Abstract
The mandible is a critical structure of the lower facial skeleton which plays an important role in several vital functions. Segmental resection of the mandible is at times required in patients with advanced oral cavity malignancies, primary mandibular tumors, and radiation or medication induced osteonecrosis. Mandibulectomy can significantly decrease quality of life, and thus mandibular reconstruction is an important aspect of the operative plan. Mandibular reconstruction is challenging due to the complex three‐dimensional anatomy of the mandible, and the precision required to restore dental occlusion in dentate patients. Significant advances have been made over the past decade in the ability to reconstruct and rehabilitate patients after a segmental mandibulectomy. This review will highlight these advances and discuss the timing of dental implantation.
Keywords: 3D modeling, CAMR, fibular free flap, mandibulectomy

1. INTRODUCTION
The mandible is a critical structure of the lower facial skeleton which plays an important role in several vital functions, including feeding, speech production and breathing. Segmental resection of the mandible is at times required to ensure en bloc tumor removal in patients with advanced oral cavity malignancies. It is less commonly performed for primary tumors of the mandible or for osteonecrosis related to radiation therapy or bisphosphonates. Mandibulectomy is a procedure which can have lasting physical and psychological effects with a significant decrease in quality of life.1, 2, 3, 4, 5 Mandibular reconstruction is warranted to restore airway patency, improve speech articulation, re‐establish the lower facial contour, and restore adequate oral intake. The latter is particularly challenging given the complex three‐dimensional anatomy of the mandible. In dentate patients, reconstruction is made more challenging by the precision required to restore functional dental occlusion.6, 7, 8 Significant advances have been made over the past decade in the ability to reconstruct and rehabilitate patients after a segmental mandibulectomy. This includes composite microvascular free tissue transfer, as well as dental restoration. This review will highlight these advances, evaluate their efficacy, and discuss the timing of dental implantation.
2. HISTORY OF MANDIBULAR RECONSTRUCTION
In the first half of the twentieth century, ablative surgery for advanced oral cavity cancers requiring mandibulectomy frequently resulted in the so‐called “Andy Gump deformity.” This was a euphemism on a 1917 popular comic strip character known for his retrognathic jaw line, and refers to an anterior mandibular defect which creates the appearance of an absent chin and lower lip.9 Aside from the cosmetic implications of this deformity, anterior mandibular defects carry significant morbidity by limiting the recovery of oral feeding and intelligible speech postoperatively (Figure 1).10, 11
FIGURE 1.

This lateral, A, and AP, B, view of a patient with a “Gump” deformity demonstrates the cosmetic and functional disability
Initial attempts at mandibular reconstruction focused on promoting osteogenesis between the remaining mandibular fragments to restore the mandibular contour. This technique was associated with a high incidence of infection and fistula formation and was ultimately abandoned.12 Cortical autologous bone grafts, known for their inherent strength and slower rate of resorption were attempted with the same results.13, 14 A variety of wiring and plating techniques with local or regional soft tissue flaps were then developed and employed to secure avascular cortical bone grafts from various sites to the native mandible.15, 16, 17 While successful in small resections in larger or anterior mandibular defects, bone resorption or plate extrusion remained high (Figure 2).10
FIGURE 2.

This patient has an anterior mandibular defect reconstructed with cancellous bone and a plate. The cancellous bone has extruded and he demonstrates an exposed plate
The latter half of the twentieth century saw the advent of vascularized bony flaps, Initial reconstructive attempts described the use of pedicled osteomyocutaneous flaps.10, 18, 19, 20 While these lowered the rates of reconstructive failure by allowing faster bony union, they were imperfect three‐dimensional structures with limited reach and rotation.10
With the advent of microvascular surgery in the 1980s, free tissue transfer was quickly adapted to oral cavity reconstruction.21, 22, 23, 24, 25, 26 In 1989, David Hidalgo introduced the fibular free flap as a viable donor site for bone replacement.27 This swiftly became a popular donor site due to its ample length, low donor site morbidity, and distance from the head and neck, which allows for the use of two surgical teams.27, 28, 29
3. EVOLUTION OF THE BONY FREE FLAP
Today, rigid titanium plate fixation in conjunction with an osteomyocutaneous free tissue transfer continues to be the preferred method of mandibular rehabilitation, with overall success rates greater than 90%.10, 30, 31 The past decade has seen continued advances to this reconstructive option, which aim to increase surgical precision, improve cosmesis and function, decrease complication rates, and allow for complete dental rehabilitation.
The fibular free flap is the workhorse for large, anterior, or three‐dimensionally complex defects.10, 29, 30 It has excellent vascular supply along its length due to periosteal perforators arising from the peroneal vessels, which allows for multiple osteotomies without compromising vascular integrity. It can supply up to 25 cm of bone stock, which is adequate for dental implantation (Figure 3). Lastly, it has acceptable donor site morbidity.10, 29, 30 Its limitations include peripheral vascular disease and large intraoral soft tissue defects, the latter of which can be resolved by the use of a second soft tissue‐only flap reconstruction (Table 1).32
FIGURE 3.

The fibula osteocutaneous free flap offers supple cutaneous tissue with a long piece of bone and a consistent vascular pedicle
TABLE 1.
The anatomic characteristics of the various bony free flaps used in head and neck reconstruction are described
| Artery | Artery diameter | Vein(s) | Pedicle length | Nerve | Bone | |
|---|---|---|---|---|---|---|
| Fibular osteocutaneous flap | Peroneal artery | 1.5‐4 mm | Paired peroneal veins (may converge into one) | Up to 8 cm | Lateral sural cutaneous nerve |
Fibula Up to 25 cm length |
| Radial forearm osteocutaneous flap | Radial artery | 2‐2.5 mm | Paired venae comitantes (may converge into one) and/or cephalic vein | Up to 20 cm | Lateral antebrachial cutaneous nerve |
Radius Up to 12 cm length |
| Scapular osteocutaneous flap | Circumflex scapular artery (may be followed to subscapular artery) |
4 mm (6 mm if followed to subscapular artery) |
Paired venae comitantes |
7‐10 cm (11‐14 cm if followed to subscapular vessels) |
— (dorsal cutaneous rami of T1 and T2 experimental) |
Lateral border of scapula Up to 14 cm length |
| Iliac crest osteocutaneous flap | Deep circumflex iliac artery | 2‐3 mm | Deep circumflex iliac vein | Up to 6 cm | — |
Iliac crest Up to 16 cm length |
The scapular osteomyocutaneous free flap provides less implantable bone (only about 10‐12 cm of bone), but has the advantage of several large, reliable associated soft tissue harvest options, which can be used to reconstruct both extra‐ and intraoral defects. It has low associated donor site morbidity, allowing for preserved shoulder function.33 Short segments of the scapula have the potential for dental implantation.34 Most authors are comfortable with only a single osteotomy, however, which limits the three‐dimensional configuration and length of reconstruction. Some authors have utilized the scapula with more than 2 segments for anterior mandibular reconstruction. In our hands, the scapular free flap is thus reserved for defects less than 10 cm in length or where copious soft tissue is needed for reconstruction (Figure 4).29 Additionally, due to its anatomical proximity to the ablative site, it cannot be harvested using two teams. Thus, reconstruction cannot be undertaken until the ablative procedure has been finished.35
FIGURE 4.
