placebo, alpha2 agonists (RoM 0.65, 95% CrI 0.52–0.83; moderate certainty) probably reduce hospital length of stay. Opioids (non-short acting RoM 0.47, 95% CrI 0.27–0.80; very low certainty, or short-acting opioids RoM 0.52, 95% CrI 0.32–0.83; very low certainty), sedation interruption (RoM 0.64, 95% CrI 0.41–0.99; very low certainty), protocolized sedation (RoM 0.68, 95% CrI 0.47–0.97; very low certainty) may do so as well (Fig. 3D; eTables 13, 14 and 15), but the evidence is very uncertain. Compared with antipsychotics, opioids (non-short acting opioids RoM 0.46, 95% CrI 0.26–0.81; very low certainty) or short acting opioids RoM 0.51, 95% CrI 0.31–0.84; very low certainty), protocolized sedation (RoM 0.67, 95% CrI 0.45–0.99; very low certainty) and alpha2 agonists (RoM 0.64, 95% CrI 0.49–0.85; low certainty) may reduce hospital length of stay but the evidence is uncertain. Pairwise comparisons for single or multi-component non-pharmacological interventions found no differences compared to standard care for ICU or hospital length of stay, except for mobilization with occupational or physical therapists compared to standard care (eFigure 1).
Mortality
Nine interventions studied in 26 trials (N = 11,385) [34–36, 40, 44, 49–51, 55, 56, 59, 62, 67, 69, 73, 74, 76, 81, 82, 85, 97–99, 102, 107, 113] connected to the evidence network for mortality (Table 1, Fig. 2E, eTable 5); 25% (9/36) of the pairwise comparisons were direct evidence. No intervention reduced mortality (Fig. 3E; eTables 16, 17 and 18) compared to placebo or compared to each other. There were no differences detected for single or multi-component non-pharmacological interventions compared to standard care (eFigure 1).
Other outcomes
For delirium duration, eight interventions were reported in 13 trials (N = 2752) [34, 36, 40, 44, 56, 59, 69, 73, 74, 82, 85, 97, 102]. However, there were insufficient trials of comparable interventions to connect to an evidence network. Treatment effect estimates from pairwise meta-analyses indicated no intervention was effective for reducing delirium duration compared to placebo (eFigure 4); nor for non-pharmacological interventions compared to standard care (eFigure 1). There were insufficient trials of comparable interventions to conduct pairwise comparisons for delirium-free and coma-free days, delirium severity, incidence of sub-syndromal delirium, long-term outcomes of cognition, discharge disposition, and health-related quality of life.
Adverse events identified included device removal [34, 36, 44, 47, 56, 76, 81, 85, 95, 98, 106], reintubation [44, 56, 76, 81, 86, 97, 106], arrhythmias [35, 67, 89, 97, 99, 107, 113], tracheostomy [44, 56, 76, 81, 106], and extrapyramidal side effects [36, 40, 59, 113]. Except for arrhythmias, we identified insufficient data to conduct pairwise comparisons or form a network. For arrhythmias, four interventions reported in seven trials (N = 5761) connected to the evidence network [35, 67, 89, 97, 99, 107, 113]. Compared to placebo, there was no difference in occurrence of arrhythmia with any intervention in trials reporting this outcome (Table 1, Fig. 3F; eTables 5, 19, 20 and 21); 100% direct evidence. There was no difference in NMA estimates for any other intervention comparison.
Discussion
In this systematic review and network meta-analysis of 11 pharmacological interventions from 38 trials enrolling 11,993 critically ill participants, we found that dexmedetomidine (studied in 21/22 alpha2 agonist trials) probably reduces the odds of delirium occurrence relative to placebo. The included trials used similar dexmedetomidine dose ranges, mostly without a loading dose that has been associated with bradycardia. Relative to benzodiazepine sedation, we found dexmedetomidine and strategies to reduce sedative exposure such as analgesia-first, protocolization and daily interruption, also may reduce delirium occurrence, but the evidence is uncertain. Dexmedetomidine was the only intervention identified that probably reduces length of ICU or hospital stay relative to placebo and may also do so relative to antipsychotics, but with less certainty. Opioids, sedation strategies, and dexmedetomidine may reduce hospital length of stay compared with antipsychotics commonly used in everyday ICU practice, but the evidence is very certain. No pharmacological intervention evaluated influenced mortality or arrhythmias. Non-pharmacological interventions did not connect to the evidence network; however, pairwise comparisons did not detect differences compared to standard care.
Clinicians need to consider multiple available therapeutic interventions as part of routine decision-making, without necessarily having evidence from direct comparisons or head-to-head trials. This NMA combines direct and indirect evidence for a multitude of available delirium prevention interventions and thus fills an important evidence gap, allowing for the assessment of clinically important treatment comparisons where direct comparisons are lacking. Through the use of NMA and inclusive selection criteria for interventions of interest, this review determined that dexmedetomidine reduces delirium occurrence compared to placebo and probably compared to benzodiazepines. We note our findings regarding dexmedetomidine and the occurrence of delirium are echoed by other systematic reviews including acutely ill patients requiring non-invasive mechanical ventilation [114] and cardiac surgery patients [115]. Dexmedetomidine’s pharmacological properties of minimal impact on respiratory effort, modest sedative effects with some analgesic properties make it an attractive alternative to benzodiazepines. Since benzodiazepines can increase delirium prevalence, worsen sleep architecture by altering stage 1 and 2 sleep, and suppress respiratory drive, dexmedetomidine is an attractive alternative [5, 116, 117]. Based on these properties and evidence from this review, clinicians may wish to consider dexmedetomidine for delirium prophylaxis. Other sedation strategies that reduce sedative drug exposure, such as analgesia-first or no sedation, protocolized sedation, and daily interruption, may also be considered to reduce delirium occurrence but the evidence remains uncertain.
The evidence networks in our review provide further evidence, although very uncertain, of the lack of effect of antipsychotics on important patient outcomes including delirium occurrence, delirium duration, duration of ventilation, ICU stay or mortality. Caution should be applied when interpreting and applying these results given the very low certainty of evidence due to risk of bias (e.g., lack of blinding), indirectness, imprecision, and heterogeneity. A recent review of antipsychotics for delirium prevention similarly identified lack of effect on incident delirium or hospital length of stay compared to placebo in a mix of ICU and non-ICU hospitalized settings [118].
