Abstract
INTRODUCTION:
Metastatic progression in triple-negative breast cancer (TNBC) patients occurs primarily because of nuclear reprogramming that includes chromatin remodeling and epigenetic modifications. The existing and most successful chemotherapies available for metastatic TNBC target nuclear proteins or damage DNA. The objectives here are to investigate an undescribed role for the molecular biology of nuclear angiopoietin-like protein 4 (ANGPTL4) and to characterize the effect of ectopic overexpression of ANGPTL4 in the metastatic biology of TNBC.
MATERIALS AND METHODS:
Lentiviral-mediated transduction was used to overexpress ANGPTL4 in the TNBC cell line MD Anderson–metastatic breast cancer 231. The overexpression of ANGPTL4 was confirmed by western blot and ELISA. Subcellular fractionation, western blot, and immunofluorescence microscopy were used to characterize the intracellular localization of ANGPTL4. Mammosphere culture and the anchorage-independent growth assay analyzed the metastatic potential of the cell line. Xenograft assays assessed the effect of ANGPTL4 overexpression on TNBC metastases in vivo.
RESULTS:
The ANGPTL4 overexpressing cell line formed larger mammospheres and anchorage-independent colonies in vitro and developed larger primary tumors, more liver metastases, and brain metastatic outgrowth in vivo in comparison to a cell line that expressed endogenous levels of ANGPTL4. ANGPTL4, aurora kinase A (AURKA), a mitotic kinase, and Tat-interacting protein p60 kDa (Tip60), a lysine acetyltransferase, associated with chromatin in the ANGPTL4 overexpressing cells but not in cells that expressed endogenous levels of ANGPTL4.
CONCLUSIONS:
The ANGPTL4 overexpressing cell line showed in vitro and in vivo activities that suggest that nuclear ANGPTL4, AURKA, and Tip60 may cooperatively modulate TNBC metastases within chromatin-remodeling complexes or DNA-associated machinery.
Keywords: Angiopoietin-like protein 4, aurora kinase A, metastasis, Tat-interacting protein p60 kDa, triple-negative breast cancer
Introduction
Triple-negative breast cancer (TNBC) is an aggressive subtype of breast cancer with high incurability due to its metastatic biology. The life expectancy of patients who present with TNBC brain or liver metastases is grim. Their life expectancy is shortened to an average of 6-and 9-month post diagnosis, respectively,[1,2,3] making TNBC brain or liver metastases the most life-threatening forms of TNBC metastases.[3,4] Particularly notable is that the brain or the liver is the first site of metastatic presentation in 20% or 30% of TNBC patients, respectively.[1,5] Consequently, there is an urgent need for therapies that selectively prevent metastatic outgrowth while causing minimal damage to the brain or liver. Ideally, those therapies would extend the life expectancy of patients that present with TNBC brain or liver metastases and improve their quality of life.
Nuclear proteins are ideal targets for use in cancer therapy for TNBC patients because the metastatic potential of TNBC cells correlates with the expression of proteins that regulate the cell cycle or other nuclear events. For example the current and most effective chemotherapies available for TNBC are cell cycle specific drugs including anthracyclines and taxanes or drugs that damage DNA including cisplatin. The conundrum is that resistance to existing chemotherapies and recurrence is likely to occur within 3–5 years post treatment. Thus, the identification of chromatin or DNA-associated proteins that cooperatively regulate metastases will enable the development of chemotherapeutic agents for naïve and chemoresistant TNBC metastases.
A modulator of TNBC metastasis is angiopoietin-like protein 4 (ANGPTL4). ANGPTL4 is preferentially upregulated in patient-derived TNBC primary tumors, serum, and metastases, and by human TNBC cell line-derived xenografts in mice.[6,7,8,9,10,11,12,13,14,15] Previous studies have shown that metastatic TNBC cells secrete soluble ANGPTL4 that in turn disrupts the integrity of junctions of capillary endothelial cells within the lungs and brain giving TNBC cells access to the lung and brain parenchyma, respectively.[6,10,11
The ANGPTL4 protein contains a signal sequence for protein secretion, a coiled-coil domain at its amino terminus and a fibrinogen-like domain at its carboxyl terminus.[15] The full-length protein localizes intracellularly, is secreted into the bloodstream, or binds to the extracellular matrix.[15] ANGPTL4 is cleaved by proprotein convertases at a consensus sequence and the terminuses are secreted to function as autocrine or paracrine signaling molecules.[15,16] In TNBC cells, the secreted full length or the fibrinogen-like domain of ANGPTL4 disrupts the cell-to-cell junctions of microvascular endothelial cells for extravasation and metastatic organ colonization.[6,7] Antibody-based subcellular localization has identified ANGPTL4 as a nuclear protein (Human Protein Atlas Cell Atlas), but its nuclear localization has not been characterized in TNBC cells.
In this study, we characterized the effect of ectopic overexpression of ANGPTL4 on TNBC metastases. We identify that ANGPTL4 is a nuclear protein that affects the localization of aurora kinase A (AURKA) and Tip60. We also show that ANGPTL4 promotes the formation of anchorage-independent colonies, mammospheres, and lipid droplets in vitro as well as brain and liver metastases in vivo.
Materials and Methods
Cell culture
MD Anderson–metastatic breast cancer 231 (MDA-MB-231) cells were cultured in complete medium (Dulbecco's modified Eagle medium nutrient mixture F12 [DMEM/F12] medium containing 5% fetal bovine serum [FBS] and 0.02% gentamicin). The cells were grown at 37°C and 5% CO2. MDA-MB-231 mGFP and MDA-MB-231 ANGPTL4 mGFP cells were cultured in a complete medium supplemented with 2 μg/ml puromycin.
