Abstract
Bacterial vaginosis (BV) is one of the most common vaginal infections that affects hundreds of millions of women of reproductive age, worldwide. Traditional treatment strategies, such as oral and topical antibiotics, have shown efficacy against BV, but frequent recurrence of infection and the development of antibiotic-resistant bacteria remain as significant challenges. Alternatively, recent progress in understanding immune, microbiological, and metabolic interactions in the vaginal microbiota has prompted the consideration of administering probiotic organisms to restore and maintain vaginal health within the context of BV prevention and treatment. Given this, the objective of this review is to discuss existing and potential alternative approaches to deliver, and to potentially sustain the delivery of probiotics, to prevent and/or treat BV infections. First, a brief overview is provided regarding the probiotic species and combinatorial probiotic strategies that have shown promise in the treatment of BV and in restoring female reproductive health. Additionally, the advantages and challenges associated with current oral and intravaginal probiotic delivery platforms are discussed. Lastly, we present emerging and promising alternative dosage forms, such as electrospun fibers and 3D bioprinted scaffolds, that may be adapted as new strategies to intravaginally deliver probiotic organisms.
Keywords: Bacterial vaginosis, Lactobacillus, Probiotics, Oral delivery, Vaginal delivery
1. Introduction
The thriving female reproductive tract (FRT) is colonized by diverse microbial organisms, with the genus Lactobacillus constituting about 70% of the microbiota (1). The four most prominent Lactobacillus species present in the FRT include Lactobacillus crispatus, Lactobacillus iners, Lactobacillus jensenii, and Lactobacillus gasseri (2). This natural Lactobacillus-dominant flora is paramount in the maintenance of host homeostasis and protection against endogenous and exogenous pathogens (3, 4).
In contrast to the Lactobacillus-dominated microbiome of the healthy FRT, bacterial vaginosis (BV) is characterized by a dysbiosis and shift in the diversity of the microbiome to favor the proliferation of vaginal pathogens. While G. vaginalis has been identified as the predominant pathogenic species characterizing BV, other anaerobes including Prevotella and Atopobium species have similarly been implicated with disease initiation and progression (5). These alterations to the vaginal ecosystem can lead to severe and potentially detrimental complications such as endometriosis, pelvic inflammatory disease, and an increased risk of acquiring sexually transmitted infections (STIs) such as human immunodeficiency virus (HIV) (6, 7). Additional details regarding BV prevalence and detection are included in Supplementary Materials.
Current treatment options for BV primarily consist of antibiotics, namely metronidazole, clindamycin, and tinidazole (8, 9). Metronidazole and clindamycin are presently available in the form of oral tablets, which are typically taken twice a day for one week, and intravaginal gels/creams, which are generally applied once or twice daily for 5 days. Conversely, tinidazole is only available as an oral tablet and is customarily taken twice daily for one week (2, 10). Unfortunately, antibiotic administration may predispose the microenvironment not only to Lactobacillus regrowth, but detrimentally, to the recolonization of pathogenic bacteria as well, which can yield antibiotic-resistant strains and resulting superinfections.
In order to prevent infection recurrence, adherence to antibiotic regimens for a period of 4 to 6 months is often advised (10). However, frequent, long-term administration and the prolonged duration of retreatment regimens increase the risks of antibiotic resistance and reduce user adherence and efficacious dose administration. The mechanisms and other challenges associated with antibiotic regimens are further discussed in Supplementary Materials.
Given these challenges, ongoing research has been focused on investigating alternative and more effective approaches to prevent and treat BV. The consideration of probiotics, or “live microorganisms that, when administered in adequate amounts, confer a health benefit to the host,” have been suggested as active pharmaceutical ingredients in the treatment of BV (1, 11). Recent progress in understanding immune, microbiological, and metabolic interactions in the vaginal microbiota has also increased attention to consider probiotics for BV prevention and/or treatment. Considering this, the major objective of this review paper is to discuss existing and potential alternative approaches to provide intravaginal probiotic delivery against BV infections. First, a brief overview is provided here and in Supplementary Materials of the probiotic species and combinatorial probiotic strategies that have been used to treat BV and to restore female reproductive health. Additionally, the advantages and challenges associated with current oral and intravaginal probiotic delivery platforms are discussed. Lastly, we present emerging and promising alternative delivery vehicles for the intravaginal delivery of probiotics.
2. Probiotics for BV Prevention and Treatment
2.1. Mechanism of Action
The introduction of Lactobacillus spp. to the vaginal environment has the potential to overcome biofilm-mediated challenges to reduce and/or prevent BV recurrence (8, 12). Lactobacilli are fermentative bacteria that metabolize glycogen, present from exfoliated and lysed vaginal epithelial cells, to produce lactic acid, thereby acidifying the vaginal environment (pH ≤ 4.5)(13, 14). This increase in acidity is positively correlated with increased membrane permeability to hydrogen peroxide and other bacteriocins (both secreted by Lactobacillus), which can provide protection against invading pathogens by promoting the lysis of pathogenic species such as Gardnerella vaginalis, while maintaining a Lactobacillus-dominated vaginal flora (14, 15). The proposed mechanism of action of lactobacilli and other probiotics is cited in their ability to prevent or disrupt pathogenic biofilms through adhesion to epithelial cells, secretion of antimicrobial products, and competition for nutrients (12, 16). In addition, lactobacilli have been found to modulate host immunity, exhibit anti-inflammatory effects (16, 17), and possess autoaggregation or coaggregation properties (18), all of which are important to efficaciously treat BV.
Due to the Lactobacillus-dominance observed in the healthy FRT, lactobacilli have been the probiotic genus most investigated for BV treatment. Depending on the administration route (oral or intravaginal), probiotic doses on the order of 108 or 109 colony forming units (CFUs) per day are required to therapeutically impact BV progression (19). Another consideration in probiotic therapy is the duration of treatment course; prolonged and repeated administrations (e.g., 60 and 90 days) have been found to be more effective than short courses (e.g., 14 days) of treatment, in particular given the lack of adverse effects thus far associated with probiotic administration (20, 21).
2.2. Different Lactobacillus Species
Of the approximately 60 vaginal Lactobacillus isolates that have been investigated and characterized, L. acidophilus, L. crispatus, and L. delbrueckii have emerged as the most effective probiotics against BV based on in vitro studies (22) and have been found to produce lactic acid, secrete hydrogen peroxide, and/or demonstrate strong adhesion to the vaginal epithelium. These findings were partially corroborated by a later in vitro study that found that L. rhamnosus and L. fermentum, in addition to L. crispatus, were the most promising probiotics, given their propensity to adhere to vaginal and uroepithelial cells and to inhibit pathogen adhesion and viability (21). Additionally in vivo studies (23–26) have shown the effectiveness of different probiotic species that have been summarized in this review (27), and another in vivo study demonstrated the impact of single lactobacilli strains to prevent both Candida albicans and G. vaginalis growth (28).
In addition, clinical investigations have focused on and examined the efficacy of oral and intravaginal administration of L. acidophilus, as one of the most beneficial Lactobacillus species to promote vaginal health and to treat BV (20, 29). In general, these studies originally found that the effectiveness of L. acidophilus was attributed to its strong vaginal epithelium adhesion and high concentrations of hydrogen peroxide secretion (22). However, more recent studies have shown that the secretion of hydrogen peroxide from lactobacilli is a protection mechanism, which is more toxic to lactobacilli than pathogenic bacteria, hence pathogen activity is believed to be inhibited instead by the hypoxic vaginal environment (30).
In addition to the administration of individual species of probiotics alone, studies have explored the efficacy of administering species like L. rhamnosus with other species including L. acidophilus (17, 31–33), L. reuteri (27), and L. fermentum (11, 32). In fact, a combinatorial approach, utilizing multiple species and/or strains seems to be promising, enabling complementary benefits. Furthermore, combined probiotic and antibiotic strategies have shown increased efficacy, decreases in duration of infection, and decreased recurrent infections, relative to antibiotic treatment alone, supporting the potential of these approaches against BV(8, 34, 35). A variety of studies/strategies supporting single probiotic, multi-probiotic and probiotic-antibiotic delivery can be found in Supplementary Materials.
Considering the above-mentioned approaches, delivery strategies which incorporate multiple species may result in improved delivery to, and colonization of probiotics in vaginal tissue, in turn resulting in higher efficacy in BV treatment/prevention compared to the use of single species.
2.3. Vaginal Microbiome Transplantation
Relative to the administration of single or few probiotic species, vaginal microbiome transplantation (VMT) has recently been proposed as a novel extension of probiotic therapy, involving the transfer of the entire vaginal microbial community from healthy donor cervicovaginal secretions to BV-afflicted patients (36).
VMT seems promising, especially since a similar procedure involving transplantation of fecal microbiota has shown success in improving gastrointestinal flora and health. One clinical study investigating VMT treatment for BV reported complete remission (through a 5 to 21 month follow-up period) in 4 out of 5 patients after 1 to 3 VMT sessions (37), while the fifth patient displayed partial remission. However, due to the multiple sessions that seem to be required to obtain remission, delivery vehicles that enable more convenient dosing regimens and alternatives for the patient may be desirable.
While additional details regarding VMT considerations can be found in Supplementary Materials, the following sections detail the delivery platforms that have been used for probiotic administration and may, in future work, be extrapolated to VMT to decrease sessions and frequency of administration (36, 38).
3. Probiotic Dosage Forms
3.1. Oral Delivery of Probiotics Relevant to BV Infection and Reproductive Health
While the primary focus of this review is on intravaginal probiotic delivery, it is worth briefly reviewing some of the original dosage forms, centered on oral probiotic administration for intravaginal applications, that include yogurt, capsules, tablets, and other edibles (11). Despite these numerous dosage forms, some of the most significant challenges surrounding oral delivery are probiotic degradation and subsequent loss of activity that results upon exposure to the gastrointestinal environment. This necessitates increased dosing and administration frequency to achieve efficacious delivery to the FRT (39), which in turn has the potential to decrease user adherence. To address these challenges, new oral dosage forms that better protect and maintain probiotic activity during transport to the FRT have been developed (39); however prolonged methods of oral delivery remain unattained. While outside the scope of this review and thoroughly covered in other reviews, additional information is provided in Table 1 and the Supplementary Materials section to discuss some of the oral dosage forms that have been used to deliver probiotics for the prevention and treatment of BV infections and more broadly, for the maintenance and restoration of female reproductive health. Additionally, we discuss the challenges surrounding administration of these dosage forms.
Table 1.
