Abstract
Background and purpose:
Methamphetamine (METH) abuse has devastating consequences on the nervous system. There are limited therapy choices in METH abuse with reduced effectiveness and elevated recurrence rates. Thymoquinone (TQ), the most bioactive constituent of Nigella sativa seeds exerts neuroprotective effects mainly via antioxidant properties. This study aimed to evaluate the effect of TQ against METH-induced striatal neurotoxicity and hyperlocomotor activity in mice.
Experimental approach:
Our groups of animals received METH (10 mg/kg) four times a day with 2 h intervals. Normal saline or TQ (5, 10, or 20 mg/kg) was injected intraperitoneally 30 min before METH administration. Control and sham groups received vehicle or TQ, respectively. The rectal temperature and behavioral tests including the open field for locomotor activity and rotarod for motor coordination were evaluated. The level of superoxide dismutase (SOD), as well as pathological changes, were also assessed in the striatum region.
Findings/Results:
No significant differences in rectal temperatures were observed among treated groups. Administration of METH increased locomotor activity and did not change motor coordination. TQ co-administration with METH significantly reduced the central and total locomotion and the mean latency to fall off the rotarod in a dose-dependent manner compared with the METH group. TQ also alleviated the METH-induced decrease in the activity of SOD.TQ, especially at the high dose, reduced the METH-induced reactive gliosis level.
Conclusion and implications:
In conclusion, TQ prevents the enhanced locomotor activity, antioxidant impairment, and morphological striatal damage caused by METH in mice. TQ may be a potential candidate for the treatment of specific METH-induced brain disorders or neurological diseases.
Keywords: Adverse drug effects, Methamphetamine, Nervous system, Nigella sativa, Substance abuse, Thymoquinone
INTRODUCTION
Methamphetamine (METH), a derivative product of amphetamine with a similar stimulant effect on the central nervous system (CNS), is the second most widely used illicit drug worldwide after cannabis (1,2).
METH has a long history of use going back to the date of World War 2 when it was used by soldiers to reduce their fatigue and after that prescribed to treat a wide range of disorders such as narcolepsy, depression, obesity, alcoholism, and attention deficit hyperactivity disorder (ADHD) (3). METH abuse is rising all over the world due to reasons such as easy access to the necessary precursor chemicals like pseudoephedrine, uncomplicated synthesis, long-lasting effects (10-12 h), and low-cost price, which is about 75% cheaper in comparison with cocaine (4).
METH abuse is followed by severe, devastating consequences on individual health, specifically the nervous system. Therefore, it has been presented as an important public health issue around the world (5). Researches have shown that METH affects almost every organ and system in the body. It changes brain structure, metabolism, and neurotransmitter levels and results in various neurological problems and psychiatric disorders (6,7). Brain-imaging studies indicated structure abnormalities in METH abusers and prenatally METH-exposed children, especially in their striatum region (8).
Up to now, there are limited therapy choices for METH abusers with reduced effectiveness and elevated recurrence rates. Special consideration has been given to METH abuse all over the world, and although scientists are searching for the best treatment, there is no specific drug to reduce this substantial global issue. Most of the time, acute METH intoxication is controlled via supportive interventions (9).
N. sativa, also called black seed or black cumin has been used for the treatment of various diseases and conditions including toothache, flatulence, asthma, cough, inflammation, fever, hypertension, diabetes, obesity, back pain, headache, dizziness, and gastrointestinal disturbances. The most pharmacological properties of the N. sativa seeds are mainly attributed to thymoquinone (TQ). TQ possesses several beneficial effects including anti-inflammatory, analgesic, anticonvulsion, immunomodulatory, anticancer activities, and antioxidant activity. TQ has been shown to induce significant neuroprotection against beta-amyloid-, acrylamide-, and sodium arsenate-induced neurotoxicity (10,11). It was documented that the N. sativa extract can markedly decrease the cellularity of the hippocampus in mice that were treated with METH at the dose of 10 mg/kg (12). TQ has been suggested as a promising candidate for the treatment of amphetamine abuse through the interaction with the dopaminergic system. N. sativa and its derivatives effectively reduced the adverse health effects associated with opioids abuse such as withdrawal symptoms, dependency, and tolerance (13). In all studies, the antioxidant properties of TQ had an apparent role in its neuroprotective effects.
According to the previous studies, we aimed to investigate the effect of TQ against METH-induced striatal injury in the mouse brain.
MATERIALS AND METHODS
Chemicals
METH was donated by the Department of Medicinal Chemistry, School of Pharmacy, Mashhad University of Medical Sciences, and TQ was obtained from Sigma-Aldrich (Germany, Cat. no. 274666).
