Keywords: eupnea, gasping, respiratory rhythm, serotonin, 5-HT2 receptors
Abstract
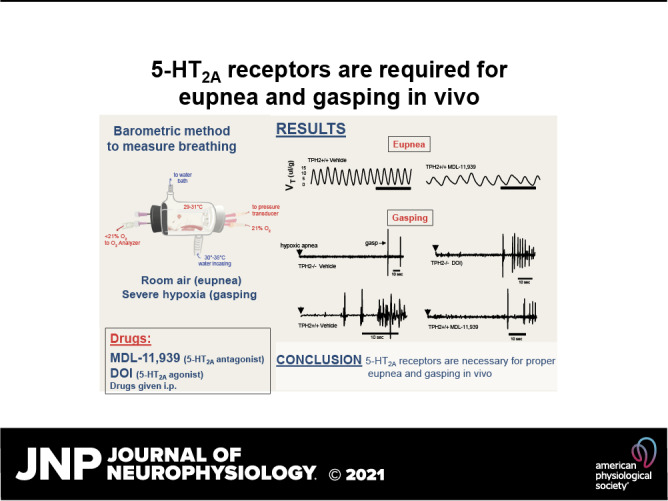
Eupnea and gasping in infancy depend on central nervous system (CNS) serotonin (5-hydroxytryptamine; 5-HT). Although previous in vitro preparations have provided some evidence that 5-HT acts through type 2 A receptors (5-HT2A) to facilitate eupnea and gasping, here the hypothesis addressed is that 5-HT2A receptor activation is necessary for eupnea and the proper generation of gasping in vivo. To test this, we administered 2,5-dimethoxy-4-iodoamphetamine (DOI; 0.25 mg/kg i.p.), a 5-HT2A agonist, 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT; 0.25 mg/kg i.p.), a 5-HT1A agonist, or vehicle (saline) to 7–9-day-old tryptophan hydroxylase 2 knockout (TPH2−/−) mice. A second experiment assessed the effect of MDL-11,939 (MDL; 10 mg/kg i.p.), the specific 5-HT2A antagonist, or vehicle (DMSO) on the gasping of wild-type (TPH2+/+) animals. Drugs were given 15 min prior to five episodes of severe hypoxia that elicited gasping. TPH2−/− breathed more slowly but had the same V̇e and V̇e/V̇o2 compared with TPH2+/+. As previously reported, the gasping of TPH2−/− was significantly delayed (P < 0.001) and occurred at a significantly lower frequency compared with TPH2+/+ (P = 0.04). For both genotypes, DOI hastened eupneic frequency but had no effect on V̇e or V̇e/V̇o2. The gasping of TPH2−/−, although unaffected by 8-OH-DPAT, was indistinguishable from the gasping of TPH2+/+ following DOI. In TPH2+/+, application of MDL led to hypoventilation (P = 0.01), a delay in the appearance of gasping (P = 0.005), and reduced gasp frequency (P = 0.05). These data show that, in vivo, 5-HT2A receptors facilitate both eupnea and gasping. As has been shown in vitro, 5-HT2A probably promotes gasping by exciting hypoxia-resistant pacemaker neurons.
NEW & NOTEWORTHY Previous in vitro studies suggest that 5-HT2A receptors contribute to eupnea and are necessary for fictive gasping. The current study shows that the impaired gasping displayed by neonatal TPH2−/− mice, deficient in CNS serotonin, is restored by 5-HT2A receptor activation. Following 5-HT2A blockade, wild-type mice hypoventilated and their gasping resembled that of TPH2−/− mice. This study shows that both eupnea and gasping in vivo rely on the activation of 5-HT2A receptors.
INTRODUCTION
Experiments conducting in a variety of conscious rodents lacking 5-HT neurons, or specifically 5-HT, have provided strong evidence that 5-HT is critical for eupnea and the generation of gasping in response to severe hypoxia. For example, newborn mice with a partial (Pet-1−/−) or complete (Lmx1b−/−) loss of 5-HT neurons have reduced respiratory frequency and an unstable respiratory pattern characterized by frequent apneas (1–3). This effect can be ascribed at least partially to an acute or chronic loss of drive provided by 5-HT, given that mice deficient in tryptophan hydroxylase 2 (TPH2) and therefore specifically lacking 5-HT, have a similar phenotype (4).
Infant Pet-1−/− and TPH2−/− mice, as well as infant rats having a partial loss of 5-HT neurons, also have an altered eupneic breathing pattern as well as delayed and abnormally slow gasping in response to severe hypoxia (4–6). This phenotype is associated with a delay in cardiorespiratory recovery upon return to room air, as well as high mortality (i.e., failed autoresuscitation) (5). The abnormal eupnea and gasping of TPH2−/− mice was reversed by administration of 5-hydroxytryptophan (5-HTP), the product of TPH2 and immediate precursor to 5-HT, suggesting that 5-HT facilitates gasping through acute neuromodulation, rather than through an effect on the development of the relevant respiratory neural circuitry (4). It also strongly links the serotonergic abnormalities associated with sudden infant death syndrome (SIDS) cases—including reduced 5-HT and reduced expression of 5-HT2A and 5-HT1A receptors (7, 8)—to the severe hypoxia and failed autoresuscitation that ultimately precedes death (9–11).
Normal respiratory rhythm depends on the activity provided by two types of respiratory pacemaker neurons: cadmium-sensitive (CS) pacemakers whose bursting depends on Ca2+-activated nonspecific cation currents, and cadmium-insensitive (CI) pacemakers whose bursting depends on persistent Na+ currents (12–14). Both types of pacemakers are sufficient to drive eupnea; i.e., eupnea continues if only one type is blocked (12, 13, 15, 16). In contrast, gasping, the high amplitude respiratory pattern that emerges during severely hypoxic conditions, depends solely on the activity of CI pacemakers (13, 15). In vitro, blockade of 5-HT2A receptors selectively eliminates the activity of CI pacemakers (17, 18), leading to reduced fictive respiratory frequency and amplitude (18) and an elimination of fictive gasping (17). On the contrary, separate experiments conducted in situ suggest that 5-HT2A receptor blockade minimally impairs gasping (19, 20). The issue of whether 5-HT2A receptor activation is necessary for eupnea and the proper initiation of gasping in vivo is unresolved and is the focus here.
