Abstract
Background
Inflammatory pseudotumors (IPTs) are rare, idiopathic, and inflammatory lesions that are histopathologically benign. Here, we present three cases of labyrinthine destruction caused by an IPT.
Methods
The first patient was a 74‐year‐old male with a mass lesion extending from the inner ear to the external ear canal. The second patient was a 62‐year‐old female with a foliated polycystic lesion in the petrous bone on the dorsal side of the left internal auditory canal. The third patient was a 68‐year‐old female with a mass extending from the inner ear to the middle ear, destroying the semicircular canal and cochlea.
Results
In two cases, we performed surgical resection successfully with no recurrence. In the other case, the lesion showed shrinkage after chemotherapy for colorectal cancer incidentally found during the examination process.
Conclusion
Surgical technique and indication for IPT should be based on the location and function of the lesion. In addition, there is room to consider pharmacotherapy as a treatment option for IPT of the temporal bone.
Level of Evidence
4.
Keywords: facial nerve palsy, hearing loss, inflammatory pseudotumor, labyrinthine, temporal bone
1. INTRODUCTION
Inflammatory pseudotumors (IPTs) are rare, idiopathic, and inflammatory lesions that are histopathologically benign. Histopathologically, IPT is associated with nonspecific chronic inflammation with fibroblast proliferation and strong infiltration of inflammatory cells, such as lymphocytes, plasmocytes, and histiocytes. However, it can be locally invasive and can destroy adjacent bony tissue.1 Similar to neoplastic lesions, some IPTs show local recurrence as well as distant metastasis. Several reports have revealed that IPT can present as a single lesion or multiple inflammatory masses.2
IPT is most commonly found in the lung but can occur throughout the body, including the retroperitoneum, mediastinum, spleen, liver, limbs, and pelvis. Approximately 5% of cases involve the head and neck.3, 4 In the head and neck region, it is most commonly identified in the orbit, with some reports in the parapharyngeal space, sinuses, major salivary glands, and thyroid glands4; it is rare in the temporal bone. To date, less than 50 cases of IPT involving the temporal bone have been reported (Table 1).2, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 Therefore, the clinical physiology of IPT in the temporal bone has not been fully understood, and no standard treatment has been established.
TABLE 1.
Previous case reports and sites of destruction by inflammatory pseudotumor of the temporal bone
| Year | Case | Sites of destruction | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mastoid | Middle ear | Facial nerve | Sigmoid | Petrous apex | Intracranial/dura | Labyrinth | Internal auditory canal | |||
| This report | 2021 | 3 | 1 | 2 | 2 | 2 | 2 | 3 | 3 | |
| Yanagihara et al5 | 1991 | 1 | 1 | 1 | ||||||
| Benton et al6 | 1992 | 1 | 1 | 1 | ||||||
| Nam et al7 | 1994 | 1 | 1 | 1 | ||||||
| Wiseman et al8 | 1995 | 1 | 1 | 1 | 1 | |||||
| Mulder et al9 | 1995 | 3 | 3 | 3 | 3 | |||||
| Janicki et al10 | 1995 | 1 | 1 | 1 | 1 | |||||
| Schönermark et al11 | 1996 | 3 | 3 | 3 | 3 | |||||
| Gasparotti et al12 | 2003 | 1 | 1 | 1 | ||||||
| Williamson et al13 | 2003 | 1 | 1 | 1 | 1 | 1 | ||||
| Cho et al14 | 2004 | 1 | 1 | 1 | 1 | |||||
| Cho et al15 | 2007 | 1 | 1 | 1 | ||||||
| Lee et al16 | 2007 | 1 | 1 | 1 | ||||||
| Lee et al2 | 2007 | 1 | 1 | 1 | ||||||
| Strasnick and Vaughan17 | 2008 | 2 | 2 | 1 | 1 | |||||
| Coulson et al18 | 2008 | 1 | 1 | 1 | 1 | |||||
| Lee et al19 | 2008 | 1 | 1 | 1 | ||||||
| Santaolalla‐Montoya et al20 | 2008 | 1 | 1 | 1 | 1 | 1 | ||||
| Galindo et al21 | 2008 | 1 | 1 | 1 | ||||||
| Allona et al22 | 2009 | 1 | 1 | 1 | ||||||
| Goh et al23 | 2009 | 1 | 1 | 1 | ||||||
| Ajibade et al24 | 2010 | 1 | 1 | 1 | ||||||
| Curry et al25 | 2010 | 1 | 1 | 1 | ||||||
| Papanikolaou et al26 | 2012 | 1 | 1 | |||||||
| Das et al27 | 2013 | 1 | 1 | |||||||
| Tian et al28 | 2013 | 3 | 3 | 2 | 1 | |||||
| Jung et al29 | 2014 | 1 | 1 | |||||||
| Wang et al30 | 2015 | 1 | 1 | 1 | 1 | |||||
| Ortlip et al31 | 2017 | 7 | 3 | 4 | 1 | 2 | 2 | |||
| Sakano et al32 | 2018 | 1 | 1 | |||||||
Most IPTs involving the temporal bone are located in the mastoid, and usually affect only the mastoid cavity or middle ear.33 IPTs with accompanying destruction of the bony labyrinth are extremely rare. To the best of our knowledge, 11 cases of IPT arising from the inner ear and involving the internal auditory canal and labyrinth have been reported in the literature to date.9, 11, 13, 22, 29, 31 Thus, the diagnosis and treatment of IPTs accompanying the destruction of the bony labyrinth is still challenging.