Animal treatments
Thirty-six male mice weighing 25-35 g, were provided from the Animals Research Center, Mashhad University of Medical Sciences, Mashhad, Iran, maintained at a controlled temperature (23 ± 2 °C), 12/12-h light/dark cycle, and allowed free access to food and water. Animals were divided into six groups, 6 each, in separated cages. All experimental procedures were conducted according to the ethical standard protocols approved by the Animal Experiments Committee of Mashhad University of Medical Sciences, Iran (Ethics No. IR.MUMS.PHARMACY.REC.1397.088).
Four groups of animals received METH at 10 mg/kg four times a day with 2 h intervals. They also received TQ (5, 10, and 20 mg/kg) or vehicle (10 mL/kg) 30 min before each METH injection. In the control group, animals received normal saline plus dimethyl sulfoxide (as TQ solvent); and in the sham group, normal saline plus dimethyl sulfoxide and TQ (20 mg/kg). All injections were done intraperitoneally. The behavioral tests were conducted on the day after the last treatment.
Body temperature
The rectal temperature was measured 30 min before the first METH injection and 30 min after each METH administration.
Behavioral studies
Rotarod test
The rotarod test is generally applied to assess motor coordination in rodents. In brief, mice were scheduled to remain for 5 min on the rod for three constitutive days. On test day, animals were placed on the rod rotating with a maximum time of 300 s and a speed of 20 rpm. Then, the time that each animal stayed on the rod (latency) was measured (14).
Open field test
The open-field test is carried out to measure behavioral responses including locomotor activity, hyperactivity, and exploratory behaviors. Briefly, each mouse was placed in the center of a cage made of white wood (45 × 45 × 45 cm) and the movement was recorded with a digital camera for 10 min. Motor activity was evaluated by measuring the number of total and central square crossings. The two measures are referred to as central and total locomotion (CL and TL) (15).
Superoxide dismutase activity
The enzyme activity of superoxide dismutase activity (SOD) was measured in 5-10 μg of striatum protein with a commercial kit (706002, Ann Arbor, MI, USA). Briefly, the tetrazolium salt is used to quantify the superoxide radicals created from xanthine oxidase and hypoxanthine. One unit of SOD was defined as the amount of enzyme catalyzing 50% of the superoxide radicals into ordinary oxygen molecules. The standard curve was obtained using quality-controlled SOD standards.
Reduced glutathione assay
In brief, the tissue homogenate was mixed with 10% tricolor acetic acid and vortexed. After centrifugation, the supernatant was mixed with reaction mixtures including 500 μL 5,5′ di thiobis- (2-nitrobenzoic acid) indicator (DTNB) and 2 mL phosphate buffer (pH 8). Within 10 min, the absorbance was recorded at 412 nm using a spectrophotometer (Jenway 6105 UV/Vis, UK). Commercially available reduced glutathione (GSH) was used to calculate the standard curve. GSH levels were reported as nmol/g tissue.
Histopathological examination
On the third day following drug administration, the animal’s brain was removed after euthanasia and fixed by immersion in 10% formalin. At the next step, the tissues were embedded into paraffin blocks and were cut at a thickness of 5 μm. After that, they were stained with hematoxylin and eosin (H&E). A pathologist who was blind to the groups evaluated the striatum section for the level of neuronal damage.
Statistical analysis
All statistical analyses were performed using SPSS (Version 16.0. Chicago, SPSS Inc) and reported as mean ± SEM and analyzed using ANOVA, followed by Tukey’s test; for non-parametric data, Kruskal Wallis followed by Dunn’s test was used for data analysis. Statistically, the significant level was considered at the P < 0.05.
RESULTS
Body temperature
There was no significant difference between rectal temperatures of various treated groups before and after treatments (data not shown).
Motor coordination (rotarod test)
METH injections did not change the latency to fall in comparison with the respective control group (the animals did not fall off by 300 s). TQ administration to the sham group, at 20 mg/kg, significantly reduced the remaining time of animals on the rotating rod compared to the control group (P < 0.001). The mean latency to fall off the rotarod in groups receiving METH plus TQ, at 10 and 20 mg/kg, was significantly less than the METH-treated group (P < 0.001). There were no significant differences between METH + TQ (5 mg/kg) and the control group (Fig. 1).
Fig. 1.