The hypothesis tested is that eupnea and gasping in vivo rely on the activation of 5-HT2A receptors. If the altered eupnea and delayed, slow gasping of TPH2−/− mice (4) is due to a lack of drive provided through 5-HT2A receptors, then a 5-HT2A agonist should normalize both rhythms. Likewise, the application of a 5-HT2A antagonist to a wild-type animal should recapitulate the altered eupneic breathing and hindered gasping of the TPH2−/− mice. In addition to 5-HT2A receptors, this study also tested the role of 5-HT1A receptors in eupnea and gasping, given that others have shown they can potently stimulate breathing through an inhibition of glycinergic neurons [i.e., via disinhibition (21, 22)], and because reduced 5-HT1A expression is highly associated with SIDS (7, 8). The findings of this study support the hypothesis, with respect to the role of 5-HT2A receptors, in that the delayed and slower gasping of TPH2−/− mice was normalized by 5-HT2A receptor activation, whereas in wild-type mice 5-HT2A blockade led to hypoventilation as well as delayed and slowed gasping.
MATERIALS AND METHODS
Ethical Approval
All animal experiments were approved by the University of Missouri Institutional Animal Care and Use Committee and performed in accordance with guidelines set forth by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, 2011).
Animals and Treatments
The generation and characteristics of the TPH2−/− mice used in this study have been previously described (23). This is the same line used for our previous study, in which we confirmed a loss of central 5-HT in the TPH2−/− animals (4). Genotyping has also been previously described (4). Pups from heterozygous (TPH2+/−) breeders maintained on a mixed C57Bl/6 and 129 Sv genetic background were used in this study. All animals were provided food and water ad libitum and were housed with a 12-h light-dark cycle and a room temperature of 21–23°C. For all experiments, we used TPH2−/− pups and littermate controls. Control data were obtained by combining data from TPH2+/+ and TPH2+/− pups (hereafter referred to as “wild-type” or “TPH2+/+”), as we found no difference in tissue 5-HT levels. In addition, gasping and other autoresuscitation variables are not different between TPH2+/+ and TPH2+/− animals, including those measured in this study. To avoid litter effects, pups were obtained from multiple litters from multiple breeding pairs.
Experiment 1 tested the effects of 5-HT2A and 5-HT1A activation and inhibition in postnatal day 8–10 TPH2−/− and TPH2+/+, respectively. A total of 47 pups were tested. Ten TPH2−/− and 10 wild-type mice were injected with vehicle (saline); nine TPH2−/− and seven TPH2+/+ were treated with 2,5-dimethoxy-4-iodoamphetamine (DOI; 0.25 µg/mg, 20–30 µL volume, i.p.), a commonly used 5-HT2A agonist. The affinity of DOI for 5-HT2A receptors is ∼3-fold higher than 5-HT2C receptors and ∼30-fold higher than 5-HT2B receptors (24). Nine TPH2−/− and five TPH2+/+ pups were treated with 8-hydroxy-2-(di-n-propylamino)tetralin (8-OH-DPAT; 0.25 µg/mg, 20–30 µL volume, i.p.), a commonly used 5-HT1A receptor agonist that also has some affinity for 5-HT7 receptors (25). Experiment 2 was performed at a later date with pups from different breeding pairs. Ten TPH2+/+ pups were treated with MDL-11,939 (MDL; 10 µg/mg, 20–30 µL volume, i.p.), a highly selective 5-HT2A antagonist, and eight TPH2+/+ were given vehicle alone (100% DMSO). The affinity of MDL for 5-HT2A receptor is ∼800-fold greater than its affinity for 5-HT2C receptors, and it has almost no affinity for 5-HT2B receptors (26). Pups were randomly assigned to either drug or vehicle. Drug concentrations were chosen based on minimum concentrations producing physiological effects in previous experiments (27).
Experimental Setup
Data were recorded from unrestrained animals kept within a water-perfused glass plethysmographic chamber (volume: 100 mL), attached to a programmable water bath/pump to maintain chamber temperature at 31–32°C, within the thermoneutral range for pups this age (28). Air (21% O2, balance N2) from a gas cylinder passed through a flowmeter before entering the chamber via a 20-G needle pushed through one of the rubber stoppers that seal the chamber. Chamber pressure was kept near atmospheric by pulling the gas from the opposite end of the chamber with the pump from a O2/CO2 analyzer (model GA-200, iWorx Systems, Inc., Dover, NH), also through a 20-G needle. Air flow through the chamber was held constant at 150 mL/min. The analog signal from the respiratory pressure transducer was fed into a Powerlab data acquisition system (ADInstruments, Colorado Springs, CO) and analyzed in LabChart V8 (ADInstruments).
Experimental Protocol
While blinded to genotype, pups were removed from the litter and weighed. Pups were then placed in the plethysmographic chamber and allowed a 15-min settling period. For experiment 1, after the 15-min settling period, pups were injected i.p. with saline, DOI, or DPAT. For experiment 2, pups were injected with either 100% DMSO or MDL. Following injection, pups were allowed to breathe room air for another 15 min to allow for the determination of drug effects on eupnea, at which point the gas flowing into the chamber was switched from room air to 0% O2 balanced with 3% CO2 to elicit gasping. At the onset of hypoxic apnea, the gas was switched back to room air to allow autoresuscitation once gasping ensued. To assess survival, each animal was exposed to a total of five episodes of severe hypoxia, with 5 min of intervening room air between each episode.
Data and Statistical Analyses
The experimenter remained blinded to genotype until after the data were analyzed. Respiratory activity was determined with LabChart 8 using peak detection on the raw pressure trace. Variables during the baseline period of both experiments 1 and 2 were combined to assess the influence of genotype during normoxic conditions. Variables measured included tidal volume (VT; mL/kg), frequency (f) and eupneic ventilation (V̇e; mL/min/kg), metabolic rate [V̇o2; mL/min/kg; (0.21 − fractional O2 in the effluent gas) × flow (150 mL·min−1)/mass (kg)], and the ventilatory equivalent (V̇e/V̇o2). VT was calculated from the amplitude of the respiratory signal using the method described by Drorbaugh and Fenn (29). For both vehicle and drug groups, resting (i.e., normoxic) ventilatory and metabolic variables were collected and analyzed before and after the injection, and effects of drug, genotype, and their interaction were assessed with a two-way repeated measures ANOVA with Tukey’s post hoc comparisons using GraphPad Prism (GraphPad Software, San Diego, CA).
In each group (vehicle and drug), the five episodes of severe hypoxia to elicit gasping were given only once, following the injection, as severe hypoxia has secondary effects on metabolic rate and blood pressure, which can potently affect the timing and frequency of gasping (5, 30). For this reason, gasping was analyzed only during the first of the five hypoxic episodes. We measured the delay to the emergence of the first gasp following the apnea produced by the first episode of severe hypoxia (i.e., gasp latency) and the instantaneous frequency of the first gasp (gasp f; 60/period between the first and second gasps). For gasping variables, significant differences were determined using one-way ANOVA and Tukey’s multiple comparisons post hoc analyses, or two-tailed t tests where appropriate.