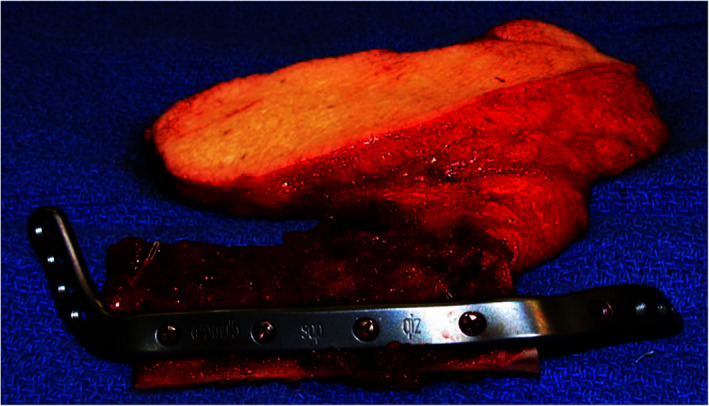
The scapular osteocutaneous free flap provides a nice bony cutaneous segment for reconstruction
The radial forearm osteocutaneous free flap remains an option when reconstructing small volume defects with large intraoral mucosal components. It has a considerable amount of well vascularized, thin skin which can be used to line the oral cavity. It is limited in that only 8‐10 cm of bone can be harvested and a single osteotomy is potentially available (Figure 5). The bone is thin and cannot accommodate implantation, thus complete dental rehabilitation is not possible unless additional bone grafting is undertaken.29, 32, 36 It has been found, however, to have similar functional outcomes to the fibular free flap,32, 37 and remains a good alternative for short defects, particularly in edentulous patients or patients with peripheral vascular disease.
FIGURE 5.

The radial forearm osteocutaneous free flap provides soft tissue and a small segment of bone for reconstruction
The iliac crest free flap was one of the first osteomyocutaneous flaps utilized for bony reconstruction. It provides a substantial amount of bone that is thick, available for osteotomy and takes implants readily. Historically the skin paddle has been suboptimal due to short perforators and copious adipose tissue that is not a good fit for reconstruction of composite tissue defects of the oral cavity. A modification utilizing the internal oblique muscle with a skin graft was used to overcome this shortcoming. Pedicle length is short compared to other bony osteocutaneous flaps. In experienced hands, this is an excellent reconstructive modality. It use has diminished as other osteocutaneous flaps have become more prevalent (Table 2).
TABLE 2.
The table contrasts the advantages and disadvantages of the various bony free flaps available for reconstruction
| Flap | Advantages | Disadvantages |
|---|---|---|
|
Fibular Osteocutaneous Flap |
Up to 25 cm of bone available Large caliber, long vascular pedicle Ideal bone height Multiple osteotomies possible May be used for total mandibular reconstruction Accepts endosseous dental implants Two team approach possible |
Usually requires skin graft Potential for vascular compromise of lower extremity Skin paddle fixed to bone Moderate donor site morbidity May be difficult to fit with dental prostheses (dentures) |
|
Radial forearm Osteocutaneous Flap |
Thin, pliable tissue paddle Large caliber, long vascular pedicle Consistent anatomy Versatile positioning in recipient site Supports dental prostheses (dentures) Two team approach possible |
Limited bone length and height Donor site visible Requires skin graft Potential for vascular compromise of hand Risk of radius fracture Plating of radius recommended |
|
Scapular Osteocutaneous Flap |
Large skin paddle available Skin paddle may be oriented independent of bone axis Versatile in volume Multiple osteotomies possible Primary closure usually possible Minimal atrophy Good color match |
Repositioning patient in operating room Limited bone height Bulky, thick skin Potential for long term shoulder dysfunction Low potential for transfer as sensate flap Two team approach difficult |
|
Iliac Crest Osteocutaneous Flap |
Large skin paddle available Contour similar to mandible Donor site easily hidden Primary closure usually possible Supports endosseous dental implants Two team approach |
Bulky skin paddle Short pedicle Not pliable in three dimensions Donor site morbidity—pain, hernia Requires abdominal wall reconstruction Technically challenging Small caliber, short vascular pedicle Poor color match |
Despite the excellent reported success rates of mandibular reconstruction using bony free tissue transfer, this technique does have inherent challenges. Creating sufficient bony contact between the transplanted bone and the native mandible is required for osseointegration of the transplanted segment. Additionally, ensuring plate to bone contact is necessary for proper stabilization of the bony transplant. The absence of either can lead to non‐ or malunion, plate destabilization and extrusion, chronic infection/orocutaneous fistulas, and bone exposure.38, 39 The shaping of the osseous free flap for mandibular reconstruction was historically performed based on an intraoperative hand‐made template of the mandibular arch. Reconstructive plates were bent intraoperatively using a combination of this template and the native mandible.40 Obtaining perfect bone to bone contact with wedge osteotomies performed in this manner is time consuming, of variable precision, and requires extensive experience. The same can be said for bending the fixation plate, which assumes a certain amount of knowledge of the load‐bearing forces at play. The advent of 3D printing and three‐dimensional computer modeling has helped diminish this variability and improve surgical outcomes, all while optimizing both form and function.40, 41
Three‐dimensional printing technology was first used in craniomaxillofacial surgery in a process known as contour modeling, in which 3D printed models of patient specific anatomy are made and used for bending of titanium plates preoperatively. This helps the surgeon more closely replicate the patient's normal mandibular contour by improving the accuracy of plate bending and guiding osteotomies for the transplanted bone to match the pre‐bent plates. This removes intraoperative challenges such as gaining enough exposure and removing adjacent soft tissue to accurately form the reconstruction plates on the native mandible, all while decreasing surgical time.42 In a study of 28 patients undergoing segmental mandibulectomy and simultaneous mandibular reconstruction, Azuma et al compared surgical and aesthetic outcomes of patients treated with conventional intraoperative plate bending vs pre‐bent reconstruction plates modeled based on patient specific 3D printed mandibular models. They found, via postoperative pantography, that patients in the 3D modeling group had improved mandibular contour and more symmetric mandibular angles when compared to the non‐affected side.43
One challenge with this method, as with conventional plate bending, is that the reconstruction plate follows the natural curvature of the native mandible. Given that the fibula is a straight bone, dead space is created between the plate and the bone, increasing the risk of plate exposure and extrusion (Figure 6). Further 3‐D modeling advancements have sought to resolve this and over the last decade, the technology has expanded to yield what we now term computer assisted mandibular reconstruction (CAMR). CAMR is a full‐service reconstructive planning technology that uses preoperative virtual surgical planning (VSP) to generate customized cutting jigs and reconstruction plates based on three‐dimensional computer models of both resection and reconstructive goals. CAMR begins with a VSP session, a web‐based conference attended by the ablative and reconstructive surgeons, prosthodontist, and biomedical engineers from a third‐party vendor. The group uses preoperative CT scans of the mandible and the lower extremities to discuss the proposed resection/osteotomies, laterality of the donor site, number of bony segments, plate location, number and location of screw holes, and shape/position of bony cutting guides. After the VSP session, rapid prototype modeling (RPM) is used to create highly accurate three‐dimensional polymer models of the resection specimen, planned reconstruction outcome, as well as precise cutting guides to assist with flap harvest.44, 45 Reconstruction plates are either pre‐bent based on these models, or in some cases custom milled titanium plates are designed which perfectly adapt to the transplanted bone (Figure 7).44
FIGURE 6.

