Abstract
Background and purpose:
Microemulsions are gaining an increased interest in transdermal drug delivery. Microemulsions are stable, easy to prepare, and provide high solubilizing capacity for various drugs. The aim of this work was to prepare microemulsions from jojoba oil for transdermal delivery of ketorolac and lidocaine HCl with improved permeation.
Experimental approach:
Microemulsions based on jojoba oil as the oil phase were formulated for transdermal delivery of lidocaine HCl and ketorolac. Brij 97 was selected as surfactant and hexanol as cosurfactant. Pseudoternary phase diagrams were constructed. Selected microemulsion formulations were characterized for their physical properties and in vitro drug permeation.
Findings/Results:
Water-in-oil microemulsions were obtained with droplet sizes not more than 220 nm. The viscosity of the microemulsions was linked to the viscosity of the surfactant used. Improved drug permeation rates were observed for both model drugs. The significant increase in permeation rate in presence of hexanol was due to its impact on skin integrity as indicated by the histopathological study. Drug permeation enhancements were caused by the surfactant, the cosurfactant used, jojoba oil itself, and the microemulsion formulation. Higher surfactant content showed lower lag times and better flux.
Conclusion and implications:
Jojoba oil microemulsions are considered promising vehicles for transdermal delivery of ketorolac and lidocaine HCl with improved drug permeation. Jojoba oil-based microemulsion would present a safe and effective means for delivering drugs through the skin.
Keywords: Jojoba oil, Ketorolac, Lidocaine HCl, Microemulsion, Transdermal delivery
INTRODUCTION
Microemulsions have gained wide interest in pharmaceutical formulations as potential drug delivery systems because of their stability, ease of preparation, low viscosity, and high solubilizing capacity for various drugs including hydrophilic and lipophilic molecules. They also may enhance drug stability, prevent irritation caused by some drugs, and may prolong their release (1,2). Microemulsions are composed of mixtures of oil, water, surfactant, and cosurfactant. Cosurfactants are frequently added in order to further decrease the surface tension and thus reduce the required surfactant concentration in the final microemulsion formulation, which is known to be toxic in high levels (3).
Recently, there has been increased attention towards the development of transdermal dosage systems due to the advantages they have over other routes of administration (4). Transdermal drug delivery is viable for administering low molecular weight potent drugs that are susceptible to the first-pass metabolism. It also provides a non-invasive route of administration, prolonged drug release, and reduced side effects.
Improved bioavailability, better patient compliance, and ease of drug termination are also of interest. However, the barrier function of human skin presents a major limitation for transdermal drug delivery. Therefore, increasing the permeability of drugs into the skin without inducing a significant irreversible change in skin barrier function is a challenge.
Microemulsions are promising vehicles for transdermal drug delivery due to their high capacity for incorporating both lipophilic and hydrophilic drugs and high penetration enhancing ability and the tendency of incorporated drugs to favor partitioning into the stratum corneum (5,6). The choice of oil and surfactant constituents in microemulsions is an important factor in drug release and in the ability of microemulsions to improve skin permeation (6,7). Most commonly used oils include saturated and unsaturated fatty acids, which also act as penetration enhancers in transdermal delivery. Surfactants and cosurfactants may also enhance the permeability through the skin.
A major limitation of microemulsions is the possibility of skin irritation due to the utilization of high concentrations of surfactants. Attempts have been made to reduce the possibility of skin irritation by using a combination of surfactants and by utilizing low irritant constituents (8).
Natural oils including jojoba oil (9,10,11), cottonseed oil (12), coconut oil (13), and linseed oil (14) have attracted many investigators due to their biocompatibility, low irritation potential, and safety. Natural oils have been employed as non-aqueous vehicles and permeation enhancers in dermal and transdermal preparations (15). Jojoba oil differs from true oils in that it is a liquid wax ester composed mainly of esters of long-chain fatty acids and alcohols (Fig. 1). Jojoba oil has unique properties which makes it even more interesting for use in cosmetics and pharmaceutical preparations. It resembles the oil secreted by human skin, so it is less likely to build upon the skin and clog pores. It is hypoallergic, moisturizing, and has potential applications in cosmetics and topical formulations to protect, soothe, lubricate the skin (16) and reduce inflammation commonly associated with dermatological formulations (17). Jojoba oil contains natural antioxidants alpha, gamma, and delta tocopherols, which present high stability toward oxidation and hydrolysis and help stabilize other unstable ingredients in the formulation. It has other promising physical properties including its pleasant feel on the skin and excellent skin penetration capability (18). There is an increased interest in the employment of jojoba oil for the preparation of microemulsions in transdermal drug delivery.
Fig. 1.
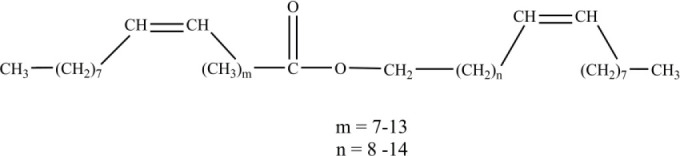
General structure of Jojoba oil components.
Examples of the research in which jojoba oil has been used in micro emulsion-based transdermal drug delivery systems is its incorporation in formulations for potential topical applications of fluconazole (19), lycopene (20), diclofenac sodium (21), and aceclofenac (22) with promising results.
In more recent work microemulsions based on jojoba oil showed improved dermal deposition for tazarotene. The highest permeation results were seen for the formulation with 40% oil content (11). Clotrimazole emulgels prepared using jojoba oil as the oil phase also showed improved antifungal activity compared to a commercial product (23).
The objective of this work was to examine jojoba oil dual usage as the potential vehicle in microemulsion with penetration enhancement capabilities and its effect on stratum corneum using ketorolac and lidocaine as model drugs. Ketorolac is a non-steroidal anti-inflammatory agent used for the management of moderate to severe pain (24). It has a short half-life of 4-6 h, which requires frequent administration in order to maintain its effect.
The duration of treatment with ketorolac is restricted to a short period of time due to the severity of the upper gastrointestinal tract side effects associated with its oral use. Transdermal delivery of ketorolac maintains prolonged therapeutic levels of the drug in the body, avoids hepatic first-pass metabolism, and eliminates the upper gastrointestinal side effects associated with the drug. Lidocaine is a local anesthetic with a rapid onset and intermediate action, which requires frequent administration in order to alleviate pain. There is increased interest in transdermal delivery of lidocaine to enhance the therapeutic outcomes and prolong the drug action.
MATERIALS AND METHODS
Lidocaine HCl was obtained from Sigma Aldrich (Munchen, Germany). Ketorolac tromethamine was kindly donated by Al-Hikma Pharmaceuticals, Jordan. Lidocaine HCl and isopropyl myristate were kindly donated by the Jordanian Pharmaceutical Manufacturing Co., Jordan. Brij 97 (polyoxyethylene-10 EO-oleyl alcohol) was obtained from Uniqema (DE, USA). Hydrochloric acid (HCl) and high-performance liquid chromatography (HPLC) grade water and acetonitrile were obtained from Acros Organics, Belgium. Water for preparation was double distilled and deionized. Jojoba oil was obtained from the seeds of jojoba plants planted at the farms of Jordan University of Science and Technology and filtered through a 0.45 μm Millipore filter.