Lentiviral-mediated angiopoietin-like protein 4 overexpression
MDA-MB-231 cells were seeded at a density of 1 × 105 cells per well in a 24-well plate and grown in complete medium at 37°C under 5% CO2 for 24 h. For lentiviral-mediated transduction, lentiviral particles (multiplicity of infection of 2) expressing the pLenti-C-mGFP-P2A-Puro plasmid with the ANGPTL4 gene or the pLenti-C-mGFP-P2A-Puro plasmid were added to cells and grown in complete medium supplemented with 8 μg/ml polybrene for 16 h. The virus-containing medium was removed and replaced with a complete medium and grown at 37°C under 5% CO2 for 24 h. Puromycin selection at 2 μg/ml was initiated 24 h post transduction and continued for 2 weeks. Single cells were cloned and propagated for 3 weeks. A single colony of MDA-MB-231 cells expressing mGFP or ANGPTL4 mGFP with robust GFP expression as determined by fluorescence microscopy and fluorescence-activated cell sorting was propagated and used in all experiments.
Mammosphere culture
MDA-MB-231 mGFP and MDA-MB-231 ANGPTL4 mGFP cells were cultured for 7 days at 37°C and 5% CO2 in ultra-low attachment plates or flasks that contained MammoCult Medium (STEMCELL™ Technologies). After 7 days, the colonies were counted and photographed with a camera attached to an inverted light microscope, used for western blotting or stained with Oil Red O.
Oil red O staining
Mammospheres were stained with Oil Red O stain (60% 0.5% Oil Red O solution in 40% ddH2O) according to the manufacturer's instructions.
Anchorage-independent growth assay
MDA-MB-231 ANGPTL4 mGFP or mGFP cells (1.25 × 103 cells) suspended in complete medium supplemented with 2 μg/ml puromycin (Sigma) and 3% noble agarose were seeded on the top of a semisolid layer of DMEM/F12 medium containing 5% FBS, 0.02% gentamicin, 2 μg/ml puromycin, and 0.6% noble agarose in six wells plates. Medium was added twice per week to maintain humidity and to prevent desiccation. After 7 days, the colonies were counted and photographed with a camera attached to an inverted light Nikon Ti2 microscope.
Enzyme-linked immunosorbent assay
Cells were seeded in 100 mm × 20 mm plates for 24 h, rinsed thrice with phosphate-buffered saline (PBS), and grown in phenol red-free DMEM/F12 medium containing 0.02% gentamicin for 48 h. The amount of secreted ANGPLT4 into the cell culture medium was determined using the human ANGPTL4 ELISA kit (cat. EHANGPTL4, Thermo Fisher Scientific) according to the manufacturer's instructions.
Western blotting
Westerns blots were performed as described by Bailey et al., 2014.[17]
Subcellular protein fractionation
Cell fractions were obtained using the Thermo Fisher Scientific Subcellular Protein Fractionation Kit for Cultured Cells according to the manufacturer's instructions.
Confocal immunofluorescence microscopy
MDA-MB-231 mGFP or MDA-MB-231 ANGPTL4 mGFP cells were prepared for confocal immunofluorescence microscopy, as previously described by Bailey et al.[17] Images were captured with a Leica confocal microscope at the indicated magnifications. Merged fluorescence pictures were generated using Fiji-ImageJ.
Mouse human tumor xenograft model
All mouse studies were approved by the Institutional Care and Animal Use Committee at the University of Arkansas. Four-week-old female athymic nude mice weighing approximately 20 g were purchased from the Jackson Laboratory. The mice were housed at the Central Laboratory Animal Facilities (CLAF). No more than five mice were housed in a single cage of dimensions 11.5” × 7.5'× 5'. Cage changes were performed twice a week. Mice were routinely monitored (at least once a day) for injury to the surgical site, weight loss, tumor burden, and sluggishness. All cages had cage cards that indicated age, sex, strain, and date of arrival. Mice were provided unrestricted access to food and water. Standard 12-h light/dark cycles were maintained. Mice were acclimated to CLAF at least 1 week prior to experimentation.
Athymic nude mice were subcutaneously injected without ultrasonography with either the MDA-MB-231 mGFP cell line or the MDA-MB-231 ANGPTL4 mGFP cell line. The mice were weighed and monitored each day post injection for symptoms of neurological involvement and distress until euthanasia. Tumor areas were measured every 3 days using an electric caliper and were quantitated with the formula length multiplied by the width (L × W). Four weeks after subcutaneous injection with breast cancer cells, the mice were anesthetized with isoflurane mixed with compressed air/oxygen. Primary tumors and organs were harvested for histopathology, imaging with fluorescence stereomicroscopy to identify GFP-positive tumors and GFP-positive metastases, and immunohistochemistry. Eight animals were used per cell line.
In a separate experiment, athymic nude mice were injected in the left heart ventricles without ultrasonography with either the MDA-MB-231 mGFP cell line or the MDA-MB-231 ANGPTL4 mGFP cell line or PBS. The mice were weighed and monitored each day post injection for symptoms of neurological involvement and distress until euthanasia. Four weeks after injections, the mice were anesthetized with isoflurane mixed with compressed air/oxygen. Brains were harvested for histopathology. Brains were imaged using fluorescence stereomicroscopy to identify GFP-positive metastases. Three animals were used per cell line.
Results
Ectopically expressed angiopoietin-like protein 4 is localized to the nucleus and binds to chromatin
To evaluate the role of ANGPTL4 in TNBC metastases, we overexpressed mGFP-tagged ANGPTL4 (ANGPTL4 mGFP) or tag alone (mGFP, control) in the human TNBC cell line MDA-MB-231. Immunoblotting and ELISAs confirmed the overexpression of the ANGPTL4 mGFP protein in the MDA-MB-231 cell line [Figure 1a and b]. Confocal micrographs confirmed the presence of mGFP in the cell lines [Figure 1c]. The MDA-MB-231 cell lines ectopically expressing mGFP or ANGPTL4 mGFP were designated MDA-MB-231 mGFP or MDA-MB-231 ANGPTL4 mGFP, respectively.
Figure 1.
