Abstract
Objective:
The aims of this brief report were to examine the extent to which early onset of cannabis use by parents and their children, and intergenerational continuity in early-onset cannabis use by parents and children, differ as a function of parent age at birth of first child.
Method:
A total of 795 parent–child dyads (57% male parents and 49% male children) were compiled from three intergenerational studies: Oregon Youth Study–Three Generational Study (OYS–3GS), Rochester Youth Development Study and Rochester Intergenerational Study (RYDS–RIGS), and Seattle Social Development Project–The Intergenerational Project (SSDP–TIP). Parents and children identified as non-Hispanic White (29% and 22%, respectively), Black (55% and 47%), and Hispanic (8% and 11%). Early-onset cannabis use was defined as initiation at or before age 15. Time-varying effect models were fit to examine the research questions.
Results:
Among parents, earlier initiation of cannabis use was associated with an earlier entry into parenthood. Moreover, parents’ later age at first birth was predictive of a lower prevalence of early-onset cannabis use among their children. Last, regarding intergenerational continuity, parental early onset of cannabis use increased the likelihood of child early-onset use, but only among older parents (i.e., later age at first birth).
Conclusions:
We provide a nuanced examination of the associations between parental and child early-onset cannabis use as a function of parents’ age at first birth and describe a novel approach to incorporating parent's age at first birth into models of intergenerational continuity.
Tiberio and colleagues (2020) pooled data from three U.S.-based intergenerational (IG) studies and identified a small, but significant, degree of IG continuity in adolescent cannabis use. Results suggested that preventing adolescent cannabis use in one generation can delay onset in the next. Although many studies have considered parents’ concurrent cannabis use as a key risk factor for their children's use of the drug, the present study is one of the few to examine IG continuity in behaviors measured prospectively in the same developmental period for parent and child (Thornberry, 2016).
We built on these findings in two ways. First, we focused specifically on early-onset cannabis use, operationalized here as use that begins at or before age 15. Growth in the prevalence of substance use is relatively slow in early adolescence and accelerates rapidly thereafter (Capaldi et al., 2009). Furthermore, initiation of substance use at age 15 or earlier is associated with numerous ill consequences (Odgers et al., 2008). Second, we considered the extent to which IG continuity in early-onset cannabis use varies as a function of parents’ age when their first child was born.
The timing of entry into parenthood can have important implications for the family environment and the development of the child. Delaying parenthood, particularly until after adolescence, can afford the individual time to “accumulate relevant economic, psychological, or social resources,” all of which may improve the family context once the child is born and increase the likelihood of positive child development (Hawkes & Joshi, 2012, p. R53). Parents’ age at child's birth also dictates the amount of time that has elapsed between the parent's and child's adolescence; substantial heterogeneity in this occurs in IG studies, where 15 to more than 25 years could separate the generations. The sheer temporal proximity of adolescence may be relevant in studies of IG continuity in adolescent behaviors for a variety of reasons, including factors specific to the parent–child dyad (e.g., a teen parent may still be engaged in substance use experimentation) and to period or cohort effects (e.g., the substance use of a parent and child closer in age may be more similar because of temporal trends in substance use). A closer investigation of the extent to which early onset of cannabis use by parents and their children, and IG continuity in early-onset cannabis use by parents and children, shows that the difference as a function of parental age at first birth may have important implications for prevention and shed light on the best approach for incorporating this phenomenon in conceptual and analytic models of IG transmission.
In this study, we aggregated data from three intergenerational studies—the Oregon Youth Study–Three Generational Study (OYS–3GS), the Rochester Youth Development Study and Rochester Intergenerational Study (RYDS–RIGS), and the Seattle Social Development Project–The Intergenerational Project (SSDP–TIP)—to examine three hypotheses. We expected that (a) youth who showed early-onset cannabis use would become parents at a younger age than youth who did not, (b) an earlier timing of parenthood would be associated with a stronger likelihood of early-onset cannabis use by the firstborn child, and (c) the association between early-onset cannabis use by parents and their offspring would be stronger for parents who transitioned to parenthood at a younger age compared with those who became parents at an older age.