For all data, the average ± 95% confidence intervals are presented, with α being set to 0.05. Log-rank (Mantel–Cox) tests within GraphPad Prism were used to determine whether survival curves over the five hypoxic episodes were affected by genotype or drug treatment.
RESULTS
5-HT2A Receptor Activation Contributes to Eupnea In Vivo
During baseline conditions, the breathing pattern of TPH2−/− was slightly but significantly different compared with TPH2+/+. Specifically, f of TPH2−/− was on average 23 breaths/min slower than TPH2+/+ (t = 2.0; P = 0.04). However, neither V̇e nor V̇e/V̇o2 were affected by genotype, as the VT of TPH2−/− (12.9 ± 0.5 mL/kg) tended to be greater than that of TPH2+/+ (11.8 ± 0.6 mL/kg; t = 1.7; P = 0.15).
In our first experiment, we applied DOI and DPAT to TPH2−/− and TPH2+/+ mice to target 5-HT2A and 5-HT1A receptors, respectively. The 15 min before and after the injection of drug or saline allowed for the assessment of drug effects on the eupneic breathing pattern. In both genotypes, DPAT reduced overall V̇e, but this effect was significantly greater in TPH2−/− (n = 10) compared with TPH+/+ (n = 5; genotype × drug: F = 6.3; P = 0.027; Fig. 1A). This effect was mostly due to the inhibitory effect of DPAT on VT (genotype × drug: P = 0.046), whereas frequency (f) was spared; VT decreased from an average of 14.8 mL/kg to 12.1 mL/kg in TPH2+/+ (P = 0.06), and from 14.0 mL/kg to 8.2 mL/kg in TPH2−/− (P < 0.001). Although this finding suggests an effect of 5-HT1A receptor activation on the eupneic breathing pattern, DPAT also strongly suppressed V̇o2 (by ∼50% in both genotypes; F = 23.2; P = 0.0004; Fig. 1A). In this way, for both genotypes, DPAT had no influence on V̇e/V̇o2, suggesting no effect of 5-HT1A activation on eupnea and therefore blood gases.
Figure 1.
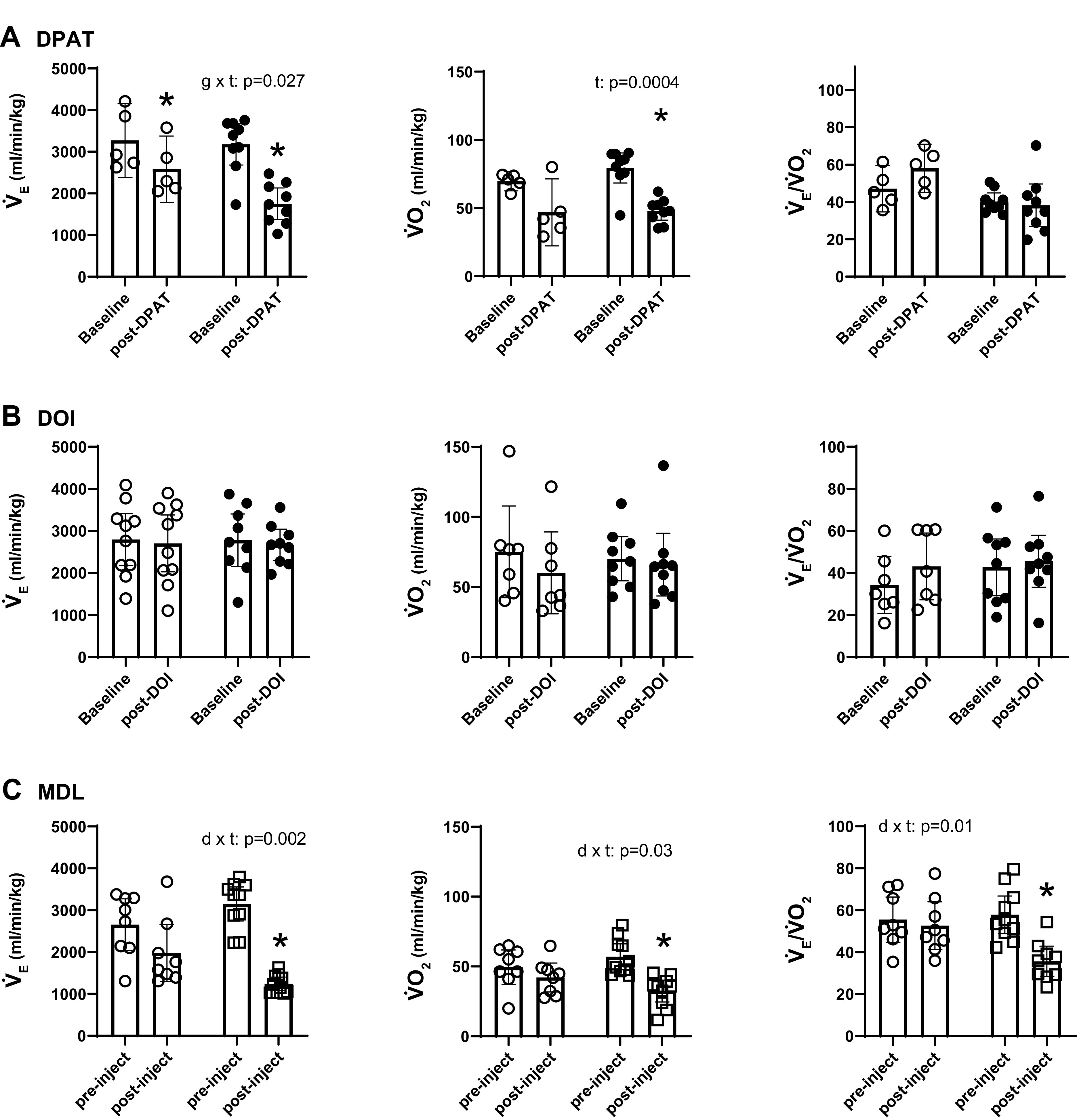
Effects of 8-OH-DPAT (A) and DOI (B) on ventilation (V̇e), metabolic oxygen consumption (V̇o2), and the ventilatory equivalent (V̇e/V̇o2) of TPH2+/+ (open circles, n = 5) and TPH2−/− littermates (closed circles; n = 9) under baseline, resting conditions. C: effects of vehicle (DMSO; open circles; n = 8) or MDL-11,939 (MDL, open squares; n = 10) on these variables in TPH2+/+ mice. Significant effects of genotype (g) (in A and B) or drug (d) (in C) and time (t) assessed with a two-way repeated measures ANOVA, with Tukey’s post hoc tests. *Significant effect of drug (post hoc: P < 0.05). DOI, 2,5-dimethoxy-4-iodoamphetamine; DPAT, 8-OH-DPAT; TPH2, tryptophan hydroxylase 2; TPH2+/+ mice, wild-type mice; 8-OH-DPAT, 8-hydroxy-2-(di-n-propylamino)tetralin.
