Abstract
Vagal afferent neurons abundantly express excitatory transient receptor potential (TRP) channels, which strongly influence afferent signaling. Cannabinoids have been identified as direct agonists of TRP channels, including TRPA1 and TRPV1, suggesting that exogenous cannabinoids may influence vagal signaling via TRP channel activation. The diverse therapeutic effects of electrical vagus nerve stimulation also result from administration of the nonpsychotropic cannabinoid, cannabidiol (CBD); however, the direct effects of CBD on vagal afferent signaling remain unknown. We investigated actions of CBD on vagal afferent neurons, using calcium imaging and electrophysiology. CBD produced strong excitatory effects in neurons expressing TRPA1. CBD responses were prevented by removal of bath calcium, ruthenium red, and the TRPA1 antagonist A967079, but not the TRPV1 antagonist SB366791, suggesting an essential role for TRPA1. These pharmacological experiments were confirmed using genetic knockouts where TRPA1 KO mice lacked CBD responses, whereas TRPV1 knockout (KO) mice exhibited CBD-induced activation. We also characterized CBD-provoked inward currents at resting potentials in vagal afferents expressing TRPA1 that were absent in TRPA1 KO mice, but persisted in TRPV1 KO mice. CBD also inhibited voltage-activated sodium conductances in A-fiber, but not in C-fiber afferents. To simulate adaptation, resulting from chronic cannabis use, we administered cannabis extract vapor daily for 3 wk. Cannabis exposure reduced the magnitude of CBD responses, likely due to a loss of TRPA1 signaling. Together, these findings detail a novel excitatory action of CBD at vagal afferent neurons, which requires TRPA1 and may contribute to the vagal mimetic effects of CBD and adaptation following chronic cannabis use.
NEW & NOTEWORTHY CBD usage has increased with its legalization. The clinical efficacy of CBD has been demonstrated for conditions including some forms of epilepsy, depression, and anxiety that are also treatable by vagus nerve stimulation. We found CBD exhibited direct excitatory effects on vagal afferent neurons that required TRPA1, were augmented by TRPV1, and attenuated following chronic cannabis vapor exposure. These effects may contribute to vagal mimetic effects of CBD and adaptation after chronic cannabis use.
Keywords: cannabis, CBD, nodose ganglia, transient receptor potential, vagus
INTRODUCTION
Increasing cannabis potency, use frequency, legal availability, and decreasing risk perception have recently contributed to increased cannabis exposure in the United States. In particular, cannabis use among adults over the age of 50 has more than tripled in one decade (SAMHSA 2013), resulting in greater coincidence of cannabis use with chronic diseases including type-II diabetes, metabolic syndrome, and cardiovascular disease, conditions influenced both by vagal afferent signaling and cannabis (Benowitz et al. 1979; Benowitz and Jones 1981; Berthoud 2008; De Ferrari et al. 2011; Derbenev and Zsombok 2016; Gidron et al. 2007; Guo et al. 1987; Rohner-Jeanrenaud et al. 1983; Stanley et al. 2013; Thayer et al. 2010; Weiss et al. 2006, 2008; Zhang et al. 2009). The primary phytocannabinoids D9-tetrahydrocannabinol (THC) and cannabidiol (CBD), as well as the majority of more than 100 other phytocannabinoids in cannabis are transient receptor potential (TRP) channel agonists (Janero and Makriyannis 2014; Meotti et al. 2014; Turner et al. 2017). This finding has recently generated interest in the role of TRP channels in the effects of both endogenous and exogenous cannabinoids. CBD is an intriguing target for study, as it lacks the CB1 receptor-mediated psychotropic effects that are characteristic of THC, yet is broadly therapeutic. CBD has a wide array of biological targets, and the therapeutic effects of CBD are similarly varied; it ameliorates insulin resistance (Kaneto et al. 1967; Rohner-Jeanrenaud et al. 1983), atherosclerosis (Gidron et al. 2007; Steffens et al. 2005), cardiovascular disease (Benowitz and Jones 1981; Stanley et al. 2013), chronic pain and inflammation (Costa et al. 2007), emesis (Rock et al. 2012), seizures (Leo et al. 2016), anxiety, depression (Ashton et al. 2005; de Mello Schier et al. 2014), and psychotic disorders including schizophrenia (Makiol and Kluge 2019; Zuardi et al. 2006a, 2006b). Remarkably, all of these effects are also elicited by electrical vagus nerve stimulation with comparable efficacy (Borovikova et al. 2000; De Ferrari et al. 2011; Groves and Brown 2005; Kirchner et al. 2000; Li et al. 2004; Rohner-Jeanrenaud et al. 1983; Zhang et al. 2009). However, the contribution of the vagus nerve to the therapeutic effects of CBD is not yet known.
The vagus nerve is a major component of the autonomic nervous system, comprising sensory afferent and parasympathetic efferent fibers that control cardiopulmonary, glucoregulatory, gastrointestinal, and other functions (Dockray 2009). Vagal afferent neurons consist of myelinated A-fiber and unmyelinated C-fiber phenotypes. A-fibers are generally thought to fine tune autonomic reflexes and can synergistically enhance more powerful reflexes evoked by TRPV1-containing C-fibers (Coleridge and Coleridge 1984; Fan et al. 1999). Although cannabinoids are canonically inhibitory, TRP channels have recently emerged as excitatory effectors of endogenous and exogenous cannabinoids in recombinant systems (De Petrocellis et al. 2011; Pertwee 2008). CBD has also been reported to activate nonvagal primary sensory neurons via activation of unidentified ruthenium red-sensitive TRP channel(s) or TRPV2 (Price et al. 2004; Qin et al. 2008). Although determining the effects of cannabinoids on vagal afferent signaling may identify useful therapeutic targets, the direct effects of cannabinoids on the vagus nerve have not yet been investigated.
In this study, we characterize the excitatory effects of CBD on vagal afferent neurons and determine the contribution of TRPA1 and TRPV1 in these effects. Ratiometric fluorescent calcium imaging and whole cell patch-clamp recordings of cultured nodose ganglia neurons revealed that CBD results in depolarization, action potential firing, and increased intracellular calcium from a ruthenium red-sensitive membrane conductance in a subpopulation of vagal afferents. CBD calcium concentration-response profiles indicate that functional expression of TRPA1 confers high sensitivity to CBD. The magnitude of these responses was synergistically enhanced by TRPV1, although TRPV1 alone was not associated with sensitivity to CBD. Although the TRPV1 antagonist SB366791 did not prevent CBD calcium responses, the TRPA1 antagonist A967079 and TRPA1-knockout (KO) both prevented CBD responses. Furthermore, CBD significantly inhibited voltage-activated sodium channel current in A-fibers, but not in TRPV1-expressing C-fibers that express the majority of vagal afferent TRPA1 and exert the most influence on vagal reflexes.
MATERIALS AND METHODS
Animals.
Male Sprague-Dawley rats (120–250 g, Simonsen Laboratories), male TRPA1 KO mice (20–30 g) (B6.129P-Trpa1tm1Kykw/J, Jackson Laboratories), and male TRPV1 KO mice (B6.129S4-Trpv1tm1Jul/J) were used under procedures approved by the Institutional Animal Care and Use Committee at Washington State University and in accordance with the Guide for the Care and Use of Laboratory Animals. For these initial studies, male animals were used, as TRP channel expression is regulated by gonadal hormones including estrogen (Kumar et al. 2018). Animals were housed under normal 12:12-h light/dark conditions and fed standard pellet chow ad libitum.
Nodose ganglia isolations and primary neuronal cultures.
For primary rat and mouse neuronal cultures, we removed and pooled both the left and right nodose using aseptic surgical protocols as previously reported (Kinch et al. 2012). Using high magnification and blunt dissection, the vagal trunk was dissociated from the common carotid artery. All surgeries and euthanasia were performed under a deep plane of anesthesia (ketamine, 25 mg/100 g; with xylazine, 2.5 mg/100 g). Once isolated, nodose ganglia was digested in Ca2+/Mg2+-free Hanks’ balanced salt solution containing 1 mg/mL of both dispase II (Hoffmann-La Roche) and collagenase type 1A (Sigma-Aldrich Corp., St. Louis, MO) (90 min at 37°C in 95% air-5% CO2). Following enzymatic digestion, the neurons were dissociated by gentle trituration through siliconized pipettes, and then washed in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin. Dissociated neurons were plated onto poly-l-lysine-coated coverslips maintained in DMEM+10% FBS (37°C in 95% air-5% CO2) and used after 24–48 h, following isolation for ratiometric fluorescent calcium imaging or patch-clamp electrophysiology.