These images demonstrate the results that one gets with bending the plate to the native mandible, A. Note the space between the plate and the gap in the osteotomies. This plate has been computer generated and fits the bone with no gap, B. The osteotomies are also perfect
FIGURE 7.

CAMR is used to model resection and free fibula mandibular reconstruction for a left anterior mandibular tumor. The defect is first determined and highlighted, A. Cutting guides are designed to aid with the mandibulectomy, B. The reconstruction is planned, ensuring a precise fit between the fibular graft and remaining mandible, C,D. A custom pre‐fabricated plate is designed, using appropriate angulation to fit the straight neo‐mandible and the curvature of the existing mandible, E. The fibular cuts are planned to precisely match the reconstructive model, and custom cutting guides are created to aid with the fibular osteotomies, F
Several groups have reported improved precision of mandibular reconstruction with the use of CAMR relative to conventional reconstructive techniques.41, 46, 47 Hanasono et al, in a study of 38 patients that underwent fibula free flap using computer‐assisted design between 2005 and 2011, calculated the cumulative mean difference of five bony landmarks between preoperative and postoperative imaging—the left gonion, left condyle, gnathion, right gonion, and right condyle. They found a significant decrease in this difference with the use of CAMR as opposed to conventional mandibular reconstruction.44 In a similar fashion, in a cohort of 50 patients, Weitz et al compared the mean difference in gonion angle and in the distance from the mandibular angle to the anterior nasal spine before and after mandibulectomy and reconstruction with either CAMR or conventional methods. They found a significant decrease in both of these values with the use of computer‐assisted modeling.41 Lastly, Pucci et al conducted a systematic review of studies comparing traditional free‐hand surgery and VSP computer‐based reconstructive techniques over a 10 year period. In 6 studies published between 2014 and 2018, CAMR/VSP was found to have significantly improved accuracy as measured by the change in intercondylar distance and gonion angle between pre‐ and postoperative CT scans.48 Furthermore, this improvement also held true when comparing CAMR with contour modeling. Chang et al, in a retrospective study of 92 patients who underwent osteocutaneous free flap mandibular reconstruction using either prefabricated stereolithic models and pre‐bent reconstruction plates or VSP/cutting guides, found that the use of the CAMR process resulted in fewer osteotomy revisions, and less bone burring of the neomandible and associated need for intraoperative bone grafting.49
While the overall early postoperative complication rates are similar between patients in whom CAMR is used as compared to conventional reconstruction,40, 41, 49, 50 there is a suggestion that the improved precision afforded by this technology could potentially help prevent late complications.41, 49 Weitz et al, in the aforementioned study, saw a higher rate of bony union in the CAMR group at both 1 week and 6 months after surgery, which allowed for less iliac bone grafting (performed commonly by their group in cases of non‐union).41 Chang et al found similar results on postoperative CT scans obtained at least one year after reconstruction.49 Furthermore, CAMR overwhelmingly led to a significant decrease in operative time across studies,40, 42, 48, 49, 50 which indirectly affects outcomes by decreasing perioperative morbidity and mortality. In a study published recently, Krane et al described the use of a single osteocutaneous fibular free flap to reconstruct a large defect involving the maxilla and mandible, limiting donor site morbidity while restoring masticatory function (Figure 8).51
FIGURE 8.

Note here how the fibular osteotomy is perfectly aligned and the plate is flush to the entire bone
Despite the many benefits of this technology, there are some limitations that merit discussion. VSP does not account for tumor or osteoradionecrosis progression between the planning session and the day of surgery (Table 3).52 One possible limitation is that the predicted bony cuts may not be adequate for tumor resection when the patient is asleep and the tumor is assessed. This occurs rarely as communication between the reconstructive and ablative surgeon during the planning session considers the bone to be resected and the placement of osteotomies in the reconstructed bone. In the occasional osteoradionecrosis or where doubts about being able to assess bony invasion preoperatively exist, one can design 2 or 3 case scenarios with different osteotomies. This entails multiple plates being made. In the senior authors hands of over 200 preoperative patient specific plates and 3D modeling this occurs less than 2% of the time. In cases where neural or bone marrow is involved or the bony segment to be excised is larger than planned, reconstruction is performed as it was prior to computer modeling being available. Another limitation is that modeling cannot adequately consider the soft tissue elements of reconstruction, including the exact location of the harvested skin paddle, which can be an issue when a planned osteotomy overlies a skin perforator. Lastly, VSP does come at an increased monetary cost, which must be weighed against the overall complexity of the reconstruction and the benefits the technology may or may not provide.41
TABLE 3.
