Abstract
Background Context:
Back and neck pain secondary to disc degeneration is a major public health burden. There is a need for therapeutic treatments to restore intervertebral disc (IVD) composition and function.
Purpose:
To quantify ALK3, BMP-2, pSMAD1/5/8 and MMP-13 expression in IVD specimens collected from patients undergoing surgery for disc degeneration, to correlate ALK3, BMP-2, pSMAD1/5/8 and MMP-13 expression in IVD specimens to the 5-level Pfirrmann MRI grading system, and to compare ALK3, BMP-2, pSMAD1/5/8 and MMP-13 expression between cervical and lumbar degenerative disc specimens.
Study Design:
An immunohistochemical study assessing ALK3, BMP-2, pSMAD1/5/8, and MMP-13 expression levels in human control and degenerative IVD specimens.
Methods:
Human IVD specimens were collected from surgical patients who underwent discectomy and interbody fusion at our institution between 1/2015 and 8/2017. Each patient underwent MRI prior to surgery. The degree of disc degeneration was measured according to the 5-level Pfirrmann MRI grading system. Patients were categorized into either the 1) control group (Pfirrmann grades I-II) or 2) degenerative group (Pfirrmann grades III-V). Histology slides of the collected IVD specimens were prepared and immunohistochemical staining was performed to assess ALK3, BMP-2, pSMAD1/5/8, and MMP-13 expression levels in the control and degenerative specimens. Expression levels were also correlated to the Pfirrmann criteria. Lastly, the degenerative specimens were stratified according to their vertebral level and expression levels between the degenerative lumbar and cervical discs were compared.
Results:
Fifty-two patients were enrolled; however, 2 control and 2 degenerative patients were excluded due to incomplete data sets. Of the remaining 48 patients, there were 12 control and 36 degenerative specimens. Degenerative specimens had increased expression levels of BMP-2 (p = 0.0006) and pSMAD1/5/8 (p < 0.0001). Pfirrmann grade 3 (p = 0.0365) and grade 4 (p = 0.0008) discs had significantly higher BMP-2 expression as compared to grade 2 discs. Pfirrmann grade 4 discs had higher pSMAD1/5/8 expression as compared to grade 2 discs (p < 0.0001). There were no differences in ALK3 or MMP-13 expression between the control and degenerative discs (p > 0.05). Stratifying the degenerative specimens according to their vertebral level showed no significant differences in expression levels between the lumbar and cervical discs (p > 0.05).
Conclusions:
BMP-2 and pSMAD1/5/8 signaling activity was significantly upregulated in the human degenerative specimens, while ALK3 and MMP-13 expression were not significantly changed. The expression levels of BMP-2 and pSMAD1/5/8 correlate positively with the degree of disc degeneration measured according to the Pfirrmann MRI grading system.
Clinical Significance:
BMP-SMAD signaling represents a promising therapeutic target to restore IVD composition and function in the setting of disc degeneration.
Keywords: Bone morphogenic protein, BMP-2, pSMAD1/5/8, ALK3, MMP-13, disc degeneration, Pfirrmann, MRI, proteoglycan, disc herniation
INTRODUCTION
Back and neck pain as a result of disc degeneration is a major public health burden [1, 2]. Up to 80% of the population will be affected with back pain at some point in their lifetime and 10% may become chronically disabled [3, 4]. In 2005, an estimated $86 billion was spent in the United States for the management of neck and back problems [5]. Etiology of disc degeneration has been attributed to a multitude of factors including environment, atherosclerosis, genetics, nicotine use, obesity, occupation, and low vitamin D levels [6, 7]. Clinically, the management of axial neck and back pain is with physical therapy and non-steroidal anti-inflammatory medications [8]. There is a limited role for spinal fusion for axial symptoms [9–13]. Thus, there is currently a need for therapeutic treatments to restore intervertebral disc (IVD) composition and function in the setting of disc degeneration.
The IVD, which functions as a shock absorber between adjacent vertebral bodies and allows for motion of the spine, is composed of an annulus fibrosus, nucleus pulposus (NP), and two cartilaginous endplates [6]. The annulus fibrosus has an inner and outer layer and is predominantly composed of type I collagen. It is responsible for tensile strength. The NP is composed mainly of NP cells and proteoglycan aggrecans enmeshed in a loose type II collagen matrix that provides compressive strength to the disc [14, 15]. Throughout development and aging, the extracellular matrix (ECM) within the IVD undergoes extensive remodeling via anabolic and catabolic processes. A disruption in ECM homeostasis leading to increased catabolic activity (mediated by matrix metalloproteinases [MMPs], disintegrins, metalloproteinases with thrombospondin motifs [ADAMTS], and positively regulated by inflammatory cytokines such as interleukin 1- ß [IL-1 ß], IL-6, IL-8, prostaglandin E2 and nitric oxide) and decreased anabolic activity (mediated by bone morphogenetic proteins [BMPs], transforming growth factor-ß [TGF-ß] and insulin-like growth factor [IGF]) ultimately results in disc degeneration characterized by a loss of proteoglycans and dehydration of the NP [16–18]. As disc degeneration progresses, nociceptive nerve fibers and blood vessels can grow on the annulus fibrosus resulting in back pain [19, 20]. Fissures can progress through the annulus fibrosus, leading to disc herniation and subsequent radiculopathy.
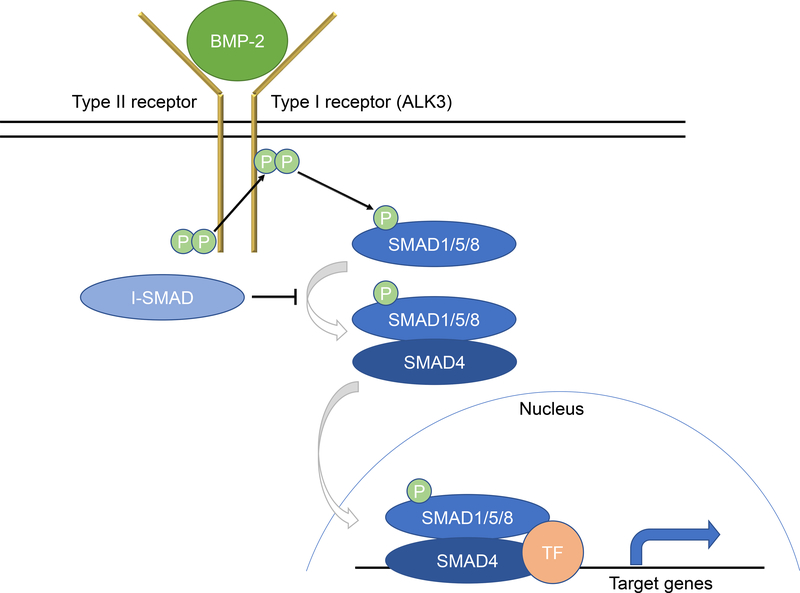
BMPs are members of the TGF-ß superfamily and are critical in the growth and differentiation of osteoblasts, chondroblasts, neurons, and epithelial cells [21]. BMP signal transduction occurs through two cell surface serine/threonine kinase type I and type II receptors (Fig. 1) [22–25]. Of the type I receptors, Activin receptor like-kinase 3 (ALK3) is responsible for binding BMP-2 [25]. There are three type II receptors (BMPR II, ActR-II, ActR-IIB). Once BMP-2 binds, the type II receptor phosphorylates the type I ALK3 receptor. The ALK3 receptor in turn initiates downstream signaling via the SMAD pathway. pSMAD1/5/8 binds to SMAD4 and together translocate to the nucleus where transcription of downstream target genes is regulated [26].
Figure 1. Schematic of the BMP-2 signaling pathway.
Upon BMP-2 binding to two serine/threonine kinase receptors (type I and type II), the type II receptor transphosphorylates the type I receptor (e.g., ALK3). After becoming active, the type I receptor phosphorylates SMAD1/5/8 to propagate the signal into the cell. pSMAD1/5/8 then forms a hetero-oligomeric complex with SMAD4, where together they translocate to the nucleus to interact with specific transcription factors (TF) to regulate target gene expression.
MMPs are members of the zinc-dependent endopeptidase family and are believed to be a primary driver of catabolic activity during disc degeneration [27]. MMPs can be categorized into 6 groups: 1) collagenases (MMP-1, −8, −13, and −18), 2) gelatinases (MMP-2 and −9), 3) stromelysins (MMP-3, −10, and −11), 4) matrilysins (MMP-7 and −26), 5) membrane-type MMPs (MMP-14, −17, −24, and −25), and 6) other non-classified MMPs (MMP-12, −19, −20, −21, −23, −27, and −28). They are secreted in an inactive form and require extracellular activation by regulatory proteins to have physiologic effects. In particular, MMP-13 is secreted by cells as a 60-kDA precursor form (ProMMP-13), and then activated to a 48-kDA form by plasmin and other MMPs [28, 29]. MMP-13 has demonstrated the ability to degrade a variety of ECM proteins, including type I and type II collagen, aggrecan, fibronectin, tenascin, osteonectin, laminin, perlecan, and gelatin [30].