Strengths and limitations
The main strength of this review is the inclusion of a broad range of interventions in a NMA. Compared to previous reviews, we did not apply any restrictions on language, sample size, types of interventions, types of delirium assessment tools, or types of ICU patient populations enrolled, with the intent of increasing the generalizability of findings. However, this decision introduces clinical heterogeneity, and appraising the transitivity assumption inherent to NMA, therefore, becomes more complex. Patient populations ranged from mechanically ventilated participants with high illness severity and high risk of delirium (for example, in trials of sedation-minimization strategies) to non-ventilated participants, with lower illness acuity and lower risk of delirium (for example, in trials of a single drug for delirium prevention). We extracted covariates that may influence delirium occurrence and response to treatment such as age, severity of illness, and exposure to treatments for pain, sedation, and agitation, but were unable to adjust for these. Thus, the lack of adjustment for effect modifiers has unknown implications on our results. Except for sedation strategies, which are studied only in mechanically ventilated patients, the other interventions could be applied to mixed ICU patients. Included trials rarely controlled for co-interventions such as analgesics, co-sedative, agitation, or non-pharmacological treatments. We used GRADE to downgrade the evidence for risk of bias related to lack of blinding and differential co-interventions wherever applicable.
We were unable to conduct comparisons and rankings of single or multi-component non-pharmacological interventions compared with pharmacological interventions due to the number of studies reporting diverse interventions and no trials that permitted connection to evidence networks. Thus, we were limited to direct pairwise comparisons only for non-pharmacological strategies. While we found no effect of these strategies, similar to another review [9], further investigation is warranted given their common use. Finally, outcomes recently recommended as part of a core set, such delirium severity, time to delirium resolution, health-related quality of life, and emotional distress were generally not reported [18].
Conclusions
Given no known effective interventions to treat delirium and the high incidence of delirium in the ICU, this review provides clinicians with evidence on pharmacological, sedation management, and non-pharmacological strategies to prevent ICU delirium. Important take-home messages are that compared to placebo or benzodiazepines, dexmedetomidine probably prevents delirium; a sedation-minimization strategy that targets reduced exposure to sedatives might prevent delirium; and antipsychotics may not prevent delirium.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We would like to acknowledge E. Wes Ely for his advice and support as a Knowledge Expert for our Canadian Institutes of health Research grant application which shaped the design of this review.
Author contributions
LB, BH and LR generated the research question and designed and lead the conduct of the review. WC, LB, and BH lead the statistical analysis. DW, NA, SK, IE, CM contributed to the protocol, extracted data, and interpretation of the results. All the authors approved of the final manuscript and had final responsibility for the decision to submit for publication. LB is the guarantor.
Funding
This study was funded by the Canadian Institutes of Health Research (CIHR) (Grant No. CIHRFRN144048). The funding body did not have input into the design, conduct or reporting of this systematic review.
Declarations
Conflicts of interest
All the authors declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. BH has previously provided methodologic advice to Eversana Inc for the conduct of systemic reviews and meta-analysis on unrelated topics.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Lisa D. Burry, Email: lisa.burry@sinaihealth.ca
Wei Cheng, Email: w.cheng@yale.edu.
David R. Williamson, Email: David.williamson@umontreal.ca
Neill K. Adhikari, Email: Neill.adhikari@utoronto.ca
Ingrid Egerod, Email: Ingrid.egerod@regionh.dk.
Salmaan Kanji, Email: skanji@toh.ca.
Claudio M. Martin, Email: Cmartin1@uwo.ca
Brian Hutton, Email: bhutton@ohri.ca.
Louise Rose, Email: Louise.rose@kcl.ac.uk.
References
- 1.Wilson JE, Mart MF, Cunningham C, Shehabi Y, Girard TD, MacLullich AMJ, Slooter AJC, Ely EW. Delirium. Nat Rev Dis Primers. 2020;6:90. doi: 10.1038/s41572-020-00223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devlin JW, Skrobik Y, Gelinas C, Needham DM, Slooter AJC, Pandharipande PP, Watson PL, Weinhouse GL, Nunnally ME, Rochwerg B, Balas MC, van den Boogaard M, Bosma KJ, Brummel NE, Chanques G, Denehy L, Drouot X, Fraser GL, Harris JE, Joffe AM, Kho ME, Kress JP, Lanphere JA, McKinley S, Neufeld KJ, Pisani MA, Payen JF, Pun BT, Puntillo KA, Riker RR, Robinson BRH, Shehabi Y, Szumita PM, Winkelman C, Centofanti JE, Price C, Nikayin S, Misak CJ, Flood PD, Kiedrowski K, Alhazzani W. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 3.Salluh JI, Wang H, Schneider EB, Nagaraja N, Yenokyan G, Damluji A, Serafim RB, Stevens RD. Outcome of delirium in critically ill patients: systematic review and meta-analysis. BMJ. 2015;350:h2538. doi: 10.1136/bmj.h2538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reade MC, Finfer S. Sedation and delirium in the intensive care unit. N Engl J Med. 2014;370:444–454. doi: 10.1056/NEJMra1208705. [DOI] [PubMed] [Google Scholar]
