Abstract
Objective
We desired to establish an active surveillance clinic for head and neck cancer. In this review we examined.
Methods
We examined the natural history of human oral carcinogenesis, the types of preneoplastic lesions, and efforts at oral chemoprevention over the past decades for presentation here.
Results
We established a clinic and program for patients with oral premalignant lesions approximately over 15 years ago based on an unmetneed for this service. We have completed over 4000 outpatient visits for this cohort and have a place for referrals of difficult oral lesions. We have leveraged this population for multiple federally funded trials on oral cancer prevention as well as specimen banking.
Conclusion
There is need for routine active surveillance for oral preneoplastic conditions in patients at high risk for conversion to cancer. There are no effective durable treatment or preventions for these individuals and we have attempted to fill this unmet need with our program.
Keywords: active surveillance, oral leukoplakia, preneoplasia, squamous cell carcinoma
Oral preneoplasia presents an opportunity for earlier diagnosis and treatment of head and neck squamous cell carcinoma. In this review we present the most recent literature in diagnosis and treatment in oral leukoplakia, as well as our program of Active Surveillance for head and neck squamous cell carcinoma.
1. INTRODUCTION
Upper aerodigestive cancers of the head and neck are a significant public health problem. Worldwide, roughly 800 000 cancers in this anatomic region are diagnosed annually.1, 2 This is a malignancy that affects the oral cavity, oropharynx, hypopharynx, and larynx with tobacco, alcohol, and HPV infection as principal risk factors. Tobacco use has been declining in the United States, but it remains a significant global risk factor with over 20% of adults in the world using tobacco.3
The overall long‐term survival of head and neck cancer has improved somewhat, but significantly lags behind other solid tumor malignancies like breast, colon, and prostate cancer.2 The lag in cure rate improvements is a multifactorial problem in the upper aerodigestive organ disease basin contributed to by a variety of factors. These range from the complex mutated epithelial landscape that develops from longstanding exposures to tobacco carcinogens and alcohol. Furthermore, routine robust screening programs for this malignancy do not exist. In 1953, Slaughter published his observations on field cancerization or “field effect.” This concept asserts that multiple malignancies can arise in a given mucosal space due to the fact that a combination of factors has resulted in the mucosa being “condemned.” This means it is able to achieve the development of cancer and able to achieve multiple and recurrent malignancies.4 As a corollary to this concept, patients who have undergone treatment for head and neck cancer are at significant risk for second primary disease in both the head and neck as well as other organs that have higher risk for tobacco‐associated malignancies (lung, esophagus, bladder, among others.) The concept of field carcinogenesis has directed otolaryngologists to keep patients afflicted with head and neck cancer under a protocol that includes frequent physical and endoscopic examination, as well as imaging evaluations to discover recurrences or second primaries at the earliest stage to allow for best outcomes and longevity. Currently, patients are followed at 1 to 2 mo intervals for the first year, and approximately every 2 to 4 mo for the second and third years. After year 3, patients are generally followed every 6‐12 mo. With the emergence of chemotherapy and radiochemotherapy to the treatment regimen, there are additional long‐term toxicities that are potentially addressed at these follow‐up visits.
This level of surveillance by otolaryngologists for those previously afflicted by head and neck cancer is among the most robust of any medical subspecialty. However, standard practices for how to perform surveillance for those at high risk for developing carcinoma of the head and neck are not well established. In a variety of malignancies, there has been robust and effective screening for patients who are at high risk for those diseases. For example, colonic preneoplastic disease (polyps) constitutes a significant portion of the subspecialty of gastroenterology.5 With the advent of mammography, there has been a 30‐y crusade for the increasingly earlier discovery of breast cancer and advanced preneoplastic disease. This work has contributed to significantly improved survival in breast carcinoma over this time.6 In addition to invasive procedures (colonoscopy and CT‐guided breast biopsies for preneoplastic disease), there is an emergence of non‐invasive diagnostic technologies (eg, ColoGuard) to allow for at home cancer screening.7 These measures in screening and early detection all contribute to enhanced survival in colon and breast cancer.