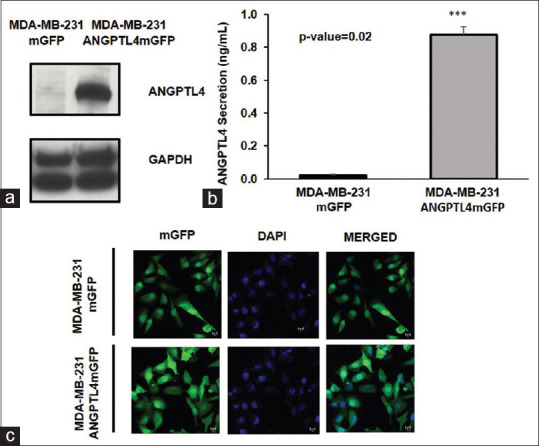
Lentiviral-mediated transduction of the MD Anderson–metastatic breast cancer 231 cell line to overexpress angiopoietin-like protein 4-mGFP or mGFP. (a) Ectopic expression of angiopoietin-like protein 4 mGFP in the MD Anderson–metastatic breast cancer 231 cell line assessed by Western blot. (b) Secreted angiopoietin-like protein 4 from the MD Anderson–metastatic breast cancer 231 mGFP and MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 mGFP cell lines, respectively, was measured by ELISA. The mean concentration of angiopoietin-like protein 4 secreted from MD Anderson–metastatic breast cancer 231 mGFP and MD Anderson–metastatic breast cancer 231 Angiopoietin-like protein 4 mGFP measured 0.022 ng/ml and 0.87725 ng/ml, respectively. n = 3; P value was calculated with the Student's t-test using GraphPad Prism version 8 for Windows, GraphPad Software, La Jolla California USA, www.graphpad.com. The error bars indicate the standard deviation. (c) Micrographs of mGFP expressed by MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 –mGFP
Data deposited within the Human Protein Atlas Cell Atlas and the National Center for Biotechnology Information databases indicate that ANGPTL4 is a nuclear protein. To determine whether ANGPTL4 localizes to the nucleus, the cells were immunostained for ANGPTL4. Immunofluorescence microscopy confirmed the nuclear localization of ANGPTL4 mGFP [Figure 2a]. To determine whether ANGPTL4 binds chromatin, chromatin-enriched lysates were fractionated from the MDA-MB-231 mGFP or MDA-MB-231 ANGPTL4 mGFP cells. Immunoblotting revealed that ANGPTL4 mGFP was present in the chromatin fractions [Figure 2b]. These results indicated that ANGPTL4 can be a chromatin-bound protein. Data deposited in the Biological General Repository for interaction datasets listed AURKA and Tat Interacting Protein, 60 kDa (Tip60), also named K (Lysine) acetyltransferase 5 (KAT5) as interactors of ANGPTL4. Immunofluorescence and immunoblot analysis showed nuclear and chromatin localization of AURKA and Tip60 within the ANGPTL4 mGFP cells [Figure 2a and b].
Figure 2.
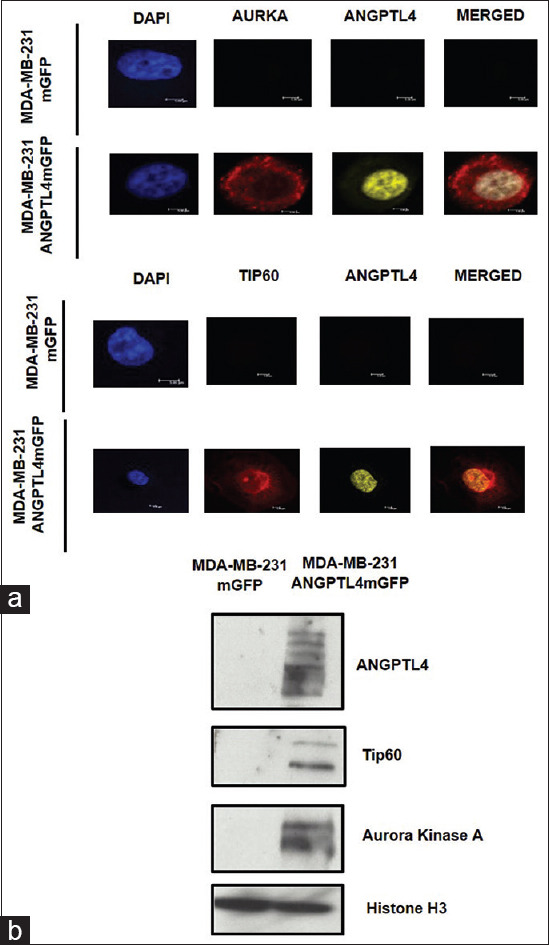
Chromatin-bound angiopoietin-like protein 4 and Tat-interacting protein p60 kDa. (a) Micrographs of angiopoietin-like protein 4, aurora kinase A, and Tat-interacting protein p60 kDa expressed by MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 -mGFP. (b) Chromatin fractions were isolated, quantified, and 25 μg of total protein was resolved by gradient SDS-PAGE followed by immunoblotting with anti-angiopoietin-like protein 4, anti-aurora kinase A, anti-Tat interacting protein p60 kDa, and anti-Histone H3 (loading control) antibodies
Angiopoietin-like protein 4 promotes triple-negative breast cancer stem cell formation
The MDA-MB-231 ANGPTL4 mGFP and MDA-MB-231 mGFP cell lines were grown in mammosphere culture for 7 days. The number and size of the mammospheres were quantified with microscopy. The MDA-MB-231 ANGPTL4 mGFP cell line formed significantly more ≥200 μm mammospheres in comparison to the MDA-MB-231 mGFP cell line [Figure 3a]. The mammospheres derived from the MDA-MB-231 ANGPT4 mGFP cell line were large, loosely compacted cell aggregates [Figure 3a]. In contrast, the mammospheres derived from the MDA-MB-231 mGFP cell line were small, compacted cell aggregates [Figure 3a]. Mammospheres derived from MDA-MB-231 ANGPTL4 mGFP and MDA-MB-231 mGFP cell lines expressed less of the stem cell marker cluster of differentiation 24 (CD24) in comparison to adherent cells, as shown in immunoblots [Figure 3b]. The expression of stem cell marker CD44 was comparable between the MDA-MB-231 mGFP and MDA-MB-231 ANGPTL4 mGFP mammospheres and adherent cells [Figure 3b].