The roles of BMP-2 and MMP-13 in the etiology or prevention of disc degeneration are areas of active investigation. In-vitro and in-vivo studies have used adenoviruses expressing BMP-2 to evaluate the anabolic reaction on IVDs [7, 21, 31–34]. These studies have shown that BMP-2 has anabolic effects and represent a potential agent for treating disc degeneration. Alternatively, other studies have proposed inhibiting MMP-13 activity as a strategy to slow the progression of disc degeneration [27]. Therefore, the objective of this study was threefold: 1) To quantify ALK3, BMP-2, pSMAD1/5/8, and MMP-13 expression in IVD specimens collected from patients undergoing surgery for disc degeneration; 2) To correlate ALK3, BMP-2, pSMAD1/5/8, and MMP-13 expression in IVD specimens to the Pfirrmann MRI grading system; and 3) To compare ALK3, BMP-2, pSMAD1/5/8, and MMP-13 expression between cervical and lumbar degenerative disc specimens.
MATERIAL & METHODS
Patients and Intervertebral Disc Specimens
This study was approved by our institution’s Institutional Review Board, STUDY00000577. Human IVD specimens (including annulus fibrosus and NP) were collected from patients who underwent discectomy and interbody fusion at our institution between 1/2015 and 8/2017. The dissected discs were placed into a sterile container and then transferred into a separate container with 4% paraformaldehyde. Patients were categorized into one of two groups based on their clinical presentation: 1) patients with disc degeneration presenting with cervical or lumbar disc herniation, spinal stenosis, or spondylolisthesis (degenerative group) or 2) patients without disc degeneration being treated for spinal fracture, tumor, or scoliosis (control group). Exclusion criteria for both groups included patients with a history of infection of the spine. Patient characteristics were collected. Magnetic resonance imaging (MRI) was taken of all patients prior to surgery.
Pfirrmann MRI Grading System
The degree of disc degeneration was assessed for each patient using the 5-level Pfirrmann MRI grading system on the T-2 weighted sagittal MRI sequences. The Pfirrmann grades are summarized in Table 1 [35]. Patients with grades I and II, which indicate non-degenerative discs on T2-weighted MRI, were assigned to the control group. Patients with grades III, IV, and V, which indicate degenerative discs, were assigned to the degenerative group. Immunohistochemical expression levels of ALK3, BMP-2, pSMAD1/5/8, and MMP-13 in the control and degenerative specimens were compared. The expression levels were also compared across the Pfirrmann grades. This was done to evaluate whether the degree of disc degeneration that can be assessed on T2-weighted MRI correlates with BMP-2 receptor signaling or MMP-13 activity. In addition, the degenerative specimens were stratified according to their vertebral level and expression levels between the degenerative lumbar and cervical discs were compared. This analysis was done to determine whether BMP-2 receptor signaling or MMP-13 activity in the degenerative specimens vary significantly based on the vertebral level.
Table 1:
Pfirrmann Classification of Intervertebral Disc Degeneration
| Grade | Structure | Distinction of Nucleus and Anulus | Signal Intensity | Height of Intervertebral Disc |
|---|---|---|---|---|
| I | Homogenous, bright white | Clear | Hyperintense, isointense to cerebrospinal fluid | Normal |
| II | Inhomogenous with or without horizontal bands | Clear | Hyperintense, isointense to cerebrospinal fluid | Normal |
| III | Inhomogenous, gray | Unclear | Intermediate | Normal to slightly decreased |
| IV | Inhomogenous, gray to black | Lost | Intermediate to hypointense | Normal to moderately decreased |
| V | Inhomogenous, black | Lost | Hypointense | Collapsed disc space |
Histology and Immunohistochemistry
After collection, the human IVD specimens were fixed in 4% paraformaldehyde, decalcified in 10% ethylenediaminetetraacetic acid (EDTA), and embedded in paraffin. Three-μm sections were prepared and immunohistochemistry was performed using goat polyclonal anti-ALK3 (BMPR-1A) (Santa Cruz Biotechnology, Dallas, TX), goat polyclonal anti-BMP-2 (Abcam, Cambridge, MA), rabbit polyclonal anti-phosphorylated-SMAD1/5/8 (Cell Signaling Technology, Danvers, MA), and human MMP-13 antibody (R&D Systems, Minneapolis, MN). Permeabilization was done with 0.1% Triton in a TRIS-citrate retrieval buffer at 95°C for 15 min. To block endogenous peroxidase activity, sections were treated with methanol/1% H2O2 for 30 min. After the wash, sections were blocked with TBS/0.1% triton/10% normal serum for 1 hr, followed by incubation with the primary antibody for 1 hr and with the corresponding HRP-conjugated secondary antibody. Signal was developed with DAB substrate and slides counterstained with hematoxylin. Slides were photographed. ALK3, BMP-2, and pSMAD1/5/8 slides were analyzed for positive and total cell count using OsteoMeasure software (Osteometrics, Decatur, Georgia). Expression levels are expressed as a percent ratio of positive cell count to total cell count. MMP-13 slides were analyzed for total signal intensity per area using ImageJ software (National Institutes of Health, Bethesda, MD).
Statistical Analysis
All statistical analyses were performed in GraphPad Prism 9.0 (GraphPad Software, San Diego, CA). A Mann-Whitney U test was used to analyze patient characteristic data and to compare expression levels of ALK3, BMP-2, pSMAD1/5/8, and MMP-13. A Kruskal-Wallis test and Dunn’s multiple comparison was used to correlate the Pfirrmann grades with IVD expression levels of ALK3, BMP-2, pSMAD1/5/8, and MMP-13. Significance level was set at p < 0.05.
RESULTS
Patient Characteristics
Fifty-two patients were enrolled in the study; however, 2 control and 2 degenerative patients were excluded due to incomplete data sets. Of the 48 remaining patients, 36 IVD specimens were collected from patients with disc degeneration (15 males and 21 females; range 19–82 years) and 12 IVD specimens were collected from control patients (6 males and 6 females; range 14–66 years). There were no significant differences in age, weight, height, body mass index, or vitamin D levels between the two groups (p > 0.05). Patient characteristics are summarized in Table 2.