There has been a second trend in a variety of cancers to avoid invasive, often morbid, treatments (eg, mastectomy, prostatectomy) once an accurate assessment of advanced premalignancy or indolent malignancy has occurred. For example, in prostate cancer, histologic biopsies (Gleason grade, prostatic intraepithelial neoplasia) and serology (eg, PSA testing and level trending in elderly gentlemen) may guide treatment. Risk stratification of those with positive screening examinations may allow de‐escalation of care. In otolaryngology, this is being performed in thyroid carcinoma.8
There is a low amount of attention on screening for preneoplastic diseases of the oral cavity or pharynx and larynx. There is a lack of organized surveillance clinics for oral precancerous lesions. The screening that is performed is often performed outside of otolaryngology, generally by a dental professional. These lesions often come to the attention of an otolaryngologist only after a histologic biopsy of invasive cancer or when the precancerous lesion pathology is confusing histologically. This process of lesion screening, risk stratification, and seamless follow‐up for preneoplastic disease is not yet cohesive in our field. Even when recognized, oral premalignancy remains difficult to treat. Simply excising precancers (leukoplakia) is not a successful long term strategy, with lesion recurrences occurring up to 40% after excision.9 Chemoprevention remains a holy grail for this malignancy, despite 40 y of clinical trials.10 Finally, the gold standard diagnostic modality remains histologic biopsy, as the field lacks an oral cancer equivalent of Cologuard.
The notable exception to screening in aerodigestive cancer is the recent work in lung cancer. Clinical trial efforts in low‐dose CT scanning of smokers have resulted in identification of more early‐stage and curable lung cancer being discovered.11 This is now a test that is covered by insurance for individuals at risk for lung cancer.
In 2000, the senior author launched an active surveillance program for oral cavity cancer that has been leveraged into the majority of the NCI sponsored chemoprevention clinical trials in the principal investigator role. So far these clinical trials have not yielded a standard chemoprevention treatment for preneoplastic disease. However, we do have a large population of “at risk” individuals with a regional catchment of approximately 3.5 million persons who are potential surveillance patients. In this article, we will examine issues regarding the complexities of preneoplastic disease, its surveillance, and putative therapies, as well as potentially emerging non‐invasive diagnostic technologies. Hopefully this summary will be a call for better head and neck cancer active surveillance practices in the otolaryngology community.
2. DEFINITIONS
Oral preneoplasia (leukoplakia) is a common finding that confers a significant risk of future oral squamous cell carcinoma. The worldwide prevalence of oral leukoplakia is reported between 1.5% and 4.1%12, 13, 14 with a risk of malignant transformation commonly reported at 2% to 3% annually.15, 16 There is no gender predilection and generally it is more commonly seen in older age. The most commonly described risk factors are tobacco use, including cigarettes and chewing tobacco, alcohol, betel nut, UV light exposure, immunosuppression, and hereditary syndromes like dyskeratosis congenita.12, 17 Defining and differentiating leukoplakia from other oral lesions is important. The World Health Organization defines leukoplakia as: “a white plaque of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer.”18 Others posit similar definitions like “a predominantly white lesion of the oral mucosa that cannot be characterized as any other definable lesion,” but these lack the implication of precancer, which should be emphasized.19 The lesions are white from keratin overproduction (hyperkeratosis), epithelial thickening (acanthosis), or intrinsic genodermatoses.18 Benign traumatic leukoplakia from friction from dentures or biting is technically termed morsicatio mucosae oris (MMO) but is more commonly called friction ridge or benign alveolar ridge keratosis when found along the gingiva. When these lesions are found along in the buccal mucosa it is commonly called linea alba. Villa and Woo18 report that these highly benign gingival and buccal mucosa lesions make up 75% of all white lesions submitted for pathological evaluation18, 20, 21 Other common white lesions of the oral cavity include thrush (candida) and oral lichen planus. Candida can be scraped off and is surrounded by a ring of erythema, and lichen planus may be differentiated from leukoplakia by reticulations and bilaterality.18 Over time, authors have subcategorized leukoplakia to better assess the risk of a single lesion for malignant transformation.
