Abstract
Anthrax, caused by the spore-forming bacterium Bacillus anthracis, is a zoonosis affecting animals and humans globally. In the United States, anthrax outbreaks occur in wildlife and livestock, with frequent outbreaks in native and exotic wildlife species in Texas, livestock outbreaks in the Dakotas, and sporadic mixed outbreaks in Montana. Understanding where pathogen and host habitat selection overlap is essential for anthrax management. Resource selection and habitat use of ungulates may be sex-specific and lead to differential anthrax exposure risks across the landscape for males and females. We evaluated female elk (Cervus canadensis) resource selection in the same study areas as male elk in a previous anthrax risk study to identify risk of anthrax transmission to females and compare transmission risk between females and males. We developed a generalized linear mixed-effect model to estimate resource selection for female elk in southwest Montana during the June to August anthrax transmission risk period. We then predicted habitat selection of female and male elk across the study area and compared selection with the distribution of anthrax risk to identify spatial distributions of potential anthrax exposure for the male and female elk. Female and male elk selected different resources during the anthrax risk period, which resulted in different anthrax exposure areas for females and males. The sex-specific resource selection and habitat use could infer different areas of risk for anthrax transmission, which can improve anthrax and wildlife management and have important public health and economic implications.
Keywords: anthrax, Cervus canadensis, disease risk, elk, habitat use, resource selection function, sex-specific
Summary for online Table of Contents:
Female and male elk are under different resource selection pressures during the anthrax risk period, which resulted in different potential anthrax exposure areas. The areas highly selected by male and female elk overlapping with anthrax areas should be prioritized for carcass detection and anthrax surveillance efforts.
Anthrax, caused by the spore-forming bacterium Bacillus anthracis, is a globally distributed zoonotic disease, primarily affecting wildlife and livestock, and secondarily humans (Alexander et al. 2012, Carlson et al. 2019). The spores of anthrax are usually environmentally maintained and can remain viable in specific soil environments for an extended period of time ranging from days to years, possibly decades (Van Ness 1971, Hugh-Jones and Blackburn 2009). In the United States, anthrax outbreaks occur in wildlife and livestock. After an infected animal dies from anthrax, spores from the carcass contaminate the surrounding environment (Turner et al. 2014). When susceptible hosts, especially grazing herbivores, ingest the spores within the contaminated soils, grasses, rhizosphere, animal hides, and bones, anthrax transmission could occur (Blackburn et al. 2014, Turner et al. 2014). Currently, anthrax control and management in the wildlife population still focus on the surveillance and decontamination of carcasses during the anthrax risk season (Bellan et al. 2013, Morris et al. 2016a). Therefore, identifying the distribution of anthrax in the environment and where anthrax distribution overlaps with susceptible wildlife populations is important to estimate the spatial distribution of anthrax transmission risk (Blackburn et al. 2015, Morris et al. 2016a). Delineating the potential places where the transmissions occur and quantifying the anthrax exposure risk across the landscape can identify the priority areas for effective disease surveillance and management (Blackburn et al. 2015).
A significant, multi-species outbreak of anthrax occurred in southwestern Montana, USA, in 2008. Because no cases have been reported in southwestern Montana since the 1980s, the outbreak was considered a re-emergence of the disease from contaminated soils that infected domestic plains bison (Bison bison bison) and free-ranging elk (Cervus canadensis; Blackburn et al. 2014a, Morris et al. 2016a, Nekorchuk et al. 2018). The reported anthrax cases in the bison and elk populations suggested significant male-biased prevalence rates of anthrax exposure in both species, with no female elk anthrax cases being reported and a skew towards the male population for anthrax mortalities in bison (i.e., 28% of male bison died from anthrax vs. 8% of females; Bagamian et al. 2013, Nekorchuk et al. 2018). The differences in the disease exposure between sexes may arise because of the differences in movement behaviors, resource selection, and activity space between male and female animals during the anthrax risk season.
Habitat preferences of ungulates are sex-specific (Geist 1971, Varland et al. 1978, Franklin and Leib 1979). Animals usually respond to the changes in the environmental space through decisions in movements to meet demands for refuge and resources (Nathan et al. 2008, Van Moorter et al. 2016). Female ungulates select habitat that enhances offspring survival to maximize their fitness and males compromise security to increase nutrient intake and breeding success (Geist 1982, McCorquodale 2003). The morphological and behavioral differences between sexes also allow the individuals in some ungulate species to occupy different niches (Bowyer 1981). For example, it is typical that female elk and calves live together in groups in summer following calving and during the lactation period, whereas male elk live away from the females in solitary or small groups (Martinka 1969). Different preferences in elevation, density of canopy stands, slope, aspect, and responses to roads have been identified and documented between male and female elk (Marcum 1975, Leptich and Zager 1994, Smith and Anderson 2001, McCorquodale 2003, Montgomery et al. 2013). In addition, elk resource selection varies by location (i.e., herds), given the different spatial heterogeneity in environments and resources (McCorquodale 2003, Proffitt et al. 2011, Van Moorter et al. 2016). Morris et al. (2016) previously studied male elk resource selection during the anthrax risk period (i.e., Jun–Aug) in southwestern Montana and observed significant spatial overlap between preferred elk use areas and predicted anthrax risk areas. Because Morris et al. (2016) were limited to male-only data and given the known sexual segregation in ungulate behaviors, their findings prioritized anthrax management areas for male elk and may not directly apply to female elk. In the United States, most wildlife are managed by the state government, but with limited state manager access to wildlife on private lands controlled by landowners (Watson 2012). Elk usually have a relatively large seasonal home range in summer and can move freely across public and private land to commingle with livestock, which can results in disease spillover to livestock (Blackburn et al. 2014a, Schmitt et al. 2002, Proffitt et al. 2011).
We were interested in resource selection differences between male and female elk during the anthrax risk period in southwestern Montana. We hypothesized that female and male elk have different resource selection patterns, which could lead to different geographical extents, likelihoods of anthrax exposure, and disease management strategies. We were also interested in the distribution of different land ownership across the anthrax exposure areas for wildlife to inform disease management.
STUDY AREA
This study focused on the 1,652-km2 Northern Madison Study Area (NMSA) in southwestern Montana, following Morris et al. (2016) for comparability (Fig. 1). Atwood et al. (2007) provide additional details on the ecology of the region. Briefly, the NMSA is located ~50 km northwest of Yellowstone National park, bordered by the Gallatin River to the west and the Madison River to the east. Habitat varies by elevation from dry savannah (juniper, Juniperus scopulorum) at lower elevation (1300 m) to closed canopy Douglas-fir (Psueotsuga menziesii)- lodgepole pine (Pinus contorta) forests at higher elevation moist areas and dry sage (Artemesia tridentata tridentata)-grassland mosaic in higher dry areas (south facing slopes). Temperatures range from winter lows of ‒34° C to 21–32° C in summer. Land ownership in the study area consists of 57% private lands, 39% United States Forest Service (USFS) lands, and 4% Montana state lands (Morris et al. 2016a). This area includes a 380-km2 privately owned ranch managed for grazing domestic bison and wildlife conservation. The private ranch was the center of the study area because the elk population in the NMSA usually aggregate on the ranch. In summer, elk tend to move within a larger geographical area around the ranch. The potential commingling with bison and other wildlife inside the ranch increases the likelihood of wildlife disease burdens (Proffitt et al. 2011, Morris et al. 2016a, Nekorchuk et al. 2018). Land cover in the study area includes 36% coniferous forests, 31% grassland, 27% shrubland, and 5% deciduous forests (Morris et al. 2016a). Hunting opportunities are available on some part of both public and private lands in the study region. Wildlife populations include grizzly bears (Ursus arctos horribilis), coyotes (Canis latrans), mountain lions (Puma concolor), black bears (Ursus americanus), wolves (Canis lupus), elk, mule deer (Odocoileus hemionus), white-tailed deer (O. virginianus), moose (Alces alces), and scavenging birds (Atwood 2006, Morris et al. 2016a).