Table 2:
Patient Characteristics
| Control (n=12) | Degenerative (n=36) | P-value | |
|---|---|---|---|
| Age, years (mean ± SD) | 39.8 ± 17.6 | 44.2 ± 15.2 | 0.4763 |
| Male:Female | 6:6 | 15:21 | |
| Race | |||
| Caucasian | 12 | 28 | |
| African American | 0 | 6 | |
| Asian American | 0 | 1 | |
| Other | 0 | 1 | |
| Weight, kg (mean ± SD) | 81.9 ± 28.5 | 88.9 ± 20.7 | 0.1066 |
| BMI, kg/m2 (mean ± SD) | 27.3 ± 7.5 | 31.2 ± 7.5 | 0.0984 |
| Height, m (mean ± SD) | 1.7 ± 0.1 | 1.7 ± 0.1 | 0.2620 |
| Vitamin D, ng/mL (mean ± SD) | 34.9 ± 14.1 | 25.3 ± 9.7 | 0.2657 |
| Smoking | |||
| Current | 2 | 9 | |
| Former | 3 | 7 | |
| Never | 7 | 20 | |
| EBL | 384.1 ± 526.3 | 163.5 ± 370.6 | 0.0989 |
| Pfirrmann grade | 1.75 ± 0.5 | 3.9 ± 0.6 | < 0.0001 |
| Diagnosis | |||
| Facet slip/fracture | 3 | 0 | |
| Vertebral body fracture | 4 | 0 | |
| Tumor | 3 | 0 | |
| Scoliosis | 1 | 0 | |
| Other | 1 | 0 | |
| Disc herniation | 0 | 28 | |
| Spondylolisthesis | 0 | 6 | |
| Stenosis | 0 | 2 | |
| Procedure | |||
| Anterior cervical fusion | 11 | 9 | |
| Lumbar discectomy | 0 | 21 | |
| Lumbar posterior spinal fusion | 1 | 6 | |
| Immunohistochemical Staining (mean ± SD) | |||
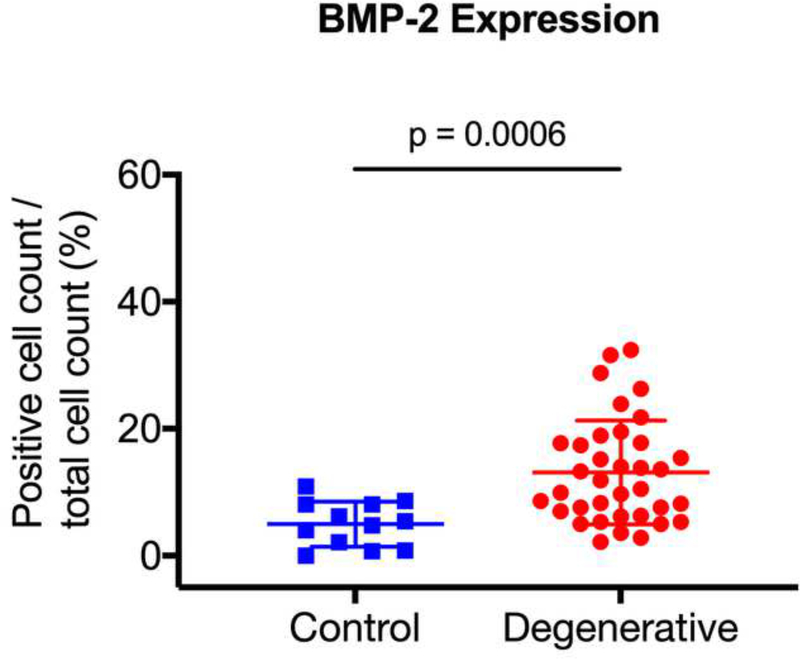
| BMP-2 | 5.0 ± 0.03 | 13.1 ± 0.08 | 0.0006 |
| SMAD1/5/8 | 3.9 ± 0.04 | 15.4 ± 0.09 | < 0.0001 |
| ALK3 | 7.5 ± 0.1 | 8.5 ± 0.1 | 0.7903 |
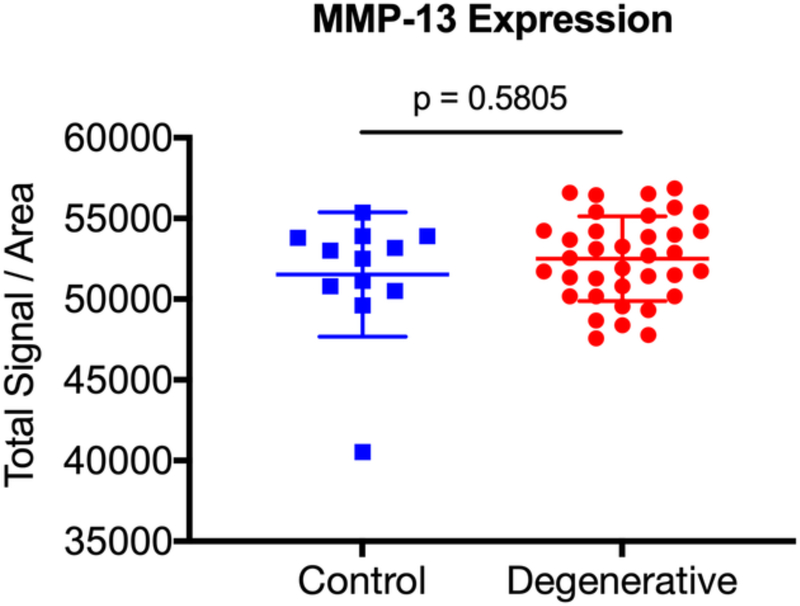
| MMP-13 | 51526.3 ± 3855 | 52509.6 ± 2617 | 0.5805 |
Abbreviations: BMI, body mass index; SD, standard deviation; BMP-2, bone morphogenic protein-2; ALK3, Activin receptor like-kinase 3; MMP-13, matrix metalloproteinase-13.
Increased Expression of BMP-2 and pSMAD1/5/8 in Human Degenerated Discs
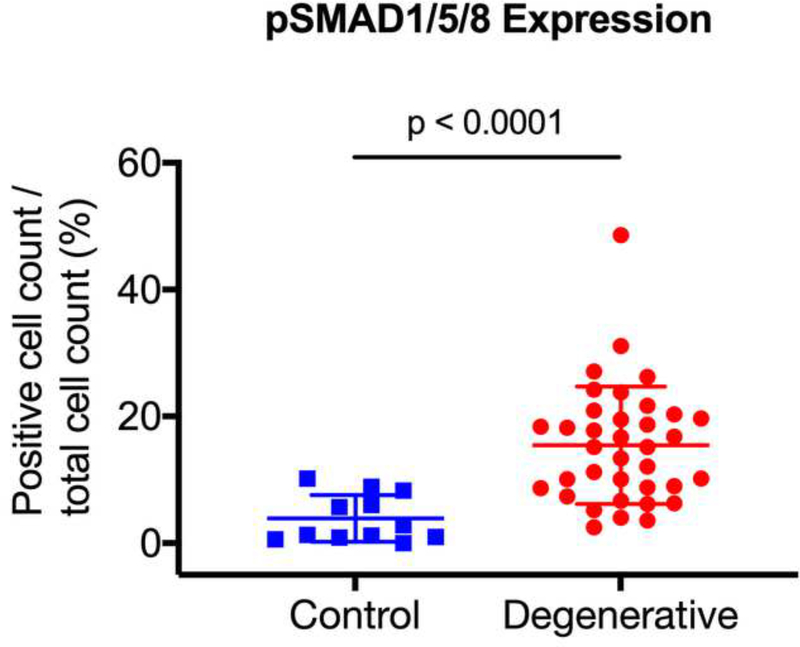
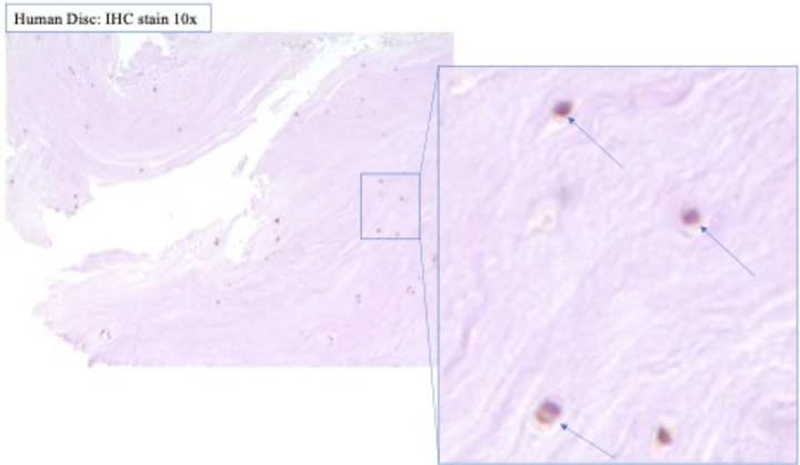
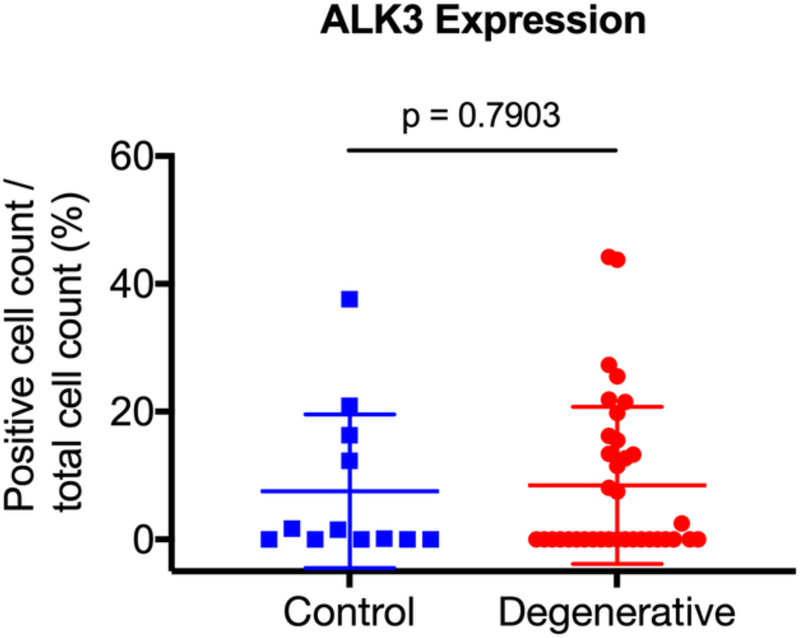
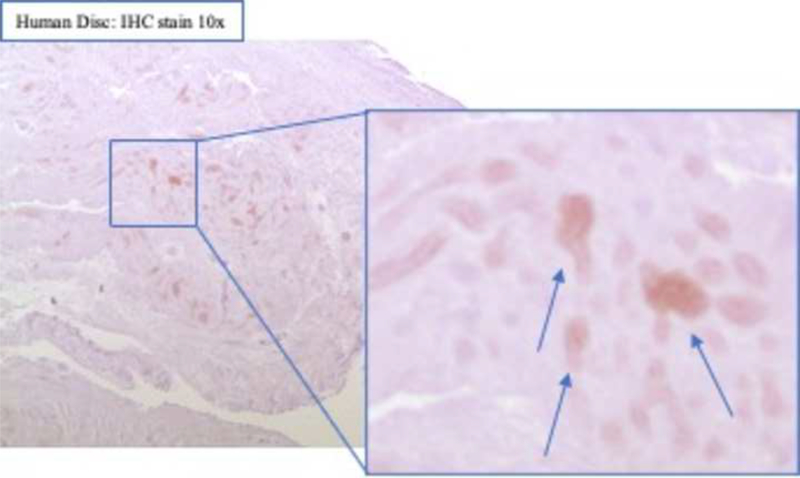
After surgical collection of the IVD specimens, immunohistochemical staining for ALK3, BMP-2, pSMAD1/5/8, and MMP-13 was performed (Fig. 2a). Staining revealed positive cell cluster formation in the degenerative specimens for BMP-2 (Fig. 2b). BMP-2 expression was significantly elevated in the degenerative specimens as compared to the control specimens (p = 0.0006) (Fig. 2c). Staining also revealed positive cell cluster formation in the degenerative specimens for pSMAD1/5/8 (Fig. 3a), which was significantly elevated as compared to the control specimens (p < 0.0001) (Fig. 3b). However, no significant difference in ALK3 expression was observed between the degenerative and control specimens (p = 0.7903 (Fig. 4a-b). Similarly, no significant difference in MMP-13 expression was present between the degenerative and control specimens (p = 0.5805) (Fig. 5a-b). Altogether, these results suggest that BMP-SMAD signal activity is upregulated during disc degeneration, however, catabolic activity, measured by MMP-13 expression levels, was not significantly changed in the degenerative specimens.