3. GROSS AND VISIBLE CHARACTERIZATIONS OF LEUKOPLAKIA
Leukoplakia should be described by anatomical location, homogeneity, color, border, and texture, as there are associations of greater risk of malignant transformation with each variable. Therefore, one can learn a significant amount about the risk of a lesion based on visual inspection alone. A commonly reported point of clinical differentiation is homogeneous and non‐homogeneous leukoplakia. Homogeneous leukoplakia is typically thin, flat, uniform and has at least one area that is well‐defined.18 Non‐homogeneous leukoplakia may be speckled, nodular, and multifocal. Differentiating these entities is significant, as homogeneous leukoplakia has a 0.6% to 5% chance of transformation, compared to 20% to25% with nonhomogeneous.14, 22, 23 Some authors group erythroleukoplakia, or leukoplakia with areas of erythema, with nonhomogeneous leukoplakia.12 Similarly, some authors also include verrucous leukoplakia within nonhomogeneous, while others describe it as a third entity.14, 18 As a separate entity, proliferative verrucous leukoplakia confers a very high risk of transformation: 10% annually and 70% to 100% overall.14 Regardless of whether they are described independently or as part of nonhomogeneous leukoplakia, erythroplakia and verrucous leukoplakia are negative prognostic indicators. Anatomic subsite is also important. Tongue, especially ventral, floor of mouth, and soft palate have higher risks of malignant transformation as well as higher rates of genetic abnormalities, including aneuploidy and loss of heterozygosity.20, 21 It is suggested that pooling of carcinogens in these dependent areas with thin mucosa allows carcinogen penetration that predisposes them to transformation. It should be noted that despite these useful clinical characteristics, an alarming proportion of “low risk lesions,” for example, homogeneous leukoplakia of the buccal mucosa, may transform into cancer over time.22 Therefore, biopsy of any suspicious white oral lesion is required for initial management.
4. BIOPSY
Biopsy of oral leukoplakia is required at initial diagnosis to assess for dysplasia, carcinoma‐in‐situ, or carcinoma. Histological dysplasia and of course carcinoma‐in‐situ are significant risk factors for transformation to carcinoma.18, 24, 25 Excisional vs incisional biopsy are debated in the literature, but the important aspect is that the biopsy includes a representative sample of the most advanced pathology within the lesion. Lee et al. reported that incisional biopsy underdiagnosed dysplasia in 29% of cases, and that in 200 patients with no malignancy on incisional biopsy, 24 (12%) patients had a malignancy on excisional biopsy. The percentage was even higher for patients with severe dysplasia.24, 25 Not surprisingly, multiple‐site biopsy has a lower rate of underdiagnosis. There is also the challenge of malignant transformation outside the boundaries of the visible lesion.15 Many authors suggest that induration, erythema, or ulceration should be used as guides for where to biopsy. A less invasive option for monitoring is the brush biopsy, which Mehrotra et al. reported has a very high positive predictive value (PPV) and negative predictive value (84% and 98%, respectively.)26 It also allows rapid assessment of several sites at once. However, the gold standard remains tissue biopsy at this time.14
5. PATHOLOGY, IMMUNOHISTOCHEMISTRY, AND MARKERS
Formal evaluation of oral leukoplakia relies on histological examination. The World Health Organization enumerates mild, moderate, and severe dysplasia. Higher levels of dysplasia correspond to a greater risk of transformation to malignancy. On histology, dysplasia is characterized by “architectural and cytonuclear changes of epithelium with hyperchromasia and nuclear enlargement, decreased N‐C ratio, mitoses in suprabasal layers, and loss of differentiation of keratinocytes toward the surface.”13 Despite the ostensible objectivity of pathology, several authors note that interobserver variability among pathologists is an important factor with respect to grading.24, 25, 26 Van der Waals suggests that clinicians should submit specimens to pathology with the specific prompt to the pathologist to “rule out dysplasia” to decrease report ambiguity. Wils et al. recently reported on differentiated dysplasia, which is a subtype of dysplasia that is separate from the classic WHO definition and may be a more sensitive indicator to rule out concerning oral lesions. This dysplasia is characterized by “a basal layer of small cells with hyperchromatic or open nuclei with small nucleoli with an abrupt transition to suprabasal large cells with abundant, eosinophilic cytoplasm with differences in eosinophilia, intercellular edema, with clearly visible desmosomes, and large open nuclei with prominent nucleoli.”13 In their study, when classic (WHO) dysplasia was ruled out, 11 of 56 progressed to cancer, but only two of 30 progressed to cancer when differentiated dysplasia was also ruled out. This is a new application to oral leukoplakia and it is not clear if it will become applied widely. A more sensitive marker is needed, because many cases of leukoplakia with no evidence for dysplasia still go on to progress to cancer.27
