Keywords: mouse hippocampus, ouabain, single channels, sodium-activated potassium
Abstract
Potassium channels play an important role regulating transmembrane electrical activity in essentially all cell types. We were especially interested in those that determine the intrinsic electrical properties of mammalian central neurons. Over 30 different potassium channels have been molecularly identified in brain neurons, but there often is not a clear distinction between molecular structure and the function of a particular channel in the cell. Using patch-clamp methods to search for single potassium channels in excised inside-out (ISO) somatic patches with symmetrical potassium, we found that nearly all patches contained non–voltage-inactivating channels with a single-channel conductance of 100–200 pS. This conductance range is consistent with the family of sodium-activated potassium channels (Slo2.1, Slo2.2, or collectively, KNa). The activity of these channels was positively correlated with a low cytoplasmic Na+ concentration (2–20 mM). Cell-attached recordings from intact neurons, however, showed little or no activity of this K+ channel. Attempts to increase channel activity by increasing intracellular sodium concentration ([Na+]i) with bursts of action potentials or direct perfusion of Na+ through a whole cell pipette had little effect on KNa channel activity. Furthermore, excised outside-out (OSO) patches across a range of intracellular [Na+] showed less channel activity than we had seen with excised ISO patches. Blocking the Na+/K+ pump with ouabain increased the activity of the KNa channels in excised OSO patches to levels comparable with ISO-excised patches. Our results suggest that despite their apparent high levels of expression, the activity of somatic KNa channels is tightly regulated by the activity of the Na+/K+ pump.
NEW & NOTEWORTHY We studied KNa channels in mouse hippocampal CA1 neurons. Excised inside-out patches showed the channels to be prevalent and active in most patches in the presence of Na+. Cell-attached recordings from intact neurons, however, showed little channel activity. Increasing cytoplasmic sodium in intact cells showed a small effect on channel activity compared with that seen in inside-out excised patches. Blockade of the Na+/K+ pump with ouabain, however, restored the activity of the channels to that seen in inside-out patches.
INTRODUCTION
Potassium-conducting ion (K+) channels play an important role in the nature of the electrical activity of neurons. They can determine the probability of action potential initiation and the shape of the action potential and regulate repetitive spiking or bursts of action potentials (1). Several studies have shown that changes in the activity of certain potassium channels affect the intrinsic properties of hippocampal neurons (2–4). Molecular investigations identified at least 30 different K+ channels expressed in the mammalian brain, and more if you count heteromers and splice variants (5–7). The functional properties of only a few of these molecularly defined channels are known. In this study, we used single-channel recordings to search for K+ channels that might play a role in regulating intrinsic excitability in central nervous system (CNS) neurons.
Many K+ channels can be identified based on their single-channel conductance. For example, Ca2+-activated K+ channels (BK) with their large single-channel conductance (>250 pS) are readily discernable from A-, D-, and DR-type K+ channels (<20 pS). While investigating the properties of various K+ channels in the mammalian CNS, we often recorded channels of a relatively larger amplitude, but with smaller conductance than the BK channel. Excised inside-out patches with symmetrical K+ concentrations on both sides of the membrane (a standard method to compare conductance properties among different K+ channels) revealed a single-channel conductance of 100–200 pS. Published descriptions of K+ channels with a single-channel conductance in this range suggested that they might belong to the class of sodium-activated potassium channels (KNa) (7–9). Potassium ion channels that are activated by sodium ions (Na+) were first described in mammalian cardiac myocytes (10) and cat neocortical neurons (11) and subsequently found in a wide variety of cell types. The molecular properties of KNa channels have been studied extensively by Kaczmarek and colleagues (12–19).
We observed the putative KNa channels on rat and mouse hippocampal CA1 neurons, mouse dentate granule cells, and mouse layer 5 neurons from prefrontal cortex. Further examination of KNa channels in mouse CA1 neurons revealed that their activity was strongly correlated to the cytoplasmic Na+ concentration. In intact neurons and outside-out patches, block of the Na+/K+ ATPase increased the activity of KNa channels. We chose to investigate them in more detail exclusively in wild-type mouse CA1 hippocampal neurons. We found that although these channels can be activated by relatively low concentrations of intracellular Na+, their overall activity is tightly regulated by the Na+/K+ pump, even in excised outside-out membrane patches.
METHODS
Animals
All data presented, other than Fig. 1 which included multiple cell types, were collected from mouse hippocampus CA1 pyramidal neurons. Animal procedures were approved by The University of Texas at Austin Institutional Animal Care and Use Committee. Male wild-type mice (8–16 wk old) from either The Jackson Laboratory or an in house colony were used for all experiments. Mice were anesthetized with isoflurane exposure followed by injection of ketamine/xylazine cocktail (100/10 mg/kg i.p.). Mice were then perfused through the heart with ice-cold saline consisting of (in mM): 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 0.5 CaCl2, 7 MgCl2, 7 dextrose, 205 sucrose, 1.3 ascorbate, and 3 sodium pyruvate (bubbled with 95% O2-5% CO2 to maintain pH at 7.4). The brain was removed, trimmed, and a vibrating tissue slicer (Vibratome 3000, Vibratome) was used to make 300-µm-thick transverse sections of hippocampus or coronal sections containing the prefrontal cortex. Slices were held for 30 min at 35°C in a chamber filled with artificial cerebral spinal fluid (aCSF) consisting of (in mM) 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 25 NaHCO3, 2 CaCl2, 2 MgCl2, 10 dextrose, and 3 sodium pyruvate (bubbled with 95% O2-5% CO2) and then at room temperature until the time of recording.
Figure 1.