Ratiometric fluorescent calcium measurements.
Intracellular calcium concentrations were monitored using fluorescent Ca2+ indicator Fura-2 AM (Molecular Probes, Eugene, OR) on an inverted Nikon Eclipse Ti microscope (Nikon Instruments, Melville, NY), with an Andor Zyla digital camera (Andor Technology Ltd, Belfast, Northern Ireland). Neurons plated on coverslips the day before were loaded with Fura-2 AM (1 μM) for 1 h, rinsed, and then mounted onto a closed chamber while constantly perfused with physiological bath (in mM: 140 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 6 glucose, 10 HEPES with pH adjusted to 7.4 with Tris Base). Cells loaded with Fura-2 AM were alternatively excited with 340 nm and 380 nm light with fluorescence monitored at 510 nm. The mean fluorescence ratio of each cell was collected with Nikon Elements software at 6-s time intervals and ratios of fluorescence intensity were converted to Ca2+ nM concentrations using a standard curve. The calcium calibration curve was determined previously using a sequential calcium titration with Fura-2 in the bath. The resulting fluorescence ratios were fit to determine the binding parameters using protocols previously summarized (Helmchen 2011). The calibration curve is then saved in the imaging software for conversion of fluorescence ratios to calcium concentrations. Drugs were perfused via a common manifold, and all manipulations were performed at room temperature. Neuron viability was confirmed through depolarization with a high-potassium (HiK) bath, where 55 mM KCl was used with an equimolar reduction of NaCl to 90 mM.
Whole cell patch-clamp recordings.
Whole cell recordings were performed on dissociated nodose ganglion neurons using an inverted Nikon Eclipse FN1 microscope. Recording electrodes (3–4.5 MΩ) were filled with K+ internal or Cs2+ internal. K+ internal contained (in mM) 6 NaCl, 4 NaOH, 130 K-gluconate, 11 EGTA, 1 CaCl2, 1 MgCl2, 10 HEPES, 2 Na2ATP, and 0.2 Na2GTP. Cs2+ internal contained (in mM) 10 CsCl, 110 Cs-methanesulfonate, 11 EGTA, 1 CaCl2, 2 MgCl2, 10 HEPES, 2 Na2ATP, and 0.2 Na2GTP. Cs2+ internal solution was used to reduce potassium currents and to better discriminate changes in currents at −60 mV relative to K+ internal solution. The intracellular solution was adjusted to pH 7.4 and 285–295 mosmol/L. The extracellular solution contained (in mM) 125 NaCl, 3 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 2 CaCl2, and 10 dextrose. The pH of external solution was 7.4 and 295–305 mosmol/L adjusted to 7–10 mosmol/L higher than the internal solution with dextrose. We studied neurons under voltage-clamp and current-clamp conditions with a MultiClamp 700B or AxoClamp 200A amplifier (Molecular Devices, Union City, CA) held initially at Vm = −60 mV, using pipettes in whole cell patch configuration. Current-voltage relationships were generated using a voltage-step protocol where voltage was stepped by 10 mV from −100 to +80 mV. Signals were filtered at 10 kHz and sampled at 30 kHz using p-Clamp software (version 10.3, Molecular Devices). Liquid junctional potentials were not corrected.
Chronic cannabis vapor exposure.
Eight vapor administration chambers (La Jolla Alcohol Research Inc.) measuring 13.5 in. × 9.0 in. × 8.25 in. (16.4 L) were programmed using MED-Associates IV software to deliver discrete puffs of vapor daily for a minimum of 21 days. For each puff, an uninterrupted flow of air entered through a port in the front of the chamber and was removed by vacuum in the rear of the chamber. The air intake port pulled air through tubing connected to an air flow meter (1 L/min constant air flow) and tubing connected to a commercial e-cigarette cartridge (SMOK Tank Baby Beast TFV8 with 0.2Ω M2 atomizer) filled with either vehicle or cannabis extract. Each cartridge was connected to a vaporizer box (La Jolla Alcohol Research Inc.) and 3 s puffs were delivered every 3 min through the air intake port during daily 45-min exposure sessions. Chamber air was evacuated through an activated charcoal filter (Carbatrol Corporation; Bridgeport, CT). For preparation, cannabis extract was heated to 60°C under constant stirring and suspended in an 80% propylene glycol/20% vegetable glycerin vehicle (VEH) at a concentration of 400 mg/mL. Based on the certificate of analysis provided by the supplier (NIDA), the final concentrations of THC and CBD in the cannabis extract were ∼117 mg/mL and ∼4 mg/mL, respectively. These concentrations were chosen based on previous studies (Javadi-Paydar et al. 2018). Sprague-Dawley rats (n = 10 rats, 200–300 g, 10–12 wk) were assigned to a vehicle group (n = 4 rats) or cannabis extract group (n = 6 rats), and their identities were double blinded until after experimentation and analyses were completed.
Circulating cannabinoid concentration quantification.
Immediately following the final vapor exposure session, 100–150 μL of blood was collected via tail nick to determine the circulating concentration of THC and primary metabolites at the end of the exposure session. Blood was collected in sterile tubes containing 10 mL EDTA, centrifuged at 4°C at 4,000 g for 15 min, and stored at −20°C. Sample THC (mean = 15.3 ± 3.2 ng/mL) and 11-OH-THC (mean = 6.0 ± 0.5 ng/mL) concentrations in plasma were determined using an ultra-performance liquid chromatography system (Waters ACQUITY I-Class UPLC, Milford, MA), coupled with a quadrupole time of flight mass spectrometer (Waters Xevo G2, Manchester, UK) as described previously (Britch et al. 2017).
Reagents.
The following reagents were purchased from Sigma-Aldrich, St. Louis, MO: allyl isothiocyanate (AITC) (Cat. No. 203408-Sample-K), capsaicin (Cap) (Cat. No. M2028), ruthenium red (RuR, Cat. No. R2751). A967079 (A96, Cat. No. 4716) and SB366791 (SB, Cat. No. 3420) were purchased from Tocris, Minneapolis, MN. Cannabidiol (CBD) was purchased from Iso International, Manchester, England (ISOC121216A). Cannabis extract containing ∼27.4% THC and ∼1.2% CBD was provided by the NIDA Drug Supply Program.
Statistical analysis.
Data for each experiment were collected from three or more biological replicates. When possible, we designed within-subject protocols and analyzed data using paired t tests or repeated-measures ANOVA followed by Bonferroni’s post hoc t test comparisons against control. Normality was tested by Shapiro–Wilk’s test, and nonnormally distributed data were compared with nonparametric tests including Mann–Whitney rank sum and Kruskal–Wallis ANOVA. Linear correlations were generated with a linear regression fit regimen. Data are expressed as the average ± SE with all statistical analysis performed using SigmaStat software (Systat Software Inc., San Jose, CA). Statistical significance was defined as α < 0.05.
