Abstract
Objective:
We examined whether gender is associated with heavy drinking in three cohorts of people living with HIV (PLWH) in Mbarara, Uganda; St. Petersburg, Russia; and Boston, Massachusetts.
Method:
We conducted secondary analyses of baseline data collected from three cohorts in the Uganda Russia Boston Alcohol Network for Alcohol Research Collaboration on HIV/AIDS (URBAN ARCH) consortium. We used multiple logistic regression models to evaluate the association between gender and heavy drinking (defined in combination with self-report and phosphatidylethanol [PEth]) within each cohort.
Results:
In unadjusted logistic regression models, we found no significant association between gender and heavy drinking in Russia or Boston. In Uganda, women were less likely than men to engage in heavy drinking (odds ratio = 0.38, 95% CI [0.26, 0.58], p < .01). These findings were invariant to adjustment for covariates.
Conclusions:
We did not detect associations between gender and heavy drinking in cohorts of PLWH in Russia or Boston, suggesting that heavy drinking may be as common in women living with HIV as in men living with HIV in these locations. Although these cohorts were enriched with heavy drinking participants, which limits broad extrapolation to PLWH in those settings, nonetheless the findings are concerning given the significant morbidity associated with alcohol use among PLWH and women in particular.
Alcohol use and hiv often co-occur and have synergistic negative consequences on health outcomes, as alcohol use affects HIV disease progression, disease management, and risk of onward transmission (Azar et al., 2010; Marshall et al., 2017; Williams et al., 2016). Alcohol use has been significantly associated with mortality among people living with HIV (PLWH) in Russia, worse HIV outcomes in India and Vietnam, and poorer antiretroviral treatment adherence in Uganda (Fairbairn et al., 2016; Fatch et al., 2017; Wagman et al., 2020).
Globally, men are more likely than women to consume alcohol, drink in larger quantities, and experience behavioral problems related to their drinking (Bobak et al., 2004; Erol & Karpyak, 2015; Wilsnack et al., 2009). The differences by gender in patterns and consequences of alcohol use may be attributable to both biological and sociocultural factors. Compared with men, on average women are at greater risk for cardiovascular disease and cancer at lower levels and shorter duration of alcohol use (Erol & Karpyak, 2015). Women also experience decreased fertility and other reproductive consequences as a result of unhealthy alcohol use (Erol & Karpyak, 2015). Although differences in drinking patterns by gender are more likely to be observed in settings where gender roles are clearly divided, drinking is becoming less taboo among women globally because of changing social norms (Erol & Karpyak, 2015; Simons-Morton et al., 2009; Wilsnack et al., 2009). In the context of HIV, alcohol use has been implicated in poor outcomes for women: Females with HIV who use alcohol are less likely to engage in HIV care and receive antiretroviral treatment, have worse quality of life, and are more likely to be exposed to gender-based violence compared with males with HIV who use alcohol (Leddy et al., 2018; Matson et al., 2018; Pokhrel et al., 2018). However, there is limited research on gender differences in drinking behaviors among PLWH across settings.
This analysis aims to explore whether gender is associated with heavy drinking in three distinct cohorts of PLWH in Mbarara, Uganda; St. Petersburg, Russia; and Boston, MA. Understanding and documenting gender differences in drinking patterns could inform the design of interventions that address alcohol use and improve HIV care and prevention.
Method
We conducted secondary analyses of baseline data from three cohorts in the Uganda Russia Boston Alcohol Network for Alcohol Research Collaboration on HIV/AIDS (URBAN ARCH) consortium: Uganda ARCH in Mbarara, Uganda; Russia ARCH in St. Petersburg, Russia; and Boston ARCH in Boston, Massachusetts. URBAN ARCH is an ongoing consortium in its second wave of cohort studies. The current analysis was performed on data collected as part of the first wave of URBAN ARCH research projects.
Uganda ARCH cohort
The first wave of the Uganda ARCH cohort was an observational cohort study conducted to determine the effect of alcohol consumption on HIV disease progression before antiretroviral treatment initiation (Hahn et al., 2018). Uganda ARCH enrolled 447 participants between September 2011 and August 2014 from the Immune Suppression Syndrome Clinic of the Mbarara Regional Referral Hospital in Uganda. All participants provided informed consent. Key eligibility criteria were age 18 years or older; antiretroviral treatment–naive; diagnosed with World Health Organization Stage I or II HIV disease; and cluster of differentiation 4 (CD4) count greater than 350 cells/mm3 (>500 as of February 19, 2014). This study was approved by institutional review boards of Boston Medical Center and Boston University Medical Campus (BMC/BUMC); University of California, San Francisco; Mbarara University of Science and Technology; and the Uganda National Council of Science and Technology. The study aimed to enroll approximately equal numbers of people with heavy drinking (defined below) versus others (including people with moderate drinking and abstainers). To achieve this, starting in 2013, the study limited recruitment to patients reporting prior-year alcohol use at clinic enrollment.