Figure 3.
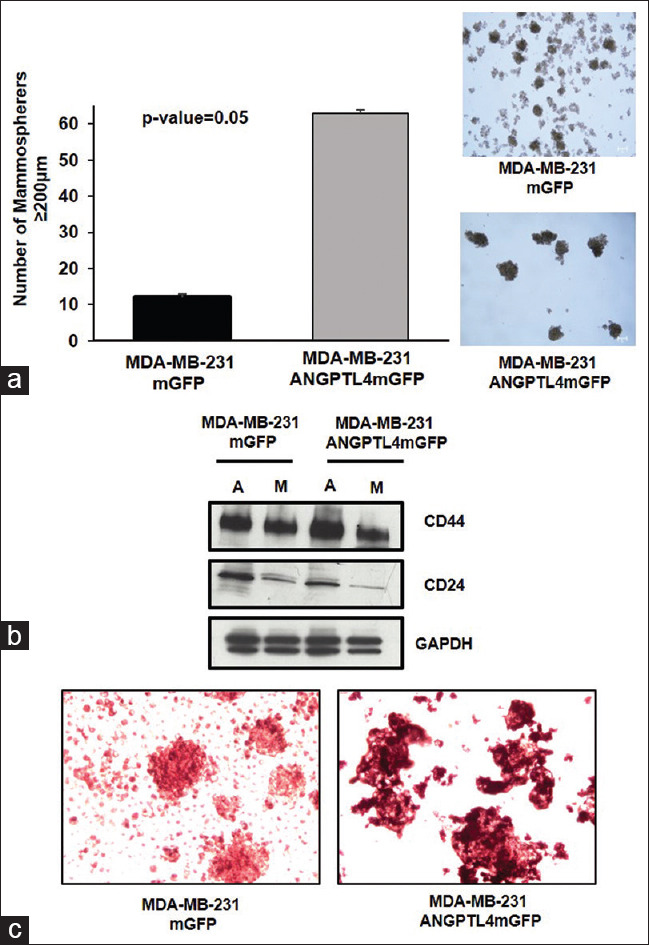
Mammospheres derived from the MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 mGFP cell lines. (a) The number and morphology of ≥200 mm primary mammospheres. The mean number of MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 Angiopoietin-like protein 4-mGFP mammospheres ≥200 mm is 12 and 63, respectively, n = 3. The error bars depict the standard deviation. (b) Comparison of CD44 and cluster of differentiation 24 expression in adherent cells and mammospheres. (A = adherent, M = mammospheres). (c) Mammospheres stained with Oil Red O to detect triglycerides and cholesterol esters
Next, the MDA-MB-231 mGFP or MDA-MB-231 ANGPTL4 mGFP-derived mammospheres were stained with Oil Red O to detect triglycerides and esterified cholesterol. Microscopic observation of the mammospheres derived from the MDA-MB-231 ANGPT4 mGFP cell line exhibited a deeper red color indicative of lipid droplets in comparison to mammospheres derived from the MDA-MB-231 mGFP cell line [Figure 3c].
Angiopoietin-like protein 4 promotes anchorage-independent growth
To assess anchorage-independent growth, the MDA-MB-231 mGFP or MDA-MB-231 ANGPTL4 mGFP cell lines were propagated in suspension in a semisolid medium containing 0.3% noble agar. ANGPTL4 overexpression by the MDA-MB-231 ANGPTL4 mGFP cell line increased colony formation in comparison to the MDA-MB-231 mGFP cell line [Figure 4a]. The MDA-MB-231ANGPTL4 mGFP cell line formed large, loosely compacted nonspherical cell colonies that were similar to the morphology observed with MDA-MB-231 ANGPTL4 mGFP mammospheres [compare Figure 3a and Figure 4b].
Figure 4.
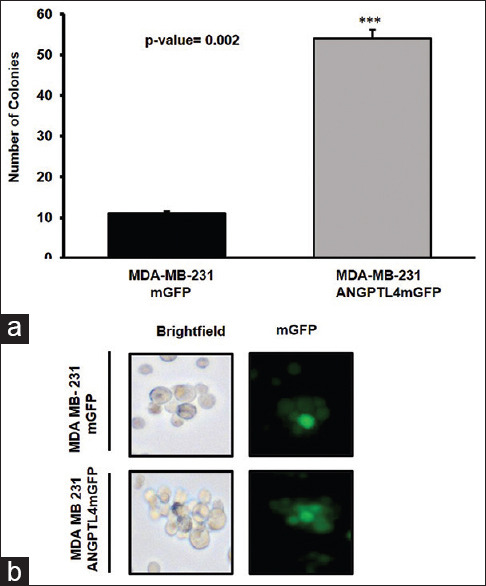
Anchorage-independent growth of cells derived from the MD Anderson– metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 mGFP cell lines, respectively. (a) The number and (b) morphology of ≥200 mm colonies derived from MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 mGFP cells grown in suspension in semisolid medium containing 0.3% noble agar, respectively. The mean number of MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 mGFP colonies ≥200 mm is 11 and 54, respectively, n = 3. The error bars depict the standard deviation
Angiopoietin-like protein 4 enhances triple-negative breast cancer primary tumor growth and metastatic liver tropism
To evaluate the tumorigenicity and the metastatic potential of the MDA-MB-231 mGFP or MDA-MB-231 ANGPTL4 mGFP TNBC cell lines, the cells were injected into the subcutaneous region of the right flank of nude mice and grown for 4 weeks. All mice developed primary tumors; however, the mean tumor area of the ANGPTL4-driven primary tumors was significantly greater than the mean tumor area of primary tumors that expressed mGFP [Figure 5a and b]. The weights of the mice did not vary despite the significantly larger ANGPTL4 mGFP primary tumors [Figure 5c]. The brains, lungs, spleen, and livers of all mice were evaluated for mGFP-positive metastases. A comparison of the livers identified that seven of the eight mice subcutaneously injected with the MDA-MB-231 ANGPTL4 mGFP cell line developed liver macrometastases, whereas one of the eight mice subcutaneously injected with the MDA-MB-231 mGFP cell line developed a single micrometastatic lesion [Figure 6a and b]. No metastases were observed in the brains, spleen, and lungs of the mice (data not shown).