RESULTS
Current-clamp recordings of cultured vagal afferent neurons revealed that bath application of 10 μM CBD elicited membrane depolarization and action potential firing from approximately half of vagal afferents recorded (54% from n = 13 of 24 neurons/3 rats) (Fig. 1, A–C). The mean depolarization provoked by CBD was 42 ± 6 mV, and the mean action potential frequency for the first 5 min of responses was 2.2 ± 0.6 Hz. Using ratiometric fluorescent calcium imaging, we established a concentration-response relationship for CBD-induced activation of cultured vagal afferent neurons (Fig. 1D). Increasing concentrations of CBD increased the amplitude and duration of calcium responses (Fig. 1, E and F). Overall, CBD activated a large subset of vagal afferent neurons using ratiometric fluorescent calcium measurements (33% from n = 48 of 144 neurons/6 rats) that approximated the response rate from the electrical recordings with the distributions not significantly different via chi-square comparison (χ2 = 3.86, P = 0.08). Functional expression of TRPA1 and TRPV1 was simultaneously assessed in this experiment using the agonists allyl isothiocyanate (AITC, 300 μM) and capsaicin (Cap, 1 μM), respectively. Sensitivity to CBD-induced activation was always found in AITC-responsive afferents, suggesting functional expression of TRPA1. All vagal afferents activated by AITC were also responsive to CBD, whereas those lacking TRPA1 only responded at the highest concentration tested (100 μM CBD) and then only to a small degree (Fig. 2, A–C). Statistical comparison between AITC-responsive (TRPA1+, n = 18 neurons/4 rats) and AITC-nonresponsive neurons (TRPA1−, n = 19 neurons/4 rats) across CBD concentrations confirmed the large difference in responsiveness between these groups (***P < 0.001, two-way ANOVA). Linear regression of peak calcium responses from CBD and AITC indicated that AITC response magnitude accounts for nearly half of CBD response magnitude variability (R2 = 0.41, Fig. 2D). Functional expression of TRPV1 alone did not influence CBD calcium concentration-response profiles relative to afferents functionally lacking both TRPV1 and TRPA1; yet coexpression of both channels resulted in considerably larger calcium responses (Fig. 3). Both capsaicin-responsive (TRPV1+) and capsaicin-nonresponsive (TRPV1−) neurons responsive to AITC produced significant concentration-dependent increases in cytosolic calcium (TRPV1−: n = 8, *P = 0.004, Kruskal–Wallis one-way ANOVA and TRPV1+: n = 21, ***P < 0.001, Kruskal–Wallis one-way ANOVA); however, post hoc comparisons against controls revealed statistical significance at lower concentrations for the TRPV1+/TRPA1+ group. Furthermore, there was a significant difference in average responses across concentrations between the TRPV1−/TRPA1+ and TRPV1+/TRPA1+ groups (*P = 0.017, two-way ANOVA).
Fig. 1.
Cannabidiol (CBD) provokes depolarization and increases calcium in dissociated vagal afferent neurons. A: cultured vagal afferent neuron cell body with recording pipette in whole cell configuration. B: representative voltage trace of CBD-provoked depolarization and action potential firing. C: quantification of membrane potential at baseline and following CBD-provoked depolarization (n = 13 of 24 neurons/3 rats, ***P < 0.001, paired t test). D: representative images of intracellular calcium concentrations in dissociated vagal afferent neurons under control (left), following bath application of CBD (10 μM, middle), and with potassium-induced depolarization as a positive control (right). Intracellular calcium concentrations are shown with pseudo color by Fura-2 fluorescence ratio according to legend. E: representative calcium trace showing CBD concentration-response relationship in vagal afferents. F: quantification of CBD concentration-response relationship (n = 48 of 144 neurons/6 rats, *P < 0.05, **P < 0.01, and ***P < 0.001, repeated-measures ANOVA with post hoc Holm–Sidak comparison against control). G: population distribution of CBD responsivity.
Fig. 2.
Vagal afferent sensitivity to cannabidiol (CBD) is associated with functional expression of TRPA1. A: representative calcium traces of CBD concentration responses from AITC− (left) and from AITC+ (right) vagal afferent neurons. B: average calcium responses across CBD concentrations in TRPA1-lacking (TRPA1−, left) and TRPA1-containing (TRPA1+, right). There was a large difference in CBD responsiveness between TRPA1+ (n = 18 neurons/4 rats) and TRPA1− neurons (n = 19 neurons/4 rats) (P < 0.001, two-way ANOVA). Post hoc comparison against control revealed only the highest concentration of CBD (100 μM) produced a statistically significant increase in calcium in TRPA1– neurons (*P < 0.05); whereas CBD significantly increased calcium concentrations by 1 μM in TRPA1+ neurons (*P < 0.05). Only the highest concentration of CBD (100 μM) produced a statistically significant increase in calcium in TRPA1− neurons. C: population distributions of CBD-nonresponsive and CBD-responsive afferents with regard to their expression of TRPA1 (A1) and TRPV1 (V1) as functionally assayed with AITC (300 μM) and Cap (1 μM). D: scatterplot and linear regression correlating the magnitude of CBD and AITC calcium responses (n = 48 neurons/6 rats, slope = 0.56 ± 0.12, R2 = 0.41, P < 0.0001). AITC, allyl isothiocynate; Cap, capsaicin.
Fig. 3.
Vagal afferent cannabidiol (CBD) calcium responses vary according to functional expression of TRPA1 and TRPV1. A: representative calcium trace of CBD responses from a vagal afferent functionally lacking TRPV1 but expressing TRPA1 (TRPV1−/TRPA1+). B: quantification of peak CBD calcium response amplitude from vagal afferents functionally lacking TRPV1 but expressing TRPA1. CBD produced a significant concentration-dependent increase in calcium (n = 8, *P = 0.004, Kruskal–Wallis one-way ANOVA). C: representative calcium trace of CBD responses from a vagal afferent neuron expressing both TRPV1 and TRPA1 (TRPV1+/TRPA1+). D: quantification of peak CBD calcium response amplitude from vagal afferents containing both TRPV1 and TRPA1. There was a main effect of CBD to increase calcium levels across CBD concentrations (n = 21, P < 0.001, Kruskal–Wallis one-way ANOVA) that was qualified by a statistically significant interaction compared with different TRPV1−/TRPA1+ group of neurons (*P = 0.02, two-way ANOVA). AITC, allyl isothiocynate; Cap, capsaicin.
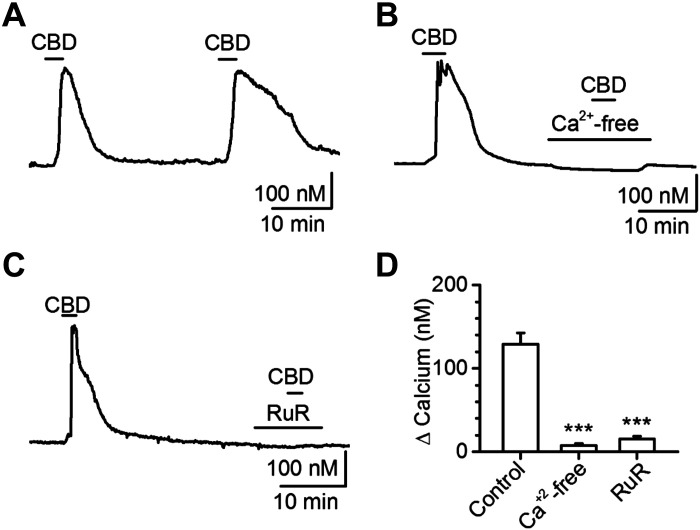
To determine the contributions of TRPV1 and TRPA1 to this CBD-evoked calcium response, we used a combination of pharmacological and genetic approaches with ratiometric fluorescent calcium imaging. First, the repeatability of CBD responses was tested in a time-control experiment; we found that 10 μM CBD responses did not desensitize, following a 25-min washout period, and we used a 30-min washout for the remaining experiments (Fig. 4A). To verify that CBD calcium responses depend on an external calcium source consistent with gating of membrane ion channel(s), we removed extracellular calcium and effectively eliminated the CBD response (Fig. 4B). The broad-spectrum TRP channel blocker, ruthenium red (RuR,10 μM), also prevented CBD-provoked calcium responses (Fig. 4C). Ruthenium red is effective at blocking the TRPV family, TRPM6, and TRPA1, as well as P/Q-, N-, and L-type calcium channels (Cibulsky and Sather 1999; Clapham 2003). To selectively determine the putative contributions of TRPV1 and TRPA1 on the CBD responses, we used the channel-specific antagonists SB366791 (SB, 3 μM) for TRPV1 and A967079 (A96, 10 μM) for TRPA1. Although SB366791 failed to prevent the CBD calcium response (Fig. 5A), A967079 pretreatment eliminated CBD responses (Fig. 5B). These findings are summarized in Fig. 5C. Although pharmacology is a useful approach and permits a paired experimental design, off-target effects cannot always be ruled out. Therefore, we conducted a series of experiments with genetic knockout mice and found that deletion of TRPA1, but not TRPV1, prevented all CBD calcium responses (Fig. 5, D and E).
Fig. 4.
Cannabidiol (CBD) activation of vagal afferent neurons requires external calcium and is prevented by the nonspecific transient receptor potential (TRP) channel blocker ruthenium red. A: representative calcium trace of 30 μM CBD calcium responses, illustrating lack of desensitization with >25 min washout periods (n = 61 neurons/6 rats, P = 0.64, paired t test). B: representative calcium trace illustrating lack of CBD-calcium response in the absence of extracellular calcium (n = 42 neurons/3 rats, ***P < 0.001, Mann–Whitney rank sum). C: representative calcium trace illustrating lack of CBD-calcium response with the nonspecific TRP-channel pore blocker ruthenium red (RuR; n = 101 neurons/3 rats, ***P < 0.001, Mann–Whitney rank sum). D: quantifications for experiments illustrated in A–C.