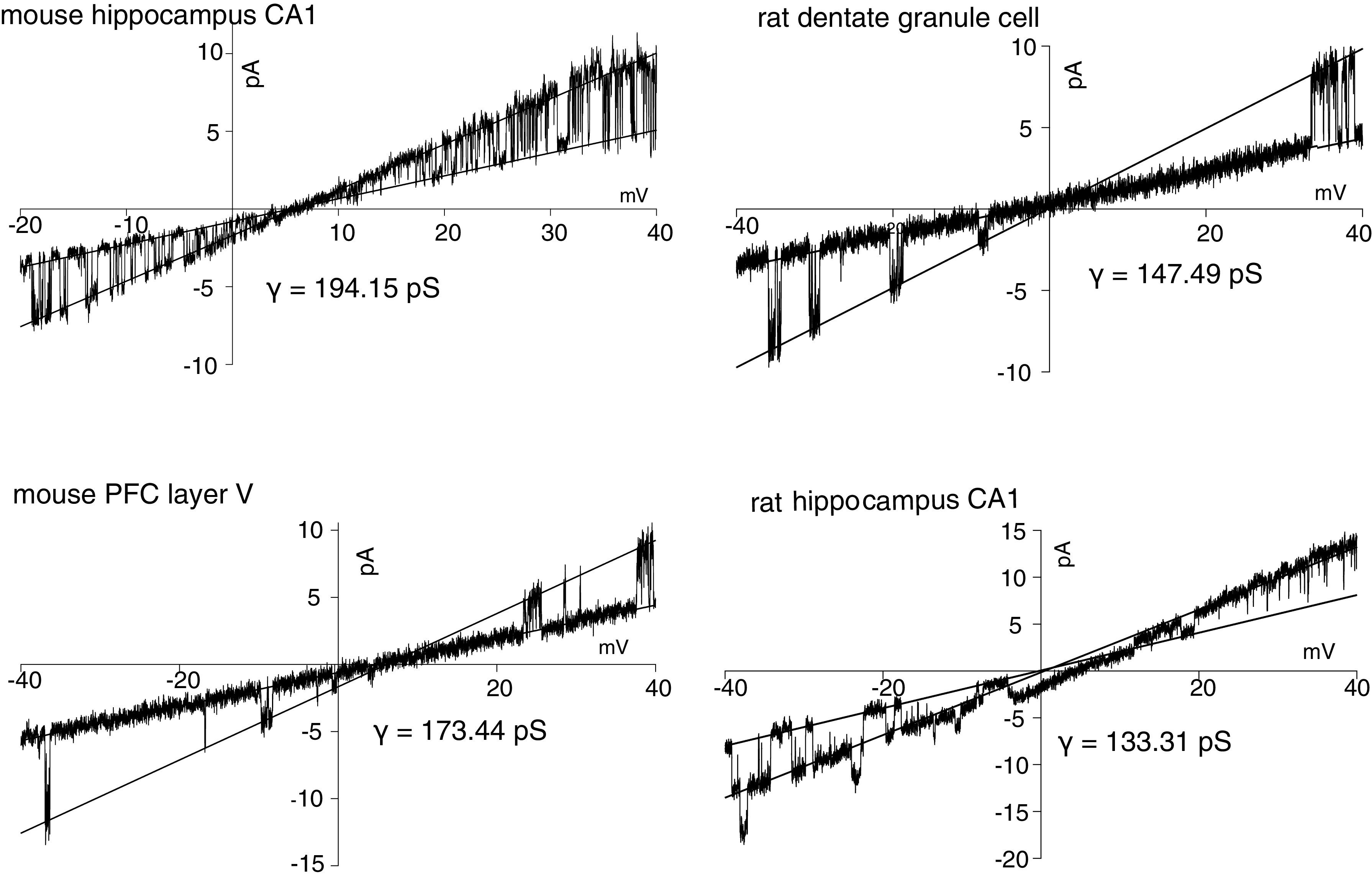
Single-channel currents in response to ramp voltage commands for a variety of cell types. Ramp duration was 1 s. Single-channel conductance, γ, was measured as the difference in slopes between the closed and open states. The open state has the greater slope. CA1, pyramidal neuron; PFC, prefrontal cortex.
Electrophysiology
Patch clamp recordings were made with 1 or 2 Axopatch 200B amplifiers (Molecular Devices) using 2-mm-thick-walled glass (Sutter BF200-116-10) coated with Sylgard. Electrodes were fire polished to 10–14 MegOhms to increase the chances of obtaining only one or two channels in the patch.
Bath saline for inside-out patches and pipette saline for outside-out patches (in mM): 120 K-gluconate, 5 EGTA, 10 HEPES, sucrose 13, to which was added NaCl at 1–20 mM with KCl from 4–23 mM to bring the sum of added Na+ + K+ concentration to 24 mM and the solution osmolality to 305 mOsm.
Pipette saline for excised inside-out patches (in mM): KCl 40, K-gluconate 100, MgCl2 2, HEPES 10, and CaCl2 2. The K+ equilibrium potential (EK) for cell-attached and excised outside-out patches varied from −95 to −99 mV.
Pipette saline for cell-attached patches (in mM): 140 NaCl, 2.5 KCl, 2 CaCl2, 1 MgCl2, 10 HEPES.
The transmembrane potential for outside-out and cell attached recordings was set to 0 mV so that the single-channel currents were outward and of amplitude 10–20 pA. Current traces were inverted as needed to display outward currents as upward in all plots. Holding the patch at 0 mV inactivated any voltage-gated channels that might be in the patch although a small-amplitude noninactivating channel was sometimes visible but below the threshold amplitude for measuring KNa channel activity.
Synaptic blockers (in mM) were used in the bath saline for cell-attached and outside-out patch recordings: 0.025 d-APV, 0.02 DNQX, and 0.002 gabazine. TTX (0.5 µM) was used in the extracelluar saline for all excised patch experiments.
Extracellular stimulation of antidromic action potentials were made with a bipolar tungsten stimulating electrode placed in stratum oriens or closer to the soma. Stimuli were varied from a duration of 0.2–1 ms and applied at 10–100 Hz. Stimulation parameters were chosen so that each stimulus elicited an action potential and stayed the same for each patch recording.
Analysis
Single-channel conductance was measured as the difference of slopes of closed-state and open-state currents of the channels during voltage ramps, and fractional open time was measured with QUB to determine occupancy (20, 21). Statistics for Figs. 2B, 6B, and 7 were performed with Prism 8 (Graphpad). Two-way repeated measures (RM) ANOVA was applied to experiments with multiple test variables for each condition. The Holm-Sidak multiple comparison test was used to compare row means between groups and to adjust P values. Data are presented as mean ± SE. Alpha was set to 0.05 for all experiments.
RESULTS
Excised ISO Patches Showing Channel Openings with Single-Channel Conductance of 100–200 pS Were Common in Various Cell Types
In initial experiments excised inside-out patches (ISO) were used to measure single channels in multiple brain regions. At this stage, brain slices were obtained from fellow laboratory members from rat hippocampus, mouse prefrontal cortex layer 5, or mouse hippocampus CA1. [K+] was symmetrical (150 mM) on both sides of the membrane in all cases. In ISO-excised patches, with a low concentration of Na+ (5 mM) on the cytoplasmic side, channel openings with single-channel conductance of 100–200 pS were seen in a majority of all patches from all neurons [mouse CA1: 80 of 100 patches; rat dentate: 3 of 3; mouse prefrontal cortex (PFC): 4 of 6; rat hippocampus: 5 of 8] during these initial experiments (Fig. 1).