Figure 1.
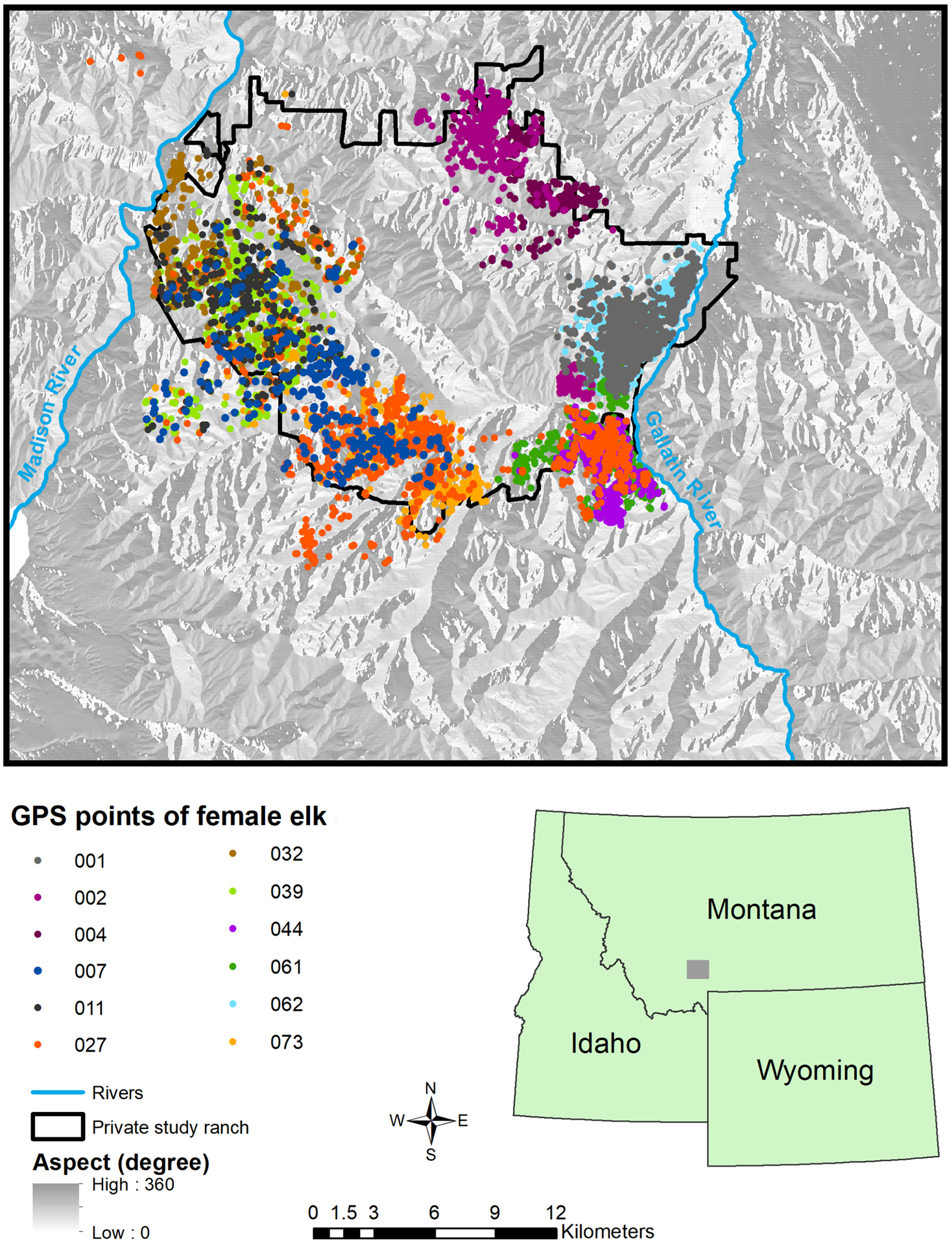
The northern Madison study area, Montana, USA, and global positioning system (GPS) fixes of female elk recorded during anthrax risk season, 2014–2017. Each dot color represents a separate animal.
METHODS
Elk Locations and Environment Data
We captured adult female elk via helicopter net-gunning (Jacques et al. 2009) around the private ranch in the study area, and fitted them with global positioning system (GPS)-collars following Montana Fish Wildlife and Parks protocols (Montana Fish Wildlife & Parks 2015, 2016) and with approval from the University of Florida Institutional Animal Care and Use Committee (201308206). Yang et al. (2019) provide details of animal capture. We programmed collars to record GPS fixes for 1–3 years from 2014 to 2016 with 30-minute intervals. There was 1 collar programmed to record locations with 2-hour intervals. The collars were programmed to record GPS fixes with 4-hour intervals in 2017. Morris et al. (2016a) captured male elk on the ranch and fitted them with GPS-collars in April 2010 and January 2012. To analyze female elk resource selection during the anthrax risk period, we trimmed the GPS fixes to focus on the anthrax period (i.e., 1 Jun–31 Aug) for all female elk with approximately 25-m accuracy.
Studies on female elk resource selection have previously included measures of wolf density or predation, elevation, slope, habitat (land cover type), distance to roads, and distance to water. Covariates used in this study reflect those variables for our study area (Table 1). We measured the environmental covariates slope, aspect (i.e., southerly = 134–224°; not southerly = 0–135°, 225°–360°), elevation, distance to primary roads (National Highway System [NHS] interstate, NHS non‐interstate, and state roads), distance to secondary roads (main ranch roads and county roads), distance to tertiary roads (2-track and logging roads), and density of wolf kills, which have also been measured for male resource selection (Morris et al. 2016a). For the land cover data, we used the 2011 Montana Spatial Data Infrastructure (MSDI) land cover data (http://geoinfo.msl.mt.gov/Home/msdi/land_use_land_cover, accessed 21 Sep 2018). We considered 2 land cover types, forested versus non-forested (i.e., shrublands and grasslands) to be included in model development. We also included distance to streams, as another potential predictor. We downloaded MSDI stream data (https://mslservices.mt.gov/Geographic_Information/Data/DataList/datalist_Details.aspx?did={5e706ec0-aa27-11e3-a5e2-0800200c9a66}, accessed 21 Sep 2018) and calculated the Euclidean distance to streams via ArcMap version 10.3 (ESRI, Redland, CA, USA). Consequently, we considered 9 possible environmental variables and all covariates were generated or resampled to a spatial resolution of 30 m.
Table 1.
Environmental covariates used in the resource selection function models to predict female elk habitat use, Montana, USA, 2014–2017.
| Variable | Description | Sources |
|---|---|---|
| Wolf | Density of wolf kills (per 30m pixel) | Laundré et al. (2001), Hebblewhite et al. (2005), Atwood (2006), Creel et al. (2009) |
| Elevation | Elevation (m) | Altmann (1951), Leptich and Zager (1994), McCorquodale (2003) |
| Forest | Forests (compared to grasslands and shrublands) | McCorquodale (1993), Leptich and Zager (1994), Unsworth et al. (1998), Proffitt et al. (2011) |
| Aspect | Southerly (134–224°), non-southerly (0–135° or 225°–360°) | Marcum (1975), Leptich and Zager (1994), Proffitt et al. (2011) |
| Slope | Slope | Marcum (1975), Leptich and Zager (1994), Proffitt et al. (2011) |
| Stream | Distance to stream (km) | McCorquodale (2003) |
| Primary road | Distance to primary road (km) | Irwin and Peek (1983), Edge et al. (1988), McCorquodale (2003), Montgomery et al. (2013) |
| Secondary road | Distance to secondary road (km) | |
| Tertiary road | Distance to tertiary road (km) |
Female Resource Selection and Anthrax Exposure
We employed a similar use-available modeling framework as in Morris et al. (2016) to estimate selection for resources within the population-level seasonal home range during the anthrax risk season (Johnson 1980, Blackburn et al. 2014a). We defined the available area as the population-level seasonal home range during the risk season. To be consistent with Morris et al. (2016), we used a 100%, herd-level, minimum convex polygon around all GPS fixes to estimate available areas. We randomly selected 4 GPS fixes/elk/day from 1 June to 31 August in 2014–2017 to represent the used points and then generated 5 random points/each used point within the available areas to represent the available points. We screened variables for multicollinearity (Pearson’s correlation coefficient |r| ≥ 0.7) and significance in univariate analyses (P < 0.1; Morris et al. 2016a). We standardized continuous variables (elevation, slope, distances to roads and streams) using the scale function in R (Morris et al. 2016a).