Fig. 5.
Cannabidiol (CBD) activation of vagal afferent neurons requires TRPA1. A: representative calcium trace illustrating a persistent CBD-calcium response with 3 μM of the TRPV1 antagonist. B: representative calcium trace illustrating elimination of the CBD-calcium response with 10 μM of the TRPA1 antagonist A967079. C: quantifications of peak calcium response magnitude from CBD under control conditions and following pretreatment with SB366791 (n = 28 neurons/3 rats, P = 0.36, Mann–Whitney rank sum) or A967079 (n = 87 neurons/4 rats, ***P < 0.001, Mann–Whitney rank sum). D: representative calcium trace illustrating persistent CBD-calcium response in TRPV1 KO. E: representative calcium trace illustrating loss of CBD-calcium response in TRPA1 KO. F: quantification of CBD, AITC, and capsaicin responses from control, TRPV1 KO, and TRPA1 KO mice. Only deletion of TRPA1 prevented CBD-calcium responses (n = 66 neurons/3 mice, ***P < 0.001, Bonferroni corrected t test) as well as AITC-calcium responses as expected (n = 66 neurons/3 mice, ***P < 0.001, Bonferroni corrected t test). AITC, allyl isothiocynate; Cap, capsaicin.
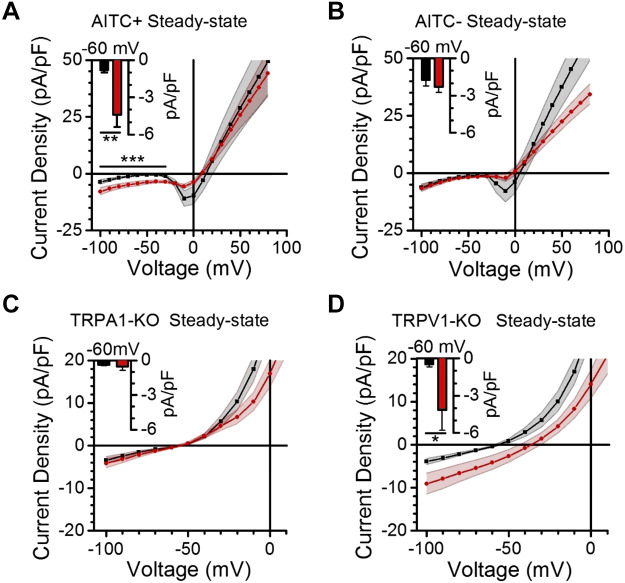
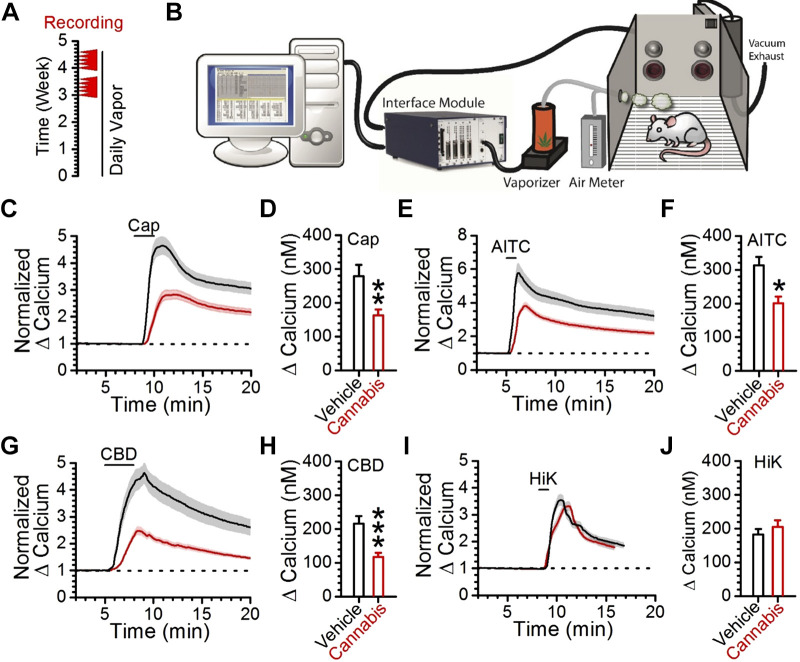
Next, we conducted whole cell patch-clamp electrophysiology experiments to more comprehensively assess the effects of CBD on cultured vagal afferent neurons. Current-voltage (IV) profiles were generated in 10 mV steps from −100 mV to 80 mV (Fig. 6). This allowed for comparisons of voltage-activated sodium channel (Nav) and steady-state currents in response to CBD (Figs. 6 and 7). Our assessment of Nav currents demonstrated that they were inactive at hyperpolarized potentials and activated as the membrane was depolarized from −40 to −20 mV. We found that CBD consistently and significantly inhibited Nav currents in putative A-fibers but to a much smaller extent in putative C-fibers, as identified with the absence or presence of capsaicin responses (Fig. 6, D and E). We also observed a shift in the voltage threshold of activation in both groups of neurons following CBD exposure. Analysis of the steady-state currents showed that CBD exposure produced significant nondesensitizing inward currents from −100 mV to −30 mV in afferents sensitive to the TRPA1 agonist AITC, but not in AITC-insensitive afferents (Fig. 7). We observed that CBD also inhibited voltage-gated potassium and calcium currents, an effect most evident in AITC-insensitive afferents above 50 mV (Fig. 7B). However, these effects were highly variable across neurons, which are consistent with reported heterogeneity in channel expression (Lancaster et al. 2002; Zhao et al. 2010). Steady-state IV profiles of CBD in cultured vagal afferents lacking TRPA1 or TRPV1 confirmed that only deletion of TRPA1 prevents the significant inward currents generated by CBD at negative potentials (Fig. 7, C and D). Finally, we assessed how a model of chronic cannabis use influenced CBD- and AITC-provoked calcium responses (Fig. 8, A and B). We found that >3 wk of daily cannabis vapor exposure decreased the magnitude of the CBD-, AITC-, and Cap-induced calcium responses (Fig. 8, C–H), with no change in the depolarization-induced calcium influx (Fig. 8, I and J).
Fig. 6.
Cannabidiol (CBD) inhibits voltage-activated sodium channels in capsaicin-insensitive vagal afferent neurons. A: illustration of voltage step protocol used to produce current-voltage curves. B: representative baseline current-voltage traces resulting from step protocol shown in A. Highlighted are the regions used to isolate the steady-state and voltage-activated sodium channel (NaV) component of the trace to generate current-voltage curves, such as the NaV curves in D and E. NaV currents are activated as the neuron is depolarized and not present at very hyperpolarized potentials. C: representative current-voltage traces produced following bath application of 30 μM CBD. D: quantification of NaV current-voltage profiles of dissociated vagal afferents sensitive to capsaicin (Cap). Baseline current-voltage is plotted in black, whereas CBD current-voltage is plotted in red. E: quantification of NaV current-voltage profiles of vagal afferents insensitive to capsaicin. CBD application significantly inhibited NaV selectively in this population (n = 16 neurons/5 rats, P < 0.001, two-way ANOVA; *P < 0.01, Bonferroni’s t tests).
Fig. 7.
Cannabidiol (CBD) provokes depolarizing current via TRPA1. A and B: quantifications of steady-state current-voltage profiles of dissociated vagal afferents at baseline in black and following application of 30 μM CBD in red, with the population divided according to sensitivity to the TRPA1 agonist AITC. Current at resting membrane potential (−60 mV) is illustrated with column plots (inset) (n = 16 neurons/8 rats , **P < 0.01, paired t test). CBD provoked significant inward currents at resting potentials, compared with bath control, exclusively in afferents functionally expressing TRPA1 (n = 16 neurons/8 rats, ***P = 0.005, paired t test) but not in those lacking TRPA1 (n = 12 neurons/7 rats, P = 0.14, paired t test). C and D: quantifications of steady-state current-voltage profiles of dissociated vagal afferents, at baseline in black and following application of 30 μM CBD in red. TRPA1 knockout (KO) prevented CBD-provoked inward currents at resting potentials (n = 13 neurons/4 mice, P = 0.36, paired t test) that were evident in TRPV1 KO (n = 15 neurons/3 mice, *P = 0.04, paired t test). AITC, allyl isothiocynate.
