Abstract
Background
IgA nephropathy (IgAN) is most common primary glomerulopathy. There are variations in prevalence of IgAN and its clinical features in different studies from India.
Aim
To summarize overall scenario of IgAN in India.
Methods
In this systematic review, studies related to IgAN and related renal disease were included. Data searched were PubMed, EMBASE, Google scholar, and Cochrane Database from inception to 31st January 2019
Results
Total 49 studies (N=2480) were included: 21 studies (N=2309) of primary IgAN; 19 studies (N=21) of Secondary IgAN; four studies (N=133) of IgA vasculitis nephropathy (IgAVN); and five studies (N=17) of IgA dominant nephropathy (IgADN). Prevalence of IgAN was 16.5% in India. Age of affected persons was ranging from 27.2±16.7 to 48.6±21.3 years . Male female ratio was 1.8:1. Clinical features of Primary IgAN, IgAVN, IgADN & Secondary IgAN were microscopic hematuria (49.6%, 44.4%, 15.6% & 59.5%), macroscopic hematuria (5.1%, 0.4%,40.9%,& 35.7%), Subnephrotic proteinuria (42.1%, 29.4%, 23.2%, & 52.3%), nephrotic proteinuria (16.0%, 4.4%, 76.8%,& 47.6%), and hypertension (25.8%,18.3%, 35.5%,& 47.6%).. The 24 hours proteinuria was ranging from 2.6±1.5 to 4.7±2.3 gm/day and serum creatinine (mg/dl) was ranging from 0.9±0 to 3.5±3.9 mg/dl. Histolomorphologically, all type of IgAN showed mesangial hypercellularity and Immunofluorescence revealed IgA deposition..
Conclusion
The overall prevalence of primary IgAN in India was 16.5%. The subnephrotic proteinuria and microscopic hematuria were common clinical features.
Keywords: IgA Nephropathy, histomorphology, prevalence, India
Introduction
IgA nephropathy (IgAN) was first demonstrated by Berger in 1768.1 It is characterized by persistent microscopic hematuria, sub-nephrotic proteinuria, episodic gross hematuria, normal to severe impairment of renal function and hypertension.1,2
Histomorphology varies from normal to chronic glomerulonephritis on renal biopsy of these patients. The most common histomorphology is focal glomerulonephritis. 1,2 On immunofluorescence microscopy (IF), all cases had mesangial deposition of IgA dominantly and less commonly weak staining for IgG and C3. Theelectron microscopy (EM) had presence of mesangial immune complex deposits.1,2 IgA nephropathy is most common primary glomerulonephritis world-wide.3,4 IgA nephropathy mostly diagnosed in certain Asian countries, e.g. Japan, Mainland China, and Singapore.5,6 The incidence of IgA nephropathy ranges from 4.0% to 35.5% in the world.7 IgA nephropathy occurs in all age groups from children to elderly peoples, but it mostly affected age group is 10 to 40 years..8 Clinically features of patients with IgA nephropathy include microscopic hematuria or gross hematuria after one to two days or at a time of fever or upper respiratory tract infection (sore throat), but occasionally it may be associated with gastroenteritis, pneumonia, or urinary tract infection.9 It may remain asymptomatic and diagnosed incidentally or during routine screening of urine.9 The incidence and prevalence of IgA nephropathy is varied in different studies from India.7 Aim of index study was to evaluate the scenario of IgA nephropathy in India.
Methods
We performed a systematic review and meta-analysis to assess the overall scenario of IgAN in India. We included studies of IgA nephropathy and IgA related renal diseases reported from India. Type of studies included were observational studies. We excluded randomized controlled trials and studies from outside of India. Studies including both children and adults were eligible for inclusion in the review. Renal biopsy was considered as reference standard. We searched PubMed (inception to 31st January 2019), EMBASE (inception to 31st January 2019), Google scholar (inception to 31st January 2019) and Cochrane Database Reviews on 31st January 2019. Search strategy for PubMed included (IgA nephropathy) OR IgAN)). We searched EMBASE with key words /exp IgA GN OR IgA nephropathy AND (IgAN), Google scholar with IgA GN or IgA nephropathy and Cochrane Database Reviews with IgA nephropathy. References of included studies were hand searched for additional studies. We assessed the studies for inclusion and extracted data for review.