Some of the commercially available oral probiotic dosage forms available to treat BV and/or improve vaginal health.
| Oral Dosage Form | Brand | Probiotic Strains |
|---|---|---|
| Capsule | Hum - Private Party | L. acidophilus, L. reuteri, L. rhamnosus |
| Capsule | Love Wellness - Good Girl Probiotics | L. acidophilus, L. rhamnosus, L. plantarum, L. salivarius, L. casei, L. paracasei, L. brevis, L. gasseri |
| Capsule | Fair Haven Health - IsoFresh Probiotic | L. acidophilus, L. reuteri, L. rhamnosus, B. lactis, B. longum |
| Capsule | Renew Life - Ultimate Flora Women’s Care Probiotic | L. acidophilus, L. rhamnosus, L. plantarum, L. salivarius, L. casei, L. paracasei, L. brevis, L. gasseri, B. lactis, B. longum |
| Capsule | Renew Life - Women’s Daily | L. acidophilus, L. rhamnosus, L. casei, L. paracasei, L. plantarum, L. salivarius, L. gasseri, B. lactis |
| Capsule | Wholesome Wellness -Women’s RAW Probiotic | L. acidophilus, B. bifidum, B. lactis, L. paracasei, B. longum, L. brevis, L. bulgaricus, L. casei, L. fermentum, L. helveticus, L. kefirano-faciens, L. kefirgranum, L. rhamnosus, L. lactis, L. cremoris, S. thermophilus, Lactobacillus kefir, L. parakefir, L. plantarum, L. lactis biovar diacetylactis, leuconostoc lactis, Leuconostoc mesenteroides, Leuconostoc cremoris, Leuconostoc dextranicum, Kluyvero-myces marxianus, Brettanomyces anomalus, Debaryomyces hansenii, Saccharomyces unisporus, Saccharomyces turicensis, Saccharomyces cerevisiae, Saccharomyces exiguus, Torulaspora delbrueckii, B. breve |
| Capsule | Garden of Life - RAW Probiotics Vaginal Care | L. acidophilus, L. reuteri, L. rhamnosus, L. fermentum, L. paracasei, L. salivarius, L. gasseri, L. brevis, L. bulgaricus, L. casei, L. helveticus, L kefiranofaciens, L. kefirgranum, Lactococcus lactis, Lactococcus lactis biovar diacetylactis, Lactococcus cremoris, L. kefir, L. parakefir, B. lactis, B. longum, S. thermophilus, Leuconostoc lactis, Leuconostoc mesenteroides, Leuconostoc cremoris, Leuconostoc dextranicum, Kluyveromyces marxianus, Brettanomyces anomalus, Debaryomyces hansenii, Saccharomyces unisporus, Saccharomyces turicensis, Saccharomyces cerevisiae, Saccharomyces exiguus, Torulaspora delbrueckii |
| Capsule | Garden of Life - Dr. Formulated Probiotics - Women’s Daily Care | L. acidophilus, L. rhamnosus, L. reuteri, L. paracasei, L. casei, L. plantarum, L. bulgaricus, L. brevis, L. salivarius, L. gasseri, L. fermentum, B. lactis, B. bifidum, B. breve, B. infantis, B. longus, |
| Capsule | Super Smart - Vaginal Health | L. acidophilus, L. rhamnosus, L. casei, L. salivarius, B. lactis |
| Capsule | Physician’s Choice Women’s Probiotic | L. acidophilus, L. paracasei, L. plantarum, L. gasseri, B. lactis |
| Capsule | Jarrow Formulas - Jarrow Dophilus Women, Vaginal Probiotic | L. rhamnosus, L. crispatus, L. gasseri, L. jensenii |
| Capsule | Jarrow Formulas - Fem Dophilus | L. rhamnosus, L. reuteri |
| Capsule | Intimate Rose - Flora Bloom Probiotics for Women | L. acidophilus, L. rhamnosus, L. reuteri, L. plantarum |
| Capsule | Intelligent Labs - Women’s Probiotics and Prebiotics with Sunfiber and FOS | L. acidophilus, B. lactis, B. bifidum, B. longum |
| Capsule | WonderVites - Women’s 15 Billion Probiotic Formula | L. acidophilus, L. rhamnosus, L. plantarum, L. salivarius, L. bulgaricus, B. lactis, B. bifidum, B. longum |
| Capsule | WonderVites - Women’s 50 Billion Probiotic Formula | L. acidophilus, L. casei, L. lactis, L. salivarius, L. plantarum, B. lactis, B. breve |
| Capsule | RepHresh Pro-B | L. rhamnosus, L. reuteri |
| Capsule | vH Essentials | L. acidophilus, L. rhamnosus, Bacillus coagulans |
| Capsule | Queen V | L. acidophilus, L. rhamnosus, Bacillus coagulans, B. boulardi, Saccharomyces boulardi |
| Capsule | Ora - Lady Bugs | L. acidophilus, L. rhamnosus, L. reuteri, L. crispatus, L. fermentum, L. plantarum |
| Capsule | AZO - Complete Feminine Balance | L. rhamnosus, L. crispatus, L. jensenii, L. gasseri |
| Capsule | MegaFood - MegaFlora Women’s Probiotic | L. acidophilus, L. casei, L. plantarum, L. rhamnosus, L. salivarius, L. brevis, L. bulgaricus, L. gasseri, L. lactis, B. lactis, B. longum, B. bifidum, B. infantis, S. thermophilus |
| Capsule | UP4 Women’s Probiotic | L. acidophilus, L. plantarum, L. rhamnosus, L. gasseri, B. lactis |
| Capsule | Vita Miracle - Ultra 30 Women’s Probiotic | L. acidophilus, L. rhamnosus, L. fermentum, B. bifidum, B. longum, B. infantis, L. gasseri, B. coagulans, B. lactis, L. reuteri, L. casei, L. paracasei, L. bulgaricus, L. plantarum, L. salivarius, S. thermophilus, B. breve, and L. helveticus |
| Capsule | Zentastic | L. acidophilus, L. rhamnosus, L. gasseri, L. paracasei, L. plantarum, L. reuteri, B. bifidum, B. infantis, B. lactis, B. longum |
| Capsule | Integrative Therapeutics | L. rhamnosus, L. reuteri |
| Capsule | Routine | B. lactis, L. rhamnosus, L. acidophilus |
| Capsule | Biom Probiotics - Feminine Support | L. acidophilus, L. gasseri, L. crispatus, L. reuteri, L. fermentum, L. plantarum, L. rhamnosus, Bacillus coagulans, B. brevis, B. lactis, B. bifidum |
| Capsule | DrFormulas - Nexabiotic - Probiotics for Women | Saccharomyces boulardi, L. helveticus, L. delbrueckii, L. reuteri, B. bifidum, B. longum, Bacillus coagulans, L. rhamnosus, L. plantarum, L. acidophilus, B. infantis, L. brevis, L. gasseri, B. subtilis, L. lactis, L. casei, L. salivarius, B. breve, L. paracasei, L. plantarum, S. thermophilus, B. lactis |
| Capsule | Nature’s Way - Primadophilus - Optima - Women’s | L. rhamnosus, L. casei, L. acidophilus, L. paracasei, L. salivarius, L. reuteri, B. breve, B. longum, B. bifidum |
| Capsule | Nature’s Way - Fortify - Optima - Women’s Probiotic | L. acidophilus, L. gasseri, B. lactis, B. bifidum, B. infantis |
| Capsule | Nature’s Way - Fortify - Women’s Probiotic + Prebiotic | L. paracasei, L. acidophilus, L. rhamnosus, L. plantarum, L. casei, L. salivarius, B. lactis |
| Capsule | Innate Vitality - Women’s Probiotics | L. acidophilus, L. rhamnosus, L. reuteri, L. plantarum, L. crispatus, L. fermentum, L. gasseri, L. paracasei, L. helveticus, L. casei, Pediococcus acidilactici, B. bifidum, B. infantis, B. lactis, B. breve, B. adolescentis, B. longum |
| Capsule | Health Labs Nutra - Women’s Probiotic | L. plantarum, L. fermentum, L. acidophilus, L. reuteri, L. rhamnosus, B. bifidum |
| Capsule | Bluebonnet - Advanced Choice - Ladies’ Single Daily Probiotic | L. plantarum, L. casei, L. rhamnosus, L. helveticus |
| Capsule | Florajen Acidophilus | L. acidophilus |
| Capsule | Florajen 3 | L. acidophilus, B. lactis, B. longum |
| Capsule | New Chapter - Women’s Daily Probiotic | L. acidophilus, L. rhamnosus |
| Capsule | Carlson Women’s Probiotic | L. acidophilus, L. plantarum, L. gasseri, L. rhamnosus, B. lactis |
| Capsule | Feminine Balance - Balance Complex | L. acidophilus |
| Capsule | Culturelle - Women’s Healthy Balance | L. rhamnosus, L. crispatus, L. gasseri, L. jensenii |
| Capsule | Spring Valley - Women’s Probiotic | B. bifidum, B. longum, L. acidophilus, L. casei, L. paracasei, L. rhamnosus, L. reuteri, S. thermophilus |
| Capsule | Spring Valley - Women’s Total Care Probiotic | B. lactis, L. acidophilus, L. casei, L. gasseri, L. plantarum, L. rhamnosus |
| Capsule | Swanson - FemFlora | L. reuteri, L. rhamnosus, L. acidophilus, L. casei, L. fermentum, L. lactis, L. plantarum |
| Capsule | Nature’s Truth - Women’s Probiotic + Cranberry | L. acidophilus, L. plantarum, L. paracasei, L. rhamnosus, B. bifidum, L. casei, L. salivarius, B. lactis |
| Capsule | Maxi Healthy - Maxi 7M Supreme | L. acidophilus, B. bifidum |
| Capsule | 1MD - BiomeMD for Women | B. lactis, L. acidophilus, L. rhamnosus, L. reuteri, B. longum, B. bifidum, L. casei, L. plantarum, L. gasseri, L. salivarius, L. bulgaricus, Bacillus coagulans |
| Capsule | Flora Pro - Feminine Probiotic for Vaginal Health | L. rhamnosus, L. casei, L. acidophilus, B. bifidum, B. longum, L. brevis, L. salivarius, L. bulgaricus, L. plantarum |
| Capsule | Flora Cap | L. rhamnosus, L. casei, L. reuteri, L. acidophilus, B. bifidum, B. longum, L. brevis, L. salivarius, L. bulgaricus, L. plantarum |
| Capsule | Vital Planet - Vital Flora Pro | L. acidophilus, L. plantarum, L. paracasei, L. rhamnosus, L. brevis, L. bulgaricus, L. casei, L. reuteri, L. salivarius, L. fermentum, L. gasseri, L. helveticus, L. delbrueckii, B. lactis, B. infantis, B. bifidum, B. longum, B. breve |
| Capsule | Her Secret - Vaginal Support Probiotic Blend | Bacillus subtilis, Bacillus coagulans, L. acidophilus, B. lactis, B. longum, L. rhamnosus, L. salivarius, L. casei, B. breve, L. plantarum |
| Capsule | The Vitamin Shoppe | L. acidophilus, L. rhamnosus, L. casei, L. plantarum, B. bifidum, L. bulgaricus, B. longum, L. salivarius, S. thermophilus, B. breve |
| Capsule | Andrew Lessman - Procaps - Ultimate Friendly Flora | L. acidophilus, L. plantarum, L. paracasei, L. lactis |
| Capsule | NextLevel Probiotics | L. acidophilus, L. fermentum, B. longum, B. bifidum, L. rhamnosus, L. paracasei, L. plantarum, Saccharomyces boulardi, Bacillus coagulans |
| Capsule | Probiology by Belle+Bella - Flora Fem | L. paracasei, L. rhamnosus, L. helveticus, B. bifidum |
| Capsule | NutriCelebrity - NutriFlora-Pro | L. acidophilus, L. plantarum, L. gasseri, L. rhamnosus, B. lactis, L. crispatus |
| Capsule | Viva Naturals - Probiotics for Women | L. paracasei, L. casei, L. rhamnosus, L. helveticus, B. bifidum, B. breve, B. lactis, B. animalis lactis, L. acidophilus, L. brevis, L. plantarum, L. lactis, L. reuteri |
| Capsule | Now Foods - Women’s Probiotic | B. lactis, L. rhamnosus, L. acidophilus |
| Capsule | Advanced Naturals - Ultimate Flora Max - Vaginal Balance | L. acidophilus, L. rhamnosus, L. plantarum, L. gasseri, L. casei, L. paracasei, L. salivarius, L. brevis, B. lactis, B. longum |
| Capsule | Vitanica - FemEcology - Vaginal Probiotic Support | L. acidophilus, L. plantarum. L. reuteri, L. rhamnosus, L. salivarius |
| Tablet | LoveBug Probiotics | L. reuteri, L. plantarum, L. gasseri, L. fermentum, L. brevis |
| Tablet | Naturewise - Women’s Care | B. lactis, L. acidophilus, L. fermentum, L. plantarum, L. reuteri, B. bifidum, B. breve, S. thermophilus |
| Softgel | Pearls Women’s Probiotic | L. acidophilus, L. rhamnosus, L. plantarum |
| Liquid Drops | Humarian - Probonix | L. fermentum, L. gasseri, L. parcasei, L. plantarum, L. rhamnosus, L. acidophilus, B. bifidum, B. infantis, L. brevis, L. casei, L. salivarius, S. thermophilus |
3.2. Intravaginal Delivery of Probiotics Relevant to BV Infection and Reproductive Health
The intravaginal delivery of lactobacilli and other probiotics may help circumvent some of the challenges associated with oral delivery for BV treatment. Most notably, localized intravaginal delivery ensures greater probiotic viability since it avoids the hepatic first-pass effect and passage through the harsh gastrointestinal environment. Furthermore, unlike oral dosage forms that rely on gastrointestinal and subsequent rectal absorption, intravaginal delivery localizes probiotics to the FRT, often resulting in increased probiotic concentrations, relative to oral delivery (40).