Here, we report three cases of IPT that affected inner ear structures (Table 2).
TABLE 2.
Summary of our cases
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age (Years old) | 74 | 62 | 68 |
| Sex | Male | Female | Female |
| Past medical history | None | Diabetes, postoperative endometrial cancer | Untreated diabetes |
| Primary symptoms | Hearing loss, vertigo | Hearing loss | Hearing loss, vertigo |
| Other symptoms | Otorrhea | Vertigo | Facial nerve paralysis |
| Hearing of affected side | No measurable hearing | No measurable hearing | No measurable hearing |
| Postoperative hearing | No change | Slight improvement of bone conduction hearing | No change |
| Location of the lesion | Middle ear – Facial nerve – Petrous apex – Middle cranial fossa – Dura – Labyrinth – Internal auditory canal | Jugular bulb – Dura – Labyrinth – Internal auditory canal | Mastoid – Middle ear – Facial nerve – Petrous apex – Labyrinth – Internal auditory canal |
| Destruction of the perimeter | Otic capsule, vestibule, cochlea, ossicles, facial neural tube, and internal carotid artery canal | Otic capsule, posterior semicircular canal, vestibule, the base of the skull | Vestibule, cochlea, semicircular canal |
| MRI | Strong contrast effect, T2 low | Slight contrast effect, T1 high, T2 high, Lobular multifocal cystic lesions | Mild contrast effect, T1 iso‐low |
| Treatment | Resection (transotic approach) | Resection (retrolabyrinthine approach) | Transcanal biopsy (chemotherapy for the colorectal cancer) |
| Progress | Total removal and no recurrence | Subtotal removal and no regrowth | Shrinkage and no regrowth |
Abbreviation: MRI, magnetic resonance imaging.
2. CASE REPORTS
2.1. Case 1
A 74‐year‐old male with no significant medical history presented to his previous doctor with a complaint of right hearing loss and vertigo 6 months before his first visit to our department. Pure tone audiogram (PTA) showed no measurable hearing on the right. The previous doctor did not point out any abnormal findings on computed tomography (CT) and magnetic resonance imaging (MRI) scans. Following a diagnosis of sudden onset hearing loss with vertigo, he was treated with corticosteroid therapy but his hearing did not improve. Four months later, right‐sided otorrhea appeared, and he was noted to have granulomatous changes in the tympanic membrane and was referred to our department. On observation of the tympanic membrane, a pulsatile protruding lesion from the tympanic cavity was observed (Figure 1A). PTA showed no measurable hearing on the right (Figure 1B). There was no vertigo, facial nerve palsy, or other significant neurological abnormalities at the time of examination. Blood tests revealed no unusual findings. CT imaging showed a mass lesion extending from the inner ear to the internal auditory canal, tympanic cavity, and external ear canal. The lesion had destroyed the inner ear labyrinth (Figure 1C,D). Contrast‐enhanced MRI showed a lesion extending around the inner ear that enhanced avidly with contrast. This was accompanied by the destruction of the bones surrounding the lesion (Figure 1E,F). We carefully reviewed the MRI that was performed 6 months before the patient's first visit to our department, and found a mildly contrast‐enhanced lesion around the fundus of the inner auditory canal.
FIGURE 1.
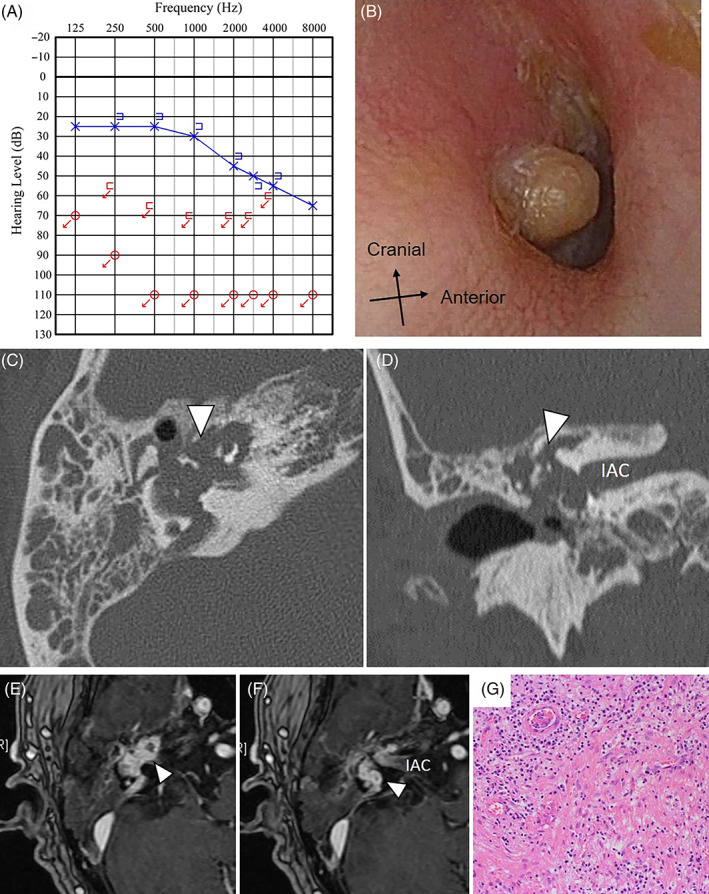
Case 1: A, PTA showed no measurable hearing on the right. B, On observation of the right tympanic membrane, a pulsatile protruding lesion is seen from the tympanic cavity. C,D, CT shows a mass lesion extending from the inner ear to the internal auditory canal, tympanic cavity, and external ear canal (arrowhead in C and D). It is accompanied by the destruction of bones (C: axial image, D: coronal Image). E,F, Contrast‐enhanced MRI revealing an avidly enhancing lesion extending around the inner ear (arrowhead in E and F). The longest diameter of the tumor was 20 mm. G, There is hyperinflammatory granulation tissue growth and fibrosis. The overall picture is consistent with nonspecific inflammation, with no obvious neoplastic lesions observed. CT, computed tomography; IAC, internal auditory canal; MRI, magnetic resonance imaging; PTA, pure tone audiogram
Transcanal biopsy of the protruding granuloma from the tympanic cavity was performed. The result indicated inflammatory cell infiltration by mostly plasmacytoid cells and no neoplastic lesions. We suspected an IPT of the primary internal auditory canal, but planned a total resection of the lesion to rule out the diagnosis of a malignant tumor.