The advantages and disadvantages of VSP are addressed here
| Ref. | Senior author | Institution | Cases | Objective measures | Operative time | Intraoperative improvement | Long‐term benefits | Intraoperative complications | Postoperative complications | Cost |
|---|---|---|---|---|---|---|---|---|---|---|
| 40 | Jamie Levine, MD | NYU Langone | 54 | Fibula segment number, operative time, dental implantation rates | Decreased with VSP | Increase in case complexity, including double‐barreling of flaps with VSP | Higher rates of dental implantation with VSP, improved denture fitting | Not discussed | No change with VSP | Less |
| 41 | MR Kesting | Technical University of Munich | 50 | Bony consolidation, change in mandibular angle, number of osteotomies, ischemic time, operative time | Decreased with VSP | Higher number of osteotomies and complexity of reconstruction with VSP | Less change in mandibular angle after VSP recon, improved consolidation of bony segments with VSP | Increased fracture of miniplates, which were used more commonly in VSP cases | No change with VSP | N/A |
| 44 | Matthew Hanasono, MD | University of Texas, MD Anderson | 38 | Position of 4 bony landmarks, postoperative length of fibula used, angles between fibula segments, operative time | Decreased with VSP | Not discussed | Higher accuracy with VSP (more accurate position of 4 bony landmarks compared to preop) | Need for longer fibular segment and revised osteotomies due to tumor progression | No change with VSP | N/A |
| 46 | Xudong Wang, DDS | Shanghai Jiao Tong School of Medicine | 22 | Deviation in fibular segment length, position of 4 bony landmarks, duration of ischemia time | Decreased with VSP | Decreased ischemia time, decreased time required for osteotomies | Improved precision as measured by postoperative position of 4 bony landmarks | None reported (benign disease only) | No change with VSP | N/A |
| 47 | Derek Steinbacher, DMD, MD | Yale University School of Medicine | 19 | Gap distances between fibular segments and between fibula and native mandible, symmetry as measured by mandibular body‐symphysis angles | Decreased with VSP | Smaller fibular segment gap distance with VSP | Trend toward increased symmetry of reconstruction with VSP | Not discussed | Not discussed | N/A |
| 49 | Eric Chang, MD | Fox Chase Cancer Center, PA | 92 |
Accuracy of osteotomies, operative time, postoperative bone gap size/non‐union rates |
Decreased with VSP | Less burring, fewer osteotomy revisions, less bone grating with VSP | Decreased bone gap size/non‐union rates with VSP | No change with VSP | No change with VSP | N/A |
| 50 | Neal Topham, MD | Fox Chase Cancer Center, PA | 57 | Cost, operative time, complication rates | Decreased with VSP | More accurate plate bending with exophytic lesions | Not discussed | No change with VSP | No change with VSP | Less |
4. DENTAL IMPLANTATION
Dental rehabilitation following segmental mandibulectomy plays a major role in facial aesthetics, resumption of oral diet, and overall quality of life.8, 11, 53 Conventional dentures often have a poor fit given the significant alterations in intraoral anatomy,54 leaving osseointegrated dental implants as the best option for dental restoration when a large segment of the mandible has been resected. Dental implantation in this setting can be a challenging process given the surgical precision required to restore functional occlusion,40 as well as the anatomic changes in both the bony and soft tissue components of the implant environment.8, 55, 56 Free osteocutaneous flaps have been shown to be the most appropriate recipient of dental implants after mandibulectomy, with the fibular graft carrying the lowest rate of bony resorption as compared to vascularized ilium, scapula, or radial forearm grafts.57, 58 Bone loss, peri‐implant infection, and mobile or hyperplastic implant‐adjacent soft tissue are common causes of failed dental implantation in the reconstructed population, occurring in up to 30% of patients within 10 years.8, 54, 55 Radiotherapy has been shown to reduce soft tissue and bony vascularization, lower infection resistance, and promote local inflammation, and is thought to exacerbate these factors, leading to increased rates of implant failure.54, 55, 59 For these reasons, implantation was historically performed at least 6 months after mandibular reconstruction, with the delay extended to 12 months if the patient underwent adjuvant radiotherapy.60, 61 As a result, rates of dental implant placement following mandibular reconstruction have historically been low, ranging in the literature from 15 to 45%, with even lower rates of successful implant‐associated denture placement.31, 55, 62, 63
Recent surgical and technological advances have allowed surgeons to overcome some of the above factors, which has led to improved outcomes and increasing confidence in performing earlier dental rehabilitation. First, several techniques have been employed to overcome the soft tissue deficiencies of the neo‐mandible, all with the goal of creating attached neo‐gingiva to promote implant success. One such technique is vestibuloplasty with thinning of the intraoral skin paddle and introduction of connective tissue, skin grafts, or both to the implant bed. Using this technique 2‐3 months prior to implantation, Pellegrino et al were able to improve both implant survival and success by approximately 20%.54 Kumar et al (2015) described the use of SD‐DGER (sub‐periosteal dissection with denture guided epithelial regeneration), which involves making a subperiosteal incision over the fibular graft, creating buccal and lingual flaps, stripping the bone of excess overlying fat, and laying down the flaps to create lingual and buccal vestibules. An implant is then placed, and immediately loaded with an interim denture to guide epithelial regeneration.56 In a study of 52 patients with healed fibular free flaps undergoing implantation, SD‐DGER was found to significantly increase the amount of oral lining attached to the neo‐mandible as compared to conventional techniques.8 It is thus far unclear if either of these methods is truly superior, given that all of these studies have limited power. It is clear that careful attention to the soft tissue envelope of the implant is paramount to implant success, particularly in radiated patients.
Increasing bone stock and vertical height is another consideration for promoting implant success, given unavoidable post‐implantation bone resorption and the impact of height mismatch between the native and neo‐mandible on the surrounding soft tissues. Several techniques have been attempted to accommodate for this, including supplementing the peri‐implant region with avascular bone grafts or bone substitutes, vertical distraction osteogenesis (VDO), and “double barreling” of fibular free flaps (DBF). VDO refers to the use of a distractor device to vertically advance a partial thickness segment of the mandible slowly over time, allowing for osteogenesis between the remaining mandible and this segment, and thus increasing bone stock and height.64 DBF is a technique which involves harvesting double the length of fibular graft needed to reconstruct a mandibular defect, halving it, and folding the two segments on top of one another to increase the vertical height.66 A systematic review evaluating several randomized controlled trials that used different methods, including avascular bone grafting, use of bone substitute, and VDO to increase bone stock in native atrophic mandibles found that VDO provided more vertical bone gain as compared to other methods, although without any statistically significant improvement in implant success rates.65 Wang et al applied this method to fibular grafts, and compared the results to DBF. In their study of 19 patients who received 51 implants and were followed over a course of 3 years, they found higher implant stability with the DBF method but no significant difference in bony resorption or implant success rates.67 These results were concordant with a similar study by Chang et al, which showed no significant differences in osseointegration between DBF and VDO and only a slight advantage in decreasing bony resorption if a mucosal graft was applied around the implant at the time of DBF reconstruction.58 Unfortunately, limited follow up and low patient numbers plague all of the available studies, which limits our ability to conclude which method of bony augmentation is superior. DBF has gained particular popularity in recent reports given its lower reported complication rate,58, 68 and given that it requires fewer surgical interventions and can be performed at the time of primary reconstruction, thus shortening the time to complete dental rehabilitation.