Primary outcome of review was prevalence of IgA nephropathy in India. Secondary outcomes included clinical features, laboratory findings, histopathological pattern, immunofluorescences findings and electron microscopic findings of IgAN from India in comparison to foreign studies.
Statistical methods
The data were entered in excel sheet and analyzed using STATA 12.0 and Cochrane Rev Man 5.1. Continuous outcome data were reported as mean and dichotomous data as percentages.
The Newcastle-Ottawa Scale (NOS) was used for assessing quality of nonrandomized studies (cohort and case-control studies).10
Results
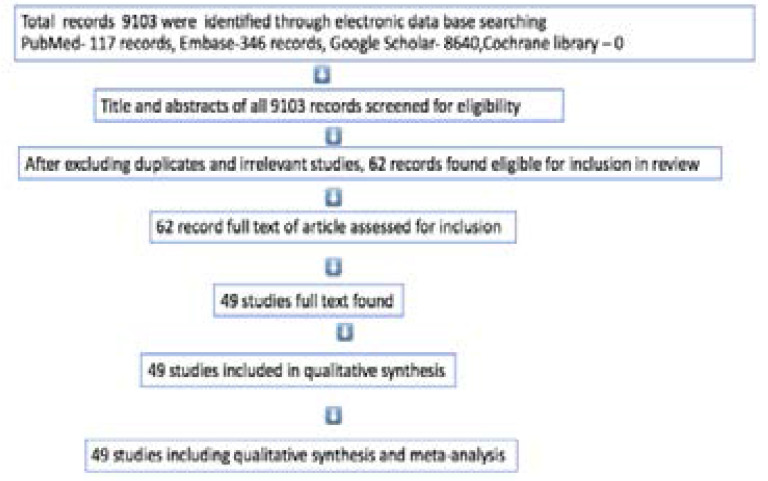
The study selection flow diagram is shown in Figure 1. By electronic database search we found a total of 9103 records and screened titles and/or abstracts of all records. After excluding duplicates and irrelevant studies, 62 eligible studies were assessed for full texts. Full texts were found for 49 studies and these were including for qualitative synthesis and meta-analysis.
Figure 1.
Study selection flow diagram
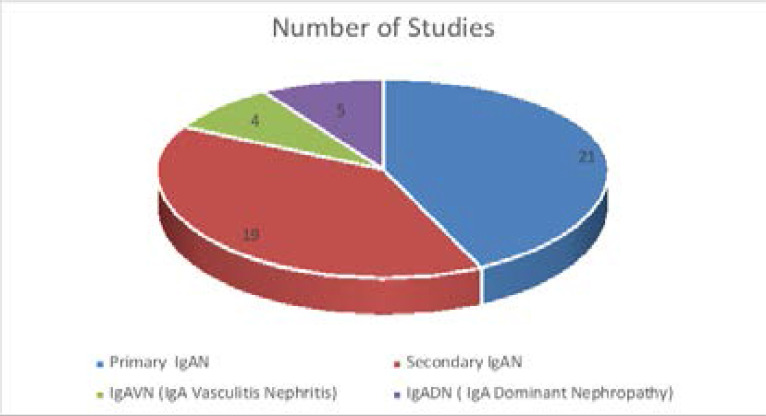
Out of 49 studies, 21 studies (2309 cases) were related to Primary IgAN, 19 studies (21 cases) were related to secondary IgA nephropathy (Secondary IgAN), four studies (133 cases) were related to Henoch-Schönlein purpura (HSP) with IgA nephropathy or IgA vasculitis nephritis (IgAVN), and five studies (17 cases) were related to IgA dominant nephropathy (IgADN) (figure 2). The frequency/ prevalence of IgA nephropathy was reported in 14 studies and is shown in Table 1.
Figure 2.
Subgrouping of the 49 studies in four
Table 1.