We chose a transotic approach for excision. In addition, subtotal petrosectomy and a blind sac closure of the external auditory canal were performed. The lesion was continuous from the fundus of the internal auditory canal to the vestibular organ and cochlea, with the destruction of the bone surrounding the internal carotid artery. The facial nerve was firmly adherent to the lesion. Following a tumor removal, the internal auditory canal was filled with small pieces of gelfoam soaked with corticosteroid. The facial nerve, from the geniculate ganglion to the vertical portion, was treated as per the aforementioned procedure. Vertigo remained the same because the loss of inner ear function was present preoperatively and did not improve postoperatively.
Histopathological examination revealed only nonspecific inflammation, which led to a definite diagnosis of an IPT (Figure 1G). No IgG4 positive cells were observed. The patient developed facial nerve palsy (House‐Brackmann grade V) right after the operation, which recovered to grade III 1 year later. MRI scans revealed total resection of the lesion 3 months after the operation, and the patient was followed‐up for 2 years during which there was no recurrence.
2.2. Case 2
A 62‐year‐old woman presented to her previous doctor with a complaint of left‐sided hearing loss, one and a half years before her first visit to our department. She was given a prescription for vitamin B12 and adenosine triphosphate, and her symptoms had improved. Five months before her first visit to our department, she developed left‐sided hearing loss and vertigo again, and was referred to our department as CT and MRI scans revealed a mass around the endolymphatic sac.
Upon her first visit to our department, PTA showed severe sensorineural hearing loss on the left side (Figure 2A), and an otoacoustic emission test was poorly evoked on the left. She had rightward unidirectional grade II nystagmus. Blood tests revealed no unusual findings. Contrast‐enhanced MRI revealed a foliated polycystic lesion in the petrous bone on the dorsal side of the left internal auditory canal and showed slight contrast enhancement and hyperintensity on T1‐ and T2‐weighted sequences (Figure 2C,D). It had progressed to the bony wall of the cranial base, posterior semicircular canal, and jugular bulb. It showed a nibbling osteoclastic appearance with sclerosis around the lesion on CT (Figure 2E). Positron emission tomography‐CT (PET‐CT) revealed that the temporal bone was difficult to evaluate due to overlap with cerebral accumulation. Her previous medical history included diabetes mellitus and postoperative endometrial cancer.
FIGURE 2.