The application of DOI had no effect on V̇e (Fig. 1B). Although DOI significantly increased f in both genotypes (by ∼60 breaths/min in TPH2+/+ and ∼80 breaths/min in TPH2−/−; F = 26.5; P = 0.0001), this effect was met with a concurrent decrease in VT [by ∼2 mL/kg in TPH2+/+ (n = 7) and 3 mL/kg in TPH2−/− (n = 9); F = 28.3; P = 0.0001]. Given also that there was no effect of DOI on V̇o2, for both genotypes there was no effect of the drug on V̇e/V̇o2 (Fig. 1B). The injection of saline (vehicle) had no effect on any of the measured variables (not shown).
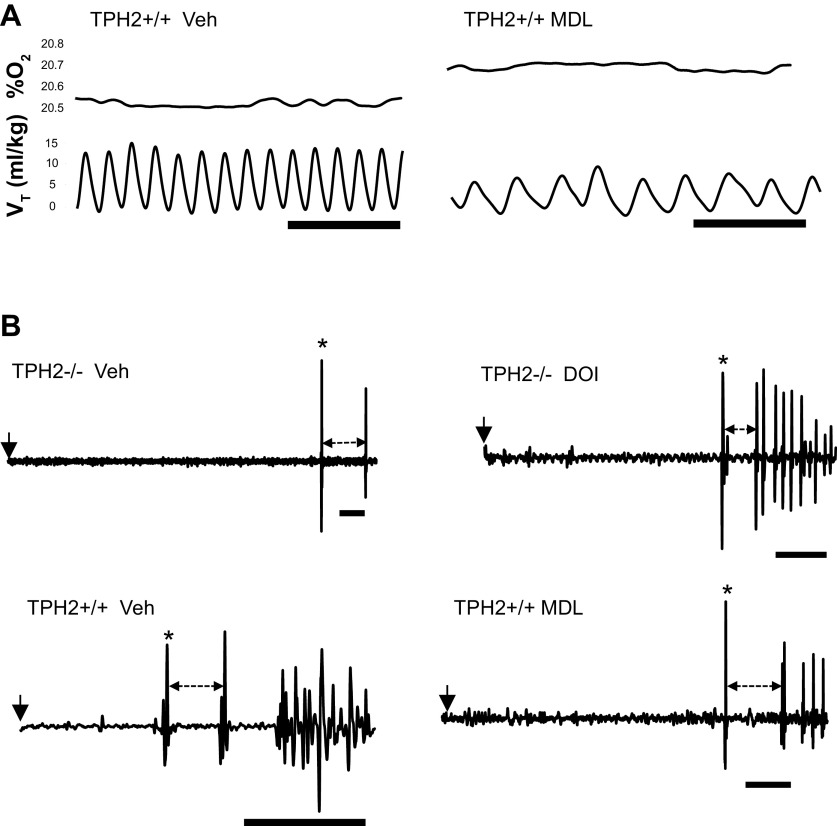
In the second experiment, the highly selective 5-HT2A antagonist MDL, or vehicle (DMSO) alone, was administered to TPH2+/+ pups to further address the hypothesis that 5-HT2A receptor activation is required for proper eupnea in vivo. In normoxic conditions, MDL potently suppressed eupneic V̇e; although there was no difference in V̇e between the vehicle (DMSO; n = 8) and MDL (n = 10) groups before the injection, the V̇e of TPH2+/+ pups was significantly reduced by MDL compared with those given vehicle alone (drug × time: F = 13.2; P = 0.002; Fig. 1C and Fig. 2A). MDL suppressed V̇e through inhibitory effects on both f (by 88 breaths/min; drug × time: F = 19.6; P = 0.0004; Fig. 2A) and VT (by 4.8 mL/kg; drug × time: F = 6.7; P = 0.02; Fig. 2A). MDL also led to a greater fall in V̇o2 compared with vehicle alone (drug × time: F = 5.5; P = 0.03; Fig. 1B and Fig. 2A). However, the fall in V̇e in response to MDL was proportionally larger than the fall in V̇o2, such that MDL resulted in a significant fall in V̇e/V̇o2 (i.e., hypoventilation; drug × time: F = 7.7; P = 0.014).
Figure 2.
A: representative respiratory (tidal volume, VT) and metabolic rate (reflected in the % O2 leaving the chamber; %O2) traces from a TPH2+/+ pup following administration of vehicle (DMSO) or MDL-11,939 (MDL); scale bar = 1 s. B: raw traces of gasping in a TPH2−/− given vehicle (saline; top left), a TPH2−/− given DOI (top right), a TPH2+/+ given vehicle (DMSO; bottom left) and a TPH2+/+ given MDL (bottom right). Start of hypoxic apnea denoted by arrow. First gasp denoted by *. Dashed arrow indicates the period between the first and second gasp, used to calculate gasping frequency. Scale bar = 10 s in all panels of B. Note the delayed gasping in TPH2−/− and its hastening following DOI. MDL delayed and slowed the gasping of TPH2+/+. DOI, 2,5-dimethoxy-4-iodoamphetamine; TPH2, tryptophan hydroxylase 2; TPH2+/+ mice, wild-type mice; TPH2−/− mice, tryptophan hydroxylase 2 knockout mice; Veh, vehicle.