While the unique features of the FRT, which include its large surface area and low enzymatic activity, make it a favorable site for topical administration (39), similar to oral delivery, characteristics specific to the intravaginal environment must be considered to provide efficacious probiotic delivery and distribution to maintain and restore healthy vaginal flora. First, the safety of different probiotics and their associated dosage forms must be considered to negligibly impact other eukaryotic and prokaryotic cell types in the vaginal milieu and to promote the viability of a lactobacilli-dominant environment. Furthermore, the role of the menstrual cycle, reproductive status, and contraceptive agents including hormones such as estradiol and progesterone, which have a tremendous impact on the physiology of and regulation of epithelial cell function in the FRT, should also be considered when evaluating the potential effectiveness of probiotics and intravaginal delivery vehicles (40, 41).
As with oral probiotic administration, one of the initial methods investigated for vaginal probiotic delivery involved the administration of probiotic-containing yogurt formulations. While topical application of yogurt provided an initial method of intravaginally delivering probiotics, since then, other dosage forms including vaginal tablets, capsules, suppositories, microparticles, and intravaginal rings (IVRs) have been developed and used to locally deliver probiotics to the FRT (7, 33, 42–44). These delivery technologies, in the context of BV and female reproductive health, are more thoroughly reviewed in Supplementary Materials and are briefly summarized in Figure 1. We also highlight some of the commercially available intravaginally-administered probiotic products to improve vaginal health and BV infection (Table 2).
Figure 1.
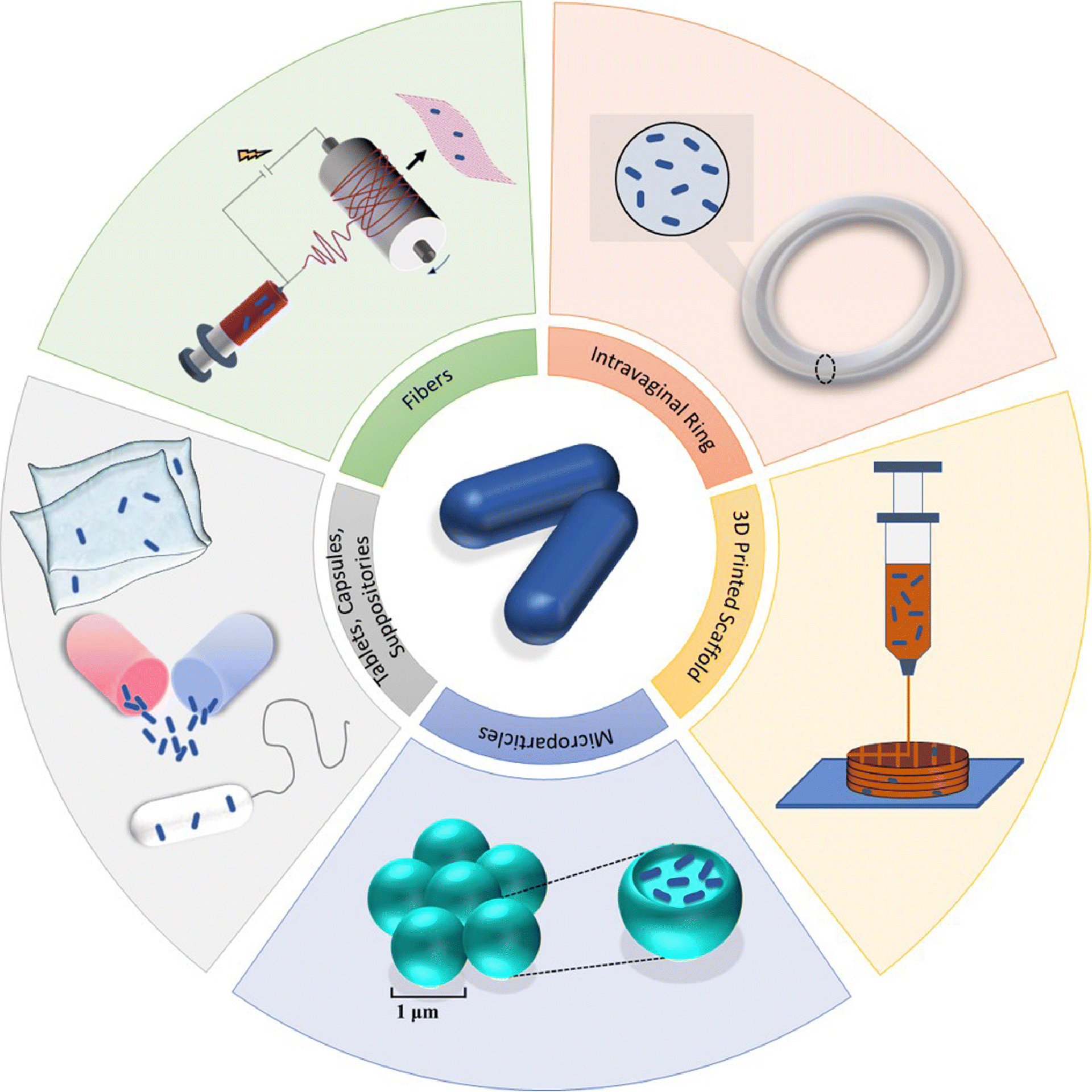
Schematic depiction of the different vaginal dosage forms used to deliver probiotics for BV treatment. Existing platforms include tablets, capsules, suppositories, microparticles, and intravaginal rings, while dosage forms including electrospun fibers and 3D printed scaffolds are either in development or may be considered to intravaginally deliver probiotics.
Table 2.
Some of the commercially available vaginal probiotic dosage forms available to treat BV and/or improve vaginal health.
| Vaginal Dosage Form | Brand | Probiotic Strains |
|---|---|---|
| Capsule | Physioflor | L. crispatus |
| Capsule | Canesten/ Canesflor | L. plantarum |
| Suppository | PurO3 | L. acidophilus, L. rhamnosus, L. reuteri, L. casei, L. parcasei, L. plantarum, L. bulgaricus, L. salivarius, L. delbrueckii, L. helveticus, L. brevis, L. gasseri, S. thermophilus, B. bifidum, B. infantis, B. breve, B. longum, B. lactis, B. coagulans |
| Suppository | Peachlife - Peachbody Probiotic Vaginal Suppository | B. longum, B. lactis, L. salivarius, L. casei, L. acidophilus, L. brevis, L. fermentum, L. parcasei, L. plantarum, L. reuteri, L. rhamnosus |
| Suppository | Good Clean Love - BiopHresh Vaginal Homeopathic Suppository | L. crispatus, L. acidophilus, L. gasseri, L. fermentum, L. plantarum, L. rhamnosus, L. salivarius |
| Suppository | NFH Flora SAP Vaginal Flora | L. rhamnosus, L. acidophilus, L. fermentum, L. casei, S. salivarius spp. thermophilus |
| Suppository | BYOSkin Bar - Probiotic Vaginal Suppository | L. reuteri, L. rhamnosus, L. acidophilus |
| Suppository | FloraFemme - Homeopathic Vaginal Probiotic Suppository | B. lactis, B. longum, L. acidophilus, L. brevis, L. bulgaricus, L. casei, L. paracasei, L. plantarum, L. reuteri, L. rhamnosus, L. salivarius |
| Suppository | Biom Probiotics - Vaginal Health Suppositories | L. crispatus, L. gasseri, L. acidophilus, Bacillus coagulans |
| Suppository | Biom Probiotics - Vaginal Health Suppositories | Bacillus coagulans, L. acidophilus |
4. Future Directions in Probiotic Delivery for BV Treatment
4.1. Electrospun Fibers
Electrospinning has been extensively explored for a variety of medical applications to date (45–50), with more recent consideration for the intravaginal delivery of therapeutic agents against viral (51–61) and bacterial pathogens (62–66). Fibers possess a multitude of favorable characteristics including cost-effectiveness, ease of scale-up, and the ability to fabricate a variety of geometries and sizes (67, 68), making them promising options as new probiotic delivery platforms. Furthermore, the potential of fibers to provide sustained-delivery − arising from their high surface-area-to-volume ratio, degree of interconnected porosity, tunable pore sizes, interchangeable polymer options, and diverse fiber architectures − is of particular interest to intravaginal applications (69), in which the sustained delivery of a variety of active agents (live cells in particular) is highly desirable. Lastly, fibers have been shown to enhance encapsulant stability (70), which is especially important to the viable incorporation and delivery of live probiotic cells.
While electrospun fibers have been explored as a practical and promising dosage form to incorporate and deliver bacteria for food engineering and biomedical applications, to date only a few studies have investigated the feasibility of probiotic incorporation in electrospun fibers that are, or may be relevant to, intravaginal delivery applications (70–76). Furthermore, a scarcity of studies has evaluated how to achieve sustained delivery of incorporated probiotics. Given this, the goal of these sections is to review studies that have incorporated live probiotics into fibers and discuss their future potential to integrate and modulate the release of probiotics for reproductive health applications such as BV. We first review studies focused on probiotic incorporation within electrospun fibers from food engineering and biomedical applications outside of female reproductive delivery, the knowledge of which may be applied to develop similar technologies for female reproductive health. Subsequently, we elaborate on the few studies that have incorporated bacteria in fibers for intravaginal applications. Overall, we highlight the promise fibers hold as efficacious, probiotic delivery platforms for BV treatment and identify considerations such as prebiotic incorporation, fiber architecture, and the incorporation of different bacterial species to enhance the viability and subsequent release of probiotics.
4.1.1. Probiotic Incorporation in Fibers
Some of the first studies to demonstrate the feasibility of electrospinning probiotics pertained to food processing and biomedical applications. One of these studies focused on food industry applications that incorporated L. plantarum and prebiotic fructo-oligosaccharides in polyvinyl alcohol (PVA) fibers to improve probiotic-resistance to moist heat (77), demonstrating the ability to finely tune fiber conditions such that probiotic viability could be maintained across different temperature ranges. In another study focused instead on fiber architecture, the effectiveness of two different fiber designs was investigated to improve the survivability of L. plantarum. This study found that probiotic incorporation in coaxial PVA:sodium alginate fibers improved the survivability and heat resistance of probiotic L. plantarum, relative to uniaxial PVA-only fibers (78). Lastly, another study evaluated the feasibility of incorporating lactic acid-producing bacteria of different sizes with bacteriocins in poly(ethylene oxide) (PEO) fibers to assess the impact of bacteria type on post-spun viability, suggesting that smaller Gram-positive bacteria such as M. luteus may be more resistant to the high voltages involved in the electrospinning process (72). These studies emphasize the promising potential of electrospun fibers in probiotic delivery when factors such as prebiotic incorporation, fiber structure, and bacteria type are considered.
Taking cues from applications other than intravaginal delivery, more recent work has investigated methods to increase the incorporation and viability of various lactobacilli species into electrospun fibers by varying the polymer ratio (71), showing that probiotic viability varied as a function of polyvinylpyrrolidone (PVP) content. Another study utilized lyoprotectants to improve the long-term storage viability (24 wk) of probiotic dosage forms for vaginal delivery applications, finding that the most crucial parameter in achieving high cell viability was the concentration of the probiotic itself and lyoprotectant, as opposed to applied voltage or relative humidity (75).
A more recent study investigated the potential viability of incorporating ten different Lactobacillus species (at 1010 CFU probiotic/mg) in fibers for future intravaginal delivery applications (73). The results from this study indicated that lactobacilli remained viable after incorporation in 4% w/w PEO fibers, with a species-dependent 0.1 to 3 log CFU/mg decrease in viability after electrospinning. The highest viability was achieved for L. gasseri, which had a 0.1 log-decrease in post-spun viability. Furthermore, additional recent studies have highlighted parameters including bacterial morphology and surface properties that significantly impact cell viability (73) and conditions that improve probiotic fiber stability (76).
