Abstract
Influenza A virus (IAV) has been raising public health and safety concerns worldwide. Cyanovirin-N (CVN) is a prominent anti-IAV candidate, but both cytotoxicity and immunogenicity have hindered the development of this protein as a viable therapy. In this article, linker-CVN (LCVN) with a flexible and hydrophilic polypeptide at the N-terminus was efficiently produced from the cytoplasm of Escherichia coli at a >15-l scale. PEGylation at the N-terminal α-amine of LCVN was also reformed as 20 kDa PEGylated linkered Cyanovirin-N (PEG20k–LCVN). The 50% effective concentrations of PEG20k–LCVN were 0.43 ± 0.11 µM for influenza A/HK/8/68 (H3N2) and 0.04 ± 0.02 µM for A/Swan/Hokkaido/51/96 (H5N3), dramatically lower than that of the positive control, Ribavirin (2.88 ± 0.66 × 103 µM and 1.79 ± 0.62 × 103 µM, respectively). A total of 12.5 µM PEG20k–LCVN effectively inactivate the propagation of H3N2 in chicken embryos. About 2.0 mg/kg/day PEG20k–LCVN increased double the survival rate (66.67%, P = 0.0378) of H3N2 infected mice, prolonged the median survival period, downregulated the mRNA level of viral nuclear protein and decreased (attenuated) the pathology lesion in mice lung. A novel PEGylated CVN derivative, PEG20k–LCVN, exhibited potent and strain-dependent anti-IAV activity in nanomolar concentrations in vitro, as well as in micromolar concentration in vivo.
Keywords: Cyanovirin-N, influenza A virus, mice, PEGylation, polypeptide linker
The enveloped negative-sense single-strand segmented influenza A virus (IAV) was categorized into different subtypes according to the type of viral surface envelope proteins, haemagglutinin (HA) and neuraminidase (NA). IAV usually causes a high level of morbidity and mortality in birds and certain mammals (human, pig, dog, etc.). The most severe influenza pandemic resulted in ∼40 million deaths worldwide in 1918 (1). The mortality rate of the highly pathogenic avian influenza virus, H5N1, was up to 61% in infected individuals from November 2003 to June 2009 (2). World Health Organization reported that the explosion of the H1N1 pandemic in 2009 killed more than 18,000 people in 1 year from April 2009 to April 2010.
Cyanovirin-N (CVN) is a 101 amino-acid protein with a special amino acid sequence and 2 intra-molecular disulphide bonds derived from Nostoc ellipsosporum. It represents a new generation of microbicides due to its novel mechanism, phenomenal chemicophysical stability and potent broad spectrum anti-viral activity (HIV, influenza virus, hepatitis C virus, Ebola virus and Herpes simplex virus). CVN inhibits virus replication during the early stage of viral infection. It usually blocks viral entry by specifically binding to high-mannose oligosaccharides, which sit on the surface glycoprotein of enveloped viruses. CVN possesses potent antiviral activity against most strains of influenza A and B, including clinical isolates and an NA inhibitor-resistant strain (3). CVN has the potential for prophylactic and early treatment of IAV infections. In mice and ferret influenza virus models, treatment with CVN before viral infection caused a significant and dose-dependent reduction of viral titres in the lungs and nasal washes (4). But due to its cyanobacterial origin, CVN also has unavoidably typical drawbacks in pharmaceutical applications, such as a short plasma half-life, proteolysis and immunogenicity. PEGylation of CVN could improve its pharmacokinetic and pharmacodynamic properties. However, this only occurred for the mutant, Q62C, in which glutamine 62 was replaced with cysteine and the extra free sulfhydryl was able to be site-specifically PEGylated with maleimide-activated PEG (Fig. 1A). The in vitro anti-HIV activity of Q62C was ∼50% of that of wild type (WT) CVN, and the 20 kDa PEG conjugate of Q62C showed ∼80% activity of WT CVN, whereas the 30 kDa conjugate showed almost no activity.
Fig. 1.
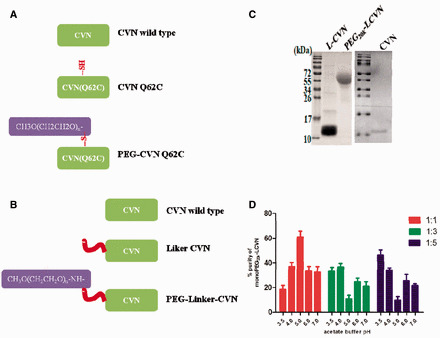
Strategies for the PEGylation of CVN and the preparation of recombinant linker CVN (LCVN) and PEG20k–LCVN. (A) The reported strategy for the PEGylation of CVN with a point mutation. CVN was site-specifically PEGylated at the free –SH of the mutation, CVN (G62C). (B) Schematic for the PEGylation of CVN with a N-terminus extension. A 15-aa linker sequence was fused to the N-terminus of CVN by DNA recombination to obtain LCVN. Then, the LCVN was PEGylated by 20-kDa mPEG-ALD to obtain a monoPEGylated CVN derivative, PEG20k–LCVN. (C) SDS-PAGE analysis of the purity of CVN, LCVN and PEG20k–LCVN. The recombinant CVN and LCVN without any tag fusion were purified from a recombinant His6-SUMO-CVN and His6-SUMO-LCVN produced in E. coli by two rounds of Ni2+-chelating chromatography and gel filtration. The PEG20k–LCVN was purified by ion exchange chromatography after the PEG-conjugation reaction. (D) Optimization of the PEGylation efficiency of LCVN. Different molar ratio of LCVN and 20-kDa mPEG-ALD was incubated 3 h in pH 3.5–7.0 acetate buffer at room temperature. The relative level (purity) of monoPEGylated LCVN was determined by SDS-PAGE and grey density analysis.
To avoid potential druggability modification resulting from the point mutation in the primary amino acid sequence of CVN and the complicated downstream purification process of the non–site-specific PEGylation, we extended the N-terminus of CVN with a hydrophilic flexible polypeptide, (GGGGS)3, to make the linker-CVN (LCVN) and tried site-specific PEGylation of LCVN with polyethylene glycol-propionaldehyde (mPEG-ALD) at the amino-terminal group (Fig. 1B). We hypothesized that the construction of the ‘PEG–LCVN’ might preserve CVN’s anti-viral bioactivity by separating the large PEG molecular group from CVN’s active sites and allow the synthesis of PEGylated products with higher homogeneity. In this article, the preparation of the 20-kDa derivative, PEG20k–LCVN, was described, and its bioactivities against IAV were also demonstrated in vitro and in vivo.