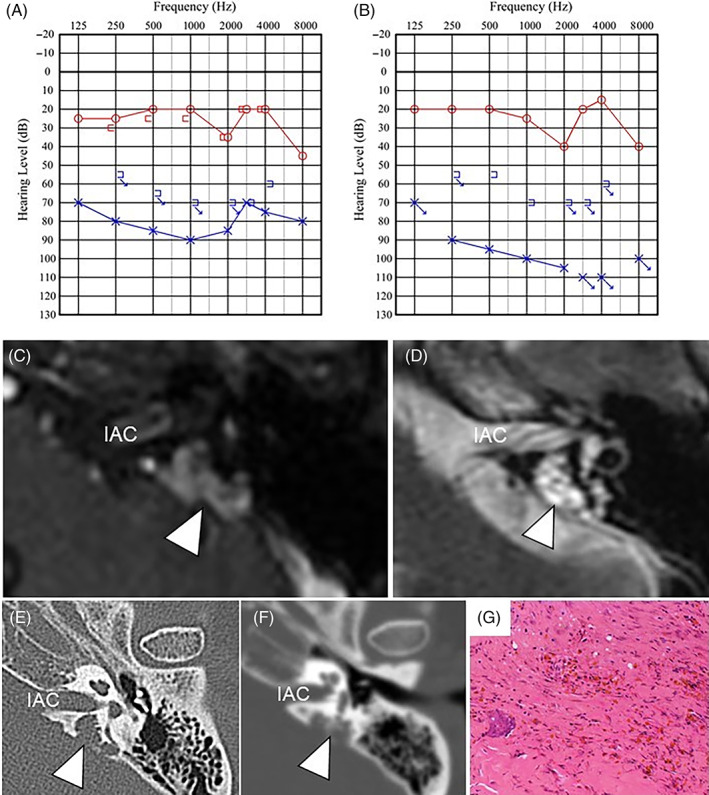
Case 2: A, PTA at the initial visit to our department. B, PTA at 7 months after the surgery. C, Contrast‐enhanced MRI at the initial visit to our department. It shows a foliated polycystic cystic lesion in the petrous bone on the dorsal side of the left internal auditory canal (arrowhead). D, T2‐weighted MRI performed at the initial visit to our department (arrowhead). It shows a slight contrast effect and hyperintense signal on both T1‐ and T2‐weighted studies. The longest diameter of the tumor was 12 mm. E, CT at the initial visit to our department shows a mass lesion around an endolymphatic sac showing bone destruction (arrowhead). F, On the CT performed the day before surgery, the destruction of the bone was advanced (arrowhead). G, Dilated vasculature is seen, with fibrous connective tissue, bony tissue, inflammatory cell infiltration, and hemosiderosis. CT, computed tomography; IAC, internal auditory canal; MRI, magnetic resonance imaging; PTA, pure tone audiogram
Surgical resection was performed for diagnostic treatment because the lesion was accompanied by bone destruction and a malignant tumor diagnosis could not be excluded. On CT imaging performed on the day prior to surgery, destruction of the bony labyrinth was slightly advanced (Figure 2F). The surgery was performed using a retrolabyrinthine approach with auditory brainstem response (ABR) hearing monitoring. There was a dark blue lesion posterior and inferior to the posterior semicircular canal. The endolymphatic sac was compressed but not visually contiguous with the lesion. The wall of the lesion was a hard‐fibrous tissue with some dark brown reservoirs inside. Rapid intraoperative histopathology indicated no neoplastic lesions. To preserve the cochlear function, we decided not to perform a total resection of the lesion near the vestibule. We were able to detach the lesion from the wall of the jugular bulb. Intraoperatively, ABR waveforms became to show higher amplitude. Finally, more identifiable waveforms could be detected compared to the waveforms at the start of the operation. Overall, a subtotal resection was achieved with more than 95% of the lesions removed. The postoperative period passed without complications such as facial nerve palsy.
Histopathological examination revealed nonspecific inflammatory cell infiltration and fibrous connective tissue without IgG4 positive cells (Figure 2G). This result was compatible with an IPT. Hearing tests performed 8 days postoperatively showed an improvement in the bone‐conducted hearing at 250, 500, and 1000 Hz (up to 15 dB); hearing tests performed 7 months postoperatively did not differ much from those performed at 8 days postoperatively (Figure 2B). Four months after surgery, CT showed no progression of the bone destruction. Seven months after surgery, MRI scans identified small residual lesions, which did not grow 2 years after surgery. The vertigo improved over several months.
2.3. Case 3
A 68‐year‐old female patient developed vertigo and tinnitus 1 month before her first visit to our department and the symptoms self‐resolved in approximately 4 days. She was presented to our department with a complaint of left‐sided facial palsy. At that time, the facial palsy was House‐Brackmann grade V. She had rightward unidirectional nystagmus. PTA showed no measurable hearing on the left (Figure 3A). MRI scans showed weak contrast‐enhancement in the internal auditory canal, but based on the imaging findings we diagnosed Ramsay Hunt syndrome. Given that she had untreated diabetes, she was hospitalized for facial nerve palsy and was treated with intravenous steroid therapy. Four months after onset, the facial nerve palsy improved to House‐Brackmann grade III, but the left‐sided hearing did not. The vertigo had already improved. Six months after onset, the left tympanic membrane was slightly bulging in the upper posterior region, and there was poor transparency in the tympanic cavity. MRI revealed an increase in the size of the lesions which enhanced with contrast administration; the semicircular canal and cochlea were obscured (Figure 3B). CT showed a soft tissue shadow extending from the internal auditory canal to the middle ear, destroying all of the semicircular canal and cochlea (Figure 3C,D). Blood tests revealed no unusual findings.
FIGURE 3.