Fig. 8.
Chronic cannabis vapor exposure attenuates CBD and AITC calcium responses. A: illustration of chronic cannabis vapor administration protocol; cannabis or vehicle vapor was administered for at least 3 wk before tissue collection and recordings (red arrowheads). B: illustration of chronic cannabis vaporization system. C: normalization of vehicle control (n = 27 neurons/4 rats, black trace) and cannabis extract-treated (n = 31 neurons/6 rats, red trace) capsaicin responses. The standard error is represented as a shaded region over each line. D: comparison of peak capsaicin responses in control and cannabis cohorts (**P = 0.002, t test). E: normalization of vehicle control (n = 27 neurons/4 rats, black trace) and cannabis extract-treated (n = 31 neurons/6 rats, red trace) AITC responses. The standard error range is represented as a shaded region over each line. F: comparison of peak AITC calcium responses from control and cannabis vapor-treated cohorts (*P = 0.02, t test). G: normalization of vehicle control (n = 27 neurons/4 rats, black trace) and cannabis extract-treated (n = 31 neurons/6 rats, red trace) CBD responses. The standard error range is represented as a shaded region over each line. H: comparison of peak CBD responses in vehicle control and cannabis extract groups (***P < 0.001, t test). I: normalization of vehicle control (n = 27 neurons/4 rats, black trace) and cannabis extract (n = 31 neurons/6 rats, red trace) to 55 mM potassium (HiK) calcium responses. J: quantification of vehicle control and cannabis extract to 55 mM potassium (HiK) calcium responses (P = 0.47, t test). AITC, allyl isothiocynate; Cap, capsaicin; CBD, cannabidiol.
DISCUSSION
Decades have passed since the clinical characterization of the autonomic effects of cannabis and the discovery of the key cannabinoids, CBD and THC (Benowitz et al. 1979; Gaoni and Mechoulam 1964; Kanakis et al. 1979). In recent years, the therapeutic potential of THC and the nonpsychotropic cannabinoid CBD has increasingly attracted attention. Despite the opportunity to inspire new therapeutic approaches for widespread chronic diseases, the direct cellular effects of these cannabinoids have been generally understudied on viscerosensory afferents of the vagus. The present findings support a key role of TRPA1 in mediating the excitatory effects of CBD in vagal afferent neurons. Our findings indicate that CBD increases intracellular calcium from an external source and produces a depolarizing current; sensitivity to this effect requires TRPA1, but is enhanced by expression of TRPV1; selective antagonism or genetic deletion of TRPA1 prevents CBD-induced calcium and current responses from cultured afferents; afferent C-fibers, which are most responsive to the excitatory effects of CBD and disproportionately influence vagal reflexes, are also resistant to the inhibitory effects of CBD on voltage-activated sodium channels; and chronic exposure to cannabis extract vapor reduces the magnitude of CBD-, AITC-, and Cap-provoked calcium responses.
Our concentration-response experiment revealed that AITC sensitivity was associated with high sensitivity and magnitude of CBD calcium responses. Approximately half of the electrically recorded neurons were activated by CBD, whereas one third showed increases in calcium influx, with all CBD responses occurring in AITC-responsive neurons. The discrepancy between CBD percentages between electrophysiological and calcium imaging approaches likely reflects differences in samples sizes and sensitivity of each approach. A previous in vitro study with heterologously expressed TRP channels determined that CBD activates TRPV1, TRPV2, and TRPA1 (De Petrocellis et al. 2011). However, the half-maximal effective concentration (EC50) of CBD at overexpressed TRPV1 or TRPA1 channels in this report are more than 10-fold lower than what we observed at endogenous expression levels of these targets in vagal afferents; we calculated an EC50 of CBD in TRPV1+/TRPA1+ vagal afferents of 3.6 ± 2.2 μM, whereas De Petrocellis et al. (2011) report an EC50 for CBD at TRPA1 of 0.11 ± 0.05 μM in a HEK293 system. Presumably, this difference is due to increased channel density in the heterologous system; that is, the EC50 of CBD is dependent on ion channel availability. Nonetheless, the concentrations of CBD required for robust excitatory effects in vagal afferents appear relevant to the users of “medicinal” cannabis with high CBD content or oral CBD, which transiently reach low micromolar concentrations in plasma (Deiana et al. 2012; Karschner et al. 2011). Despite the lack of CBD potency on afferents functionally expressing TRPV1 but not TRPA1, coexpression of TRPV1 with TRPA1 considerably increased calcium response magnitude and duration (Fig. 3). Previously, Choi et al. (2011) observed a similar phenomenon with AITC application that was larger in Cap-sensitive versus insensitive neurons, although just missed significance due to large cell-to-cell variability. However, whole cell currents evoked by CBD were not influenced by functional TRPV1 expression (Fig. 6). This curious difference is possibly explained by the reported formation of calcium-selective TRPV1-TRPA1 heteromultimeric channels (Fischer et al. 2014). These heteromultimers would potentiate calcium responses but not whole cell current, as observed in this study.
In addition to actions at TRPA1, it is important to note that both endogenous and exogenous cannabinoids have activity at many different effectors, including cannabinoid receptors and other ion channels (De Petrocellis et al. 2011; Ghovanloo et al. 2018; Hill et al. 2014; Ross et al. 2008; Tham et al. 2019; Thomas et al. 2009). Our present findings need to be considered in context with actions at other signaling molecules (Bisogno et al. 2001; Mahgoub et al. 2013; Russo et al. 2005; Zhang and Xiong 2013). Our confirmation of CBD activity at some voltage-activated sodium channels and not others is a relevant example (Fig. 6). Furthermore, additional evidence of this can be seen in the membrane depolarization, action-potential firing, and then inhibition of firing, consistent with either depolarization block (similar to capsaicin activation of TRPV1) or inhibition of sodium channels (Fig. 1). Thus, the net effect of CBD will be dependent on the concentration and specific constellation of effectors in particular groups of neurons. Nonetheless, TRPA1 clearly contributes a large depolarizing drive to CBD activity on vagal afferent neurons.
We further demonstrated that CBD-provoked calcium responses originate from an extracellular calcium source, conducted via a ruthenium red-sensitive effector (Fig. 4). Ruthenium red is a pore blocker effective on many ion channels including the TRPV family, TRPM6, and TRPA1, as well as P/Q-, N-, and L-type calcium channels (Cibulsky and Sather 1999; Clapham 2003). As TRPV1 and TRPA1 are abundantly expressed in vagal afferents, activated by CBD, and inhibited by ruthenium red, we pharmacologically assessed their contributions to this response with the specific TRPA1 antagonist A967079 and TRPV1 antagonist SB366791. Only TRPA1 antagonism prevented the CBD-evoked calcium response (Fig. 5). To extend this finding, we used wild type and transgenic mice lacking either TRPV1 or TRPA1. We found that the CBD response was somewhat larger in wild-type mice compared with rats, and that only deletion of TRPA1 eliminated CBD-evoked calcium responses. These data indicate that TRPA1 is necessary for the excitatory effects of CBD in vagal afferents (Fig. 5). Interestingly, genetic deletion of TRPV1 did not alter CBD-evoked responses as may be expected from the differences observed in native neurons with and without TRPV1. This suggests that other channels may assume this role in the absence of TRPV1 (Vandewauw et al. 2018) or that TRPA1 expression may be enhanced in the absence of TRPV1.
