Abstract
Introduction
The efficacy of dapagliflozin for type 1 diabetes remains controversial. We conduct a systematic review and meta-analysis to explore the treatment efficacy of dapagliflozin versus placebo in patients with type 1 diabetes.
Methods
We have searched PubMed, EMbase, Web of science, EBSCO and Cochrane library databases through May 2019 for randomized controlled trials (RCTs) assessing the effect of dapagliflozin versus placebo for type 1 diabetes. This meta-analysis is performed using the random-effect model.
Results
Six RCTs are included in the meta-analysis. Overall, compared with control group for type 1 diabetes, dapagliflozin treatment shows favorable impact on glycated hemoglobin HbA1c (standard mean difference SMD=-3.93; 95% confidence interval CI =-4.44 to -3.48; P<0.00001), HbA1c reduction of ≥0.5% (risk ratio RR=1.98; 95% CI=1.65 to 2.39; P<0.00001), and fasting plasma glucose FPG (SMD=-0.93; 95% CI=-1.77 to -0.10; P=0.03). There is no statistical difference of hypoglycemia (RR=1.09; 95% CI=0.66 to 1.79; P=0.75) or adverse events (RR=1.07; 95% CI=0.96 to 1.20; P=0.20) between two groups, but the incidence of ketone-related events is higher than those in control group (RR=0.28; 95% CI=3.96 to 11.52; P=0.01).
Conclusions
Dapagliflozin treatment benefits to reduce HbA1c and FPG for type 1 diabetes.
Keywords: Dapagliflozin, type 1 diabetes, glycemic control, randomized controlled trials
Introduction
It is estimated that less than one-third of patients with type 1 diabetes obtain optimal glycemic control 1, 2, and these patients may have increased mortality even after achieving targeted HbA1c levels 3–5. Insulin therapy is widely accepted as the mainstay of treatment, but may result in hypoglycemia, weight gain and cardiovascular events 6–9. These complications negatively affect the quality of life and increase the mortality 10, 11.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors are the insulin-independent, glucose-dependent antihyperglycemic agents and demonstrate some potential in the treatment of type 1 diabetes 12–15. They were reported to result in weight loss and decreased glycemic variability in patients with type 1 diabetes. For instance, many studies revealed that dapagliflozin, an SGLT2 inhibitor held some promise in glycemic control for type 1 diabetes 16–19. In patients with inadequately controlled type 1 diabetes, dapagliflozin treatment was associated with significantly decreased HbA1c, body weight, total insulin dose and glycemic variability 20.
Current evidence is insufficient for routine clinical use of dapagliflozin for patients with type 1 diabetes. Recently, several studies have investigated the efficacy and safety of dapagliflozin for these patients, but the results are conflicting 17, 18, 21. This systematic review and meta-analysis of RCTs aims to assess the impact of dapagliflozin versus placebo on glycemic control for type 1 diabetes.
Materials and methods
This systematic review and meta-analysis were performed based on the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement and Cochrane Handbook for Systematic Reviews of Interventions 22, 23. No ethical approval and patient consent were required because all analyses were based on previous published studies.
Literature search and selection criteria
We have systematically searched several databases including PubMed, EMbase, Web of science, EBSCO, and the Cochrane library from inception to May 2019 with the following keywords: “dapagliflozin”, and “diabetes”. The reference lists of retrieved studies and relevant reviews were also hand-searched and the process above was performed repeatedly in order to include additional studies.
The inclusion criteria were presented as follows: (1) study design was RCT, (2) patients were diagnosed with type 1 diabetes, and (3) intervention treatments were dapagliflozin versus placebo.
Data extraction and outcome measures
Some baseline information was extracted from the original studies, and they included first author, number of patients, age, body mass index (BMI), duration of disease, and detail methods in two groups. Data were extracted independently by two investigators, and discrepancies were resolved by consensus. We contacted the corresponding author to obtain the data when necessary.
The primary outcomes were glycated hemoglobin (HbA1c) and HbA1c reduction of ≥0.5%. Secondary outcomes included fasting plasma glucose (FPG), hypoglycemia, ketone-related events and/span>adverse events.
Quality assessment in individual studies
The methodological quality of each RCT was assessed by the Jadad Scale which consisted of three evaluation elements: randomization (0–2 points), blinding (0–2 points), dropouts and withdrawals (0–1 points) 24. One point would be allocated to each element if they were conducted and mentioned appropriately in the original article. The score of Jadad Scale varied from 0 to 5 points. An article with Jadad score≤2 was considered to be of low quality. The study was thought to be of high quality if Jadad score≥3 25.