- 5.Fraser GL, Devlin JW, Worby CP, Alhazzani W, Barr J, Dasta JF, Kress JP, Davidson JE, Spencer FA. Benzodiazepine versus nonbenzodiazepine-based sedation for mechanically ventilated, critically ill adults: a systematic review and meta-analysis of randomized trials. Crit Care Med. 2013;41:S30–38. doi: 10.1097/CCM.0b013e3182a16898. [DOI] [PubMed] [Google Scholar]
- 6.Burry L, Hutton B, Williamson DR, Mehta S, Adhikari NK, Cheng W, Ely EW, Egerod I, Fergusson DA, Rose L. Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst Rev. 2019;9:CD011749. doi: 10.1002/14651858.CD011749.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikooie R, Neufeld KJ, Oh ES, Wilson LM, Zhang A, Robinson KA, Needham DM. Antipsychotics for treating delirium in hospitalized adults: a systematic review. Ann Intern Med. 2019;171:485–495. doi: 10.7326/M19-1860. [DOI] [PubMed] [Google Scholar]
- 8.Inouye SK, Bogardus ST, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–676. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 9.Bannon L, McGaughey J, Verghis R, Clarke M, McAuley DF, Blackwood B. The effectiveness of non-pharmacological interventions in reducing the incidence and duration of delirium in critically ill patients: a systematic review and meta-analysis. Intensive Care Med. 2019;45:1–12. doi: 10.1007/s00134-018-5452-x. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy M, Helfand BKI, Gou RY, Gartaganis SL, Webb M, Moccia JM, Bruursema SN, Dokic B, McCulloch B, Ring H, Margolin JD, Zhang E, Anderson R, Babine RL, Hshieh T, Wong AH, Taylor RA, Davenport K, Teresi B, Fong TG, Inouye SK. Delirium in older patients with COVID-19 presenting to the emergency department. JAMA Netw Open. 2020;3:e2029540. doi: 10.1001/jamanetworkopen.2020.29540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pun BT, Badenes R, Heras La Calle G, Orun OM, Chen W, Raman R, Simpson BK, Wilson-Linville S, Hinojal Olmedillo B, Vallejo de la Cueva A, van der Jagt M, Navarro Casado R, Leal Sanz P, Orhun G, Ferrer Gomez C, Nunez Vazquez K, Pineiro Otero P, Taccone FS, Gallego Curto E, Caricato A, Woien H, Lacave G, O'Neal HR, Jr, Peterson SJ, Brummel NE, Girard TD, Ely EW, Pandharipande PP, Group C-ICIS Prevalence and risk factors for delirium in critically ill patients with COVID-19 (COVID-D): a multicentre cohort study. Lancet Respir Med. 2021;9:239–250. doi: 10.1016/S2213-2600(20)30552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hshieh TT, Yue J, Oh E, Puelle M, Dowal S, Travison T, Inouye SK. Effectiveness of multicomponent nonpharmacological delirium interventions: a meta-analysis. JAMA Intern Med. 2015;175:512–520. doi: 10.1001/jamainternmed.2014.7779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu YC, Tseng PT, Tu YK, Hsu CY, Liang CS, Yeh TC, Chen TY, Chu CS, Matsuoka YJ, Stubbs B, Carvalho AF, Wada S, Lin PY, Chen YW, Su KP. Association of delirium response and safety of pharmacological interventions for the management and prevention of delirium: a network meta-analysis. JAMA Psychiatr. 2019;76:526–535. doi: 10.1001/jamapsychiatry.2018.4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12:103–111. doi: 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burry LD, Hutton B, Guenette M, Williamson D, Mehta S, Egerod I, Kanji S, Adhikari NK, Moher D, Martin CM, Rose L. Comparison of pharmacological and non-pharmacological interventions to prevent delirium in critically ill patients: a protocol for a systematic review incorporating network meta-analyses. Syst Rev. 2016;5:153. doi: 10.1186/s13643-016-0327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen JP, Mulrow C, Catala-Lopez F, Gotzsche PC, Dickersin K, Boutron I, Altman DG, Moher D. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 17.Rose L, Agar M, Burry LD, Campbell N, Clarke M, Lee J, Siddiqi N, Page VJ, Cg D. Development of core outcome sets for effectiveness trials of interventions to prevent and/or treat delirium (Del-COrS): study protocol. BMJ Open. 2017;7:e016371. doi: 10.1136/bmjopen-2017-016371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rose L, Burry L, Agar M, Campbell NL, Clarke M, Lee J, Marshall JC, Devlin JW, Blackwood B, Needham DM, Siddiqi N, Page V, Del CG (2021) A core outcome set for research evaluating interventions to prevent and/or treat delirium in critically ill adults: an international consensus study (Del-COrS). Crit Care Med. 10.1097/CCM.0000000000005028 [DOI] [PubMed]
- 19.Sud S, Friedrich JO, Adhikari NK, Fan E, Ferguson ND, Guyatt G, Meade MO (2021) Comparative effectiveness of protective ventilation strategies for moderate and severe ARDS: network meta-analysis. Am J Respir Crit Care Med 203(11):1366–1377. 10.1164/rccm.202008-3039OC [DOI] [PubMed]
- 20.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Puhan MA, Schunemann HJ, Murad MH, Li T, Brignardello-Petersen R, Singh JA, Kessels AG, Guyatt GH, Group GW. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ. 2014;349:g5630. doi: 10.1136/bmj.g5630. [DOI] [PubMed] [Google Scholar]
- 22.Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, Brignardello-Petersen R, Carrasco-Labra A, De Beer H, Hultcrantz M, Kuijpers T, Meerpohl J, Morgan R, Mustafa R, Skoetz N, Sultan S, Wiysonge C, Guyatt G, Schünemann HJ. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. 2020;119:126–135. doi: 10.1016/j.jclinepi.2019.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JP, White IR, Anzures-Cabrera J. Meta-analysis of skewed data: combining results reported on log-transformed or raw scales. Stat Med. 2008;27:6072–6092. doi: 10.1002/sim.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Mak. 2013;33:607–617. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Ser B (Stat Methodol) 2002;64:583–639. doi: 10.1111/1467-9868.00353. [DOI] [Google Scholar]