Materials and Methods
Cells and virus
Cells
Madin–Darby canine kidney (MDCK) cells (ATCC, VA) growing in minimum essential medium (MEM; Invitrogen; Life Technologies, CA) containing 10% foetal bovine serum (FBS; Zhejiang Tianhang, Zhe Jiang, China) were incubated at 37°C in a humidified 5% CO2 atmosphere.
Virus strains
The influenza virus strains A/PR/8/34 (H1N1), A/WSN/33 (H1N1) and A/HK/8/68 (H3N2) were stored in-house. A/Swan/Hokkaido/51/96 (H5N3) was obtained from the Wuhan Institute of Virology, Chinese Academy of Science. Given low mortality of A/HK/8/68(H3N2) in mice, an adapted strain was obtained as described before. All strains were propagated in 10-day-old chicken embryo eggs (Guangdong Dahuanong Animal Health Products Co., Ltd, Guangdong, China) for 3 days at 35°C in 50–60% relative humidity under forced air circulation, and then maintained at 4°C overnight. Harvested and mixed allantoic liquids with high HA titres were stored at −80°C.
Embryonated eggs and animals
Nine-day-old specific pathogen free (SPF) chicken embryo eggs (Xinxin Da-hua-nong, Inc., Guangdong, China) were cultured at 35°C in 50–60% relative humidity under forced air circulation. Six-week-old BALB/c female mice (class SPF) and 180–200 g SD rat (Guangdong Medical Laboratory Animal Center, Guangdong, China) were cultured with freely accessed to water and regular diet under in a 12-hours-light, 12-hours-dark cycle. Experiments were conducted in accordance with the guidelines of the Institute of Laboratory Animal Science, Jinan University [SCXK (Yue) 2008–0002].
DNA recombination and purification of linked CVN
Recombinant LCVN was prepared by modifying a previously reported process (5). Briefly, an oligonucleotide fragment encoding the linker sequence, (GGGGS)3, was designed and inserted into a previously constructed fusion gene to obtain his6-sumo-linker-cvn, which was then inserted into plasmid pET-3c (Merck, Darmstadt, Germany) and transformed into Escherichia coli BL21 (Invitrogen). The expression of recombinant LCVN was induced by 0.5 mM isopropyl-beta-d- thiogalactopyranoside (Sigma, MO) at 20°C for 20 h when the density of the culture reached logarithmic growth phase, that is, 0.6–1.0 OD600 in 5-ml flask tube or 8–10 OD600 in a 15-l bioreactor. Total soluble protein was extracted by ultrasonic treatment, and the His6–SUMO–LCVN (small ubiquitin-related modifier) was purified by Ni2+-NTA affinity chromatography (GE Healthcare, Uppsala, Sweden). Then, SUMO protease (prepared in house) was applied to cleave the His6–SUMO tag, and the second round of Ni+-NTA affinity chromatography was run to separate the mixture of LCVN and His6–SUMO. The collection of LCVN was polished by gel filtration chromatography, and its purity was determined by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE).
The PEGylation of LCVN and its purification
Key parameters for the PEGylation including pH, molar ratio of LCVN to mPEG-ALD and the reaction period were optimized. LCVN and 20-kDa mPEG-ALD (Kaizhen Biotech Co., Beijing, China) were conjugated with the ratio from 1:1 to 1:5 in pH 3.5–7.0 (20 mM acetate buffer) in the presence of 5 mg/ml sodium cyanoborohydride at room temperature for 3 h. Further optimization was conducted with the reaction period from 1 to 24 h. The reaction mixture was resolved in SDS-PAGE to evaluate the efficiency of PEGylation.
Ion-exchange chromatography was used to separate the mixture of PEGylation products of LCVN, which was diluted 5 times with 70-mM NaAc buffer (pH 4.0) and loaded onto equilibrated SP-sepharose Fast Flow resin (GE Healthcare); then, the column was washed using NaAc buffer with a NaCl gradient. The monoPEGylated LCVN was collected in fractions detected by Tricine SDS-PAGE, and finally, the monoPEGylated LCVN was buffer exchanged into PBS for long-term storage.
Haemagglutination inhibition assay
The haemagglutination inhibition (HI) assay was performed to evaluate the inhibitory effects of PEG20k–LCVN, LCVN and CVN on influenza virus adsorption into red blood cell (RBC). All the samples were 2-fold serially diluted in PBS, and then it was mixed with equal volume of influenza virus with four haemagglutination units. Each well was mixed with 50 μl of 1% RBC suspension and incubated for 30 min at room temperature. This experiment was replicated three times.
Enzyme-linked immunosorbent assay
The binding ability of CVN and its derivatives to viral HA was determined by enzyme-linked immunosorbent assay (ELISA). HA from A/duck/Hokkaido/167/2007 (H5N3), A/California/07/2009 (H1N1) or A/HK/8/68 (H3N2) (Sino biological, Inc., Beijing, China) in phosphate buffer (pH 8.0) was coated onto ELISA plates (Corning, NY) at 100 ng per well at 4°C overnight. After washing three times with PBS containing 0.1% Tween-20 (PBST), the plates were blocked with 15% FBS for 1.5 h at 37°C and then washed with PBST as above. Various concentrations of PEG20k–LCVN, LCVN and CVN were added to each triple well; the same concentrations of BSA were used for the negative control. The plate was incubated for 2 h at 37°C. After washing with PBST, the wells were incubated with rabbit anti-CVN polyclonal antibody (prepared in house) for 2 h at 37°C, followed by incubation with horse-radish peroxidase-conjugated goat anti-rabbit IgG antibody (GE Healthcare, UK) for 1.5 h at 37°C. The plate was washed four times with PBST, and subsequently 3,3′,5,5′-tetramethylbenzidine (TMB) (Sigma) was added in the dark room. The reaction was stopped using 2 M H2SO4, and the absorbance at 450 nm was measured using a Microplate Reader (Bio-Rad 550; Bio-Rad, CA). The experiment was repeated at least three times. At least nine parallel measurements were obtained for the mean and SD values.