Russia ARCH cohort
The first wave of the Russia ARCH cohort was an observational prospective cohort study conducted to assess the longitudinal association between alcohol consumption and biomarkers of microbial translocation and inflammation/altered coagulation (So-Armah et al., 2019). Russia ARCH enrolled 351 participants in St. Petersburg, Russia, between November 2012 and June 2015 from clinical sites and through participant referral. All participants provided informed consent. The following were key eligibility criteria: 18–70 years old as well as documented HIV-positive and antiretroviral treatment–naive status. This study was approved by the institutional review board of BMC/BUMC and First St. Petersburg Pavlov State Medical University. Russia ARCH was enriched for people with heavy drinking by including an embedded clinical trial of zinc supplementation that enrolled 254 people with heavy drinking (Freiberg et al., 2020; Gnatienko et al., 2018).
Boston ARCH cohort
The first wave of the Boston ARCH cohort was an observational cohort study to determine the effect of alcohol consumption on changes in bone health in PLWH over time (Ventura et al., 2017). Boston ARCH enrolled 250 participants between December 2012 and November 2014 from an urban hospital-based HIV primary care clinic, and an urban HIV clinic at a community health center focused on serving homeless individuals. All participants provided written informed consent. Key eligibility criteria included confirmed HIV infection and current Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV; American Psychiatric Association, 1994), diagnosis of past-12-month drug or alcohol dependence, or ever injection drug use. This study was approved by the institutional review board of BMC/BUMC.
Study measures
Outcome measure
The primary outcome measure was heavy alcohol use defined by a combination of participant self-report and a biological marker. All three studies collected information on alcohol use via self-report, as well as testing for phosphatidylethanol (PEth), a biomarker of alcohol consumption (Aradottir et al., 2006; Hahn et al., 2012; Hartmann et al., 2007). In all cohorts, PEth levels were determined from dried blood spots tested at the United States Drug Testing Laboratories (Des Plaines, IL), using previously described methods (Jones et al., 2011). PEth levels were used in combination with self-report to define the primary outcome measure of heavy alcohol use in all three cohorts.
In Uganda, heavy alcohol use was defined by past-3-month Alcohol Use Disorders Identification Test–consumption questions (AUDIT-C) scores of 4 or more for men and 3 or more for women (Bradley et al., 2007), and/or PEth of 50 ng/ml or more. In Russia and Boston, alcohol use was assessed using the 30-day Timeline Followback method (So-bell & Sobell, 1995), with heavy use defined as more than 4 drinks in a day or more than 14 drinks per week for men and more than 3 drinks in a day or more than 7 per week for women, and/or PEth of 50 ng/ml or more. To define heavy drinking in our analyses, the cutoffs used for AUDIT-C correspond with unhealthy drinking and the cutoffs used for the Timeline Followback correspond with risky drinking criteria from the National Institute on Alcohol Abuse and Alcoholism (2007). The cutoff of 50 ng/ml for PEth was chosen because this level has good documented sensitivity and high specificity to detect unhealthy drinking (Hahn et al., 2016).
Independent variable
The independent variable of interest was gender (male vs. female) as reported by the participant during their baseline assessment.
Covariates and descriptive measures
All three studies included the following self-reported measures: demographics, substance use, sexual behaviors, depressive symptoms, and social support.
Statistical analysis
Within each cohort, women and men were compared on demographic and alcohol-related characteristics using chi-square and t tests. The association between gender and baseline heavy drinking was evaluated using multiple logistic regression models, with separate analyses performed in each cohort. Covariates included in the adjusted models were age, marital status (married/living with partner vs. other), depressive symptoms (in Russia and Uganda assessed as score of 16 or more on the CES-D [Radloff, 1977]; in Boston assessed as Patient Health Questionnaire Version 2 [PHQ-2] score of 3 or more [Kroenke et al., 2003]), education (secondary or higher), and employment status. Covariates were selected a priori based on clinical knowledge. Odds ratios (ORs) and 95% confidence intervals (CIs) are reported from the logistic regression models. Data were analyzed with SAS statistical software Version 9.4 (SAS Institute Inc., Cary, NC).