- 28.Brooks SP, Gelman A. General methods for monitoring convergence of iterative simulations. J Comput Graph Stat. 1998;7:434–455. [Google Scholar]
- 29.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol. 2011;64:163–171. doi: 10.1016/j.jclinepi.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 30.Donegan S, Williamson P, D'Alessandro U, Tudur Smith C. Assessing key assumptions of network meta-analysis: a review of methods. Res Synth Methods. 2013;4:291–323. doi: 10.1002/jrsm.1085. [DOI] [PubMed] [Google Scholar]
- 31.Gelman A, Ligges U, Sturtz S (2005) R2winbugs: a package for running WinBUGS from R. J Stat Softw. 10.18637/jss.v012.i03
- 32.Lunn D, Thomas A, Best N, Spiegelhalter D. WinBUGS—a Bayesian modeling framework: concepts, structure and extensibility. Stat Comput. 2000;10:325–337. doi: 10.1023/A:1008929526011. [DOI] [Google Scholar]
- 33.Spiegelhalter DJTA, Best N, and Lunn D (2014) OpenBUGS user manual, version 3.2.3. MRC Biostatistics Unit, Cambridge, UK http://www.openbugs.net/w/Manuals
- 34.Abbasi S, Farsaei S, Ghasemi D, Mansourian M. Potential role of exogenous melatonin supplement in delirium prevention in critically ill patients: a double-blind randomized pilot study. Iran J Pharm Res. 2018;17:1571–1580. [PMC free article] [PubMed] [Google Scholar]
- 35.Abdelgalel EF. Dexmedetomidine versus haloperidol for prevention of delirium during non-invasive mechanical ventilation. Egypt J Anaesth. 2016;32:473–481. doi: 10.1016/j.egja.2016.05.008. [DOI] [Google Scholar]
- 36.Al-Qadheeb NS, Skrobik Y, Schumaker G, Pacheco MN, Roberts RJ, Ruthazer RR, Devlin JW. Preventing ICU subsyndromal delirium conversion to delirium with low-dose IV haloperidol: a double-blind, placebo-controlled pilot study. Crit Care Med. 2016;44:583–591. doi: 10.1097/CCM.0000000000001411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alvarez EA, Garrido MA, Tobar EA, Prieto SA, Vergara SO, Briceno CD, Gonzalez FJ. Occupational therapy for delirium management in elderly patients without mechanical ventilation in an intensive care unit. A pilot randomized clinical trial. J Crit Care. 2017;40:265. doi: 10.1016/j.jcrc.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 38.Azeem TMA, Yosif NE, Alansary AM, Esmat IM, Mohamed AK. Dexmedetomidine vs morphine and midazolam in the prevention and treatment of delirium after adult cardiac surgery; a randomized, double-blinded clinical trial. Saudi J Anaesth. 2018;12:190–197. doi: 10.4103/sja.SJA_303_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azuma K, Takaesu Y, Soeda H, Iguchi A, Uchida K, Ohta S, Mishima S, Inoue T, Inoue Y, Oda J. Ability of suvorexant to prevent delirium in patients in the intensive care unit: a randomized controlled trial. Acute Med Surg. 2018;5:362–368. doi: 10.1002/ams2.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van den Boogaard M, Slooter AJC, Bruggemann RJM, Schoonhoven L, Beishuizen A, Vermeijden JW, Pretorius D, de Koning J, Simons KS, Dennesen PJW, Van Der Voort PHJ, Houterman S, van der Hoeven JG, Pickkers P, Reduce SI. Effect of haloperidol on survival among critically ill adults with a high risk of delirium: The REDUCE randomized clinical trial. JAMA. 2018;319:680–690. doi: 10.1001/jama.2018.0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Brummel NE, Girard TD, Ely EW, Pandharipande PP, Morandi A, Hughes CG, Graves AJ, Shintani A, Murphy E, Work B, Pun BT, Boehm L, Gill TM, Dittus RS, Jackson JC. Feasibility and safety of early combined cognitive and physical therapy for critically ill medical and surgical patients: the activity and cognitive therapy in ICU (ACT-ICU) trial. Intensive Care Med. 2014;40:370–379. doi: 10.1007/s00134-013-3136-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burkhart CS, Dell-Kuster S, Siegemund M, Pargger H, Marsch S, Strebel SP, Steiner LA. Effect of n-3 fatty acids on markers of brain injury and incidence of sepsis-associated delirium in septic patients. Acta Anaesthesiol Scand. 2014;58:689–700. doi: 10.1111/aas.12313. [DOI] [PubMed] [Google Scholar]
- 43.Chang YF, Chao A, Shih PY, Hsu YC, Lee CT, Tien YW, Yeh YC, Chen LW, Research NCoMM Comparison of dexmedetomidine versus propofol on hemodynamics in surgical critically ill patients. J Surg Res. 2018;228:194–200. doi: 10.1016/j.jss.2018.03.040. [DOI] [PubMed] [Google Scholar]
- 44.Chanques G, Conseil M, Roger C, Constantin JM, Prades A, Carr J, Muller L, Jung B, Belafia F, Cisse M, Delay JM, de Jong A, Lefrant JY, Futier E, Mercier G, Molinari N, Jaber S, investigators SO-Vs, Immediate interruption of sedation compared with usual sedation care in critically ill postoperative patients (SOS-Ventilation): a randomised, parallel-group clinical trial. Lancet Respir Med. 2017;5:795–805. doi: 10.1016/S2213-2600(17)30304-1. [DOI] [PubMed] [Google Scholar]
- 45.Chlan LL, Skaar DJ, Tracy MF, Hayes SM, Hetland BD, Savik K, Weinert CR. Safety and acceptability of patient-administered sedatives during mechanical ventilation. Am J Crit Care. 2017;26:288–296. doi: 10.4037/ajcc2017408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Damshens MSM, Javadpour S, Khaef MA, Rastgarian A (2018) The role of music on the delirium in traumatic patients: a case study in the ICU of Peymanieh Hospital of Jahrom, Fars Province, Iran. Ambient Sci. 10.21276/ambi.2018.05.sp1.ra11