Although our data suggest these channels can be found across a variety of cell types, we decided to conduct all future experiments with mouse CA1 neurons to reduce the complexity of analysis (for example, the heterogeneity of layer 5 PFC neurons) (22, 23).
Channel Activity Is a Function of Cytoplasmic [Na+]
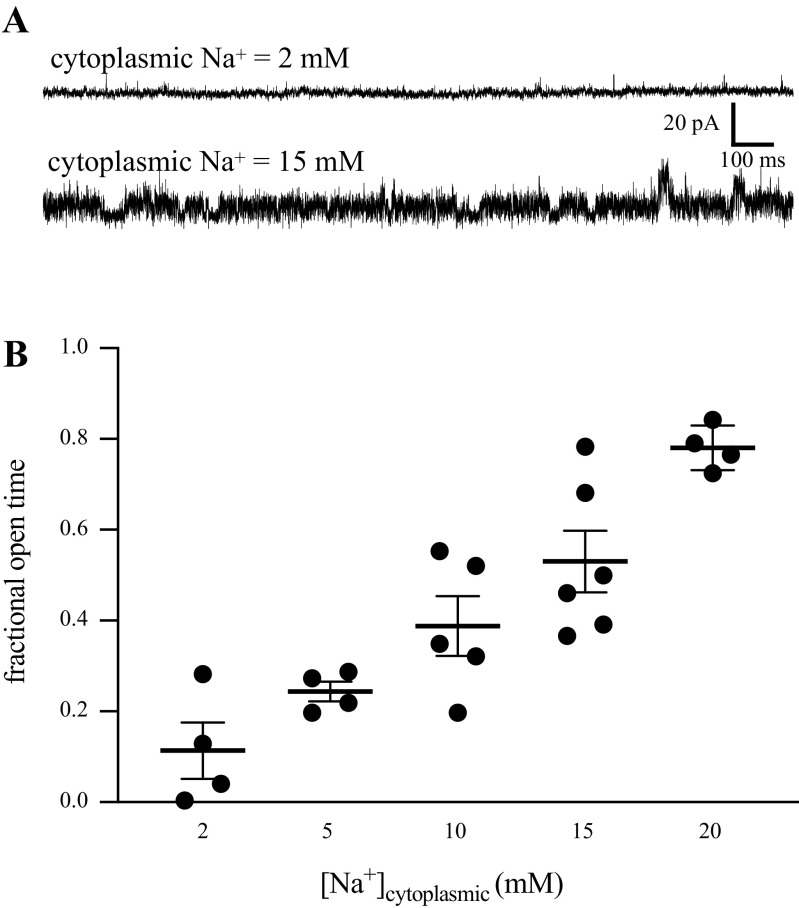
We hypothesized that this channel was likely a KNa channel based on the unique single-channel conductance. To test this, we performed experiments with various [Na+] on the cytoplasmic side of the membrane and measured the probability of channel opening (as fractional open time). Slices were bathed in normal ACSF and excised ISO patches were moved to a small perfusion pipette containing varied [Na+]. Fractional open time increased monotonically with increasing concentrations of Na+ (Fig. 2). These results support our hypothesis that the channels we observed were sodium-activated K+ channels and we refer to these channels as KNa channels.
Figure 2.
A: sample 2-s duration traces of channel activity with 2 and 15 mM cytoplasmic [Na+]. In the 15 mM, trace channels are open most of the time. The baseline current (all channels closed) noise was similar in the 2 mM and 15 mM traces. B: fractional open time as a function of cytoplasmic [Na+]. Each point was taken from a different ISO patch from 30-s epochs of channel activity. One-way ANOVA: F(1.51,6.77) = 17.33, P = 0.003. Sodium concentration had a consistent effect on the open probability of KNa in inside-out patches of membrane. ISO, inside-out; KNa, sodium-activated potassium channels.
KNa Channel Openings Were Rarely Seen in Cell-Attached Patches from Intact Neurons
Because KNa channels were seen in ∼80% of excised ISO patches, we were interested in their level of activity in intact CA1 neurons. Slices were bathed in normal ACSF and the patch pipette contained HEPES-buffered ACSF. Cell-attached recordings were made with the pipette voltage held at −60 mV, so the channels would experience a potential near 0 mV assuming a normal resting potential of −60 mV. Channel openings with an amplitude or conductance indicative of KNa were rare (11 of 120 patches) and usually at the end of long-duration experiments.
During these experiments several other types of K+ channels were observed. When the patch potential was held so that the channel saw a negative potential, voltage-gated channels could be seen during step depolarizations. The properties of these channels corresponded with those seen from cell-attached recordings from rat CA1 apical dendrites (24) and whole cell recordings from dendrites of CA1 neurons from a mouse model of Fragile X syndrome (25). Although delayed rectifiers, M-type, D-type, and A-type channels were present, most of these were fully inactivated by holding the transmembrane potential at 0 mV and did not interfere with measurement of KNa.
Trains of Action Potentials Were Rarely Able to Elicit KNa Channel Activity
Because the abundant channel activity seen in excised ISO patches was sensitive to cytoplasmic [Na+], we asked if antidromically initiating action potentials in the neuron was sufficient to raise inhibitory sodium concentration ([Na+]i) to a level sufficient to activate the channels. Cell-attached patches were recorded from CA1 neurons to measure both action potentials (seen as a capacitance current in the patch as the cell fired) and channel activity. As before, the patch electrode was held at −60 mV so the single channel currents would be outward. Repetitive 10–20 repeats of trains of 1, 2, 5, 10, and 20 spikes were initiated with an extracelluar bipolar stimulating electrode while recording single-channel activity. In most cases, spikes did not activate channels, but in a few patches many trains of 20 spikes could eventually elicit channel openings (Fig. 3). Triggering of antidromic spikes was rarely able to elicit openings of KNa channels (9 patches out of 83 experiments).
Figure 3.