Frequencies/prevalence (%) of IgA nephropathy in India
| S. No. |
Reference | Year | Study population Children, Adult |
Prevalence of IgA N (%) |
| 1. | Chacko et al | 2011 | C, A | 8.6 |
| 2 | Ganesh et al | 2018 | C, A | 21.6 |
| 3 | Siddappa S | 2011 | A | 7.8 |
| 4 | Das et al | 2015 | A, C | 7.5 |
| 5 | Agrawal et al | 2017 | A | 2.6 |
| 6 | Mittal et al | 2012 | A | 8.1 |
| 7 | Chandrika et al | 2009 | A, C | 14.2 |
| 8 | Ramakrishan | 2016 | A | 11.5 |
| 9 | Chacko et al, | 2007 | A,C | 32 |
| 10 | Vanikar et al, | 2005 | A | 16.5 |
| 11. | Bhuyan et al | 1992, 87 | A,C | 7.24 |
| 12. | Tiwari et al | 2016 | A,C | 14.3 |
| 13 | Chowdary et al | 2018 | A | 42.0 |
| 14 | Dhanapriya et al | 2018 | A | 5 |
| After pooling the data total prevalence/frequencies | 16.5% | |||
| C-children, A-Adult, | ||||
Clinical and laboratory features of Primary IgA nephropathy, IgA vasculitis nephritis (HSP), IgA dominant nephritis and Secondary IgAN are shown in Table 2. Histomorphology (MEST-C& Haas's classification), Immunofluorescences, and Electron microscopic findings of Primary IgAN, IgAVN (HSP), IgADN and Secondary IgAN are described in Table 3.
Table 2.
Clinical and Laboratory features of Primary IgA nephropathy, IgA vasculitis nephritis (HSP), IgA dominant nephritis and Secondary IgAN
| S.No. | Main clinical features | Primary IgA nephropathy |
IgA vasculitis nephritis(HSP) |
IgA dominant nephritis |
Secondary IgAN |
| 1. | Mean age (years) | 31.7±13.8 | 37.5±15.8 | 48.6±21.3 | 27.2±16.7 |
| 2. | Male : female | 1.93:1 | 1.07:1 | 1.64:1 | 2:1 |
| 3. | Asymptomatic urinary abnormalities (%) |
5.5 | 1.4 | 0 | 9.5 |
| 4. | Macroscopic hematuria(%) | 5.1 | 0.44 | 40.94 | 35.71 |
| 5. | Microscopic hematuria(%) | 49.6 | 44.49 | 15.64 | 59.57 |
| 6. | Subnephrotic proteinuria (>1g/d) (%) |
42.1 | 29.42 | 23.29 | 52.38 |
| 7 | Nephrotic range proteinuria (>3g/d) (%) |
16.04 | 4.44 | 76.88 | 47.61 |
| 8. | Loin or abdominal pain(%) | 1.8 | 61.72 | 0 | 4.7 |
| 9. | Hypertension(%) | 25.8 | 18.39 | 35.5 | 47.61 |
| 10. | Infection-related exacerbations(%) |
10.3 | 19.14 | 47.05 | 4.7 |
| 11. | Skin Rashes(%) | 0 | 99.24 | 0 | 0 |
| 12. | GIT manifestation(%) | 0 | 54.83 | 5.8 | 0 |
| 13. | Arthritis/arthralgia(%) | 0 | 78.58 | 0 | 0 |
| 14. | Duration of symptoms | 5.40±3.2 | 3.91±0.59 | 2.87±1.84 | 2.81±3.9 |
| 15. | Raised serum creatinine | 3.04±2.6 | 0.9±0 | 3.21±20 | 3.5±3.9 |
| 16. | Reduced e-GFR | 59.54±19.3 | - | 63.6±0 | 78±0 |
| 17. | 24 h urine protein (g/d) | 2.6±1.5 | 3.2±0 | 3.5±0.2 | 4.7±2.3 |
| 18. | Serum C3 level reduced (%) | 0 | 0.06 | 88.47 | 0 |
Table 3.