Our patch-clamp electrophysiology experiments confirmed that the excitatory effects of CBD on vagal afferents require TRPA1. Only afferents responsive to the TRPA1 agonist AITC exhibited CBD-provoked depolarizing current at resting potentials, and genetic deletion of TRPA1, but not TRPV1, prevented CBD-provoked depolarizing current (Fig. 7). In addition to known direct and CB2 receptor-mediated inhibitory effects of CBD on voltage-gated potassium and calcium channels (Ghovanloo et al. 2018; Ross et al. 2008; Tham et al. 2019; Thomas et al. 2009), these experiments revealed a fiber-type specific effect of CBD on voltage-gated sodium channels. CBD significantly inhibited voltage-gated sodium channel (NaV) currents and shifted the voltage of activation in Cap-nonresponsive (putative A-fibers), but not in Cap-responsive TRPV1-expressing C-fibers (Fig. 6). The NaV currents in Cap-responsive neurons were reduced to some degree and also exhibited the shift in threshold voltage of activation; however, this effect was much smaller in this population of neurons. It is remarkable that afferents functionally coexpressing both TRPV1 and TRPA1 are the most abundant and CBD-sensitive afferent phenotype, with the most robust influence on vagal reflexes, and are largely resistant to this key inhibitory effect of CBD. Previously it has been reported that CBD inhibits NaV 1.1–1.7 (Ghovanloo et al. 2018), therefore the resistant sodium currents in C-fibers are likely due to uninhibited NaV 1.8 and/or NaV 1.9, which are prominently expressed in C-fiber afferent neurons (Kwong et al. 2008; Muroi et al. 2011). Notably, NaV 1.8-expressing vagal afferents are crucial for many reflex pathways including vagally mediated satiety, acute-phase responses to dietary fat, and baroreflex function (Schild and Kunze 2012; Udit et al. 2017). Facilitation of signaling from this population by CBD may contribute to attenuation of weight gain and cardiovascular responses (Ishiguro et al. 2010; Ignatowska-Jankowska et al. 2011; Jadoon et al. 2017; Riedel et al. 2009; Stanley et al. 2013).
Although the direct effects of cannabis on vagal afferents have not previously been reported, the results of chronic cannabis use on certain autonomic reflexes have been clinically investigated (Benowitz et al. 1979; Benowitz and Jones 1981). A remarkable dichotomy is present between the acute and chronic autonomic effects of cannabis. Acute cannabis use results in a variety of deleterious effects associated with decreased vagal tone, such as inhibition of the baroreflex, tachycardia, hypertension, and increased risk of stroke (Aryana and Williams 2007; Benowitz et al. 1979; Benowitz and Jones 1981; Gómez et al. 2002; Malit et al. 1975; Thomas et al. 2014). In contrast, chronic cannabis use results in therapeutic effects consistent with increased vagal tone, such as reduced body mass index and insulin resistance and improved outcomes for atherosclerosis and cardiovascular disease (Penner et al. 2013; Steffens et al. 2005). To examine physiological changes following chronic cannabis exposure that may contribute to these differences, we modeled chronic cannabis use by exposing rats to daily cannabis vapor administration sessions for 3 wk. We found that chronic cannabis vapor administration significantly reduced the magnitude of CBD-, AITC-, and Cap-provoked calcium responses from dissociated vagal afferents (Fig. 8). Given that the excitatory effects of CBD require TRPA1 and are enhanced with TRPV1, we posited that its reduced efficacy is due to the loss of TRPA1 and possibly TRPV1 signaling. However, quantitative PCR failed to detect a significant change in levels of CB1, TRPV1, or TRPA1 mRNA between control and cannabis vapor groups but trended toward a decrease for each transcript (data not shown). If this change in mRNA is not sufficient to impact protein levels then the observed functional desensitization of TRPA1 may be a result of protein modification or localization, such as phosphorylation and membrane trafficking.
The acute excitatory effects of CBD on vagal afferent neurons that we have observed in this study are not inconsistent with the clinically observed acute inhibitory effects of cannabis on vagal reflexes; Although both CBD and THC activate TRP channels including TRPA1, CBD lacks the CB1 receptor-dependent inhibitory effects of THC (Tham et al. 2019; Thomas et al. 2009). Cannabis may acutely prevent vagal reflexes by either CB1 receptor-dependent inhibitory effects or by depolarization block from TRP channel activation. Desensitization of CBD-provoked responses after chronic treatment with cannabis extract indicates that cannabis extract exposure and CBD treatment activate overlapping excitatory targets, in particular TRPA1. Future studies will be necessary to determine the mechanisms by which cannabis acutely impairs vagal reflexes.
This study presents a cellular investigation of CBD actions on vagal afferent signaling. The high abundance of TRP channels including TRPA1 renders vagal afferents uniquely sensitive to excitation by CBD, and likely other cannabinoids. Moreover, the population of C-fiber vagal afferents that is most sensitive to CBD and exerts strong influence on vagal reflexes is also insensitive to its inhibition of voltage-gated sodium channels. The similarities between the therapeutic effects of CBD and vagus nerve stimulation suggest that the vagus may be a target for the therapeutic effects of CBD. This study advances our understanding of the autonomic effects of CBD and the nature of the adaptations resulting from chronic cannabis exposure. Future studies will be required to investigate the combinatorial effects of CBD and THC on vagal afferent signaling, as well as the impact in the brain at the level of the nucleus of the solitary tract. These studies may identify novel mechanisms influencing widespread chronic diseases including cardiovascular disease and diabetes to inspire new approaches for their treatment.
GRANTS
This work was supported by the National Institutes of Health and the National Institute of Diabetes and Digestive and Kidney Diseases (Grant DK-092651) and WSU Alcohol and Drug Abuse Program-Dedicated Marijuana Account (ADARP-DMAc) Award (#127413).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
C.W.K., R.J.M., and J.H.P. conceived and designed research; C.W.K., F.J.R., J.E.M.L., B.P., J.M.L., R.J.M., and J.H.P. performed experiments; C.W.K., F.J.R., J.E.M.L., B.P., R.J.M., and J.H.P. analyzed data; C.W.K., F.J.R., J.E.M.L., B.P., R.J.M., and J.H.P. interpreted results of experiments; C.W.K. and J.H.P. prepared figures; C.W.K., R.J.M., and J.H.P. drafted manuscript; C.W.K., F.J.R., R.J.M., and J.H.P. edited and revised manuscript; C.W.K., F.J.R., R.J.M., and J.H.P. approved final version of manuscript.
REFERENCES
- Aryana A, Williams MA. Marijuana as a trigger of cardiovascular events: speculation or scientific certainty? Int J Cardiol 118: 141–144, 2007. doi: 10.1016/j.ijcard.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Ashton CH, Moore PB, Gallagher P, Young AH. Cannabinoids in bipolar affective disorder: a review and discussion of their therapeutic potential. J Psychopharmacol 19: 293–300, 2005. doi: 10.1177/0269881105051541. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jones RT. Cardiovascular and metabolic considerations in prolonged cannabinoid administration in man. J Clin Pharmacol 21, S1: 214S–223S, 1981. doi: 10.1002/j.1552-4604.1981.tb02598.x. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Rosenberg J, Rogers W, Bachman J, Jones RT. Cardiovascular effects of intravenous delta-9-tetrahydrocannabinol: autonomic nervous mechanisms. Clin Pharmacol Ther 25: 440–446, 1979. doi: 10.1002/cpt1979254440. [DOI] [PubMed] [Google Scholar]
- Berthoud H-R. The vagus nerve, food intake and obesity. Regul Pept 149: 15–25, 2008. doi: 10.1016/j.regpep.2007.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisogno T, Hanuš L, De Petrocellis L, Tchilibon S, Ponde DE, Brandi I, Moriello AS, Davis JB, Mechoulam R, Di Marzo V. Molecular targets for cannabidiol and its synthetic analogues: effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 134: 845–852, 2001. doi: 10.1038/sj.bjp.0704327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, Wang H, Abumrad N, Eaton JW, Tracey KJ. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405: 458–462, 2000. doi: 10.1038/35013070. [DOI] [PubMed] [Google Scholar]