Characterization of jojoba oil
Jojoba oil was characterized according to the American Society of Testing and Material (ASTM) procedures for acid number (ASTM D1386-98) (25), saponification value (ASTM D1387-89) (26), iodine number (ASTM D5768-02) (27), specific gravity (ASTM D287-92) (28), refractive index (ASTM D 1218-02) (29) and surface tension (ASTM D1331-89) (30). The viscosity of jojoba oil was measured using an A&D Vibro digital viscometer (SV-10, A&D Co. Ltd., Japan). All measurements were performed at room temperature.
Preparation of microemulsions
Construction of pseudoternary phase diagrams
Mixtures of different volume ratios of Brij 97 and three different oil/hexanol blends (at 1:0, 2:1 and 1:1 v/v) were prepared by adding the appropriate amount of the surfactant to the jojoba oil/hexanol blend. The volume fraction of surfactant to oil/hexanol mixture was varied from 0.9 to 0.1. Mixtures were agitated using a magnetic stirrer until clear systems were obtained. Water was then added to each mixture in small aliquots while mixing under magnetic stirring, to reach equilibrium quickly. The concentration of water at which the ternary mixture appeared cloudy was obtained from the volume measurements and the boundaries corresponding to the chosen values of oil/hexanol and surfactant mixing ratios were accordingly determined. All experiments were carried out at room temperature. Experiments were repeated three times and mean results were taken. Pseudoternary phase diagrams were constructed. The surfactants examined were Cremophore, Labrasol®, cetostearyl alcohol, Tween, Plurol Oleique and Brij 97. Phase diagrams were also constructed using coconut oil, palm oil and cottonseed oil with the same surfactant/cosurfactant systems in order to compare the regions of microemulsion formation with that for jojoba oil.
Preparation of drug-loaded w/o microemulsions
A volume of 5 mL of each water-in-oil jojoba oil microemulsion, containing 4 mg/mL drug, was prepared by dissolving an accurately weighed 20 mg of drug in an appropriate amount of Brij 97 or Tween® 80 then adding the jojoba oil (with or without hexanol) at appropriate concentration while stirring until a clear solution was obtained. The drugs were solubilized in the surfactant system since they were insufficiently soluble in jojoba oil. The oily phase was then micro emulsified by the addition of water, with continuous stirring, to make 5 mL. Microemulsions were homogenized using a Janke and Kunkel homogenizer (Staufen, Germany) to facilitate mixing.
Characterization of microemulsions
The electrical conductivity and pH of microemulsions were measured using a Mettler Toledo pH-conductivity meter (Switzerland). The refractive indices of microemulsion formulations were determined using a Metler Toledo refractometer (Switzerland). Microemulsion viscosity was determined using an A&D Vibro digital viscometer (SV-10, A&D Co. Ltd., Japan).
Microemulsion droplet size was determined at 25 °C, by dynamic light scattering at an observation angle of 90° with vertical polarization of the analyzing polarizer, operating at 633 nm, using a Malvern Zetasizer (Nano-ZS), England. Microemulsions were suitably diluted with isopropyl myristate to avoid multi-scattering phenomena. All measurements were carried out in triplicates. Results were expressed as average ± standard deviation.
Drug loading in microemulsion formulations was assessed as percent drug content. The drug-loaded microemulsions were ultra-centrifuged at 15000 rpm. Samples were then taken from the supernatant and analyzed for drug content using HPLC. Each experiment was repeated in triplicate and data were presented as mean ±RSD.
Stability of microemulsions
The stability of the studied microemulsions based on jojoba oil and Brij 97 was assessed visually for clarity and phase separation at 5, 25, and 40 °C for six months. To assess their physical stability, the microemulsions were also centrifuged using a Hermle Z300 centrifuge (Hermle Labortechnik, Germany) for 30 min at 4000 rpm immediately after preparation and storage for six months in a dark place at the mentioned temperatures (31).
Skin irritation test
The use of animals in this study was in accordance with approval from the Animal Care and Use Committee of Jordan University of Science and Technology (Ethics No. 4719), which is in full compliance with local, national, ethical, and regulatory principles and local licensing regulations, per the spirit of the Association for Assessment and Accreditation of Laboratory Animal Care International’s expectations for animal care and use/ethics committees.
The irritancy of microemulsions was tested on male rats (200-300 g). The dorsal part of rat skin was carefully shaved using an electric razor. A plastic frame with an area of 4 cm2 was mounted onto shaved rat skin. Samples of 5 mL of each microemulsion preparation were applied topically inside the plastic frame and secured in place with hypoallergic adhesive tape for 24 h. Rats were then sacrificed, and pieces of microemulsion-treated skin were collected and stained with hematoxylin/eosin (H&E). The skin was then examined under a light microscope (Nikon, Japan) for histopathological changes; microphotographs were taken and compared to a control skin (untreated with microemulsion).
HPLC analysis of drugs
HPLC was used for drug analysis. The HPLC system consisted of an LC system (LC-10AD vp, Shimadzu, Japan), an automatic injector (SIL-10AD vp, Shimadzu, Japan), a UV-visible detector (SPD-10AV vp, Shimadzu, Japan), a degasser (DGU-12A, Shimadzu, Japan), and a system controller (SCL-10A vp, Shimadzu, Japan). The HPLC was attached to a computer with appropriate software (Class VP, V 6.2, Shimadzu, Japan). HPLC analyses were conducted under isocratic reversed-phase conditions using Hypersil BDS-C18 column (4.5 μm, 150 χ 4.6 mm). The mobile phase consisted of a mixture of 10% acetic acid in water and methanol (75:25 v/v) for lidocaine HCl analysis (32), and a mixture of acetate buffer and acetonitrile (70:30 v/v) for ketorolac analysis (33). Mobile phases were filtered through Millipore cellulose acetate membranes (0.45μm) under vacuum and degassed. The injection volume was 50 μL and the mobile phase flow rate was 1.25 mL/min. The detection wavelength was 254 nm for lidocaine HCl and 320 nm for ketorolac. The HPLC methods of analysis were fully validated and all HPLC measurements were carried out in triplicates.
in vitro skin permeation studies
in vitro-permeation studies of lidocaine HCl and ketorolac were investigated from different jojoba oil formulations (Table 1) using rat skin.
Table 1.
Composition of jojoba oil microemulsions.
| Components | Jojoba oil formulations (%v/v) | ||||
|---|---|---|---|---|---|
|
| |||||
| M1 | M2 | M3 | M4 | M5 | |
| Tween 80 | 80 | 80 | 0 | 0 | 0 |
| Brij 97 | 0 | 0 | 50 | 70 | 70 |
| Jojoba oil | 7 | 5 | 40 | 20 | 10 |
| Water | 10 | 10 | 10 | 10 | 10 |
| Hexanol | 3 | 5 | 0 | 0 | 10 |
Rat skin was obtained from the whole dorsal area of male rats (350 ± 20 g) after being carefully shaved with an electric razor. Subcutaneous fat was carefully removed with forceps. The skin was then cut into 2 χ 2 cm pieces, placed in filter papers wetted with phosphate buffer saline (PBS, pH 7.4), wrapped in cling film, and stored at 4 °C until use. The skin was used within 3 days of storage (34).
in vitro-permeation experiments were conducted at 37 ± 1 °C using Franz diffusion cells with a diffusional area of 1.77 cm2 and a receptor compartment volume of 15 ± 0.01 mL. The receiver solution, consisting of fresh phosphate buffer PBS (pH 7.4), was stirred with a small magnetic bar to ensure adequate mixing, decrease lag time and maintain sink conditions.