Results
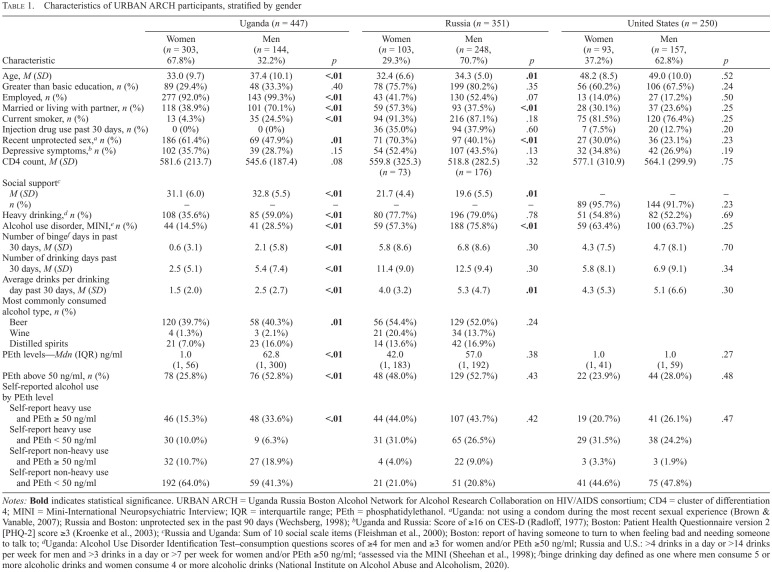
Women comprised the majority of the PLWH cohort in Uganda (303/447; 67.8%) but a minority in Russia (103/351, 29.3%) and Boston (93/250, 37.2%) (Table 1). By design, the prevalence of heavy alcohol use was high in the three cohorts, with 43% of participants meeting the definition of heavy alcohol use (self-reported heavy alcohol use, plus PEth ≥50 ng/ml) in Uganda (36% of women and 59% of men), 79% in Russia (78% of women and 79% of men), and 53% in Boston (55% of women and 52% of men). In unadjusted logistic regression models, no association was detected between gender and heavy drinking in Russia (OR = 0.92, 95% CI [0.53, 1.61]) and Boston (OR = 1.11, 95% CI [0.66, 1.86]). In Uganda, women were less likely than men to engage in heavy drinking (OR = 0.38, 95% CI [0.26, 0.58], p < .01). After adjusting for potential confounders (age, marital status, depressive symptoms, education, employment), there was still no association found between gender and heavy alcohol use in Russia (adjusted OR = 0.89, 95% CI [0.50, 1.60]) and Boston (adjusted OR = 1.04, 95% CI [0.61, 1.78]), whereas the relationship between these variables remained significant in Uganda (adjusted OR = 0.38, 95% CI [0.24, 0.59], p < .01). There were no statistically significant differences between female and male PLWH enrolled in Boston ARCH (Table 1). In both Uganda and Russia, women were less likely than men to have an alcohol use disorder diagnosis as assessed by the MINI (Uganda: 14.5% vs. 28.5%, p < .01; Russia: 57.3% vs. 75.8%, p < .01) and reported fewer drinks per drinking day in the past month (Uganda: 1.5 vs. 2.5, p < .01; Russia: 4.0 vs. 5.3, p = .01) (Table 1).
TABLE 1.
Characteristics of URBAN ARCH participants, stratified by gender
| Characteristic | Uganda (n = 447) | Russia (n = 351) | United States (n = 250) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Women (n = 303, 67.8%) | Men (n = 144, 32.2%) | P | Women (n = 103, 29.3%) | Men (n = 248, 70.7%) | P | Women (n = 93, 37.2%) | Men (n = 157, 62.8%) | P | |
| Age, M (SD) | 33.0(9.7) | 37.4(10.1) | <.01 | 32.4(6.6) | 34.3 (5.0) | .01 | 48.2(8.5) | 49.0 (10.0) | .52 |
| Greater than basic education, n (%) | 89 (29.4%) | 48 (33.3%) | .40 | 78 (75.7%) | 199(80.2%) | .35 | 56 (60.2%) | 106 (67.5%) | .24 |
| Employed, n (%) | 277 (92.0%) | 143 (99.3%) | <.01 | 43 (41.7%) | 130 (52.4%) | .07 | 13 (14.0%) | 27 (17.2%) | .50 |
| Married or living with partner, n (%) | 118 (38.9%) | 101 (70.1%) | <.01 | 59 (57.3%) | 93 (37.5%) | <.01 | 28 (30.1%) | 37 (23.6%) | .25 |
| Current smoker, n (%) | 13 (4.3%) | 35 (24.5%) | <.01 | 94(91.3%) | 216(87.1%) | .18 | 75 (81.5%) | 120 (76.4%) | .25 |
| Injection drug use past 30 days, n (%) | 0 (0%) | 0 (0%) | 36 (35.0%) | 94 (37.9%) | .60 | 7 (7.5%) | 20 (12.7%) | .20 | |
| Recent unprotected sex,a n (%) | 186 (61.4%) | 69 (47.9%) | .01 | 71 (70.3%) | 97 (40.1%) | <.01 | 27 (30.0%) | 36 (23.1%) | .23 |
| Depressive symptoms,b n (%) | 102 (35.7%) | 39 (28.7%) | .15 | 54 (52.4%) | 107 (43.5%) | .13 | 32 (34.8%) | 42 (26.9%) | .19 |
| CD4 count, M (SD) | 581.6 (213.7) | 545.6 (187.4) | .08 | 559.8 (325.3) (n = 73) | 518.8 (282.5) (n= 176) | .32 | 577.1 (310.9) | 564.1 (299.9) | .75 |
| Social supportc | |||||||||