- Britch SC, Wiley JL, Yu Z, Clowers BH, Craft RM. Cannabidiol-Δ9-tetrahydrocannabinol interactions on acute pain and locomotor activity. Drug Alcohol Depend 175: 187–197, 2017. doi: 10.1016/j.drugalcdep.2017.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M-J, Jin Z, Park YS, Rhee YK, Jin YH. Transient receptor potential (TRP) A1 activated currents in TRPV1 and cholecystokinin-sensitive cranial visceral afferent neurons. Brain Res 1383: 36–42, 2011. doi: 10.1016/j.brainres.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Cibulsky SM, Sather WA. Block by ruthenium red of cloned neuronal voltage-gated calcium channels. J Pharmacol Exp Ther 289: 1447–1453, 1999. [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature 426: 517–524, 2003. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol 99: 1–110, 1984. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Costa B, Trovato AE, Comelli F, Giagnoni G, Colleoni M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur J Pharmacol 556: 75–83, 2007. doi: 10.1016/j.ejphar.2006.11.006. [DOI] [PubMed] [Google Scholar]
- De Ferrari GM, Crijns HJGM, Borggrefe M, Milasinovic G, Smid J, Zabel M, Gavazzi A, Sanzo A, Dennert R, Kuschyk J, Raspopovic S, Klein H, Swedberg K, Schwartz PJ; CardioFit Multicenter Trial Investigators . Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J 32: 847–855, 2011. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]
- de Mello Schier AR, de Oliveira Ribeiro NP, Coutinho DS, Machado S, Arias-Carrión O, Crippa JA, Zuardi AW, Nardi AE, Silva AC. Antidepressant-like and anxiolytic-like effects of cannabidiol: a chemical compound of Cannabis sativa. CNS Neurol Disord Drug Targets 13: 953–960, 2014. doi: 10.2174/1871527313666140612114838. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, Stott CG, Di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 163: 1479–1494, 2011. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deiana S, Watanabe A, Yamasaki Y, Amada N, Arthur M, Fleming S, Woodcock H, Dorward P, Pigliacampo B, Close S, Platt B, Riedel G. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Δ9-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology (Berl) 219: 859–873, 2012. doi: 10.1007/s00213-011-2415-0. [DOI] [PubMed] [Google Scholar]
- Derbenev AV, Zsombok A. Potential therapeutic value of TRPV1 and TRPA1 in diabetes mellitus and obesity. Semin Immunopathol 38: 397–406, 2016. doi: 10.1007/s00281-015-0529-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray GJ. The versatility of the vagus. Physiol Behav 97: 531–536, 2009. doi: 10.1016/j.physbeh.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Fan W, Schild JH, Andresen MC. Graded and dynamic reflex summation of myelinated and unmyelinated rat aortic baroreceptors. Am J Physiol Regul Integr Comp Physiol 277: R748–R756, 1999. doi: 10.1152/ajpregu.1999.277.3.R748. [DOI] [PubMed] [Google Scholar]
- Fischer MJM, Balasuriya D, Jeggle P, Goetze TA, McNaughton PA, Reeh PW, Edwardson JM. Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch 466: 2229–2241, 2014. doi: 10.1007/s00424-014-1497-z. [DOI] [PubMed] [Google Scholar]
- Gaoni Y, Mechoulam R. Isolation, structure, and partial synthesis of an active constituent of hashish. J Am Chem Soc 86: 1646–1647, 1964. doi: 10.1021/ja01062a046. [DOI] [Google Scholar]
- Ghovanloo M-R, Shuart NG, Mezeyova J, Dean RA, Ruben PC, Goodchild SJ. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J Biol Chem 293: 16546–16558, 2018. doi: 10.1074/jbc.RA118.004929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidron Y, Kupper N, Kwaijtaal M, Winter J, Denollet J. Vagus-brain communication in atherosclerosis-related inflammation: a neuroimmunomodulation perspective of CAD. Atherosclerosis 195: e1–e9, 2007. doi: 10.1016/j.atherosclerosis.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Gómez R, Navarro M, Ferrer B, Trigo JM, Bilbao A, Del Arco I, Cippitelli A, Nava F, Piomelli D, Rodríguez de Fonseca F. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 22: 9612–9617, 2002. doi: 10.1523/JNEUROSCI.22-21-09612.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev 29: 493–500, 2005. doi: 10.1016/j.neubiorev.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Guo YP, McLeod JG, Baverstock J. Pathological changes in the vagus nerve in diabetes and chronic alcoholism. J Neurol Neurosurg Psychiatry 50: 1449–1453, 1987. doi: 10.1136/jnnp.50.11.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmchen F. Calibration of fluorescent calcium indicators. Cold Spring Harb Protoc 2011: 923–930, 2011. [DOI] [PubMed] [Google Scholar]
- Hill AJ, Jones NA, Smith I, Hill CL, Williams CM, Stephens GJ, Whalley BJ. Voltage-gated sodium (NaV) channel blockade by plant cannabinoids does not confer anticonvulsant effects per se. Neurosci Lett 566: 269–274, 2014. doi: 10.1016/j.neulet.2014.03.013. [DOI] [PubMed] [Google Scholar]
- Ignatowska-Jankowska B, Jankowski MM, Swiergiel AH. Cannabidiol decreases body weight gain in rats: involvement of CB2 receptors. Neurosci Lett 490: 82–84, 2011. doi: 10.1016/j.neulet.2010.12.031. [DOI] [PubMed] [Google Scholar]
- Ishiguro H, Carpio O, Horiuchi Y, Shu A, Higuchi S, Schanz N, Benno R, Arinami T, Onaivi ES. A nonsynonymous polymorphism in cannabinoid CB2 receptor gene is associated with eating disorders in humans and food intake is modified in mice by its ligands. Synapse 64: 92–96, 2010. doi: 10.1002/syn.20714. [DOI] [PubMed] [Google Scholar]
- Jadoon KA, Tan GD, O’Sullivan SE. A single dose of cannabidiol reduces blood pressure in healthy volunteers in a randomized crossover study. JCI Insight 2: e93760, 2017. doi: 10.1172/jci.insight.93760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janero DR, Makriyannis A. Terpenes and lipids of the endocannabinoid and transient-receptor-potential-channel biosignaling systems. ACS Chem Neurosci 5: 1097–1106, 2014. doi: 10.1021/cn5000875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi-Paydar M, Nguyen JD, Kerr TM, Grant Y, Vandewater SA, Cole M, Taffe MA. Effects of Δ9-THC and cannabidiol vapor inhalation in male and female rats. Psychopharmacology (Berl) 235: 2541–2557, 2018. doi: 10.1007/s00213-018-4946-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanakis C, Pouget M, Rosen KM. Lack of cardiovascular effects of delta-9-tetrahydrocannabinol in chemically denervated men. Ann Intern Med 91: 571–574, 1979. doi: 10.7326/0003-4819-91-4-571. [DOI] [PubMed] [Google Scholar]
- Kaneto A, Kosaka K, Nakao K. Effects of stimulation of the vagus nerve on insulin secretion. Endocrinology 80: 530–536, 1967. doi: 10.1210/endo-80-3-530. [DOI] [PubMed] [Google Scholar]
- Karschner EL, Darwin WD, Goodwin RS, Wright S, Huestis MA. Plasma cannabinoid pharmacokinetics following controlled oral delta9-tetrahydrocannabinol and oromucosal cannabis extract administration. Clin Chem 57: 66–75, 2011. doi: 10.1373/clinchem.2010.152439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch DC, Peters JH, Simasko SM.. Comparative pharmacology of cholecystokinin induced activation of cultured vagal afferent neurons from rats and mice. PLoS One 7: e34755, 2012. doi: 10.1371/journal.pone.0034755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner A, Birklein F, Stefan H, Handwerker HO. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology 55: 1167–1171, 2000. doi: 10.1212/WNL.55.8.1167. [DOI] [PubMed] [Google Scholar]
- Kumar S, Singh O, Singh U, Goswami C, Singru PS. Transient receptor potential vanilloid 1-6 (Trpv1-6) gene expression in the mouse brain during estrous cycle. Brain Res 1701: 161–170, 2018. doi: 10.1016/j.brainres.2018.09.005. [DOI] [PubMed] [Google Scholar]