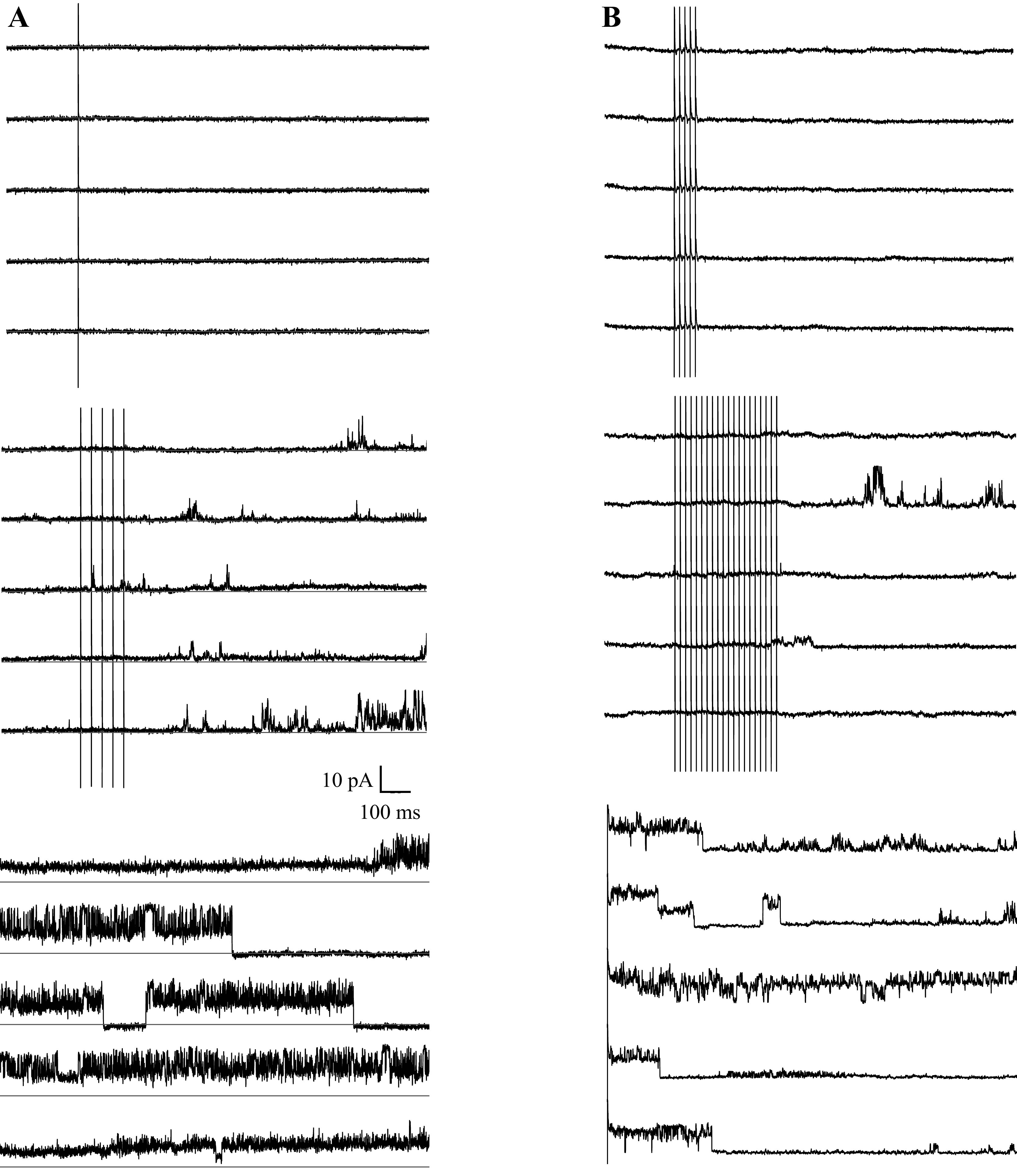
Examples of patches where channel activity was elicited by antidromically stimulated action potentials. A: 20 repeats of one triggered spike per trace did not elicit channel openings, but during the last five traces of 20 containing five spikes per trace at 50 Hz did show channel activity. B: 20 traces each containing 1, 2, 5, or 10 triggered spikes at 100 Hz did not elicit channel activity, but the first five traces containing 20 trigged spikes did elicit channel activity. Channel activity was only seen after several trains of 20 spikes. Stimulus frequency was chosen so each stimulus elicited an action potential.
Elevating Bulk Cytoplasmic [Na+] Had Little Effect on KNa Activity
We attempted to elevate bulk cytoplasmic [Na+] to see if that was able to increase the activity of KNa channels. Two patch electrodes were used: one was filled with HEPES-buffered ACSF for cell-attached recording, and another filled with internal saline with varying amounts of Na+ for whole cell access. Both electrodes started out as cell-attached recordings, and after baseline measurements were made, the membrane under the whole cell electrode was ruptured and the cytoplasm dialyzed with the specific Na+ concentration. In only three (2 with 5 mM and 1 with 8 mM) out of 27 patches with [Na+]i of 2–10 mM was channel activity seen after breaking in when there was none before (Fig. 4).
Figure 4.