Together these studies show that to best achieve high cell viability and stability, parameters such as polymer/solution choice, fiber material and architecture, addition of lyoprotectants and additives, bacterial morphology/type, and moisture barrier properties must be considered, in particular to achieve conditions amenable to prolonged probiotic release (79). Specifically, polymer and solvent choices are often restricted as bacteria require aqueous environments for viability and processing; furthermore, for long-term release on the order of weeks to months, selected materials must enable relevant time frames of release and degradation to facilitate the desired cell release kinetics. Thus far, fiber designs have primarily consisted of uniaxial architectures, in which a single hydrophilic polymer type is used, due to the constraints of incorporating live cells in non-aqueous formulations. However, one of the attributes of fiber-based technologies is that advanced fiber architectures may be designed to provide differential stimuli-responsive, rapid, or sustained-release kinetics. Furthermore, the ability to formulate fibers in various shapes, sizes, and textures, combined with microscale properties including pore size, degree of porosity, surface modifications, surface-area-to-volume ratio, and interchangeable polymer selection, enables a platform with finely tunable capabilities (39, 80). The studies highlighted in the next section discuss important considerations and different architectures that may be applied to further enhance cell viability and modulate cell release.
4.1.2. Architectural Lessons from the Intravaginal Delivery of Active Agents from Fibers
The ability of electrospun fibers to be fabricated in a variety of architectures is a prominent feature which may impact probiotic incorporation, therapeutic co-administration, sustained- or staged-release, and overall functionality. Conventionally, uniaxial architectures have provided high encapsulation of a variety of active agents ranging from antiretrovirals to antibiotics (68, 81, 82) and have been tailored to exhibit sophisticated release patterns in response to environmental conditions such as pH (56).
Despite the use of uniaxial fibers to deliver active agents to the female reproductive tract, most uniaxial fibers consist of hydrophilic polymers such as PVP and PEO which rapidly dissolve and release active agents within relatively short time frames of minutes to hours (83). In contrast, hydrophobic polymers can provide sustained release of agents; however, hydrophobic polymers often require the use of harsh solvents for electrospinning, which may limit the application of uniaxial fibers in probiotic delivery (57, 84). However, blends of hydrophobic and hydrophilic polymers may be used to modulate active agent release (85–87). While polymer blends are effective in tuning the release of active agents, the use of discrete architectures, in which hydrophilic and hydrophobic polymers are spun in parallel or sequentially, may provide additional versatility in incorporating different agents and modulating release. Recently, more complex architectures including coaxial, multilayered, and composite fiber architectures have been explored to modulate the release of active agents for intravaginal delivery (45–50) and have the potential to incorporate probiotics in discrete fiber sections for female reproductive health applications.
Coaxial Electrospun Fibers
Coaxial fibers, derived from uniaxial electrospinning, are comprised of an outer protective shell which surrounds an inner core (39). While the inner core serves to provide favorable conditions for the incorporation of active-agents/encapsulants, the primary functionality of the outer layer is to protect encapsulants in the inner core from potentially harsh conditions (e.g., the FRT) and to modulate their release (88). Additionally, the ability to select polymers with different properties (such as hydrophobicity or hydrophilicity) for any given layer of the coaxial fiber yields further control over the release properties. Furthermore, varying the thickness ratio of the outer versus inner shell enables additional tunability that is lacking in uniaxial fibers (89). The benefit of these characteristics was highlighted in a study that compared ethyl cellulose (EC) shell and PVP core coaxial fibers to uniaxial EC fibers for localized vaginal administration. The coaxial fibers demonstrated improved sustained-release of maraviroc relative to uniaxial EC fibers, highlighting the relationship between shell thickness and decreased drug release (59). Additional study details and the attributes of stimuli-responsive coaxial fibers are discussed in Supplementary Materials.
Given the benefits that coaxial fibers have shown in intravaginal delivery applications, one can envision their potential to also incorporate probiotics with high viability and sustained-release. A hydrophilic core, electrospun in an aqueous solution with high nutrient content, may be envisioned to benefit bacterial viability and proliferation. Furthermore, an outer hydrophobic shell may act to minimize exposure and protect bacteria (facultative or anaerobic) from oxygen, high temperatures, and other harsh conditions, while simultaneously serving as a barrier to prolong probiotic release. Additionally, release may be further modulated by changing the polymer composition and pore size of the outer shell.
Multilayered Fibers
Building upon the benefits provided by coaxial fiber architectures, multilayered fibers have been recently used to further sustain the release of active agents for intravaginal and other dosage forms. Multilayered architectures − comprised of various individual layers arranged on top of each other − conveniently allow for the encapsulation of different active agents within each layer; their release can then be easily tuned, with substantial control, by modifying the porosity, thickness, and composition of each layer. Multilayered fibers offer simplicity in design, cost-effectiveness, and perhaps the greatest flexibility in finely tuning release properties. Importantly, spatially-specific release advantageously characterizes this particular architecture, allowing for distinct release profiles from separate layers (39).
The primary methods to fabricate multilayered fibers include sequential layering, stacking, and interweaving of fibers (90–92). To date, few studies have explored multilayered fibers for intravaginal applications, and have primarily focused on multilayered architectures that provide short-term and intermediate levels of release (240 hr)(93). However, research in other drug delivery applications has demonstrated the ability of multilayered fibers to modulate the release of active agents by altering the number and thickness of drug-loaded layers (94). One can envision that the use of multilayered fiber architectures may be extended to incorporate probiotics, perhaps within a hydrophilic layer sandwiched between hydrophobic layer(s) to tailor release. Since release from multilayered fibers is governed by many parameters including polymer composition, number of layers, and layer thickness and porosity, these architectures seem to offer the greatest versatility in modulating release kinetics, in particular for applications that require discrete aqueous-based polymer solutions for probiotic incorporation.
Particle-Fiber Composite
A potentially promising platform, that may be effective for probiotic delivery, may be found in the integration of nanoparticles (NPs) with electrospun fibers. The resulting composite may be desirable for FRT delivery because NPs and fibers offer complementary delivery features. For example, NPs may serve as mobile platforms to better facilitate active agent transport across intravaginal barriers and subsequent retention in target tissue (39), while fibers may offer a more stationary platform that serves as a reservoir to enhance agent stability and modulate release (95). Both delivery platforms have been extensively used for intravaginal delivery of more “traditional” therapeutics including antiretrovirals and proteins (96–98). While it may be infeasible to incorporate probiotics in synthetic hydrophobic polymers, natural polymers such as chitosan and gelatin are more likely candidates that may be applied for probiotic incorporation (99, 100). However, the constant shedding of the mucus is known to pose a significant obstacle to NP retention and corresponding efficaciousness (101–103). One way to address this limitation may be to formulate NP-fiber composites, in which fibers serve as reservoirs for NPs and consequently improve NP retention. Furthermore, the pre-established long-term release potential of multilayered fibers, may allow for a significant range of kinetic tunability. A summary of some recent composite systems, envisioned for intravaginal delivery, can be found in Supplementary Materials.
While future work may imagine incorporating probiotics in particle-fiber composites, due to the larger size of probiotics, microparticles fabricated from natural hydrophilic polymers (perhaps, alginate-based particles) may be used instead to provide high levels of viable probiotic encapsulation. The fiber component of the structure may similarly serve as a reservoir to improve particle/probiotic retention and modulate the release of probiotics for BV treatment, when needed. Conversely, one can imagine that particles may incorporate different active agents to offer complementary mechanisms of activity to probiotic organisms in restoring or maintaining vaginal health. Regardless of vision, the use of advanced fiber architectures has the potential to elevate probiotic delivery by providing additional options to protect cells from unfavorable conditions, tuning sustained- and/or stimuli-responsive release and offering multi-mechanistic approaches to combat the multitude of bacterial and viral infections experienced by women globally.
4.2. 3D Bioprinting
In addition to the potential offered by electrospinning to incorporate and modulate the release of probiotics, 3D bioprinting may offer a promising alternative by which to incorporate and deliver probiotic organisms for female reproductive infections, including BV. With respect to other technologies, 3D bioprinting is a relatively new technique that involves the fabrication of complex 3D architectures through the layer-by-layer deposition of cells and biomaterials (104). As opposed to traditional 3D printers, bioprinters operate using processing conditions and temperature ranges that are conducive to the survival of cell-laden bioinks (105, 106). The low cost, potential for constructing personalized patient-specific designs, and the high precision and degree of control that bioprinting lends to the design process have helped it to become a method of rapid prototyping for tissue engineering applications in the research and medical communities (104). In particular, 3D bioprinting has shown tremendous promise in tissue engineering and regenerative medicine due in part to its potential to construct complex, functional tissues and organs. In addition, the development of complex 3D cell culture architectures has enabled researchers to develop more accurate in vitro disease models for toxicology studies and to better customize in vivo therapies (107). In parallel, bioprinted architectures have been used to target drug release to specific sites within the body in a controlled manner and in response to specific stimuli (108, 109). Extrapolating these features, we envision that 3D bioprinting may be used to incorporate and release live probiotics to promote and restore intravaginal health. To our knowledge, such a 3D bioprinted scaffold has not yet been developed for female reproductive health applications. Here we will summarize some of the strides made in 3D bioprinting for intravaginal delivery and in 3D bioprinting bacteria for other biomedical applications, that may be applied to FRT delivery.
4.2.1. 3D Bioprinting Methods/Technologies
A variety of 3D bioprinting technologies exist, with the most popular being based on inkjet, laser-assisted, and extrusion printing methodologies. A brief synopsis of these methodologies can be found in Supplementary Materials. Of all the 3D printing technologies, extrusion printing offers the widest selection of biomaterials – from hydrogels to cell spheroids. More importantly, it is capable of printing very high cell densities and also exhibits fast printing speeds (110, 111). The primary concern with extrusion bioprinting is that cell damage may be induced by the high shear stress, but this can be circumvented in part through the use of bioinks that have shear-thinning or thermal gelation properties (109, 112). Multiple studies have used extrusion-based printing to successfully print bacterial scaffolds (106, 113–117).
Regardless of the specific technology used, the basic process of bioprinting remains the same. The 3D construct is first modeled in silico through a computer-aided design (CAD) software and uploaded to the printer. The scaffold is then printed using a customized bioink and undergoes post-processing steps such as crosslinking to provide additional mechanical features (105, 106).
Careful adjustment of many parameters including printing speed, pressure, nozzle diameter, nozzle shape, and bioink composition are necessary to optimize printing resolution. Oftentimes, speed and pressure are directly dependent, and have to be adjusted in parallel to ensure the extrusion of a continuous, even flow of bioink filament (106, 118).
4.2.2. Parameters for Bioink Selection for Probiotic Incorporation
The evaluation and selection of appropriate bioinks for a particular application is one of the most important considerations of the bioprinting process, due to its impact on the structure and function of the printed architectures, and the growth and differentiation of incorporated cells. A variety of bioinks are available, ranging from acellular thermoplastics to polymer solutions that are comprised of live cells and/or biomaterials capable of supporting cell adhesion, proliferation, and differentiation.
The ideal bioink should possess biological properties such as biocompatibility and low cytotoxicity, as well mechanical properties including good printability, which is defined by high mechanical stability and fine printing resolution. However, this can be challenging since the properties that enhance printability typically diminish biological properties (107). For example, although highly viscous solutions better maintain their structure upon extrusion, exhibiting the desired printability, the increased pressure needed for extrusion induces high shear stress on encapsulated cells, decreasing viability. To overcome this challenge, materials that possess shear-thinning effects (i.e., non-Newtonian solutions) are typically chosen as bioinks and have included sodium alginate and gelatin methacrylamide/gellan gum blends (104, 107).
Additional desirable bioink properties include in situ gelation, viscoelasticity, low cost, ease of availability, industrial scalability, short maturation time after printing, immunological compatibility, and permeability of nutrients/oxygen and waste (107). Furthermore, scaffolds should elicit negligible immune responses from the host, especially for applications, such as BV, which involve increased susceptibility to infection. A summary of some of the most widely used bioinks is provided in Supplementary Materials.