Many authors have examined immunohistochemical staining and genetic markers in an attempt to augment histological studies, but thus far none have gained significant acceptance in the literature or clinical practice. Despite this, clinicians should be aware of some of these previous studies as they do provide information on leukoplakia pathophysiology. In a study by Wils et al., patients with no histological dysplasia who retained cytokeratin 13 (CK13) staining had significantly lower risk of progression to cancer.13 Podoplanin is a lymphatic endothelial marker expressed in some cancers like esophageal cancer. Kawaguchi et al. reported that greater expression is associated with greater degree of dysplasia and risk of oral cancer development.27 Bagan et al. showed that oral leukoplakia and oral cancer had significantly higher copy numbers of epidermal growth factor receptor (EGFR), a cell‐surface receptor associated with cellular replication.28 Oral and laryngeal cancer were shown to develop more quickly in leukoplakia from patients with p27 loss, a tumor suppressor gene, and e‐cadherin loss.29 Multiple authors have examined chromosomal and genetic characteristics of leukoplakia. Castagnola et al. showed that oral leukoplakia of the tongue had a higher degree of DNA aneuploidy compared to other subsites. Several loci have been shown to be associated with transformation to cancer. By studying chromosomal abnormalities extensively, loss of heterozygosity of leukoplakic lesions at chromosome 3p, 9p, and 17p are each associated with a risk of oral cancer development. Zhang et al. report that the 9p21 locus includes the p16INK4A gene, which produces a cyclin‐dependent kinase (CDK) inhibitor. The 17p13 locus includes the gene for TP53 and 17p11.1‐p12 includes the CHRNB1 gene, both important tumor suppressors.20, 21 However, contrasting reports in the literature exist. VanZyl et al. report that chromosomal aneuploidy is associated with degree of dysplasia, while Bremmer et al. report that greater lesion aneuploidy is associated with risk of progression to cancer and not with dysplasia. Micro RNA (miRNA) is another area of interest. Several investigators have shown that presence of specific miRNA may be predictive of leukoplakia progression, and that it may actually be recovered from sputum.30, 31, 32 However, the abundance of studies in this area have thus far yielded few clinically useful markers. In a paper in 2001, Lippman and Hong concluded that there were no biomarkers that were clinically helpful or predictive of malignant transformation. Two decades later unfortunately, authors from the World Workshop on Oral Diseases evaluated multiple studies and concluded there is still no evidence any biomarkers are clinically predictive of malignant transformation.14
6. CHEMOPREVENTION
Chemoprevention is the use of drugs, vitamins, or other agents to try to reduce or delay the occurrence or recurrence of cancer. Pharmaceuticals have so far not shown durable efficacy for the reversal of high‐risk lesions or the prevention of oral carcinoma. Early studies with retInoids showed some efficacy and were advanced to larger randomized trials. In a widely cited phase III trial published in 1990, Hong et al. showed that isotretinoin is effective for prevention of second primary tumors in HNSCC.33 However, the majority of patients treated with retinoids required dose‐reduction due to toxicity which significanrtly reduced efficacy. A more recent phase III trial failed to confirm any effect of isotretinoin on second primary formation.34 Other molecules that have been investigated include COX inhibitors, topical bleomycin, and phytochemicals that can be found in fruits like black raspberries.34, 35, 36 Small molecule tyrosine kinase inhibitor, erlotinib, was used to selectively target the EGFR overexpressed in some oral squamous cell carcinoma in a recent randomized placebo‐controlled clinical trial. Unfortunately this trial did not show benefit on