5-HT2A Receptor Activity Is Required for the Proper Initiation and Rhythm of Gasping
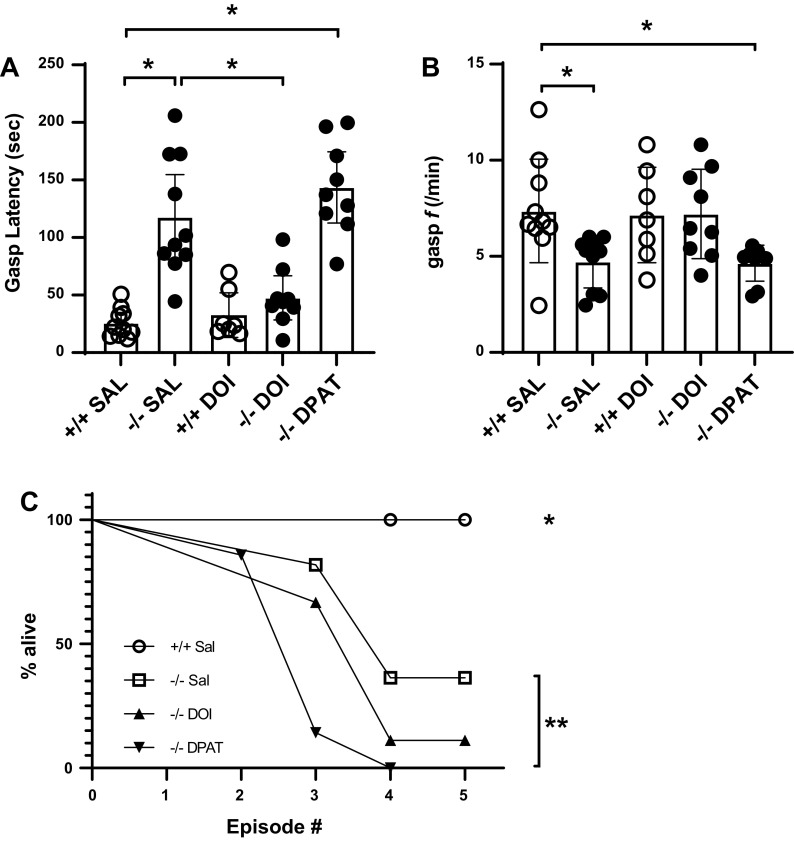
To test the hypothesis that 5-HT2A receptor activity is necessary for the proper timing and rhythm of gasping, we tested TPH2−/− animals following the systemic administration of either vehicle or DOI, as well as TPH2+/+ following the administration of MDL. As we previously reported (4), the initiation of gasping was significantly delayed in TPH2−/− pups; gasp latency was ∼5 times longer than that of TPH2+/+ (overall F = 22.5; P < 0.0001; post hoc comparison of genotypes: P < 0.0001; Fig. 2B and Fig. 3A). Although DOI had no significant effect on the gasp latency of TPH2+/+ pups, it significantly hastened the appearance of the first gasp in TPH2−/− pups, such that following treatment their gasp latency was no different than TPH2+/+ (post hoc comparison of TPH2+/+ given vehicle and TPH2−/− given DOI: P = 0.63; Fig. 3A). DPAT, on the other hand, had no significant effect on the gasp latency of TPH2−/− (Fig. 3A). Similarly, gasp frequency was significantly reduced in TPH2−/− (overall P = 0.005; post hoc between genotypes given saline: P = 0.047; Fig. 3B). DOI tended to increase the frequency of TPH2−/− gasping (P = 0.08; Fig. 3B) such that following treatment, their gasp frequency was indistinguishable from that of TPH2+/+ (P = 0.99; Fig. 3B). The gasp frequency of TPH2−/− was not affected by DPAT; following treatment their gasping was still slower than that of TPH2+/+ (post hoc between TPH2−/− given DPAT and TPH2+/+ given saline: P = 0.047). All TPH2+/+ survived the five episodes of severe hypoxia, compared with 36% of TPH2−/− (overall chi square: 27; P < 0.0001; Fig 3C). Surprisingly, although DOI significantly hastened the gasping of TPH2−/−, it had no effect on their ability to survive all five hypoxic episodes (P = 0.19; Fig. 3C). DPAT, on the other hand, reduced the ability of TPH2−/− to survive all five episodes (P = 0.004; Fig. 3C).
Figure 3.
Gasp latency (A) and frequency (B) in TPH2+/+ given vehicle (saline; n = 10), TPH2−/− given vehicle (n = 10), TPH2+/+ given DOI (n = 7), TPH2−/− given DOI (n = 9), and TPH2−/− given DPAT (n = 9). *Significant difference (P < 0.05), assessed using a one-way ANOVA with Tukey’s post hoc analysis. C: survival curves of TPH2+/+ (open circles), TPH2−/− (open squares), TPH2−/− given DOI (closed triangles), and TPH2−/− given DPAT (inverted closed triangles) across the five episodes of severe hypoxia. *Significant difference in survival curve of TPH2+/+ compared with all other groups. **Significant difference in the survival curve of TPH2−/− given DPAT compared with TPH2−/− given saline. Survival curves analyzed using Log-rank (Mantel–Cox) tests. DOI, 2,5-dimethoxy-4-iodoamphetamine; DPAT, 8-OH-DPAT; f, frequency; SAL, saline; TPH2, tryptophan hydroxylase 2; TPH2+/+ mice, wild-type mice; TPH2−/− mice, tryptophan hydroxylase 2 knockout mice; 8-OH-DPAT, 8-hydroxy-2-(di-n-propylamino)tetralin.
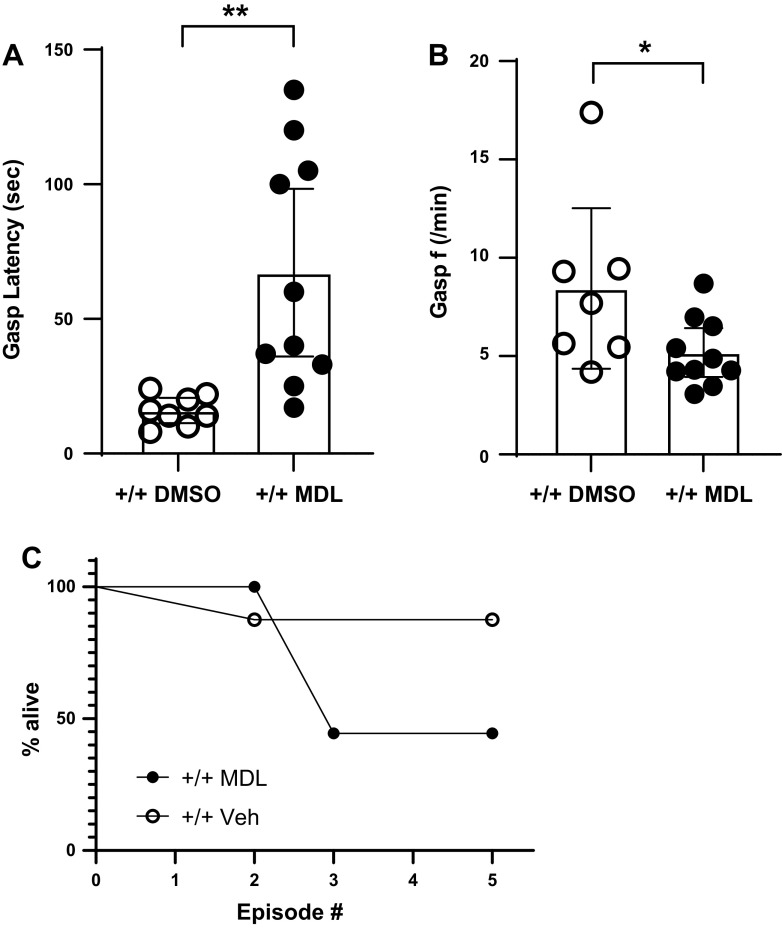
MDL significantly delayed the appearance of gasping in TPH2+/+ during severely hypoxic conditions [t test comparing vehicle (DMSO) to MDL: t = 3.3; P = 0.0047; Fig. 4A]. This effect was unrelated to the hypometabolic effect of MDL (regression analysis of gasp latency and V̇o2: R2 < 0.01). In addition, MDL marginally slowed the gasping frequency of TPH2+/+ (t = 2.1; P = 0.05; Fig. 4B). Compared with vehicle alone, MDL tended to reduce the ability of TPH2+/+ pups to survive the five hypoxic episodes, but this effect did not reach statistical significance (P = 0.17; Fig. 4C).