For resource selection function (RSF) model development, we used the generalized linear mixed-effect model (GLMM) with individual elk and year as random effects (Manly et al. 2002). The RSF model compared environmental conditions at the locations selected by the female elk with those at a set of randomly selected points within available areas (Manly et al. 2002). The RSF is described in a logistic regression model framework:
where w(x) is the relative probability of a location being selected by female elk, β0 is the intercept, and βi is the estimated coefficient for the potential environmental covariates that could influence elk resource selection (Manly et al. 2002). We extracted the values of covariates (Xi) to the pixel centroid. We built the RSF models using the glmer function from the lme4 R package, optimized using the lnbobyqa algorithm from the nloptr R package, under the assumption that the response is binomially distributed (Bates et al. 2007, Powell 2009, Johnson 2014, R Core Team 2017). R code for the analyses are available online in Supporting Information.
To evaluate whether male and female elk used the landscape in a similar fashion, we first fit the best model for resource selection of male elk as identified by Morris et al. (2016) to female elk data. Our model parameter estimates from that model for female elk, however, had poor model performance and predictive accuracy based on cross validation (Table 2; Table S1, available online in Supporting Information), which indicated different resource use or different responses to the environment between male and female elk. We then modified the RSF models by adding an additional predictor (distance to stream) reflecting the selection of riparian areas in summer as found in previous work on female elk biology (McCorquodale 1993). We generated all additive combinations of covariates and selected the top model based on the lowest Akaike’s Information Criterion (ΔAIC) and the highest Akaike weight (wi; Anderson et al. 2000, Burnham and Anderson 2003).
Table 2.
Competing models predicting female elk resource selection (RSF) during anthrax risk season in southwestern Montana, USA, 2014–2017. We report the model performance based on the difference of Akaike’s Information Criterion (ΔAIC) and Akaike weights (wi).
| Numbera | Model terms | Variable numbers | ΔAIC | w i | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female elk RSF model with new combinations | ||||||||||||
| 1 | Wolf | Elevation | Forest | Aspect | Stream | Slope | Primary road | Secondary road | Tertiary road | 9 | 0 | 0.99 |
| 2 | Wolf | Elevation | Aspect | Stream | Slope | Primary road | Secondary road | Tertiary road | 8 | 44 | <0.001 | |
| 3 | Elevation | Forest | Aspect | Stream | Slope | Primary road | Secondary road | Tertiary road | 8 | 48 | <0.001 | |
| 4 | Wolf | Elevation | Forest | Aspect | Stream | Primary road | Secondary road | Tertiary road | 8 | 77 | <0.001 | |
| 5 | Wolf | Elevation | Forest | Aspect | Stream | Primary road | Secondary road | Tertiary road | 7 | 88 | <0.001 | |
| 6 | Elevation | Aspect | Stream | Slope | Primary road | Secondary road | Tertiary road | 7 | 94 | <0.001 | ||
| 7 | Wolf | Elevation | Forest | Aspect | Slope | Primary road | Secondary road | Tertiary road | 7 | 156 | <0.001 | |
| 8 | Elevation | Forest | Aspect | Slope | Primary road | Secondary road | Tertiary road | 7 | 207 | <0.001 | ||
| 9 | Wolf | Elevation | Forest | Stream | Slope | Primary road | Secondary road | Tertiary road | 8 | 294 | <0.001 | |
| 10 | Wolf | Elevation | Forest | Aspect | Stream | Slope | Primary road | Secondary road | 8 | 361 | <0.001 | |
| Female elk RSF model adopted from the best male elk RSF model | ||||||||||||
| 11 | Elevation | Forest | Slope | Secondary road | Tertiary road | 5 | 1,988 | <0.001 | ||||
Models 1–10 are the top 10 competing RSF models for female elk with new variable combinations that have relatively lower ΔAIC among all possible models. Model 11 is the best RSF for male elk reported in Morris et al. (2016a) and adapted to females.
We assessed the predictive performance of the top selected model by k-fold cross validation (Boyce et al. 2002). We used 5-fold cross-validation and withheld 20% of the data each of 5 times. We refitted the model each time with the unstandardized variables using the fixed effect from the GLMMs to predict and map resource selection on the landscape. We scaled the values from the RSF output based on Boyce et al. (2002) via 10 equal-area bins, with 1 as the lowest probability of selection and 10 as the highest. The equal-area classification partitions the values into groups that cover the same amount of the area on the landscape (Morris et al. 2016b). The median value of the RSF was 5; therefore, all pixels >5 represented the 50% of the landscape with the highest predicted values of suitability (or areas selected by) female elk. We calculated Spearman rank correlation coefficients (rs) based on the number of points per bin and the bin rank (Boyce et al. 2002).
To identify the geographical extent of potential anthrax transmission for female elk, we adopted the definitions of potential risk zones for B. anthracis from a previously published ecological niche model (Genetic Algorithm for Ruleset Prediction [GARP]; Morris et al. 2016a). Most ecological niche models predict distributions of species based on non-random relationships between species occurrence and environmental variables; that is, each pixel on the landscape represents the likelihood of species presence. The GARP method employs a random walk approach based on rules of genetics to influence the rule selection process (Stockwell and Peters 1999). These rules are Boolean strings in the form of if-then logic statements applying 1 of 4 rule types (range rules, negated range rules, logistic regression rules, and atomic [bioclimatic] rules) to presence or absence. These rules are then fit to the landscape to estimate presence (1) and absence (0) with binary maps. Any GARP experiment generates multiple models and binary maps and a best subset procedure is used to select and summate the top models into a final prediction (Anderson et al. 2003). We recently defined the GARP process in detail (Yang et al. 2020a). We calculated the geographic extent of the presence of B. anthracis to generate the moderate anthrax risk surface based on the commonly used threshold of 5 out of 10 best model agreements from that final subset. In other words, for any pixel predicted by ≥5 best models, we assumed the (potential) presence of the pathogen (Blackburn et al. 2007, Fitzpatrick et al. 2013, Yang et al. 2020b). We overlaid the resource selection surface (RSF values ≥ 5; ≥ fifth equal-area bin) with the moderate anthrax risk surface to identify where female elk potentially overlap with B. anthracis. In addition, we also adopted another 2 cutoffs to estimate the liberal (model agreement ≥1 of 10 best models) and conservative (model agreement ≥9 of 10 best models) anthrax risk surfaces from Morris et al. (2016) and overlapped male and female predicted high suitability with those 2 anthrax risk surfaces.
Compare Resource Selection and Anthrax Exposure between Female and Male Elk
We employed a niche overlap similarity estimate (Warren’s I metric; Warren and Seifert 2011) twice in this study to quantify the differences in resource selection and anthrax exposure areas between male and female elk. The statistic ranging from zero (no overlap) to 1 (perfect overlap) has been used to estimate spatial overlap of niche model predictions (Warren and Seifert 2011, Börger and Fryxell 2012, Broennimann et al. 2012). First, to evaluate the similarity of the space use between female and male elk during the anthrax risk period, we transformed the RSF results for male (adopted from Morris et al. [2016]) and female elk into a binary raster layer with the top 6 RSF bins (RSF ≥5) reclassified as 1 and the rest as zero (Nekorchuk 2017) and compared the overlaps of the 2 habitat selection surfaces using the Warren’s I metric calculated in the SDMTools R package (VanDerWal et al. 2014).