Although other bioinks have not been specifically shown to incorporate prokaryotic organisms, they have demonstrated promise with eukaryotic cells and their application could potentially be extended to the printing of prokaryotic organisms including lactobacilli. Recent research has discovered bioinks with supramolecular functionality, reversible crosslinking polymers, and stimuli-responsive hydrogels, expanding the possibilities that bioprinting can offer to the medical community (110).
4.2.3. Potential of 3D printing for Probiotic Incorporation
Although there has been extensive research regarding the 3D printing of eukaryotic cells for tissue engineering applications (119), there are comparatively fewer studies that have printed prokaryotic organisms (120), in particular probiotics. Given the dearth of probiotic bioprinting in general, and none-to-date targeting female reproductive health, the focus here will be to discuss technologies that have been employed to print bacterial communities for other applications, and that may be extrapolated to design probiotic structures for BV treatment. Furthermore, unlike the majority of 3D cell printing studies that focus on developing scaffolds capable of supporting cell growth throughout its architecture, here we focus on how the technology may be extended to release and deliver cells to the FRT.
3D printed scaffolds embedded with bacteria have been used to induce chemical reactions, manufacture biologically important substances, break down pollutants/toxic chemicals, and study biofilm structure and formation (106, 115, 116). Bacterial constructs containing Staphylococcus aureus, methicillin-resistant S. aureus, and Pseudomonas aeruginosa have also been printed for antibiotic testing purposes (114, 121).
Other more complex designs, that encapsulate multiple bacterial species, have been printed for biomedical and bioremediation applications (122). In one study, structures containing both Pseudomonas putida and Acetobacter xylinum were 3D bioprinted, using a direct ink writing technique, for bioremediation and dermatological applications. The same study described another example in which both P. putida and B. subtilis were printed into a single grid pattern, but each strain was spatially separated into different columns of the grid, thus demonstrating the ability of bioprinted technologies to control distinct spatial organization and composition of bacterial architectures. Furthermore, this study illustrated the ability to combine multiple bacteria, each with varying characteristics, within one functional structure (113). These findings suggest the possibility of constructing probiotic scaffolds capable of releasing multiple different lactobacilli species/strains to more closely mimic the microbial diversity of the healthy FRT.
3D printed scaffolds not only offer control over composition and spatial organization, but they can also be genetically manipulated to enhance desirable characteristics. In one study, E. coli biofilms were printed and subsequently genetically induced to form curli fibers (a natural extracellular component of E.coli biofilms); this combination of 3D printing and genetic manipulation of the resulting platform improved scaffold stability and resilience, and induced adherent biofilm-mimicking structures for water filtration applications (115).
While bacterial 3D printing has been explored for a variety of applications outlined above, very few studies have focused specifically on probiotic printing. In one study, Lactobacillus plantarum was incorporated into a 3D printed cereal-based food, a category of food which comprises approximately two-thirds of human food consumption. In this printing application, probiotic viability of ~106 CFU/g was achieved, and increased survival may have been achieved without high temperature baking of dough formulations (117). In a different study, Bifidobacterium animalis were incorporated (with a viability of around 1010 log CFU/g) as a probiotic food source for gastrointestinal health applications. Given these findings, in combination with the successes of highly incorporating viable bacteria in the aforementioned studies, we anticipate that 3D printed probiotic scaffolds for intravaginal BV treatment have the potential to meet the dosing requirements of ~108 viable organisms per day for clinical BV treatment (19). Although these are among the few studies that demonstrate 3D printing of probiotics to date, they in conjunction with other bacterial 3D printing studies, support the possibility of using similar bioinks and technologies to incorporate and print probiotic lactobacilli for intravaginal applications.
4.2.4. Applying 3D Bioprinted Scaffolds to Customize Probiotic Release
To date, 3D cell printing studies have largely focused on developing scaffolds capable of supporting and facilitating cell growth throughout their architectures, rather than releasing and delivering cells to specific locations such as the FRT. Regardless, given the variety of 3D printed drug delivery platforms that have already been developed to modulate the release of non-living pharmaceutical agents, we predict that similar technologies and architectures may be employed to deliver probiotics for female reproductive health issues like BV.
3D printed sustained-release drug (non-cell) delivery platforms for vaginal applications are limited. Some have been briefly discussed in a prior review (123). One particular study printed suppository molds using a castable resin and injected non-biodegradable silicone elastomers to produce non-dissolvable suppositories that tailored shape and size to fit individual needs, while facilitating efficacious analgesic release for cancer, post-surgical, and postpartum pain management for more than 30 days (124).
In another study, suppository shell molds were 3D printed using PVA, a water-soluble polymer that disintegrated to release incorporated progesterone (125). This study discovered that modification of the number, position, and size of holes in the suppository shell affected hormone release rates in a predictable pattern, further emphasizing that modifications to the architecture can affect drug release characteristics and be used to personalize delivery. Similar 3D printed molding processes have been used to develop unique tablet architectures capable of complex release kinetics including pulsatile release (126). The tablets consisted of 3 layers: an impermeable outer layer, surface eroding middle layer without drugs, and a surface eroding internal layer with drugs. Modifications to the shapes/thickness of each layer altered the surface area exposed to the external environment, thereby allowing for the precise tuning of release profiles. Although not specifically designed for vaginal applications, this study highlighted the potential of 3D printing to provide complex release profiles through unique architectures. Release of probiotics could be modulated through similar scaffold designs, although increased porosity/hole sizes may be needed to facilitate the release of cells since they are much larger than drugs (micrometers for cells vs. nanometers for drugs).
Drug-eluting 3D constructs of differing geometries have also been fabricated through direct 3D printing (without the use of molds) as well. Progesterone and estrogen laden PCL polymers were printed in a multitude of designs including surgical meshes, subdermal rods, intrauterine devices, and pessaries, each demonstrating release over the course of one week (127). Mesh architectures were also reported in a study in which antibiotics were incorporated into thermoplastic polyurethane vaginal meshes and displayed bacteriostatic activity on S. aureus and E. coli with release lasting 3 days (128). Another study printed T-shaped intrauterine devices and subcutaneous rods with ethylene vinyl acetate showing release of indomethacin longer than 30 days (129). While drug solubility upon release, drug/polymer crystallinity, drug loading concentration, and extrusion temperature were the primary factors contributing to the differential release profiles, these studies help to demonstrate how the architectural flexibility of 3D printing can customize devices that fit individual patient anatomy.
As another example and utilizing a gold-standard product of sustained intravaginal delivery, intravaginal rings were 3D printed to topically deliver progesterone with sustained-release for up to 7 days. Here progesterone was mixed with PEG, and subsequently printed into a hydrophobic matrix consisting of poly(lactic acid) (PLA) and PCL, serving to restrict progesterone release, while enabling pore formation for progesterone release (130). A similar concept, involving the integration of hydrophilic and hydrophobic polymers, may be potentially used to sustain probiotic release in a similar environment. We anticipate that such a development would be key to solving problems involving BV recurrence and user adherence since it can offer prolonged local delivery of probiotics at high concentrations.
Innumerable other drug delivery platforms have been 3D printed with the purpose to sustain active agent delivery, although not for female reproductive health applications like BV and none (to our knowledge) involving the release of live cells. Release profiles of active agents from printed scaffolds can be modulated by controlling the polymer composition, architecture, and infill densities. Given this, similar factors may be investigated to tune the release of prokaryotic cells from 3D bioprinted vaginal scaffolds.
4.2.5. Architectural Lessons from other 3D Printing Applications
While our focus has primarily been on 3D printed vaginal drug delivery platforms and discussing how those technologies may be extended to release probiotics in the FRT, important architectural ideas can also be gleaned from other 3D printing applications. Here we expand on a few 3D printed designs used in non-vaginal applications, to discuss how they may benefit intravaginal delivery.
Two of the most utilized architectures that have been used to incorporate L. plantarum are “concentric” and “honeycomb” geometries with advanced surface-to-volume ratio and infill patterns of 60% and 30%, respectively (117). The major advantage of these printed structures is their ability to incorporate increased concentrations of viable probiotics (106 CFU/g) and to improve cell survival. In addition, in a previously noted study, cylindrical architectures were used to incorporate Bifidobacterium animalis in 3D printed mashed potatoes as a probiotic food source for gastrointestinal health applications. Overall, the incorporation of probiotics within a cylindrical scaffold resulted in the improved stability of Bifidobacterium animalis (131).
A more complex 3D printed architecture was also investigated for its role in improving gastrointestinal microbial health. A coaxial 3D printing configuration was used to design a core-shell fiber, with each layer incorporating one of two drugs to either improve Bifidobacterium proliferation or inhibit the growth of pathogenic E. coli (132). The design resulted in a biphasic release profile of both active agents, stachyose and proteoglycan, that were incorporated in the outer and inner layers, respectively, providing both burst and sustained-release. It was found that release could be modulated by changing the location of drug loading (either within shell or core layers) and the geometry of the fiber matrix (either a square, semi-circular, or circular patterned grid).
In a study that explored the impact of 3D printed scaffold geometry on the pathogenicity of incorporated bacteria, a more complex geometry – a 3D gelatin-based pyramid-shape – was designed to incorporate and investigate the resistance of S. aureus to β-lactam antibiotics in the presence of Pseudomonas aeruginosa (121). This study showed that physically distinct, yet chemically interactive populations of defined shape, size, and density can be organized into limitless arrangements. Additionally, a previously mentioned study which printed E.coli biofilms, showed that distinct bacterial layers can be printed with spatial separation in the z-axis (115), while a different study investigated the spatiotemporal characteristics of 3D biofilms by bioprinting E. coli, S. aureus (MSSA), methicillin-resistant S. aureus (MRSA), and P. aeruginosa into solid or lattice geometries using an alginate-based bioink (114).
A similar study utilized a 3D printing process known as “freeform reversible embedding of suspended hydrogels” to design complex 3D structures that were otherwise impossible to fabricate with traditional fabrication methods. Square lattices with rectilinear infill, more complex patterns with octagonal infill, and nonplanar helices were printed using an alginate-based bioink containing C2C12 myoblast cells (133).
Expanding beyond scaffolds that are entirely 3D printed, 3D printing can also be combined with other techniques such as electrospinning (134) and nanoparticle encapsulation (135) to create more complex architectures that facilitate sustained release. For instance, since direct incorporation of growth factors in 3D bioprinted tissue scaffolds has been shown to lead to degradation and quick elimination, growth factors have been embedded in nanocarriers, which are printed into the final tissue scaffold. Sustained release of the growth factors was observed as the nanocarrier shell slowly degraded (118). Similar to this work, one may envision the potential of probiotic-containing microparticles to be incorporated in a bioprinted platform. This method has the potential to provide the benefits of highly tunable 3D bioprinted release while maintaining high probiotic viability (136). To address concerns of decreased probiotic bioavailability upon direct mixing with polymer bioink, a different study microencapsulated probiotics into 3-layered capsules to shield the organisms from external forces (137). These microcapsules can be subsequently delivered through a bioprinted platform, thereby providing the benefits of 3D bioprinted release platforms, while maintaining high probiotic viability (136).
Another study used a combination of electrospinning and 3D bioprinting to design a structure with controlled porosity and nanoscale features that mimic the ECM for tissue engineering applications (138). The design consisted of an orthogonal array of 3D bioprinted gel microfibers, with electrospun PCL nanofibers intercalated between every two microfiber layers. This design enhanced control over porosity relative to 3D printing alone, ensuring adequate pore sizes for proper cell migration and diffusion of nutrients/waste. At the same time, the nanofiber web functioned as a cell entrapment system, enabling greater cell attachment and proliferation than a normal bioprinted gel scaffold. Similar concepts could be used to design scaffolds that offer sufficient controlled porosity for cell release while promoting cell proliferation within the scaffold. Such an integration of different technologies may help to overcome the weaknesses of each technique to design complex functional probiotic scaffolds that suit a variety of female reproductive needs.