The timing of dental implantation following mandibular reconstruction has also evolved over the last decade, with increasing trends toward immediate implantation. This has the benefit of faster prosthetic rehabilitation, with associated positive impacts on emotional and social well‐being. It does carry a theoretical higher risk of implant failure due to imprecise implant positioning, tumor recurrence, or other post‐treatment complications.57, 69 Mizbah et al, in a retrospective study of 109 patients undergoing mandibular reconstruction with a fibular free flap and either immediate or delayed implant placement, found no difference in rates of implant loss between the two groups, but did show a higher number of non‐functional implants (implants with no loaded denture) in the immediate implantation group.69 This was overwhelmingly related to patient factors, such as tumor recurrence, unfavorable soft tissue profiles, and radiotherapy‐induced trismus, but speaks to the overall unpredictable response to treatment in oral cavity malignancies. This finding has been corroborated across the literature, particularly in malignant indications, with more nonfunctional implants reported in immediate dental implantation due to poor positioning or tumor recurrence.57
CAMR and virtual surgical planning are promising advances which can help overcome some of the challenges of immediate dental implantation, namely to eliminate non‐functional implants due to poor positioning and malocclusion. 3D modeling can be used preoperatively to plan the location of implant placement so as to ensure adequate occlusion of the overlying denture and avoid interference with reconstruction screws and plates as well as perforators to overlying soft tissue (Figure 9). The planning also provides surgeons with a visual assistance in performing more complex reconstructions, such as double barreling of the fibular free flap. In a study of 54 fibular based mandible reconstructions performed by the NYU Langone group between 2009 and 2012 with the use of VSP, Avraham et al reported a significantly higher rate of dental implantation as compared to conventional reconstructive methods (62.5% vs 22.2%). They also reported an overall higher rate over achieving functional dentition in this group as compared to literature‐reported rates, with 47% of the VSP patients receiving implant‐associated dentures. Lastly, they reported no cases of implant malposition, indicating that pre‐surgical planning was accurate and reliable.40 Okay et al, in a study of 28 patients who underwent mandibular reconstruction with a fibular free flap, with computer assisted immediate dental implantation of 116 implants, found an 85.4% rate of both osseointegration and of achieving functional dentition in their cohort,70 surpassing any previously reported rates in the literature.
FIGURE 9.

3D modeling is used to provide for dental implantation. An ameloblastoma of the anterior mandible is going to be resected, A. Planning demonstrates a 3 segment bony reconstruction, B. After a suitable healing time implants have been placed in each of the bony segments, C. A prosthesis has been made and fitted, D
While there are still unmodifiable risk factors of implant loss after immediate reconstruction despite the aforementioned advances (such as tumor recurrence or radiotherapy‐induced soft tissue alterations) immediate dental implantation has led to larger overall numbers of patients undergoing dental rehabilitation after segmental mandibulectomy.40, 69, 70 Studies show that one year after undergoing treatment for oral cancer, far fewer patients are motivated to be rehabilitated, due to both financial and psychological reasons.69 Thus, the cost of potential failure of immediate dental implantation must be carefully weighed against the risk of losing the opportunity for restoration altogether, especially in patients with benign indications for surgery or a long life‐expectancy, in whom dentition may provide a meaningful QOL benefit.
5. JAW IN A DAY/SINGLE STAGE MANDIBULAR AND DENTAL REHABILITATION
The considerable advances in both 3D modeling and dental implantation described above have allowed reconstructive surgeons to reach the theoretical pinnacle of mandibular reconstruction—the ability to restore both form and function immediately following ablative surgery. The technique was first described by the NYU Langone group in 2012, and involves using VSP and CAMR to plan, design, fabricate and deliver a comprehensive reconstruction of an ablative mandibular defect in one operation.71
The preoperative preparation is similar to the previously described VSP process, except particular attention is given to creating an ideal dental prosthesis and precisely restoring dental occlusion. The reconstruction is then planned in a “backwards” fashion, using dental occlusion to guide fibular osteotomies and plate bending. A 3D model of the resection and reconstruction is made, based on which cutting jigs, an occlusal splint, and a customized dental prosthesis are manufactured. The fibular cutting jigs are made to include drill guides for precise dental implant placement, while the mandibular cutting jigs and dental prosthesis are created to attach to an occlusal splint that preserves proper dental relationships intraoperatively.71, 72
The surgery begins with the ablative mandibulectomy and application of pre‐bent reconstruction plates to the remaining native mandible. The fibular free flap is then harvested, and the fibular cutting jig is secured to the fibula. Dental implants are then placed in the fibula using the provided drill guides, after which osteotomies are made and the customized dental prosthesis is mounted and secured to the dental implants. The vascular pedicle is then divided and the flap inset into jaw, taking particular care to ensure appropriate dental occlusion via maxillomandibular fixation. Once the desired occlusion is achieved, the fibula is secured to the pre‐bent reconstructive plate, and reconstruction is complete.71, 72