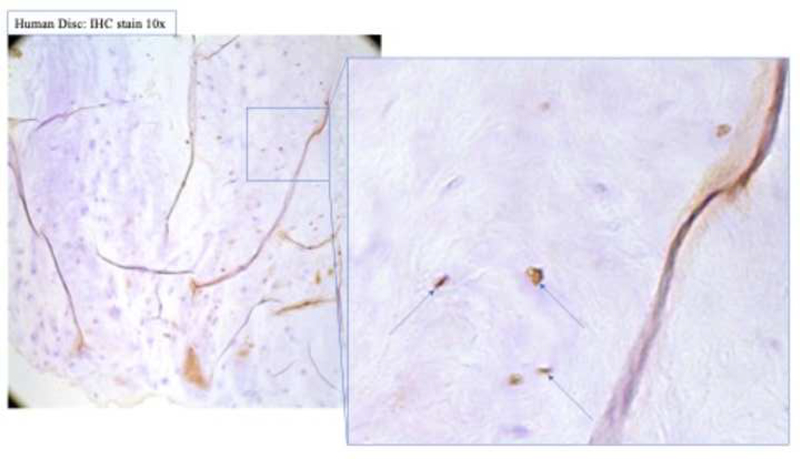
Figure 2. BMP-2 expression was significantly upregulated in human degenerated discs.
(a) Surgically collected IVD specimens were fixed in 4% paraformaldehyde, decalcified in 10% ethylenediaminetetraacetic acid, and embedded in paraffin. Immunohistochemical staining for ALK3, BMP-2, pSMAD1/5/8, and MMP-13 expression was performed. (b) Staining revealed positive cell cluster formation in the degenerative specimens for BMP-2. (c) BMP-2 expression was significantly upregulated in the degenerative specimens as compared to the control specimens.
Figure 3. pSMAD1/5/8 expression was significantly upregulated in human degenerated discs.
(a) Staining revealed positive cell cluster formation in the degenerative specimens for pSMAD1/5/8. (b) pSMAD1/5/8 expression was significantly upregulated in the degenerative discs as compared to the control specimens.
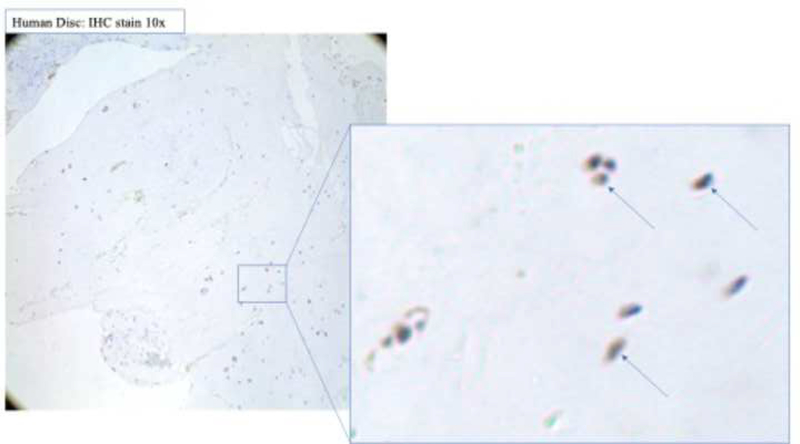
Figure 4. ALK3 expression does not significantly change in human degenerated discs.
(a) Staining revealed positive cell cluster formation in the degenerative specimens. (b) ALK3 expression was not significantly different in the degenerative discs as compared to the control specimens.
Figure 5. MMP-13 expression does not significantly change in human degenerated discs.
(a) Staining revealed positive signal intensity in the degenerative specimens. (b) MMP-13 expression was not significantly different between the degenerative discs and the control specimens.
Expression of BMP-2 and pSMAD1/5/8 Correlate with Pfirrmann MRI Grades
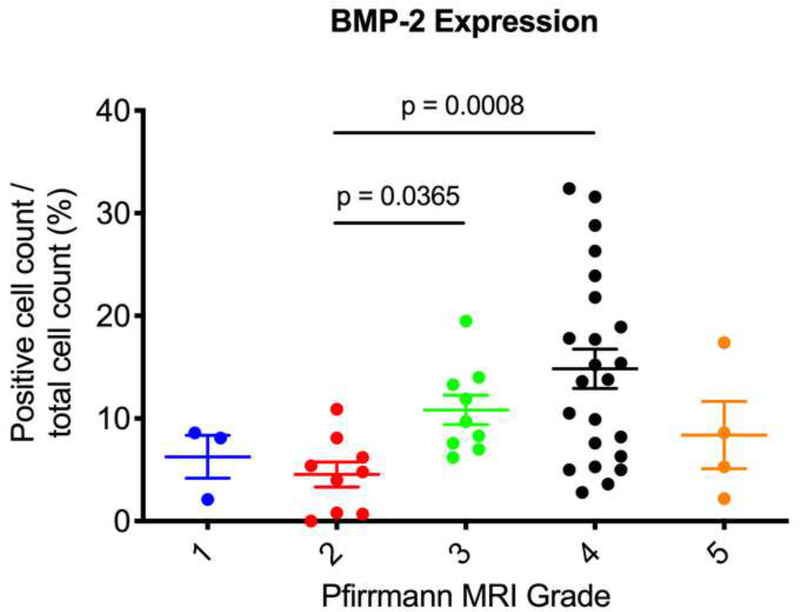
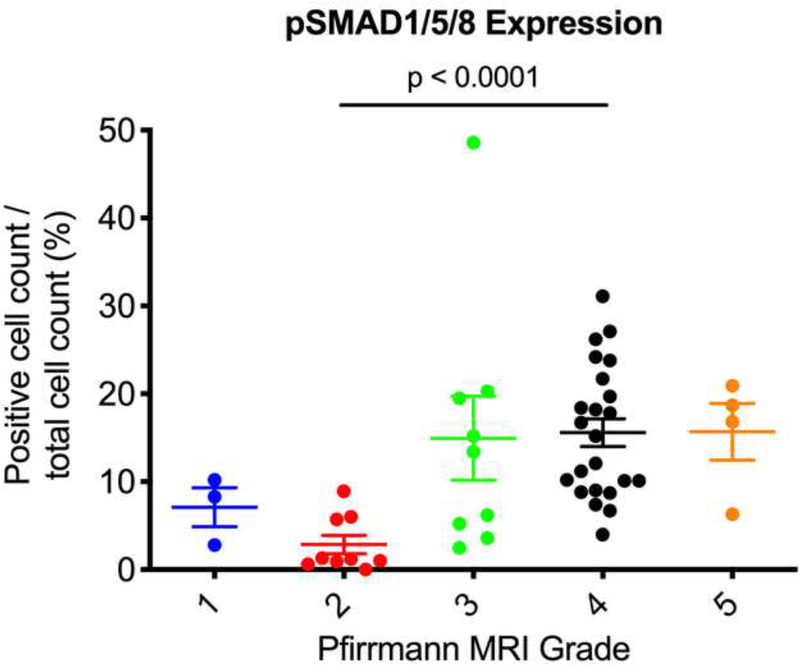
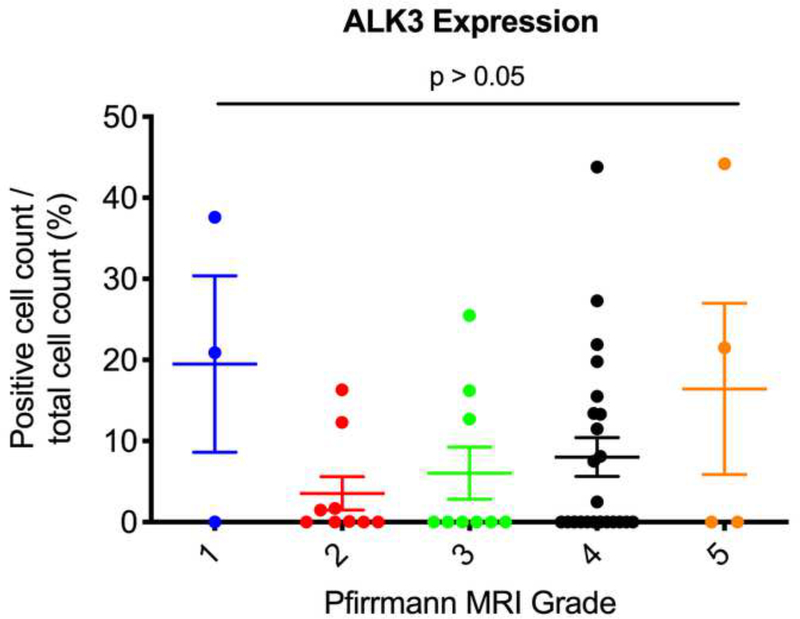
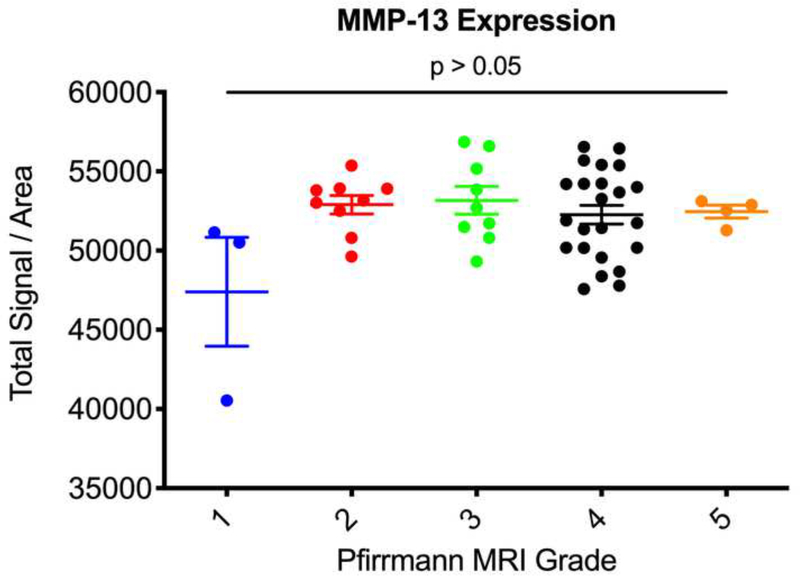
Next, we used the Pfirrmann MRI grading system to correlate the degree of disc degeneration to expression levels of ALK3, BMP-2, pSMAD1/5/8, and MMP-13. We found that Pfirrmann grade 3 discs (n = 9) had significantly upregulated BMP-2 expression as compared to grade 2 discs (n = 9) (p = 0.0365) (Fig. 6a). In addition, Pfirrmann grade 4 discs (n = 23) had significantly upregulated expression of BMP-2 as compared to grade 2 discs (p = 0.0008). Grade 4 discs had increased BMP-2 expression as compared to grade 3 discs, though this was nonsignificant (p = 0.6288). In regard to pSMAD1/5/8, Pfirrmann grade 4 degenerated discs had significantly higher expression levels as compared to grade 2 discs (p < 0.0001) (Fig. 6b). Though nonsignificant, Pfirrmann grade 3 discs had increased pSMAD1/5/8 expression as compared to grade 2 discs (p = 0.2430). Also, there were no significant differences in ALK3 or MMP-13 expression across any of the Pfirrmann grades (p > 0.05) (Fig. 6c-d). Altogether, these findings suggest that BMP-2 and pSMAD1/5/8 expression correlate positively with the degree of disc degeneration, while ALK3 and MMP-13 activity do not change depending on the severity of disc degeneration, measured according to the Pfirrmann MRI grading system.
Figure 6. BMP-2 and pSMAD1/5/8 expression correlate with Pfirrmann MRI grades.
(a) Pfirrmann grade 3 and grade 4 discs had significantly higher BMP-2 expression as compared to Pfirrmann grade 2 discs. (b) Pfirrmann grade 4 discs had significantly higher pSMAD1/5/8 expression as compared to Pfirrmann grade 2 discs. (c) No significant differences in ALK3 expression were observed across the Pfirrmann grades. (d) No significant differences in MMP-13 expression were observed across the Pfirrmann grades.
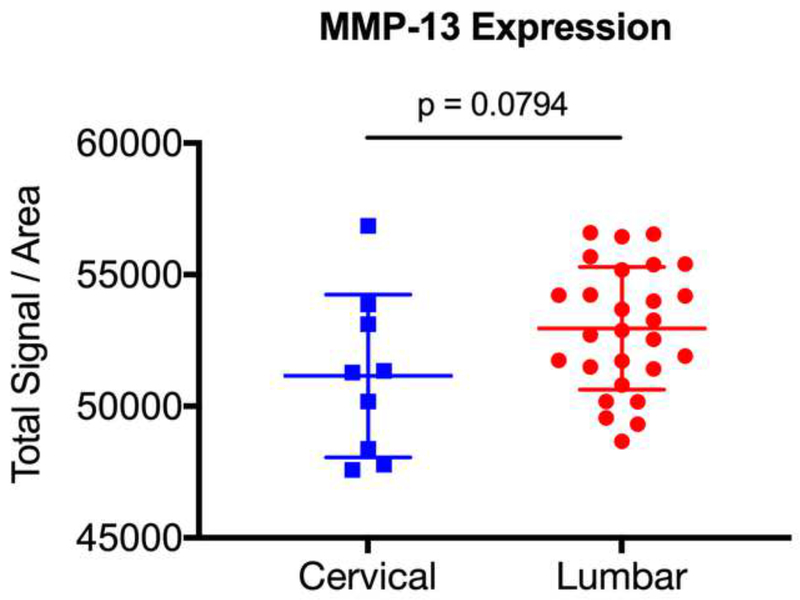
Expression Among Degenerated Discs Do Not Differ Based on Vertebral Level
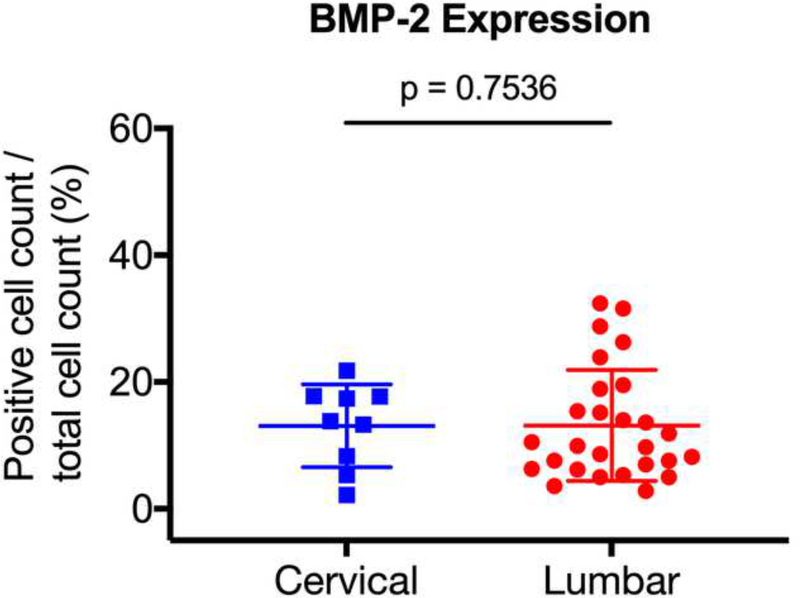
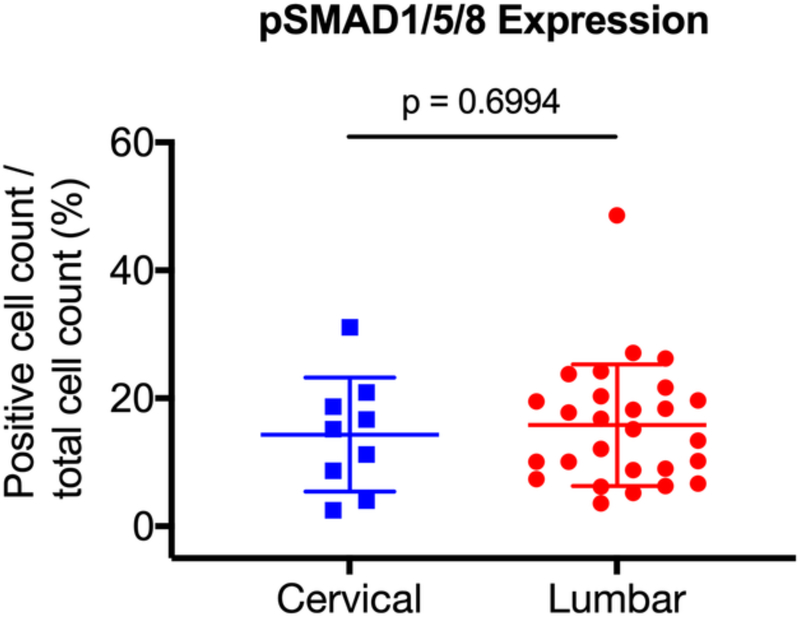
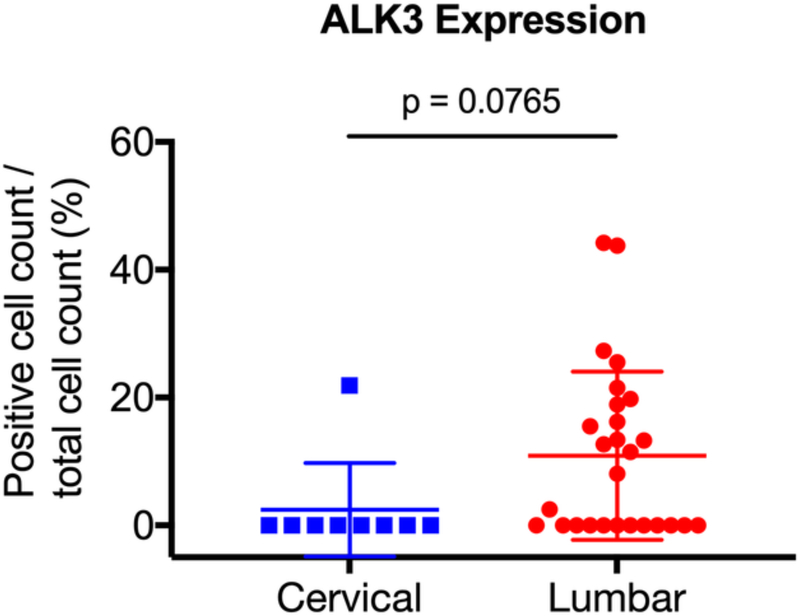