Cytotoxicity test
MDCK cells were seeded into 96-well plates at a concentration of 1.5 × 105 cells per well and incubated for 18–24 h. Then, the cells were treated with various concentrations of PEG20k–LCVN, LCVN, CVN or Ribavirin (Huazhong Pharmaceutical Co., Ltd, Hubei, China), each three replication wells a concentration, then incubated at 37°C for 72 h, 10 μL MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide, 5 mg/ml) was added to each well. After further incubation for 4 h, the supernatant was discarded carefully. Hundred microlitres of DMSO was added to each well to solubilize the formazan crystals. The absorbance was recorded by an ELISA plate reader at the test wavelength of 490 nm and a reference wavelength of 630 nm; subsequently, 50% cytotoxic concentrations (CC50) was calculated (6). The experiment was repeated at least three times. At least nine parallel measurements were obtained for the mean and SD values.
EC50 of PEG20k–LCVN in influenza virus
PEG20k–LCVN and Ribavirin were 2-fold diluted from concentrations less than the maximum non-toxic concentration. The mixtures of 50 µl of each dilution and 50 µl of 100 TCID50 of each influenza virus A/PR/8/34 (H1N1), A/WSN/33 (H1N1), A/HK/8/68 (H3N2) and A/Swan/Hokkaido/51/96 (H5N3) were added to the single layer of MDCK cells seeded in a 96-well cell culture plate. Moreover, the virus control (virus free of PEG20k–LCVN) and negative control group (medium without FBS) were set and incubated for 48 h. Then, 10-µl MTT was placed into each well to read the optical density (OD) values at 490 and 630 nm. The inhibition rate was calculated as follows = (ODsample−ODvirus control)/(ODnegative control−ODvirus control)%. Thus, EC50 and select index (SI) values were calculated. Each concentration was conducted with three replications and three independent experiments.
Immunofluorescence
Each of the PEG20k–LCVN dilutions mixed with 100 TCID50 A/HK/8/68 (H3N2) per 50 µl was attached to a monolayer of MDCK cells grown in 96-well plates, incubated at 37°C under 5% CO2 for 48 h. CVN and LCVN were used as control group. The cells were washed with PBS three times and treated with 4% polyformaldehyde for fixation, 0.1% Triton-X100 for permeation and 5% BSA for blocking. Mouse anti-influenza A nucleus protein (NP) (5d8) monoclonal immunoglobulin (IgG) (1:150, Santa Cruz, TX) and goat anti-mouse IgG (Fab2) Alexa Fluor (R) 488 (1:1000, Cell Signaling Technology, MA) were sequentially used to probe the NP protein. A total of 5 μM TRITC-phalloidin (Sigma Aldrich, MO) and 1 mg/ml DAPI-PBS were applied to label the F-actin and nuclei, respectively. Fluorescence was observed using an Olympus fluorescence microscope (Olympus IX71; Olympus Corporation, Tokyo, Japan), and images were processed using ImageJ software. Three independent experiments were performed, in which at least three photographs were randomly taken for each well.
Testing antiviral activity of PEG20k–LCVN in chicken embryos
IAV HK/8/68 (H3N2) was diluted from 10−1 to 10−6 and injected into 10-day-old chicken embryo eggs (0.1 ml per egg) for an additional incubation at 35°C for 7 days. Then, 50% egg infective dose (EID50) of the virus was determined to be 10−4.5 using the Reed-Muench method.
To test the antiviral activity of PEG20k–LCVN in embryos, 100 EID50 of virus was inoculated into the embryonic allantoic cavity, and then 2-fold serially diluted PEG20k–LCVN was also inoculated into the allantoic fluid. CVN and LCVN groups were used as compared experiments. The virus control, negative control (0.1-ml PBS), positive control groups (Ribavirin 0.5 mg) were set at the same time. After incubating at 35°C for 72 h, then 4°C for 12 h, allantoic fluid from the eggs was collected for haemagglutination titration.
Survival rate assay
Female BALB/c mice was randomly divided into five groups. (i) In the virus group, the mice were intranasally driped with 10 LD50/50 µl H3N2 mouse-adapted-strain before anesthetized with diethyl ether (n = 6). (ii) In the PEG20k–LCVN groups, the mice were treated with 0.5, 1.0 or 2.0 mg/kg/day PEG20k–LCVN in sterile PBS by nasal dripping twice per day for 4 days (n = 6). On Day 1, the mice were treated with the compound 1 h before the virus challenging. (iii) In the positive control group, the mice were treated with 75 mg/kg/day Ribavirin (n = 6). Survival rate of the mice were recorded post 14 days infection.
Lung parameters
At 3 days post-infection, mice from the virus group (n = 5), 2.0 mg/kg/day PEG20k–LCVN group (n = 5) and Ribavirin group (n = 5) were sacrificed to record body weight and lung index. Part of the lung was fixed with 4% paraformaldehyde for HE staining. A pair of lungs was respectively homogenized and centrifuged at 4,000 rpm for 10 min at 4°C. The total RNA was extracted using Trizol (Invitrogen) according to the protocols recommended by the manufacturer.
Reverse transcription polymerase chain reaction (RT-PCR) was performed in a 10-μl reaction with 1-μg RNA and Prime Script RT enzyme mix I (Takara) for 15 min at 37°C, 3 s at 85°C and 4°C forever. The relative mRNA level of NP was measured by quantitative real-time RT-PCR, using the Bio-Rad detective system and SYBR green dye (TAKARA). GAPDH was used as an endogenous control. Triple independent experiments were carried out. Below is the primers for the amplification. GAPDH (215 bp): forward: 5′-GTCATTGAGAGCAATGCCAG-3′, reverse: 5′-GTGTTCCTACCCCCAATGTG-3′; NP (202 bp): forward: 5′-AAATCATGGCGTCCCAAGGCACC-3′, reverse: 5′-TCTTCCCGACGGATGCCCTAA-3′.
Mucosa toxicity assessment
A total of 180- to 200-g SD rats were divided into 2 groups (placebo and PEG20k–LCVN, n = 3). About 1.0 mg PEG20k–LCVN was delivered into the rat vagina once a day for 7 days. The morphology of vagina isolated from rats at Day 9 was observed after HE staining.
Statistical analysis
All data are presented as the mean ± standard deviation (SD). Statistical analysis of the results was performed using Mann–Whitney test and log-rank (Mantel–Cox) test of GraphPad prism 5 software. P < 0.05 was considered to be statistically significant.