| M (SD) | 31.1 (6.0) | 32.8(5.5) | <.01 | 21.7 (4.4) | 19.6 (5.5) | .01 | — | — | — |
| n (%) | — | — | — | — | — | — | 89 (95.7%) | 144(91.7%) | .23 |
| Heavy drinking,d n (%) | 108 (35.6%) | 85 (59.0%) | <.01 | 80 (77.7%) | 196(79.0%) | .78 | 51 (54.8%) | 82 (52.2%) | .69 |
| Alcohol use disorder, MINI,e n (%) | 44(14.5%) | 41 (28.5%) | <.01 | 59 (57.3%) | 188 (75.8%) | <.01 | 59 (63.4%) | 100 (63.7%) | .25 |
| Number of bingef days in past 30 days, M (SD) | 0.6 (3.1) | 2.1 (5.8) | <.01 | 5.8 (8.6) | 6.8 (8.6) | .30 | 4.3 (7.5) | 4.7 (8.1) | .70 |
| Number of drinking days past 30 days, M (SD | 2.5 (5.1) | 5.4 (7.4) | <.01 | 11.4(9.0) | 12.5 (9.4) | .30 | 5.8 (8.1) | 6.9 (9.1) | .34 |
| Average drinks per drinking day past 30 days, M (SD) | 1.5 (2.0) | 2.5 (2.7) | <.01 | 4.0 (3.2) | 5.3 (4.7) | .01 | 4.3 (5.3) | 5.1 (6.6) | .30 |
| Most commonly consumed alcohol type, n (%) | |||||||||
| Beer | 120 (39.7%) | 58 (40.3%) | .01 | 56 (54.4%) | 129(52.0%) | .24 | |||
| Wine | 4(1.3%) | 3 (2.1%) | 21 (20.4%) | 34(13.7%) | |||||
| Distilled spirits | 21 (7.0%) | 23 (16.0%) | 14(13.6%) | 42 (16.9%) | |||||
| PEth levels—Mdn (IQR) ng/ml | 1.0 | 62.8 | <.01 | 42.0 | 57.0 | .38 | 1.0 | 1.0 | .27 |
| (1.56) | (1, 300) | (1, 183) | (1, 192) | (1.41) | (1.59) | ||||
| PEth above 50 ng/ml, n (%) | 78 (25.8%) | 76 (52.8%) | <.01 | 48 (48.0%) | 129 (52.7%) | .43 | 22 (23.9%) | 44 (28.0%) | .48 |
| Self-reported alcohol use by PEth level | |||||||||
| Self-report heavy use and PEth ≥ 50 ng/ml | 46 (15.3%) | 48 (33.6%) | <.01 | 44 (44.0%) | 107 (43.7%) | .42 | 19 (20.7%) | 41 (26.1%) | .47 |
| Self-report heavy use and PEth < 50 ng/ml | 30 (10.0%) | 9 (6.3%) | 31 (31.0%) | 65 (26.5%) | 29 (31.5%) | 38 (24.2%) | |||
| Self-report non-heavy use and PEth ≥ 50 ng/ml | 32 (10.7%) | 27 (18.9%) | 4 (4.0%) | 22 (9.0%) | 3 (3.3%) | 3 (1.9%) | |||
| Self-report non-heavy use and PEth < 50 ng/ml | 192 (64.0%) | 59 (41.3%) | 21 (21.0%) | 51 (20.8%) | 41 (44.6%) | 75 (47.8%) | |||
Notes: Bold indicates statistical significance. URBAN ARCH = Uganda Russia Boston Alcohol Network for Alcohol Research Collaboration on HIV/AIDS consortium; CD4 = cluster of differentiation 4; MINI = Mini-International Neuropsychiatric Interview; IQR = interquartile range; PEth = phosphatidyl ethanol.
aUganda: not using a condom during the most recent sexual experience (Brown & Vanable, 2007); Russia and Boston: unprotected sex in the past 90 days (Wechsberg, 1998);
bUganda and Russia: Score of ≥16 on CES-D (Radloff, 1977); Boston: Patient Health Questionnaire version 2 [PHQ-2] score ≥3 (Kroenke et al., 2003);
cRussia and Uganda: Sum of 10 social scale items (Fleishman et al., 2000); Boston: report of having someone to turn to when feeling bad and needing someone to talk to;
dUganda: Alcohol Use Disorder Identification Test-consumption questions scores of ≥4 for men and ≥3 for women and/or PEth ≥50 ng/ml; Russia and U.S.: >4 drinks in a day or >14 drinks per week for men and >3 drinks in a day or >7 per week for women and/or PEth ≥50 ng/ml:
eassessed via the MINI (Sheehan et al., 1998);
fbinge drinking day defined as one where men consume 5 or more alcoholic drinks and women consume 4 or more alcoholic drinks (National Institute on Alcohol Abuse and Alcoholism, 2020).