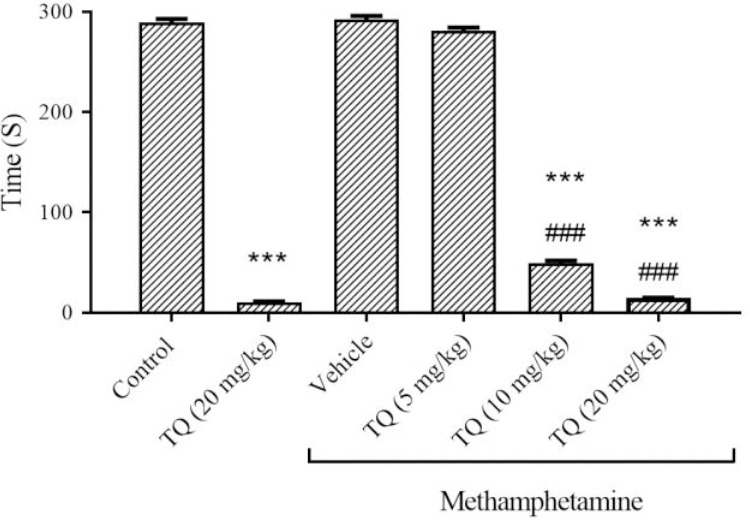
The effect of methamphetamine and TQ on motor coordination of mice in the rotarod test. Mice received intraperitoneal methamphetamine (10 mg/kg) four times a day in 2-h intervals and TQ at 5, 10, and 20 mg/kg, 30 min before each methamphetamine injection. The results are presented as mean ± SEM, n = 6. ***P < 0.001 Indicates significant difference compared to the control group; and ###P < 0.001 versus the vehicle + methamphetamine group. TQ, Thymoquinone.
Locomotor activity (open field test)
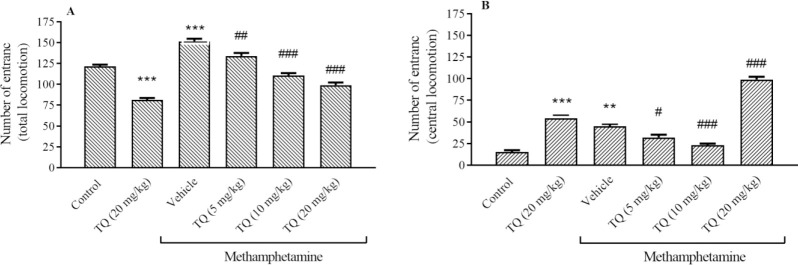
METH increased the open field test factors, TL and CL activities in comparison with the control group (P < 0.001 and P < 0.01, respectively). However, the mice exposed to the TQ crossed the total squares significantly less than the METH group (Fig. 2A). TQ administration at 5 and 10 mg/kg also could decrease the CL activities compare to METH group. Although, a high dose of TQ could not decrease the number of the entrance to the central square in comparison with METH (Fig. 2B).
Fig. 2.
Effect of methamphetamine and TQ on the (A) total and (B) central locomotion in the open field test. Mice received intraperitoneal methamphetamine (10 mg/kg) four times a day in 2-h intervals and TQ at 5, 10, and 20 mg/kg, 30 min before each METH injection. The results are presented as mean ± SEM, n = 6. **P < 0.01 and ***P < 0.001 indicate significant differences compared to the control group; and #P < 0.05, ##P < 0.01, and ###P < 0.001 against the vehicle + methamphetamine group. The median of groups was analyzed by Kruskal-Wa Wallis followed by the Dunn test. TQ, Thymoquinone.
Determination SOD activity in the striatum
METH was able to significantly decrease the SOD activity in the striatum region compared to the control group (P < 0.001). The level of SOD was increased in the group receiving METH plus TQ at 10 and 20 mg/kg in comparison with METH + vehicle-treated animals (P < 0.05 and P < 0.01, respectively). The higher dose of TQ reversed the METH-induced decrease in SOD activity to normal (Fig. 3).
Fig. 3.
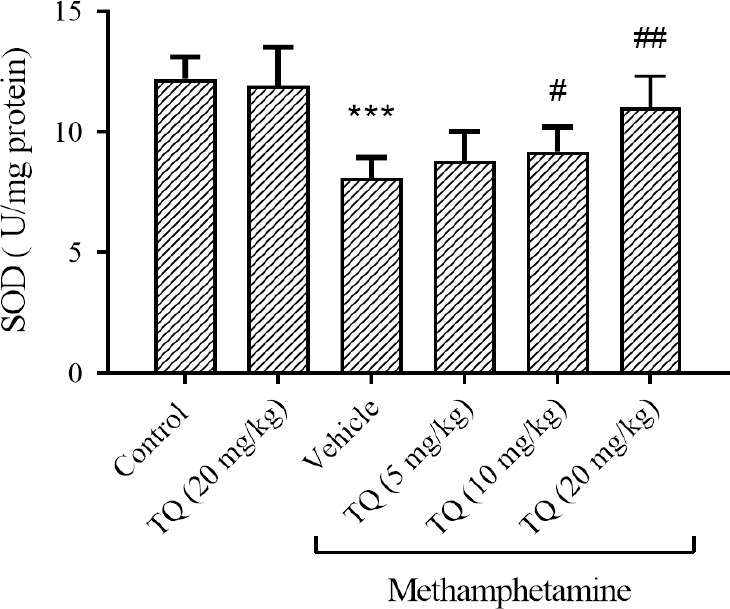
Effect of methamphetamine and TQ on SOD enzyme activity in the striatum region. Mice received intraperitoneal methamphetamine (10 mg/kg) four times a day in 2-h intervals and TQ at 5, 10, and 20 mg/kg, 30 min before each methamphetamine injection. The results are presented as mean ± SEM, n = 3. ***P < 0.001 Indicates significant differences compared to the control group; and #P < 0.05 and ###P < 0.001 against the vehicle + methamphetamine group. TQ, Thymoquinone, SOD, superoxide dismutase.