Statistical analysis
We assessed standard mean difference (SMD) with 95% confidence interval (CI) for continuous outcomes (HbA1c, and FPG), and risk ratio (RR) with 95% CI for dichotomous outcomes (HbA1c reduction of ≥0.5%, hypoglycemia, ketone-related events and adverse events). Heterogeneity was evaluated using the I2 statistic, and I2 > 50% indicated significant heterogeneity26. The random-effects model used for all meta-analysis. We searched for potential sources of heterogeneity when encountering significant heterogeneity. Sensitivity analysis was performed to detect the influence of a single study on the overall estimate via omitting one study in turn or performing the subgroup analysis. Owing to the limited number (<10) of included studies, publication bias was not assessed. Results were considered as statistically significant for P <0.05. All statistical analyses were performed using Review Manager Version 5.3 (The Cochrane Collaboration, Software Update, Oxford, UK).
Results
Literature search, study characteristics and quality assessment
Figure 1 showed the detail flowchart of the search and selection results. 628 potentially relevant articles were identified initially. Finally, six RCTs were included in the meta-analysis 16–18, 21, 27, 28.
Figure. 1.
Flow diagram of study searching and selection process.
The baseline characteristics of six included RCTs were shown in Table 1. These studies were published between 2015 and 2018, and the total sample size was 1159. Two included RCTs reported the same patient sample at the follow-up of 24 weeks and 52 weeks 17, 20.
Table 1.
Characteristics of included studies
| NO. | Author | Dapagliflozin group | Control group | Jada scores | ||||||||||
| Number | Age | Female (n) | BMI (kg/m2) | Duration of disease (year) | Methods | Number | Age | Female (n) | BMI (kg/m2) | Duration of disease (year) | Methods | |||
| 1 | Watada 2018 | 14 | 37.1±10.2 | 7 | 22.2±2.1 | 14.7±12.4 | dapagliflozin 10 mg once daily for 7 days | 14 | 42.6±10.6 | 11 | 22.9±3.4 | 16.9±10.5 | placebo | 4 |
| 2 | Melmer 2018 | 6 | 38.12±12.41 | - | 26.31±4.29 | 8.34±14.67 | 10 mg of dapagliflozin for 3 days | 6 | 38.12±12.41 - | 26.31±4.29 | 8.34±14.67 | placebo | 3 | |
| 3 | Mathieu 2018 | 270 | 42.4±12.80 | 149 | 27.80±5.53 | 19.45±11.90 | dapagliflozin 10 mg once daily for 24 weeks | 272 | 43.0±13.73 | 153 | 27.62±5.41 | 18.98±11.65 | placebo | 5 |
| 4 | Dandona 2018 | 259 | 42.7±14.1 | 129 | 28.1±5.1 | 19.9±11.1 | dapagliflozin 10 mg once daily for 52 weeks | 260 | 42.7±13.6) | 128 | 28.6±5.2) | 21.2±12.2) | placebo | 5 |
| 5 | Kuhadiya 2016 | 20 | 55±3 | 10 | 31±1 | 25±3 | dapagliflozin 10 mg daily for 12 weeks | 10 | 52±3 | 6 | 27±2 | 31±5 | placebo | 5 |
| 6 | Henry 2015 | 15 | 37.5±15.2 | 7 | 25.8±4.8 | 18.1±14.0 | dapagliflozin 10 mg daily for 2 weeks | 13 | 34.5±12.2 | 5 | 25.3±3.0 | 16.2±9.7 | placebo | 4 |
BMI = body mass index.
Among the six included RCTs, two RCTs reported HbA1c 17, 28, two RCTs reported HbA1c reduction of ≥0.5% 17, 18, two RCTs reported FPG 21, 28, four RCTs reported hypoglycemia 16–18, 28, three RCTs reported ketone-related events 17, 18, 28 and four RCTs reported adverse events 16–18, 21. Jadad scores of the six included studies varied from 3 to 5, and all six studies had high-quality based on the quality assessment.