Skin pieces were allowed to hydrate in PBS for 1 h at 37 °C and checked for integrity prior to use. The skin was then mounted between donor and receptor compartments of the diffusion cell with the dermis side facing the receiver solution. An O-ring was then placed over the skin. The two compartments were fitted with a clamp and left a sufficient time to equilibrate. Air bubbles under the skin were drained out by bending the cell aside and returning it. An amount of 5 mL of each drug-loaded formulation (total initial drug content = 20 mg) was carefully placed over the skin and the donor compartment was covered to prevent evaporation. At appropriate intervals, 1 mL of the receiver solution was removed for drug content determination and replaced immediately with an equal volume of fresh medium. No interference of other formulation components was observed. All experiments were performed in triplicate and results were expressed as mean ± RSD.
Data treatment
The cumulative amount of drug permeated through a unit surface area of excised rat skin was plotted as a function of time. Drug permeation rate at steady state was obtained from the slope of the regression line of the linear portion of the plot. The lag time was measured as the x-intercept of the regression line at a steady state. The skin permeation coefficient was calculated by dividing the flux at steady-state by the initial drug concentration in the donor compartment. The percent cumulative drug permeated after 24 h was also calculated from the ratio of the cumulative amount of drug released at 24 h to the initial drug amount in the donor compartment.
Statistical analysis
Data from three independent experiments were obtained. Statistically significant differences were assessed using Students t-test and two-way Analysis Of Variance (ANOVA) using GraphPad Prism version 8.0.0 for Mac, GraphPad Software, San Diego, California USA. P values of < 0.05 were considered significant.
RESULTS
Selection of surfactants for microemulsion formation
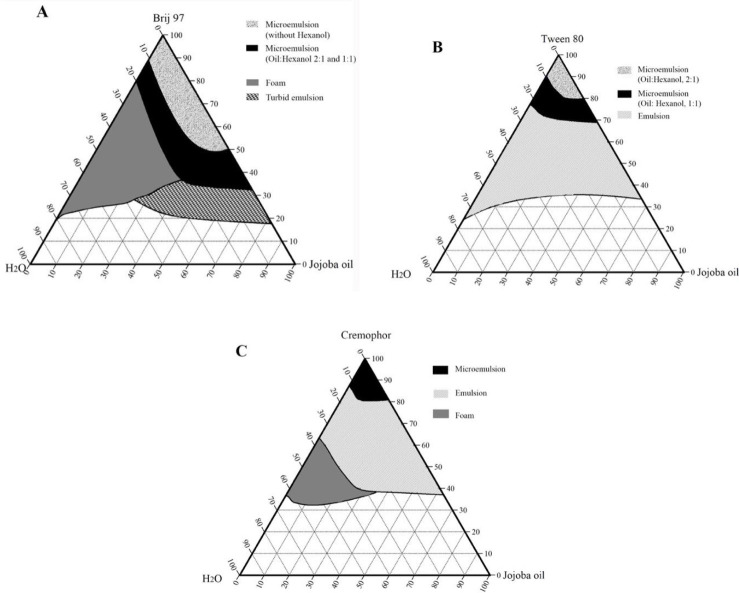
Preliminary work has been carried out on a range of non-ionic surfactant/cosurfactant systems including Cremophore, Labrasol®, cetostearyl alcohol, Tween, Plurol Oleique, Brij 97, and short-chain alcohols. Among which, Brij 97, Tween 80, and Cremophore were found reasonably suitable surfactants and hexanol was selected as a cosurfactant. Pseudoternary phase diagrams of jojoba oil, water, and surfactant/cosurfactant systems are shown in Fig. 2.
Fig. 2.
Pseudoternary phase diagrams of jojoba oil, water, and surfactant: (A) Brij 97, (B) Tween 80, and (C) cremophor, with and without hexanol, at room temperature.
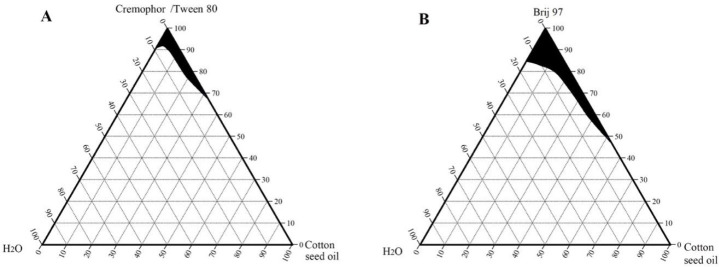
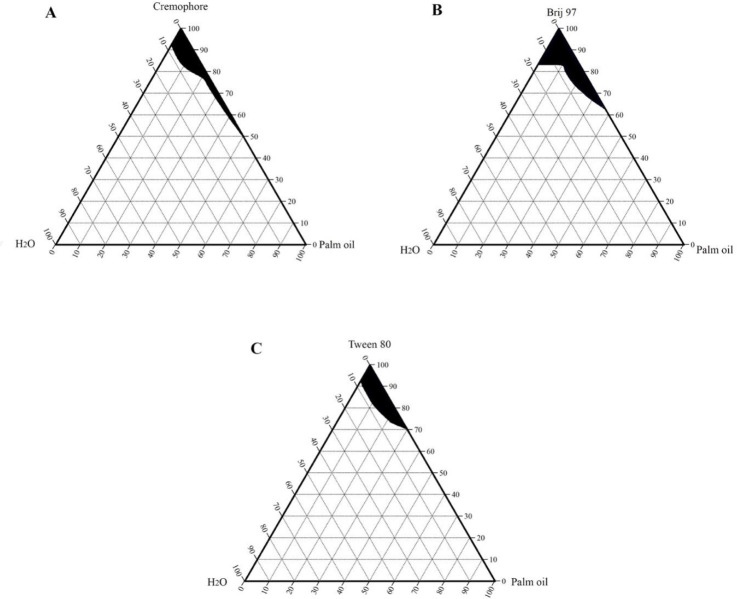
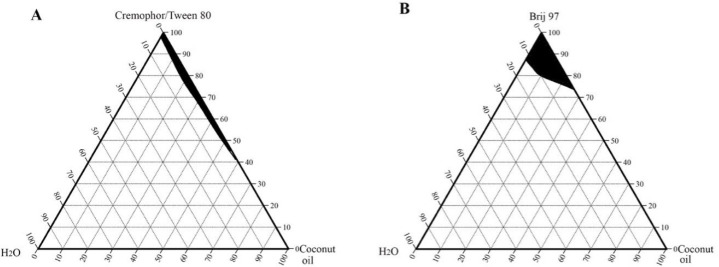
Phase diagrams of cottonseed oil, coconut oil, and palm oil were also constructed using the same non-ionic surfactants and are shown in Figs. 3-5, respectively.
Fig. 3.
Pseudoternary phase diagrams of cottonseed oil, water, and surfactant: (A) cremophor or Tween 80 and (B) Brij 97, at room temperature. The shaded region represents a clear microemulsion area.
Fig. 5.
Pseudo-ternary phase diagrams of palm oil, water, and surfactant: (A) cremophor, (B) Brij 97 and (C) Tween 80, at room temperature. The shaded region represents a clear microemulsion area.
Fig. 4.