Determination of GSH in the striatum
The GSH content was significantly reduced by METH in comparison with the control group (P < 0.001). Simultaneous usage of TQ at different doses could improve the GSH content compared to the group that received METH. Administration of TQ (20 mg/kg) alone did not change the GSH level when compared to the control group (Fig. 4).
Fig. 4.
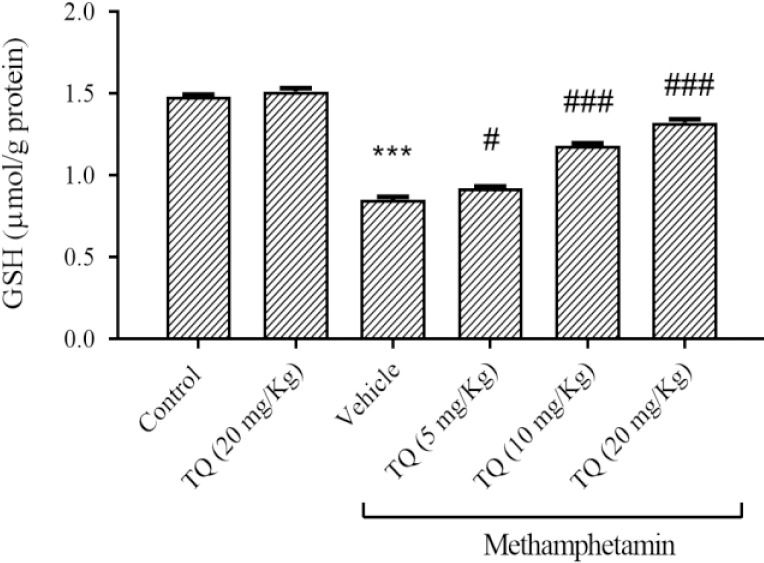
Effect of methamphetamine and TQ on GSH content in the striatum region. Mice received intraperitoneal methamphetamine (10 mg/kg) four times a day in 2-h intervals and TQ at 5, 10, and 20 mg/kg, 30 min before each methamphetamine injection. The results are presented as mean ± SEM, n = 6. ***P < 0.001 Indicates significant differences compared to the control group; and #P < 0.05 and ###P < 0.001 against the vehicle + methamphetamine group. GSH, Reduced glutathione; TQ, thymoquinone.
Histopathological examination
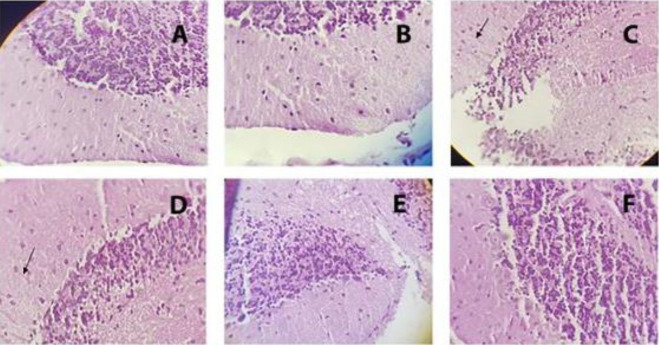
Examination of mice brain striatum following METH exposure showed marked gliosis in the striatum. TQ at 10 and 20 mg/kg reduced the METH-induced reactive gliosis response (moderate to mild gliosis). No pathological finding was observed in the control and sham groups (Fig. 5).
Fig. 5.