Primary outcomes: HbA1c and HbA1c reduction of ≥0.5%
The random-effect model was used for the analysis of primary outcomes. The results revealed that compared to control group in patients with type 1 diabetes, dapagliflozin treatment could significantly reduce HbA1c (SMD=-3.93; 95% CI=-4.44 to -3.48; P<0.00001) with low heterogeneity among the studies (I2=13%, heterogeneity P=0.28, Figure 2) and improve HbA1c reduction of ≥0.5% (RR=1.98; 95% CI=1.65 to 2.39; P<0.00001 with no heterogeneity among the studies (I2=0%, heterogeneity P=0.68, Figure 3).
Figure. 2.
Forest plot for the meta-analysis of HbA1c.
Figure. 3.
Forest plot for the meta-analysis of HbA1c reduction of ≥0.5%.
Sensitivity analysis
There was low or no heterogeneity for the primary outcomes, and thus we did not perform the meta-analysis via omitting one study or subgroup analysis to detect the heterogeneity.
Secondary outcomes
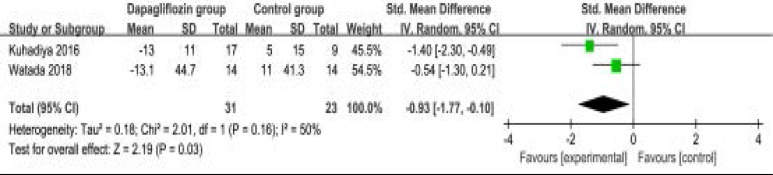
In comparison with control intervention for type 1 diabetes, dapagliflozin treatment was associated with the decrease in FPG (SMD=-0.93; 95% CI=-1.77 to -0.10; P=0.03; Figure 4), but showed no obvious impact on the incidence of hypoglycemia (RR=1.09; 95% CI=0.66 to 1.79; P=0.75; Figure 5). However, the incidence of ketone-related events was higher than those in control group (RR=0.28; 95% CI=3.96 to 11.52; P=0.01; Figure 6). There was no statistical difference of adverse events between two groups (RR=1.07; 95% CI=0.96 to 1.20; P=0.20; Figure 7).
Figure. 4.
Forest plot for the meta-analysis of FPG.
Figure. 5.
Forest plot for the meta-analysis of hypoglycemia.
Figure. 6.
Forest plot for the meta-analysis of ketone-related events.
Figure. 7.
Forest plot for the meta-analysis of adverse events.
Discussion
SGLT2-inhibition has shown the efficacy for the treatment of type 1 and 2 diabetes. In patients with type 2 diabetes, dapagliflozin treatment was reported to increase muscle insulin sensitivity, and reduce endogenous glucose production and fasting glucagon 29. Several studies revealed that SGLT2-inhibition was able to reduce fasting and postprandial glucose concentrations, as well as HbA1c in type 1 diabetes 20, 30. In addition, dapagliflozin treatment resulted in insulin reduction of more than 30% in type 1 diabetes21.
Our meta-analysis suggests that dapagliflozin at the dose of 10 mg daily shows the significant benefits in HbA1c, HbA1c reduction of ≥0.5% and FPG in patients with type 1 diabetes. Furthermore, treatment with dapagliflozin 5 mg and 10 mg as adjunct therapy to insulin may improve glycemic control in these patients and reduce insulin dose by −19.3% (95% CI, −30.1 to −6.8) and − 16.2% (95% CI, −29.4 to −0.5), respectively 16. There is no significant difference of hypoglycemia and adverse events between two groups based on the results of this meta-analysis. These adverse events are well tolerated.
However, our meta-analysis reveals that dapagliflozin treatment appears to increase the ketone-related events compared to placebo. In DEPICT-2 study, the incidence of diabetic ketoacidosis was reported to be higher compared with DEPICT-1 study (dapagliflozin 5 mg versus dapagliflozin 10 mg versus placebo: 5.83, 4.99, and 0 per 100 patient-years in DEPICT-2, respectively; 3.29, 3.78, and 2.64 per 100 patient-years in DEPICT-1) 17, 18, 20. The variability may be caused by the small number of events, and different risk factors to develop diabetic ketoacidosis. The events of diabetic ketoacidosis were generally resolved using conventional treatments 18
Several limitations exist in this meta-analysis. Firstly, our analysis is based on only six RCTs, and more RCTs with large samplsize should be conducted to explore this issue. Although there is low heterogeneity, different treatment duration of dapagliflozin may lead to some bias. Finally, some unpublished and missing data may lead to some bias to the pooled effect.
Conclusion
Dapagliflozin treatment can provide some benefits for glycemic control in patients with type 1 diabetes, as evidenced by the reduction in HbA1c and FPG.