Several small series have been published demonstrating the feasibility of this method.71, 72, 73, 74 The series are limited to young patients with benign indications, all of whom had uneventful immediate postoperative recovery with good bony union, good restoration of facial contour and ideal occlusion. To date, there has been no meaningful information published regarding long‐term implant outcomes of this reconstruction, and the technique has not yet been adapted to malignant indications, or instances in which soft tissue reconstruction is also required. However, it is a promising step toward immediate complete rehabilitation after segmental mandibulectomy.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Lilly GL, Petrisor D, Wax MK. Mandibular rehabilitation: From the Andy Gump deformity to jaw‐in‐a‐day. Laryngoscope Investigative Otolaryngology. 2021;6(4):708–720. 10.1002/lio2.595
BIBLIOGRAPHY
- 1.Rigoni L, Bruhn RF, De Cicco R, Kanda JL, Matos LL. Quality of life impairment in patients with head and neck cancer and their caregivers: a comparative study. Braz J Otorhinolaryngol. 2016;82(6):680‐686. 10.1016/j.bjorl.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vartanian JG, Kowalski LP. Acceptance of major surgical procedures and quality of life among long‐term survivors of advanced head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135(4):376‐379. 10.1001/archoto.2009.5. [DOI] [PubMed] [Google Scholar]
- 3.Rogers SN, Lowe D, Fisher SE, Brown JS, Vaughan ED. Health‐related quality of life and clinical function after primary surgery for oral cancer. Br J Oral Maxillofac Surg. 2002;40(1):11‐18. 10.1054/bjom.2001.0706. [DOI] [PubMed] [Google Scholar]
- 4.Rogers SN. Quality of life perspectives in patients with oral cancer. Oral Oncol. 2010;46(6):445‐447. 10.1016/j.oraloncology.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Van Cann EM, Dom M, Koole R, Merkx MA, Stoelinga PJ. Health related quality of life after mandibular resection for oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2005;41(7):687‐693. 10.1016/j.oraloncology.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Smolka K, Kraehenbuehl M, Eggensperger N, et al. Fibula free flap reconstruction of the mandible in cancer patients: evaluation of a combined surgical and prosthodontic treatment concept. Oral Oncol. 2008;44(6):571‐581. 10.1016/j.oraloncology.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 7.Ch'ng S, Skoracki RJ, Selber JC, et al. Osseointegrated implant‐based dental rehabilitation in head and neck reconstruction patients. Head Neck. 2016;38(suppl 1):E321‐E327. 10.1002/hed.23993. [DOI] [PubMed] [Google Scholar]
- 8.Kumar VV, Ebenezer S, Kämmerer PW, et al. Implants in free fibula flap supporting dental rehabilitation – implant and peri‐implant related outcomes of a randomized clinical trial. J Craniomaxillofac Surg. 2016;44(11):1849‐1858. 10.1016/j.jcms.2016.08.023. [DOI] [PubMed] [Google Scholar]
- 9.Aziz SR. Andy Gump and his deformity. J Oral Maxillofac Surg. 2010;68(3):651‐653. 10.1016/j.joms.2009.03.044. [DOI] [PubMed] [Google Scholar]
- 10.Bak M, Jacobson AS, Buchbinder D, Urken ML. Contemporary reconstruction of the mandible. Oral Oncol. 2010;46(2):71‐76. 10.1016/j.oraloncology.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 11.Kansara S, Wang T, Koochakzadeh S, et al. Prognostic factors associated with achieving total oral diet following osteocutaneous microvascular free tissue transfer reconstruction of the oral cavity. Oral Oncol. 2019;98:1‐7. 10.1016/j.oraloncology.2019.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adamo AK, Szal RL. Timing, results, and complications of mandibular reconstructive surgery: report of 32 cases. J Oral Surg. 1979;37(10):755‐763. [PubMed] [Google Scholar]
- 13.Roberts TT, Rosenbaum AJ. Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing. Organogenesis. 2012;8(4):114‐124. 10.4161/org.23306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raghuram A, Singh A, Chang DK, Nunez M, Reece EM. Bone grafts, bone substitutes, and orthobiologics: applications in plastic surgery. Semin Plast Surg. 2019;33(3):190‐199. 10.1055/s-0039-1693020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Skolnik EM, Yee KF, Keyes GR. Flap reconstruction in major surgery of the head and neck. Laryngoscope. 1976;86(10):1584‐1593. 10.1288/00005537-197610000-00014. [DOI] [PubMed] [Google Scholar]
- 16.Rana M, Warraich R, Kokemüller H, et al. Reconstruction of mandibular defects – clinical retrospective research over a 10‐year period. Head Neck Oncol. 2011;3:23. 10.1186/1758-3284-3-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krause CJ. Reconstruction in head and neck cancer patients. J Iowa Med Soc. 1977;67(3):83‐87. [PubMed] [Google Scholar]
- 18.Conley J. Use of composite flaps containing bone for major repairs in the head and neck. Plast Reconstr Surg. 1972;49(5):522‐526. 10.1097/00006534-197205000-00008. [DOI] [PubMed] [Google Scholar]
- 19.Panje W, Cutting C. Trapezius osteomyocutaneous island flap for reconstruction of the anterior floor of the mouth and the mandible. Head Neck Surg. 1980;3(1):66‐71. 10.1002/hed.2890030112. [DOI] [PubMed] [Google Scholar]
- 20.Cuono CB, Ariyan S. Immediate reconstruction of a composite mandibular defect with a regional osteomusculocutaneous flap. Plast Reconstr Surg. 1980;65(4):477‐484. 10.1097/00006534-198004000-00012. [DOI] [PubMed] [Google Scholar]
- 21.MacLeod AM, Robinson DW. Reconstruction of defects involving the mandible and floor of mouth by free osteo‐cutaneous flaps derived from the foot. Br J Plast Surg. 1982;35(3):239‐246. 10.1016/s0007-1226(82)90108-4. [DOI] [PubMed] [Google Scholar]
- 22.Bell MS, Barron PT. A new method of oral reconstruction using a free composite foot flap. Ann Plast Surg. 1980;5(4):281‐287. 10.1097/00000637-198010000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Duncan MJ, Manktelow RT, Zuker RM, Rosen IB. Mandibular reconstruction in the radiated patient: the role of osteocutaneous free tissue transfers. Plast Reconstr Surg. 1985;76(6):829‐840. 10.1097/00006534-198512000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Taylor GI, Townsend P, Corlett R. Superiority of the deep circumflex iliac vessels as the supply for free groin flaps. Plast Reconstr Surg. 1979;64(5):595‐604. [PubMed] [Google Scholar]
- 25.Sanders R, Mayou BJ. A new vascularized bone graft transferred by microvascular anastomosis as a free flap. Br J Surg. 1979;66(11):787‐788. 10.1002/bjs.1800661111. [DOI] [PubMed] [Google Scholar]
- 26.Swartz WM, Banis JC, Newton ED, Ramasastry SS, Jones NF, Acland R. The osteocutaneous scapular flap for mandibular and maxillary reconstruction. Plast Reconstr Surg. 1986;77(4):530‐545. 10.1097/00006534-198604000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Hidalgo DA. Fibula free flap: a new method of mandible reconstruction. Plast Reconstr Surg. 1989;84(1):71‐79. [PubMed] [Google Scholar]