Light photomicrograph of hematoxylin and eosin-stained sections of the mice striatum that were exposed to different doses of METH and TQ (original magnification 400×). Mice received intraperitoneal METH (10 mg/kg) four times a day in 2-h intervals and TQ at 5, 10, and 20 mg/kg, 30 min before each METH injection. Arrows indicate Rosenthal fibers. (A) Control group, no gliosis; (B) TQ at 20 mg/kg, no gliosis; (C) METH, high grad gliosis; (D) METH + TQ 5 mg/kg, high grad gliosis; (E) METH + TQ 10 mg/kg, moderate gliosis; (F) METH + TQ 20 mg/kg, mild gliosis. METH, Methamphetamine; TQ, thymoquinone
DISCUSSION
The present study evaluated the effects of TQ in METH-induced striatal neurotoxicity and hyperlocomotor activity in mice. METH is an addictive stimulant that affects monoamine concentrations especially dopamine, in the synaptic cleft and leading to long-term neurotoxicity in a variety of species including rodents, nonhuman primates, and humans. The predominant alteration in dopaminergic neurotransmission occurs in the striatum. Repeated METH administration can cause deficits in memory, executive, and motor function (16).
Depending on the dosage and duration, METH administration can cause different open field test results. In this study, METH caused a significant increase in locomotor activity after four times injections. Crossing the central squares by METH-treated mice more than the control group implied that had an anxiety-like behavior. It has been reported that METH-induced behavioral sensitization occurs after repeated administration in rodents (17). In line with our results, Struntz and Siegel reported increased locomotor activity and anxiety-like behavior in adult mice that were treated with a single low dose METH, 4 mg/kg (18). However, in our previous work, animals treated with a single high dose of METH (45 mg/kg), lost the ability to cross the lines of open field (15).
The result of this study showed no motor coordination deficits were observed in the mice that received METH. It maybe shows that the animals were hyperactive at the time of evaluation and if the cutoff point or speed could be increased, the results would change and expressed this increased activity.
In our study, the pathological findings also revealed marked gliosis in the striatum. METH exposure, especially at high doses, damaged primarily dopamine-rich striatal neurons in the brain and resulted in nerve terminal degeneration, apoptosis, and gliosis (19).
In our previous study, a high dose of METH-induced hyperthermia in rats (15). However, in this study, METH did not affect the core body temperature of mice. The effect of METH on body temperature, via both central and peripheral nervous systems may be influenced by several factors including strain, genetic differences, ambient temperature, dose, and the method of temperature measurement (20).
For decades, multiple molecular mechanisms have been reported as responsible for the neurotoxic effects of METH including oxidative stress and apoptosis (21,22). In the present study, the activity of SOD, as an indicator of antioxidant was measured. The results indicated that METH significantly decreased the SOD activity in the striatum that is in agreement with the previous studies. Evaluation of the effect of METH-induced toxicity in copper/zinc-SOD transgenic mice showed the key role of oxygen-based radicals, as well as antioxidant enzymes such as SOD, in the negative consequences of the drug (23). It was reported that pretreatment with SOD inhibitors enhanced the METH-induced toxicity in the striatum and depleted stores of dopamine and serotonin. On the other hand, pretreatment with antioxidants or over-expression of SOD attenuated the striatal neurotoxicity (5,24,25). METH administration also decreased the GSH content, while it considerably increased by TQ up to the concentration of 1.32 μmol. GSH, a non-enzymatic antioxidant defends the cell against various forms of oxidative injury. It is well documented that METH selectively alters the brain, especially the striatum, GSH antioxidant system (5,22,26). The reduced GSH level in the current study was restored by TQ treatment. TQ has been extremely studied for its antioxidant activities. TQ exerts its antioxidant properties through the inhibition of lipid peroxidation, myeloperoxidase, lipid peroxidation, reactive oxygen species, and nitric oxide levels. Improvement of catalase (CAT) and SOD activity and GSH level is also considered an important TQ-neuroprotective mechanism (27). These studies indicated the beneficial effects of TQ against METH-induced neurotoxicity. Numerous studies have suggested TQ as a useful therapy in the prevention of many diseases and conditions such as depression, epilepsy, Parkinson’s and Alzheimer’s, ischemia, brain injury, encephalomyelitis, anxiety, and brain cancers (28). In 2018, TQ was proposed as a good candidate for the treatment of amphetamine abuse via its interaction with dopamine neurotransmission (29). Our findings indicated that TQ markedly reduced oxidative stress and locomotor activity and reversed brain changes in the striatum associated with METH exposure. Previous studies have reported this natural product with a strong sedative effect. It has been proposed that TQ has an affinity to GABAA receptors and enhances the inhibitory actions of GABA. So, it can cause muscular relaxation, motor incoordination, and a reduction in locomotor activity. On the other hand, GABAergic, as well as dopaminergic systems play important roles in amphetamine-type stimulant use disorders. Therefore, TQ may exert its beneficial effects via activation of this pathway (30).