Previous studies have suggested that degenerative disc pathology differs according to the vertebral level [36]. As such, we sought to determine whether there was differential expression of ALK3, BMP-2, pSMAD1/5/8, and MMP-13 between the degenerative lumbar and cervical discs. The degenerative specimens were stratified into lumbar (n = 27) and cervical (n = 9) discs, and expression levels were compared. We found no significant difference in expression between the degenerative lumbar and cervical discs for BMP-2 (p = 0.7536), pSMAD1/5/8 (p = 0.6994), ALK3 (p = 0.0765), or MMP-13 (p = 0.0794) (Fig. 7a-d). These findings suggest that the BMP-SMAD signal and MMP-13 activity during disc degeneration do not significantly differ based on the vertebral level.
Figure 7. BMP-SMAD expression in degenerated discs do not vary based on the vertebral level.
The degenerative specimens were stratified into lumbar (n = 27) and cervical (n = 9) discs, and expression levels were compared. No significant differences in expression were observed between the degenerative lumbar and cervical discs for (a) BMP-2, (b) pSMAD1/5/8, (c) ALK3, and (d) MMP-13.
DISCUSSION
Disc degeneration is characterized by a loss of proteoglycans from the ECM of the IVD, changing the compressive load on the vertebral bodies and causing injurious changes to the spine [37, 38]. It has been proposed that as IVDs age and degenerate, there is a physiologic compensatory upregulation of BMPs and their downstream signaling receptors as an anabolic response. This notion is supported by two studies comparing IVD gene expression between young and old New Zealand white (NZW) rabbits and reporting significantly higher mRNA levels of BMP-2 in the older rabbits [39, 40]. Similarly, an immunohistochemical study of rodent cervical spines found a differential expression of BMP-2 between young and old mice [41]. Furthermore, investigations using an external fixation device to induce lumbar disc degeneration in NZW rabbits found upregulated BMP-2 gene expression [42, 43].
In our study, we compared BMP-2 receptor signaling expression between human degenerative and non-degenerative IVDs. Our results indicate a higher expression level of both BMP-2 and pSMAD1/5/8 in human degenerative discs as compared to control specimens. Consistent with previous studies, we believe that this increased expression represents a protective and anabolic response to disc injury and degeneration. Of significant note, we found no upregulated ALK3 expression in the degenerative specimens. Though ALK3 represents the type IA receptor that binds BMP-2, it is possible that the upregulated BMP-SMAD signal activity in response to disc degeneration is mediated via a type IB receptor or another yet to be determined pathway. It is also possible that in these conditions, ALK3 gene simply does not transcriptionally respond to increased BMP-SMAD signaling.
In addition to quantifying expression levels in IVDs of control and degenerative patients, we also attempted to correlate ALK3, BMP-2, pSMAD1/5/8, and MMP-13 expression to the Pfirrmann MRI grading system. The Pfirrmann grading system is a validated tool to evaluate degenerative abnormalities on T2-weighted sagittal MRI. It classifies IVD degeneration based on signal intensity, disc structure, distinction between nucleus and annulus, and disc height [44]. In our study, Pfirrmann grade 3 IVD specimens had significantly higher BMP-2 expression as compared to the healthier grade 2 discs. Also, Pfirrmann grade 4 IVD specimens showed significantly higher BMP-2 and pSMAD1/5/8 expression as compared to grade 2 discs. No significant differences were observed across the other Pfirrmann grades. Our results demonstrate a positive correlation between BMP-SMAD activity and the degree of disc degeneration measured on MRI according to the Pfirrmann criteria. Interestingly, it has been reported that MRI signal change correlates directly with proteoglycan concentration within the IVD [45]. While degenerated discs are known to have proteoglycan loss, the upregulated BMP-SMAD signaling that we observed in the degenerative discs likely represents an insufficient anabolic response to injury. The control discs, on the other hand, had lower BMP-2 and pSMAD1/5/8 expression likely because there was less inciting injury prompting a compensatory anabolic response.