Compliance with Ethical Standards
Research involving human participants and/or animals Not applicable.
Declaration of conflict of interest
None.
References
- 1.McKnight JA, Wild SH, Lamb MJ, Cooper MN, Jones TW, Davis EA, et al. Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: an international comparison. Diabetic Medicine: A Journal of the British Diabetic Association. 2015;32:1036–1050. doi: 10.1111/dme.12676. [DOI] [PubMed] [Google Scholar]
- 2.Miller KM, Foster NC, Beck RW, Bergenstal RM, Du-Bose SN, DiMeglio LA, et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D Exchange clinic registry. Diabetes Care. 2015;38:971–978. doi: 10.2337/dc15-0078. PubMed. [DOI] [PubMed] [Google Scholar]
- 3.Lind M, Svensson AM, Kosiborod M, Gudbjornsdottir S, Pivodic A, Wedel H, et al. Glycemic control and excess mortality in type 1 diabetes. The New England Journal of Medicine. 2014;371:1972–1982. doi: 10.1056/NEJMoa1408214. [DOI] [PubMed] [Google Scholar]
- 4.Patton SR, Noser AE, Youngkin EM, Majidi S, Clements MA. Early Initiation of Diabetes Devices Relates to Improved Glycemic Control in Children with Recent-Onset Type 1 Diabetes Mellitus. Diabetes Technology & Therapeutics. 2019 doi: 10.1089/dia.2019.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Juhl B, Lauszus FF, Lykkesfeldt J. Glycemic Control during Pregnancy-A Predictor of Vitamin C Status at Labor in Type 1 Diabetic Women? Antioxidants. 2019;8 doi: 10.3390/antiox8060153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnelly LA, Morris AD, Frier BM, Ellis JD, Donnan PT, Durrant R, et al. Frequency and predictors of hypoglycaemia in Type 1 and insulin-treated Type 2 diabetes: a population-based study. Diabetic Medicine: A Journal of the British Diabetic Association. 2005;22:749–755. doi: 10.1111/j.1464-5491.2005.01501.x. [DOI] [PubMed] [Google Scholar]
- 7.Gubitosi-Klug RA, Group DER The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: summary and future directions. Diabetes Care. 2014;37:44–49. doi: 10.2337/dc13-2148. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Group UKHS, author. Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia. 2007;50:1140–1147. doi: 10.1007/s00125-007-0599-y. PubMed. [DOI] [PubMed] [Google Scholar]
- 9.Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care. 2011;34(Suppl 2):S132–S137. doi: 10.2337/dc11-s220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalra S, Mukherjee JJ, Venkataraman S, Bantwal G, Shaikh S, Saboo B, et al. Hypoglycemia: The neglected complication. Indian Journal of Endocrinology and Metabolism. 2013;17:819–834. doi: 10.4103/2230-8210.117219. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis RE, Morrissey M, Peters JR, Wittrup-Jensen K, Kennedy-Martin T, Currie CJ. Impact of hypoglycaemia on quality of life and productivity in type 1 and type 2 diabetes. Current Medical Research and Opinion. 2005;21:1477–1483. doi: 10.1185/030079905X61929. [DOI] [PubMed] [Google Scholar]
- 12.El Masri D, Ghosh S, Jaber LA. Safety and efficacy of sodium-glucose cotransporter 2 (SGLT2) inhibitors in type 1 diabetes: A systematic review and meta-analysis. Diabetes Research and Clinical Practice. 2018;137:83–92. doi: 10.1016/j.diabres.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Takebayashi K, Inukai T. Effect of Sodium Glucose Cotransporter 2 Inhibitors With Low SGLT2/SGLT1 Selectivity on Circulating Glucagon-Like Peptide 1 Levels in Type 2 Diabetes Mellitus. Journal of Clinical Medicine Research. 2017;9:745–753. doi: 10.14740/jocmr3112w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rieg T, Vallon V. Development of SGLT1 and SGLT2 inhibitors. Diabetologia. 2018;61:2079–2086. doi: 10.1007/s00125-018-4654-7. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danne T, Biester T, Kordonouri O. Combined SGLT1 and SGLT2 Inhibitors and Their Role in Diabetes Care. Diabetes Technology & Therapeutics. 2018;20:S269–S277. doi: 10.1089/dia.2018.0081. [DOI] [PubMed] [Google Scholar]