Figure 5.
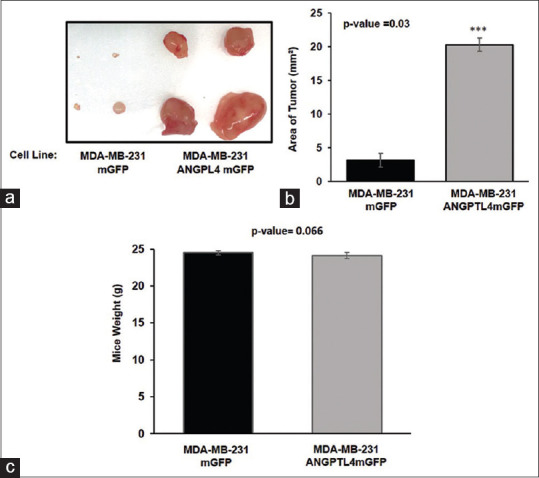
Angiopoietin-like protein 4 driven primary tumor proliferation. (a) Photographs of primary tumors. (b) The mean primary tumor area and (c) mean mice body weight measured before necropsy. The mean primary tumor area for MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 mGFP is 3.14 mm2 and 20.32 mm2, respectively. Average combined mGFP tumor area = 1.16 mm2, Average combined angiopoietin-like protein 4 mGFP tumor area = 8.84 mm2. The mean weight of mice bearing MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson– metastatic breast cancer 231 Angiopoietin-like protein 4 mGFP xenografts is 25 and 24, respectively. The error bars represent the standard deviation. (n = eight mice per group)
Figure 6.
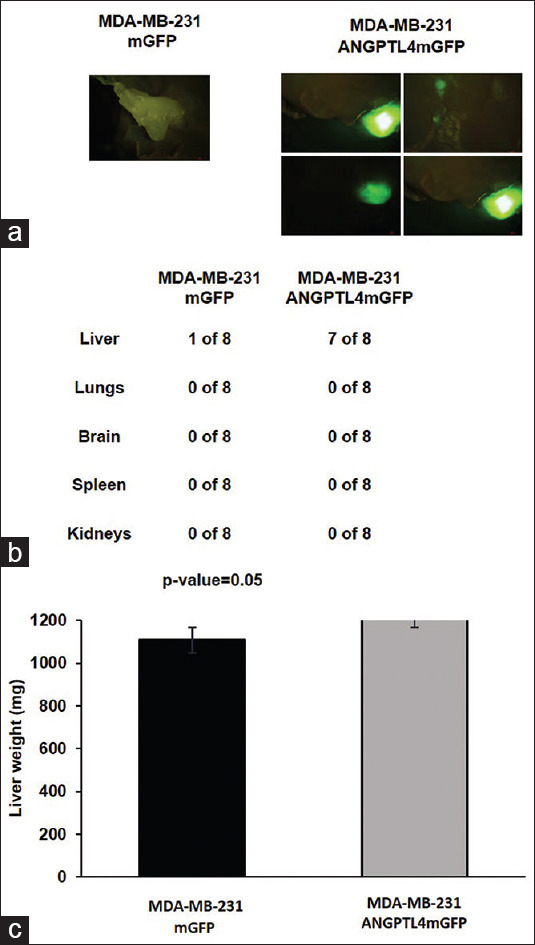
Metastatic liver tropism by MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 mGFP cells. (a and b) Livers were resected 4 weeks after subcutaneous injection of MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 -mGFP cells into the right flank of nude mice (n = 8). (a) mGFP expressing human triple-negative breast cancer liver metastases in mice livers, (b) the number of mice with mGFP expressing metastases derived from the MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 mGFP cell lines, (c) mean liver weight. Mice liver mean for MD Anderson– metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 Angiopoietin-like protein 4 mGFP is 1276.15 mg and 1227.14 mg, respectively. The error bars represent the standard error
Angiopoietin-like protein 4 promotes brain metastasis of triple-negative breast cancer cells
To characterize the effect of ANGPTL4 overexpression on TNBC brain metastases, the MDA-MB-231 ANGPTL4 mGFP cell lines or the MDA-MB-231 mGFP cell lines were injected in the left heart ventricles of mice. After 4 weeks, the brains were assessed for the development of brain metastases. A comparison of the brains showed that three of the three mice injected with the MDA-MB-231 ANGPTL4 mGFP cell line developed brain metastatic lesions, whereas one of the three mice injected with the MDA-MB-231 mGFP cell line developed a single micrometastatic lesion [Figure 7a and b]. ANGPTL4 mGFP overexpression increased the number of metastatic TNBC brain lesions by 89% in comparison to the mGFP overexpressing cells [Figure 7a and b].
Figure 7.