Second, to estimate the similarity of the anthrax exposure risk between female and male elk, we used a similar process with the Warren’s I metric to quantitatively compare the female anthrax exposure surface with male elk anthrax exposure areas (i.e., male elk habitat overlapping with moderate anthrax risk surface) adopted from Morris et al. (2016). Additionally, to evaluate how transmission risk varied across land ownerships, we overlaid the distribution of anthrax transmission risk for female elk with land ownership parcel data adopted from Morris et al. (2016) for disease management for female elk, and then compared it to results for male elk. Following Morris et al. (2016), we considered 3 types of ownership: USFS land, Montana state land, and privately owned land.
RESULTS
We captured 12 adult female elk in the study area in 2014–2017, including 3 individuals with 1-year GPS-collar data collected in 2014, 5 elk with 2-year GPS-collar data recorded in 2014–2015, 3 elk with 3-year movement data in 2014–2016, and 1 with 2014–2017 data collected. This resulted in 26 animal-years used in estimating RSF during the anthrax risk season. The 13 mature male elk data adopted from Morris et al. (2016) included 8 elk collected in 2010, 2 elk collected in 2010 and 2011, and 3 individuals recorded in 2012, resulting in 15 animal-years in that study. The GPS fix success rates of location data for female elk in each animal-year ranged from 0.86–0.99 (Table S2, available online in Supporting Information).
Female Resource Selection and Anthrax Exposure
We did not detect multicollinearity among the environmental covariates or significant correlations. We used all 9 covariates as possible predictors in the female RSF model development. We tested all additive combinations of covariates and identified the top 10 ranked models with relatively low ΔAIC (i.e., higher Akaike weights; Table 2).
We selected the model with the structure of w(x) ~ exp (wolf + elevation + forest + aspect + stream + slope + primary road + secondary road + tertiary road) as the best model to describe resource selection for female elk during anthrax season of 2014–2017 (Table 2). The mean Spearman rank correlation coefficient (rs) estimating the predictive accuracy from the 5-fold cross validations for the best model was 0.93 ± 0.03 (SE). The standardized coefficients of the best model indicated that female elk during the anthrax season selected areas away from wolf predation events with high elevation, forested land cover (compared to grasslands and shrublands), farther distances to streams, gentler slopes, non-southerly aspects, and closer distances to primary roads, secondary roads, and tertiary roads (Table 3). The conditional variances for random effects were 0.014 for individuals and 0.001 for years.
Table 3.
Estimates of coefficients of standardized variables with respective 95% confidence intervals in parenthesis for the final selected resource selection function model for female elk during anthrax risk season, southwestern Montana, USA, 2014–2017.
| Variable | Estimated coefficient |
|---|---|
| Density of wolf kills | −0.11 (−0.12, −0.09) |
| Elevation | 0.68 (0.65, 0.70) |
| Forest (compared to grassland and shrubland)a | 0.20 (0.17, 0.23) |
| Aspect | −0.62 (−0.66, −0.58) |
| Slope | −0.12 (−0.13, −0.11) |
| Distance to stream | 0.14 (0.13, 0.15) |
| Distance to primary road | −0.55 (−0.57, −0.53) |
| Distance to secondary road | −0.56 (−0.58, −0.54) |
| Distance to tertiary road | −0.26 (−0.27, −0.25) |
The coefficients for the standardized continuous variables cannot be directly compared to the coefficients for the (unstandardized) categorical variables, forest and aspect.
The odds ratio for distance to streams indicated that the probability of female elk selection increased by 15% when distance increased by 1 km. Female elk selection was expected to decrease by 42%, 43%, and 23% for every 1-km increase in distance from primary, secondary, and tertiary roads, respectively. For every 1-degree increase in slope, there was an expected 11% decline in probability of female elk selection. For every 1-km increase in elevation, the probability of female elk selection was expected to increase by 97%. The expected probability of female elk selection was 22% greater in forests than in grasslands or shrublands (Fig. S1, available online in Supporting Information). The prediction of the final selected RSF model on the landscape showed the highest probability of selection at the southeast part of the private bison ranch in the study area (Fig. 2).
Figure 2.
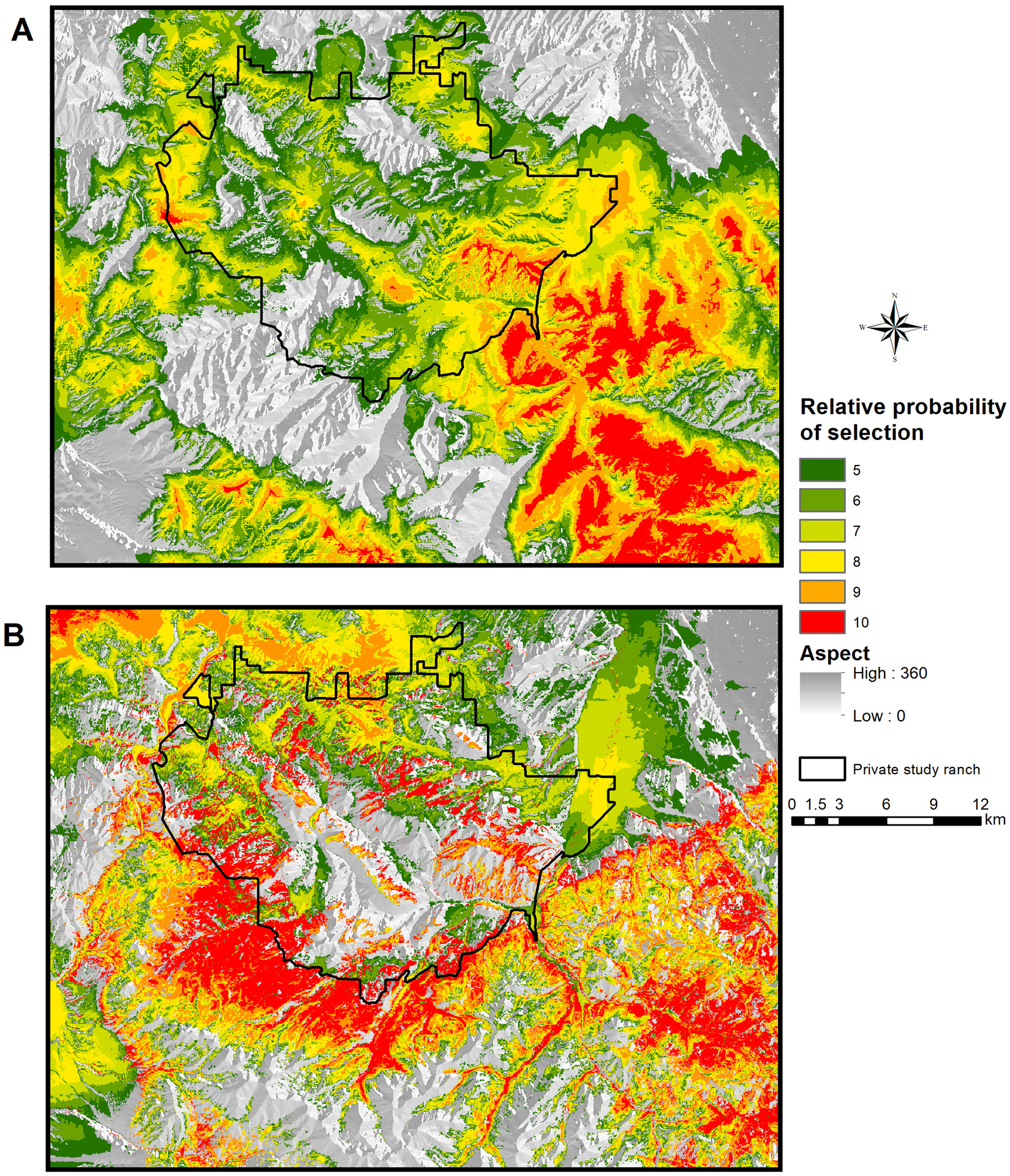
The relative probability (top 6 bins out of 10 equal-area bins) of female elk resource selection during anthrax season in the northern Madison Study area, southwestern Montana, USA, 2014–2017 based on a resource selection function (RSF).