The anabolic role of BMP-2 in the IVD has been well documented in animal models. In a study of NZW rabbits that received stab injuries to their lumbar spine, there was a reported delayed yet upregulated expression of BMP-2, suggesting an inability of the IVDs to mount an early anabolic response to disc injury [46]. The authors proposed that early use of exogenous BMPs in the setting of disc degeneration may be beneficial in promoting reparative processes. Several studies have shown in vitro that human IVD cells transfected with recombinant human BMP-2 promote a chondrocyte-like phenotype with increased production of aggrecan and type II collagen, as well as an increase in proteoglycan synthesis [32, 47]. Similarly, a study utilizing simvastatin containing polymer gels injected into a rat tail degenerative disc model to upregulate BMP-2 expression found an increase in aggrecan expression, as well as a significant improvement in NP weight, T2 signal intensity, and MRI index [33]. In addition to promoting cellular proliferation within the IVD [48], BMP-2 has also been shown to have anti-catabolic effects in human NP cells by antagonizing IL-18 mediated disc degeneration [49]. Accordingly, these studies in conjunction with our results demonstrate the potential utility of upregulating BMP-SMAD signaling as a therapeutic strategy for treatment of disc degeneration. Given that we found no significant difference in upregulated BMP-SMAD activity between the degenerative lumbar and cervical discs, we propose that promoting BMP-SMAD signaling would be beneficial in both lumbar and cervical degenerative disc pathology.
Our study also found no significant difference in MMP-13 expression when comparing between the control and degenerative specimens, as well as when comparing across the Pfirrmann grades. However, the grade 1 discs had noticeably lower expression levels compared to the grade 2–5 discs. While the low sample size for grade 1 discs precluded our ability to infer statistical significance, our results may suggest that the mildest degree of disc degeneration is associated with increased catabolic activity, though beyond Pfirrmann grade 2 discs, MMP-13 activity is not significantly upregulated. These results are supported by Le Maitre et al. [50] who reported increased MMP-13 expression in discs with early degeneration, however in discs with higher grades of disc degeneration, MMP-13 levels did not significantly change. Similarly, Klawitter et al. [51] reported increased MMP-28 expression in cases with disc degeneration, but found no significant correlation between the severity of disc degeneration and MMP-28 expression. One possible explanation for these and our results is that upregulated BMP-2-mediated anabolism counteracts the catabolic signals during disc degeneration. Several studies have described the inhibitory effects of BMPs on MMPs [52–54]. In fact, in a study examining the effect of BMP-2 on the human carcinoma cell line TSU-Pr1, Kumagai et al. [55] showed that BMP-2 inhibits the activity, migration, and secretion of MMP-9. It should be noted though that other studies have reported significant correlations between MMP expression and the degree of disc degeneration [56–58], and as such, future work will be needed to further elucidate the interactions of BMPs and MMPs in the setting of disc degeneration. In addition, understanding the temporal pattern of change in anabolic and catabolic markers during the development and progression of disc degeneration will be crucial for the discovery of therapeutic treatments.