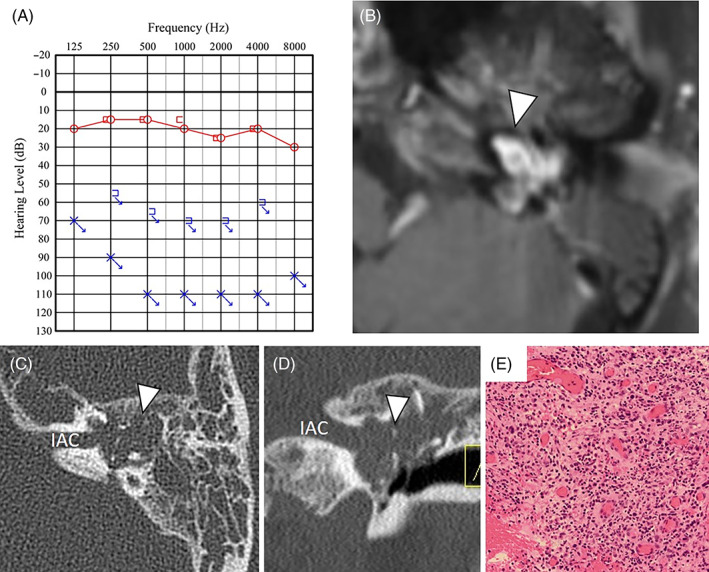
Case 3: A, PTA showed no measurable hearing on the left. B, Contrast‐enhanced MRI shows a lesion extending from the internal auditory canal to the middle ear (arrowhead). The longest diameter of the tumor was 16 mm. C,D, The lesion was found to extend from the internal auditory canal to the middle ear with destruction of the semicircular canal and cochlea (arrowhead) (C: axial image, D: coronal Image). E, There is a dense infiltration of inflammatory cells, mainly lymphocytes and plasmacytes, and increased capillary growth. The tissue is inflammatory granulation tissue, with no findings suggestive of a tumor. IAC, internal auditory canal; MRI, magnetic resonance imaging; PTA, pure tone audiogram
We suspected a neoplastic lesion and performed a transcanal biopsy. The middle ear was filled with granulomatous tissue, and the cochlea and vestibule had been destroyed. Biopsies were performed for the mass in the middle ear, vestibule, and cochlea. On rapid intraoperative examination, none of the findings were clear enough to determine a tumor component. Histopathological examination revealed dense infiltration of inflammatory cells, mainly lymphocytes and plasmocytes, and the growth of capillaries. It was confirmed as inflammatory granulation tissue and there were no findings suggestive of a tumor (Figure 3E). There were only a few IgG4 positive cells. We diagnosed the lesion as an IPT.
We performed PET‐CT for additional inspection, which confirmed a sclerotic bone lesion and mild hyperaccumulation in the left middle ear, and hyperaccumulation in the anterior cecum and anterior colon lymph nodes. She was diagnosed with colorectal cancer, and underwent surgery and chemotherapy (XELOX regimen) under the care of the Gastrointestinal Surgery Department. Four months later, MRI revealed shrinkage in mass in the temporal bone. The mass subsequently maintained a reduction in size, and was planned to be monitored by regular MRI scans. The status of the facial nerve palsy and hearing remained unchanged from the preoperative period.
This study was conducted in accordance with the approval of the ethics committee of the Keio University School of Medicine (approval no. 20200033). Informed consent for publication was obtained from all the patients.
3. DISCUSSION
IPT is a destructive lesion but represents a benign disease.1 Destruction of the cochlea or vestibular organ can be caused by primary temporal bone malignancy, metastatic malignancy, malignant lymphoma, vestibular schwannoma, facial nerve schwannoma, cholesteatoma, paraganglioma, or infectious diseases (eg, otitis media tuberculosis or gangrenous otitis media). Other differential diagnoses of temporal bone mass lesions include cholesterin granuloma, angiectopia, malignant otitis externa, and sarcoidosis. To confirm the diagnosis, a comprehensive evaluation of the subjective symptoms, tympanic findings, neurological findings, blood tests, bacterial culture tests, tuberculosis tests, and imaging tests are required.
IPT of the temporal bone can cause various symptoms depending on its origin in the temporal bone and occurs most frequently in the mastoid. Spinazzi et al reported that the most frequent subjective symptom of IPT was hearing loss (53.8%), followed by earache/headache (38.5%), otorrhea (33.3%), tinnitus (20.5%), dizziness (15.4%), and facial paralysis (10.3%).33 Subjective symptoms were seen depending on the degree of extension of the lesion, with extension to the middle ear present on imaging in 48.7%, the inner ear in 23.1%, and the outer ear in 10.2%.33 Table 1 shows the reported cases with lesions in the inner ear. The chief complaints in all of our cases were hearing loss and vertigo. One patient had otorrhea, and another patient suffered from facial nerve palsy.
No specific findings on blood tests have been reported for IPT, but they are useful for ruling out other diseases. To differentiate from other diseases, peripheral blood count, C‐reactive protein, β‐D glucan, serologic tests for syphilis (rapid plasma reagin card agglutination test and treponema pallidum antibody hemagglutination test), tuberculosis, and human immunodeficiency virus testing can be performed to identify inflammatory/infective etiologies; squamous cell carcinoma‐related antigen and carcinoembryonic antigen can be performed for malignancy testing; lactate dehydrogenase and soluble interleukin‐2 receptor can identify hematologic diseases; and complement C3/C4/CH50, ferritin, rheumatoid factor, antinuclear antibody, proteinase3‐anti‐neutrophil cytoplasmic antibody, myeloperoxidase‐anti‐neutrophil cytoplasmic antibody, immunoglobulin G4 (IgG4), and angiotensin‐converting enzyme can confirm autoimmune diseases. High serum IgG4 levels are considered to be consistent with a diagnosis of IgG4 sclerosing disease, a subtype of IPT. However, it has been reported that no difference exists in the site of involvement, clinical course, or steroid response between IgG4‐positive and IgG4‐negative patients.34 All three cases in the present study had negative serum IgG4 levels.