Method
Overview of studies
The three studies were initiated in the 1980s and have core features that create advantages for hypothesis tests involving pooled data. Each study initially recruited a focal participant in late childhood or adolescence (Generation 2; G2) and their primary caregiver(s) (Generation 1; G1). The studies assessed G2 repeatedly throughout adolescence and into adulthood, and later initiated a second prospective study of Generation 3 (G3) offspring. Study details are presented elsewhere (Bailey et al., 2018; Capaldi et al., 2018; Thornberry et al., 2018), and the initiative to pool these data is described in Tiberio et al. (2020). IG studies are time intensive and expensive and have small sample sizes. By pooling data, we were able to capitalize on these rich data sources and to conduct more fine-grained analyses than are possible with a single IG study.
We used all G2–G3 parent–child dyads in which the G3 child was the biological firstborn and was assessed through age 15. This included 795 parent–child dyads—81 from OYS–3G, 457 from RYDS–RIGS, and 257 from SSDP–TIP. The G2 parent sample was 57% male, 29% non-Hispanic White, 55% Black, and 8% Hispanic, and the G3 child sample was 49% male, 22% non-Hispanic White, 47% Black, and 11% Hispanic. Race/ethnic identities were self-identified by the families. The average birth years of the parents and children were 1974 (SD = .8) and 1995 (SD = 3.6), respectively. The average age of the parent when the child was born was 21 years (SD = 3.3).
Prospectively measured early-onset cannabis use for each generation was defined as initiation at or before their age-15 interview (coded 1) and was compared with those with later or no onset (coded 0). Parents’ age at the child's birth was recorded as a continuous variable. Few parents in the defined subsample had their first child before age 16 (n = 37) or after age 28 (n = 15). Therefore, we censored at these ages, recoding age of the youngest parents to age 16 and that of the oldest parents to age 28.
Analysis plan
Time-varying effect modeling (TVEM), a statistical technique used to evaluate change in a variable over time and/or how associations between variables change over time (Lanza et al., 2016), is an ideal analytic approach for the present research questions. The time variable in the models was parents’ age at the birth of their first child, and the outcomes were parent and child early onset of cannabis use. We used the p-spline estimation method for fitting the TVEM, allowing up to 10 knots and specifying a logit link function for the outcomes. Statistical significance was determined by whether the 95% confidence interval (CI) around the estimate(s) across time (the shaded regions in the TVEM plots) did not contain 0.
To begin, we fitted two TVEMs to describe the prevalence of early-onset cannabis use by parent (Model 1) and by child (Model 2) as a function of parents’ age at child's birth. In these models, the only time-varying effect was the intercept, and we controlled for parent and child sex, parent race/ethnicity, study, and birth year of the parent as time-independent covariates, all centered at the mean in the cross-study sample. Next, to Model 2, we added G2 parent early onset of cannabis use as a time-varying predictor (Model 3). This model examined the extent to which IG continuity in early-onset cannabis use (the slope relating child early onset to parent early onset) differed as a function of parents’ age at child's birth.