In summary, 3D bioprinting has been based on a variety of materials that are able to form layer-by-layer structures. By applying computer aided software, bioprinting has demonstrated the ability to generate a wide range of high-resolution biological architectures with complex and controlled designs and shapes (113). These capabilities have advanced 3D bioprinting in the fields of tissue engineering (139), regenerative medicine (140, 141), biomedical sciences, and laboratory and commercial settings (142). Scaffold architecture is one of its most important features since it influences its mechanical support, biological response, and transport of important substances throughout its structure (118). Modification of the 3D scaffold geometry may be advantageous in designing a vehicle that can sustain probiotic delivery for BV treatment. Although there is no existing research exploring this, it may be a promising option by which to promote female reproductive health and prevent infections such as BV.
5. Conclusions
Bacterial vaginosis is a dysbiotic condition caused by an imbalance of naturally occurring bacterial flora. BV has emerged as a public health concern given its serious implications, some of which include endometriosis, pelvic inflammatory disease, impact on preterm birth, and increased risk of acquiring sexually transmitted infections. In the first portion of this review, we focus on the devastating impact of BV on female reproductive health, and highlight the limitations of traditional antibiotic approaches to provide a long-term “cure” for BV. Furthermore, we discuss the emergence of probiotic organisms as an alternative therapy to reinstate the healthy lactobacilli-dominant vaginal flora and to treat and/or prevent BV recurrence. Toward this we discuss research studies and clinical trials that have investigated the current methods of frequent, often daily, oral or vaginal probiotic administration and the associated dosage forms to date, that deliver probiotics alone or in combination with antibiotics. In the final sections of this review paper, we aimed to explore the potential utilization of other technologies that have begun to be employed for the intravaginal delivery of active agents, namely electrospun fibers and 3D bioprinted scaffolds. We have highlighted different materials, parameters, formulations, and architectures in both electrospinning and 3D bioprinting that may be optimized to provide the viability and release necessary for future alternative and innovative intravaginal probiotic delivery approaches. Ultimately, we envision that the implementation of these innovative strategies has significant potential to improve not only BV, but to impact broader female reproductive health applications.
Supplementary Material
Acknowledgments
Funding for this work was provided by NIH NIAID R01-AI139671.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Basavaprabhu H, Sonu K, Prabha R. Mechanistic insights into the action of probiotics against bacterial vaginosis and its mediated preterm birth: An overview. Microbial Pathogenesis. 2020:104029. [DOI] [PubMed] [Google Scholar]
- 2.Nader-Macías MEF, Tomás MSJ. Profiles and technological requirements of urogenital probiotics. Advanced drug delivery reviews. 2015;92:84–104. [DOI] [PubMed] [Google Scholar]
- 3.Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ. Characterization of vaginal microbial communities in adult healthy women using cultivation-independent methods. Microbiology. 2004;150(Pt 8):2565–73. [DOI] [PubMed] [Google Scholar]
- 4.Burton JP, Cadieux PA, Reid G. Improved understanding of the bacterial vaginal microbiota of women before and after probiotic instillation. Appl Environ Microbiol. 2003;69(1):97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onderdonk AB, Delaney ML, Fichorova RN. The human microbiome during bacterial vaginosis. Clinical microbiology reviews. 2016;29(2):223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vujic G, Knez AJ, Stefanovic VD, Vrbanovic VK. Efficacy of orally applied probiotic capsules for bacterial vaginosis and other vaginal infections: a double-blind, randomized, placebo-controlled study. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2013;168(1):75–9. [DOI] [PubMed] [Google Scholar]
- 7.Anukam KC, Osazuwa E, Osemene GI, Ehigiagbe F, Bruce AW, Reid G. Clinical study comparing probiotic Lactobacillus GR-1 and RC-14 with metronidazole vaginal gel to treat symptomatic bacterial vaginosis. Microbes and Infection. 2006;8(12–13):2772–6. [DOI] [PubMed] [Google Scholar]
- 8.Machado D, Castro J, Palmeira-de-Oliveira A, Martinez-de-Oliveira J, Cerca N. Bacterial Vaginosis Biofilms: Challenges to Current Therapies and Emerging Solutions. Frontiers in Microbiology. 2016;6(1528). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menard J-P. Antibacterial treatment of bacterial vaginosis: current and emerging therapies. International journal of women’s health. 2011;3:295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones A Bacterial Vaginosis: A Review of Treatment, Recurrence, and Disparities. The Journal for Nurse Practitioners. 2019;15(6):420–3. [Google Scholar]
- 11.Falagas M, Betsi G, Athanasiou S. Probiotics for the treatment of women with bacterial vaginosis. Clinical microbiology and infection. 2007;13(7):657–64. [DOI] [PubMed] [Google Scholar]
- 12.Nader-Macias ME, de Ruiz CS, Ocaña VS, Juarez Tomas MS. Advances in the knowledge and clinical applications of lactic acid bacteria as probiotics in the urogenital tract. Current Women’s Health Reviews. 2008;4(4):240–57. [Google Scholar]
- 13.Buchta V Vaginal microbiome. Ceska Gynekol. 2018;83(5):371–9. [PubMed] [Google Scholar]
- 14.Amabebe E, Anumba DOC. The Vaginal Microenvironment: The Physiologic Role of Lactobacilli. Frontiers in Medicine. 2018;5(181). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van de Wijgert JH, Verwijs MC. Lactobacilli- containing vaginal probiotics to cure or prevent bacterial or fungal vaginal dysbiosis: a systematic review and recommendations for future trial designs. BJOG: An International Journal of Obstetrics & Gynaecology. 2020;127(2):287–99. [DOI] [PubMed] [Google Scholar]
- 16.Mastromarino P, Vitali B, Mosca L. Bacterial vaginosis: a review on clinical trials with probiotics. New Microbiol. 2013;36(3):229–38. [PubMed] [Google Scholar]
- 17.Jang S-E, Jeong J-J, Choi S-Y, Kim H, Han M, Kim D-H. Lactobacillus rhamnosus HN001 and Lactobacillus acidophilus La-14 attenuate Gardnerella vaginalis-infected bacterial vaginosis in mice. Nutrients. 2017;9(6):531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pessoa WFB, Melgaço ACC, de Almeida ME, Ramos LP, Rezende RP, Romano CC. In Vitro Activity of Lactobacilli with Probiotic Potential Isolated from Cocoa Fermentation against Gardnerella vaginalis. Biomed Res Int. 2017;2017:3264194–. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reid G, Beuerman D, Heinemann C, Bruce AW. Probiotic Lactobacillus dose required to restore and maintain a normal vaginal flora. FEMS Immunology & Medical Microbiology. 2001;32(1):37–41. [DOI] [PubMed] [Google Scholar]
- 20.Reid G, Charbonneau D, Erb J, Kochanowski B, Beuerman D, Poehner R, et al. Oral use of Lactobacillus rhamnosus GR-1 and L. fermentum RC-14 significantly alters vaginal flora: randomized, placebo-controlled trial in 64 healthy women. FEMS Immunology & Medical Microbiology. 2003;35(2):131–4. [DOI] [PubMed] [Google Scholar]
- 21.Reid G, Bruce AW. Selection of Lactobacillus Strains for Urogenital Probiotic Applications. The Journal of Infectious Diseases. 2001;183(Supplement_1):S77–S80. [DOI] [PubMed] [Google Scholar]
- 22.McLEAN NW, ROSENSTEIN IJ. Characterisation and selection of a Lactobacillus species to recolonise the vagina of women with recurrent bacterial vaginosis. Journal of medical microbiology. 2000;49(6):543–52. [DOI] [PubMed] [Google Scholar]
- 23.Pascual L, Ruiz F, Giordano W, Barberis IL. Vaginal colonization and activity of the probiotic bacterium Lactobacillus fermentum L23 in a murine model of vaginal tract infection. Journal of medical microbiology. 2010;59(3):360–4. [DOI] [PubMed] [Google Scholar]
- 24.Liao H, Liu S, Wang H, Su H, Liu Z. Enhanced antifungal activity of bovine lactoferrin-producing probiotic Lactobacillus casei in the murine model of vulvovaginal candidiasis. BMC microbiology. 2019;19(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sabbatini S, Monari C, Ballet N, Mosci P, Decherf AC, Pélerin F, et al. Saccharomyces cerevisiae–based probiotic as novel anti-microbial agent for therapy of bacterial vaginosis. Virulence. 2018;9(1):954–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zárate G, Santos V, Nader-Macias ME. Protective effect of vaginal Lactobacillus paracasei CRL 1289 against urogenital infection produced by Staphylococcus aureus in a mouse animal model. Infectious Diseases in Obstetrics and Gynecology. 2007;2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MacPhee RA, Hummelen R, Bisanz JE, Miller WL, Reid G. Probiotic strategies for the treatment and prevention of bacterial vaginosis. Expert opinion on pharmacotherapy. 2010;11(18):2985–95. [DOI] [PubMed] [Google Scholar]
- 28.Drutz DJ. Lactobacillus prophylaxis for Candida vaginitis. American College of Physicians; 1992. [DOI] [PubMed] [Google Scholar]
- 29.Sieber R, Dietz U-T. Lactobacillus acidophilus and yogurt in the prevention and therapy of bacterial vaginosis. International Dairy Journal. 1998;8(7):599–607. [Google Scholar]
- 30.O’Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PloS one. 2013;8(11):e80074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bertuccini L, Russo R, Iosi F, Superti F. Effects of Lactobacillus rhamnosus and Lactobacillus acidophilus on bacterial vaginal pathogens. International Journal of Immunopathology and Pharmacology. 2017;30(2):163–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Homayouni A, Bastani P, Ziyadi S, Mohammad-Alizadeh-Charandabi S, Ghalibaf M, Mortazavian AM, et al. Effects of probiotics on the recurrence of bacterial vaginosis: a review. Journal of lower genital tract disease. 2014;18(1):79–86. [DOI] [PubMed] [Google Scholar]
- 33.Ya W, Reifer C, Miller LE. Efficacy of vaginal probiotic capsules for recurrent bacterial vaginosis: a double-blind, randomized, placebo-controlled study. American journal of obstetrics and gynecology. 2010;203(2):120.e1–.e6. [DOI] [PubMed] [Google Scholar]
- 34.Bodean O, Munteanu O, Cirstoiu C, Secara D, Cirstoiu M. Probiotics-a helpful additional therapy for bacterial vaginosis. Journal of medicine and life. 2013;6(4):434. [PMC free article] [PubMed] [Google Scholar]
- 35.Recine N, Palma E, Domenici L, Giorgini M, Imperiale L, Sassu C, et al. Restoring vaginal microbiota: biological control of bacterial vaginosis. A prospective case–control study using Lactobacillus rhamnosus BMX 54 as adjuvant treatment against bacterial vaginosis. Archives of gynecology and obstetrics. 2016;293(1):101–7. [DOI] [PubMed] [Google Scholar]
- 36.Ma D, Chen Y, Chen T. Vaginal microbiota transplantation for the treatment of bacterial vaginosis: a conceptual analysis. FEMS Microbiology Letters. 2019;366(4). [DOI] [PubMed] [Google Scholar]
- 37.Lev-Sagie A, Goldman-Wohl D, Cohen Y, Dori-Bachash M, Leshem A, Mor U, et al. Vaginal microbiome transplantation in women with intractable bacterial vaginosis. Nat Med. 2019;25(10):1500–4. [DOI] [PubMed] [Google Scholar]