We detected an extensive spatial overlap between female elk resource selection surface and anthrax risk surface (Fig. 3; Fig. S2, available online in Supporting Information). The areas selected by female elk (RSF value ≥5) that overlapped with the pathogen were distributed across the study area with greater overlap on the private ranch and in the east of the study area outside the ranch.
Figure 3.
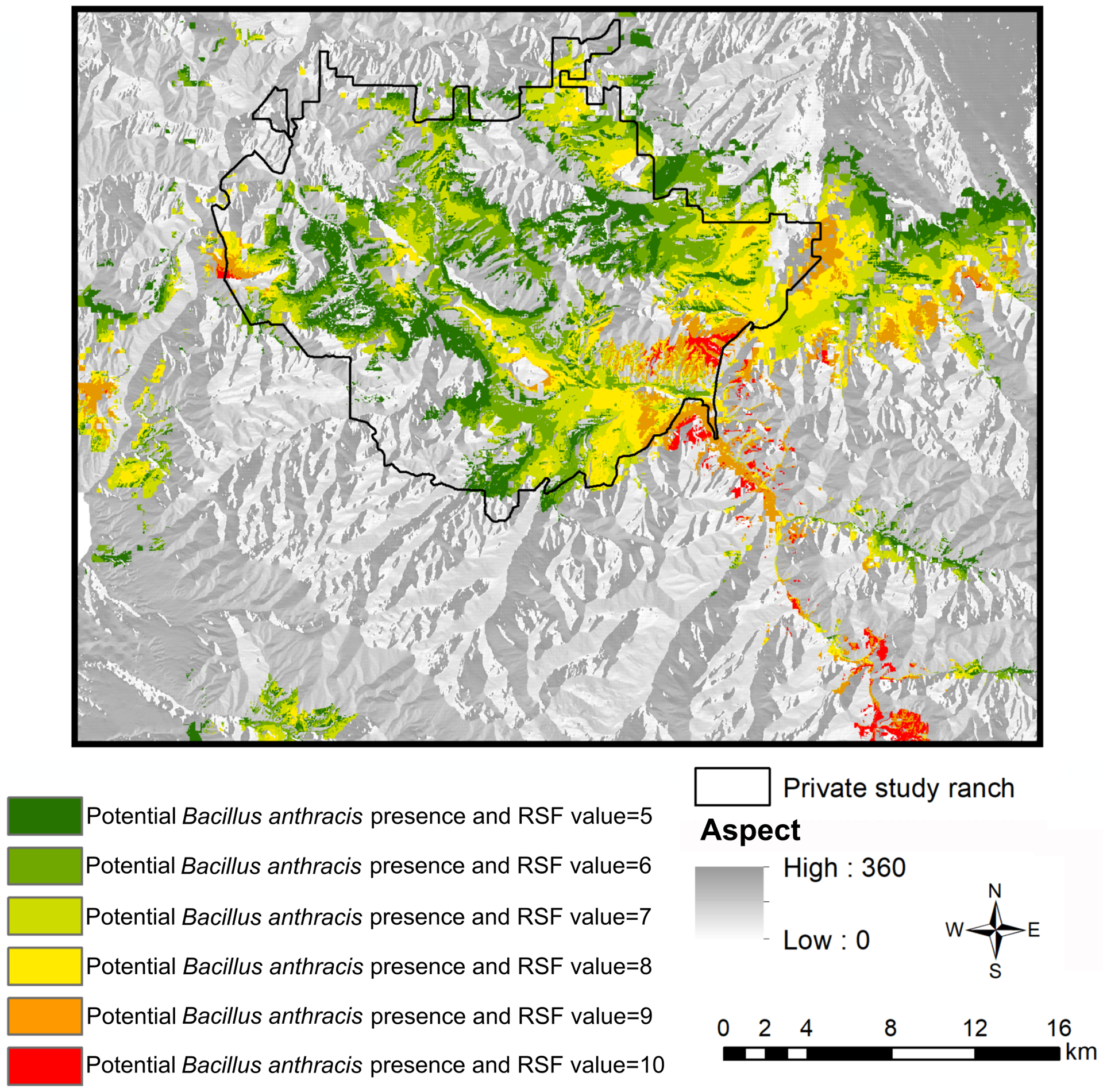
The predicted relative probability of female elk overlapping with the moderate anthrax risk surfaces during the anthrax risk period in northern Madison study areas, southwestern Montana, USA, 2014–2017. Elk probability estimated from a resource selection function (RSF) value ≥5.
Comparisons between Male and Female Elk
The best RSF model to describe female elk resource selection was different than the best model for males, suggesting different resource selection pressures on the sexes during the anthrax risk period. Male elk in the study area during the anthrax risk period selected habitat closer to tertiary roads, with gentler slopes, in forested land, at lower elevations, and farther from secondary roads (Morris et al. 2016a). Female elk selected areas farther from wolf predation areas, closer to all types of roads, with gentler slope, at higher elevation, farther from streams, on hillsides with non-southerly aspect, and in forested lands (Table 3).
The high use areas (RSF values ≥ 5) for female elk covered 1,026 km2 of 1,652 km2 across the study area, and the high use areas (RSF values ≥ 5) for male elk was reported to cover 1,056 km2 in the previous study (Morris et al. 2016; Fig. 2). The spatial differences of the high use areas between male and female elk had a Warren’s I metric of 0.632, which suggested that high use habitat of male and female (RSF values ≥ 5) was different during the anthrax risk period with 63.2% overlap.
The moderate anthrax exposure areas (i.e., overlap of high use areas and anthrax risk surface from the ecological niche model) for female elk covered 332 km2, whereas the anthrax exposure areas for male elk covered 285 km2 (Morris et al. 2016a; Fig. 3). The Warren’s I metric to quantify the spatial differences between male and female elk anthrax exposure areas was 0.659, which indicated a 65.9% overlap between the 2 surfaces. We also found similar patterns with more anthrax exposure areas for female elk than for male elk under the liberal and conservative cutoffs of the anthrax risk surfaces (Table S3 and Fig. S2, available online in Supporting Information).
Female elk primarily selected for private lands and USFS lands (Table 4). Because B. anthracis was predicted across the study areas on USFS lands, state lands, and private lands but with high proportions on the private lands (Blackburn et al. 2014, Morris et al. 2016), most overlap between female elk preferred habitat and anthrax risk were identified on private lands, followed by the USFS lands and state lands (Fig. 4, Table 4). This pattern was similar patterns to the relationship of male elk selection and anthrax risk areas and land ownership (Morris et al. 2016a). Similarly, anthrax exposure areas for female and male elk were largest on private land compared to other land ownership categories under the liberal and conservative cutoffs of the anthrax risk surfaces (Table S4 and Fig. S3, available online in Supporting Information).
Table 4.
Summary of area (km2) classified as high risk for male and female elk anthrax exposure to the moderate anthrax risk surfaces in 3 land ownership classifications during the anthrax season in southwestern Montana, USA, 2014–2017. Area of land used by elk determined from resource selection function models (RSFs). Anthrax exposure areas determined from overlap with RSF values ≥ 5.
| Land ownership | RSF surface (RSF values ≥ 5) | Anthrax exposure areas | |||
|---|---|---|---|---|---|
| Female | Malea | Female | Male | ||
| U.S. Forest Service | 444.9 | 579.0 | 32.1 | 32.2 | |
| Private land | 559.1 | 37.7 | 292.4 | 253.3 | |
| Montana state land | 21.4 | 439.6 | 7.5 | 9.1 | |
The results for male elk are adopted from Morris et al. (2016a).