Results
Preparation of LCVN and PEG20k–LCVN
CVN has a unique primary sequence, and it is very difficult to efficiently produce soluble and biologically functional recombinant CVN from E. coli or other hosts. Using the strategy of hexahistidine-SUMO double-tag fusion we developed for the production of CVN recombinant, LCVN was produced efficiently from the cytoplasm of E. coli (Fig. 1). Differing from the PEGylation strategy of a point mutation (Fig. 1A), the coding sequence of the CVN core in PEG20k–LCVN does not contain a point mutation (Fig. 1B). The purity of CVN, LCVN and PEGylated LCVN products were confirmed by SDS-PAGE to be >95% (Fig. 1C). The highest yield of monoPEGylated LCVN was produced at pH 5.0 with molar ratio 1:1 of LCVN to 20 kDa mPEG-ALD (Fig. 1D and Fig. S1A, See online supplementary material for a colour version of this figure). But PEGylation efficiency was not significantly changed in reaction period different from 1 h to 24 h (Fig. S1B, See online supplementary material for a colour version of this figure). Finally, the optimum condition for LCVN PEGylation was conducted in 5-ml acetate buffer (pH 5.0) with 1:1 molar ratio of LCVN and 20-kDa mPEG-ALD by 2 h incubation at room temperature.
PEG20k–LCVN inhibits haemagglutination and binding to the HA protein
The IAV envelope protein, HA, is able to bind to RBC surface receptors and cause chicken RBC agglutination. First, to quickly identify whether the PEG20k–LCVN has anti-IAV bioactivities, an RBC HI assay was performed (Fig. 2B). Differing from the cellular control group, the RBC of the virus control group was uniformly distributed in each well and exhibited 100% agglutination. CVN, LCVN and PEG20k–LCVN disturbed IAV-forming agglutination. RBC agglutination phenomenon in these three experiment groups disappeared and was similar to the cellular control group. These data revealed that CVN and its derivatives, especially PEG20k–LCVN, inhibited chicken RBC agglutination which was induced by the influenza virus.
Fig. 2.
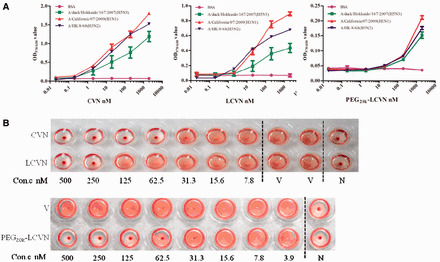
CVN and its derivatives binding to HA and inhibiting agglutinantion induced by IAV. (A) The interaction of PEG20k–LCVN with three types of influenza HA protein. Serial dilutions of samples or BSA were incubated with 100 ng/well of each HA protein from A/duck/Hokkaido/167/2007 (H5N3), A/California/07/2009 (H1N1) and A/HK/8/68 (H3N2). Rabbit anti-CVN polyclonal antibody was used to detect bound CVN. Absorbance at 450 nm was measured to evaluate the binding potency of PEG20k–LCVN to HA. (B) PEG20k–LCVN, CVN and LCVN inhibit the agglutination of chicken erythrocyte caused by the influenza A/HK/8/68(H3N2) virus. Two-fold serial dilutions of the three samples were mixed with IAV (4× haemagglutination units) and 1% chicken erythrocyte suspension and then incubated at room temperature for 30 min to observe the aggregation of red cells.
HA plays a key role in the procession of the influenza virus attached to RBC. It can recognize and bind to the host cell membrane surface of sialic acid receptor. We further determined whether PEG20k–LCVN could directly bind with HA protein (Fig. 2A). Three subtypes, HA from A/duck/Hokkaido/167/2007 (H5N3), A/California/07/2009 (H1N1) and A/HK/8/68(H3N2), were tested. The OD value of each HA protein increased according to the CVN, LCVN or PEG20k–LCVN gradient from 0.02 to 2.0 µM. These data show that all these recombinant proteins could bind to HA in a concentration-dependent manner. But their binding abilities were different. CVN has the strongest ability to bind with HA protein than others. More than 0.02 µM PEG20k–LCVN exhibited interaction with the HA protein.
PEG20k–LCVN inhibited IAV replication in vitro
PEG20k–LCVN exhibited HA binding bioactivity. This activity inspired us to evaluate its in vitro cytotoxicity and antiviral activity. The cytotoxicity assay demonstrated that cells treated with PEG20k–LCVN, up to 100 μM, did not show any morphological alteration compared with the negative control cells (treated with MEM medium). Therefore, the CC50 of PEG20k–LCVN must be higher than 100 μM, which was the highest concentration of this protein we prepared here. While comparing with CVN and LCVN, PEG20k–LCVN’s has the weakest cytotoxicity. These data suggested that the cytotoxicity of PEGylated LCVN reduced more than 100 times compared with native CVN (Table I).
Table I.
The EC50, CC50 and SI of CVN, LCVN and PEG20k–LCVN against influenza virus strains in vitro.
| CVN | LCVN | PEG20k–LCVN | Ribavirina | ||
|---|---|---|---|---|---|
| Maximum non-toxicity concentration (µM) | 0.26 | 4.25 | >100.00 | 2.06 × 104 | |
|
| |||||
| CC50 (µM) | 4.00 ± 1.03 | 58.00 ± 5.88 | >100.00 | (9.62 ± 0.58) × 104 | |
|
| |||||
| EC50 (µM) | A/WSN/33 | >2.50 | >2.50 | >2.50 | (1.76 ± 1.17) × 103 |
| A/PR/8/34 | >2.50 | >2.50 | >2.50 | (0.97 ± 0.07) × 103 | |
| A/HK/8/68 | 0.04 ± 0.01 | 0.19 ± 0.09 | 0.43 ± 0.11 | (2.88 ± 0.66) × 103 | |
| A/Swan/Hokkaido/51/96 | 0.02 ± 0.01 | 0.07 ± 0.01 | 0.04 ± 0.02 | (1.79 ± 0.62) × 103 | |
|
| |||||
| SI | A/HK/8/68 | 104.41 | 294.02 | >233.56 | 33.40 |
| A/Swan/Hokkaido/51/96 | 173.38 | 804.88 | >2,564.10 | 53.73 | |
The values are the mean ± SD of three independent experiments, and each independent experiment comprises triplicate wells.
CC50: 50% toxicity to MDCK cells determined using an MTT assay.
EC50: 50% effective concentration against influenza strain.
SI: select index was calculated as the ratio of CC50 to EC50.
aPositive control.