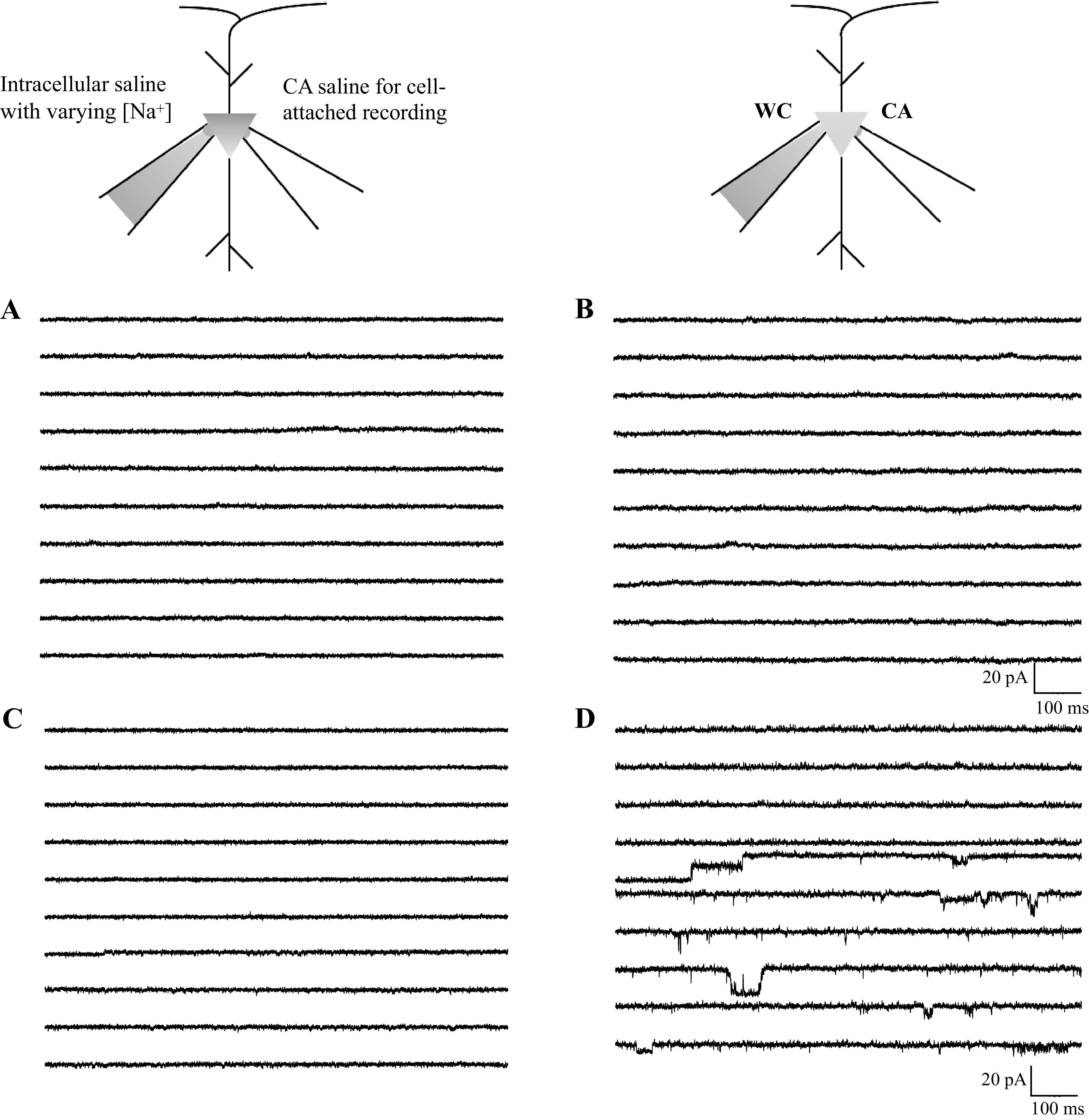
Dual-electrode recordings. A: 10 sample traces of 100 before breaking in with the whole cell electrode. B: 10 sample traces of 100 after breaking in with whole cell electrode containing 5 mM Na+. No KNa channel activity was seen. C: a different cell with 10 sample traces before breaking in. D: by the 24th trace after breaking in with 5 mM Na+, channel activity appeared. Three channels were apparent, and two channels were constantly open. CA, cell-attached; WC, whole-cell.
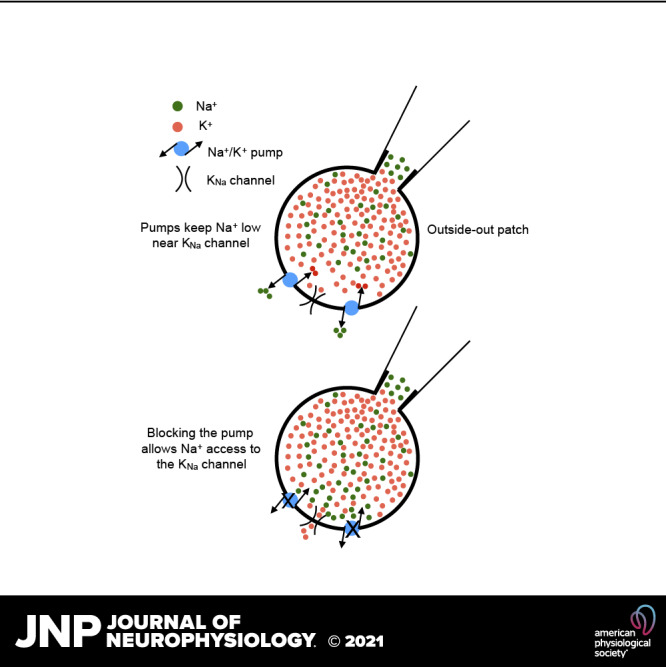
Could the Na+/K+ Pump Be Controlling the Amount of Na+ Available to the KNa Channel?
The inability to increase the activity of KNa channels by directly increasing [Na+]i through a patch electrode was puzzling. We were aware that the Na+/K+ ATPase could be playing a role in regulating [Na+]i but were unsure of the effectiveness of that regulation. We applied the Na+/K+ ATPase inhibitor ouabain (1 µM) to an intact slice under normal conditions and measured the resting potential with a current-clamp recording. The cell depolarized to approximated −50 mV as seen for other cell types (26) (Fig. 5A). A cell-attached recording from another cell showed a high level of KNa channel activity in the presence of ouabain (Fig. 5B).
Figure 5.