Pseudoternary phase diagrams of coconut oil, water, and surfactant: (A) Cremophorec or Tween 80 and (B) Brij 97, at room temperature. The shaded region represents a clear microemulsion area.
The microemulsion region of cottonseed oil and coconut oil produced with Tween 80 or with Cremophore were identical. Jojoba oil microemulsions were spontaneously formed and showed larger microemulsion regions than the other studied vegetable oils. This enlightened the suitability of using jojoba oil over the other selected vegetable oils and supported the use of jojoba oil in the microemulsion formation of this work.
Characterization of jojoba oil
The measured acid value, saponification number, iodine number, specific gravity, refractive index, surface tension, and viscosity of the obtained jojoba oil are shown in Table 2. Jojoba oil produced by Jordan University of Science and Technology had the same physicochemical properties as those previously reported in the literature (35,36). The values indicated that jojoba oil was of high quality.
Table 2.
Physical characteristics of jojoba oil.
| Physical measurement | Measured value |
|---|---|
| Acid number | 0.37 |
| Saponification value | 88 |
| Iodine number | 81 |
| Specific gravity | 0.861 |
| Refractive index | 1.4612 |
| Surface tension | 32 dyne/cm2 |
| Viscosity | 33 mPa |
Characterization of jojoba oil microemulsions
All jojoba oil microemulsions prepared in this work were isotropic, transparent, and slightly viscous with low conductivity. The conductivity of microemulsions at the highest water content was 7.42 ± 0.02 μS/cm. The refractive indices of investigated microemulsions were in the range of 1.431 to 1.465, increasing as the content of oil increased. These values were closer to the refractive index value of jojoba oil (1.461) than to the refractive index value of water (1.3336). The low microemulsion conductivity together with their refractive index values indicated the formation of water-in-oil microemulsions. The pH of Brij 97-based microemulsions was 5.30 ± 0.40, whereas the pH of Tween 80 microemulsions was 6.58 ± 0.03.
The addition of ketorolac slightly increased the pH values (6.53 ± 0.01 for Brij 97-, and 7.10 ± 0.32 for Tween 80-based microemulsions), while lidocaine HCl slightly reduced the pH (4.70 ± 0.60 for Brij 97-, and 5.80 ± 0.07 for Tween 80-based microemulsions). The viscosity of Brij 97-based microemulsions ranged between 70-130 mPa, being lowest at higher hexanol contents and higher at higher water contents. Tween 80-based microemulsions had higher viscosities than those based on Brij 97. The viscosity of Tween 80 microemulsions ranged between 370-630 mPa, increasing as the content of water increased and decreasing as the content of hexanol increased.
Microemulsion droplet size was determined by dynamic light scattering, at 25 °C. The droplet size of Brij 97 microemulsion was 122 ± 0.08 nm for M3 and 220 ± 0.22 nm for M4 (without hexanol) and 59 ± 0.35 nm for M5 (when hexanol was used at the 1:1 volume ratio). The droplet size of Tween 80 based microemulsions was 10 ± 0.45 nm. Cremophore-based microemulsions had a droplet size of only 3 ± 0.14 nm, which was considered very low and out of the generally recognized range of microemulsion size (37).
The percent drug content in microemulsion formulations ranged from 95.05 ± 2.69% to 98.73 ± 0.88% for ketorolac, and from 96.96 ± 2.69% to 99.29 ± 1.78% for lidocaine HCl.
Stability studies
The investigated jojoba oil microemulsions (Table 1) remained as transparent single-phase and did not show phase separation for six months when stored in a dark place at 25 or 40 °C. The centrifugation tests also showed that the microemulsions were physically stable at 25 and 40 °C. On the other hand, phase separation and turbidity were observed with microemulsions stored at 5 °C. However, unlike emulsions, they were easily recovered by heating up.
Skin irritation test
The effect of jojoba oil microemulsions based on Brij 97 (containing 70% v/v Brij 97) on rat skin was investigated and skin micrographs were presented in Fig. 6. A normal uniformly layered stratum corneum, epidermis, and loosely textured collagen in the dermis could be observed in untreated control rat skin (Fig. 6A). The stratum corneum of 24 h-treated skin remained intact when microemulsion formulation without hexanol was applied to rat skin (Fig. 6B). However, disruption of stratum corneum organization was observed after 24 h of treatment with microemulsion containing hexanol at 1:1 oil to hexanol volume ratio (Fig. 6C).
Fig. 6.
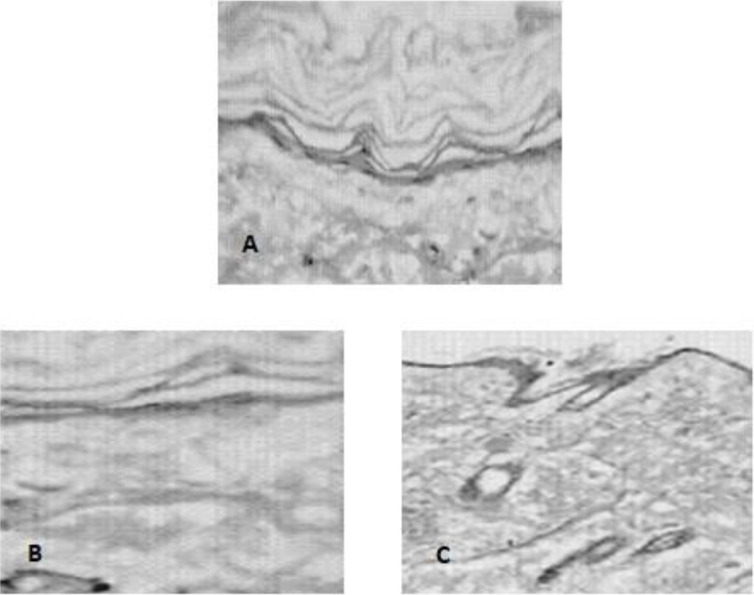
Microscopic images of (A) untreated control rat skin, and 24 h-treated rat skin with jojoba oil microemulsion formulations based on Brij 97 (B) without hexanol (M4) and (C) with hexanol (M5).
in vitro permeation studies
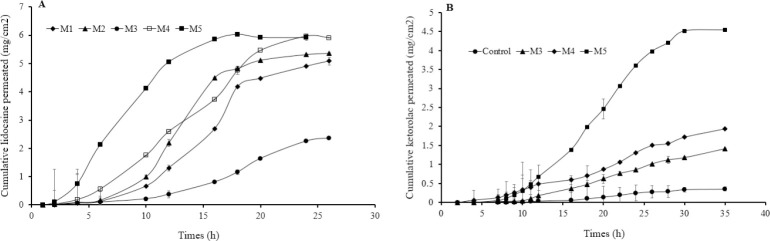
The in vitro permeation profiles of lidocaine HCl and ketorolac from different jojoba oil formulations into PBS, at 37 °C, are shown in Fig. 7. Their permeation parameters are summarized in Tables 3 and 4, respectively. Jojoba oil-Brij 97 at 90:10 volume ratio was used as a control in ketorolac permeation in order to solubilize the drug, which was insoluble in either water or jojoba oil.
Fig. 7.
Cumulative amount of (A) lidocaine HCl and (B) ketorolac per unit area permeating through rat skin against time from a control (oil and Brij 97 at 90:10 v/v ratio) and different jojoba oil microemulsion formulations into PBS (pH 7.4), at 37 °C.