- Kwong K, Carr MJ, Gibbard A, Savage TJ, Singh K, Jing J, Meeker S, Undem BJ. Voltage-gated sodium channels in nociceptive versus non-nociceptive nodose vagal sensory neurons innervating guinea pig lungs. J Physiol 586: 1321–1336, 2008. doi: 10.1113/jphysiol.2007.146365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster E, Oh EJ, Gover T, Weinreich D. Calcium and calcium-activated currents in vagotomized rat primary vagal afferent neurons. J Physiol 540: 543–556, 2002. doi: 10.1113/jphysiol.2001.013121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leo A, Russo E, Elia M. Cannabidiol and epilepsy: rationale and therapeutic potential. Pharmacol Res 107: 85–92, 2016. doi: 10.1016/j.phrs.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Li M, Zheng C, Sato T, Kawada T, Sugimachi M, Sunagawa K. Vagal nerve stimulation markedly improves long-term survival after chronic heart failure in rats. Circulation 109: 120–124, 2004. doi: 10.1161/01.CIR.0000105721.71640.DA. [DOI] [PubMed] [Google Scholar]
- Mahgoub M, Keun-Hang SY, Sydorenko V, Ashoor A, Kabbani N, Al Kury L, Sadek B, Howarth CF, Isaev D, Galadari S, Oz M. Effects of cannabidiol on the function of α7-nicotinic acetylcholine receptors. Eur J Pharmacol 720: 310–319, 2013. doi: 10.1016/j.ejphar.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Makiol C, Kluge M. Remission of severe, treatment-resistant schizophrenia following adjunctive cannabidiol. Aust N Z J Psychiatry 53: 262–265, 2019. doi: 10.1177/0004867418815982. [DOI] [PubMed] [Google Scholar]
- Malit LA, Johnstone RE, Bourke DI, Kulp RA, Klein V, Smith TC. Intravenous delta9-tetrahydrocannabinol: effects of ventilatory control and cardiovascular dynamics. Anesthesiology 42: 666–673, 1975. doi: 10.1097/00000542-197506000-00008. [DOI] [PubMed] [Google Scholar]
- Meotti FC, Lemos de Andrade E, Calixto JB. TRP modulation by natural compounds. Handb Exp Pharmacol 223: 1177–1238, 2014. doi: 10.1007/978-3-319-05161-1_19. [DOI] [PubMed] [Google Scholar]
- Muroi Y, Ru F, Kollarik M, Canning BJ, Hughes SA, Walsh S, Sigg M, Carr MJ, Undem BJ. Selective silencing of Na(V)1.7 decreases excitability and conduction in vagal sensory neurons. J Physiol 589: 5663–5676, 2011. doi: 10.1113/jphysiol.2011.215384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penner EA, Buettner H, Mittleman MA. The impact of marijuana use on glucose, insulin, and insulin resistance among US adults. Am J Med 126: 583–589, 2013. doi: 10.1016/j.amjmed.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br J Pharmacol 153: 199–215, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Patwardhan A, Akopian AN, Hargreaves KM, Flores CM. Cannabinoid receptor-independent actions of the aminoalkylindole WIN 55,212-2 on trigeminal sensory neurons. Br J Pharmacol 142: 257–266, 2004. doi: 10.1038/sj.bjp.0705778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin N, Neeper MP, Liu Y, Hutchinson TL, Lubin ML, Flores CM. TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci 28: 6231–6238, 2008. doi: 10.1523/JNEUROSCI.0504-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel G, Fadda P, McKillop-Smith S, Pertwee RG, Platt B, Robinson L. Synthetic and plant-derived cannabinoid receptor antagonists show hypophagic properties in fasted and non-fasted mice. Br J Pharmacol 156: 1154–1166, 2009. doi: 10.1111/j.1476-5381.2008.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rock EM, Bolognini D, Limebeer CL, Cascio MG, Anavi-Goffer S, Fletcher PJ, Mechoulam R, Pertwee RG, Parker LA. Cannabidiol, a non-psychotropic component of cannabis, attenuates vomiting and nausea-like behaviour via indirect agonism of 5-HT(1A) somatodendritic autoreceptors in the dorsal raphe nucleus. Br J Pharmacol 165: 2620–2634, 2012. doi: 10.1111/j.1476-5381.2011.01621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohner-Jeanrenaud F, Hochstrasser AC, Jeanrenaud B. Hyperinsulinemia of preobese and obese fa/fa rats is partly vagus nerve mediated. Am J Physiol 244: E317–E322, 1983. doi: 10.1152/ajpendo.1983.244.4.E317. [DOI] [PubMed] [Google Scholar]
- Ross HR, Napier I, Connor M. Inhibition of recombinant human T-type calcium channels by delta9-tetrahydrocannabinol and cannabidiol. J Biol Chem 283: 16124–16134, 2008. doi: 10.1074/jbc.M707104200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo EB, Burnett A, Hall B, Parker KK. Agonistic properties of cannabidiol at 5-HT1a receptors. Neurochem Res 30: 1037–1043, 2005. doi: 10.1007/s11064-005-6978-1. [DOI] [PubMed] [Google Scholar]
- SAMHSA . Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings. NSDUH Ser H-48, HHS Publ. No. 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2013. [Google Scholar]
- Schild JH, Kunze DL. Differential distribution of voltage-gated channels in myelinated and unmyelinated baroreceptor afferents. Auton Neurosci 172: 4–12, 2012. doi: 10.1016/j.autneu.2012.10.014. [DOI] [PubMed] [Google Scholar]
- Stanley CP, Hind WH, O’Sullivan SE. Is the cardiovascular system a therapeutic target for cannabidiol? Br J Clin Pharmacol 75: 313–322, 2013. doi: 10.1111/j.1365-2125.2012.04351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard J-L, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature 434: 782–786, 2005. [Erratum in Nature 435: 528, 2005] doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- Tham M, Yilmaz O, Alaverdashvili M, Kelly MEM, Denovan-Wright EM, Laprairie RB. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br J Pharmacol 176: 1455–1469, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Yamamoto SS, Brosschot JF. The relationship of autonomic imbalance, heart rate variability and cardiovascular disease risk factors. Int J Cardiol 141: 122–131, 2010. doi: 10.1016/j.ijcard.2009.09.543. [DOI] [PubMed] [Google Scholar]
- Thomas A, Baillie GL, Phillips AM, Razdan RK, Ross RA, Pertwee RG. Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br J Pharmacol 150: 613–623, 2009. doi: 10.1038/sj.bjp.0707133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G, Kloner RA, Rezkalla S. Adverse cardiovascular, cerebrovascular, and peripheral vascular effects of marijuana inhalation: what cardiologists need to know. Am J Cardiol 113: 187–190, 2014. doi: 10.1016/j.amjcard.2013.09.042. [DOI] [PubMed] [Google Scholar]
- Turner SE, Williams CM, Iversen L, Whalley BJ. Molecular pharmacology of phytocannabinoids. Prog Chem Org Nat Prod: 103: 61–101, 2017. doi: 10.1007/978-3-319-45541-9_3. [DOI] [PubMed] [Google Scholar]
- Udit S, Burton M, Rutkowski JM, Lee S, Bookout AL, Scherer PE, Elmquist JK, Gautron L. Nav1.8 neurons are involved in limiting acute phase responses to dietary fat. Mol Metab 6: 1081–1091, 2017. doi: 10.1016/j.molmet.2017.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandewauw I, De Clercq K, Mulier M, Held K, Pinto S, Van Ranst N, Segal A, Voet T, Vennekens R, Zimmermann K, Vriens J, Voets T. A TRP channel trio mediates acute noxious heat sensing. Nature 555: 662–666, 2018. [Erratum in Nature 559(7713): E7]. doi: 10.1038/nature26137. [DOI] [PubMed] [Google Scholar]
- Weiss L, Zeira M, Reich S, Har-Noy M, Mechoulam R, Slavin S, Gallily R. Cannabidiol lowers incidence of diabetes in non-obese diabetic mice. Autoimmunity 39: 143–151, 2006. doi: 10.1080/08916930500356674. [DOI] [PubMed] [Google Scholar]
- Weiss L, Zeira M, Reich S, Slavin S, Raz I, Mechoulam R, Gallily R. Cannabidiol arrests onset of autoimmune diabetes in NOD mice. Neuropharmacology 54: 244–249, 2008. doi: 10.1016/j.neuropharm.2007.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Xiong W. Nonpsychoactive cannabinoid action on 5–HT3 and glycine receptors. In: endoCANNABINOIDS. Springer, 2013, p. 199–218. [Google Scholar]
- Zhang Y, Popović ZB, Bibevski S, Fakhry I, Sica DA, Van Wagoner DR, Mazgalev TN. Chronic vagus nerve stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail 2: 692–699, 2009. doi: 10.1161/CIRCHEARTFAILURE.109.873968. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sprunger LK, Simasko SM. Expression of transient receptor potential channels and two-pore potassium channels in subtypes of vagal afferent neurons in rat. Am J Physiol Gastrointest Liver Physiol 298: G212–G221, 2010. doi: 10.1152/ajpgi.00396.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Crippa JAS, Hallak JEC, Moreira FA, Guimarães FS. Cannabidiol, a Cannabis sativa constituent, as an antipsychotic drug. Braz J Med Biol Res 39: 421–429, 2006a. doi: 10.1590/S0100-879X2006000400001. [DOI] [PubMed] [Google Scholar]
- Zuardi AW, Hallak JEC, Dursun SM, Morais SL, Sanches RF, Musty RE, Crippa JAS. Cannabidiol monotherapy for treatment-resistant schizophrenia. J Psychopharmacol 20: 683–686, 2006b. doi: 10.1177/0269881106060967. [DOI] [PubMed] [Google Scholar]