Histomorphology (MEST & Haas's classification, Immunofluorescences and Electron microscopic findings in primary IgA nephropathy, IgA vasculitis nephritis (HSP), IgA dominant nephritis and Secondary IgAN
| S.No. | Types IgAN | Investigations | |||||||||||
| Histomorphology(%) | Immunofluorescence (%) | Electron microscopy(%) | |||||||||||
| MEST-C classification |
Haas's classification |
Skin biopsy |
IgG | IgA | IgM | C3 | F.H. | MeD | SubEnD | SubEpD | Both | ||
| Renal biopsy | |||||||||||||
| 1. | Primay IgAN | Me-59 En-10.4 SG-20.9 C1–3.6 C2–5.3 T0-2.8 T1-11.5 T2-9.6 N-1.03 |
Cl I -11.1 Cl II-10.6 Cl III-13 Cl IV-7.5 Cl V-16.2 |
- | 1.6 | 100 | 4.5 | 4.6 | 0.2 | 26.4 | 0.58 | 0 | 17.3 |
| 2. | IgAVN | Me-9.7 En-6.7 SG-0.7 C1-2.2 T0-2.2 |
- | LC-33.8 | 0 | 100 | 0 | 22.5 | 0 | - | - | - | - |
| 3. | IgADN | Me-29.4 En-47.0 C2-58.8 |
- | - | 12.2 | 100 | 1 | 89.7 | 0 | 0 | 5.8 | 11.7 | 5.8 |
| 4. | Secondary IgAN |
Me-95.2 En-23.8 SG-33.3 C1-19 T0-14.2 |
- | - | 16.6 | 100 | 5.9 | 9.5 | 0 | 9.5 | 0 | 4.7 | 0 |
Me-Mesangial proliferation, En-Endocapillary proliferation, SG-Segmental Glomerulosclerosis, C1- <25% Cellular/fibrocellular crescent, C2- >25%Cellular/Fibrocellular crescent, T0- ≤25%TA/IF T1- 26% to 50% TA/IF, T2->50%TA/IF, N-Normal, Cl- Class, MeD-mesangial deposition, SubEnD-subenothelial deposition, SubepD-subepithelial deposition, Both-MeD+EnD, ,HistoM-histomorphology, LC- leukocytoclastic vasculitis ,F.H.-Full House
Discussion
We found combined prevalence of IgAN as 16.5% of in India. The prevalence of IgA nephropathy showed considerable variations (0.8–47%) among geographic regions with different renal biopsy practices.3,6,25–26 The prevalence in India was lower than certain Asian counties e.g. Japan (47.4%) and China(45%).25–26 We found higher prevalence of IgAN in comparison to study by Seedate et al (13.3%) from African country Natal done on Indians.27 The IgA nephropathy was uncommon (0.8%) in African blacks.27 The frequencies of IgAN was higher in India than the studies from United States that had prevalence of 10.8% and 9.4%.28 We were unable to pool the data for incidence because the studies were not accurately defined the incidence. Primary IgAN and Secondary IgAN was found in younger age groups and it is similarly reported by other studies29,30 Males were more commonly effected than females and our findings corroborated with other studies27,29 We found that IgADN was presented in higher age group (48.6±21.3) than Primary IgAN, IgAVN, and Secondary IgAN. Similarly, Nasr et al reported IgADN in elderly age group.31 We also found rapid onset of symptoms in primary IgAN and Secondary IgAN as described by other studies.9,32 It was different from PIGN (post infection glomerulonephritis), which had a period of latency (1–2 weeks) after the symptoms of infection.33 Infection related exacerbations were associated maximum with IgADN (47%) followed by IgAVN (19%). The similar observation was reported in a study by Satoskar et al.34 Episodic macroscopic hematria, nephrotic range proteinuria and hypertension had been typically associated with IgADN.35 We found the similar observations. Microscopic hematuria (49.6–59.5%) and subnephrotic proteinuria (42.1–52.3%) were more frequent in primary and secondary IgAN and it correlated with other studies.36–38 We found that rapidly progressive renal failure was associated with IgADN and secondary IgAN than primary IgAN and IgAVN and similar trend had been demonstrated by other studies.35,37 Similar to Nasr et al., we also found hypocomplementemia more in IgADN in comparison to primary and secondary IgAN and IgAVN.39 Histomorphology of primary and secondary IgAN ranged from normal to chronic glomerulonephritis and it was similar to various other studies.29, 36, 40,41 On IF, most biopsies showed predominantly IgA deposition in primary and secondary IgAN at mesangial region and same had been demonstrated by many studies.42–44 Electron microscopy depicted mesangial electron-dense deposition followed by both (mesangial and subendothelial) deposition in primary and secondary IgAN, and similar findings were reported by Haas et al.45 IgAVN (HSP) showed highly variable histomorphology of glomeruli ranging from normal to diffuse proliferative and crescentic glomerulonephritis. 46 Skin biopsy demonstrated leukocytoclastic vasculitis in (33.8%) cases, which was less than other study.47
The limitation of this study was that we were unable to pool incidence of IgAN because none of studies clearly defined the data.