- 28.Mehta RP, Deschler DG. Mandibular reconstruction in 2004: an analysis of different techniques. Curr Opin Otolaryngol Head Neck Surg. 2004;12(4):288‐293. 10.1097/01.moo.0000131444.50445.9d. [DOI] [PubMed] [Google Scholar]
- 29.Cordeiro PG, Disa JJ, Hidalgo DA, Hu QY. Reconstruction of the mandible with osseous free flaps: a 10‐year experience with 150 consecutive patients. Plast Reconstr Surg. 1999;104(5):1314‐1320. 10.1097/00006534-199910000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Hidalgo DA, Pusic AL. Free‐flap mandibular reconstruction: a 10‐year follow‐up study. Plast Reconstr Surg. 2002;110(2):438‐449; discussion 450‐1. 10.1097/00006534-200208000-00010. [DOI] [PubMed] [Google Scholar]
- 31.van Gemert JT, van Es RJ, Rosenberg AJ, van der Bilt A, Koole R, Van Cann EM. Free vascularized flaps for reconstruction of the mandible: complications, success, and dental rehabilitation. J Oral Maxillofac Surg. 2012;70(7):1692‐1698. 10.1016/j.joms.2011.08.024. [DOI] [PubMed] [Google Scholar]
- 32.Virgin FW, Iseli TA, Iseli CE, et al. Functional outcomes of fibula and osteocutaneous forearm free flap reconstruction for segmental mandibular defects. Laryngoscope. 2010;120(suppl 4):S190. 10.1002/lary.21654. [DOI] [PubMed] [Google Scholar]
- 33.Clark JR, Vesely M, Gilbert R. Scapular angle osteomyogenous flap in postmaxillectomy reconstruction: defect, reconstruction, shoulder function, and harvest technique. Head Neck. 2008;30(1):10‐20. 10.1002/hed.20649. [DOI] [PubMed] [Google Scholar]
- 34.Solis RN, Mahaney J, Mohhebali R, et al. Digital imaging evaluation of the scapula for prediction of endosteal implant placement in reconstruction of oromandibular defects with scapular free flaps. Microsurgery. 2019;39(8):730‐736. 10.1002/micr.30466. [DOI] [PubMed] [Google Scholar]
- 35.Mitsimponas KT, Iliopoulos C, Stockmann P, et al. The free scapular/parascapular flap as a reliable method of reconstruction in the head and neck region: a retrospective analysis of 130 reconstructions performed over a period of 5 years in a single department. J Craniomaxillofac Surg. 2014;42(5):536‐543. 10.1016/j.jcms.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez‐Castro J, Petrisor D, Ballard D, Wax MK. The double‐barreled radial forearm osteocutaneous free flap. Laryngoscope. 2016;126(2):340‐344. 10.1002/lary.25388. [DOI] [PubMed] [Google Scholar]
- 37.Dean NR, Wax MK, Virgin FW, Magnuson JS, Carroll WR, Rosenthal EL. Free flap reconstruction of lateral mandibular defects: indications and outcomes. Otolaryngol Head Neck Surg. 2012;146(4):547‐552. 10.1177/0194599811430897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Disa JJ, Cordeiro PG. Mandible reconstruction with microvascular surgery. Semin Surg Oncol. 2000;19(3):226‐234. . [DOI] [PubMed] [Google Scholar]
- 39.Swendseid B, Kumar A, Sweeny L, et al. Long‐term complications of osteocutaneous free flaps in head and neck reconstruction. Otolaryngol Head Neck Surg. 2020;162(5):641‐648. 10.1177/0194599820912727. [DOI] [PubMed] [Google Scholar]
- 40.Avraham T, Franco P, Brecht LE, et al. Functional outcomes of virtually planned free fibula flap reconstruction of the mandible. Plast Reconstr Surg. 2014;134(4):628e‐634e. 10.1097/PRS.0000000000000513. [DOI] [PubMed] [Google Scholar]
- 41.Weitz J, Bauer FJ, Hapfelmeier A, Rohleder NH, Wolff KD, Kesting MR. Accuracy of mandibular reconstruction by three‐dimensional guided vascularised fibular free flap after segmental mandibulectomy. Br J Oral Maxillofac Surg. 2016;54(5):506‐510. 10.1016/j.bjoms.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 42.Ghai S, Sharma Y, Jain N, Satpathy M, Pillai AK. Use of 3‐D printing technologies in craniomaxillofacial surgery: a review. Oral Maxillofac Surg. 2018;22(3):249‐259. 10.1007/s10006-018-0704-z. [DOI] [PubMed] [Google Scholar]
- 43.Azuma M, Yanagawa T, Ishibashi‐Kanno N, et al. Mandibular reconstruction using plates prebent to fit rapid prototyping 3‐dimensional printing models ameliorates contour deformity. Head Face Med. 2014;10:45. 10.1186/1746-160X-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hanasono MM, Skoracki RJ. Computer‐assisted design and rapid prototype modeling in microvascular mandible reconstruction. Laryngoscope. 2013;123(3):597‐604. 10.1002/lary.23717. [DOI] [PubMed] [Google Scholar]
- 45.Succo G, Berrone M, Battiston B, et al. Step‐by‐step surgical technique for mandibular reconstruction with fibular free flap: application of digital technology in virtual surgical planning. Eur Arch Otorhinolaryngol. 2015;272(6):1491‐1501. 10.1007/s00405-014-3078-3. [DOI] [PubMed] [Google Scholar]
- 46.Zhang L, Liu Z, Li B, Yu H, Shen SG, Wang X. Evaluation of computer‐assisted mandibular reconstruction with vascularized fibular flap compared to conventional surgery. Oral Surg Oral Med Oral Pathol Oral Radiol. 2016;121(2):139‐148. 10.1016/j.oooo.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 47.Stirling Craig E, Yuhasz M, Shah A, et al. Simulated surgery and cutting guides enhance spatial positioning in free fibular mandibular reconstruction. Microsurgery. 2015;35(1):29‐33. 10.1002/micr.22229. [DOI] [PubMed] [Google Scholar]
- 48.Pucci R, Weyh A, Smotherman C, Valentini V, Bunnell A, Fernandes R. Accuracy of virtual planned surgery versus conventional free‐hand surgery for reconstruction of the mandible with osteocutaneous free flaps. Int J Oral Maxillofac Surg. 2020;49(9):1153‐1161. 10.1016/j.ijom.2020.02.018. [DOI] [PubMed] [Google Scholar]
- 49.Chang EI, Jenkins MP, Patel SA, Topham NS. Long‐term operative outcomes of preoperative computed tomography‐guided virtual surgical planning for osteocutaneous free flap mandible reconstruction. Plast Reconstr Surg. 2016;137(2):619‐623. 10.1097/01.prs.0000475796.61855.a7. [DOI] [PubMed] [Google Scholar]
- 50.Toto JM, Chang EI, Agag R, Devarajan K, Patel SA, Topham NS. Improved operative efficiency of free fibula flap mandible reconstruction with patient‐specific, computer‐guided preoperative planning. Head Neck. 2015;37(11):1660‐1664. 10.1002/hed.23815. [DOI] [PubMed] [Google Scholar]
- 51.Krane NA, Fagin A, Ghanem TA, Cannady SB, Petrisor D, Wax MK. Simultaneous maxillary and mandibular reconstruction with a single Osteocutaneous fibula free flap: a description of three cases. Microsurgery. 2021;41(1):79–83. 10.1002/micr.30652. [DOI] [PubMed] [Google Scholar]