- 38.DeLong K, Zulfiqar F, Hoffmann DE, Tarzian AJ, Ensign LM. Vaginal Microbiota Transplantation: The Next Frontier. The Journal of Law, Medicine & Ethics. 2019;47(4):555–67. [DOI] [PubMed] [Google Scholar]
- 39.Tyo KM, Minooei F, Curry KC, NeCamp SM, Graves DL, Fried JR, et al. Relating advanced electrospun fiber architectures to the temporal release of active agents to meet the needs of next-generation intravaginal delivery applications. Pharmaceutics. 2019;11(4):160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ensign LM, Cone R, Hanes J. Nanoparticle-based drug delivery to the vagina: a review. Journal of Controlled Release. 2014;190:500–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wira CR, Grant-Tschudy KS, Crane-Godreau MA. Epithelial cells in the female reproductive tract: a central role as sentinels of immune protection. American journal of reproductive immunology. 2005;53(2):65–76. [DOI] [PubMed] [Google Scholar]
- 42.Mastromarino P, Macchia S, Meggiorini L, Trinchieri V, Mosca L, Perluigi M, et al. Effectiveness of Lactobacillus-containing vaginal tablets in the treatment of symptomatic bacterial vaginosis. Clinical microbiology and infection. 2009;15(1):67–74. [DOI] [PubMed] [Google Scholar]
- 43.Senok AC, Verstraelen H, Temmerman M, Botta GA. Probiotics for the treatment of bacterial vaginosis. Cochrane Database of Systematic Reviews. 2009(4). [DOI] [PubMed] [Google Scholar]
- 44.Eriksson K, Carlsson B, Forsum U, Larsson P. A double-blind treatment study of bacterial vaginosis with normal vaginal lactobacilli after an open treatment with vaginal clindamycin ovules. Acta dermato-venereologica. 2005;85(1):42–6. [DOI] [PubMed] [Google Scholar]
- 45.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue engineering. 2006;12(5):1197–211. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed FE, Lalia BS, Hashaikeh R. A review on electrospinning for membrane fabrication: challenges and applications. Desalination. 2015;356:15–30. [Google Scholar]
- 47.Chronakis IS. Novel nanocomposites and nanoceramics based on polymer nanofibers using electrospinning process—a review. Journal of materials processing technology. 2005;167(2–3):283–93. [Google Scholar]
- 48.Schiffman JD, Schauer CL. A review: electrospinning of biopolymer nanofibers and their applications. Polymer reviews. 2008;48(2):317–52. [Google Scholar]
- 49.Huang Z-M, Zhang Y-Z, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites science and technology. 2003;63(15):2223–53. [Google Scholar]
- 50.Teo WE, Ramakrishna S. A review on electrospinning design and nanofibre assemblies. Nanotechnology. 2006;17(14):R89. [DOI] [PubMed] [Google Scholar]
- 51.Ball C, Woodrow KA. Electrospun fibers for microbicide drug delivery. Drug Delivery and Development of Anti-HIV Microbicides. 2014:459–507. [Google Scholar]
- 52.Grooms TN, Vuong HR, Tyo KM, Malik DA, Sims LB, Whittington CP, et al. Griffithsin-modified electrospun fibers as a delivery scaffold to prevent HIV infection. Antimicrobial agents and chemotherapy. 2016;60(11):6518–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krogstad EA, Woodrow KA. Manufacturing scale-up of electrospun poly (vinyl alcohol) fibers containing tenofovir for vaginal drug delivery. International journal of pharmaceutics. 2014;475(1–2):282–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blakney AK, Ball C, Krogstad EA, Woodrow KA. Electrospun fibers for vaginal anti-HIV drug delivery. Antiviral research. 2013;100:S9–S16. [DOI] [PubMed] [Google Scholar]
- 55.Kuhn JC, Zussman E. Encapsulation of bacteria and viruses in electrospun fibers. Google Patents; 2015. [DOI] [PubMed] [Google Scholar]
- 56.Huang C, Soenen SJ, van Gulck E, Vanham G, Rejman J, Van Calenbergh S, et al. Electrospun cellulose acetate phthalate fibers for semen induced anti-HIV vaginal drug delivery. Biomaterials. 2012;33(3):962–9. [DOI] [PubMed] [Google Scholar]
- 57.Tyo KM, Vuong HR, Malik DA, Sims LB, Alatassi H, Duan J, et al. Multipurpose tenofovir disoproxil fumarate electrospun fibers for the prevention of HIV-1 and HSV-2 infections in vitro. International journal of pharmaceutics. 2017;531(1):118–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hua D, Liu Z, Wang F, Gao B, Chen F, Zhang Q, et al. pH responsive polyurethane (core) and cellulose acetate phthalate (shell) electrospun fibers for intravaginal drug delivery. Carbohydrate Polymers. 2016;151:1240–4. [DOI] [PubMed] [Google Scholar]
- 59.Ball C, Chou S-F, Jiang Y, Woodrow KA. Coaxially electrospun fiber-based microbicides facilitate broadly tunable release of maraviroc. Materials Science and Engineering: C. 2016;63:117–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ball C, Woodrow KA. Electrospun solid dispersions of maraviroc for rapid intravaginal preexposure prophylaxis of HIV. Antimicrobial agents and chemotherapy. 2014;58(8):4855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tyo KM, Lasnik AB, Zhang L, Mahmoud M, Jenson AB, Fuqua JL, et al. Sustained-release Griffithsin nanoparticle-fiber composites against HIV-1 and HSV-2 infections. Journal of Controlled Release. 2020;321:84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reise M, Wyrwa R, Müller U, Zylinski M, Völpel A, Schnabelrauch M, et al. Release of metronidazole from electrospun poly (L-lactide-co-D/L-lactide) fibers for local periodontitis treatment. Dental Materials. 2012;28(2):179–88. [DOI] [PubMed] [Google Scholar]
- 63.Liakos IL, Holban AM, Carzino R, Lauciello S, Grumezescu AM. Electrospun fiber pads of cellulose acetate and essential oils with antimicrobial activity. Nanomaterials. 2017;7(4):84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mahmoud MY. Development of BAR-peptide nanoparticles and electrospun fibers for the prevention and treatment of oral biofilms. 2019.
- 65.El-Newehy MH, Al-Deyab SS, Kenawy E-R, Abdel-Megeed A. Fabrication of electrospun antimicrobial nanofibers containing metronidazole using nanospider technology. Fibers and Polymers. 2012;13(6):709–17. [Google Scholar]
- 66.Zamani M, Morshed M, Varshosaz J, Jannesari M. Controlled release of metronidazole benzoate from poly ε-caprolactone electrospun nanofibers for periodontal diseases. European Journal of Pharmaceutics and Biopharmaceutics. 2010;75(2):179–85. [DOI] [PubMed] [Google Scholar]
- 67.Zamani M, Prabhakaran MP, Ramakrishna S. Advances in drug delivery via electrospun and electrosprayed nanomaterials. International journal of nanomedicine. 2013;8:2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hu X, Liu S, Zhou G, Huang Y, Xie Z, Jing X. Electrospinning of polymeric nanofibers for drug delivery applications. Journal of controlled release. 2014;185:12–21. [DOI] [PubMed] [Google Scholar]
- 69.Jain KK. Drug delivery systems: Springer; 2008. [Google Scholar]
- 70.Amna T, Hassan MS, Pandeya DR, Khil M-S, Hwang I. Classy non-wovens based on animate L. gasseri-inanimate poly (vinyl alcohol): upstream application in food engineering. Applied microbiology and biotechnology. 2013;97(10):4523–31. [DOI] [PubMed] [Google Scholar]
- 71.Nagy ZK, Wagner I, Suhajda Á, Tobak T, Harasztos AH, Vigh T, et al. Nanofibrous solid dosage form of living bacteria prepared by electrospinning. 2014.
- 72.Heunis T, Botes M, Dicks L. Encapsulation of Lactobacillus plantarum 423 and its bacteriocin in nanofibers. Probiotics and antimicrobial proteins. 2010;2(1):46–51. [DOI] [PubMed] [Google Scholar]
- 73.Zupančič Š, Škrlec K, Kocbek P, Kristl J, Berlec A. Effects of Electrospinning on the Viability of Ten Species of Lactic Acid Bacteria in Poly (Ethylene Oxide) Nanofibers. Pharmaceutics. 2019;11(9):483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Soares J, da Costa MM, de Oliveira HP. Encapsulation and Bioavailability of Lactobacillus spp. in Electrospun Fibers. Current Biotechnology. 2020;9(1):15–22. [Google Scholar]
- 75.Škrlec K, Zupančič Š, Mihevc SP, Kocbek P, Kristl J, Berlec A. Development of electrospun nanofibers that enable high loading and long-term viability of probiotics. European Journal of Pharmaceutics and Biopharmaceutics. 2019;136:108–19. [DOI] [PubMed] [Google Scholar]
- 76.Silva JA, De Gregorio PR, Rivero G, Abraham GA, Nader-Macias MEF. Immobilization of vaginal Lactobacillus in polymeric nanofibers for its incorporation in vaginal probiotic products. Eur J Pharm Sci. 2021;156:105563. [DOI] [PubMed] [Google Scholar]
- 77.Feng K, Zhai M-Y, Zhang Y, Linhardt RJ, Zong M-H, Li L, et al. Improved viability and thermal stability of the probiotics encapsulated in a novel electrospun fiber mat. Journal of agricultural and food chemistry. 2018;66(41):10890–7. [DOI] [PubMed] [Google Scholar]
- 78.Feng K, Huang R-m, Wu R-q, Wei Y-s, Zong M-h, Linhardt RJ, et al. A novel route for double-layered encapsulation of probiotics with improved viability under adverse conditions. Food Chemistry. 2020;310:125977. [DOI] [PubMed] [Google Scholar]
- 79.Hemamalini T, Dev VRG. Comprehensive review on electrospinning of starch polymer for biomedical applications. International journal of biological macromolecules. 2018;106:712–8. [DOI] [PubMed] [Google Scholar]
- 80.Tao J, Shivkumar S. Molecular weight dependent structural regimes during the electrospinning of PVA. Materials letters. 2007;61(11–12):2325–8. [Google Scholar]
- 81.Kim TG, Lee DS, Park TG. Controlled protein release from electrospun biodegradable fiber mesh composed of poly (ɛ-caprolactone) and poly (ethylene oxide). International journal of pharmaceutics. 2007;338(1–2):276–83. [DOI] [PubMed] [Google Scholar]
- 82.Ji W, Sun Y, Yang F, van den Beucken JJ, Fan M, Chen Z, et al. Bioactive electrospun scaffolds delivering growth factors and genes for tissue engineering applications. Pharmaceutical research. 2011;28(6):1259–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tuğcu-Demiröz F, Saar S, Tort S, Acartürk F. Electrospun Metronidazole-Loaded Nanofibers for Vaginal Drug Delivery. Drug Development and Industrial Pharmacy. 2020(just-accepted):1–37. [DOI] [PubMed] [Google Scholar]
- 84.Aniagyei SE, Sims LB, Malik DA, Tyo KM, Curry KC, Kim W, et al. Evaluation of poly (lactic-co-glycolic acid) and poly (dl-lactide-co-ε-caprolactone) electrospun fibers for the treatment of HSV-2 infection. Materials Science and Engineering: C. 2017;72:238–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zong S, Wang X, Yang Y, Wu W, Li H, Ma Y, et al. The use of cisplatin-loaded mucoadhesive nanofibers for local chemotherapy of cervical cancers in mice. European Journal of Pharmaceutics and Biopharmaceutics. 2015;93:127–35. [DOI] [PubMed] [Google Scholar]
- 86.Li W, Luo T, Yang Y, Tan X, Liu L. Formation of controllable hydrophilic/hydrophobic drug delivery systems by electrospinning of vesicles. Langmuir. 2015;31(18):5141–6. [DOI] [PubMed] [Google Scholar]
- 87.Ball C, Krogstad E, Chaowanachan T, Woodrow KA. Drug-eluting fibers for HIV-1 inhibition and contraception. PloS one. 2012;7(11):e49792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.He CL, Huang ZM, Han XJ, Liu L, Zhang HS, Chen LS. Coaxial electrospun poly (L- lactic acid) ultrafine fibers for sustained drug delivery. Journal of Macromolecular Science, Part B. 2006;45(4):515–24. [Google Scholar]
- 89.Wang C, Yan K-W, Lin Y-D, Hsieh PC. Biodegradable core/shell fibers by coaxial electrospinning: processing, fiber characterization, and its application in sustained drug release. Macromolecules. 2010;43(15):6389–97. [Google Scholar]
- 90.Huang L-Y, Branford-White C, Shen X-X, Yu D-G, Zhu L-M. Time-engineeringed biphasic drug release by electrospun nanofiber meshes. International journal of pharmaceutics. 2012;436(1–2):88–96. [DOI] [PubMed] [Google Scholar]