- 47.de Jong B, Schuppers AS, Kruisdijk-Gerritsen A, Arbouw MEL, van den Oever HLA, van Zanten ARH. The safety and efficacy of nicotine replacement therapy in the intensive care unit: a randomised controlled pilot study. Ann Intensive Care. 2018;8:70. doi: 10.1186/s13613-018-0399-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Demoule A, Carreira S, Lavault S, Pallanca O, Morawiec E, Mayaux J, Arnulf I, Similowski T. Impact of earplugs and eye mask on sleep in critically ill patients: a prospective randomized study. Crit Care. 2017;21:284. doi: 10.1186/s13054-017-1865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.de Jonghe B, Leger J, Giraudeau B, Aissaoui N, Ehrmann S, SRLF Trial Group et al. Impact of oversedation prevention in ventilated critically ill patients: a randomized trial-the AWARE study. Ann Intensive Care. 2018;8:93. doi: 10.1186/s13613-018-0425-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devlin JW, Al-Qadheeb NS, Chi A, Roberts RJ, Qawi I, Garpestad E, Hill NS. Efficacy and safety of early dexmedetomidine during noninvasive ventilation for patients with acute respiratory failure. Chest. 2014;145:1204–1212. doi: 10.1378/chest.13-1448. [DOI] [PubMed] [Google Scholar]
- 51.Djaiani G, Silverton N, Fedorko L, Carroll J, Styra R, Rao V, Katznelson R. Dexmedetomidine versus propofol sedation reduces delirium after cardiac surgery: a randomized controlled trial. Anesthesiology. 2016;124:362–368. doi: 10.1097/ALN.0000000000000951. [DOI] [PubMed] [Google Scholar]
- 52.Eghbali-Babadi M, Shokrollahi N, Mehrabi T. Effect of family-patient communication on the incidence of delirium in hospitalized patients in cardiovascular surgery ICU. Iran J Nurs Midwifery Res. 2017;22:327–331. doi: 10.4103/1735-9066.212985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Finotto S, Artioli G, Davoli L, Barbara B. Nursing interventions for the prevention of the delirium in intensive care unit (ICU): a randomized study. ProfInferm. 2006;59:228–232. [PubMed] [Google Scholar]
- 54.Foreman B, Westwood AJ, Claassen J, Bazil CW. Sleep in the neurological intensive care unit: feasibility of quantifying sleep after melatonin supplementation with environmental light and noise reduction. J Clin Neurophysiol. 2015;32:66–74. doi: 10.1097/WNP.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 55.Gandolfi JV, Di Bernardo APA, Chanes DAV, Martin DF, Joles VB, Amendola CP, Sanches LC, Ciorlia GL, Lobo SM. The effects of melatonin supplementation on sleep quality and assessment of the serum melatonin in ICU patients: a randomized controlled trial. Crit Care Med. 2020;48:e1286–e1293. doi: 10.1097/CCM.0000000000004690. [DOI] [PubMed] [Google Scholar]
- 56.Girard TD, Kress JP, Fuchs BD, Thomason JWW, Schweickert WD, Pun BT, Taichman DB, Dunn JG, Pohlman AS, Kinniry PA, Jackson JC, Canonico AE, Light RW, Shintani AK, Thompson JL, Gordon SM, Hall JB, Dittus RS, Bernard GR, Ely EW. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (awakening and breathing controlled trial): a randomised controlled trial. Lancet. 2008;371:126–134. doi: 10.1016/S0140-6736(08)60105-1. [DOI] [PubMed] [Google Scholar]
- 57.Giraud K, Pontin M, Sharples LD, Fletcher P, Dalgleish T, Eden A, Jenkins DP, Vuylsteke A. Use of a structured mirrors intervention does not reduce delirium incidence but may Improve factual memory encoding in cadiac surgical ICU patients aged over 70 years: a pilot time-cluster randomized controlled trial. Front Aging Neurosci. 2016;8:228. doi: 10.3389/fnagi.2016.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo Y, Sun L, Li L, Jia P, Zhang J, Jiang H, Jiang W. Impact of multicomponent, nonpharmacologic interventions on perioperative cortisol and melatonin levels and postoperative delirium in elderly oral cancer patients. Arch Gerontol Geriatr. 2016;62:112–117. doi: 10.1016/j.archger.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 59.Hakim SM, Othman AI, Naoum DO. Early treatment with risperidone for subsyndromal delirium after on-pump cardiac surgery in the elderly: a randomized trial. Anesthesiology. 2012;116:987–997. doi: 10.1097/ALN.0b013e31825153cc. [DOI] [PubMed] [Google Scholar]
- 60.Hu ZL, Zhang ZC, Li DW, Shuai WZ, Zou JF. The use of dexmedetomidine combined with propofol in mechanically ventilated patients. Med J Chin People's Lib Army. 2015;40:479–483. [Google Scholar]
- 61.Huang F, Wang J, Yang X, Xu H, Kong J, Liu S, Jin J. Sedative effects of dexmedetomidine in post-operative elder patients on mechanical ventilation. Chung Hua I Hsueh Tsa Chih. 2014;94:3211–3215. [PubMed] [Google Scholar]
- 62.Hughes CG, Mailloux PT, Devlin JW, Swan JT, Sanders RD, Anzueto A, Jackson JC, Hoskins AS, Pun BT, Orun OM, Raman R, Stollings JL, Kiehl AL, Duprey MS, Bui LN, O'Neal HR, Jr, Snyder A, Gropper MA, Guntupalli KK, Stashenko GJ, Patel MB, Brummel NE, Girard TD, Dittus RS, Bernard GR, Ely EW, Pandharipande PP, Investigators MS. Dexmedetomidine or propofol for sedation in mechanically ventilated adults with sepsis. N Engl J Med. 2021;384:1424–1436. doi: 10.1056/NEJMoa2024922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Javaherforoosh Zadeh F, Janatmakan F, Shafaeebejestan E, Jorairahmadi S. Effect of melatonin on delirium after on-pump coronary artery bypass graft surgery: a randomized clinical trial. Iran J Med Sci. 2021;46:120–127. doi: 10.30476/ijms.2020.82860.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johansson L, Lindahl B, Knutsson S, Ogren M, Persson WK, Ringdal M (2017) Evaluation of a sound environment intervention in an ICU: a feasibility study. Aust Crit Care 31(2):59–70. 10.1016/j.aucc.2017.04.001 [DOI] [PubMed]