Our study contributes to the growing literature describing the anabolic role of BMP-2 in response to disc degeneration. One limitation to our study is the small sample size for Pfirrmann grade 1 and grade 5 discs. In addition, while we report upregulated expression of both BMP-2 and pSMAD1/5/8 using immunohistochemistry, we cannot draw any conclusions regarding the mechanism underlying this anabolic response. Due to the practicality of intraoperative discectomy, we also did not discern between the annulus fibrosus and NP. Lastly, while we used MMP-13 as a catabolic marker, others exist which are also known to contribute to the development of disc degeneration.
To our knowledge, this is the first study to evaluate the expression of BMP-2 type I receptor ALK3 in human degenerative discs. While several studies have utilized the Pfirrmann grading system to investigate expression of genes associated with disc degeneration [59, 60] and their response to BMP-2 treatment [61], here we report that BMP-2 and pSMAD1/5/8 have significantly upregulated expression in human degenerative discs as compared to the non-degenerative control discs. Interestingly, we did not find any difference in ALK3 expression between the control and degenerative specimens, suggesting that an alternative receptor or pathway was activated to increase BMP-SMAD signaling or that ALK3 gene simply does not transcriptionally respond to BMP-2 signaling in these conditions. Pfirrmann grade 3 and grade 4 IVD specimens were associated with higher levels of BMP-2 and pSMAD1/5/8 expression as compared to Pfirrmann grade 2 discs. In addition, MMP-13 expression levels were not significantly different between the control and degenerative specimens. This is potentially related to upregulated BMP-2 activity counteracting MMP-13 signal. In future work, we plan to elucidate the mechanism of upregulated BMP-2 signaling in the setting of disc degeneration, as well as evaluate the protective effects of gain-of-function BMP signaling in a mouse model of disc degeneration.
Acknowledgments:
We would like to thank The Histology Core at the Center for Musculoskeletal Research and Mrs. Loralee McMahon from the URMC Surgical Pathology Core for help with immunostaining, and Dr. Jennifer Jonason at the Center for Musculoskeletal Research for valuable advice.
Funding Disclosure Statement: Funding for this project was provided by grants from the Orthopaedic Research and Education Foundation, Corelink, and LES Society to AM. Additional funding came from NIH/NIAMS R01 AR072601 grant to RE.
University of Rochester IRB Approval No RSRB00000577
Abbreviations:
- IVD
intervertebral disc
- NP
nucleus pulposus
- ECM
extracellular matrix
- IL
interleukin
- BMP
bone morphogenic protein
- TGF-ß
transforming growth factor-ß
- ALK3
Activin receptor like-kinase 3
- MMP
matrix metalloproteinase
- MRI
magnetic resonance imaging
- NZW
New Zealand white
REFERENCES
- 1.Hoy DG, Protani M, De R, Buchbinder R. The epidemiology of neck pain. Best practice & research Clinical rheumatology. 2010;24(6):783–92. [DOI] [PubMed] [Google Scholar]
- 2.Ferrari R, Russell AS. Regional musculoskeletal conditions: neck pain. Best practice & research Clinical rheumatology. 2003;17(1):57–70. [DOI] [PubMed] [Google Scholar]
- 3.Rubin DI. Epidemiology and risk factors for spine pain. Neurologic clinics. 2007;25(2):353–71. [DOI] [PubMed] [Google Scholar]
- 4.Andersson GB. Epidemiological features of chronic low-back pain. Lancet (London, England). 1999;354(9178):581–5. [DOI] [PubMed] [Google Scholar]
- 5.Martin BI, Deyo RA, Mirza SK, Turner JA, Comstock BA, Hollingworth W, et al. Expenditures and health status among adults with back and neck problems. Jama. 2008;299(6):656–64. [DOI] [PubMed] [Google Scholar]
- 6.Chan WC, Sze KL, Samartzis D, Leung VY, Chan D. Structure and biology of the intervertebral disk in health and disease. The Orthopedic clinics of North America. 2011;42(4):447–64, vii. [DOI] [PubMed] [Google Scholar]
- 7.Zhang D, Jin L, Reames DL, Shen FH, Shimer AL, Li X. Intervertebral disc degeneration and ectopic bone formation in apolipoprotein E knockout mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2013;31(2):210–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Förster M, Mahn F, Gockel U, Brosz M, Freynhagen R, Tölle TR, et al. Axial low back pain: one painful area--many perceptions and mechanisms. PLoS One. 2013;8(7):e68273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou R, Loeser JD, Owens DK, Rosenquist RW, Atlas SJ, Baisden J, et al. Interventional therapies, surgery, and interdisciplinary rehabilitation for low back pain: an evidence-based clinical practice guideline from the American Pain Society. Spine (Phila Pa 1976). 2009;34(10):1066–77. [DOI] [PubMed] [Google Scholar]
- 10.Xu W, Ran B, Luo W, Li Z, Gu R. Is lumbar fusion necessary for chronic low back pain associated with degenerative disc disease? A meta-analysis. World Neurosurg. 2020. [DOI] [PubMed] [Google Scholar]
- 11.Fairbank J, Frost H, Wilson-MacDonald J, Yu LM, Barker K, Collins R. Randomised controlled trial to compare surgical stabilisation of the lumbar spine with an intensive rehabilitation programme for patients with chronic low back pain: the MRC spine stabilisation trial. Bmj. 2005;330(7502):1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brox JI, Nygaard Ø P, Holm I, Keller A, Ingebrigtsen T, Reikerås O. Four-year follow-up of surgical versus non-surgical therapy for chronic low back pain. Ann Rheum Dis. 2010;69(9):1643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mannion AF, Brox JI, Fairbank JC. Comparison of spinal fusion and nonoperative treatment in patients with chronic low back pain: long-term follow-up of three randomized controlled trials. Spine J. 2013;13(11):1438–48. [DOI] [PubMed] [Google Scholar]
- 14.Urban JP, McMullin JF. Swelling pressure of the lumbar intervertebral discs: influence of age, spinal level, composition, and degeneration. Spine. 1988;13(2):179–87. [DOI] [PubMed] [Google Scholar]
- 15.Wan Y, Feng G, Shen FH, Balian G, Laurencin CT, Li X. Novel biodegradable poly(1,8-octanediol malate) for annulus fibrosus regeneration. Macromolecular bioscience. 2007;7(11):1217–24. [DOI] [PubMed] [Google Scholar]
- 16.Roughley PJ, Alini M, Antoniou J. The role of proteoglycans in aging, degeneration and repair of the intervertebral disc. Biochemical Society transactions. 2002;30(Pt 6):869–74. [DOI] [PubMed] [Google Scholar]
- 17.Le Maitre CL, Pockert A, Buttle DJ, Freemont AJ, Hoyland JA. Matrix synthesis and degradation in human intervertebral disc degeneration. Biochemical Society transactions. 2007;35(Pt 4):652–5. [DOI] [PubMed] [Google Scholar]
- 18.Mwale F Molecular therapy for disk degeneration and pain. Global spine journal. 2013;3(3):185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freemont AJ, Peacock TE, Goupille P, Hoyland JA, O’Brien J, Jayson MI. Nerve ingrowth into diseased intervertebral disc in chronic back pain. Lancet. 1997;350(9072):178–81. [DOI] [PubMed] [Google Scholar]
- 20.Freemont AJ. The cellular pathobiology of the degenerate intervertebral disc and discogenic back pain. Rheumatology (Oxford). 2009;48(1):5–10. [DOI] [PubMed] [Google Scholar]
- 21.Than KD, Rahman SU, Vanaman MJ, Wang AC, Lin CY, Zhang H, et al. Bone morphogenetic proteins and degenerative disk disease. Neurosurgery. 2012;70(4):996–1002; discussion [DOI] [PubMed] [Google Scholar]
- 22.Zhang D, Schwarz EM, Rosier RN, Zuscik MJ, Puzas JE, O’Keefe RJ. ALK2 functions as a BMP type I receptor and induces Indian hedgehog in chondrocytes during skeletal development. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2003;18(9):1593–604. [DOI] [PubMed] [Google Scholar]
- 23.Nohe A, Keating E, Underhill TM, Knaus P, Petersen NO. Effect of the distribution and clustering of the type I A BMP receptor (ALK3) with the type II BMP receptor on the activation of signalling pathways. Journal of cell science. 2003;116(Pt 16):3277–84. [DOI] [PubMed] [Google Scholar]
- 24.Graham H, Peng C. Activin receptor-like kinases: structure, function and clinical implications. Endocrine, metabolic & immune disorders drug targets. 2006;6(1):45–58. [DOI] [PubMed] [Google Scholar]
- 25.Miyazono K Signal transduction by bone morphogenetic protein receptors: functional roles of Smad proteins. Bone. 1999;25(1):91–3. [DOI] [PubMed] [Google Scholar]