Figure 4.
Gasp latency (A) and frequency (B) in TPH2+/+ given vehicle (DMSO; open circles; n = 8) or MDL-11,939 (MDL; closed circles; n = 10). Significant differences between groups indicated by * (P < 0.05) and ** (P < 0.01), assessed using a two-tailed t tests. C: survival curves of TPH2+/+ given vehicle (open circles) and TPH2+/+ given MDL (closed circles) across the five episodes of severe hypoxia. f, frequency; TPH2, tryptophan hydroxylase 2; TPH2+/+, wild-type mice; Veh, vehicle.
DISCUSSION
These experiments show that the delayed and slow gasping of TPH2−/− mice can be normalized by DOI, an agonist of 5-HT2A receptors. In addition, 5-HT2A blockade in wild-type animals resulted in hypoventilation as well as delayed and slowed gasping. These results support previous in vitro and in situ studies showing that the endogenous activation of the 5-HT2A receptor is required for proper eupneic ventilation (3, 18, 31). 5-HT2A receptor blockade reduced both frequency and tidal volume in wild-type mice, mirroring the effects of Ptak and colleagues (31) who used the same antagonist (MDL) in experiments performed in situ. Our data are also in keeping with in vitro data showing that 5-HT2A is necessary for fictive gasping in severe hypoxia (17). Although 5-HT2A likely promotes gasping via a stimulatory effect on cadmium-insensitive, hypoxia-resistant pacemaker neurons (i.e., those relying on persistent sodium currents) (17), its effect on eupnea in vivo may be more complex, involving several regions of the ventral respiratory column; indeed, serotonergic neurons project throughout the respiratory network (32). That said, a loss of 5-HT2A drive solely to pacemakers within the pre-Botzinger that rely on persistent sodium currents is sufficient to decrease the frequency and amplitude of fictive respiratory activity in vitro (18) and could also underlie the in vivo findings reported here.
The effect of DOI on the eupneic breathing of TPH2−/− mice (increased frequency and decreased tidal volume) was not the exactly the opposite of the effect of MDL in TPH2+/+ mice (decreased frequency and tidal volume). TPH2−/− animals have been without 5-HT their entire lives, likely eliciting plasticity within the respiratory network to maintain ventilation (e.g., they tend to have higher tidal volume). Thus, it is perhaps not surprising that the effects of acute activation of 5-HT2A receptors in TPH2−/− did not precisely mirror the effect of 5-HT2A blockade in the TPH2+/+. It is also acknowledged that blocking 5-HT2A receptors did not eliminate gasping altogether; rather, it delayed its initiation and frequency. Substance P has been shown to increase the burst frequency of CI pacemaker neurons (33) and may function to partially compensate for a loss of serotonergic drive (34).
Surprisingly, despite their hastened gasping, the survival of TPH2−/− mice was not improved by DOI. Likewise, although the survival of TPH2+/+ tended to be decreased by 5-HT2A blockade, this effect did not reach statistical significance. Moreover, activating 5-HT1A receptors by DPAT actually decreased the survival of TPH2−/−. This latter effect may be due to the inhibition of TRH and Substance P neurons by the activation of somato-dendritic 5-HT1A autoreceptors, halting the release of these neuromodulators at target sites. What is clear from these data is that 5-HT2A receptor activation, while facilitating gasping, is not sufficient for withstanding multiple episodes of severe hypoxia. The maintenance of arterial blood pressure by 5-HT is also critical for surviving multiple episodes of severe hypoxia (30). Although purely speculative, this effect may depend on serotonergic signaling through multiple 5-HT receptors, not just 5-HT2A.
Methodological Considerations
It is acknowledged that the approach used here cannot resolve the site of action whereby 5-HT2A activation facilitates eupnea and gasping. Targeted application of drugs in TPH2−/− mice at this age is exceedingly difficult due to their small size (<4 g) and sensitivity to anesthesia. However, based on previous data from in vitro and in situ studies, it is highly likely that 5-HT2A receptor activation on hypoxia-resistant, CI pacemaker neurons within the Pre-Botzinger Complex promotes the gasp in vivo (18, 31, 35).
Exposure to severe hypoxia undoubtedly would have led to a suppression of metabolic O2 consumption and CO2 production, which in theory could have affected the timing of the gasp following hypoxic apnea. Some observations argue against this possibility. First, DPAT potently reduced metabolic drive but did not alter the latency to gasping or the gasp frequency of TPH2−/− pups. Second, DOI had no effect on metabolic drive, yet expedited the gasping of TPH2−/− pups. Finally, we could find no hint of a correlation between the underlying metabolic drive and gasp latency or frequency. A drug-induced change in arterial blood pressure could have also confounded the central effects of our pharmacological manipulations. However, 5-HT2A receptor activation in the periphery generally leads to vasoconstriction (36) and increased left ventricular contractility (37, 38), potentially increasing blood pressure and central blood flow. If anything, this would delay the initiation of the gasp because of a relative preservation of tissue PO2. The opposite would occur following 5-HT2A blockade: reduced blood pressure, a relative decrease in tissue PO2 centrally, and expedited gasping (30). The current results, however, are the opposite of these predicted changes in gasping: activating 5-HT2A in TPH2−/− expedited gasping, whereas blocking them in wild-type animals delayed gasping. Taken together, it seems unlikely that the effects of the drugs used were due to, or obfuscated by, any effects in the periphery.