- 65.Johnson K, Fleury J, McClain D. Music intervention to prevent delirium among older patients admitted to a trauma intensive care unit and a trauma orthopaedic unit. Intensive Crit Care Nurs. 2018;47:7–14. doi: 10.1016/j.iccn.2018.03.007. [DOI] [PubMed] [Google Scholar]
- 66.Karadas C, Ozdemir L. The effect of range of motion exercises on delirium prevention among patients aged 65 and over in intensive care units. Geriatr Nurs. 2016;37:180–185. doi: 10.1016/j.gerinurse.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 67.Kawazoe Y, Miyamoto K, Morimoto T, Yamamoto T, Fuke A, Hashimoto A, Koami H, Beppu S, Katayama Y, Itoh M, Ohta Y, Yamamura H, Dexmedetomidine for Sepsis in Intensive Care Unit Randomized Evaluation Trial I Effect of dexmedetomidine on mortality and ventilator-free days in patients requiring mechanical ventilation with sepsis: a randomized clinical trial. JAMA. 2017;317:1321–1328. doi: 10.1001/jama.2017.2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Keh D, Trips E, Marx G, Wirtz SP, Abduljawwad E, Bercker S, Bogatsch H, Briegel J, Engel C, Gerlach H, Goldmann A, Kuhn SO, Huter L, Meier-Hellmann A, Nierhaus A, Kluge S, Lehmke J, Loeffler M, Oppert M, Resener K, Schadler D, Schuerholz T, Simon P, Weiler N, Weyland A, Reinhart K, Brunkhorst FM, SepNet-Critical Care Trials G Effect of hydrocortisone on development of shock among patients with severe sepsis: The HYPRESS randomized clinical trial. JAMA. 2016;316:1775–1785. doi: 10.1001/jama.2016.14799. [DOI] [PubMed] [Google Scholar]
- 69.Khan BA, Perkins AJ, Campbell NL, Gao S, Khan SH, Wang S, Fuchita M, Weber DJ, Zarzaur BL, Boustani MA, Kesler K. Preventing postoperative delirium after major noncardiac thoracic surgery-a randomized clinical trial. J Am Geriatr Soc. 2018;66:2289–2297. doi: 10.1111/jgs.15640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan SH, Xu C, Purpura R, Durrani S, Lindroth H, Wang S, Gao S, Heiderscheit A, Chlan L, Boustani M, Khan BA. Decreasing delirium through music: a randomized pilot trial. Am J Crit Care. 2020;29:e31–e38. doi: 10.4037/ajcc2020175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li J, Dong C, Zhang H, Zhang H, Song R, Yang Z, Feng F, Qi Y, Yang J. Study of prevention and control of delirium in ventilated patients by simulating blockage of circadian rhythm with sedative in intensive care unit. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2016;28:50–56. doi: 10.3760/cma.j.issn.2095-4352.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 72.Litton E, Elliott R, Ferrier J, Webb SAR. Quality sleep using earplugs in the intensive care unit: the QUIET pilot randomised controlled trial. Crit Care Resusc. 2017;19:128–133. [PubMed] [Google Scholar]
- 73.Liu D, Lyu J, Zhao H, An Y. The influence of analgesic-based sedation protocols on delirium and outcomes in critically ill patients: a randomized controlled trial. PLoS ONE. 2017;12(e0184310):0182017. doi: 10.1371/journal.pone.0184310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lyu J, Liu D, An Y, Feng Y. The influence of the sedation based on remifentanil analgesia on the occurrence of delirium in critically ill patients. Chin Crit Care Med. 2015;27:845–849. [PubMed] [Google Scholar]
- 75.Mahrose R, Elserwi H, Maurice A, Elsersi M. Postoperative delirium after coronary artery bypass graft surgery: dexmedetomidine infusion alone or with the addition of oral melatonin. Egypt J Anaesth. 2021;37:62–68. doi: 10.1080/11101849.2021.1885956. [DOI] [Google Scholar]
- 76.Mehta S, Burry L, Cook D, Fergusson D, Steinberg M, Granton J, Herridge M, Ferguson N, Devlin J, Tanios M, Dodek P, Fowler R, Burns K, Jacka M, Olafson K, Skrobik Y, Hebert P, Sabri E, Meade M, Sleap I, Canadian Critical Care Trials G Daily sedation interruption in mechanically ventilated critically ill patients cared for with a sedation protocol: a randomized controlled trial. JAMA. 2012;308:1985–1992. doi: 10.1001/jama.2012.13872. [DOI] [PubMed] [Google Scholar]
- 77.Mitchell ML, Kean S, Rattray JE, Hull AM, Davis C, Murfield JE, Aitken LM. A family intervention to reduce delirium in hospitalised ICU patients: a feasibility randomised controlled trial. Intensive Crit Care Nurs. 2017;40:77–84. doi: 10.1016/j.iccn.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 78.Mohammadi M, Ahmadi M, Khalili H, Cheraghchi H, Arbabi M. Cyproheptadine for the prevention of postoperative delirium: a pilot study. Ann Pharmacother. 2016;50:180–187. doi: 10.1177/1060028015624938. [DOI] [PubMed] [Google Scholar]
- 79.Mokhtari M, Farasatinasab M, Jafarpour Machian M, Yaseri M, Ghorbani M, Ramak Hashemi SM, Nikoobakht M, Golchin N, Mohammadi G, Sistanizad M. Aripiprazole for prevention of delirium in the neurosurgical intensive care unit: a double-blind, randomized, placebo-controlled study. Eur J Clin Pharmacol. 2020;76:491–499. doi: 10.1007/s00228-019-02802-1. [DOI] [PubMed] [Google Scholar]
- 80.Moon KJ, Lee SM. The effects of a tailored intensive care unit delirium prevention protocol: a randomized controlled trial. Int J Nurs Stud. 2015;52:1423–1432. doi: 10.1016/j.ijnurstu.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 81.Nassar Junior AP, Park M. (2014) Daily sedative interruption versus intermittent sedation in mechanically ventilated critically ill patients: a randomized trial. Ann Intensive Care. 2014;4:14. doi: 10.1186/2110-5820-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishikimi M, Numaguchi A, Takahashi K, Miyagawa Y, Matsui K, Higashi M, Makishi G, Matsui S, Matsuda N (2018) Effect of administration of ramelteon, a melatonin receptor agonist, on the duration of stay in the ICU: a single-center randomized placebo-controlled trial. Crit Care Med 46(7):1099–1105. 10.1097/CCM.0000000000003132 [DOI] [PMC free article] [PubMed]