The importance of imaging tests for IPT has been established.35 Park et al reported that MRI findings in cases of IPT located at the base of the skull and temporal bone have low signal intensity on T2‐weighted images and homogenous contrast enhancement.35 Both T1‐ and T2‐weighted signals are reported to be low in the pseudotumor of orbital inflammation,35 and the spleen is also reported to show T1 iso‐low and T2 iso‐low intensity signal. The lack of mobile protons due to the fibrotic background and/or the high cellularity of the lesions maybe the reason for their hypointensity and weaker enhancement on MR images.36 All three of our cases showed contrast enhancement but a variable pattern on T1‐ and T2‐weighted MRI. Our experience indicates that contrast enhancement on MRI is still useful as part of the diagnostic workup if suspecting IPT, but IPT can show a variable pattern and it is not possible to confirm a diagnosis of IPT using only T1‐ or T2‐weighted imaging.
Some previous studies on IPT of the lung reported that F‐18 fluorodeoxyglucose PET showed high uptake in the IPT.37 In case 3, light accumulation in the middle ear was observed; however, in case 2, evaluation was difficult because it overlapped with the accumulation of the brain. PET‐CT may be of little value because of the increased accumulation of malignancy and inflammation. Although uncommon, there have been reports of cases with distant metastasis, so it may be worthwhile to use PET for the detection of distant metastasis.1
The treatment of IPT remains controversial. Surgical resection, radiotherapy, steroid treatment, and chemotherapy have been tried.33 There are several differences in the treatment of IPT, depending on the primary organ.38 Surgical treatments have been preferred in most cases involving the temporal bone.33 In lung lesions, which are the most frequently reported type of IPT, surgical resection is often the first choice.39 IPT is the third most common intraorbital tumor; steroid therapy is usually the treatment of choice, with the complete resolution with steroid therapy in about 50% of cases.40, 41 Radiation therapy is the treatment of choice for patients with contraindications to steroid use or with a poor response to steroids.42 In the case of sinus lesions, although steroids are less effective than for orbital lesions, both steroid therapy and surgery are the choice of treatment.35, 43 In case 1, the patient was initially given steroids for acute sensorineural hearing loss with vertigo. An effect of steroids in the size of the mass was suspected because the progression of the lesion was observed after the steroid administration. In case 2, no steroids were administered due to a history of diabetes. In case 3, steroids were initially administered for facial nerve palsy, and were also administered as antiemetic agents during the chemotherapy for colorectal cancer. More steroids were administered for this case than in the other cases, and its role in reduction of the size may have been observed; however, the possibility of chemotherapy effects or simply spontaneous regression cannot be ruled out. Other cases of spontaneous resolution of IPT at other sites have also been reported,44, 45 and temporal bone lesions may also spontaneously resolve. There have also been cases in which surgery was combined with chemotherapy.33
There have been no previous studies reporting an appropriate method or duration of the postoperative follow‐up for patients with IPT. Our experience suggests that contrast‐enhanced MRI is useful for checking tumor recurrence, and CT is useful for checking progressive bone destruction. Contrast‐enhanced MRI may not be suitable for detecting early lesions, but the contrast effect can become stronger as the lesion progresses and can be compared between preoperative and postoperative imaging findings. In case 1, we confirmed that all of the tumors had been resected on MRI 3 months later, and the same was evident on MRI scans performed 2 years later. In case 2, even though the bone destruction had progressed 2.5 months before the surgery, the CT scan performed 4 months after the surgery revealed no progressive bone destruction. In case 3, 4 months after the biopsy and treatment for colorectal cancer the lesion had reduced in size. The prognosis of tumor reduction after chemotherapy without resection remains unclear, and long‐term follow‐ups are necessary.
In conclusion, IPTs originating from the temporal bone are rare, and there are few reports of those that destroy the inner ear. Surgical removal has a possibility to improve the prognosis, but the indication should be judged based on the location and function of the lesion. In addition, there is room to consider pharmacotherapy as a treatment option for temporal bone IPT.
CONFLICT OF INTEREST
The authors declare no potential conflict of interest.
AUTHOR CONTRIBUTIONS
All authors contributed to the creation and revision of the manuscript. All authors have read and approved the final manuscript.