All models were conducted in SAS 9.3 (SAS Institute Inc., Cary, NC) using the TVEM SAS macro (Li et al., 2014). The results were collated and graphed using R 4.0.2 and RStudio 1.2.5, with the tidyverse 1.3.0 suite of packages (Wickham et al., 2019).
Results
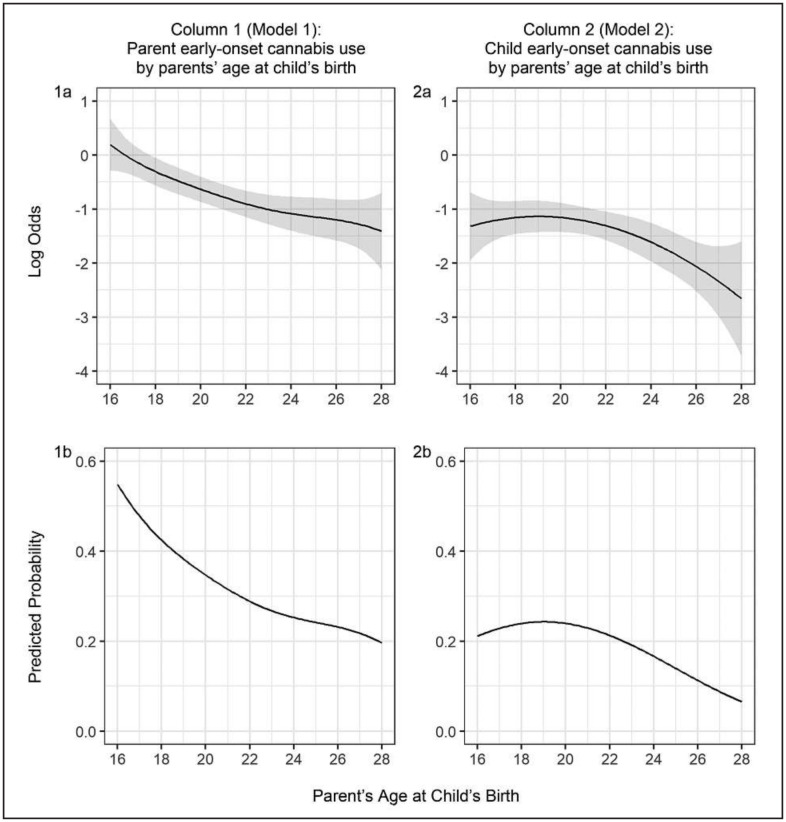
The results of the first two TVEMs are presented in Figure 1. The first column shows the extent to which prevalence of parents’ early-onset cannabis use was associated with their age at the child's birth. The top panel of Column 1 (1a) depicts the log odds of parents’ early onset, along with the corresponding 95% CI (the shaded area); the bottom panel of Column 1 (1b) translates the log odds to predicted probabilities. The declining trend indicates that individuals who initiated cannabis use at an early age tended to enter parenthood at an earlier age than those who did not do so. For example, 55% of those who became a parent at age 16 or younger used cannabis at or before age 15, compared with 20% of those who became a parent at age 28 or older.
FIGURE 1.
Early onset of cannabis by parent and child as a function of parents’ age at child's birth. Note: Results of time-varying effect models (TVEM) for Hypotheses a and b. Columns 1 and 2 examine the log odds (top) and predicted probability (bottom) of parent (Column 1) and child (Column 2) early-onset cannabis use as a function of parents’ age at the birth of the child. Shaded areas of row 1 represent 95% confidence intervals. The x-axis of all graphs represents parent's age at child's birth. All covariates are held at the mean in the cross-study sample.
The second column presents the prevalence of children's early-onset cannabis use as a function of their parent's age at first birth. As in Column 1, the top panel (2a) shows the log odds of early onset, and the bottom panel depicts the corresponding predicted probabilities. First, comparing Columns 1 and 2 makes apparent the lower prevalence of early-onset cannabis use for children than for parents. Across the entire range of parent age at first birth, the probability of early onset of cannabis use is higher for parents than for children (comparison of Figure 1, panel 1b and 2b). A declining trend is also seen for the children's early onset, which is visible at about parent age 20 and older. That is, children of older parents had a lower probability of early-onset cannabis use.
Figure 2 presents the results of Model 3, which considers IG continuity in early-onset cannabis use as a function of parents’ age at child's birth. Panel a presents the log odds slope and corresponding 95% CI relating parents’ early onset to children's early onset. In regions of the graph where the CI does not cross 0 on the y-axis (the dashed horizontal line), IG continuity is statistically significant. Although a positive slope is estimated across the range of parents’ age at child's birth (indicating higher odds of child early onset if the parent began using cannabis early), the IG effect is not significant before age 22 and then begins to increase steadily. This indicates that IG continuity is more likely among older parents.
FIGURE 2.