- 91.Meinel AJ, Germershaus O, Luhmann T, Merkle HP, Meinel L. Electrospun matrices for localized drug delivery: current technologies and selected biomedical applications. European Journal of Pharmaceutics and Biopharmaceutics. 2012;81(1):1–13. [DOI] [PubMed] [Google Scholar]
- 92.Blakney AK, Krogstad EA, Jiang YH, Woodrow KA. Delivery of multipurpose prevention drug combinations from electrospun nanofibers using composite microarchitectures. International journal of nanomedicine. 2014;9:2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chou S-F, Woodrow KA. Relationships between mechanical properties and drug release from electrospun fibers of PCL and PLGA blends. Journal of the mechanical behavior of biomedical materials. 2017;65:724–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cortez Tornello PR, Feresin GE, Tapia A, Cuadrado TR, Abraham GA. Multilayered electrospun nanofibrous scaffolds for tailored controlled release of embelin. Soft Materials. 2018;16(1):51–61. [Google Scholar]
- 95.Falde EJ, Freedman JD, Herrera VL, Yohe ST, Colson YL, Grinstaff MW. Layered superhydrophobic meshes for controlled drug release. Journal of Controlled Release. 2015;214:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Destache CJ, Belgum T, Christensen K, Shibata A, Sharma A, Dash A. Combination antiretroviral drugs in PLGA nanoparticle for HIV-1. BMC infectious diseases. 2009;9(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang H, Li J, Patel SK, Palmer KE, Devlin B, Rohan LC. Design of poly (lactic-co-glycolic acid)(PLGA) nanoparticles for vaginal co-delivery of griffithsin and dapivirine and their synergistic effect for HIV prophylaxis. Pharmaceutics. 2019;11(4):184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.das Neves J, Sarmento B. Precise engineering of dapivirine-loaded nanoparticles for the development of anti-HIV vaginal microbicides. Acta Biomaterialia. 2015;18:77–87. [DOI] [PubMed] [Google Scholar]
- 99.Gebara C, Chaves KS, Ribeiro MCE, Souza FN, Grosso CR, Gigante ML. Viability of Lactobacillus acidophilus La5 in pectin–whey protein microparticles during exposure to simulated gastrointestinal conditions. Food research international. 2013;51(2):872–8. [Google Scholar]
- 100.Ivanovska TP, Petruševska-Tozi L, Kostoska MD, Geškovski N, Grozdanov A, Stain C, et al. Microencapsulation of Lactobacillus casei in chitosan-Ca-alginate microparticles using spray-drying method. Macedonian Journal of Chemistry and Chemical Engineering. 2012;31(1):115–23. [Google Scholar]
- 101.Gu J, Yang S, Ho EA. Biodegradable film for the targeted delivery of siRNA-loaded nanoparticles to vaginal immune cells. Molecular Pharmaceutics. 2015;12(8):2889–903. [DOI] [PubMed] [Google Scholar]
- 102.Wang S, Zhao Y, Shen M, Shi X. Electrospun hybrid nanofibers doped with nanoparticles or nanotubes for biomedical applications. Therapeutic delivery. 2012;3(10):1155–69. [DOI] [PubMed] [Google Scholar]
- 103.El-Hammadi MM, Arias JL. Nanotechnology for Vaginal Drug Delivery and Targeting. Nanotechnology and Drug Delivery, Volume Two: Nano-Engineering Strategies and Nanomedicines against Severe Diseases. 2016:191. [Google Scholar]
- 104.Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomaterials research. 2018;22(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Munaz A, Vadivelu RK, John JS, Barton M, Kamble H, Nguyen N-T. Three-dimensional printing of biological matters. Journal of Science: Advanced Materials and Devices. 2016;1(1):1–17. [Google Scholar]
- 106.Lehner BA, Schmieden DT, Meyer AS. A straightforward approach for 3D bacterial printing. ACS synthetic biology. 2017;6(7):1124–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Prendergast ME, Solorzano RD, Cabrera D. Bioinks for biofabrication: current state and future perspectives. Journal of 3D printing in medicine. 2017;1(1):49–62. [Google Scholar]
- 108.Costa PF. Bone tissue engineering drug delivery. Current Molecular Biology Reports. 2015;1(2):87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hong N, Yang GH, Lee J, Kim G. 3D bioprinting and its in vivo applications. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2018;106(1):444–59. [DOI] [PubMed] [Google Scholar]
- 110.Bedell ML, Navara AM, Du Y, Zhang S, Mikos AG. Polymeric Systems for Bioprinting. Chemical Reviews. 2020. [DOI] [PubMed] [Google Scholar]
- 111.Kačarević ŢP, Rider PM, Alkildani S, Retnasingh S, Smeets R, Jung O, et al. An Introduction to 3D Bioprinting: Possibilities, Challenges and Future Aspects. Materials (Basel). 2018;11(11):2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li J, Chen M, Fan X, Zhou H. Recent advances in bioprinting techniques: approaches, applications and future prospects. Journal of translational medicine. 2016;14(1):271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schaffner M, Rühs PA, Coulter F, Kilcher S, Studart AR. 3D printing of bacteria into functional complex materials. Science advances. 2017;3(12):eaao6804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ning E, Turnbull G, Clarke J, Picard F, Riches P, Vendrell M, et al. 3D bioprinting of mature bacterial biofilms for antimicrobial resistance drug testing. Biofabrication. 2019;11(4):045018. [DOI] [PubMed] [Google Scholar]
- 115.Schmieden DT, Basalo V zquez SJ, Sang esa Hc, Van Der Does M, Idema T, Meyer AS. Printing of patterned, engineered E. coli biofilms with a low-cost 3D printer. ACS synthetic biology. 2018;7(5):1328–37. [DOI] [PubMed] [Google Scholar]
- 116.Balasubramanian S, Aubin-Tam M-E, Meyer AS. 3D printing for the fabrication of biofilm-based functional living materials. ACS Publications; 2019. [DOI] [PubMed] [Google Scholar]
- 117.Zhang L, Lou Y, Schutyser MA. 3D printing of cereal-based food structures containing probiotics. Food structure. 2018;18:14–22. [Google Scholar]
- 118.Di Marzio N, Eglin D, Serra T, Moroni L. Bio-Fabrication: Convergence of 3D Bioprinting and Nano-Biomaterials in Tissue Engineering and Regenerative Medicine. Frontiers in Bioengineering and Biotechnology. 2020;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tytgat L, Dobos A, Markovic M, Van Damme L, Van Hoorick J, Bray F, et al. High-Resolution 3D Bioprinting of Photo-Cross-linkable Recombinant Collagen to Serve Tissue Engineering Applications. Biomacromolecules. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shavandi A, Jalalvandi E. Biofabrication of Bacterial Constructs: New Three-Dimensional Biomaterials. Bioengineering. 2019;6(2):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Connell JL, Ritschdorff ET, Whiteley M, Shear JB. 3D printing of microscopic bacterial communities. Proceedings of the National Academy of Sciences. 2013;110(46):18380–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Murphy SV, Atala A. 3D bioprinting of tissues and organs. Nature biotechnology. 2014;32(8):773–85. [DOI] [PubMed] [Google Scholar]
- 123.Lim SH, Kathuria H, Tan JJY, Kang L. 3D printed drug delivery and testing systems—a passing fad or the future? Advanced drug delivery reviews. 2018;132:139–68. [DOI] [PubMed] [Google Scholar]
- 124.Sun Y, Ruan X, Li H, Kathuria H, Du G, Kang L. Fabrication of non-dissolving analgesic suppositories using 3D printed moulds. International journal of pharmaceutics. 2016;513(1–2):717–24. [DOI] [PubMed] [Google Scholar]
- 125.Tagami T, Hayashi N, Sakai N, Ozeki T. 3D printing of unique water-soluble polymer-based suppository shell for controlled drug release. International journal of pharmaceutics. 2019;568:118494. [DOI] [PubMed] [Google Scholar]
- 126.Sun Y, Soh S. Printing tablets with fully customizable release profiles for personalized medicine. Advanced materials. 2015;27(47):7847–53. [DOI] [PubMed] [Google Scholar]
- 127.Tappa K, Jammalamadaka U, Ballard DH, Bruno T, Israel MR, Vemula H, et al. Medication eluting devices for the field of OBGYN (MEDOBGYN): 3D printed biodegradable hormone eluting constructs, a proof of concept study. PLoS One. 2017;12(8):e0182929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Domínguez-Robles J, Mancinelli C, Mancuso E, García-Romero I, Gilmore BF, Casettari L, et al. 3D printing of drug-loaded thermoplastic polyurethane meshes: A potential material for soft tissue reinforcement in vaginal surgery. Pharmaceutics. 2020;12(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Genina N, Holländer J, Jukarainen H, Mäkilä E, Salonen J, Sandler N. Ethylene vinyl acetate (EVA) as a new drug carrier for 3D printed medical drug delivery devices. European Journal of Pharmaceutical Sciences. 2016;90:53–63. [DOI] [PubMed] [Google Scholar]
- 130.Fu J, Yu X, Jin Y. 3D printing of vaginal rings with personalized shapes for controlled release of progesterone. International journal of pharmaceutics. 2018;539(1–2):75–82. [DOI] [PubMed] [Google Scholar]
- 131.Liu Z, Bhandari B, Zhang M. Incorporation of probiotics (Bifidobacterium animalis subsp. Lactis) into 3D printed mashed potatoes: Effects of variables on the viability. Food Research International. 2020;128:108795. [DOI] [PubMed] [Google Scholar]
- 132.Zhu L-F, Chen X, Ahmad Z, Peng Y, Chang M-W. A core–shell multi-drug platform to improve gastrointestinal tract microbial health using 3D printing. Biofabrication. 2020;12(2):025026. [DOI] [PubMed] [Google Scholar]
- 133.Hinton TJ, Jallerat Q, Palchesko RN, Park JH, Grodzicki MS, Shue H-J, et al. Three-dimensional printing of complex biological structures by freeform reversible embedding of suspended hydrogels. Science advances. 2015;1(9):e1500758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen T, Bakhshi H, Liu L, Ji J, Agarwal S. Combining 3D printing with electrospinning for rapid response and enhanced designability of hydrogel actuators. Advanced Functional Materials. 2018;28(19):1800514. [Google Scholar]
- 135.Lee S-J, Zhu W, Heyburn L, Nowicki M, Harris B, Zhang LG. Development of novel 3-D printed scaffolds with core-shell nanoparticles for nerve regeneration. IEEE Transactions on Biomedical Engineering. 2016;64(2):408–18. [DOI] [PubMed] [Google Scholar]
- 136.Sun J, Zhou W, Huang D, Fuh JY, Hong GS. An overview of 3D printing technologies for food fabrication. Food and bioprocess technology. 2015;8(8):1605–15. [Google Scholar]
- 137.Wu L, Qin W, He Y, Zhu W, Ren X, York P, et al. Material distributions and functional structures in probiotic microcapsules. European Journal of Pharmaceutical Sciences. 2018;122:1–8. [DOI] [PubMed] [Google Scholar]
- 138.Yu Y-Z, Zheng L-L, Chen H-P, Chen W-H, Hu Q-X. Fabrication of hierarchical polycaprolactone/gel scaffolds via combined 3D bioprinting and electrospinning for tissue engineering. Advances in Manufacturing. 2014;2(3):231–8. [Google Scholar]
- 139.Zhu W, Ma X, Gou M, Mei D, Zhang K, Chen S. 3D printing of functional biomaterials for tissue engineering. Current opinion in biotechnology. 2016;40:103–12. [DOI] [PubMed] [Google Scholar]
- 140.Atala A, Forgacs G. Three- Dimensional Bioprinting in Regenerative Medicine: Reality, Hype, and Future. Stem cells translational medicine. 2019;8(8):744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ghidini T Regenerative medicine and 3D bioprinting for human space exploration and planet colonisation. Journal of thoracic disease. 2018;10(Suppl 20):S2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Vijayavenkataraman S, Yan W-C, Lu WF, Wang C-H, Fuh JYH. 3D bioprinting of tissues and organs for regenerative medicine. Advanced drug delivery reviews. 2018;132:296–332. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