Conclusion
The IgA nephropathy was more prevalent in India with most common subgroup being primary IgAN. Subnephrotic proteinuria and microscopic hematuria were common clinical presentation of IgAN. Secondary IgAN and IgADN had more rapidly-progressive renal failure than primary IgAN and IgAVN.
Grants and/or financial support
Nil.
Conflict of interest
Nil.
References
- 1.Berger J. IgAglomerular deposits in renal disease. Transplant Proc. 1969;1:939–944. [PubMed] [Google Scholar]
- 2.Berger J, Hinglais N. Les depots intercapillaires d'IgAIgG. J Urol Nephrol. 1968;74:694–695. [PubMed] [Google Scholar]
- 3.D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Q J Med. 1987;64:709–727. PubMed. [PubMed] [Google Scholar]
- 4.Julian BA, Waldo FB, Rifai A, et al. IgA nephropathy, the most common glomerulonephritis worldwide. A neglected disease in the United States? Am J Med. 1988;84:129–132. doi: 10.1016/0002-9343(88)90019-8. PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Utsunomiya Y, Koda T, Kado T, et al. Incidence of pediatric IgA nephropathy. Pediatr Nephrol. 2003;18:511–515. doi: 10.1007/s00467-003-1127-z. [DOI] [PubMed] [Google Scholar]
- 6.Ueda Y, Sakai O, Yamagata M, et al. IgA glomerulonephritis in Japan. Contrib Nephrol. 1975;4:37–47. [PubMed] [Google Scholar]
- 7.Nickeleit V, Mengel M, Colvin RB. Renal Transplant Pathology. In: Jennette JC, Olson JL, Silva FG, Vivette D, D'Agati, editors. Heptinstall's Pathology of the Kidney. 7th ed. Wolters Kluwer; 2015. pp. 1321–1431. [Google Scholar]
- 8.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–842. doi: 10.1016/s0272-6386(97)90456-x. PubMed. [DOI] [PubMed] [Google Scholar]
- 9.Clarkson AR, Seymour AE, Thompson AJ, et al. IgA nephropathy: a syndrome of uniform morphology, diverse clinical features and uncertain prognosis. Clin Nephrol. 1977;8:459–471. [PubMed] [Google Scholar]
- 10.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta analyses. [2015 Sep 17]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm. [Google Scholar]
- 11.Chacko B. IgA nephropathy in India: what we do know. Ren Fail. 2011;33(1):102–107. doi: 10.3109/0886022X.2010.523486. [DOI] [PubMed] [Google Scholar]
- 12.Ganesh K, Nair RR, Seethalekshmy NV, Kurian G, Mathew A, et al. A Study of Clinical Presentation and Correlative Histopathological Patterns in Renal Parenchymal Disease. IJN. 2018 Jan-Feb;28(1):28–34. doi: 10.4103/ijn.IJN_256_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Siddappa S, Kowsalya R, Mythri K. IgA nephropathy in a tertiary care center from south India. Indian Journal of Nephrology. 2011;21(4):230–234. doi: 10.4103/0971-4065.82635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das U, Dakshinamurty KV, Prayaga A, Uppin M. Spectrum of IgA Nephropathy in a Single Center. Saudi J Kidney Dis Transpl. 2015;26(5):1057–1063. doi: 10.4103/1319-2442.164612. [DOI] [PubMed] [Google Scholar]
- 15.Agrawal V, Singh A, Kaul A, Verma R, Jain M, Pandey R. Utility of Oxford Classification in Post-Transplant Immunoglobulin A Nephropathy. Transplantation Proceedings. 2017;49:2274–2279. doi: 10.1016/j.transproceed.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 16.Mittal N, Joshi K, Rane S, Nada R, Sakhuja V. Primary IgA nephropathy in north India: is it different? Postgrad Med J. 2012 Jan;88(1035):15–20. doi: 10.1136/postgradmedj-2011-130077. [DOI] [PubMed] [Google Scholar]