The in vitro antiviral activity of PEG20k–LCVN was determined using four strains of influenza virus (Table I). The MTT assay suggested that PEG20k–LCVN had no significant antiviral activity against two H1N1 strains (A/WSN/33 and A/PR/8/68), as their EC50 value was >2.5 μM. CVN and LCVN also exhibited similar strain specificity. But PEG20k–LCVN exhibited potent inhibitory activity against A/HK/8/68 (H3N2) and A/Swan/Hokkaido/51/96 (H5N3), with EC50 values of 0.43 ± 0.11 and 0.04 ± 0.02 μM, respectively. Those EC50 values suggested that the anti-H3N2 and anti-H5N3 activities of PEG20k–LCVN were 5,000 or more times higher than that of Ribavirin, whose EC50 values for the 2 virus strains were 2.88 ± 0.66 and 1.79 ± 0.62 × 103 µM, respectively. Accordingly, the SIs of PEG20k–LCVN to the H3N2 and H5N3 strains were dramatically higher than that of CVN, LCVN and Ribavirin (Table I).
To further visibly confirm the activity of CVN and its derivatives, the morphology of cells infected by a representative IAV strain, A/HK/8/68 (H3N2), and treated with CVN, LCVN and PEG20k–LCVN was observed by immunofluorescence microscopy (Fig. 3). Cells of the virus group exhibited significant alteration compared with the non-infected cells. The infected cells became rounder and larger, and then cell fusion or poly-nuclear cells appeared. Most of the infected cells died off from the culture plate. Contrarily, there was a dose-dependent recovery of the cytopathic effects in PEG20k–LCVN treated cells. The higher the dose of PEG20k–LCVN, the more cells survived in the 0.125- to 0.5-μM PEG20k–LCVN treated groups. This morphological data strengthened the in vitro anti-IAV activity of PEG20k–LCVN and suggested that the activity was even more potent than Ribavirin.
Fig. 3.
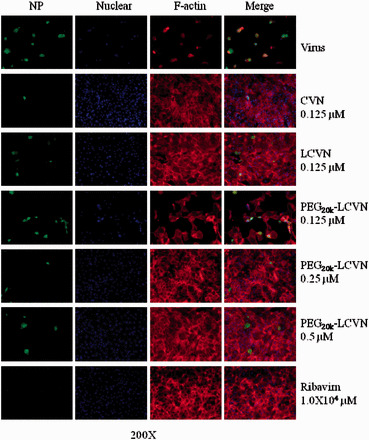
Immunofluorescence of the protective effects of PEG20k–LCVN on MDCK cells from infection by IAV. Monolayer MDCK cells were infected with a mixture of 100 TCID50 A/HK/8/68 (H3N2) and PEG20k–LCVN. Ribavirin (5.0 × 103 µM), CVN and LCVN were set as control. NP of influenza virus was visualized in green by mouse anti-NP McAb under an immunofluorescence microscope. The nuclear material was stained blue by DAPI. F-actin was stained by phalloidin in red. Pictures were taken randomly from one representative experiment of those performed in triplicate at ×200 magnified microscopy.
Anti-IAV activity of PEG20k–LCVN in chicken embryos
Because chicken embryos are living, low cost, integral individuals that can be easily cultured, they are one of the best hosts for influenza virus propagation. The anti-influenza activities of CVN, LCVN and PEG20k–LCVN were assayed by detecting the haemagglutination titre of the allantoic liquid after the chicken embryo was infected by the virus (Table II). We found that 2.5-µM CVN completely inhibited IAV replication in chicken embryos. Simultaneously, 2.5-µM LCVN significantly attenuated virus proliferation. Moreover, 100-µM PEG20k–LCVN completely suppressed the propagation of the virus in chicken eggs. Each egg injected with this dose of PEG20k–LCVN did not exhibit any RBC haemagglutination, and the haemagglutination titre was almost as zero. About 12.5-µM PEG20k–LCVN also strongly inhibited virus replication (P < 0.05).
Table II.
Three proteins inhibited the propagation of influenza virus in chicken embryos by haemagglutination assay ( ± s).
| Groups | n | Concentration (µM) | Haemagglutination titre | P value | |
|---|---|---|---|---|---|
| Mock | 5 | 0 ± 0 | |||
|
| |||||
| Virus | 5 | 2.9868 ± 0.1649 | |||
|
| |||||
| CVN | 5 | 5.0 | 0 ± 0 | ||
| 5 | 2.5 | 0 ± 0 | |||
| 5 | 1.0 | 3.1072 ± 0 | |||
|
| |||||
| LCVN | 4 | 5.0 | 1.4299 ± 0.2882 | 0.0175* | |
| 4 | 2.5 | 2.4288 ± 0.1526 | 0.0175* | ||
| 5 | 1.0 | 2.8664 ± 0.2519 | 0.4884 | ||
|
| |||||
| PEG20k–LCVN | 5 | 100.0 | 0 ± 0 | ||
| 5 | 50.0 | 1.4817 ± 0.3432 | 0.0109* | ||
| 5 | 25.0 | 1.9633 ± 0.5790 | 0.0052** | ||
| 5 | 12.5 | 2.5125 ± 0.2000 | 0.0109* | ||
|
| |||||
| Ribavirin | 5 | 2.0 × 104 | 0.5419 ± 0.1256 | 0.0109* | |
*P < 0.05, **P < 0.01 compared with virus group by Mann–Whitney test.
Anti-IAV activity of PEG20k–LCVN in mice
The remarkable bioactivity of PEG20k–LCVN in vitro and in embryo encouraged us to assess its anti-IAV activity in animal models. The death protection activity of PEG20k–LCVN on H3N2-adapted strain-infected mice was shown in Fig. 4A. The mouse survival rates in 0.2, 1.0 or 2.0 mg/kg/day PEG20k–LCVN groups were 16.7%, 50.0% and 66.7% respectively. 2.0 mg/kg/day PEG20k–LCVN treatment significantly increased the mice survival rate than virus control (16.7%, P = 0.0378, log-rank [Mantel–Cox] test). The median survival times of mice treated with three doses of PEG20k–LCVN were 7.0, 11.0 and >14.0 days, respectively, much longer than that of the viral control group (5.5 days).
Fig. 4.