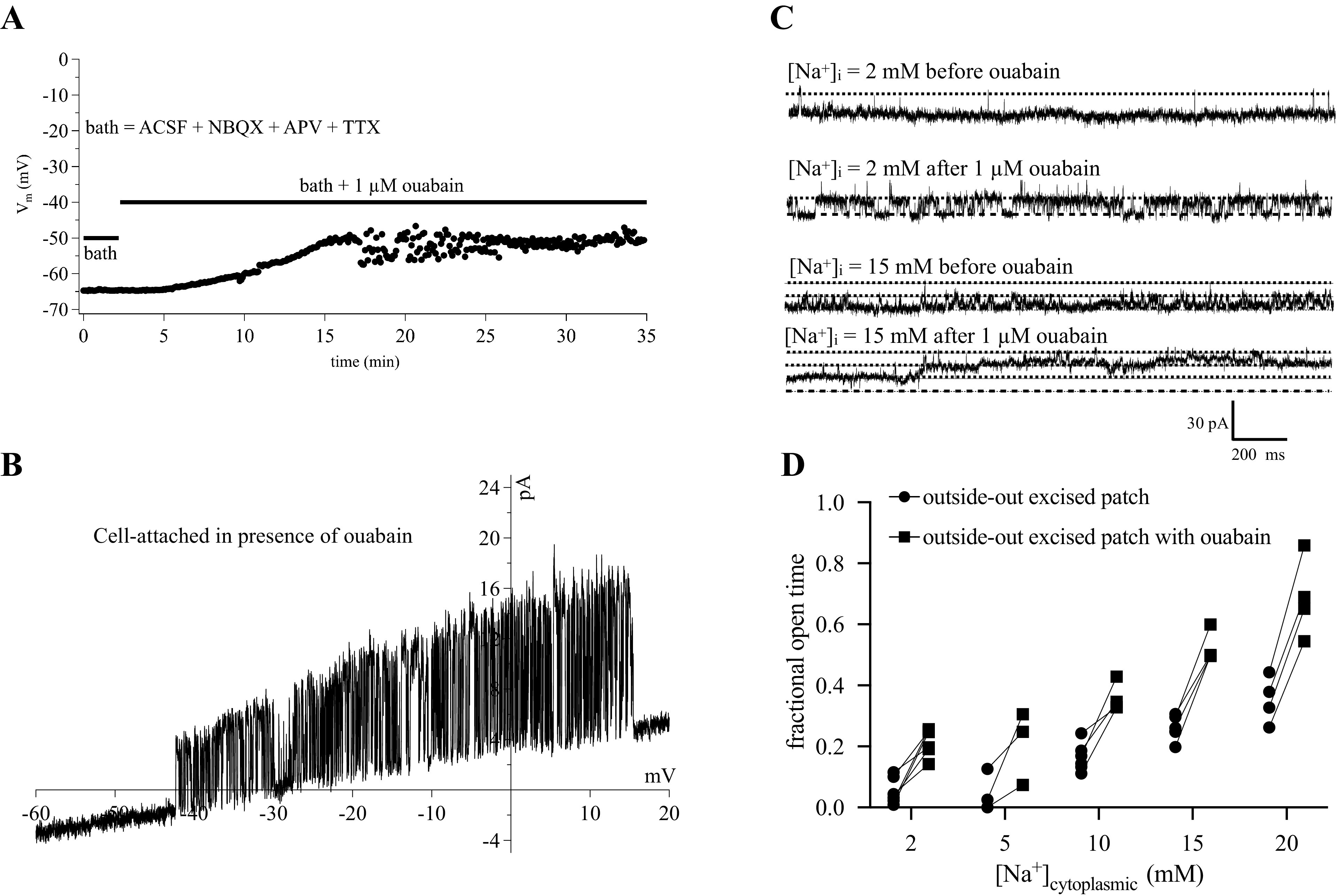
Effects of ouabain. A: a current-clamp recording shows the cell to depolarize to −50 mV in the presence of ouabain (N = 1 cell). B: a cell-attached ramp shows a high degree of activity of a KNa channel (N = 1 cell). Recordings of outside-out excised patches with various concentrations of cytoplasmic Na+. C: example traces before and after application of 1 µM ouabain. D: summary of all experiments from OSO patches before and after ouabain. Repeated measures two-way ANOVA indicated ouabain application increased fractional open time at all Na+ concentrations [F(1,10) = 115.1, P = 0.0001]. ACSF, artificial cerebral spinal fluid; KNa, sodium-activated potassium channels; [Na+]i, intracellular sodium concentration; OSO, outside-out.
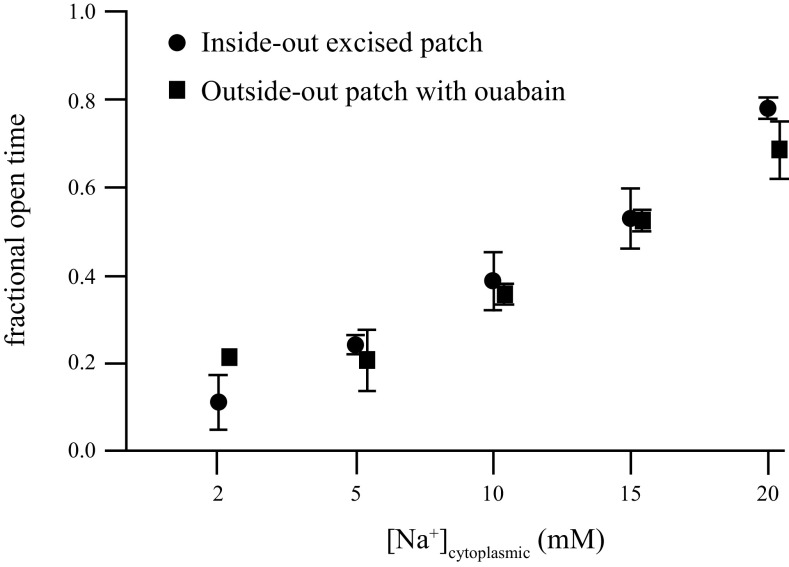
Because flooding the bulk phase of the cytoplasm with Na+ during the previous whole cell recordings had only small effects, we switched to outside-out patches where we thought we would be better able to control [Na+]in. We made outside-out patch recordings from CA1 somata with varying concentrations of Na+ in the pipette, and applied ouabain at 1 µM after measuring baseline KNa channel activity. At every concentration of internal Na+ ouabain rapidly (within seconds) increased the activity of KNa channels (Fig. 5, C and D). Comparing the channel activity seen in excised ISO patches with that from OSO patches in ouabain showed that the activity was largely restored (Fig. 6).
Figure 6.
Comparisons of channel activity from excised inside-out patches to outside-out excised patches in the presence of 1 µM ouabain with defined concentrations of cytoplasmic Na+. A repeated measures two-way ANOVA was used to compare ISO and OSO values: F(1,34) = 0.16, P = 0.69, indicating no difference between the groups. ISO, inside-out; OSO, outside-out.
DISCUSSION
In this study, we used single channel recording to investigate sodium-activated potassium channels in the mammalian CNS. We found that 1) KNa channels are expressed in many different excitatory neurons; 2) KNa channels are relatively inactive at rest and remain inactive even with moderate levels of activity; and 3) KNa channel activity is tightly regulated by the Na+/K+ pump. Our results show that KNa channels are densely located on the soma of hippocampal neurons because it was rare to not see channel activity in excised ISO patches. Their low activity in unaltered neurons is enigmatic: why are they expressed at relatively high density yet have such a low level of activity? The inability to reliably activate KNa channels with antidromic spikes is not surprising. Using Na-sensitive dyes, Konnerth only saw increases in somatic [Na]+ of up to 5 mM with trains of spikes, whereas synaptic stimulation could cause increases up to 30–40 mM in the dendrites (27). This suggests the intriguing possibility that KNa channels may play a more prominent role in the dendrites relative to the soma. Indeed, KNa mRNA and protein is found in the dendrites of auditory neurons (28), and overlapping and nonoverlapping expression of Slick and Slack have been described in various cell types and regions in rat brain (29).