- 17.Chandrika B K. IgA nephropathy in Kerala, India: A retrospective study. IJPM. 2009;52(1):14–16. doi: 10.4103/0377-4929.44954. [DOI] [PubMed] [Google Scholar]
- 18.Ramakrishnan S, Patro KC, Dilip R. Outcome of renal transplantation in IgA nephropathy. Indian Journal of Transplantation. 2016;10(4):84–85. [Google Scholar]
- 19.Chacko B, George JT, Neelakantan N, Korula A, Chakko JK. Outcomes of renal transplantation in patients with immunoglobulin A nephropathy in India. JPGM. 2007;53:92–95. doi: 10.4103/0022-3859.32207. [DOI] [PubMed] [Google Scholar]
- 20.Vanikar AV, Trivedi HL, Kanodia KV, Patel RD, Shah PR. Pathological spectrum of primary immunoglobulin: A nephropathy in western India. Gazzetta Medica Italiana Archivio per le Scienze Mediche. 2005;164(6):465–471. [Google Scholar]
- 21.Bhuyan UN, Dash SC, Srivastava RN, Tiwari SC, Malhotra KK. IgA associated glomerulonephritis. Journal of the Association of Physicians of India. 1992;40:310–313. [PubMed] [Google Scholar]
- 22.Tewari CR, Nada R, Kaur M, et al. Correlates of hematuria on glomerular histology and electron microscopy in IgA nephropathy. Med J Armed Forces India. 2016 doi: 10.1016/j.mjafi. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chowdry AM, Najar MS, Mir MM, et al. Primary IgA Nephropathy in the Kashmiri Population. Saudi J Kidney Dis Transpl. 2018;29(3):680–688. doi: 10.4103/1319-2442.235167. [DOI] [PubMed] [Google Scholar]
- 24.Dhanapriya J, Balasubramaniyan T, Maharajan SP, et al. IgA-dominant Infection-related Glomerulonephritis in India: A Single-center Experience. Indian Journal of Nephrology. 2017;27:435–439. doi: 10.4103/ijn.IJN_337_16. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levy M, Berger J. Worldwide perspective of IgA nephropathy. Am J Kidney Dis. 1988;12:340–347. doi: 10.1016/s0272-6386(88)80021-0. PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Li L-S, Liu Z-H. Epidemiologic data of renal diseases from a single unit in China: analysis based on 13,519 renal biopsies. Kidney Int. 2004;66:920–923. doi: 10.1111/j.1523-1755.2004.00837.x. PubMed. [DOI] [PubMed] [Google Scholar]
- 27.Seedat YK, Nathoo BC, Parag KB, et al. IgA nephropathy in blacks and Indians of Natal. Nephron. 1988;50:137–141. doi: 10.1159/000185144. PubMed. [DOI] [PubMed] [Google Scholar]
- 28.Galla JH, Kohaut EC, Alexander R, et al. Racial difference in the prevalence of IgA-associated nephropathies [Letter] Lancet. 1984;2:522. doi: 10.1016/s0140-6736(84)92599-6. [DOI] [PubMed] [Google Scholar]
- 29.Koyama A. Nationwide and long-term survey of primary glomerulonephritis in Japan as observed in 1,850 biopsied cases. Nephron. 1999;82:205–213. doi: 10.1159/000045404. PubMed. [DOI] [PubMed] [Google Scholar]
- 30.Briganti EM, Dowling J, Finlay M, et al. The incidence of biopsy-proven glomerulonephritis in Australia. Nephrol Dial Transplant. 2001;16:1364–1367. doi: 10.1093/ndt/16.7.1364. PubMed. [DOI] [PubMed] [Google Scholar]
- 31.Nasr SH, Vivette D, Agati IgA-Dominant Postinfectious Glomerulonephritis: A New Twist on an Old Disease. Nephron Clin Pract. 2011;119:c18–c26. doi: 10.1159/000324180. [DOI] [PubMed] [Google Scholar]
- 32.Galla JH. IgA nephropathy. Kidney Int. 1995;47:377–387. doi: 10.1038/ki.1995.50. PubMed. [DOI] [PubMed] [Google Scholar]