- 83.Nydahl P, Gunther U, Diers A, Hesse S, Kerschensteiner C, Klarmann S, Borzikowsky C, Kopke S. PROtocol-based MObilizaTION on intensive care units: stepped-wedge, cluster-randomized pilot study (pro-motion) Nurs Crit Care. 2020;25:368–375. doi: 10.1111/nicc.12438. [DOI] [PubMed] [Google Scholar]
- 84.Ono H, Taguchi T, Kido Y, Fujino Y, Doki Y. The usefulness of bright light therapy for patients after oesophagectomy. Intensive Crit Care Nurs. 2011;27:158–166. doi: 10.1016/j.iccn.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Pandharipande PP, Pun BT, Herr DL, Maze M, Girard TD, Miller RR, Shintani AK, Thompson JL, Jackson JC, Deppen SA, Stiles RA, Dittus RS, Bernard GR, Ely EW. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–2653. doi: 10.1001/jama.298.22.2644. [DOI] [PubMed] [Google Scholar]
- 86.Park JB, Bang SH, Chee HK, Kim JS, Lee SA, Shin JK. Efficacy and safety of dexmedetomidine for postoperative delirium in adult cardiac surgery on cardiopulmonary bypass. Korean J Physiol Pharmacol Thorac Cardiovasc Surg. 2014;47:249–254. doi: 10.5090/kjtcs.2014.47.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Perbet S, Verdonk F, Godet T, Jabaudon M, Chartier C, Cayot S, Guerin R, Morand D, Bazin JE, Futier E, Pereira B, Constantin JM. Low doses of ketamine reduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: a randomised double-blind control trial. Anaesth Crit Care Pain Med. 2018;37:589–595. doi: 10.1016/j.accpm.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 88.Potharajaroen S, Tangwongchai S, Tayjasanant T, Thawitsri T, Anderson G, Maes M. Bright light and oxygen therapies decrease delirium risk in critically ill surgical patients by targeting sleep and acid-base disturbances. Psychiatry Res. 2018;261:21–27. doi: 10.1016/j.psychres.2017.12.046. [DOI] [PubMed] [Google Scholar]
- 89.Prakanrattana U, Prapaitrakool S. Efficacy of risperidone for prevention of postoperative delirium in cardiac surgery. Anaesth Intensive Care. 2007;35:714–719. doi: 10.1177/0310057X0703500509. [DOI] [PubMed] [Google Scholar]
- 90.Priye S, Jagannath S, Singh D, Shivaprakash S, Reddy DP. Dexmedetomidine as an adjunct in postoperative analgesia following cardiac surgery: a randomized, double-blind study. Saudi J Anaesth. 2015;9:353–358. doi: 10.4103/1658-354X.154715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rice KL, Bennett MJ, Berger L, Jennings B, Eckhardt L, Fabre-LaCoste N, Houghton D, Vidal G, Gropen T, Diggs E, Barry E, John J, Mathew S, Egger A, Ryan S, Egger R, Galarneau D, Gaines K, Ely EW. A pilot randomized controlled trial of the feasibility of a multicomponent delirium prevention intervention versus usual care in acute stroke. J Cardiovasc Nurs. 2017;32:E1–E10. doi: 10.1097/JCN.0000000000000356. [DOI] [PubMed] [Google Scholar]
- 92.Robinson TN, Dunn CL, Adams JC, Hawkins CL, Tran ZV, Raeburn CD, Moss M. Tryptophan supplementation and postoperative delirium—a randomized controlled trial. J Am Geriatr Soc. 2014;62:1764–1771. doi: 10.1111/jgs.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rubino AS, Onorati F, Caroleo S, Galato E, Nucera S, Amantea B, Santini F, Renzulli A. Impact of clonidine administration on delirium and related respiratory weaning after surgical correction of acute type-A aortic dissection: results of a pilot study. Interact Cardiovasc Thorac Surg. 2010;10:58–62. doi: 10.1510/icvts.2009.217562. [DOI] [PubMed] [Google Scholar]
- 94.Ruokonen E, Parviainen I, Jakob SM, Nunes S, Kaukonen M, Shepherd ST, Sarapohja T, Bratty JR, Takala J. Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med. 2009;35(2):282–290. doi: 10.1007/s00134-008-1296-0. [DOI] [PubMed] [Google Scholar]
- 95.Schaller SJ, Anstey M, Blobner M, Edrich T, Grabitz SD, Gradwohl-Matis I, Heim M, Houle T, Kurth T, Latronico N, Lee J, Meyer MJ, Peponis T, Talmor D, Velmahos GC, Waak K, Walz JM, Zafonte R, Eikermann M, International Early S-gMRI Early, goal-directed mobilisation in the surgical intensive care unit: a randomised controlled trial. Lancet. 2016;388:1377–1388. doi: 10.1016/S0140-6736(16)31637-3. [DOI] [PubMed] [Google Scholar]
- 96.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, Spears L, Miller M, Franczyk M, Deprizio D, Schmidt GA, Bowman A, Barr R, McCallister KE, Hall JB, Kress JP. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shehabi Y, Grant P, Wolfenden H, Hammond N, Bass F, Campbell M, Chen J. Prevalence of delirium with dexmedetomidine compared with morphine based therapy after cardiac surgery: a randomized controlled trial (DEXmedetomidine COmpared to Morphine-DEXCOM Study) Anesthesiology. 2009;111:1075–1084. doi: 10.1097/ALN.0b013e3181b6a783. [DOI] [PubMed] [Google Scholar]
- 98.Shehabi Y, Bellomo R, Reade MC, Bailey M, Bass F, Howe B, McArthur C, Murray L, Seppelt IM, Webb S, Weisbrodt L, Sedation Practice in Intensive Care Evaluation Study I, Australian, New Zealand Intensive Care Society Clinical Trials G Early goal-directed sedation versus standard sedation in mechanically ventilated critically ill patients: a pilot study*. Crit Care Med. 2013;41:1983–1991. doi: 10.1097/CCM.0b013e31828a437d. [DOI] [PubMed] [Google Scholar]