Evaluation of the effect of TQ on chemical and electrical stimulation of skeletal muscle revealed significant inhibition of contractile responses on the skeletal muscle and reduction in maintenance time of animals in rotarod test (31). In the last decade, the neuroprotective effects of TQ have attracted much more attention and it was mainly attributed to the antioxidant and anti-inflammatory effects (28). TQ impacts oxidative stress by increasing antioxidant capacities, including CAT, GSH S-transferases, SOD, GSH, and by decreasing lipid oxidation in the brain tissue (28). In the present study, TQ significantly increased the SOD activity in mice that were exposed to METH. The SOD is considered to be the front line of antioxidant defense since it converts superoxide radicals to molecular oxygen and hydrogen peroxide that were subsequently decomposed by CAT into H2O. Our data are in agreement with the previous findings that showed the neuroprotective effect of TQ on cerebral ischemia-reperfusion injury by decreasing the neuronal cell death and malondialdehyde level as well as increasing the GSH, CAT, and SOD activities (32). TQ also exhibited neuroprotection against 6-hydroxydopamine-induced experimental Parkinson disease. TQ significantly reduced malondialdehyde and elevated-SOD level in midbrain and attenuated substantia nigra pars compacta neuronal loss and resulted in improved 6-hydroxydopamine-induced behavioral abnormalities (33). According to our results, TQ particularly at a high dose was able to abolish the METH-induced gliosis in the mice striatum. Gliosis refers to the reactive change of glial cells in response to CNS damage and is identified by the expression of glial-specific markers and morphological changes. In a rat model of Alzheimer’s disease, treatment with TQ decreased plaque formation in the hippocampal CA1 region (34). TQ also abolished the vascular and neuronal degeneration, microglial reaction, demyelination of the axon, and neuronophagia in the rat hippocampus and frontal cortex that were exposed to lead (Pb) through drinking water (10).
CONCLUSION
TQ may prevent the enhanced locomotor activity, oxidative impairment, and morphological striatal damage caused by METH. TQ may be a potential candidate for the treatment of specific METH-induced brain disorders or neurological diseases. Although complementary experimental studies including the evaluation of METH and TQ effects on brain structure and function are needed to confirm these effects.
Conflict of interest statement
The authors declared no conflicts of interest in this study.
Authors’ contributions
L. Etemad and A. Roohbakhsh conceived the original; A. Salehi Kakhki, M. Iranshahy, and F. Amin performed the experiments; L. Etemad, A. Roohbakhsh, and M. Moshiri supervised the research; L. Etemad and M. Moshiri analyzed the data; A. Salehi Kakhki, M. Iranshahy, and F. Amin prepared the original draft of the manuscript; L. Etemad, A. Roohbakhsh reviewed and edited the manuscript. All authors have read and approved the final version of the article.
Acknowledgments
The study was a Pharm.D. thesis, submitted by Azam Salehi Kakhki, which was financially supported by the Research Council of Mashhad University of Medical Sciences under Grant No. 970899.
REFERENCES
- 1.Rahmati M, Eskandari MR. Cytotoxic effects of methamphetamine in rat hepatocytes. Res Pharm Sci. 2012;7(5):S186. [Google Scholar]
- 2.Bananej A, Völkl-Kernstock S, Lesch O, Walter H, Skala K. No evidence of subgroups found in amphetamine consumers in Iran. Neuropsychiatr. 2018;32(2):69–74. doi: 10.1007/s40211-018-0259-0. DOI: 10.1007/s40211-018-0259-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prakash MD, Tangalakis K, Antonipillai J, Stojanovska L, Nurgali K, Apostolopoulos V. Methamphetamine: effects on the brain, gut and immune system. Pharmacol Res. 2017;120:60–67. doi: 10.1016/j.phrs.2017.03.009. DOI: 10.1016/j.phrs.2017.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Skowronska M, McDonald M, Velichkovska M, Leda AR, Park M, Toborek M. Methamphetamine increases HIV infectivity in neural progenitor cells. J Biol Chem. 2018;293(1):296–311. doi: 10.1074/jbc.RA117.000795. DOI: 10.1074/jbc.RA117.000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moshiri M, Roohbakhsh A, Talebi M, Iranshahy M, Etemad L. Role of natural products in mitigation of toxic effects of methamphetamine: a review of in vitro and in vivo studies. Avicenna J Phytomed. 2020;10(4):334–351. [PMC free article] [PubMed] [Google Scholar]