- 33.Silva FG. Acute postinfectious glomerulonephritis and glomerulonephritis complicating persistent bacterial infection. In: Jennette JC, Olson JL, Schwartz MM, et al., editors. Heptinstall's Pathology of the Kidney. 5th ed. Philadelphia: Lippincott-Raven; 1998. pp. 389–453. [Google Scholar]
- 34.Satoskar AA, Nadasdy G, Plaza JA, Sedmak D, Shidham G, Hebert L, et al. Staphylococcus infection associated glomerulonephritis mimicking IgA nephropathy. Clin J Am Soc Nephrol. 2006;1:1179–1186. doi: 10.2215/CJN.01030306. [DOI] [PubMed] [Google Scholar]
- 35.Koyama A, Kobayashi M, Yamaguchi N. Glomerulonephritis associated with MRSA infection: a possible role of bacterial superantigen. Kidney Int. 1995;47:207. doi: 10.1038/ki.1995.25. [DOI] [PubMed] [Google Scholar]
- 36.Ibels LS, Gyory AZ. IgA nephropathy: analysis of the natural history, important factors in the progression of renal disease, and a review of the literature. Medicine. 1994;73:79–102. PubMed. [PubMed] [Google Scholar]
- 37.Nakamoto Y, Iida H, Kobayashi K, et al. Hepatic glomerulonephritis: characteristics of hepatic IgA glomerulonephritis as the major part. Virchows Arch A Pathol Anat Histol. 1981;392:45–54. doi: 10.1007/BF00430547. [DOI] [PubMed] [Google Scholar]
- 38.Saulsbury FT. Henoch-Schonlein purpura in children: report of 100 patients and review of the literature. Medicine. 1999;78:395–408. doi: 10.1097/00005792-199911000-00005. PubMed. [DOI] [PubMed] [Google Scholar]
- 39.Nasr S, Fidler M, Valeri A, et al. Postinfectious glomerulonephritis in the elderly. J Am Soc Nephrol. 2011;22:187–195. doi: 10.1681/ASN.2010060611. [DOI] [PubMed] [Google Scholar]
- 40.Haas M. Histologic subclassification of IgA nephropathy: a clinicopathologic study of 244 cases. Am J Kidney Dis. 1997;29:829–842. doi: 10.1016/s0272-6386(97)90456-x. PubMed. [DOI] [PubMed] [Google Scholar]
- 41.Alamartine E, Sabatier J-C, Guerin C, et al. Prognostic factors in mesangial IgA glomerulonephritis: an extensive study with univariate and multivariate analyses. Am J Kidney Dis. 1991;18:12–19. doi: 10.1016/s0272-6386(12)80284-8. PubMed. [DOI] [PubMed] [Google Scholar]
- 42.Lai KN, Lai FMM, Li PKT, et al. The clinicopathological characteristics of IgA nephropathy in Hong Kong. Pathology. 1988;20:15–19. doi: 10.3109/00313028809085190. PubMed. [DOI] [PubMed] [Google Scholar]
- 43.Jennette JC. The immunohistology of IgA nephropathy. Am J Kidney Dis. 1988;12:348–353. doi: 10.1016/s0272-6386(88)80022-2. PubMed. [DOI] [PubMed] [Google Scholar]
- 44.Berger J, Yaneva H, Nabarra B. Glomerular changes in patients with cirrhosis of the liver. Adv Nephrol Necker Hosp. 1978;7:3–14. PubMed. [PubMed] [Google Scholar]
- 45.Haas M. A reevaluation of routine electron microscopy in the examination of native renal biopsies. J Am Soc Nephrol. 1997;8:70–76. doi: 10.1681/ASN.V8170. [DOI] [PubMed] [Google Scholar]
- 46.Haas M, Reich HN. Morphologic markers of progressive immunoglobulin A nephropathy. Adv Chronic Kidney Dis. 2012;19:107–113. doi: 10.1053/j.ackd.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 47.Poterucha TJ, Wetter DA, Gibson LE, et al. Histopathology and correlates of systemic disease in adult Henoch-Schönlein purpura: a retrospective study of microscopic and clinical findings in 68 patients at Mayo Clinic. J Am Acad Dermatol. 2013;68:420–424. doi: 10.1016/j.jaad.2012.08.011. PubMed. [DOI] [PubMed] [Google Scholar]