Table 3.
in vitro permeation parameters for lidocaine HCl from jojoba oil microemulsions through excised rat skin into phosphate-buffered saline (pH 7.4) at 37 °C. Data represent mean ± RSD, n = 3.
| Microemulsion formulation | Flux (μg/cm2.h) | Lag time (h) | Permeation coefficient ([cm/h] × 10-2) | Cumulative drug permeated at 24 h (%) |
|---|---|---|---|---|
| M1 | 146.79 ± 0.05 | 2.92 ± 0.06 | 0.04 | 76.7 |
| M2 | 220.61 ± 1.97 | 2.99 ± 0.01 | 0.06 | 90.3 |
| M3 | 243.75 ± 0.17 | 9.09 ± 0.01 | 0.06 | 35.4 |
| M4 | 461.98 ± 0.05 | 2.87 ± 1.72 | 0.12 | 92.4 |
| M5 | 916.90 ± 0.03 | 2.07 ± 0.02 | 0.23 | 96.5 |
Table 4.
in vitro permeation parameters for ketorolac from jojoba oil microemulsions through excised rat skin into phosphate-buffered saline (pH 7.4) at 37 °C. Data represent mean ± RSD, n = 3.
| Microemulsion formulation | Flux (μg/cm2.h) | Lag time (h) | Permeation coefficient ([cm/h] × 10-2) | Cumulative drug permeated at 24 h (%) |
|---|---|---|---|---|
| Control* | 40.27 ± 0.58 | 13.50 ± 0.18 | 0.01 | 3.8 |
| M3 | 107.11 ± 0.98 | 9.59 ± 0.03 | 0.03 | 13.5 |
| M4 | 125.38 ± 0.64 | 5.22 ± 0.03 | 0.03 | 20.3 |
| M5 | 188.21 ± 0.21 | 6.86 ± 0.01 | 0.05 | 60.4 |
* Jojoba oil and Brij 97 at 90:10 volume ratio.
DISCUSSION
The aim of the construction of pseudoternary phase diagrams was to find out the existence range of microemulsions and to select suitable non-ionic surfactant/cosurfactant systems that are able to stabilize a wider range of microemulsion area. In this work, Brij 97, Tween 80, and Cremophore were selected as surfactants for microemulsion preparation, and hexanol was selected as a cosurfactant. The cosurfactant stabilizes the microemulsion by interacting with the surfactant and increasing the flexibility of the interfacial film, thus aiding microemulsion formation. Attempts were made to use other shorter chain alcohols as cosurfactants, however, stable formulations were difficult to produce. With regard to jojoba oil pseudoternary phase diagrams (Fig. 2), Brij 97 stabilized a larger area of microemulsion existence than either Tween 80 or Cremophore. The effect of hexanol on Tween 80 microemulsion could be observed as the microemulsion area increased when the volume ratio of hexanol increased (Fig. 2B). It is worth mentioning that stable microemulsions were difficult to form with Tween 80 without the addition of hexanol. The microemulsion regions obtained with jojoba oil were larger than those obtained with the other investigated vegetable oils (Figs. 3-5) when using the same surfactant/cosurfactant systems. This could be due to the simplicity of the jojoba oil structure compared with vegetable oils, which could allow better alignment of jojoba oil with the linear surfactants and cosurfactants and spontaneous formation of microemulsions. Jojoba oil is a pure liquid ester and not oil in a chemical sense. It is composed of a mixture of long-chain esters of monounsaturated fatty acids and fatty alcohols, with an average total carbon chain length of 42 carbons. It differs from vegetable oils in that it has only one alcohol group and is a straight-chain molecule (16,38).
Jojoba oil has been investigated in microemulsion formation. However, only limited research included the use of jojoba oil in drug delivery, particularly as a transdermal vehicle (21,39). Jojoba oil is not subject to oxidation and could provide a better oily phase than other vegetable oils in terms of formation and stability of microemulsions in addition to the topical benefits of jojoba oil itself, as low skin irritation potential and anti-inflammatory properties. In addition, jojoba oil mimics the sebum secreted by the skin and regulates its production. It is non-comedogenic and does not build up on the skin, which allows its use in water in oil emulsions without fear of clogging skin pores. Therefore, jojoba oil was selected as the oily phase in this work. Jojoba oil can protect skin against possible irritation induced by the high surfactant or drug content, stabilizes incorporated drugs, and enhances their percutaneous permeation.
Jojoba oil produced in Jordan University of Science and Technology showed similar physicochemical properties as those previously reported in the literature (35,36) indicating its high quality. The surface tension of jojoba oil (33 dyne/cm) is close to the surface tension of human skin (27-28 dyne/cm). This would facilitate spreading and enhance the contact between the oil and skin which could improve drug permeation through the skin. The low iodine value indicates the stability of the oil against oxidation while the acid value indicates its low irritation potential and reactivity.
Jojoba oil microemulsions were spontaneously obtained. All microemulsions were clear and isotropic. The low conductivity values of the investigated microemulsions together with their refractive index values, which were close to that of jojoba oil (1.461) rather than that of water (1.3336), indicated the formation of water-in-oil microemulsions. In this work, investigations were carried out using water-in-oil microemulsions where jojoba oil comprises the external phase due to its desirable properties and soothing effect on the skin in addition to enhanced-skin contact and drug permeation.
The pH values of all microemulsions (5.30-6.58) were close to the pH of human skin, making them suitable for transdermal applications. However, the incorporation of drugs into such microemulsions resulted in slight changes in their pH values due to the nature of each drug. A slight increase in microemulsion pH was obtained with ketorolac. On the other hand, a slight decrease in microemulsion pH was obtained with lidocaine HCl.
Microemulsion viscosity was related to the viscosity of the surfactant itself. Brij 97 is a linear nonionic surfactant poly(oxyethylene) 10 olyel ether with a viscosity of 100 mPa. Tween 80 is a polyoxyethylene (20) sorbitan monooleate having a larger and bulkier hydrophilic head and higher viscosity of 502 mPa. Therefore, microemulsions containing Tween 80 showed higher viscosities than those containing Brij 97. Increasing water content in microemulsions resulted in an increase in the hydration of the system and consequently increased the viscosity due to more hydration and swelling of the nonionic surfactants in water.
Microemulsion droplet size was determined by DLS, at 25 °C. The effect of hexanol on lowering the microemulsion droplet size could be explained by its stabilizing effect as a cosurfactant as it reduces the interfacial tension in the microemulsion systems. The droplet size of Cremophorebased microemulsions (3 ± 0.14 nm) was considered very low and was out of the generally recognized range of microemulsion size (37).
The investigated jojoba oil microemulsions were physically stable for six months when stored in a dark place at 25 or 40 °C. Microemulsions are generally produced spontaneously and are thermodynamically stable formulations. This explains the physical stability of jojoba oil microemulsions at higher temperatures and their quick regeneration when heated up after storage at 5 °C. Skin irritation due to the drug or formulation components, particularly surfactants and cosurfactants which are typically used in high concentrations in microemulsions, presents a limitation to microemulsions. The use of jojoba oil in microemulsion formulations could decrease or eliminate this irritation. Hexanol is moderately irritating to the skin upon prolonged exposure of high concentrations (more than 1%) with the skin (40). Jojoba oil microemulsion containing hexanol did not show the expected visual skin irritation but rather showed some disruption of the stratum corneum organization microscopically after 24-h exposure (Fig. 6C).