Imamura K, Hosoya M, Kasuya K, et al. Labyrinthine destruction caused by inflammatory pseudotumor of the temporal bone: A report of three cases and review of the literature. Laryngoscope Investigative Otolaryngology. 2021;6(4):857–865. 10.1002/lio2.609
REFERENCES
- 1.Naela LD, Newman B, Spottswood SS, Narla S, Kolli R. Inflammatory pseudotumor. Radiographics. 2003;23:719‐729. [DOI] [PubMed] [Google Scholar]
- 2.Lee JH, Jung MK, Song CE, et al. Concomitant inflammatory pseudotumor of the temporal bone and lung: a case report. Ear Nose Throat J. 2007;86:614‐616. [PubMed] [Google Scholar]
- 3.Bahadori M, Liebow AA. Plasma cell granulomas of the lung. Cancer. 1973;31:191‐208. [DOI] [PubMed] [Google Scholar]
- 4.Coffin CM, Watterson J, Priest JR, Dehner LP. Extrapulmonary inflammatory myofibroblastic tumor (inflammatory pseudotumor). A clinicopathologic and immunohistochemical study of 84 cases. Am J Surg Pathol. 1995;19:859‐872. [DOI] [PubMed] [Google Scholar]
- 5.Yanagihara N, Segoe M, Gyo K, Ueda N. Inflammatory pseudotumor of the facial nerve as a cause of recurrent facial palsy: case report. Am J Otol. 1991;12:199‐202. [PubMed] [Google Scholar]
- 6.Benton NC, Korol HW, Smyth LT. Plasma cell granuloma of the middle ear and mastoid. Case report. Ann Otol Rhinol Laryngol. 1992;101:92‐94. [DOI] [PubMed] [Google Scholar]
- 7.Nam BH, Rha KS, Yoo JY, Park CI. Plasma cell granuloma of the temporal bone: a case report. Head Neck. 1994;16:457‐459. [DOI] [PubMed] [Google Scholar]
- 8.Wiseman JB, Arriaga MA, Houston GD, Boyd EM. Facial paralysis and inflammatory pseudotumor of the facial nerve in a child. Otolaryngol Head Neck Surg. 1995;113:826‐828. [DOI] [PubMed] [Google Scholar]
- 9.Mulder JJ, Cremers WR, Wisersma A, Broek P. Fibroinflammatory pseudotumor of the ear. A locally destructive benign lesion. Arch Otolaryngol Head Neck Surg. 1995;121:930‐933. [DOI] [PubMed] [Google Scholar]
- 10.Janicki PT, Adams JS, McElveen JT Jr. Plasma cell granuloma of the temporal bone. Am J Otol. 1996;17:123‐126. [PubMed] [Google Scholar]
- 11.Schönermark MP, Issing P, Stöver T, Ruh S, Lenarz T. Fibroinflammatory pseudotumor of the temporal bone. Skull Base Surgery. 1998;8:45‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gasparotti R, Zanetti D, Bolzoni A, Gamba P, Morassi ML, Ungari M. Inflammatory myofibroblastic tumor of the temporal bone. AJNR Am J Neuroradiol. 2003;24:2092‐2096. [PMC free article] [PubMed] [Google Scholar]
- 13.Williamson RA, Paueksakon P, Coker NJ. Inflammatory pseudotumor of the temporal bone. Otol Neurotol. 2003;24:818‐822. [DOI] [PubMed] [Google Scholar]
- 14.Cho AH, Lee BH, Kwak KW, Kang JK. Inflammatory pseudotumor of temporal bone with pachymeningitis, cranial neuropathies and uveitis. Eur Neurol. 2004;51:238‐240. [DOI] [PubMed] [Google Scholar]
- 15.Cho KJ, Lee DH, Jung SH, Kim JH. A case of an inflammatory myofibroblastic tumor of the mastoid presenting with chronic suppurative otitis media. Auris Nasus Larynx. 2007;34:523‐526. [DOI] [PubMed] [Google Scholar]
- 16.Lee RG, Weber DE, Ness AB, Wasman JK, Megerian CA. Inflammatory pseudotumor of the middle ear masquerading as Bell's palsy. Am J Otolaryngol. 2007;28:423‐426. [DOI] [PubMed] [Google Scholar]
- 17.Strasnick B, Vaughan A. Inflammatory pseudotumor of the temporal bone: a case series. Skull Base. 2008;18:49‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coulson C, George A, Biswas A, Phelan C, Ranit D. Pseudotumour of the temporal bone: an unusual [corrected] cause of otorrhoea and facial palsy. Eur Arch Otorhinolaryngol. 2008;265:713‐715. [DOI] [PubMed] [Google Scholar]
- 19.Lee DH, Shin OR, Cho KJ, Kim JH. Inflammatory pseudotumor in the middle ear cavity. Int J Pediatr Otorhinolaryngol. 2008;72:1569‐1572. [DOI] [PubMed] [Google Scholar]
- 20.Santaolalla‐Montoya F, Ereño C, Zabala A, Carrasco A, Martínez‐Ibargüen A, Sánchez‐Fernández JM. Inflammatory myofibroblastic tumor of the temporal bone: a histologically nonmalignant lesion with fatal outcome. Skull Base. 2008;18:339‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galindo J, Lassaletta L, Garcia E, et al. Spontaneous hearing improvement in a patient with an inflammatory myofibroblastic tumor of the temporal bone. Skull Base. 2008;18:411‐415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allona M, Royo A, Lassaletta L, Moreno P, Galindo J. Inflammatory pseudotumor of the petrous apex with spontaneous improvement of the lesion. Otol Neurotol. 2009;30:245‐247. [DOI] [PubMed] [Google Scholar]