Discussion
We examined and identified gender differences in heavy drinking among PLWH in three diverse settings in Mbarara, Uganda; St. Petersburg, Russia; and Boston, MA, United States. Gender differences in heavy drinking were not detected among PLWH in Russia or Boston, even after adjusting for age, marital status, depressive symptoms, education, and employment. However, in Uganda, women were less likely than men to engage in heavy drinking.
It is of concern that women with HIV were as likely as men with HIV to engage in heavy drinking in Boston and St. Petersburg. Women may have gender-specific motivations for initiating drinking and unique biological responses to and consequences of drinking, and they are more likely to experience gender-specific barriers when seeking and remaining engaged in alcohol treatment (Greenfield et al., 2007); thus, providers should routinely focus on identifying and treating unhealthy alcohol use among women. This is particularly important for women with HIV, for whom the consequences of unhealthy alcohol use could have implications for HIV care access and HIV transmission. In one study, women in the Veteran's Health Administration system were less likely to reach each step of the HIV care cascade and had worse outcomes at all levels of alcohol use, compared with men (Matson et al., 2018). Given that women have been reported to be more likely to seek care for an alcohol use disorder in non–alcohol-specific care settings (Erol & Karpyak, 2015), co-location of alcohol and HIV services that are culturally appropriate and tailored to engaging and treating women who have HIV and unhealthy alcohol use may be warranted.
Our findings are consistent with the evidence of a closing gender gap in alcohol use in the United States and with literature noting that as the division of gender roles diminishes, drinking patterns tend to converge (Keyes et al., 2011; Slade et al., 2016). It is important to note that although levels of alcohol use remain higher in men, adult women are experiencing greater increases in alcohol use, heavy episodic drinking, alcohol use disorders, and alcohol-related mortality, but these differences are dependent on age and birth cohort (Keyes et al., 2019; White et al., 2020). Similarly, data from Russia show that alcohol use has decreased overall from 2010 to 2016, but this decrease was proportionally larger for men than for women (Rehm & Ferreira-Borges, 2018). Although we did not detect gender differences in the occurrence of heavy alcohol use in our Russia cohort, we did observe that men were more likely to have an alcohol use disorder and consumed on average more drinks per drinking day than women.
In Uganda, women were significantly less likely than men to engage in heavy drinking and were less likely to have an alcohol use disorder. This is consistent with published data on alcohol use among adults and PLWH in Uganda and could be attributed to gender role distinctions (Kabwama et al., 2016; Wandera et al., 2015). One qualitative study explored gender norms around alcohol use in Uganda, including the expectation that women should not drink in bars (partly because of their responsibilities in the home) or appear drunk in public (to avoid the danger of rape) and should only drink during certain hours of the day (Kafuko & Bukuluki, 2008). Alcohol use among men in Uganda was found to enhance the perception of masculinity and showcase independence but be detrimental to women's maternal and spousal roles (Wolff et al., 2006).
This study had limitations. All three cohorts were enriched for people with heavy drinking. Because of this potential selection bias, the proportions of individuals with heavy alcohol use in these cohorts, and thus the study conclusions, should not be extrapolated to all PLWH in these settings. A total of 79% of the Russian cohort included in this analysis comprised people recruited on the basis of self-reported heavy drinking, resulting in low numbers of non–heavy drinking individuals. Moreover, given the high prevalence of risky behaviors in the cohorts of PLWH described here, the results reported may not be generalizable to the general populations at the study sites but may still be representative of people who drink alcohol at those locations. The interpretation of comparisons between the number of drinking days and drinks per day must take into account that the definition of heavy drinking requires for men to consume alcohol in larger quantity and frequency than women. With the exception of PEth, all variables used in the analysis were collected by self-report and thus are susceptible to recall and desirability biases. However, a strength of this analysis is the use of PEth, a biological marker of recent alcohol use, which allowed us to examine the relationship between gender and heavy alcohol use independent of social-desirability bias that could lead to underreporting of alcohol use, particularly in settings where acknowledging drinking could have negative consequences.
This study did not detect differences in heavy alcohol use by gender within two cohorts of PLWH in St. Petersburg, Russia, and Boston, Massachusetts. In a cohort of PLWH in Uganda, we found a statistically significant gender difference in heavy alcohol use. These findings are concerning given the health implications of heavy alcohol use among PLWH and women's increased likelihood of alcohol-related morbidity. HIV providers should consider addressing women's alcohol use in the broader context of their care.