We tested the effect of DPAT on gasping, as 5-HT1A receptor activation has been shown to stimulate breathing in other contexts (21, 22), and because of the high association between SIDS and decreased 5-HT1A expression (7, 8). The inhibitory effect of DPAT on the breathing of TPH2−/− and TPH2+/+ mice was secondary to a suppression of metabolic oxygen consumption. As DPAT also binds 5-HT7 receptors, it may be that the metabolic suppression and resulting hypopnea experienced by both genotypes in response to DPAT was not through 5-HT1A receptors but rather 5-HT7 (39). It is also acknowledged that DOI has affinity not only for 5-HT2A receptors but also 5-HT2C and, at much higher concentrations, 5-HT2B receptors (24). MDL, a drug that has much higher affinity for 5-HT2A receptors compared with 5-HT2B or 5-HT2C receptors, recapitulated the TPH2−/− gasping phenotype in wild-type animals. This strongly suggests that the effects reported here can be ascribed to a loss of serotonergic drive through 5-HT2A receptors, and not 5-HT2B or 5-HT2C receptors.
Summary
These data are in agreement with other studies showing that 5-HT2A receptor activation makes a significant contribution to eupnea. They are also the first to suggest that 5-HT2A receptor activation facilitates the generation of gasping in vivo, likely through an effect on hypoxia-resistant pacemaker neurons that drive gasping (13, 17). The role of 5-HT2A receptors in SIDS should be further investigated, given that cases experience severe hypoxia and failed gasping (10, 11) while also displaying reduced 5-HT2A receptor immunoreactivity in the ventrolateral medulla (7).
GRANTS
This research was funded by National Heart, Lung, and Blood Institute Grant 5R01HL136710-03 (to K. J. Cummings).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
K.C. conceived and designed research; K.C. performed experiments; K.C. analyzed data; K.C. interpreted results of experiments; K.C. prepared figures; K.C. drafted manuscript; K.C. edited and revised manuscript; K.C. approved final version of manuscript.
ACKNOWLEDGMENTS
Jenn Cornelius-Green is thanked for animal husbandry and genotyping.
REFERENCES
- 1.Cummings KJ, Hewitt JC, Li A, Daubenspeck JA, Nattie EE. Postnatal loss of brainstem serotonin neurones compromises the ability of neonatal rats to survive episodic severe hypoxia. J Physiol 589: 5247–5256, 2011. doi: 10.1113/jphysiol.2011.214445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson JT, Shafer G, Rossetti MD, Wilson CG, Deneris ES. Arrest of 5HT neuron differentiation delays respiratory maturation and impairs neonatal homeostatic responses to environmental challenges. Respir Physiol Neurobiol 159: 85–101, 2007. doi: 10.1016/j.resp.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hodges MR, Wehner M, Aungst J, Smith JC, Richerson GB. Transgenic mice lacking serotonin neurons have severe apnea and high mortality during development. J Neurosci 29: 10341–10349, 2009. doi: 10.1523/JNEUROSCI.1963-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Magnusson J, Karsenty G, Cummings KJ. Time- and age-dependent effects of serotonin on gasping and autoresuscitation in neonatal mice. J Appl Physiol (1985) 114: 1668–1676, 2013. doi: 10.1152/japplphysiol.00003.2013. [DOI] [PubMed] [Google Scholar]
- 5.Cummings KJ, Commons KG, Hewitt JC, Daubenspeck JA, Li A, Kinney HC, Nattie EE. Failed heart rate recovery at a critical age in 5-HT-deficient mice exposed to episodic anoxia: implications for SIDS. J Appl Physiol (1985) 111: 825–833, 2011. doi: 10.1152/japplphysiol.00336.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson JT, Sposato BC. Autoresuscitation responses to hypoxia-induced apnea are delayed in newborn 5-HT-deficient Pet-1 homozygous mice. J Appl Physiol (1985) 106: 1785–1792, 2009. doi: 10.1152/japplphysiol.90729.2008. [DOI] [PubMed] [Google Scholar]
- 7.Ozawa Y, Okado N. Alteration of serotonergic receptors in the brain stems of human patients with respiratory disorders. Neuropediatrics 33: 142–149, 2002. doi: 10.1055/s-2002-33678. [DOI] [PubMed] [Google Scholar]
- 8.Paterson DS, Trachtenberg FL, Thompson EG, Belliveau RA, Beggs AH, Darnall R, Chadwick AE, Krous HF, Kinney HC. Multiple serotonergic brainstem abnormalities in sudden infant death syndrome. JAMA 296: 2124–2132, 2006. doi: 10.1001/jama.296.17.2124. [DOI] [PubMed] [Google Scholar]
- 9.Kinney HC, Haynes RL. The serotonin brainstem hypothesis for the sudden infant death syndrome. J Neuropathol Exp Neurol 78: 765–779, 2019. doi: 10.1093/jnen/nlz062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Poets CF, Meny RG, Chobanian MR, Bonofiglo RE. Gasping and other cardiorespiratory patterns during sudden infant deaths. Pediatr Res 45: 350–354, 1999. doi: 10.1203/00006450-199903000-00010. [DOI] [PubMed] [Google Scholar]
- 11.Sridhar R, Thach BT, Kelly DH, Henslee JA. Characterization of successful and failed autoresuscitation in human infants, including those dying of SIDS. Pediatr Pulmonol 36: 113–122, 2003. doi: 10.1002/ppul.10287. [DOI] [PubMed] [Google Scholar]
- 12.Pena F, Aguileta M-A. Effects of riluzole and flufenamic acid on eupnea and gasping of neonatal mice in vivo. Neurosci Lett 415: 288–293, 2007. doi: 10.1016/j.neulet.2007.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Pena F, Parkis MA, Tryba AK, Ramirez J-M. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron 43: 105–117, 2004. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 14.Thoby-Brisson M, Ramirez JM. Identification of two types of inspiratory pacemaker neurons in the isolated respiratory neural network of mice. J Neurophysiol 86: 104–112, 2001. doi: 10.1152/jn.2001.86.1.104. [DOI] [PubMed] [Google Scholar]
- 15.Paton JFR, Abdala APL, Koizumi H, Smith JC, St-John WM. Respiratory rhythm generation during gasping depends on persistent sodium current. Nat Neurosci 9: 311–313, 2006. doi: 10.1038/nn1650. [DOI] [PubMed] [Google Scholar]