Figure 4.
The predicted areas of high risk for male and female elk exposure based on moderate anthrax risk surfaces and resource selection function (RSF) values ≥5 in 3 land ownership classifications during the anthrax season, in northern Madison study areas, southwestern Montana, USA, 2014–2017.
DISCUSSION
Our findings indicated that male and female elk selected resources differently and had different movement behaviors during summer anthrax season. There were extensive areas selected by female elk overlapping with anthrax risk areas, which were more widespread and distributed differently compared to the spatial patterns of male elk anthrax exposure. These areas where female elk potentially interact with the pathogen were located across public and private lands.
Our results found similar responses of male and female elk to land cover and slope. They both selected for forested land cover and gentler slope. Similar findings have also been suggested in previous work (McCorquodale 1993, 2003; Montgomery et al. 2013). In summer, male and female elk have a preference for mature semi-closed or closed forests and high elevation, which could potentially allow them to avoid the summer heat (McCorquodale 1993, 2003; Montgomery et al. 2013). McCorquodale (2003) also categorized the slope into 7 classes (6 classes of 10% slope categories and a seventh class of >59% slope) and reported males preferred moderate slope (20–39%) and females preferred gentle to moderate slope (0–39%) in summer.
Male and female elk responded to some resources differently. Although wolf predation risk and aspect were not the primary factors that influenced male elk summer distributions, those 2 factors significantly affected the summer resource selection of female elk in the study areas. Female elk avoided risky areas and selected areas with the non-southerly aspect. The avoidance of wolves has been reported in some other elk resource selection studies (Hebblewhite et al. 2005, Atwood 2006, White et al. 2010). Also, the selection of forested non-southerly aspects for females over southerly aspect supported the preference of forested land cover, and similar findings of forested northerly and easterly aspects have been suggested in McCorquodale (2003). Aspect was not a significant predictor in male elk resource selection. Although McCorquodale (2003) reported the preference of riparian areas for female elk at low elevation in late summer, we found that female elk selected habitat farther from streams at high elevations in the summer in the NMSA. Additionally, differences in the capture areas may also result in the differences in resource selections between female and male elk. Not all female elk were captured on the private ranch, whereas the males were, although females used the ranch extensively in summer. The differences in model selection methods from this study and Morris et al. (2016) may also explain the differences in results. Despite differences in the variables sets and best models for each sex, we identified differences in resource selection for female and male elk on the same landscape during the anthrax risk period.
Elk selection of habitat near roads may be related to the sex-age class and highly dependent on the density, types, and traffic volume of the roads, and may vary in different places and years because of different levels of human disturbance (McCorquodale 2003, Montgomery et al. 2013). Morris et al. (2016) did not detect any evidence of an effect of primary road; however, male elk selected habitat farther from secondary roads and closer to tertiary roads. Male elk may also move significantly farther from open road systems than females (Marcum 1975). In our study, female elk selected habitat closer to all 3 road types than expected from random selection. Other studies reported significant avoidance for both male and female elk to primary and secondary roads but a higher road density in female summer home ranges (McCorquodale 2003, Montgomery et al. 2013). Montgomery et al. (2013) also reported female elk increased their space use closer to tertiary roads (Montgomery et al. 2013). Additionally, multiple researchers have suggested that the resource selection for elk populations varies across different landscapes and years because of spatial heterogeneity and inter-annual changes in environmental suitability (McCorquodale 2003, Proffitt et al. 2011, Van Moorter et al. 2016). Therefore, the consideration of traffic volumes in different years may help to better understand elk response to human disturbances. Overall, the areas selected by female elk overlapped more with the potential anthrax risk areas, compared to the male elk; however, the prevalence rates and reported anthrax cases in the NMSA suggested a skew towards male elk in the study areas, which indicated male elk may be more susceptible to anthrax than the females (Blackburn et al. 2014, Morris et al. 2016). The possible reason for the inconsistency in the geographical extents of anthrax exposures and prevalence rates between male and female elk might be explained by their potential contacts with carcasses of previous anthrax deaths. Turner et al. (2014) reported that animal carcasses could alter the surrounding environment by increasing the forage quality and attracting ungulates to parasite aggregations. The hosts therefore experience trade-offs between exposure to anthrax and nutritional intake (Turner et al. 2014). A recent motion-sensitive camera study in the same study site has observed a higher frequency of interactions between male elk and carcass sites and behaviors of chewing bones of the dead animals than females, which indicated more potential contacts occurred between male elk and carcasses than females (Walker 2019). Additionally, by comparing higher values of habitat suitability predicted from the RSF models with the anthrax risk surface, there were more places highly selected by male elk (RSF values ≥ 9) located in the anthrax risk areas than female elk (Fig. 3). Although resource selection models estimated the spatial patterns of habitat use, the actual behaviors of the animals within those preferred areas, especially foraging behavior related to the anthrax transmission, cannot be inferred. Female elk may select areas within the anthrax risk zone more often, but forage less within these areas, or interact with carcasses less. A finer scale study to explore foraging behavior is needed to better understand if males and female forage differently, such as males grazing at carcasses more, in ways that may increase male exposure. Therefore, we focus our comparisons and discussion only on the different spatial patterns and geographical extents of anthrax exposure between male and female elk.
Anthrax can result in severe economic losses for livestock and wildlife. Wildlife outbreaks could affect unvaccinated livestock and other susceptible wild animals when they share the grazing areas that can support the survival of B. anthracis (Blackburn et al. 2014). Our results suggested male and female elk overlap with the pathogen differently because they select resources differently during the anthrax risk period. The differences in the potential anthrax exposure areas between sexes for the elk population indicates that anthrax surveillance for males and females should target different areas defined by our maps in this study. Carcass surveillance for anthrax control and management for this elk population should prioritize the areas where both male and female elk overlap with anthrax risk (Fig. 4). Given the significant overlap of the private ranch, anthrax risk surface, and elk habitat use (male and female), anthrax risk in the study area can be a multi-species concern. Increased monitoring for susceptible wildlife and livestock, besides elk, is therefore necessary. Once disease occurrence is confirmed, it is important to treat and decontaminate the carcass and the surrounding areas promptly (Hugh-Jones and De Vos 2002). Sometimes the exclusion (e.g., fencing) from the known risk zones are also used for protecting the susceptible hosts from the risk areas (Nekorchuk 2017).
The calls for collaboration between public and private organizations for the control and management of wildlife diseases (e.g., anthrax and brucellosis) have been suggested by multiple researchers (Proffitt et al. 2011, Morris et al. 2016a, Nekorchuk et al. 2018, Yang et al. 2019). In this study, we reiterate the need for such collaboration and consideration of all stakeholders for the implementation of effective anthrax surveillance and management for wildlife and livestock in the NMSA (Morris et al. 2016a). Areas of elk and anthrax risk overlap for all elk were distributed across privately owned lands, state lands, and USFS lands, but were primarily located on private lands (Fig. 4; Table 4). The free-ranging infected elk could still facilitate spillover events and spread the disease to other places on private and public lands based on their movements. Additionally, because some parts of the private and public lands in the NMSA allow hunting during the archery season, the potential areas where anthrax outbreaks occur in late summer predicted by our results may also have some important implications for public health concerns for humans (Morris et al. 2016a).
Supplementary Material
MANAGEMENT IMPLICATIONS.
Our study of sex-specific elk resource selection and the overlap of elk habitat and anthrax risk delineated the potential spatial extent of anthrax transmission risk to male and female elk. The areas highly selected by male and female elk that overlapped with the distribution of B. anthracis should be prioritized for carcass detection and anthrax surveillance efforts. Our predictions of potential anthrax exposure for the elk population can be used to define targeted areas for wildlife disease management in the study area. Anthrax exposure areas included lands under different types of ownership, meaning multi-stakeholder cooperation is essential to anthrax control and surveillance. We strongly encourage collaborative anthrax surveillance and disease management among federal, state, and private stakeholders in the study area, in particular private and public outreach. This outreach should include communication between wildlife managers (public agencies and private land owners managing for wildlife), publicly available information on the clinical signs of anthrax in live and dead animals, and communication between livestock owners in the risk area on the need for vaccination and surveillance.