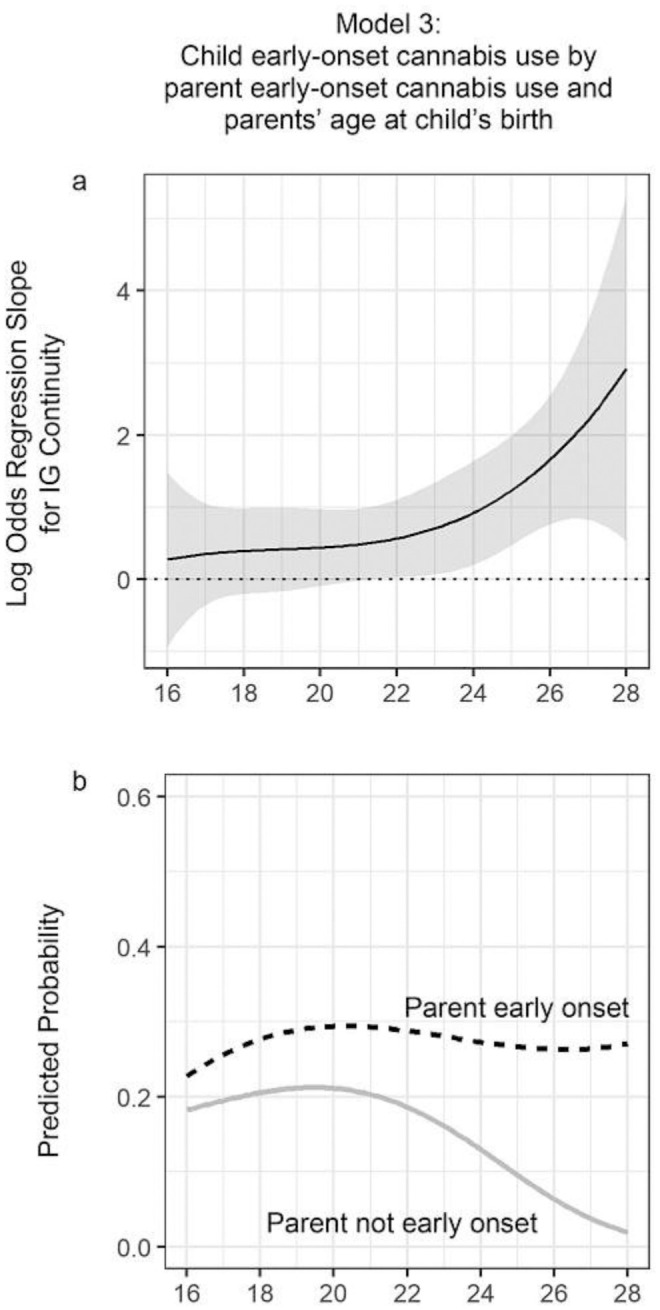
Intergenerational (IG) continuity in early-onset cannabis as a function of parents’ age at child's birth. Note: Results of time-varying effect models (TVEM) for Hypothesis c. The top panel (a) presents the log odds coefficient of IG continuity, and the bottom panel (b) translates the log odds estimates from the TVEM to the predicted probabilities of child's early onset as a function of parent's age at child's birth among parents who did (dashed line) or did not (solid line) show early onset of cannabis use. Shaded areas in the top panel represent 95% confidence intervals. The reference line at 0 (dotted line) in the top panel signifies the location of a nonsignificant slope for the effect of parental early-onset cannabis use on child early-onset cannabis use (IG effect). The x-axis of both graphs represents parent's age at child's birth. All covariates are held at the mean in the cross-study sample.
In panel b, we translated the results of the IG continuity TVEM (Model 3) to the predicted probabilities of child's early onset among parents who did and who did not have early onset of cannabis use. Among parents with an early onset of cannabis use, the probability that the child would also onset early was similar, irrespective of parents’ age at the child's birth. However, among parents who did not begin using cannabis at an early age, the probability that their child would have an early onset of cannabis use was highest for the youngest parents and began to steadily decline around age 22. Among the oldest parents, the graph shows a marked difference in the probability of the child's early onset of cannabis use when contrasting parents who were (~.27) and were not (~.02) early cannabis users. In sum, early-onset cannabis use by a parent did not appear to be related to the child's early onset among young parents, but it was substantially related among older parents.
To supplement our TVEM findings, we fitted a traditional logistic regression model, regressing child early onset of cannabis use on parental early onset of cannabis use, parent age at first birth, and the interaction of the two (we also included the control variables). The interaction was statistically significant (log odds b = .14, SE = .06, p < .05). Probing the interaction, we found the same pattern as observed in Figure 2, panel b. That is, at a young parental age (e.g., age 18), there is no effect of parental early-onset cannabis use on child early onset of cannabis use (log odds b = .24, SE = .25, NS); but, at an older parental age (e.g., age 25), there is an effect of parental early-onset cannabis use on child early-onset cannabis use (log odds b = 1.22, SE = .31, p < .05).
Discussion
An early transition to parenthood is associated with prior psychosocial risks and problem behaviors and may play a role in perpetuating these behaviors in the lives of the next generation (e.g., Fagot et al., 1998). Consistent with this notion (and Hypotheses a and b), parents who had an early onset of cannabis use tended to have their firstborn child at an earlier age than other parents, and the odds of the child's early onset of cannabis use tended to be higher when parents were younger at the time of their child's birth. However, we also found evidence of differential IG continuity as a function of parents’ age at child's birth. Specifically, and contrary to our Hypothesis c, the association of early-onset cannabis use by parents and their children was stronger among older than younger parents. We based this hypothesis on the assumption that children from the higher risk backgrounds associated with early parenthood would be more vulnerable to additional risk from their parent's early onset of cannabis use. However, this was not the case—an issue we address further below.