Footnotes
This work was supported by the following grants from the National Institutes of Health: U24AA020779, U24AA020778, U01AA020784, U01AA020780, U01AA021989, U01AA020776, K24AA022586, and P30AI042853. The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health. Data collected for the study are available to interested investigators in the URBAN ARCH Repository: www.urbanarch.org.
References
- American Psychiatric Association. (4th ed.). Washington, DC: Author; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Aradottir S., Asanovska G., Gjerss S., Hansson P., Alling C. Phosphatidylethanol (PEth) concentrations in blood are correlated to reported alcohol intake in alcohol-dependent patients. Alcohol and Alcoholism. 2006;41:431–437. doi: 10.1093/alcalc/agl027. doi:10.1093/alcalc/agl027. [DOI] [PubMed] [Google Scholar]
- Azar M. M., Springer S. A., Meyer J. P., Altice F. L. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug and Alcohol Dependence. 2010;112:178–193. doi: 10.1016/j.drugalcdep.2010.06.014. doi:10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobak M., Room R., Pikhart H., Kubinova R., Malyutina S., Pajak A., Marmot M. Contribution of drinking patterns to differences in rates of alcohol related problems between three urban populations. Journal of Epidemiology and Community Health. 2004;58:238–242. doi: 10.1136/jech.2003.011825. doi:10.1136/jech.2003.011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley K. A., DeBenedetti A. F., Volk R. J., Williams E. C., Frank D., Kivlahan D. R. AUDIT-C as a brief screen for alcohol misuse in primary care. Alcoholism: Clinical and Experimental Research. 2007;31:1208–1217. doi: 10.1111/j.1530-0277.2007.00403.x. doi:10.1111/j.1530-0277.2007.00403.x. [DOI] [PubMed] [Google Scholar]
- Brown J. L., Vanable P. A. Alcohol use, partner type, and risky sexual behavior among college students: Findings from an event-level study. Addictive Behaviors. 2007;32:2940–2952. doi: 10.1016/j.addbeh.2007.06.011. doi:10.1016/j.addbeh.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erol A., Karpyak V. M. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug and Alcohol Dependence. 2015;156:1–13. doi: 10.1016/j.drugalcdep.2015.08.023. doi:10.1016/j.drugalcdep.2015.08.023. [DOI] [PubMed] [Google Scholar]
- Fairbairn N. S., Walley A. Y., Cheng D. M., Quinn E., Bridden C., Chaisson C., Samet J. H. PLoS One. Vol. 11. Petersburg, Russia: 2016. Mortality in HIV-infected alcohol and drug users in St; p. e0166539. doi:10.1371/journal.pone.0166539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatch R., Emenyonu N. I., Muyindike W., Kekibiina A., Woolf-King S., Hahn J. A. Alcohol interactive toxicity beliefs and ART non-adherence among HIV-infected current drinkers in Mbarara, Uganda. AIDS and Behavior. 2017;21:1812–1824. doi: 10.1007/s10461-016-1429-3. doi:10.1007/s10461-016-1429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleishman J. A., Sherbourne C. D., Crystal S., Collins R. L., Marshall G. N., Kelly M., Hays R. D., the HCSUS Consortium Coping, conflictual social interactions, social support, and mood among HIV-infected persons. American Journal of Community Psychology. 2000;28:421–453. doi: 10.1023/a:1005132430171. doi:10.1023/A:1005132430171. [DOI] [PubMed] [Google Scholar]
- Freiberg M. S., Cheng D. M., Gnatienko N., Blokhina E., Coleman S. M., Doyle M. F., Samet J. H. Effect of zinc supplementation vs placebo on mortality risk and HIV disease progression among HIV-positive adults with heavy alcohol use: A randomized clinical trial. JAMA Network Open. 2020;3:e204330. doi: 10.1001/jamanetworkopen.2020.4330. doi:10.1001/jamanetworkopen.2020.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnatienko N., Freiberg M. S., Blokhina E., Yaroslavtseva T., Bridden C., Cheng D. M., Samet J. H. HIV Clinical Trials. Vol. 19. Petersburg, Russia: 2018. Design of a randomized controlled trial of zinc supplementation to improve markers of mortality and HIV disease progression in HIV-positive drinkers in St; pp. 101–111.pp. 8.1459344 doi:10.1080/15284336.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S. F., Brooks A. J., Gordon S. M., Green C. A., Kropp F., McHugh R. K., Miele G. M. Substance abuse treatment entry, retention, and outcome in women: A review of the literature. Drug and Alcohol Dependence. 2007;86:1–21. doi: 10.1016/j.drugalcdep.2006.05.012. doi:10.1016/j.drugalcdep.2006.