- 23.Goh BS, Tan SP, Husain S, Rose IM, Saim L. Metachronous inflammatory myofibroblastic tumour in the temporal bone: case report. J Laryngol Otol. 2009;123:1184‐1187. [DOI] [PubMed] [Google Scholar]
- 24.Ajibade DV, Tanaka IK, Paghdal KV, Mirani N, Lee HJ, Jyung RW. Inflammatory pseudotumor (plasma cell granuloma) of the temporal bone. Ear Nose Throat J. 2010;89:1‐13. [DOI] [PubMed] [Google Scholar]
- 25.Curry JM, King N, O'Reilly RC, Corao D. Inflammatory pseudotumor of the inner ear: are computed tomography changes pathognomonic? Laryngoscope. 2010;120:1252‐1255. [DOI] [PubMed] [Google Scholar]
- 26.Papanikolaou V, Nikitakis N, Marinakis K, Bousiotou A, Xenelis I. Inflammatory myofibroblastic tumor of the temporal bone. Otol Neurotol. 2012;33:5‐6. [DOI] [PubMed] [Google Scholar]
- 27.Das SK, Varshney H, Saha S, Chowdhury M. An unusual presentation of inflammatory pseudotumor in mastoid area of temporal bone. Indian J Otol. 2013;19:199‐201. [Google Scholar]
- 28.Tian H, Liu T, Wang C, Tang L, Chen Z, Xing G. Inflammatory pseudotumor of the temporal bone: three cases and a review of the literature. Case Rep Med. 2013;2013:480476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jung HV, Choi JW, Shin JE, Kim CH. Inner ear inflammatory pseudotumor with middle ear cholesteatoma. Otol Neurotol. 2014;37:e187‐e188. [DOI] [PubMed] [Google Scholar]
- 30.Wang J, Sun Z, Zhuo S, Wang K. Sigmoid sinus occlusion infiltrated by inflammatory myofibroblastic tumor from mastoid. Head Neck. 2014;37:E4‐E7. [DOI] [PubMed] [Google Scholar]
- 31.Ortlip TE, Drake VE, Raghavan P, et al. Inflammatory pseudotumor of the temporal bone: a case series. Otol Neurotol. 2017;38:1024‐1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sakano H, Shih CP, Jafari A, DeConde A, Harris JP. Multifocal inflammatory pseudotumor of the temporal bone, maxillary sinus, and orbit. Otol Neurotol. 2018;39:e1125‐e1128. [DOI] [PubMed] [Google Scholar]
- 33.Spinazzi EF, Desai SV, Fang CH, et al. Lateral skull base inflammatory pseudotumor: a systematic review. Laryngoscope. 2015;125:2593‐2600. [DOI] [PubMed] [Google Scholar]
- 34.Ryu G, Cho HJ, Lee KE, et al. Clinical significance of IgG4 in sinonasal and skull base inflammatory pseudotumor. Eur Arch Otorhinolaryngol. 2019;276:2465‐2473. [DOI] [PubMed] [Google Scholar]
- 35.Park SB, Lee JH, Weon YC. Imaging findings of head and neck inflammatory pseudotumor. AJR Am J Roentgenol. 2009;193:1180‐1186. [DOI] [PubMed] [Google Scholar]
- 36.Han MH, Chi JG, Kim MS, et al. Fibrosing inflammatory pseudotumors involving the skull base: MR and CT manifestations with histopathologic comparison. AJNR Am J Neuroradiol. 1996;17:515‐521. [PMC free article] [PubMed] [Google Scholar]
- 37.Slosman DO, Spiliopoulos A, Keller A, et al. Quantitative metabolic PET imaging of a plasma cell granuloma. J Thorac Imaging. 1994;9:116‐119. [DOI] [PubMed] [Google Scholar]
- 38.Alyono JC, Shi Y, Berry GJ, et al. Inflammatory pseudotumors of the skull base: meta‐analysis. Otol Neurotol. 2015;36:1432‐1438. [DOI] [PubMed] [Google Scholar]
- 39.Fabre D, Fadel E, Singhal S, et al. Complete resection of pulmonary inflammatory pseudotumors has excellent long‐term prognosis. J Thorac Cardiovasc Surg. 2009;137:435‐440. [DOI] [PubMed] [Google Scholar]
- 40.Martin CJ. Orbital pseudotumor: case report and overview. J Am Optom Assoc. 1997;68:775‐781. [PubMed] [Google Scholar]
- 41.Char DH, Miller T. Orbital pseudotumor. Fine‐needle aspiration biopsy and response to therapy. Ophthalmology. 1993;100:1702‐1710. [PubMed] [Google Scholar]
- 42.Mendenhall WM, Lessner AM. Orbital pseudotumor. Am J Clin Oncol. 2010;33:304‐306. [DOI] [PubMed] [Google Scholar]
- 43.Desai SV, Spinazzi EF, Fang CH, et al. Sinonasal and ventral skull base inflammatory pseudotumor: a systematic review. Laryngoscope. 2015;125:813‐821. [DOI] [PubMed] [Google Scholar]
- 44.Soudack M, Shechter A, Malkin L, Hayek T, Gaitini D. Inflammatory pseudotumor of the liver: sonographic and computed tomographic features with complete regression. J Ultrasound Med. 2000;19:501‐504. [DOI] [PubMed] [Google Scholar]
- 45.Nakama T, Hayashi K, Komada N, et al. Inflammatory pseudotumor of the liver diagnosed by needle liver biopsy under ultrasonographic tomography guidance. J Gastroenterol. 2000;35:641‐645. [DOI] [PubMed] [Google Scholar]