- 6.Karila L, Petit A, Cottencin O, Reynaud M. Methamphetamine dependence: consequences and complications. Presse Med. 2010;39(12):1246–1253. doi: 10.1016/j.lpm.2010.09.003. DOI: 10.1016/j.lpm.2010.09.003. [DOI] [PubMed] [Google Scholar]
- 7.Moshiri M, Rahimi P, Etemad L. Hunting meth mite by cigarette light: a case study. IJMTFM. 2020;10(1):25478,1–2. DOI: 10.32598/ijmtfm.v10i1.25478. [Google Scholar]
- 8.Chang L, Alicata D, Ernst T, Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102(Suppl 1):16–32. doi: 10.1111/j.1360-0443.2006.01782.x. DOI: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 9.Volz TJ, Hanson GR, Fleckenstein AE. The role of the plasmalemmal dopamine and vesicular monoamine transporters in methamphetamine-induced dopaminergic deficits. J Neurochem. 2007;101(4):883–888. doi: 10.1111/j.1471-4159.2006.04419.x. DOI: 10.1111/j.1471-4159.2006.04419.x. [DOI] [PubMed] [Google Scholar]
- 10.Jakaria M, Cho DY, Ezazul Haque M, Karthivashan G, Kim IS, Ganesan P, et al. Neuropharmacological potential and delivery prospects of thymoquinone for neurological disorders. Oxid Med Cell Longev. 2018;2018:1209801,1–17. doi: 10.1155/2018/1209801. DOI: 10.1155/2018/1209801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nili-Ahmadabadi A, Alibolandi P, Ranjbar A, Mousavi L, Nili-Ahmadabadi H, Larki-Harchegani A, et al. Thymoquinone attenuates hepatotoxicity and oxidative damage caused by diazinon: an in vivo study. Res Pharm Sci. 2018;13(6):500–508. doi: 10.4103/1735-5362.245962. DOI: 10.4103/1735-5362.245962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajpar F, Memon S, Goswami P, Rajpar FA. Neuroprotective role of Nigella sativa on methamphetamine induced hippocampal injury in male albino mice. J Liaquat Uni Med Health Sci. 2019;18(2):136–141. [Google Scholar]
- 13.Mohd Adnan LH, Abu Bakar NH, Simbak N, Mohamad N, Ismail R, Ahmad NZ, et al. Thymoquinone: from Nigella sativa to a protective pharmacological compound in managing opioid dependence and amphetamine type stimulant issues. Iran J Basic Med Sci. 2020;23(7):849–852. doi: 10.22038/ijbms.2020.41678.9841. DOI: 10.22038/ijbms.2020.41678.9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hosseini-Sharifabad A, Naghibzadeh S, Hajhashemi V. The effect of lead, restraint stress or their co-exposure on the movement disorders incidence in male mice. Res Pharm Sci. 2019;14(4):343–350. doi: 10.4103/1735-5362.263558. DOI: 10.4103/1735-5362.263558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghadiri A, Etemad L, Moshiri M, Moallem SA, Jafarian AH, Hadizadeh F, et al. Exploring the effect of intravenous lipid emulsion in acute methamphetamine toxicity. Iran J Basic Med Sci. 2017;20(2):138–144. doi: 10.22038/ijbms.2017.8236. DOI: 10.22038/ijbms.2017.8236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Granado N, Ares-Santos S, O’Shea E, Vicario-Abejon C, Colado MI, Moratalla R. Selective vulnerability in striosomes and in the nigrostriatal dopaminergic pathway after methamphetamine administration: early loss of TH in striosomes after methamphetamine. Neurotox Res. 2010;18(1):48–58. doi: 10.1007/s12640-009-9106-1. DOI: 10.1007/s12640-009-9106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuo CC, Shen H, Harvey BK, Yu SJ, Kopajtic T, Hinkle JJ, et al. Differential modulation of sensitization by overexpression of Mu opioid receptors in nucleus accumbens and ventral tegmental area. Psychopharmacology (Berl) 2016;233(4):661–672. doi: 10.1007/s00213-015-4134-4. DOI: 10.1007/s00213-015-4134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Struntz KH, Siegel JA. Effects of methamphetamine exposure on anxiety-like behavior in the open field test, corticosterone, and hippocampal tyrosine hydroxylase in adolescent and adult mice. Behav Brain Res. 2018;348:211–218. doi: 10.1016/j.bbr.2018.04.019. DOI: 10.1016/j.bbr.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 19.Granado N, Ares-Santos S, Moratalla R. Methamphetamine and Parkinson's disease. Parkinsons Dis. 2013;2013:308052,1–10. doi: 10.1155/2013/308052. DOI: 10.1155/2013/308052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sabol KE, Yancey DM, Speaker HA, Mitchell SL. Methamphetamine and core temperature in the rat: ambient temperature, dose, and the effect of a D2 receptor blocker. Psychopharmacology (Berl) 2013;228(4):551–561. doi: 10.1007/s00213-013-3059-z. DOI: 10.1007/s00213-013-3059-z. [DOI] [PubMed] [Google Scholar]