Strengths of the present study include, first, the synthesis of three prospective intergenerational studies, which increases statistical power and enhances confidence in generalizability across gender, ethnicity, region, and other factors specific to any single IG study (Tiberio et al., 2020). Second, several pertinent demographic variables were covaried in the analyses. Third, the study demonstrates the utility of TVEM for modeling parents’ age at child's birth in the context of IG effects and offers a framework for other explorations of IG continuity (e.g., in poly- or other substance use). Limitations include the potential for cohort effects, given that parents were born in the mid-1970s. Furthermore, the G3 children included in the study were all biologically related to the parents and were firstborns, and it is unclear whether the effects found would apply to later born or adoptive children or stepchildren.
The study findings offer some useful insight into scenarios under which children are most and least likely to begin experimenting with cannabis at an early age. It appears that, although there are general risks to offspring associated with early parenthood, parental early onset of cannabis use does not represent an additional risk for offspring early onset of cannabis use. Pears et al. (2005) found that an early age at first birth for G2 men in OYS was associated with their mother's (G1) age at first birth, G1 low family socioeconomic status, and the young men's poor academic skill, substance use, and antisocial behavior. Thus, the risk for early onset of cannabis use associated with parent's young age at childbirth may be strong enough that parental early onset of cannabis use does not affect this risk further. Overall, G3 offspring who were most protected from early onset of use were those whose parents did not begin using cannabis at an early age and who delayed parenthood to their mid- to late-20s. Panel b of Figure 2 clearly demonstrates that parents who delayed both cannabis use and childbearing had the lowest probability of having a child who began using cannabis at or before age 15. Thus, findings reiterate the importance of prevention of cannabis use in adolescence and demonstrate the potential liabilities associated with early parenthood. Of note, whereas teenage pregnancy is a well-accepted prevention target, Figure 1 implies that the liability of early parenthood was evident through the mid-20s. By this age young men and women have had more time both to mature out of adolescent problem behaviors and to establish social and economic capital (e.g., educational and vocational training). Findings also indicate, however, that children who are generally at lower risk—given their parent's older age at their birth—are nevertheless vulnerable to specific risk related to their parent's early onset of cannabis use, thus creating an IG association in early onset of cannabis use.
Future research may address the mechanisms underlying similarities between parents’ and their children's early-onset cannabis use, and the observation that these associations are a function of the timing of parenthood. Mechanisms identified in prior work on IG continuities in substance use include parents’ social and economic capital, cannabis use patterns after early or later onset including use during their child's life, cannabis use attitudes they may communicate to children, and partner use and characteristics (e.g., Bears Augustyn et al., 2020; Epstein et al., 2020; Kerr et al., 2020). Future work is also necessary to examine the role of the other parent's substance use during adolescence (albeit retrospectively reported), any ethnic or gender differences in the processes considered here (although see Tiberio et al., 2020), and whether IG continuity in early-onset cannabis use stems from IG transmission of externalizing behaviors in general. Such research will show the extent to which parents’ adolescent histories should be used to refine prevention approaches with their offspring.
Conflict-of-Interest Statement
The authors have no conflicts of interest to disclose.
Footnotes
Funding for this work was supported by the National Institutes of Health (NIH) from the National Institute on Drug Abuse (NIDA) Grant Number 5R01DA020195 awarded to Kimberly L. Henry, Grant Number R01DA015485 awarded to Deborah M. Capaldi and David C. R. Kerr, and Grant Number 5R01DA023089 awarded to Jennifer A. Bailey. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH or NIDA. NIH or NIDA had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication. These study findings have not been previously presented at any conferences or meetings or published anywhere else.