- 99.Shehabi Y, Howe BD, Bellomo R, Arabi YM, Bailey M, Bass FE, Bin Kadiman S, McArthur CJ, Murray L, Reade MC, Seppelt IM, Takala J, Wise MP, Webb SA, Group ACT, the SIIII Early sedation with dexmedetomidine in critically ill patients. N Engl J Med. 2019;380:2506–2517. doi: 10.1056/NEJMoa1904710. [DOI] [PubMed] [Google Scholar]
- 100.Shu AFY, Luo Y, Cao J, Jian W, Sun B, Tang Z. An investigation on delirium and hemodynamics in influenced by dexmedetomidine for sedating elderly patients in mechanical ventilation. Int J Clin Exp Med. 2019;12:1942–1946. [Google Scholar]
- 101.Simons KS, Laheij RJ, van den Boogaard M, Moviat MA, Paling AJ, Polderman FN, Rozendaal FW, Salet GA, van der Hoeven JG, Pickkers P, de Jager CP. Dynamic light application therapy to reduce the incidence and duration of delirium in intensive-care patients: a randomised controlled trial. Lancet Respir Med. 2016;4:194–202. doi: 10.1016/S2213-2600(16)00025-4. [DOI] [PubMed] [Google Scholar]
- 102.Skrobik Y, Duprey MS, Hill NS, Devlin JW (2018) Low-dose nocturnal dexmedetomidine prevents ICU delirium: a randomized, placebo-controlled trial. Am J Respir Crit Care Med 197(9):1147–1156. 10.1164/rccm.201710-1995OC [DOI] [PubMed]
- 103.Song R, Li J, Dong C, Yang J. A study of using dexmedetomidine in ventilator bundle treatment in an ICU. Chin Crit Care Med. 2015;27:836–840. [PubMed] [Google Scholar]
- 104.Sosnowski K, Mitchell ML, White H, Morrison L, Sutton J, Sharratt J, Lin F. A feasibility study of a randomised controlled trial to examine the impact of the ABCDE bundle on quality of life in ICU survivors. Pilot Feasibility Stud. 2018;4:32. doi: 10.1186/s40814-017-0224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Spies C, MacGuill M, Heymann A, Ganea C, Krahne D, Assman A, Kosiek HR, Scholtz K, Wernecke KD, Martin J. A prospective, randomized, double-blind, multicenter study comparing remifentanil with fentanyl in mechanically ventilated patients. Intensive Care Med. 2011;37:469–476. doi: 10.1007/s00134-010-2100-5. [DOI] [PubMed] [Google Scholar]
- 106.Strom T, Martinussen T, Toft P. A protocol of no sedation for critically ill patients receiving mechanical ventilation: a randomised trial. Lancet. 2010;375:475–480. doi: 10.1016/S0140-6736(09)62072-9. [DOI] [PubMed] [Google Scholar]
- 107.Su X, Meng ZT, Wu XH. Dexmedetomidine for prevention of delirium in elderly patients after noncardiac surgery: a randomised, double-blind, placebo-controlled trial. J Neurosurg Anesthesiol. 2017;29:178–179. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]
- 108.Susheela AT, Packiasabapathy S, Gasangwa DV, Patxot M, O'Neal J, Marcantonio E, Subramaniam B. The use of dexmedetomidine and intravenous acetaminophen for the prevention of postoperative delirium in cardiac surgery patients over 60 years of age: a pilot study. F1000Res. 2017;6:1842. doi: 10.12688/f1000research.12552.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Taguchi T, Yano M, Kido Y. Influence of bright light therapy on postoperative patients: a pilot study. Intensive Crit Care Nurs. 2007;23:289–297. doi: 10.1016/j.iccn.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 110.Takieddine SC, Droege CA, Ernst N, Droege ME, Webb M, Branson RD, Gerlach TW, Robinson BRH, Johannigman JA, Mueller EW. Ketamine versus hydromorphone patient-controlled analgesia for acute pain in trauma patients. J Surg Res. 2018;225:6–14. doi: 10.1016/j.jss.2017.12.019. [DOI] [PubMed] [Google Scholar]
- 111.Van Rompaey B, Elseviers MM, Van DW, Fromont V, Jorens PG. The effect of earplugs during the night on the onset of delirium and sleep perception: a randomized controlled trial in intensive care patients. Crit Care. 2012;16(3):R73. doi: 10.1186/cc11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wan LJ, Huang QQ, Yue JX, Lin L, Li SH. Comparison of sedative effect of dexmedetomidine and midazolam for post-operative patients undergoing mechanical ventilation in surgical intensive care unit. Zhongguo Weizhongbing Jijiuyixue. 2011;23:543–546. [PubMed] [Google Scholar]
- 113.Wang W, Li HL, Wang DX, Zhu X, Li SL, Yao GQ, Chen KS, Gu XE, Zhu SN. Haloperidol prophylaxis decreases delirium incidence in elderly patients after noncardiac surgery: a randomized controlled trial*. Crit Care Med. 2012;40:731–739. doi: 10.1097/CCM.0b013e3182376e4f. [DOI] [PubMed] [Google Scholar]
- 114.Lewis K, Piticaru J, Chaudhuri D, Basmaji J, Fan E, Moller MH, Devlin JW, Alhazzani W (2021) Safety and efficacy of dexmedetomidine in acutely ill adults requiring noninvasive ventilation: a systematic review and meta-analysis of randomized trials. Chest 159(6):2274–2288. 10.1016/j.chest.2020.12.052 [DOI] [PMC free article] [PubMed]
- 115.Wang G, Niu J, Li Z, Lv H, Cai H. The efficacy and safety of dexmedetomidine in cardiac surgery patients: a systematic review and meta-analysis. PLoS ONE. 2018;13:e0202620. doi: 10.1371/journal.pone.0202620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alexopoulou C, Kondili E, Diamantaki E, Psarologakis C, Kokkini S, Bolaki M, Georgopoulos D. Effects of dexmedetomidine on sleep quality in critically ill patients: a pilot study. Anesthesiology. 2014;121:801–807. doi: 10.1097/ALN.0000000000000361. [DOI] [PubMed] [Google Scholar]
- 117.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 118.Oh ES, Needham DM, Nikooie R, Wilson LM, Zhang A, Robinson KA, Neufeld KJ. Antipsychotics for preventing delirium in hospitalized adults: a systematic review. Ann Intern Med. 2019;171:474–484. doi: 10.7326/M19-1859. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.