- 21.Yu S, Zhu L, Shen Q, Bai X, Di X. Recent advances in methamphetamine neurotoxicity mechanisms and its molecular pathophysiology. Behav Neurol. 2015;2015:103969,1–11. doi: 10.1155/2015/103969. DOI: 10.1155/2015/103969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moshiri M, Hosseiniyan SM, Moallem SA, Hadizadeh F, Jafarian AH, Ghadiri A, et al. The effects of vitamin B12 on the brain damages caused by methamphetamine in mice. Iran J Basic Med Sci. 2018;21(4):434–438. doi: 10.22038/IJBMS.2018.23362.5897. DOI: 10.22038/IJBMS.2018.23362.5897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asanuma M, Miyazaki I, Higashi Y, Cadet JL, Ogawa N. Methamphetamine-induced increase in striatal p53 DNA-binding activity is attenuated in Cu,Zn-superoxide dismutase transgenic mice. Neurosci Lett. 2002;325(3):191–194. doi: 10.1016/s0304-3940(02)00291-4. DOI: 10.1016/s0304-3940(02)00291-4. [DOI] [PubMed] [Google Scholar]
- 24.Saeed M, Ghadiri A, Hadizadeh F, Attaranzadeh A, Alavi MS, Etemad L. Cinnamaldehyde improves methamphetamine-induced spatial learning and memory deficits and restores ERK signaling in the rat prefrontal cortex. Iran J Basic Med Sci. 2018;21(12):1316–1321. doi: 10.22038/IJBMS.2018.35368.8427. DOI: 10.22038/IJBMS.2018.35368.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maragos WF, Jakel R, Chesnut D, Pocernich CB, Butterfield DA, St Clair D, et al. Methamphetamine toxicity is attenuated in mice that overexpress human manganese superoxide dismutase. Brain Res. 2000;878(1-2):218–222. doi: 10.1016/s0006-8993(00)02707-4. DOI: 10.1016/s0006-8993(00)02707-4. [DOI] [PubMed] [Google Scholar]
- 26.Harold C, Wallace T, Friedman R, Gudelsky G, Yamamoto B. Methamphetamine selectively alters brain glutathione. Eur J Pharmacol. 2000;400(1):99–102. doi: 10.1016/s0014-2999(00)00392-7. DOI: 10.1016/s0014-2999(00)00392-7. [DOI] [PubMed] [Google Scholar]
- 27.Khader M, Eckl PM. Thymoquinone: an emerging natural drug with a wide range of medical applications. Iran J Basic Med Sci. 2014;17(12):950–957. [PMC free article] [PubMed] [Google Scholar]
- 28.Farkhondeh T, Samarghandian S, Shahri AMP, Samini F. The neuroprotective effects of thymoquinone: a review. Dose Response. 2018;16(2):1559325818761455,1–11. doi: 10.1177/1559325818761455. DOI: 10.1177/1559325818761455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Md Fauzi NFA, Abu Bakar NH, Mohamad N, Che Mat K, Syed Omar SH, Othman MS, et al. Potential therapeutic effects of thymoquinone on treatment of amphetamine abuse. Asian Pac J Trop Biomed. 2018;8(3):187–188. DOI: 10.4103/2221-1691.228001. [Google Scholar]
- 30.Jiao D, Liu Y, Li X, Liu J, Zhao M. The role of the GABA system in amphetamine-type stimulant use disorders. Front Cell Neurosci. 2015;9:162–174. doi: 10.3389/fncel.2015.00162. DOI: 10.3389/fncel.2015.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parvardeh S, Moghimi M. Skeletal muscle relaxant effects of thymoquinone, the major constituent of Nigella sativa. J Med Plants. 2015;14(54):122–133. [Google Scholar]
- 32.Hosseinzadeh H, Parvardeh S, Nasiri Asl M, Sadeghnia HR, Ziaee T. Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine. 2007;14(9):621–627. doi: 10.1016/j.phymed.2006.12.005. DOI: 10.1016/j.phymed.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Sedaghat R, Roghani M, Khalili M. Neuroprotective effect of thymoquinone, the Nigella sativa bioactive compound, in 6-hydroxydopamine-induced hemi-parkinsonian rat model. Iran J Pharm Res. 2014;13(1):227–234. [PMC free article] [PubMed] [Google Scholar]
- 34.Poorgholam P, Yaghmaei P, Hajebrahimi Z. Thymoquinone recovers learning function in a rat model of Alzheimer’s disease. Avicenna J Phytomed. 2018;8(3):188–197. [PMC free article] [PubMed] [Google Scholar]