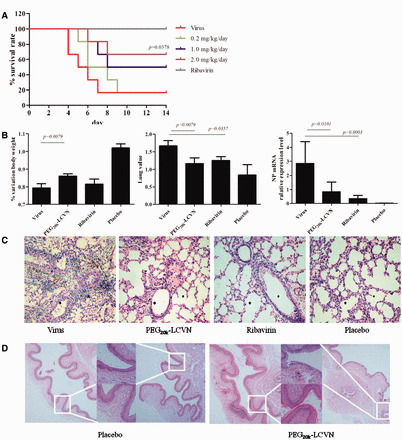
In vivo anti-IAV activity and safety of PEG20k–LCVN. (A) The survival curves of mice infected with H3N2 and treated with PEG20k–LCVN and Ribavirin. Three dose groups of PEG20k–LCVN were tested and one dose group of Ribavirin was set as positive control. (B) The bodyweight (left), lung values (middle) and NP gene expression (right) of mice. Mice treated with 2 mg/kg/day PEG20k–LCVN, 75 mg/kg/day Ribavirin or PBS (placebo) were sacrificed on Day 3 post infection (n = 5). NP mRNA level in mice lung was quantified by qPCR. (C) Pathological observations of mice lung stained by H&E, ×200. (D) Mucosa toxicity of PEG20k–LCVN in vagina of SD rat. PEG20k–LCVN in PBS was delivered into rat vagina once per day with the dose of 5.0 mg/kg for 7 days to evaluate its stimulation toxicity (H&E staining, 100 µM of each square).
The body weight variations of mice post 3 days infection were observed among the PEG20k–LCVN, Ribavirin and placebo groups (left panel in Fig. 4B). Mice body weight decreased sharply after the virus infection. The body weight of 2.0 mg/kg/day PEG20k–LCVN treated mice exhibited similar weight loss trend, but it was also significantly higher than that of the virus control group (P = 0.0079). In virus control group, the lung index increased from 0.84 ± 0.31 of the placebo group to 1.67 ± 0.15 (middle panel in Fig. 4B). For mice treated with PEG20k–LCVN, the lung index decreased to 1.17 ± 0.16. Thus, it was concluded that PEG20k–LCVN treatment dramatically decreased the lung index. To further explore the anti-viral activity of PEG20k–LCVN, mRNA levels of NP of the virus in mice lung were analysed by real-time PCR (right panel in Fig. 4B). PEG20k–LCVN treatment significantly downregulated NP level in mice lung (P = 0.0101). HE staining demonstrated that there was severe inflammatory infiltration in the lung of the virus-infected mice, but PEG20k–LCVN treatment effectively reduced the inflammatory accumulation (Fig. 4C).
Mucosa stimulate toxicity of PEG20k–LCVN
One of the major concerns of CVN is its safety in mucosa as this microbicide might be topical applied in vagina. Further safety evaluation of PEG20k–LCVN in rat demonstrated that as high as 5 mg/kg administration of PEG20k–LCVN in rat vagina did not result in swelling or other abnormal morphology in HE staining, as well as any pathological alteration such as hyperaemia, mucosa injury and inflammation (Fig. 4D).
Discussion
CVN exhibited impressive anti-viral potential for pharmaceutical applications. Additionally, different processes for manufacturing the recombinant CVN have been reported. CVN or its fusion protein has been expressed in various hosts, including E. coli (7–9), Pichia pstoris (10), transgenic Nicotiana tabacum (11) and transgenic lactic acid bacteria cultured in yogurt (12). Escherichia coli is the most commercialized and mature expression host due to its robust culture process, low cost and high efficiency. In this article, we constructed a plasmid containing a his6-sumo-linker-cvn fusion gene to prepare recombinant LCVN from an E. coli. SUMO–LCVN fusion protein was expressed in the cytoplasm in a soluble form with the help of the chaperon bioactivity of SUMO. Furthermore, a hexahistidine was tagged at the N-terminal of SUMO so that the SUMO–LCVN could be rapidly purified from the total protein extract by simply running it through a Ni-NTA column. After digestion with SUMO protease, the released LCVN could be subsequently purified to over 95% purity using the same column. Although SUMO–CVN had been successfully and efficiently expressed in E. coli, we found that the digestion efficiency of SUMO protease on the SUMO–CVN fusion was significantly lower than that of SUMO–LCVN during the purification of recombinant CVN or LCVN (data not shown).
Several strategies have emerged to improve pharmacokinetic and pharmacodynamic properties of biologic candidates, such as mutation of the amino acid sequence, fusion or conjugation to serum proteins and natural or synthetic polymers. PEG is a deeply investigated polymer for the covalent modification of biological macromolecules for pharmaceutical and biotechnical applications. More than 10 PEGylated biologics have been approved since 1990, including cytokines, growth factors, enzymes and antibody fragments, such as PEG-adenosine Deaminase, PEG-asparaginase, PEG-interferon-α2 b, PEG-interferon-αa, PEG-human growth hormone mutein antagonist, PEG-G-CSF, PEG-anti-VEGF aptamer, PEG-erytropoietin, PEG-anti-TNF Fab’ and PEG-uricase.
PEGylation is an alternative method applied to overcome the deficiencies in toxicity, pharmacodynamics and distribution of biological molecules by attaching PEG chains to polypeptides or other lead molecules (13, 14). This technique has become the leading approach for overcoming most of the aforementioned limits of the biologics. The benefits of PEGylation (i.e. the covalent attachment of PEG) include (i) shielding of antigenic and immunogenic epitopes, (ii) shielding receptor-mediated uptake by the reticuloendothelial system, (iii) preventing recognition and degradation by proteolytic enzymes and (iv) increasing the apparent size of the polypeptide, thus reducing the renal filtration and altering biodistribution. Nevertheless, a reduction in biological potency usually happened after PEGylation due to the steric entanglement of polymer chains during the protein/receptor recognition process (15). For example, directly conjugating of PEG to CVN at random or N-terminal lysine residues, or at a side group by site-specific method usually resulted in derivatives with low biological activity (16). In this study, a polypeptide linker inserted between PEG and CVN was successfully constructed. The 15-amino acid polypeptides linker separated the PEG group, and CVN domain posed little disturbance both on the bioactive domains of CVN and PEG. Meanwhile, the linker peptide facilitated both the independent folding of the SUMO and CVN subunits in the cytoplasm of E. coli and in the downstream purification process of LCVN (17). Direct fusion of functional subunits without a linker may lead to many undesirable outcomes including misfolding of the fusion proteins (18), low yield in protein production (19) or impaired bioactivity (20, 21). Usually, PEG chemical conjugation step does not have good production efficiency. It may have something to buffer pH, molar ratio or reaction time. In this job, to investigate high efficiency of PEGyaltion, the abovementioned conditions were performed, such as acetate buffer pH, molar ratios of LCVN to 20 kDa mPEG-ALD or reaction time. The buffer pH and molar ratio were vital factors for LCVN’s monoPEGylation, but reaction time did not affect PEGylation efficiency (Fig. 1S). The efficiency of LCVN's PEGylation up to 61.11 ± 4.69%, while in acetate buffer pH 5.0 and at 1:1 molar ratio of LCVN and 20 kDa mPEG-ALD for reaction of 2–3 h.