- 26.Massague J, Wotton D. Transcriptional control by the TGF-beta/Smad signaling system. The EMBO journal. 2000;19(8):1745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vo NV, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, Kang JD. Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 2013;13(3):331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knäuper V, López-Otin C, Smith B, Knight G, Murphy G. Biochemical characterization of human collagenase-3. J Biol Chem. 1996;271(3):1544–50. [DOI] [PubMed] [Google Scholar]
- 29.Knäuper V, Will H, López-Otin C, Smith B, Atkinson SJ, Stanton H, et al. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase a (MMP-2) are able to generate active enzyme. J Biol Chem. 1996;271(29):17124–31. [DOI] [PubMed] [Google Scholar]
- 30.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang C, Ruan DK, Zhang C, Wang DL, Xin H, Zhang Y. Effects of adeno-associated virus-2-mediated human BMP-7 gene transfection on the phenotype of nucleus pulposus cells. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2011;29(6):838–45. [DOI] [PubMed] [Google Scholar]
- 32.Gilbertson L, Ahn SH, Teng PN, Studer RK, Niyibizi C, Kang JD. The effects of recombinant human bone morphogenetic protein-2, recombinant human bone morphogenetic protein-12, and adenoviral bone morphogenetic protein-12 on matrix synthesis in human annulus fibrosis and nucleus pulposus cells. The spine journal : official journal of the North American Spine Society. 2008;8(3):449–56. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, Wang L, Park JB, Park P, Yang VC, Hollister SJ, et al. Intradiscal injection of simvastatin retards progression of intervertebral disc degeneration induced by stab injury. Arthritis research & therapy. 2009;11(6):R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Phillips FM, Thonar EJ, Oegema T, An HS, Roman-Blas JA, et al. Cell therapy using articular chondrocytes overexpressing BMP-7 or BMP-10 in a rabbit disc organ culture model. Spine. 2008;33(8):831–8. [DOI] [PubMed] [Google Scholar]
- 35.Griffith JF, Wang YX, Antonio GE, Choi KC, Yu A, Ahuja AT, et al. Modified Pfirrmann grading system for lumbar intervertebral disc degeneration. Spine. 2007;32(24):E708–12. [DOI] [PubMed] [Google Scholar]
- 36.Longo UG, Ripalda P, Denaro V, Forriol F. Morphologic comparison of cervical, thoracic, lumbar intervertebral discs of cynomolgus monkey (Macaca fascicularis). European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2006;15(12):1845–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Butler D, Trafimow JH, Andersson GB, McNeill TW, Huckman MS. Discs degenerate before facets. Spine. 1990;15(2):111–3. [DOI] [PubMed] [Google Scholar]
- 38.Lotz JC, Fields AJ, Liebenberg EC. The role of the vertebral end plate in low back pain. Global spine journal. 2013;3(3):153–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Murakami H, Yoon ST, Attallah-Wasif ES, Tsai KJ, Fei Q, Hutton WC. The expression of anabolic cytokines in intervertebral discs in age-related degeneration. Spine. 2006;31(16):1770–4. [DOI] [PubMed] [Google Scholar]
- 40.Sowa G, Vadala G, Studer R, Kompel J, Iucu C, Georgescu H, et al. Characterization of intervertebral disc aging: longitudinal analysis of a rabbit model by magnetic resonance imaging, histology, and gene expression. Spine. 2008;33(17):1821–8. [DOI] [PubMed] [Google Scholar]
- 41.Takae R, Matsunaga S, Origuchi N, Yamamoto T, Morimoto N, Suzuki S, et al. Immunolocalization of bone morphogenetic protein and its receptors in degeneration of intervertebral disc. Spine. 1999;24(14):1397–401. [DOI] [PubMed] [Google Scholar]
- 42.Guehring T, Omlor GW, Lorenz H, Bertram H, Steck E, Richter W, et al. Stimulation of gene expression and loss of anular architecture caused by experimental disc degeneration--an in vivo animal study. Spine. 2005;30(22):2510–5. [DOI] [PubMed] [Google Scholar]
- 43.Omlor GW, Lorenz H, Engelleiter K, Richter W, Carstens C, Kroeber MW, et al. Changes in gene expression and protein distribution at different stages of mechanically induced disc degeneration--an in vivo study on the New Zealand white rabbit. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2006;24(3):385–92. [DOI] [PubMed] [Google Scholar]
- 44.Pfirrmann CW, Metzdorf A, Zanetti M, Hodler J, Boos N. Magnetic resonance classification of lumbar intervertebral disc degeneration. Spine. 2001;26(17):1873–8. [DOI] [PubMed] [Google Scholar]
- 45.Pearce RH, Thompson JP, Bebault GM, Flak B. Magnetic resonance imaging reflects the chemical changes of aging degeneration in the human intervertebral disk. The Journal of rheumatology Supplement. 1991;27:42–3. [PubMed] [Google Scholar]
- 46.Sobajima S, Shimer AL, Chadderdon RC, Kompel JF, Kim JS, Gilbertson LG, et al. Quantitative analysis of gene expression in a rabbit model of intervertebral disc degeneration by real-time polymerase chain reaction. The spine journal : official journal of the North American Spine Society. 2005;5(1):14–23. [DOI] [PubMed] [Google Scholar]
- 47.Kim DJ, Moon SH, Kim H, Kwon UH, Park MS, Han KJ, et al. Bone morphogenetic protein-2 facilitates expression of chondrogenic, not osteogenic, phenotype of human intervertebral disc cells. Spine. 2003;28(24):2679–84. [DOI] [PubMed] [Google Scholar]
- 48.Tim Yoon S, Su Kim K, Li J, Soo Park J, Akamaru T, Elmer WA, et al. The effect of bone morphogenetic protein-2 on rat intervertebral disc cells in vitro. Spine. 2003;28(16):1773–80. [DOI] [PubMed] [Google Scholar]
- 49.Ye S, Ju B, Wang H, Lee KB. Bone morphogenetic protein-2 provokes interleukin-18-induced human intervertebral disc degeneration. Bone & joint research. 2016;5(9):412–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204(1):47–54. [DOI] [PubMed] [Google Scholar]
- 51.Klawitter M, Quero L, Bertolo A, Mehr M, Stoyanov J, Nerlich AG, et al. Human MMP28 expression is unresponsive to inflammatory stimuli and does not correlate to the grade of intervertebral disc degeneration. J Negat Results Biomed. 2011;10:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang C, Hu F, Guo S, Mi D, Shen W, Zhang J, et al. BMP-6 inhibits MMP-9 expression by regulating heme oxygenase-1 in MCF-7 breast cancer cells. J Cancer Res Clin Oncol. 2011;137(6):985–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu F, Zhang Y, Li M, Zhao L, Chen J, Yang S, et al. BMP-6 inhibits the metastasis of MDA-MB-231 breast cancer cells by regulating MMP-1 expression. Oncol Rep. 2016;35(3):1823–30. [DOI] [PubMed] [Google Scholar]
- 54.Rajaram S, Murawala H, Buch P, Patel S, Balakrishnan S. Inhibition of BMP signaling reduces MMP-2 and MMP-9 expression and obstructs wound healing in regenerating fin of teleost fish Poecilia latipinna. Fish Physiol Biochem. 2016;42(2):787–94. [DOI] [PubMed] [Google Scholar]
- 55.Kumagai T, Shimizu T, Takeda K. Bone morphogenetic protein-2 suppresses invasiveness of TSU-Pr1 cells with the inhibition of MMP-9 secretion. Anticancer Res. 2006;26(1a):293–8. [PubMed] [Google Scholar]
- 56.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11(4):308–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bachmeier BE, Nerlich A, Mittermaier N, Weiler C, Lumenta C, Wuertz K, et al. Matrix metalloproteinase expression levels suggest distinct enzyme roles during lumbar disc herniation and degeneration. Eur Spine J. 2009;18(11):1573–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Crean JK, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine (Phila Pa 1976). 1997;22(24):2877–84. [DOI] [PubMed] [Google Scholar]
- 59.Rodrigues LM, Oliveira LZ, Pinhal MA. Expression of heparanase isoforms in intervertebral discs classified according to Pfirrmann grading system for disc degeneration. Spine. 2013;38(13):1112–8. [DOI] [PubMed] [Google Scholar]
- 60.Park JY, Kuh SU, Park HS, Kim KS. Comparative expression of matrix-associated genes and inflammatory cytokines-associated genes according to disc degeneration: analysis of living human nucleus pulposus. Journal of spinal disorders & techniques. 2011;24(6):352–7. [DOI] [PubMed] [Google Scholar]
- 61.Park JY, Yoon YS, Park HS, Kuh SU. Molecular response of human cervical and lumbar nucleus pulposus cells from degenerated discs following cytokine treatment. Genetics and molecular research : GMR. 2013;12(1):838–51. [DOI] [PubMed] [Google Scholar]