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. A., Cheng D. M., Emenyonu N. I., Lloyd-Travaglini C., Fatch R., Shade S. B., Samet J. H. Alcohol use and HIV disease progression in an antiretroviral naive cohort. Journal of Acquired Immune Deficiency Syndromes. 2018;77:492–501. doi: 10.1097/QAI.0000000000001624. doi:10.1097/QAI.0000000000001624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. A., Dobkin L. M., Mayanja B., Emenyonu N. I., Kigozi I. M., Shiboski S., Wurst F. M. Phosphatidylethanol (PEth) as a biomarker of alcohol consumption in HIV-positive patients in sub-Saharan Africa. Alcoholism: Clinical and Experimental Research. 2012;36:854–862. doi: 10.1111/j.1530-0277.2011.01669.x. doi:10.1111/j.1530-0277.2011.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J. A., Emenyonu N. I., Fatch R., Muyindike W. R., Kekiibina A., Carrico A. W., Shiboski S. Declining and rebounding unhealthy alcohol consumption during the first year of HIV care in rural Uganda, using phosphatidylethanol to augment self-report. Addiction. 2016;111:272–279. doi: 10.1111/add.13173. doi:10.1111/add.13173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann S., Aradottir S., Graf M., Wiesbeck G., Lesch O., Ramskogler K., Wurst F. M. Phosphatidylethanol as a sensitive and specific biomarker: Comparison with gamma-glutamyl transpeptidase, mean corpuscular volume and carbohydrate-deficient transferrin. Addiction Biology. 2007;12:81–84. doi: 10.1111/j.1369-1600.2006.00040.x. doi:10.1111/j.1369-1600.2006.00040.x. [DOI] [PubMed] [Google Scholar]
- Jones J., Jones M., Plate C., Lewis D. The detection of 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethanol in human dried blood spots. Analytical Methods. 2011;3:1101–1106. doi:10.1039/c0ay00636j. [Google Scholar]
- Kabwama S. N., Ndyanabangi S., Mutungi G., Wesonga R., Bahendeka S. K., Guwatudde D. Alcohol use among adults in Uganda: Findings from the countrywide non-communicable diseases risk factor cross-sectional survey. Global Health Action. 2016;9:31302. doi: 10.3402/gha.v9.31302. doi:10.3402/gha.v9.31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafuko A., Bukuluki P.Qualitative research in Uganda on knowledge, attitudes and practices concerning alcohol. 2008. Retrieved from https://www.k4health.org/sites/default/files/Alcohol Study Report FINAL March 13th.pdf.
- Keyes K. M., Jager J., Mal-Sarkar T., Patrick M. E., Rutherford C., Hasin D. Is there a recent epidemic of women's drinking? A critical review of national studies. Alcoholism: Clinical and Experimental Research. 2019;43:1344–1359. doi: 10.1111/acer.14082. doi:10.1111/acer.14082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes K. M., Li G., Hasin D. S. Birth cohort effects and gender differences in alcohol epidemiology: A review and synthesis. Alcoholism: Clinical and Experimental Research. 2011;35:2101–2112. doi: 10.1111/j.1530-0277.2011.01562.x. doi:10.1111/j.1530-0277.2011.01562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R. L., Williams J. B. W. The Patient Health Questionnaire-2: Validity of a two-item depression screener. Medical Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. doi:10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- Leddy A. M., Kerrigan D., Kennedy C. E., Mbwambo J., Likindikoki S., Underwood C. R. ‘You already drank my beer, I can decide anything’: Using structuration theory to explore the dynamics of alcohol use, gender-based violence and HIV risk among female sex workers in Tanzania. Culture, Health & Sexuality. 2018;691058.2018.1438667;20:1409–1423. doi: 10.1080/13691058.2018.1438667. doi:10.1080/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall B. D. L., Tate J. P., McGinnis K. A., Bryant K. J., Cook R. L., Edelman E. J., Justice A. C. Long-term alcohol use patterns and HIV disease severity. AIDS. 2017;31:1313–1321. doi: 10.1097/QAD.0000000000001473. doi:10.1097/QAD.0000000000001473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matson T. E., McGinnis K. A., Rubinsky A. D., Frost M. C., Czarnogorski M., Bryant K. J., Williams E. C. Gender and alcohol use: Influences on HIV care continuum in a national cohort of patients with HIV. AIDS. 2018;32:2247–2253. doi: 10.1097/QAD.0000000000001946. doi:10.1097/QAD.0000000000001946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. Helping patients who drink too much: A clinician's guide. 2007 Retrieved from https://pubs.niaaa.nih.gov/publications/practitioner/cliniciansguide2005/guide.pdf.
- National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. 2020 Retrieved from https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking.