The drug-loaded microemulsions were isotropic and clear. The amount of drug-loaded was based on almost complete drug solubilization, where the percent drug content in microemulsion formulations was larger than 95%. No drug precipitation or microemulsion phase separation was observed after ultra-centrifugation at 15000 rpm. Microemulsions are thermodynamically stable formulations and are considered as one-phase systems, despite the fact that they are colloidal dispersions. Therefore, it will be difficult to attempt to separate the internal and external phases of microemulsions. On the other hand, microemulsions are meant to dissolve drugs rather than encapsulate them and drug solubilizing capacity is one of the paramount features of microemulsions in general. Therefore, drug loading was assessed as percent drug content in the prepared microemulsions. In our formulations, almost all of the drug has been dissolved or partitioned in one of the major phases of the formulation (oil/surfactant mixture in case of ketorolac and water/surfactant mixture in case of lidocaine HCl).
Percutaneous drug permeation depends on many factors as drug concentration, drug partition coefficient, droplet size, viscosity, and excipients used. Microemulsions are able to incorporate large quantities of drugs thus would improve the thermodynamic activity. They have small droplet sizes which allow more contact area with the skin, in addition to the added benefits of the microemulsion components in improving drug permeation through the skin. Generally, the drug in the external phase is first released. In order to restore equilibrium, the drug in the internal phase then partitions into the external phase and then released. The in vitro permeation of lidocaine HCl was dependent on the type of surfactant used and microemulsion composition (Fig. 7A and Table 3). Jojoba oil microemulsions (M2, M4, and M5) showed good lidocaine HCl permeation profiles through rat skin with about 90.3%-96.5% drug permeated over 24 h and short lag times of about 2-3 h. The enhanced-permeation rates of microemulsion preparations may be due to the effect of the type and content of the surfactant. Higher drug permeation was observed when Brij 97 was used as a surfactant (M4 and M5) than when Tween 80 was used (M2). Brij 97 enhances drug permeation through skin probably due to changes in barrier properties of skin and vehicle-stratum corneum partition coefficient. The lower enhancement effect of Tween 80-based jojoba oil microemulsion preparations compared with those of Brij 97 could also be attributed to the longer “tail” length of Tween 80 and the higher viscosity resulting in slower drug escape. Moreover, the addition of hexanol in M5 resulted in higher drug permeation and shorter lag time compared with M4 (without hexanol). The effect of hexanol was also observed in jojoba oil microemulsion containing Tween 80, where lower drug permeated over 24 h was obtained when the hexanol content was reduced in M1 (76.7%) compared with M2 (90.3%). On the other hand, Microemulsion M3, which contained lower surfactant concentration without hexanol, showed the lowest drug permeation. The high permeation rates of drugs may also be attributed to the small droplet size of microemulsions. Small droplet size increases the contact area with the skin thus allowing drugs to partition and diffuse more easily through the skin. M4 and M5 showed a lidocaine flux of 461.98 and 916.90 mg/cm2.h, respectively, which was comparable or better than those obtained by Yuan et al. (41,42) and Wang et al. (43). This could be explained by the differences in the microemulsion composition and by the penetration enhancement effect of jojoba oil itself.
With regard to ketorolac permeation, Tween 80 microemulsions (M1 and M2) showed no ketorolac in vitro release and permeation within 24 h. The release retardant effect of Tween 80 on ketorolac could be explained by the low partitioning of the drug to the oily phase. On the other hand, Brij 97-based jojoba oil microemulsions (M3, M4, and M5) improved ketorolac permeation through rat skin. It is worth mentioning that M3 and M4 showed substantial drug permeation of the poorly permeable ketorolac compared with the control solution (Fig. 7B and Table 4). The improvement of drug permeation was primarily correlated with the Brij 97 concentration and microemulsion formation despite the absence of hexanol in these formulations. The presence of hexanol in M5 resulted in further improvement of drug permeation (flux = 188.21 ± 0.21mg/cm2.h) with about 60% of drug permeated over 24 h.
Transdermal delivery of ketorolac has been investigated and its permeation was improved using different formulations and enhancers (8,24,44,45,46,47). Nasseri et al. demonstrated the penetration-enhancement potential of lecithin stabilized microemulsions-based organogels on the permeation of ketorolac tromethamine (a more soluble salt) through Guinea pig skin (44). Their results were comparable to those obtained in this work. Differences in the flux and permeation rates were related to variations in the formulations and experimental design. Cho and Gwak demonstrated a significant increase in ketorolac tromethamine permeation through hairless mice skin when the drug was used at concentrations higher than its solubility. They also investigated the effect of different fatty acids and solvents on the drug permeation rate with a maximum flux of 113.6 mg/cm2.h obtained when 10% caprylic acid in propylene glycol was used (24). Chandra et al. investigated the effects of alcohols, pH, and chemical enhancers on ketorolac permeation from different hydrogels through abdominal rat skin. Ketorolac flux (18.04 mg/cm2.h) was improved with a maximum flux of 90.56 mg/cm2.h obtained when 15% w/w eucalyptus oil was used (46). More recently, Salimi et al. highlighted the significance of microemulsion composition on the physicochemical properties of the microemulsion and ketorolac permeation through rat skin. All microemulsions investigated greatly improved drug permeation, which was about 8-9 times higher than that of a control 2% ketorolac solution in water. A maximum flux of 114 mg/cm2.h was obtained with microemulsion formulation composed of 20% water, 20% surfactant (Labrasol®-Tween 80), 10% cosurfactant (pleurol oleic-PEG 400), and 50% oil (isopropyl myristate), which was primarily correlated with the amount of the cosurfactant used (47).
It is worth mentioning that higher permeation rates were observed with lidocaine HCl due to its higher partition coefficient (Log P = 2.5) compared with ketorolac which has a lower partition coefficient (Log P = 1.04). Moreover, the significant increase in drug permeation and decrease in lag time observed with jojoba oil microemulsions containing hexanol were primarily due to the effect of hexanol on skin integrity as evident by the histopathological study (Fig. 6C).
CONCLUSIONS
Jojoba oil is a promising vehicle for transdermal drug delivery. The formation and stabilization of microemulsions depend on the type and content of surfactant and cosurfactant being used, which greatly influenced the physical properties of the microemulsion. In addition, the increase in water content resulted in more hydration and swelling of the nonionic surfactants with a consequent increase in the viscosities of microemulsions. Microemulsions could improve topical delivery of drugs due to small droplet size and the permeation enhancing the ability of jojoba oil and Brij 97. Drug permeation enhancements were caused by the surfactant, the cosurfactant used, jojoba oil itself, and the microemulsion formulation. Histopathological investigations illustrated some disruption of stratum corneum after 24 h of exposure due to the presence of hexanol in microemulsion, which also contributed to its permeation enhancement effect.
Conflict of interest statement
The authors declared no conflicts of interest in this study.
Authors’ contribution
S.M. Assaf designed and supervised the experimental work and analyzed the data; wrote, and submitted the manuscript. K.T. Maaroof Helped in the experimental work and data analysis and editing of the manuscript. B.M. Altaani contributed to the design and supervision of the experimental work and data. M.M. Ghareeb helps in data analysis and manuscript preparation. A.A. Abu Alhayyal performed the experimental work and data presentation.