Some cell types have been shown to have a large component of K+ current in response to Na+ entry. Tufted-mitral cells and spiny cells of the striatum have a large KNa current elicited by both voltage-gated and persistent Na+ channels (30).
KNa currents were first described in cardiac myocytes (10) and subsequently in cat cortical neurons (11). Two genes are known to encode the KNa channels (Slo2.1 or Slick and Slo2.2 or Slack) and their molecular properties have been extensively studied by Kaczmarek and colleagues (9, 13). There are five different subunit transcripts for Slo2.2 channels that can form channels with differing properties, whereas Slo2.1 channels are more homogeneous. Additionally, Slo2.1 and Slo2.2 subunits can coassemble to form heteromeric channels. Because we do not know the molecular structure of the native channels in our neurons, we refer to them as generic KNa channels. Among our many recordings we have not been able to discern different populations of KNa channels that may give a hint of their molecular composition.
The Na+-K+ ATPase is known to be heavily expressed in nearly all cells and accounts for 30%–80% of the energy consumption of the brain (31). The enzyme is equally expressed in all areas of all cells. The pump is made up of two subunits, α and β. Ouabain binds to the α subunit and completely blocks the enzyme at 1 µM. It was recently shown that an endogenous ouabain is made in the adrenal cortex and circulates in plasma (32). It is possible that endogenous ouabain could affect the activity of KNa channels. These experiments did not address how this endogenous ouabain might be activated during action potential firing or other activity in the neuron.
The results of this study suggest that KNa channels are expressed in many different excitatory cell types, are relatively inactive at rest or with moderate levels of activity, and are tightly regulated by the Na+/K+ pump. Because the KNa channels we observed were so prevalent in the different cell types we studied, we assume that regulation of that channel might be a powerful site of modulation of neuronal activity.
Mutation of the pump has been implicated in several diseases, including behavioral abnormalities, familial hemiplegic migraine, and rapid-onset dystonia-parkinsonism (33); perhaps altered endogenous ouabain could have similar pathological effects or modulate KNa channels.
It is possible that resistance to ion flow through the patch electrode might affect the amount of Na+ available to the KNa channels in cell-attached recordings. The observations that outside-out patches show KNa activity as a function of [Na+] is similar to that of excised patches (where access of Na+ to the channels is unimpeded) would argue against this. The observation that the dual-electrode experiments didn’t show similar levels of activity in cell-attached recordings when the cell body was flooded with an elevated [Na+] suggest that ion access to the channel could be impeded in this configuration, although electrode tip size was the same in all patch configurations. Calculations of diffusion of Na+ ions from the pipette to the cytoplasm suggest that within seconds the cytoplasmic concentration approaches that in the pipette (34) even with high access resistance.
There are several components of the interaction of cytoplasmic Na+ and the pump that are unknown, such as the density of pump molecules, the distance between pump molecules and the KNa channel, and the availability of ATP to the pump.
A number of different K+ channels affect the firing and integrative properties of central neurons. A transient A-type current is differentially expressed along the dendrites with a higher density at more distal locations and affects the amplitude and duration of back-propagated action potentials (2). Slowly inactivating D-type currents regulate the threshold of Ca2+ spike initiation in the somata of CA1 pyramidal neurons (35). Because the KNa channels we observed were prevalent in the different cell types studied, we suggest that regulation of KNa channels might be a powerful site of modulation of neuronal activity. We consider this study of KNa at the single-channel level in mouse CA1 neurons to be a baseline for future work for their potential function and modulation.
GRANTS
This work was supported by National Institute of Mental Health NS084473 and McKnight Endowment Fund for Neuroscience.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.G. conceived and designed research; R.G. performed experiments; R.G. analyzed data; R.G. and D.J. interpreted results of experiments; R.G. prepared figures; R.G. drafted manuscript; R.G. and D.J. edited and revised manuscript; R.G. and D.J. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank all members of the Johnston and Brager labs for comments, suggestions, and for tissue slices.
REFERENCES
- 1.Storm JF. Functional diversity of K+ currents in hippocampal pyramidal neurons. Semin Neurosci 5: 79–92, 1993. doi: 10.1016/S1044-5765(05)80002-8. [DOI] [Google Scholar]
- 2.Hoffman D, Magee J, Colbert C. K channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons [Online]. Nature 387: 869–875, 1997.doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- 3.Ordemann GJ, Apgar CJ, Brager DH. D-type potassium channels normalize action potential firing between dorsal and ventral CA1 neurons of the mouse hippocampus. J Neurophysiol 121: 983–995, 2019. doi: 10.1152/jn.00737.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Watanabe S, Hoffman DA, Migliore M, Johnston D. Dendritic K+ channels contribute to spike-timing dependent long-term potentiation in hippocampal pyramidal neurons. Proc National Acad Sci 99: 8366–8371, 2002. doi: 10.1073/pnas.122210599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gutman GA, Chandy KG, Grissmer S, Lazdunski M, McKinnon D, Pardo LA, Robertson GA, Rudy B, Sanguinetti MC, Stühmer W, Wang X. International Union of Pharmacology. LIII. Nomenclature and molecular relationships of voltage-gated potassium channels. Pharmacol Rev 57: 473–508, 2005. doi: 10.1124/pr.57.4.10. [DOI] [PubMed] [Google Scholar]
- 6.Kubo Y, Adelman JP, Clapham DE, Jan LY, Karschin A, Kurachi Y, Lazdunski M, Nichols CG, Seino S, Vandenberg CA. International Union of Pharmacology. LIV. Nomenclature and molecular relationships of inwardly rectifying potassium channels. Pharmacol Rev 57: 509–526, 2005. doi: 10.1124/pr.57.4.11. [DOI] [PubMed] [Google Scholar]
- 7.Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev 57: 463–472, 2005. doi: 10.1124/pr.57.4.9. [DOI] [PubMed] [Google Scholar]