- Pokhrel K. N., Gaulee Pokhrel K., Neupane S. R., Sharma V. D.2018Harmful alcohol drinking among HIV-positive people in Nepal: An overlooked threat to anti-retroviral therapy adherence and health-related quality of life Global Health Action 111441783.doi:10.1080/16549716.2018.1441783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. S. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. doi:10.1177/014662167700100306. [Google Scholar]
- Rehm J., Ferreira-Borges C. Risk factor policies, morbidity, and mortality in Russia. The Lancet. 2018;392:1094–1095. doi: 10.1016/S0140-6736(18)32043-9. doi:10.1016/S0140-6736(18)32043-9. [DOI] [PubMed] [Google Scholar]
- Sheehan D. V., Lecrubier Y., Sheehan K. H., Amorim P., Janavs J., Weiller E., Dunbar G. C. The Mini-International Neuro-psychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59(Supplement 20):22–57. [PubMed] [Google Scholar]
- Simons-Morton B. G., Farhat T., ter Bogt T. F. M., Hublet A., Kuntsche E., Nic Gabhainn S., Kokkevi A., the HBSC Risk Behaviour Focus Group Gender specific trends in alcohol use: Cross-cultural comparisons from 1998 to 2006 in 24 countries and regions. International Journal of Public Health. 2009;54(Supplement 2):199–208. doi: 10.1007/s00038-009-5411-y. doi:10.1007/s00038-009-5411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slade T., Chapman C., Swift W., Keyes K., Tonks Z., Teesson M. Birth cohort trends in the global epidemiology of alcohol use and alcohol-related harms in men and women: Systematic review and metaregression. BMJ Open. 2016;6:e011827. doi: 10.1136/bmjopen-2016-011827. doi:10.1136/bmjopen-2016-011827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So-Armah K. A., Cheng D. M., Freiberg M. S., Gnatienko N., Patts G., Ma Y., Samet J. H. Association between alcohol use and inflammatory biomarkers over time among younger adults with HIV-The Russia ARCH Observational Study. PLoS One. 2019;14:e0219710. doi: 10.1371/journal.pone.0219710. doi:10.1371/journal.pone.0219710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell L. C., Sobell M. B. Birmingham, AL: Addiction Research Foundation; 1995. Alcohol Timeline Followback (TLFB) users’ manual. [Google Scholar]
- Ventura A. S., Winter M. R., Heeren T. C., Sullivan M. M., Walley A. Y., Holick M. F., Saitz R. Lifetime and recent alcohol use and bone mineral density in adults with HIV infection and substance dependence. Medicine. 2017;96:e6759. doi: 10.1097/MD.0000000000006759. doi:10.1097/MD.0000000000006759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagman J. A., Wynn A., Matsuzaki M., Gnatienko N., Metsch L. R., Del Rio C., Samet J. H.2020Hazardous alcohol use, antiretroviral therapy receipt, and viral suppression in people living with HIV who inject drugs in the United States, India, Russia, and Vietnam AIDS 342285–2294.doi:10.1097/QAD.0000000000002716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandera B., Tumwesigye N. M., Nankabirwa J. I., Kambugu A. D., Parkes-Ratanshi R., Mafigiri D. K., Sethi A. K. Alcohol consumption among HIV-infected persons in a large urban HIV clinic in Kampala Uganda: A constellation of harmful behaviors. PLoS One. 2015;10:e0126236. doi: 10.1371/journal.pone.0126236. doi:10.1371/journal.pone.0126236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsberg W. M. Research Triangle; Park, NC: Research Triangle Institute; 1998. Revised risk behavior assessment, part I and part II. [Google Scholar]
- White A. M., Castle I. P., Hingson R. W., Powell P. A. Using death certificates to explore changes in alcohol-related mortality in the United States, 1999 to 2017. Alcoholism: Clinical and Experimental Research. 2020;44:178–187. doi: 10.1111/acer.14239. doi:10.1111/acer.14239. [DOI] [PubMed] [Google Scholar]
- Williams E. C., Hahn J. A., Saitz R., Bryant K., Lira M. C., Samet J. H. Alcohol use and human immunodeficiency virus (HIV) infection: Current knowledge, implications, and future directions. Alcoholism: Clinical and Experimental Research. 2016;40:2056–2072. doi: 10.1111/acer.13204. doi:10.1111/acer.13204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilsnack R. W., Wilsnack S. C., Kristjanson A. F., Vogeltanz-Holm N. D., Gmel G. Gender and alcohol consumption: Patterns from the multinational GENACIS project. Addiction. 2009;104:1487–1500. doi: 10.1111/j.1360-0443.2009.02696.x. doi:10.1111/j.1360-0443.2009.02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff B., Busza J., Bufumbo L., Whitworth J. Women who fall by the roadside: Gender, sexual risk and alcohol in rural Uganda. Addiction. 2006;101:1277–1284. doi: 10.1111/j.1360-0443.2006.01516.x. doi:10.1111/j.1360-0443.2006.01516.x. [DOI] [PubMed] [Google Scholar]