- 52.Deek NF, Wei FC. Computer‐assisted surgery for segmental mandibular reconstruction with the osteoseptocutaneous fibula flap: can we instigate ideological and technological reforms? Plast Reconstr Surg. 2016;137(3):963‐970. 10.1097/01.prs.0000479998.49928.71. [DOI] [PubMed] [Google Scholar]
- 53.Pappalardo M, Tsao CK, Tsang ML, Zheng J, Chang YM, Tsai CY. Long‐term outcome of patients with or without osseointegrated implants after resection of mandibular ameloblastoma and reconstruction with vascularized bone graft: functional assessment and quality of life. J Plast Reconstr Aesthet Surg. 2018;71(7):1076‐1085. 10.1016/j.bjps.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 54.Pellegrino G, Tarsitano A, Ferri A, Corinaldesi G, Bianchi A, Marchetti C. Long‐term results of osseointegrated implant‐based dental rehabilitation in oncology patients reconstructed with a fibula free flap. Clin Implant Dent Relat Res. 2018;20(5):852‐859. 10.1111/cid.12658. [DOI] [PubMed] [Google Scholar]
- 55.Iizuka T, Häfliger J, Seto I, Rahal A, Mericske‐Stern R, Smolka K. Oral rehabilitation after mandibular reconstruction using an osteocutaneous fibula free flap with endosseous implants. Factors affecting the functional outcome in patients with oral cancer. Clin Oral Implants Res. 2005;16(1):69‐79. 10.1111/j.1600-0501.2004.01076.x. [DOI] [PubMed] [Google Scholar]
- 56.Kumar VV, Jacob PC, Kuriakose MA. Sub‐periosteal dissection with denture‐guided epithelial regeneration: a novel method for peri‐implant soft tissue management in reconstructed mandibles. J Maxillofac Oral Surg. 2016;15(4):449‐455. 10.1007/s12663-015-0854-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Khadembaschi D, Brierly GI, Chatfield MD, Beech N, Batstone MD. Systematic review and pooled analysis of survival rates, success, and outcomes of osseointegrated implants in a variety of composite free flaps. Head Neck. 2020;42(9):2669‐2686. 10.1002/hed.26238. [DOI] [PubMed] [Google Scholar]
- 58.Chang YM, Wallace CG, Hsu YM, Shen YF, Tsai CY, Wei FC. Outcome of osseointegrated dental implants in double‐barrel and vertically distracted fibula osteoseptocutaneous free flaps for segmental mandibular defect reconstruction. Plast Reconstr Surg. 2014;134(5):1033‐1043. 10.1097/PRS.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 59.da Cruz Vegian MR, Costa BCA, de Fátima S‐MG, et al. Systemic and local effects of radiotherapy: an experimental study on implants placed in rats. Clin Oral Investig. 2020;24(2):785‐797. 10.1007/s00784-019-02946-5. [DOI] [PubMed] [Google Scholar]
- 60.Javed F, Al‐Hezaimi K, Al‐Rasheed A, Almas K, Romanos GE. Implant survival rate after oral cancer therapy: a review. Oral Oncol. 2010;46(12):854‐859. 10.1016/j.oraloncology.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 61.Claudy MP, Miguens SA, Celeste RK, Camara Parente R, Hernandez PA, da Silva AN. Time interval after radiotherapy and dental implant failure: systematic review of observational studies and meta‐analysis. Clin Implant Dent Relat Res. 2015;17(2):402‐411. 10.1111/cid.12096. [DOI] [PubMed] [Google Scholar]
- 62.Hundepool AC, Dumans AG, Hofer SO, et al. Rehabilitation after mandibular reconstruction with fibula free‐flap: clinical outcome and quality of life assessment. Int J Oral Maxillofac Surg. 2008;37(11):1009‐1013. 10.1016/j.ijom.2008.05.021. [DOI] [PubMed] [Google Scholar]
- 63.Urken ML, Buchbinder D, Costantino PD, et al. Oromandibular reconstruction using microvascular composite flaps: report of 210 cases. Arch Otolaryngol Head Neck Surg. 1998;124(1):46‐55. 10.1001/archotol.124.1.46. [DOI] [PubMed] [Google Scholar]
- 64.Mohanty R, Kumar NN, Ravindran C. Vertical alveolar ridge augmentation by distraction osteogenesis. J Clin Diagn Res. 2015;9(12):ZC43‐ZC46. 10.7860/JCDR/2015/15976.6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Esposito M, Grusovin MG, Felice P, Karatzopoulos G, Worthington HV, Coulthard P. The efficacy of horizontal and vertical bone augmentation procedures for dental implants – a Cochrane systematic review. Eur J Oral Implantol. 2009;2(3):167‐184. [PubMed] [Google Scholar]
- 66.Bähr W, Stoll P, Wächter R. Use of the “double barrel” free vascularized fibula in mandibular reconstruction. J Oral Maxillofac Surg. 1998;56(1):38‐44. 10.1016/s0278-2391(98)90914-4. [DOI] [PubMed] [Google Scholar]
- 67.Wang F, Huang W, Zhang C, Sun J, Kaigler D, Wu Y. Comparative analysis of dental implant treatment outcomes following mandibular reconstruction with double‐barrel fibula bone grafting or vertical distraction osteogenesis fibula: a retrospective study. Clin Oral Implants Res. 2015;26(2):157‐165. 10.1111/clr.12300. [DOI] [PubMed] [Google Scholar]
- 68.Kokosis G, Schmitz R, Powers DB, Erdmann D. Mandibular reconstruction using the free vascularized fibula graft: an overview of different modifications. Arch Plast Surg. 2016;43(1):3‐9. 10.5999/aps.2016.43.1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mizbah K, Dings JP, Kaanders JH, et al. Interforaminal implant placement in oral cancer patients: during ablative surgery or delayed? A 5‐year retrospective study. Int J Oral Maxillofac Surg. 2013;42(5):651‐655. 10.1016/j.ijom.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 70.Okay DJ, Buchbinder D, Urken M, Jacobson A, Lazarus C, Persky M. Computer‐assisted implant rehabilitation of maxillomandibular defects reconstructed with vascularized bone free flaps. JAMA Otolaryngol Head Neck Surg. 2013;139(4):371‐381. 10.1001/jamaoto.2013.83. [DOI] [PubMed] [Google Scholar]
- 71.Patel A, Levine J, Brecht L, Saadeh P, Hirsch DL. Digital technologies in mandibular pathology and reconstruction. Atlas Oral Maxillofac Surg Clin North Am. 2012;20(1):95‐106. 10.1016/j.cxom.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 72.Patel A, Harrison P, Cheng A, Bray B, Bell RB. Fibular reconstruction of the maxilla and mandible with immediate implant‐supported prosthetic rehabilitation: jaw in a day. Oral Maxillofac Surg Clin North Am. 2019;31(3):369‐386. 10.1016/j.coms.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 73.Qaisi M, Kolodney H, Swedenburg G, Chandran R, Caloss R. Fibula jaw in a day: state of the art in maxillofacial reconstruction. J Oral Maxillofac Surg. 2016;74(6):1284.e1‐1284.e15. 10.1016/j.joms.2016.01.047. [DOI] [PubMed] [Google Scholar]
- 74.Sukato DC, Hammer D, Wang W, Shokri T, Williams F, Ducic Y. Experience with “jaw in a day” technique. J Craniofac Surg. 2020;31(5):1212‐1217. 10.1097/SCS.0000000000006369. [DOI] [PubMed] [Google Scholar]