- 8.Kaczmarek LK, Aldrich RW, Chandy KG, Grissmer S, Wei AD, Wulff H, Ohlstein EH. International union of basic and clinical pharmacology. C. Nomenclature and properties of calcium-activated and sodium-activated potassium channels. Pharmacol Rev 69: 1–11, 2017. doi: 10.1124/pr.116.012864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaczmarek LK. Slack, slick, and sodium-activated potassium channels. ISRN Neurosci 2013: 354262, 2013. doi: 10.1155/2013/354262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kameyama M, Kakei M, Sato R, Shibasaki T. Intracellular Na+; activates a K+; channel in mammalian cardiac cells. Nature 309: 354–356, 1984. doi: 10.1038/309354a0. [DOI] [PubMed] [Google Scholar]
- 11.Schwindt PC, Spain WJ, Crill WE. Long-lasting reduction of excitability by a sodium-dependent potassium current in cat neocortical neurons. J Neurophysiol 61: 233–244, 1989. doi: 10.1152/jn.1989.61.2.233. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharjee A, Joiner WJ, Wu M, Yang Y, Sigworth FJ, Kaczmarek LK. Slick (Slo2.1), a rapidly-gating sodium-activated potassium channel inhibited by ATP. J Neurosci 23: 11681–11691, 2003. doi: 10.1523/JNEUROSCI.23-37-11681.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+. Trends Neurosci 28: 422–428, 2005. doi: 10.1016/j.tins.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Brown MR, Kronengold J, Gazula V-R, Chen Y, Strumbos JG, Sigworth FJ, Navaratnam D, Kaczmarek LK. Fragile X mental retardation protein controls gating of the sodium-activated potassium channel Slack. Nat Neurosci 13: 819–821, 2010. doi: 10.1038/nn.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown MR, Kronengold J, Gazula V-R, Spilianakis CG, Flavell RA, von HC, Bhattacharjee A, Kaczmarek LK. Amino-termini isoforms of the Slack K+ channel, regulated by alternative promoters, differentially modulate rhythmic firing and adaptation. J Physiol 586: 5161–5179, 2008. doi: 10.1113/jphysiol.2008.160861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaczmarek L, Bhattacharjee A, Desai R, Gan L. Regulation of the timing of MNTB neurons by short-term and long-term modulation of potassium channels. Null 206: 133–145, 2005. doi: 10.1016/j.heares.2004.11.023. [DOI] [PubMed] [Google Scholar]
- 17.Kim GE, Kaczmarek LK. Emerging role of the KCNT1 Slack channel in intellectual disability. Front Cell Neurosci 8: 209, 2014. doi: 10.3389/fncel.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Markham M, Kaczmarek LA. Sodium-activated potassium channel supports high-frequency firing and reduces energetic costs during rapid modulations of action potential amplitude. Null 109: 1713–1723, 2013. doi: 10.1152/jn.00875.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang B, Desai R, Kaczmarek L. Slack and Slick KNa channels regulate the accuracy of timing of auditory neurons. Null 27: 2617–2627, 2007. doi: 10.1523/jneurosci.5308-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicolai C, Sachs F. Solving ion channel kinetics with the QuB software. Biophys Rev Lett 08: 191–211, 2013. doi: 10.1142/S1793048013300053. [DOI] [Google Scholar]
- 21.Qin F, Auerbach A, Sachs F. Estimating single-channel kinetic parameters from idealized patch-clamp data containing missed events. Biophys J 70: 264–280, 1996. doi: 10.1016/S0006-3495(96)79568-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kalmbach BE, Johnston D, Brager DH. Cell-type specific channelopathies in the prefrontal cortex of the fmr1-/y mouse model of fragile X syndrome. eNeuro 2: ENEURO.0114-15.2015, 2015.doi: 10.1523/ENEURO.0114-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu J, Anderson CT, Kiritani T, Sheets PL, Wokosin D, Wood L, Shepherd GMG. Local-circuit phenotypes of layer 5 neurons in motor-frontal cortex of YFP-H mice. Front Neural Circuit 2: 6, 2008. doi: 10.3389/neuro.04.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Johnston D. Properties of single voltage‐dependent K+ channels in dendrites of CA1 pyramidal neurones of rat hippocampus. J Physiol 559: 187–203, 2004. doi: 10.1113/jphysiol.2004.068114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Routh BN, Johnston D, Brager DH. Loss of functional A-type potassium channels in the dendrites of CA1 pyramidal neurons from a mouse model of fragile X syndrome. J Neurosci 33: 19442–19450, 2013. doi: 10.1523/JNEUROSCI.3256-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gorman ALF, Marmor MF. Long‐term effect of ouabain and sodium pump inhibition on a neuronal membrane. J Physiol 242: 49–60, 1974. doi: 10.1113/jphysiol.1974.sp010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose CR, Konnerth A. NMDA receptor-mediated Na+ signals in spines and dendrites. J Neurosci 21: 4207–4214, 2001. doi: 10.1523/JNEUROSCI.21-12-04207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JH, Kang M, Park S, Perez-Flores MC, Zhang X-D, Wang W, Gratton MA, Chiamvimonvat N, Yamoah EN. The local translation of KNa in dendritic projections of auditory neurons and the roles of KNa in the transition from hidden to overt hearing loss. Aging (Albany NY) 11: 11541–11564, 2019. doi: 10.18632/aging.102553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhattacharjee A, Hehn C. V, Mei X, Kaczmarek LK. Localization of the Na+-activated K+ channel Slick in the rat central nervous system. J Comp Neurol 484: 80–92, 2005. doi: 10.1002/cne.20462. [DOI] [PubMed] [Google Scholar]
- 30.Budelli G, Hage TA, Wei A, Rojas P, Jong Y-JI, O’Malley K, Salkoff L. Na+-activated K+ channels express a large delayed outward current in neurons during normal physiology. Nat Neurosci 12: 745–750, 2009. doi: 10.1038/nn.2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arnaiz GR de L, Ordieres MGL. Brain Na+, K+-ATPase activity in aging and disease. Int J Biomed Sci 10: 85–102, 2014. [PMC free article] [PubMed] [Google Scholar]
- 32.Hamlyn JM, Blaustein MP. Endogenous ouabain: recent advances and controversies. Hypertension 68: 526–532, 2016. doi: 10.1161/HYPERTENSIONAHA.116.06599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aperia A. New roles for an old enzyme: Na,K‐ATPase emerges as an interesting drug target. J Intern Med 261: 44–52, 2007. doi: 10.1111/j.1365-2796.2006.01745.x. [DOI] [PubMed] [Google Scholar]
- 34.Pusch M, Neher E. Rates of diffusional exchange between small cells and a measuring patch pipette. Pflügers Archiv 411: 204–211, 1988. doi: 10.1007/BF00582316. [DOI] [PubMed] [Google Scholar]
- 35.Golding NL, Jung H, Mickus T, Spruston N. Dendritic calcium spike initiation and repolarization are controlled by distinct potassium channel subtypes in CA1 pyramidal neurons. J Neurosci 19: 8789–8798, 1999. doi: 10.1523/JNEUROSCI.19-20-08789.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]