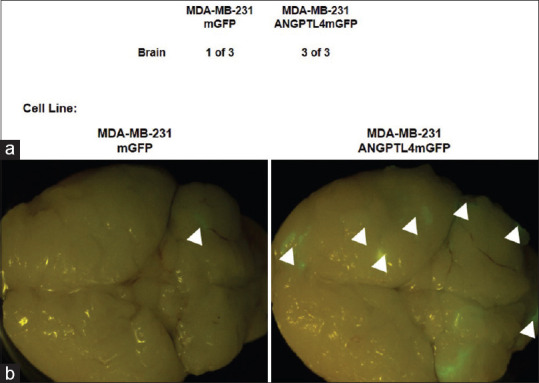
Metastatic Brain Colonization by MD Anderson–metastatic breast cancer 231 Angiopoietin-like protein 4 mGFP cells. (a and b) Brains were resected 4 weeks after intracardiac injection of MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 -mGFP cells into the left heart ventricle of nude mice (n = three mice per group). (a) The number of mice-expressing mGFP metastases derived from the MD Anderson–metastatic breast cancer 231 mGFP or MD Anderson–metastatic breast cancer 231 angiopoietin-like protein 4 mGFP cell lines and (b) fluorescence stereomicrographs mouse brains expressing mGFP human triple-negative breast cancer brain metastases. Arrowheads indicate metastatic outgrowth
Discussion
ANGPTL4 has emerged as an autocrine and paracrine modulator of metastatic TNBC in humans. It is overexpressed by primary and metastatic tumors and elevated in the serum of TNBC patients.[7,14] In human xenograft models of TNBC, ANGPTL4-dependent paracrine communication with the tumor microenvironment enhances brain and lung metastatic outgrowth.[6,7,10,11] In this study, the ANGPTL4 overexpressing cell line developed larger primary tumors, more liver metastases, and brain metastatic outgrowth in comparison to cells that express normal levels of ANGPTL4 in immunodeficient mice. Collectively, the data generated in this study confirmed previous studies that showed that ANGPTL4 is a driver of brain metastases.[10,11,18] This is the first report of ANGPTL4-driven TNBC liver metastases.
TNBC stem cells are pivotal to the metastatic biology of tumors and are especially brain and liver trophic.[19] Mammospheres derived from the MDA-MB-231 ANGPTL4 mGFP and MDA-MB-231 mGFP cell lines were CD44 + and CD24low in comparison to adherent cells. Mammospheres that express CD44 and low levels of CD24 are regarded as stem cells and possess high metastatic potential.[20,21,22,23,24,25,26,27,28,29,30,31] In this study, ANGPTL4 overexpression increased the size of mammospheres and the intensity of the Oil Red O staining of mammospheres. The difference in the intensity of the Oil Red O staining in ANGPTL4 overexpressing mammospheres in comparison to mammospheres that express endogenous levels of ANGPTL4 suggests that the lipid profile of the ANGPTL4 overexpressing cells differs from control cells. Ness et al., 2017, demonstrated the epigenetic upregulation of the transcription of ANGPTL4 and other lipogenic genes in tumor spheroids derived from uveal melanoma.[32] Köster et al., 2005 and Xu et al., 2005 reported that ANGPTL4 overexpression in mice caused hyperlipidemia and elevated triglycerides in vivo.[33,34] One mouse in our study exhibited hyperlipidemia (data not shown), indicating that ANGPTL4 secreted by TNBC may alter the lipogenic profile within an organism during tumorigenesis and metastases. This is intriguing because TNBC is regarded as a metabolic syndrome and an obesity-associated form of breast cancer.[35,36] The Carolina Breast Study linked obesity to an increased incidence of TNBC in premenopausal and postmenopausal African American women.[37,38] Considering that ANGPTL4 is a regulator of multiple metabolic pathways that are dysregulated in TNBC patients, it is plausible that ANGPTL4 may function as a pivotal node in obesity-associated TNBC pathogenesis.[33,34,39,40,41,42]
In our study, chromatin-bound ANGPTL4, AURKA, and Tip60 increased in ANGPTL4 mGFP overexpressing TNBC cells. Tip60 is a driver of TNBC tumorigenesis. Recently, Idrissou et al. showed that a Tip60 inhibitor reduced the growth of human TNBC xenograft tumors.[43] Interestingly, metabolism-associated chromatin acetylation has been identified as a driver of the aggressive metastatic biology of TNBC.[44] The promoter of ANGPTL4 is acetylated and its acetylation corresponded with hypertriglyceridemia in a mouse model.[45] Previous studies showed that a transcriptional regulator of ANGPTL4, peroxisome proliferator-activated receptor (PPAR) gamma, is acetylated by lysine acetyltransferases including Tip60 and acetylated PPAR gamma increased the transcription of lipogenic genes and fatty acid metabolism that supports breast cancer tumor growth and metastasis.[15,46,47,48,49,50] Thus, we hypothesize that ANGPTL4 and Tip60 may cooperatively alter the acetylome, especially at the promoters of lipogenic genes which will likely to alter the transcription of lipogenic genes or to lead to chromatin remodeling or DNA repair.[51
Conclusions
We report here that ANGPTL4 is a nuclear protein. Nuclear ANGPLT4 may function within chromatin-remodeling complexes or DNA repair machinery. When ANGPTL4 is overexpressed in TNBC, it enriches stem cells, alters the lipid profile of stem cells, and augments TNBC brain and liver metastases.