- 16.Henry RR, Rosenstock J, Edelman S, Mudaliar S, Chalamandaris AG, Kasichayanula S, et al. Exploring the potential of the SGLT2 inhibitor dapagliflozin in type 1 diabetes: a randomized, double-blind, placebo-controlled pilot study. Diabetes Care. 2015;38:412–419. doi: 10.2337/dc13-2955. PubMed. [DOI] [PubMed] [Google Scholar]
- 17.Dandona P, Mathieu C, Phillip M, Hansen L, Tschope D, Thoren F, et al. Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes: The DEPICT-1 52-Week Study. Diabetes Care. 2018;41:2552–2559. doi: 10.2337/dc18-1087. PubMed. [DOI] [PubMed] [Google Scholar]
- 18.Mathieu C, Dandona P, Gillard P, Senior P, Hasslacher C, Araki E, et al. Efficacy and Safety of Dapagliflozin in Patients With Inadequately Controlled Type 1 Diabetes (the DEPICT-2 Study): 24-Week Results From a Randomized Controlled Trial. Diabetes Care. 2018;41:1938–1946. doi: 10.2337/dc18-0623. PubMed. [DOI] [PubMed] [Google Scholar]
- 19.Akturk HK, Rewers A, Garg SK. SGLT inhibition: a possible adjunctive treatment for type 1 diabetes. Current Opinion in Endocrinology, Diabetes, and Obesity. 2018;25:246–250. doi: 10.1097/MED.0000000000000423. PubMed. [DOI] [PubMed] [Google Scholar]
- 20.Dandona P, Mathieu C, Phillip M, Hansen L, Griffen SC, Tschope D, et al. Efficacy and safety of dapagliflozin in patients with inadequately controlled type 1 diabetes (DEPICT-1): 24 week results from a multicentre, double-blind, phase 3, randomised controlled trial. The Lancet Diabetes & Endocrinology. 2017;5:864–876. doi: 10.1016/S2213-8587(17)30308-X. PubMed. [DOI] [PubMed] [Google Scholar]
- 21.Watada H, Shiramoto M, Ueda S, Tang W, Asano M, Thoren F, et al. Pharmacokinetics and pharmacodynamics of dapagliflozin in combination with insulin in Japanese patients with type 1 diabetes. Diabetes, Obesity & Metabolism. 2018 doi: 10.1111/dom.13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JPT G. Cochrane handbook for systematic reviews of interventions version 5.1. 0 updated March 2011. The Cochrane Collaboration. 2011 [Google Scholar]
- 24.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Controlled Clinical Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. PubMed. [DOI] [PubMed] [Google Scholar]
- 25.Kjaergard LL, Villumsen J, Gluud C. Reported Methodologic Quality and Discrepancies between Large and Small Randomized Trials in Meta-Analyses. Annals of Internal Medicine. 2001;135:982–989. doi: 10.7326/0003-4819-135-11-200112040-00010. [DOI] [PubMed] [Google Scholar]
- 26.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 27.Melmer A, Kempf P, Lunger L, Pieber TR, Mader JK, Stettler C, et al. Short-term effects of dapagliflozin on insulin sensitivity, postprandial glucose excursion and ketogenesis in type 1 diabetes mellitus: A randomized, placebo-controlled, double blind, cross-over pilot study. Diabetes, Obesity & Metabolism. 2018;20:2685–2689. doi: 10.1111/dom.13439. PubMed. [DOI] [PubMed] [Google Scholar]
- 28.Kuhadiya ND, Ghanim H, Mehta A, Garg M, Khan S, Hejna J, et al. Dapagliflozin as Additional Treatment to Liraglutide and Insulin in Patients With Type 1 Diabetes. The Journal of Clinical Endocrinology and Metabolism. 2016;101:3506–3515. doi: 10.1210/jc.2016-1451. [DOI] [PubMed] [Google Scholar]
- 29.Merovci A, Solis-Herrera C, Daniele G, Eldor R, Fiorentino TV, Tripathy D, et al. Dapagliflozin improves muscle insulin sensitivity but enhances endogenous glucose production. The Journal of Clinical Investigation. 2014;124:509–514. doi: 10.1172/JCI70704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen J, Fan F, Wang JY, Long Y, Gao CL, Stanton RC, et al. The efficacy and safety of SGLT2 inhibitors for adjunctive treatment of type 1 diabetes: a systematic review and meta-analysis. Scientific Reports. 2017;7:44128. doi: 10.1038/srep44128. [DOI] [PMC free article] [PubMed] [Google Scholar]