Acknowledgments
The authors would like to acknowledge the Deanship of Research at Jordan University of Science and Technology for financial support of this work (Grant No. 20070091). The authors would like also to acknowledge Dr. Amjad Qandil from the Department of Medicinal Chemistry at Jordan University of Science and Technology, for technical assistance in the preparation of ketorolac from ketorolac tromethamine and its NMR conformation, Dr. Adnan Badwan from the Jordanian Pharmaceutical Manufacturing Co., and Dr. Mayyas Al-Rimawi from the Faculty of Pharmacy and Medical Sciences at the University of Petra, Jordan, for their valuable comments and advice.
REFERENCES
- 1.Chaudhary S, Garg T, Murthy RS, Rath G, Goyal AK. Recent approaches of lipid-based delivery system for lymphatic targeting via oral route. J Drug Target. 2014;22(10):871–882. doi: 10.3109/1061186X.2014.950664. DOI: 10.3109/1061186X.2014.950664. [DOI] [PubMed] [Google Scholar]
- 2.Vadlamudi HC, Narendran H, Nagaswaram T, Yaga G, Thanniru J, Yalavarthi PR. Microemulsions based transdermal drug delivery systems. Curr Drug Discov Technol. 2014;11(3):169–180. doi: 10.2174/157016381103141128113034. DOI: 10.2174/157016381103141128113034. [DOI] [PubMed] [Google Scholar]
- 3.Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev. 2004;56(5):603–618. doi: 10.1016/j.addr.2003.10.025. DOI: 10.1016/j.addr.2003.10.025. [DOI] [PubMed] [Google Scholar]
- 4.Kreilgaard M. Influence of microemulsions on cutaneous drug delivery. Adv Drug Deliv Rev. 2002;54(Suppl 1):S77–S98. doi: 10.1016/s0169-409x(02)00116-3. DOI: 10.1016/s0169-409x(02)00116-3. [DOI] [PubMed] [Google Scholar]
- 5.Shukla T, Upmanyu N, Agrawal M, Saraf S, Saraf S, Alexander A. Biomedical applications of microemulsion through dermal and transdermal route. Biomed Pharmacother. 2018;108:1477–1494. doi: 10.1016/j.biopha.2018.10.021. DOI: 10.1016/j.biopha.2018.10.021. [DOI] [PubMed] [Google Scholar]
- 6.Nastiti CMR, Ponto T, Abd E, Grice JE, Benson HAE, Roberts MS. Topical nano and microemulsions for skin delivery. Pharmaceutics. 2017;9(4):37–61. doi: 10.3390/pharmaceutics9040037. DOI: 10.3390/pharmaceutics9040037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mehrpeyma M, Ravanshadi N, Jazayery Shooshtary F, Salimi A. Formulation of diclofenac microemulsion based on phase diagram. Res Pharm Sci. 2012;7(5):S303. [Google Scholar]
- 8.Amrish C, Kumar SP. Transdermal delivery of ketorolac. Yakugaku Zasshi. 2009;129(3):373–379. doi: 10.1248/yakushi.129.373. DOI: 10.1248/yakushi.129.373. [DOI] [PubMed] [Google Scholar]
- 9.Aggarwal G, Dhawan S, SL HariKumar. Natural oils as skin permeation enhancers for transdermal delivery of olanzapine: in vitro and in vivo evaluation. Curr Drug Deliv. 2012;9(2):172–181. doi: 10.2174/156720112800234567. DOI: 10.2174/156720112800234567. [DOI] [PubMed] [Google Scholar]
- 10.Shevachman M, Shani A, Garti N. Formation and investigation of microemulsions based on jojoba oil and nonionic surfactants. J Am Oil Chem Soc. 2004;81:1143–1152. DOI: 10.1007/s11746-004-1032-2. [Google Scholar]
- 11.Nasr M, Abdel-Hamid S. Optimizing the dermal accumulation of a tazarotene microemulsion using skin deposition modeling. Drug Dev Ind Pharm. 2016;42(4):636–643. doi: 10.3109/03639045.2015.1062512. DOI: 10.3109/03639045.2015.1062512. [DOI] [PubMed] [Google Scholar]
- 12.Syamasri G, Sanyal SK, Datta S, Moulik SP. Preparation of prospective plant oil derived micro-emulsion vehicles for drug delivery. Indian J Biochem Biophys. 2006;43(4):254–257. [PubMed] [Google Scholar]
- 13.Ja’afar SM, Khalid RM, Othaman R, Mokhtar WN, Suria Ramli. Coconut oil based microemulsion formulations for hair care product application. Sains Malays. 2019;48(3):599–605. DOI:10.17576/jsm-2019-4803-12. [Google Scholar]
- 14.Baboota S, ur Rahman M, Kumar A, Sharma S, Sahni J, Ali J. Submicron size formulation of linseed oil containing omega-3 fatty acid for topical delivery. J Dispers Sci Technol. 2012;33(9):1259–1266. DOI: 10.1080/01932691.2011.596339. [Google Scholar]
- 15.Shatalebi MA, Rafiei Y. Preparation and evaluation of minoxidil foamable emu oil emulsion. Res Pharm Sci. 2014;9(2):123–133. [PMC free article] [PubMed] [Google Scholar]
- 16.Pazyar N, Yaghoobi R, Ghassemi MR, Kazerouni A, Rafeie E, Jamshydian N. Jojoba in dermatology: a succinct review. G Ital Dermatol Venereol. 2013;148(6):687–691. [PubMed] [Google Scholar]
- 17.Lin TK, Zhong L, Santiago JL. Anti-inflammatory and skin barrier repair effects of topical application of some plant oils. Int J Mol Sci. 2017;19(1):70–90. doi: 10.3390/ijms19010070. DOI: 10.3390/ijms19010070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasr M, Abdel-Hamid S, Moftah NH, Fadel M, Alyoussef AA. Jojoba oil soft colloidal nanocarrier of a synthetic retinoid: preparation, characterization and clinical efficacy in psoriatic patients. Curr Drug Deliv. 2017;14(3):426–432. doi: 10.2174/1567201813666160513132321. DOI:10.2174/1567201813666160513132321. [DOI] [PubMed] [Google Scholar]
- 19.Ellaithy HM, El-Shaboury KM. The development of cutina lipogels and gel microemulsion for topical administration of fluconazole. AAPS PharmSciTech. 2002;3(4):77–85. doi: 10.1208/pt030435. DOI: 10.1208/pt030435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garti N, Shevachman M, Shani A. Solubilization of lycopene in jojoba oil microemulsion. J Am Oil Chem Soc. 2004;81(9):873–877. DOI: 10.1007/s11746-004-0994-4. [Google Scholar]
- 21.Shevachman M, Garti N, Shani A, Sintov AC. Enhanced percutaneous permeability of diclofenac using a new U-type dilutable microemulsion. Drug Dev Ind Pharm. 2008;34(4):403–412. doi: 10.1080/03639040701662479. DOI: 1080/03639040701662479. [DOI] [PubMed] [Google Scholar]
- 22.Rupali K, Mulik R, Badgujar LB, Paradkar A, Mahadik K, Bodhankar S, et al. Development and characterization of microemulsion formulations for transdermal delivery of aceclofenac: a research. IJDFR. 2010;1(1):359–386. [Google Scholar]