Financial support and sponsorship
The Bailey Lab and this study are supported by the Arkansas Breast Cancer Research Program, University of Arkansas Chancellor's Grant for Innovation and Collaboration, and the Arkansas Biosciences Institute.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Xie J, Xu Z. A population-based study on liver metastases in women with newly diagnosed breast cancer. Cancer Epidemiol Biomarkers Prev. 2019;28:283–92. doi: 10.1158/1055-9965.EPI-18-0591. [DOI] [PubMed] [Google Scholar]
- 2.Dent R, Hanna WM, Trudeau M, Rawlinson E, Sun P, Narod SA. Pattern of metastatic spread in triple-negative breast cancer. Breast Cancer Res Treat. 2009;115:423–8. doi: 10.1007/s10549-008-0086-2. [DOI] [PubMed] [Google Scholar]
- 3.Tseng LM, Hsu NC, Chen SC, Lu YS, Lin CH, Chang DY, et al. Distant metastasis in triple-negative breast cancer. Neoplasma. 2013;60:290–4. doi: 10.4149/neo_2013_038. [DOI] [PubMed] [Google Scholar]
- 4.Ma R, Feng Y, Lin S, Chen J, Lin H, Liang X, et al. Mechanisms involved in breast cancer liver metastasis. J Transl Med. 2015;13:64. doi: 10.1186/s12967-015-0425-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med. 2010;363:1938–48. doi: 10.1056/NEJMra1001389. [DOI] [PubMed] [Google Scholar]
- 6.Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77. doi: 10.1016/j.cell.2008.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ, Tan CK, et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood. 2011;118:3990–4002. doi: 10.1182/blood-2011-01-328716. [DOI] [PubMed] [Google Scholar]
- 8.Minn AJ, Gupta GP, Siegel PM, Bos PD, Weiping D, Giri DD, et al. Genes that mediate breast cancer metastasis to lung. Nature. 2005;436:518–24. doi: 10.1038/nature03799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minn AJ, Gupta GP, Padua D, Bos P, Nguyen DX, Nuyten D, et al. Lung metastasis genes couple breast tumor size and metastatic spread. Proc Natl Acad Sci U S A. 2007;104:6740–5. doi: 10.1073/pnas.0701138104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459:1005–9. doi: 10.1038/nature08021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gong X, Hou Z, Endsley MP, Gronseth E, Rarick K, Jorns J, et al. Interaction of tumor cells and astrocytes promotes breast cancer brain metastases through TGF-β2/ANGPTL4 axes. NPJ Precis Oncol. 2019;3:24. doi: 10.1038/s41698-019-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Z, Fan C, Livasy C, He X, Oh DS, Ewend MG, et al. A compact VEGF signature associated with distant metastases and poor outcomes. BMC Med. 2009;7:9. doi: 10.1186/1741-7015-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang RD, Fidler IJ, Yano S, Fidler IJ, Fujimaki T, Bucana CD. The seed and soil hypothesis: Vascularization and brain metastases. Lancet Oncol. 2011;3:53–7. doi: 10.1016/s1470-2045(01)00622-2. [DOI] [PubMed] [Google Scholar]
- 14.Shafik NM, Mohamed DA, Bedder AE, El-Gendy AM. Significance of tissue expression and serum levels of angiopoietin-like protein 4 in breast cancer progression: Link to NF-κB/P65 activity and pro-inflammatory cytokines. Asian Pac J Cancer Prev. 2015;16:8579–87. doi: 10.7314/apjcp.2015.16.18.8579. [DOI] [PubMed] [Google Scholar]
- 15.La Paglia L, Listì A, Caruso S, Amodeo V, Passiglia F, Bazan V, et al. Potential role of ANGPTL4 in the cross talk between metabolism and cancer through PPAR signaling pathway. PPAR Res. 2017;2017:8187235. doi: 10.1155/2017/8187235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei X, Shi F, Basu D, Huq A, Routhier S, Day R, et al. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J Biol Chem. 2011;286:15747–56. doi: 10.1074/jbc.M110.217638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bailey TA, Luan H, Tom E, Bielecki T, Mohapatra B, Ahmad G, et al. A kinase inhibitor screen reveals protein kinase C-dependent endocytic recycling of ErbB2 in breast cancer cells. J Biol Chem. 2014;289:30443–58. doi: 10.1074/jbc.M114.608992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Izraely S, Ben-Menachem S, Sagi-Assif O, Meshel T, Marzese DM, Ohe S, et al. ANGPTL4 promotes the progression of cutaneous melanoma to brain metastasis. Oncotarget. 2017;8:75778–96. doi: 10.18632/oncotarget.19018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erin N, Kale S, Tanrıöver G, Köksoy S, Duymuş O, Korcum AF. Differential characteristics of heart, liver, and brain metastatic subsets of murine breast carcinoma. Breast Cancer Res Treat. 2013;139:677–89. doi: 10.1007/s10549-013-2584-0. [DOI] [PubMed] [Google Scholar]
- 20.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100:3983–8. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manuel Iglesias J, Beloqui I, Garcia-Garcia F, Leis O, Vazquez-Martin A, Eguiara A, et al. Mammosphere formation in breast carcinoma cell lines depends upon expression of E-cadherin. PLoS One. 2013;8:e77281. doi: 10.1371/journal.pone.0077281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dontu G, Al-Hajj M, Abdallah WM, Clarke MF, Wicha MS. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grimshaw MJ, Cooper L, Papazisis K, Coleman JA, Bohnenkamp HR, Chiapero-Stanke L, et al. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 2008;10:R52. doi: 10.1186/bcr2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheridan C, Kishimoto H, Fuchs RK, Mehrotra S, Bhat-Nakshatri P, Turner CH, et al. CD44+/CD24- breast cancer cells exhibit enhanced invasive properties: An early step necessary for metastasis. Breast Cancer Res. 2006;8:R59. doi: 10.1186/bcr1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Honeth G, Bendahl PO, Ringnér M, Saal LH, Gruvberger-Saal SK, Lövgren K, et al. The CD44+/CD24- phenotype is enriched in basal-like breast tumors. Breast Cancer Res. 2008;10:R53. doi: 10.1186/bcr2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ricardo S, Vieira AF, Gerhard R, Leitão D, Pinto R, Cameselle-Teijeiro JF, et al. Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J Clin Pathol. 2011;64:937–46. doi: 10.1136/jcp.2011.090456. [DOI] [PubMed] [Google Scholar]