IAV is the major pathogen for most cases of epidemic influenza and has thus attracted public attention. Influenza vaccines are ∼70% effective in preventing infection and reducing morbidity and mortality (22, 23). It is currently the most effective approach for influenza pandemic prophylaxis and control. However, vaccines take more than 6 months to become effective. For example, after the swine-human virus unexpectedly broke out in the 2009 H1N1 pandemic, manufacturers made their best efforts to produce the vaccine, and it went largely to waste because it was available far too late (24). In addition, the safety of egg-based vaccines is drawing more concerns from researchers and the general public. Furthermore, some vaccines, such as the standard trivalent influenza vaccine, have relatively poor immunogenicity in elders greater than 65 years old, which is the fastest growing segment of the population. Furthermore, anti-IAV agents are indispensable means to efficiently prevent an influenza pandemic. Currently, there are only five drugs licensed worldwide, including the NA inhibitors Oseltamivir, Zanamivir and Peramivir, as well as the M2 ion-channel inhibitors Amantadine and Rimantadine. NA and M2 ion-channel inhibitors played a key role in controlling the spread of influenza virus in the past. However, an increasing number of IAV mutants resistant to NA inhibitors and/or M2 ion-channel inhibitors have been increasingly reported in recent years. The drug resistance of the IAV is a huge challenge in the section of anti-IAV drug discovery and development. Therefore, developing anti-IAV agents with novel targets and/or mechanisms is critically required for combating the seasonal or emerging influenza pandemic.
Novel anti-IAV agents are also crucial for the short-term control of pandemics. Derived from CVN, PEG20k–LCVN presented its potent binding to influenza HA protein at low concentration. At the aspect of IAV-infected MDCK, PEG20k–LCVN initially exhibited 100-times lower cytotoxicity than native CVNs, and the SI value improved significantly. In chicken embryos, PEG20k–LCVN completely inhibited A/HK/8/68 (H3N2) virus propagation in μM concentration. But PEG20k–LCVN was likely insensitive to two strains of IAV, A/PR/8/34 (H1N1) and A/WSN/33 (H1N1). In other hand, CVN was demonstrated to have potent activity for inhibiting most strains of influenza A and B viruses in vitro and in vivo, but it was also not sensitive to the two strains (3, 4). It was reported that the antiviral activity of CVN mostly depended on its oligosaccharide-binding characteristics. CVN mainly bound to high-mannose N-glycans without recognizing other oligosaccharides, including oligo-mannose, complex N-glycans and oligosaccharides with a pentasaccharide core (25). We considered that the resistance of IAV H1N1 to PEG20k–LCVN might result from the lack of the special glyco-moieties in influenza HA proteins.
A/HK/8/68 (H3N2) was firstly isolated from human body, its virulence decreased with the passage in laboratory and did not induce mice death. To further investigate the anti-IAV activity of PEG20k–LCVN in mice, we constructed a lung-adapted strain from A/HK/8/68 (H3N2). After passaged 11 times, this mouse lung adapted strain shown 83.33% mortality. Mice treated by 1.0 and 2.0 mg/kg/day PEG20k–LCVN before the virus infection 1 h dramatically increased the survival rate, prolonged survival time. Meanwhile, 2 mg/kg/day PEG20k–LCVN inhibited virus replication and attenuated inflammation factor infiltration in lung. Another toxicity experiment in SD rat implicated that high dose of PEG20k–LCVN administrated did not show mucosa stimulation toxicity.
In summary, a new 20-kDa PEGylated CVN derivative, without mutation of the primary CVN sequence, was constructed. PEG20k–LCVN exhibited potent and selective anti-IAV bioactivity in nM concentrations in vitro, as well as dramatically attenuated cytotoxicity. Furthermore, the anti-IAV activity of PEG20k–LCVN was well documented in this article. The potent bioactivity in vitro and in vivo, low toxicity, feasibility of large-scale manufacturing of LCVN and high efficiency of PEGylation suggested its potential for an anti-IAV application.
Funding
This work was supported by grants from the ‘National Project for Significant New Drugs Development’ of the Ministry of Science and Technology of China (2012ZX09103‐301‐033, 2012ZX09202301‐001), the Central University Scientific Research Funding of Jinan University (11611206) and the National Natural Science Foundation of China (30873082) to X.S.
Conflict of Interest
None declared.
Supplementary Material
Glossary
Abbreviations
- BSA
bovine serum albumin
- CC50
50% cytotoxic concentrations
- CVN
Cyanovirin-N
- EC50
50% effective concentrations
- EID50
50% egg infective dose
- ELISA
enzyme-linked immunosorbent assay
- HA
haemagglutinin
- HI
haemagglutination inhibition
- IAV
influenza A virus
- LCVN
linkered Cyanovirin-N
- MDCK
Madin–Darby canine kidney
- MEM
minimum essential medium
- MTT
(3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide
- NA
neuraminidase
- OD
optical density
- PEG-ALD
polyethylene glycol-propionaldehyde
- PEG20k–LCVN
20 kDa PEGylated linkered Cyanovirin-N
- RBC
red blood cell
- SD
standard deviation
- SI
select index
- SUMO
small ubiquitin-related modifier
- WT
wild type
References
- 1.Reid AH. Taubenberger JK. Fanning TG. The 1918 Spanish influenza: integrating history and biology. Microbes Infect. 2001;3:81–87. doi: 10.1016/s1286-4579(00)01351-4. [DOI] [PubMed] [Google Scholar]
- 2.Uyeki TM. Human infection with highly pathogenic avian influenza A (H5N1) virus: review of clinical issues. Clin. Infect. Dis. 2009;49:279–290. doi: 10.1086/600035. [DOI] [PubMed] [Google Scholar]