- 23.Shahin M, Hady SA, Hammad M, Mortada N. Novel jojoba oil-based emulsion gel formulations for clotrimazole delivery. AAPS PharmSciTech. 2011;12(1):239–247. doi: 10.1208/s12249-011-9583-4. DOI: 10.1208/s12249-011-9583-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho YA, Gwak HS. Transdermal delivery of ketorolac tromethamine: effects of vehicles and penetration enhancers. Drug Dev Ind Pharm. 2004;30(6):557–564. doi: 10.1081/ddc-120037486. DOI: 10.1081/ddc-120037486. [DOI] [PubMed] [Google Scholar]
- 25.ASTM D1386-98, Standard Test Method for Acid Number (Empirical) of Synthetic and Natural Waxes. West Conshohocken, PA: ASTM International; 1998. DOI: 10.1520/D1386-98. [Google Scholar]
- 26.ASTM D1387-89, Standard Test Method for Saponification Number (Empirical) of Synthetic and Natural Waxes. West Conshohocken, PA: ASTM International; 2019. DOI: 10.1520/D1387-89R19. [Google Scholar]
- 27.ASTM D5768-02, Standard Test Method for Determination of Iodine Value of Tall Oil Fatty Acids. West Conshohocken, PA: ASTM International; 2018. DOI: 10.1520/D5768-02R18. [Google Scholar]
- 28.ASTM D287-12b, Standard Test Method for API Gravity of Crude Petroleum and Petroleum Products (Hydrometer Method) West Conshohocken, PA: ASTM International; 2019. DOI: 10.1520/D0287-12BR19. [Google Scholar]
- 29.ASTM D1218-12, Standard Test Method for Refractive Index and Refractive Dispersion of Hydrocarbon Liquids. West Conshohocken, PA: ASTM International; 2016. DOI: 10.1520/D1218-12R16. [Google Scholar]
- 30.ASTM D1331-89, Standard Test Methods for Surface and Interfacial Tension of Solutions of Surface-Active Agents. West Conshohocken, PA: ASTM International; 1989. DOI: 10.1520/D1331-89R95. [Google Scholar]
- 31.Kantarci G, Ozguney I, Karasulu HY, Arzik S, Guneri T. Comparison of different water/oil microemulsions containing diclofenac sodium: preparation, characterization, release rate, and skin irritation studies. AAPS PharmSciTech. 2007;8(4):E91–E97. doi: 10.1208/pt0804091. DOI: 10.1208/pt0804091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zivanovic L, Zecevic M, Markovic S, Petrovic S, Ivanovic I. Validation of liquid chromatographic method for analysis of lidocaine hydrochloride, dexamethasone acetate, calcium dobesilate, buthylhydroxyanisol and degradation product hydroquinone in suppositories and ointment. J Chromatogr A. 2005;1088(1-2):182–186. doi: 10.1016/j.chroma.2005.04.049. DOI: 10.1016/j.chroma.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 33.Patri S, Patni AK, Iyer SS, Khuroo AH, Monif T, Rana S, et al. A validated high-performance liquid chromatography-tandem mass spectrometric (LC-MS/MS) method for simultaneous determination of R(+)-ketorolac and S(-)-ketorolac in human plasma and its application to a bioequivalence study. Chromatogr Res Int. 2011;2011:214793,1–11. DOI: 10.4061/2011/214793. [Google Scholar]
- 34.Takeuchi H, Mano Y, Terasaka S, Sakurai T, Furuya A, Urano H, et al. Usefulness of rat skin as a substitute for human skin in the in vitro skin permeation study. Exp Anim. 2011;60(4):373–384. doi: 10.1538/expanim.60.373. DOI: 10.1538/expanim.60.373. [DOI] [PubMed] [Google Scholar]
- 35.Shevachman M, Belfer S, Binman S, Shani A. Chemical binding of jojoba liquid wax to polyethylene. J Am Oil Chem Soc. 2001;78(3):223–238. DOI: 10.1007/s11746-001-0249-4. [Google Scholar]
- 36.Schulten HR, Murray KE, Simmleit N. Natural waxes investigated by soft ionization mass spectrometry. Z Naturforsch C. 1987;42(3):178–190. DOI: 10.1515/znc-1987-0302. [Google Scholar]
- 37.Talegaonkar S, Azeem A, Ahmad FJ, Khar RK, Pathan SA, Khan ZI. Microemulsions: a novel approach to enhanced drug delivery. Recent Pat Drug Deliv Formul. 2008;2(3):238–257. doi: 10.2174/187221108786241679. DOI: 10.2174/187221108786241679. [DOI] [PubMed] [Google Scholar]
- 38.Agarwal S, Arya D, Khan S. Comparative fatty acid and trace elemental analysis identified the best raw material of jojoba (Simmondsia chinensis) for commercial applications. Ann Agric Sci. 2018;63(1):37–45. DOI: 10.1016/j.aoas.2018.04.003. [Google Scholar]
- 39.El-Hadidy GN, Ibrahim HK, Mohamed MI, El-Milligi MF. Microemulsions as vehicles for topical administration of voriconazole: formulation and in vitro evaluation. Drug Dev Ind Pharm. 2012;38(1):64–72. doi: 10.3109/03639045.2011.590731. DOI: 10.3109/03639045.2011.590731. [DOI] [PubMed] [Google Scholar]
- 40.National Center for Biotechnology Information. PubChem Compound Summary for CID 8103, 1-Hexanol. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/1-Hexanol .
- 41.Yuan JS, Ansari M, Samaan M, Acosta EJ. Linker-based lecithin microemulsions for transdermal delivery of lidocaine. Int J Pharm. 2008;349(1-2):130–143. doi: 10.1016/j.ijpharm.2007.07.047. DOI: 10.1016/j.ijpharm.2007.07.047. [DOI] [PubMed] [Google Scholar]
- 42.Yuan JS, Yip A, Nguyen N, Chu J, Wen XY, Acosta EJ. Effect of surfactant concentration on transdermal lidocaine delivery with linker microemulsions. Int J Pharm. 2010;392(1-2):274–284. doi: 10.1016/j.ijpharm.2010.03.051. DOI: 10.1016/j.ijpharm.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Wang X, Wang X, Song Y, Wang X, Hao J. Design and development of lidocaine microemulsions for transdermal delivery. AAPS PharmSciTech. 2019;20(2):63–61. doi: 10.1208/s12249-018-1263-1. DOI: 10.1208/s12249-018-1263-1. [DOI] [PubMed] [Google Scholar]
- 44.Nasseri AA, Reza A, Zia H, Needham TE. Lecithin-stabilized microemulsion-based organogels for topical application of ketorolac tromethamine. II. In vitro release study. IJPR. 2003;2:117–123. [Google Scholar]
- 45.Choi JS, Cho YA, Chun IK, Jung SY, Gwak HS. Formulation and evaluation of ketorolac transdermal systems. Drug Deliv. 2007;14(2):69–74. doi: 10.1080/10717540600640336. DOI: 10.1080/10717540600640336. [DOI] [PubMed] [Google Scholar]
- 46.Chandra A, Sharma PK, Irchhiaya R. Effect of alcohols and enhancers on permeation enhancement of ketorolac. Asian J Pharm. 2009;3(1):37–42. DOI: 10.22377/ajp.v3i1.239. [Google Scholar]
- 47.Salimi A, Jafarinezhad S, Kalantari A. Transdermal delivery of ketorolac tromethamine using microemulsion vehicles. Jundishapur J Nat Pharm Prod. 2018;13(4):e69056,1–8. DOI: 10.5812/jjnpp.69056. [Google Scholar]