- 27.Giatromanolaki A, Sivridis E, Fiska A, Koukourakis MI. The CD44+/CD24- phenotype relates to 'triple-negative' state and unfavorable prognosis in breast cancer patients. Med Oncol. 2011;28:745–52. doi: 10.1007/s12032-010-9530-3. [DOI] [PubMed] [Google Scholar]
- 28.Guler G, Balci S, Costinean S, Ussakli CH, Irkkan C, Suren D, et al. Stem cell-related markers in primary breast cancers and associated metastatic lesions. Mod Pathol. 2012;25:949–55. doi: 10.1038/modpathol.2012.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okuda H, Xing F, Pandey PR, Sharma S, Watabe M, Pai SK, et al. miR-7 suppresses brain metastasis of breast cancer stem-like cells by modulating KLF4. Cancer Res. 2013;73:1434–44. doi: 10.1158/0008-5472.CAN-12-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abraham BK, Fritz P, McClellan M, Hauptvogel P, Athelogou M, Brauch H. Prevalence of CD44+/CD24-/low cells in breast cancer may not be associated with clinical outcome but may favor distant metastasis. Clin Cancer Res. 2005;11:1154–15. [PubMed] [Google Scholar]
- 31.Wang H, Wang L, Song Y, Wang S, Huang X, Xuan Q, et al. CD44+/CD24- phenotype predicts a poor prognosis in triple-negative breast cancer. Oncol Lett. 2017;14:5890–8. doi: 10.3892/ol.2017.6959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ness C, Garred Ø, Eide NA, Kumar T, Olstad OK, Bærland TP, et al. Multicellular tumor spheroids of human uveal melanoma induce genes associated with anoikis resistance, lipogenesis, and SSXs. Mol Vis. 2017;23:680–94. [PMC free article] [PubMed] [Google Scholar]
- 33.Köster A, Chao YB, Mosior M, Ford A, Gonzalez-DeWhitt PA, Hale JE, et al. Transgenic angiopoietin-like (angptl) 4 overexpression and targeted disruption of angptl4 and angptl3: Regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–50. doi: 10.1210/en.2005-0476. [DOI] [PubMed] [Google Scholar]
- 34.Xu A, Lam MC, Chan KW, Wang Y, Zhang J, Hoo RL, et al. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci U S A. 2005;102:6086–91. doi: 10.1073/pnas.0408452102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Davis AA, Kaklamani VG. Metabolic syndrome and triple-negative breast cancer: A new paradigm. Int J Breast Cancer. 2012;2012:809291. doi: 10.1155/2012/809291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Maiti B, Kundranda MN, Spiro TP, Daw HA. The association of metabolic syndrome with triple-negative breast cancer. Breast Cancer Res Treat. 2010;121:479–83. doi: 10.1007/s10549-009-0591-y. [DOI] [PubMed] [Google Scholar]
- 37.Dietze EC, Chavez TA, Seewaldt VL. Obesity and triple-negative breast cancer: Disparities, controversies, and biology. Am J Pathol. 2018;188:280–90. doi: 10.1016/j.ajpath.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carey L, Perou C, Dressler L, Livsay C, Geradts J, Cowan D, et al. Race and the poor prognosis basal tumor (BBT) phenotype in the population-based Carolina Breast Cancer Study (CBCS) J Clin Oncol. 2004;22:9510. [Google Scholar]
- 39.Grootaert C, Van de Wiele T, Verstraete W, Bracke M, Vanhoecke B. Angiopoietin-like protein 4: Health effects, modulating agents and structure-function relationships. Expert Rev Proteomics. 2012;9:181–99. doi: 10.1586/epr.12.12. [DOI] [PubMed] [Google Scholar]
- 40.Zhu P, Goh YY, Chin HF, Kersten S, Tan NS. Angiopoietin-like 4: A decade of research. Biosci Rep. 2012;32:211–9. doi: 10.1042/BSR20110102. [DOI] [PubMed] [Google Scholar]
- 41.Tan MJ, Teo Z, Sng MK, Zhu P, Tan NS. Emerging roles of angiopoietin-like 4 in human cancer. Mol Cancer Res. 2012;10:677–88. doi: 10.1158/1541-7786.MCR-11-0519. [DOI] [PubMed] [Google Scholar]
- 42.Liang M, Mulholland DJ. Lipogenic metabolism: A viable target for prostate cancer treatment? Asian J Androl. 2014;16:661–3. doi: 10.4103/1008-682X.132947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Idrissou M, Judes G, Daures M, Sanchez A, El Ouardi D, Besse S, et al. TIP60 Inhibitor TH1834 reduces breast cancer progression in xenografts in mice. OMICS. 2019;23:457–9. doi: 10.1089/omi.2019.0126. [DOI] [PubMed] [Google Scholar]
- 44.Senapati P, Kato H, Lee M, Leung A, Thai C, Sanchez A, et al. Hyperinsulinemia promotes aberrant histone acetylation in triple-negative breast cancer. Epigenetics Chromatin. 2019;12:44. doi: 10.1186/s13072-019-0290-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koliwad SK, Kuo T, Shipp LE, Gray NE, Backhed F, So AY, et al. Angiopoietin-like 4 (ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J Biol Chem. 2009;284:25593–601. doi: 10.1074/jbc.M109.025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tian L, Zhou J, Casimiro MC, Liang B, Ojeifo JO, Wang M, et al. Activating peroxisome proliferator-activated receptor gamma mutant promotes tumor growth in vivo by enhancing angiogenesis. Cancer Res. 2009;69:9236–44. doi: 10.1158/0008-5472.CAN-09-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Z, Kang Y. Lipid metabolism fuels cancer's spread. Cell Metab. 2017;25:228–30. doi: 10.1016/j.cmet.2017.01.016. [DOI] [PubMed] [Google Scholar]
- 48.Inoue M, Takahashi Y, Fujii T, Kitagawa M, Fukusato T. Significance of downregulation of liver fatty acid-binding protein in hepatocellular carcinoma. World J Gastroenterol. 2014;20:17541–51. doi: 10.3748/wjg.v20.i46.17541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van Beekum O, Brenkman AB, Grøntved L, Hamers N, van den Broek NJ, Berger R, et al. The adipogenic acetyltransferase Tip60 targets activation function 1 of peroxisome proliferator-activated receptor gamma. Endocrinology. 2008;149:1840–9. doi: 10.1210/en.2007-0977. [DOI] [PubMed] [Google Scholar]
- 50.Gupta G, Singhvi G, Chellappan DK, Sharma S, Mishra A, Dahiya R, et al. Peroxisome proliferator-activated receptor gamma: Promising target in glioblastoma. Panminerva Med. 2018;60:109–16. doi: 10.23736/S0031-0808.18.03462-6. [DOI] [PubMed] [Google Scholar]
- 51.Kim SA, Chatterjee N, Jennings MJ, Bartholomew B, Tan S. Extranucleosomal DNA enhances the activity of the LSD1/CoREST histone demethylase complex. Nucleic Acids Res. 2015;43:4868–80. doi: 10.1093/nar/gkv388. [DOI] [PMC free article] [PubMed] [Google Scholar]