ACKNOWLEDGMENTS
We are grateful for the assistance of Montana Fish, Wildlife & Parks (MFWP) for data collection and data sharing. Partial funding for this study was provided by the National Institutes of Health Grant 1R01GM117617-01 to JKB and SJR. Funding for data collection was supplied by United States Department of Agriculture-Animal and Plant Health Inspection Service through an agreement with Montana Department of Livestock and MFWP.
Contributor Information
ANNI YANG, Spatial Epidemiology and Ecology Research Laboratory, Department of Geography, Emerging Pathogens Institute, University of Florida, Gainesville, FL 32611, USA.
KELLY M. PROFFITT, Montana Fish, Wildlife, and Parks, Bozeman, MT 59718, USA
VALPA ASHER, Turner Enterprises, 1123 Research Drive, Bozeman, MT 59718, USA.
SADIE J. RYAN, Quantitative Disease Ecology and Conservation Laboratory, Department of Geography, Emerging Pathogens Institute, University of Florida, Gainesville, FL 32611, USA
JASON K. BLACKBURN, Spatial Epidemiology and Ecology Research Laboratory, Department of Geography, Emerging Pathogens Institute, University of Florida, Gainesville, FL 32611, USA
LITERATURE CITED
- Alexander KA, Lewis BL, Marathe M, Eubank S, and Blackburn JK. 2012. Modeling of wildlife-associated zoonoses: applications and caveats. Vector-Borne and Zoonotic Diseases 12:1005–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmann M 1951. Social behavior of elk, Cervus canadensis nelsoni, in the Jackson Hole area of Wyoming. Behaviour 4:116–142. [Google Scholar]
- Anderson DR, Burnham KP, and Thompson WL. 2000. Null hypothesis testing: problems, prevalence, and an alternative. Journal of Wildlife Management 912–923. [Google Scholar]
- Anderson RP, Lew D, and Peterson AT. 2003. Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecological Modelling 162:211–232. [Google Scholar]
- Atwood TC, Gese EM, and Kunkel KE. 2007. Comparative patterns of predation by cougars and recolonizing wolves in Montana’s Madison Range. Journal of Wildlife Management 71(4):1098–1106. [Google Scholar]
- Bagamian KH, Alexander KA, Hadfield TL, and Blackburn JK. 2013. Ante-and postmortem diagnostic techniques for anthrax: rethinking pathogen exposure and the geographic extent of the disease in wildlife. Journal of Wildlife Diseases 49:786–801. [DOI] [PubMed] [Google Scholar]
- Bates D, Sarkar D, Bates MD, and Matrix L. 2007. The lme4 package. <http://r-forge.r-project.org/projects/lme4/>. Accessed21 Sep 2017.
- Bellan SE, Gimenez O, Choquet R, and Getz WM. 2013. A hierarchical distance sampling approach to estimating mortality rates from opportunistic carcass surveillance data. Methods in Ecology and Evolution 4:361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn JK, Asher V, Stokke S, Hunter DL, and Alexander KA. 2014a. Dances with anthrax: wolves (Canis lupus) kill anthrax bacteremic plains bison (Bison bison bison) in southwestern Montana. Journal of Wildlife Diseases 50:393–396. [DOI] [PubMed] [Google Scholar]
- Blackburn JK, Kracalik IT, and Fair JM. 2015. Applying science: opportunities to inform disease management policy with cooperative research within a One Health framework. Frontiers in Public Health 3:article 276. 10.3389/fpubh.2015.00276 [DOI] [PMC free article] [PubMed]
- Blackburn JK, McNyset KM, Curtis A, and Hugh-Jones ME. 2007. Modeling the geographic distribution of Bacillus anthracis, the causative agent of anthrax disease, for the contiguous United States using predictive ecologic niche modeling. American Journal of Tropical Medicine and Hygiene 77:1103–1110. [PubMed] [Google Scholar]
- Blackburn JK, Van Ert M, Mullins JC, Hadfield TL, and Hugh-Jones ME. 2014b. The necrophagous fly anthrax transmission pathway: empirical and genetic evidence from wildlife epizootics. Vector-Borne and Zoonotic Diseases 14:576–583. [DOI] [PubMed] [Google Scholar]
- Börger L, and Fryxell J. 2012. Quantifying individual differences in dispersal using net squared displacement. Pages 222–239 in Clobert J, Baguette M, Benton TG, and Bullock JM, editors. Dispersal ecology and evolution. Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- Bowyer RT 1981. Activity, movement, and distribution of Roosevelt elk during rut. Journal of Mammalogy 62:574–582. [Google Scholar]
- Broennimann O, Fitzpatrick MC, Pearman PB, Petitpierre B, Pellissier L, Yoccoz NG, Thuiller W, Fortin M, Randin C, and Zimmermann NE. 2012. Measuring ecological niche overlap from occurrence and spatial environmental data. Global Ecology and Biogeography 21:481–497. [Google Scholar]
- Burnham KP, and Anderson DR. 2003. Model selection and multimodel inference: a practical information-theoretic approach. Springer Science & Business Media, New York, New York, USA. [Google Scholar]
- Carlson CJ, Kracalik IT, Ross N, Alexander KA, Hugh-Jones ME, Fegan M, Elkin BT, Epp T, Shury TK, Zhang W, et al. 2019. The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock and wildlife. Nature Microbiology 4:1337–1343. [DOI] [PubMed] [Google Scholar]
- Creel S, Winnie JA, and Christianson D. 2009. Glucocorticoid stress hormones and the effect of predation risk on elk reproduction. Proceedings of the National Academy of Sciences 106:12388–12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge WD, Marcum CL, and Olson-Edge SL. 1988. Summer forage and feeding site selection by elk. Journal of Wildlife Management 52:573–577. [Google Scholar]
- Fitzpatrick MC, Gotelli NJ, and Ellison AM. 2013. MaxEnt versus MaxLike: empirical comparisons with ant species distributions. Ecosphere 4:1–15. [Google Scholar]
- Franklin WL and Lieb JW. 1979. The social organization of a sedentary population of North American elk: a model for understanding other populations, p. 185–198. In: Boyce MS and Hayden-Wing LD (eds). North American elk: Ecology, behavior, and management. The University of Wyoming Press, Laramie, Wyoming, USA. [Google Scholar]
- Geist V 1971. Mountain sheep. A study in behavior and evolution. University of Chicago Press, Chicago, Illinois, USA. [Google Scholar]
- Geist V 1982. Adaptive behavioral strategies. Pages 219–277inThomas JW and Toweill DE, editors. Elk of North America: ecology ad management. Stackpole Books, Harrisburg, Pennsylvania, USA. [Google Scholar]
- Hebblewhite M, Merrill EH, and McDonald TL. 2005. Spatial decomposition of predation risk using resource selection functions: an example in a wolf–elk predator–prey system. Oikos 111:101–111. [Google Scholar]
- Hugh-Jones M, and Blackburn J. 2009. The ecology of Bacillus anthracis. Molecular Aspects of Medicine 30:356–367. [DOI] [PubMed] [Google Scholar]
- Hugh-Jones M, and De Vos V. 2002. Anthrax and wildlife. Revue Scientifique et Technique-Office International des Epizooties 21:359–384. [DOI] [PubMed] [Google Scholar]
- Irwin LL, and Peek JM. 1983. Elk habitat use relative to forest succession in Idaho. Journal of Wildlife Management 47:664–672. [Google Scholar]
- Jacques CN, Jenks JA, Deperno CS, Sievers JD, Grovenburg TW, Brinkman TJ, Swanson CC, and Stillings BA. 2009. Evaluating ungulate mortality associated with helicopter net-gun captures in the Northern Great Plains. Journal of Wildlife Management 73(8):1282–1291. [Google Scholar]
- Johnson DH 1980. The comparison of usage and availability measurements for evaluating resource preference. Ecology 61:65–71. [Google Scholar]
- Johnson SG 2014. The NLopt nonlinear-optimization package. <https://cran.r-project.org/web/packages/nloptr/index.html>. Accessed28 Nov 2017.