References
- Bailey J. A., Hill K. G., Epstein M., Steeger C. M., Hawkins J. D.2018Seattle Social Development Project—The Intergenerational Project (SSDP-TIP)In Eichelsheim V. I., van de Weijer S. G. A. (Eds.), Intergenerational continuity of criminal and antisocial behaviour. An international overview of current studies (pp. 186–206.)New York: Routledge [Google Scholar]
- Bears Augustyn M., Loughran T., Larroulet P., Fulco C. J., Henry K. L. Intergenerational marijuana use: A life course examination of the relationship between parental trajectories of marijuana use and the onset of marijuana use by offspring. Psychology of Addictive Behaviors. 2020;34:818–829. doi: 10.1037/adb0000530. doi:10.1037/adb0000530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi D. M., Kerr D. C. R., Tiberio S. S.2018The Oregon Youth Study—Three Generational Study: A review of design, theory, and findingsIn Eichelsheim V. I., van de Weijer S. G. A. (Eds.), Intergenerational continuity of criminal or antisocial behaviour: An international overview of studies (pp. 137–161.)New York: Routledge [Google Scholar]
- Capaldi D. M., Stoolmiller M., Kim H. K., Yoerger K. Growth in alcohol use in at-risk adolescent boys: Two-part random effects prediction models. Drug and Alcohol Dependence. 2009;105:109–117. doi: 10.1016/j.drugalcdep.2009.06.013. doi:10.1016/j.drugalcdep.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein M., Bailey J. A., Furlong M., Steeger C. M., Hill K. G. An intergenerational investigation of the associations between parental marijuana use trajectories and child functioning. Psychology of Addictive Behaviors. 2020;34:830–838. doi: 10.1037/adb0000510. doi:10.1037/adb0000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagot B. I., Pears K. C., Capaldi D. M., Crosby L., Leve C. S. Becoming an adolescent father: Precursors and parenting. Developmental Psychology. 1998;34:1209–1219. doi: 10.1037//0012-1649.34.6.1209. doi:10.1037/0012-1649.34.6.1209. [DOI] [PubMed] [Google Scholar]
- Hawkes D., Joshi H. Age at motherhood and child development: Evidence from the UK Millennium Cohort. National Institute Economic Review. 2012;222:R52–R66. doi:10.1177/002795011222200105. [Google Scholar]
- Kerr D. C. R., Tiberio S. S., Capaldi D. M., Owen L. D. Intergenerational congruence in adolescent onset of alcohol, tobacco, and marijuana use. Psychology of Addictive Behaviors. 2020;34:839–851. doi: 10.1037/adb0000546. doi:10.1037/adb0000546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza S. T., Vasilenko S. A., Russell M. A. Time-varying effect modeling to address new questions in behavioral research: Examples in marijuana use. Psychology of Addictive Behaviors. 2016;30:939–954. doi: 10.1037/adb0000208. doi:10.1037/adb0000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R., Tan X., Huang L., Wagner A., Yang J. University Park, PA: The Methodology Center, Penn State University; 2014. TVEM (Time-Varying Effect Model) SAS Macro Suite Users’ Guide (Version 2.1. 1)http://methodology.psu.edu Retrieved from. [Google Scholar]
- Odgers C. L., Caspi A., Nagin D. S., Piquero A. R., Slutske W. S., Milne B. J., Moffitt T. E. Is it important to prevent early exposure to drugs and alcohol among adolescents? Psychological Science. 2008;19:1037–1044. doi: 10.1111/j.1467-9280.2008.02196.x. doi:10.1111/j.1467-9280.2008.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pears K. C., Pierce S. L., Kim H. K., Capaldi D. M., Owen L. D. The timing of entry into fatherhood in young, at-risk men. Journal of Marriage and the Family. 2005;67:429–447. doi: 10.1111/j.0022-2445.2005.00126.x. doi:10.1111/j.0022-2445.2005.00126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornberry T. P.2016Three generation studies: Methodological challenges and promiseIn Shanahan M. J., Mortimer J. T., Johnson M. K. (Eds.), Handbook of the life course (1st ed.,Vol. IIpp. 571–598.)New York: Springer [Google Scholar]
- Thornberry T. P., Henry K. L., Krohn M. D., Lizotte A. J., Nadel E. L.2018Key findings from the Rochester Intergenerational StudyIn Eichelsheim V. I., van de Weijer S. G. A. (Eds.), Intergenerational continuity of criminal and antisocial behaviour: An international overview of current studies (pp. 214–234.)New York: Routledge [Google Scholar]
- Tiberio S. S., Kerr D. C. R., Bailey J. A., Henry K. L., Capaldi D. M. Intergenerational associations in onset of cannabis use during adolescence: A data synthesis approach. Psychology of Addictive Behaviors. 2020;34:877–889. doi: 10.1037/adb0000625. doi:10.1037/adb0000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H., Averick M., Bryan J., Chang W., McGowan L. D. A., François R., Yutani H. Welcome to the tidyverse. Journal of Open Source Software. 2019;4:1686. doi:10.21105/joss.01686. [Google Scholar]