- 3.O'Keefe BR. Smee DF. Turpin JA. Saucedo CJ. Gustafson KR. Mori T. Blakeslee D. Buckheit R. Boyd MR. Potent anti-influenza activity of cyanovirin-N and interactions with viral hemagglutinin. Antimicrob. Agents Chemother. 2003;47:2518–2525. doi: 10.1128/AAC.47.8.2518-2525.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Smee DF. Bailey KW. Wong MH. O'Keefe BR. Gustafson KR. Mishin VP. Gubareva LV. Treatment of influenza A (H1N1) virus infections in mice and ferrets with cyanovirin-N. Antiviral Res. 2008;80:266–271. doi: 10.1016/j.antiviral.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao X. Chen W. Guo C. Qian C. Liu G. Ge F. Huang Y. Kitazato K. Wang Y. Xiong S. Soluble cytoplasmic expression, rapid purification, and characterization of cyanovirin-N as a His-SUMO fusion. Appl. Microbiol. Biotechnol. 2010;85:1051–1060. doi: 10.1007/s00253-009-2078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng HY. Lin CC. Lin TC. Antiherpes simplex virus type 2 activity of casuarinin from the bark of Terminalia arjuna Linn. Antiviral Res. 2002;55:447–455. doi: 10.1016/s0166-3542(02)00077-3. [DOI] [PubMed] [Google Scholar]
- 7.Boyd MR. Gustafson KR. McMahon JB. Shoemaker RH. O'Keefe BR. Mori T. Gulakowski RJ. Wu L. Rivera MI. Laurencot CM. Currens MJ. Cardellina JH., 2nd Buckheit RW., Jr. Nara PL. Pannell LK. Sowder RC., 2nd Henderson LE. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: potential applications to microbicide development. Antimicrob. Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colleluori DM. Tien D. Kang F. Pagliei T. Kuss R. McCormick T. Watson K. McFadden K. Chaiken I. Buckheit RW., Jr. Romano JW. Expression, purification, and characterization of recombinant cyanovirin-N for vaginal anti-HIV microbicide development. Protein Expr. Purif. 2005;39:229–236. doi: 10.1016/j.pep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 9.Mori T. Gustafson KR. Pannell LK. Shoemaker RH. Wu L. McMahon JB. Boyd MR. Recombinant production of cyanovirin-N, a potent human immunodeficiency virus-inactivating protein derived from a cultured cyanobacterium. Protein Expr. Purif. 1998;12:151–158. doi: 10.1006/prep.1997.0838. [DOI] [PubMed] [Google Scholar]
- 10.Mori T. Barrientos LG. Han Z. Gronenborn AM. Turpin JA. Boyd MR. Functional homologs of cyanovirin-N amenable to mass production in prokaryotic and eukaryotic hosts. Protein Expr. Purif. 2002;26:42–49. doi: 10.1016/s1046-5928(02)00513-2. [DOI] [PubMed] [Google Scholar]
- 11.Sexton A. Drake PM. Mahmood N. Harman SJ. Shattock RJ. Ma JK. Transgenic plant production of Cyanovirin-N, an HIV microbicide. FASEB J. 2006;20:356–358. doi: 10.1096/fj.05-4742fje. [DOI] [PubMed] [Google Scholar]
- 12.Li M. Patton DL. Cosgrove-Sweeney Y. Ratner D. Rohan LC. Cole AM. Tarwater PM. Gupta P. Ramratnam B. Incorporation of the HIV-1 microbicide cyanovirin-N in a food product. J. Acquir. Immune Defic. Syndr. 2011;58:379–384. doi: 10.1097/QAI.0b013e31823643fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roberts MJ. Bentley MD. Harris JM. Chemistry for peptide and protein PEGylation. Adv. Drug Deliv. Rev. 2002;54:459–476. doi: 10.1016/s0169-409x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- 14.Harris JM. Chess RB. Effect of pegylation on pharmaceuticals. Nat. Rev. Drug Discov. 2003;2:214–221. doi: 10.1038/nrd1033. [DOI] [PubMed] [Google Scholar]
- 15.Pasut G. Veronese FM. State of the art in PEGylation: the great versatility achieved after forty years of research. J. Control. Release. 2012;161:461–472. doi: 10.1016/j.jconrel.2011.10.037. [DOI] [PubMed] [Google Scholar]
- 16.Bossard MJ. Zappe H. Snell ME. PEGylation of cyanovirin-N, an entry inhibitor of HIV. Adv. Drug Deliver. Rev. 2008;60:79–87. doi: 10.1016/j.addr.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Chen X. Zaro JL. Shen WC. Fusion protein linkers: property, design and functionality. Adv. Drug Deliv. Rev. 2013;65:1357–1369. doi: 10.1016/j.addr.2012.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao HL. Yao XQ. Xue C. Wang Y. Xiong XH. Liu ZM. Increasing the homogeneity, stability and activity of human serum albumin and interferon-alpha2b fusion protein by linker engineering. Protein Expr. Purif. 2008;61:73–77. doi: 10.1016/j.pep.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Amet N. Lee HF. Shen WC. Insertion of the designed helical linker led to increased expression of tf-based fusion proteins. Pharm. Res. 2009;26:523–528. doi: 10.1007/s11095-008-9767-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bai Y. Ann DK. Shen WC. Recombinant granulocyte colony-stimulating factor-transferrin fusion protein as an oral myelopoietic agent. Proc. Natl Acad. Sci. U S A. 2005;102:7292–7296. doi: 10.1073/pnas.0500062102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai Y. Shen WC. Improving the oral efficacy of recombinant granulocyte colony-stimulating factor and transferrin fusion protein by spacer optimization. Pharm. Res. 2006;23:2116–2121. doi: 10.1007/s11095-006-9059-5. [DOI] [PubMed] [Google Scholar]
- 22.Monto AS. Ohmit SE. Petrie JG. Johnson E. Truscon R. Teich E. Rotthoff J. Boulton M. Victor JC. Comparative efficacy of inactivated and live attenuated influenza vaccines. N. Engl. J. Med. 2009;361:1260–1267. doi: 10.1056/NEJMoa0808652. [DOI] [PubMed] [Google Scholar]
- 23.Fiore AE. Uyeki TM. Broder K. Finelli L. Euler GL. Singleton JA. Iskander JK. Wortley PM. Shay DK. Bresee JS. Cox NJ Centers for Disease Control and Prevention (CDC) Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm. Rep. 2010;59:1–62. [PubMed] [Google Scholar]
- 24.Shaw A. New technologies for new influenza vaccines. Vaccine. 2012;30:4927–4933. doi: 10.1016/j.vaccine.2012.04.095. [DOI] [PubMed] [Google Scholar]
- 25.Chen J. Huang D. Chen W. Guo C. Wei B. Wu C. Peng Z. Fan J. Hou Z. Fang Y. Wang Y. Kitazato K. Yu G. Zou C. Qian C. Xiong S. Linker-extended native cyanovirin-N facilitates PEGylation and potently inhibits HIV-1 by targeting the glycan ligand. PLoS One. 2014;9:e86455. doi: 10.1371/journal.pone.0086455. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