- Laundré JW, Hernández L, and Altendorf KB. 2001. Wolves, elk, and bison: reestablishing the “landscape of fear” in Yellowstone National Park, USA. Canadian Journal of Zoology 79:1401–1409. [Google Scholar]
- Leptich DJ, and Zager PE. 1994. Coeur D’Alene elk ecology. Idaho Department of Fish and Game, Boise, Idaho, USA. [Google Scholar]
- Manly BFJ, McDonald LL, and Thomas DL. 2002. Resource selection by animals: statistical design and analysis for field studies. Kluwer Academic Publishers, Secaucus, New Jersey, USA. [Google Scholar]
- Marcum CL 1975. Summer-fall habitat selection and use by a western Montana elk herd. Dissertation, University of Montana, Missoula, USA. [Google Scholar]
- Martinka C 1969. Population ecology of summer resident elk in Jackson Hole, Wyoming. Journal of Wildlife Management 465–481. [Google Scholar]
- McCorquodale SM 1993. Winter foraging behavior of elk in the shrub-steppe of Washington. The Journal of Wildlife Management 33:881–890. [Google Scholar]
- McCorquodale SM 2003. Sex-specific movements and habitat use by elk in the Cascade Range of Washington. Journal of Wildlife Management 67:729–741. [Google Scholar]
- Montana Fish Wildlife & Parks. 2015. Targeted Elk Brucellosis Surveillance Project: 2011–2015 Comprehensive Report. <http://bloximages.chicago2.vip.townnews.com/billingsgazette.com/content/tncms/assets/v3/editorial/a/3c/a3c4e560-e663-5c76-8f59-a2d2cdcdcce6/562a88e02e468.pdf.pdf>. Accessed26 Jul 2017.
- Montana Fish Wildlife & Parks. 2016. Targeted Elk Brucellosis Surveillance Project 2016 Annual Report. <http://fwp.mt.gov/fwpDoc.html?id=77742>. Accessed21 Sep 2017.
- Montgomery RA, Roloff GJ, and Millspaugh JJ. 2013. Variation in elk response to roads by season, sex, and road type. Journal of Wildlife Management 77:313–325. [Google Scholar]
- Morris LR, Proffitt KM, Asher V, and Blackburn JK. 2016a. Elk resource selection and implications for anthrax management in Montana. Journal of Wildlife Management 80:235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LR, Proffitt KM, and Blackburn JK. 2016b. Mapping resource selection functions in wildlife studies: concerns and recommendations. Applied Geography 76:173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan R, Getz WM, Revilla E, Holyoak M, Kadmon R, Saltz D, and Smouse PE. 2008. A movement ecology paradigm for unifying organismal movement research. Proceedings of the National Academy of Sciences 105:19052–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nekorchuk D 2017. Modeling indirect transmission disease risk: anthrax in bison in southwestern Montana. Dissertation, University of Florida, Gainesville, USA. [Google Scholar]
- Nekorchuk DM, Morris LR, Asher V, Hunter DL, Ryan SJ, and Blackburn JK. 2018. Potential Bacillus anthracis risk zones for male plains bison (Bison bison bison) in southwestern Montana. Journal of Wildlife Diseases 55:136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell MJ 2009. The BOBYQA algorithm for bound constrained optimization without derivatives. Technical report NA2009/06. Department of Applied Mathematics and Theoretical Physics, Cambridge, England. [Google Scholar]
- Proffitt KM, Gude JA, Hamlin KL, Garrott RA, Cunningham JA, and Grigg JL. 2011. Elk distribution and spatial overlap with livestock during the brucellosis transmission risk period. Journal of Applied Ecology 48:471–478. [Google Scholar]
- R Core Team. 2017. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- Schmitt SM, O’Brien DJ, Bruning-Fann CS, and Fitzgerald SD. 2002. Bovine tuberculosis in Michigan wildlife and livestock. Annals of the New York Academy of Sciences 969:262–268. [DOI] [PubMed] [Google Scholar]
- Smith BL, and Anderson SH. 2001. Does dispersal help regulate the Jackson elk herd? Wildlife Society Bulletin 29:331–341. [Google Scholar]
- Stockwell D, and Peters D. 1999. The GARP modelling system: problems and solutions to automated spatial prediction. International Journal of Geographical Information Science 13:143–158. [Google Scholar]
- Turner WC, Kausrud KL, Krishnappa YS, Cromsigt JP, Ganz HH, Mapaure I, Cloete CC, Havarua Z, Küsters M, and Getz WM. 2014. Fatal attraction: vegetation responses to nutrient inputs attract herbivores to infectious anthrax carcass sites. Proceedings of the Royal Society of London B: Biological Sciences 281:20141785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unsworth JW, Kuck L, Garton EO, and Butterfield BR. 1998. Elk habitat selection on the Clearwater national forest, Idaho. Journal of Wildlife Management 62:1255–1263. [Google Scholar]
- Van Moorter B, Rolandsen CM, Basille M, and Gaillard J. 2016. Movement is the glue connecting home ranges and habitat selection. Journal of Animal Ecology 85:21–31. [DOI] [PubMed] [Google Scholar]
- Van Ness GB 1971. Ecology of anthrax. Science 172:1303–1307. [DOI] [PubMed] [Google Scholar]
- VanDerWal J, Falconi L, Januchowski S, Shoo L, Storlie C, and VanDerWal MJ. 2014. Package ‘SDMTools.’ <https://CRAN.R-project.org/package=SDMTools>. Accessed 20 Nov 2017.
- Varland KL, Lovaas AL, and Dahlgren RB. 1978. Herd organization and movements of elk in Wind Cave National Park, South Dakota. Department of the Interior, National Park Service, Washington, D.C., USA. [Google Scholar]
- Walker MA 2019. Ungulate use of locally infectious zones (LIZs) in a re-emerging anthrax risk area. Thesis, University of Florida, Gainesville, USA. [Google Scholar]
- Warren DL, and Seifert SN. 2011. Ecological niche modeling in Maxent: the importance of model complexity and the performance of model selection criteria. Ecological Applications 21:335–342. [DOI] [PubMed] [Google Scholar]
- Watson R 2012. Public wildlife on private land: unifying the split estate to enhance trust resources. Duke Environmental Law and Policy Forum 23:291–321. [Google Scholar]
- White P, Proffitt KM, Mech LD, Evans SB, Cunningham JA, and Hamlin KL. 2010. Migration of northern Yellowstone elk: implications of spatial structuring. Journal of Mammalogy 91:827–837. [Google Scholar]
- Yang A, Gomez JP, and Blackburn JK. 2020a. Exploring environmental coverages of species: a new variable contribution estimation methodology for rulesets from the genetic algorithm for rule-set prediction. PeerJ 8:e8968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Gomez JP, Haase CG, Proffitt KM, and Blackburn JK. 2019. Effects of brucellosis serologic status on physiology and behavior of Rocky Mountain elk (Cervus canadensis nelsoni) in southwestern Montana, USA. Journal of Wildlife Diseases 55:304–315. [DOI] [PubMed] [Google Scholar]
- Yang A, Mullins JC, Van Ert M, Bowen RA, Hadfield TL, and Blackburn JK. 2020b. Predicting the geographic distribution of the Bacillus anthracis A1. a/Western North American sub-lineage for the continental United States: new outbreaks, new genotypes, and new climate data. American Journal of Tropical Medicine and Hygiene 102:392–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