- 16.Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci 25: 446–453, 2005. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tryba AK, Pena F, Ramirez J-M. Gasping activity in vitro: a rhythm dependent on 5-HT2A receptors. J Neurosci 26: 2623–2634, 2006. doi: 10.1523/JNEUROSCI.4186-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pena F, Ramirez J-M. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci 22: 11055–11064, 2002. doi: 10.1523/jneurosci.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St-John WM, Leiter JC. Maintenance of gasping and restoration of eupnea after hypoxia is impaired following blockers of α1-adrenergic receptors and serotonin 5-HT2 receptors. J Appl Physiol (1985) 104: 665–673, 2008. doi: 10.1152/japplphysiol.00599.2007. [DOI] [PubMed] [Google Scholar]
- 20.Toppin VAL, Harris MB, Kober AM, Leiter JC, St-John WM. Persistence of eupnea and gasping following blockade of both serotonin type 1 and 2 receptors in the in situ juvenile rat preparation. J Appl Physiol (1985) 103: 220–227, 2007. doi: 10.1152/japplphysiol.00071.2007. [DOI] [PubMed] [Google Scholar]
- 21.Dutschmann M, Waki H, Manzke T, Simms AE, Pickering AE, Richter DW, Paton JFR. The potency of different serotonergic agonists in counteracting opioid evoked cardiorespiratory disturbances. Philos Trans R Soc Lond B Biol Sci 364: 2611–2623, 2009. doi: 10.1098/rstb.2009.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang X, Dergacheva O, Kamendi H, Gorini C, Mendelowitz D. 5-Hydroxytryptamine 1A/7 and 4α receptors differentially prevent opioid-induced inhibition of brain stem cardiorespiratory function. Hypertension 50: 368–376, 2007. doi: 10.1161/HYPERTENSIONAHA.107.091033. [DOI] [PubMed] [Google Scholar]
- 23.Yadav VK, Oury F, Suda N, Liu Z-W, Gao X-B, Confavreux C, Klemenhagen KC, Tanaka KF, Gingrich JA, Guo XE, Tecott LH, Mann JJ, Hen R, Horvath TL, Karsenty G. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell 138: 976–989, 2009. doi: 10.1016/j.cell.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson DL, Lucaites VL, Wainscott DB, Glennon RA. Comparisons of hallucinogenic phenylisopropylamine binding affinities at cloned human 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn Schmiedebergs Arch Pharmacol 359: 1–6, 1999. doi: 10.1007/pl00005315. [DOI] [PubMed] [Google Scholar]
- 25.Lovenberg TW, Baron BM, de Lecea L, Miller JD, Prosser RA, Rea MA, Foye PE, Racke M, Slone AL, Siegel BW, Danielson PE, Sutcliffe JG, Erlander MG. A novel adenylyl cyclase-activating serotonin receptor (5-HT7) implicated in the regulation of mammalian circadian rhythms. Neuron 11: 449–458, 1993. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 26.Welsh SE, Romano AG, Harvey JA. Effects of serotonin 5-HT2A/2C antagonists on associative learning in the rabbit. Psychopharmacology (Berl) 137: 157–163, 1998. doi: 10.1007/s002130050605. [DOI] [PubMed] [Google Scholar]
- 27.Buchanan GF, Smith HR, MacAskill A, Richerson GB. 5-HT2A receptor activation is necessary for CO2-induced arousal. J Neurophysiol 114: 233–243, 2015. doi: 10.1152/jn.00213.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mortola JP, Naso L. Thermogenesis in newborn rats after prenatal or postnatal hypoxia. J Appl Physiol (1985) 85: 84–90, 1998. doi: 10.1152/jappl.1998.85.1.84. [DOI] [PubMed] [Google Scholar]
- 29.Drorbaugh JE, Fenn WO. A barometric method for measuring ventilation in newborn infants. Pediatrics 16: 81–87, 1955. [PubMed] [Google Scholar]
- 30.Yang HT, Cummings KJ. Brain stem serotonin protects blood pressure in neonatal rats exposed to episodic anoxia. J Appl Physiol (1985) 115: 1733–1741, 2013. doi: 10.1152/japplphysiol.00970.2013. [DOI] [PubMed] [Google Scholar]
- 31.Ptak K, Yamanishi T, Aungst J, Milescu LS, Zhang R, Richerson GB, Smith JC. Raphe neurons stimulate respiratory circuit activity by multiple mechanisms via endogenously released serotonin and substance P. J Neurosci 29: 3720–3737, 2009. doi: 10.1523/JNEUROSCI.5271-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steinbusch HW. Distribution of serotonin-immunoreactivity in the central nervous system of the rat-cell bodies and terminals. Neuroscience 6: 557–618, 1981. doi: 10.1016/0306-4522(81)90146-9. [DOI] [PubMed] [Google Scholar]
- 33.Pena F, Ramirez J-M. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci 24: 7549–7556, 2004. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Doi A, Ramirez J-M. Neuromodulation and the orchestration of the respiratory rhythm. Respir Physiol Neurobiol 164: 96–104, 2008. doi: 10.1016/j.resp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pena F. Neuronal network properties underlying the generation of gasping. Clin Exp Pharmacol Physiol 36: 1218–1228, 2009. doi: 10.1111/j.1440-1681.2009.05301.x. [DOI] [PubMed] [Google Scholar]
- 36.Watts SW, Morrison SF, Davis RP, Barman SM. Serotonin and blood pressure regulation. Pharmacol Rev 64: 359–388, 2012. doi: 10.1124/pr.111.004697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Birkeland JAK, Swift F, Tovsrud N, Enger U, Lunde PK, Qvigstad E, Levy FO, Sejersted OM, Sjaastad I. Serotonin increases L-type Ca2+ current and SR Ca2+ content through 5-HT4 receptors in failing rat ventricular cardiomyocytes. Am J Physiol Heart Circ Physiol 293: H2367–H2376, 2007. doi: 10.1152/ajpheart.01375.2006. [DOI] [PubMed] [Google Scholar]
- 38.Qvigstad E, Sjaastad I, Brattelid T, Nunn C, Swift F, Birkeland JAK, Krobert KA, Andersen GO, Sejersted OM, Osnes J-B, Levy FO, Skomedal T. Dual serotonergic regulation of ventricular contractile force through 5-HT2A and 5-HT4 receptors induced in the acute failing heart. Circ Res 97: 268–276, 2005. doi: 10.1161/01.RES.0000176970.22603.8d. [DOI] [PubMed] [Google Scholar]
- 39.Hedlund PB, Kelly L, Mazur C, Lovenberg T, Sutcliffe JG, Bonaventure P. 8-OH-DPAT acts on both 5-HT1A and 5-HT7 receptors to induce hypothermia in rodents. Eur J Pharmacol 487: 125–132, 2004. doi: 10.1016/j.ejphar.2004.01.031. [DOI] [PubMed] [Google Scholar]