oral cancer free survival.10, 37 In a systematic review, Lodi et al. were unable to conclude that any putative chemoprevention agents significantly decreased the risk of oral cancer or resolution of leukoplakia over the long term.10 Other authors have looked at the effect of diet on oral cancer risk. Greater vegetable consumption is associated with a lower risk of oral cancer, but this would likely be confounded by tobacco and alcohol use.38 The phytochemicals in green tea have also been investigated.38, 39 Recommending a healthy diet and green tea consumption are low‐risk recommendations with potential benefit, but tobacco cessation and alcohol cessation are undoubtedly the most effective lifestyle changes one can make for oral cancer prevention.34
7. SURGICAL TREATMENT
Surgical excision is the intuitive and commonly‐advised treatment for leukoplakia, as well as providing tissue for histologic assessment. However, recurrence of lesions is common and surprisingly is not demonstrated to alter whether an individual develops oral carcinoma. Similar to adenoma resection for prevention of colon adenocarcinoma, resection of leukoplakia would theoretically prevent transformation to oral squamous cell carcinoma by eliminating an autonomously replicating pre‐cancer clone. Arnaoutakis et al. argue for wide local excision, as they report that this reduced local recurrence compared to observation (P = .05.)9 Even with this recommendation, the authors described a high recurrence rate (39%) that is similarly reported elsewhere in the literature. In opinion papers, van der Waal18 advocates for excision of dysplasia or CIS, and that a recurrence rate of 0% to 35% can be expected. However, for oral leukoplakia without dysplasia there is thus far no evidence that surgical resection is effective.10, 17 Kuribayashi et al., Holmstrup et al., and Schepman et al. all showed in large retrospective studies that the risk of cancer development was actually higher in patients who underwent surgical therapy.16, 19, 23 These studies each included at least 100 patients and had robust follow‐up of at least 6 y. It should be noted that these studies are retrospective and include major differences in factors like amount of nonhomogeneous leukoplakia, lesion size, and dysplasia between surgical and nonsurgical groups. Presumably, the surgical cohort was composed of higher‐risk lesions on average. To date, there have been no prospective trials comparing surgical treatment of leukoplakia and observation.
There are multiple methods for surgical leukoplakia removal. In general, leukoplakia may be removed by wide local excision with cold knife or electrocautery. Surgical protocols vary, but generally a cuff of normal mucosa will be resected to have “negative margins.” If the lesion recurs, one author suggests re‐resecting with 2 to 3 mm margins.18 Photodynamic therapy (PDT), cryoablation, and laser ablation are other options for treatment. Photosensitizers, like aminolevulinic acid (ALA,) are reportedly taken up preferentially by dysplastic mucosal cells. When exposed to a light source, the molecule creates reactive oxygen species, leading to apoptosis and death of aberrant cells. PDT recurrence rates are similar to surgical resection (0%‐66%)30 but it is not a widely used method. Presently, the removal of a dysplastic lesion will at least allow for accurate evaluation of pathologic grade, but these lesions will commonly recur. Therefore, we believe long‐term observation is an important policy to emonitor lesions changes that may predict conversion of mucosal lesions to micro invasive or overtly invasive cancer.
8. OBSERVATION AND WATCHFUL WAITING
Patients with leukoplakia should be examined regularly. Several authors have published recommended guidelines for follow‐up. Wils et al. use an algorithm of presence of histologic dysplasia and histologic expression of CK13 to guide “intensified” or “de‐intensified” follow‐up. A definition of intensity is not provided, but the implication is that intensified follow‐up would be more frequent and thorough. Villa and Woo et al. recommend follow‐up and serial biopsy at varying intervals based on criteria like lesion size, texture (eg, verrucous), and recurrence after biopsy. Leukoplakia without dysplasia on histology is defined by the authors as “keratosis of unknown significance,” or KUS.18 Higher risk lesions, like verrucous hyperplasia, would require more frequent follow‐up and biopsy. Van der Waal is pessimistic about serial follow‐up, and without any supporting evidence concludes that “most likely, follow‐up programs will not result in improved survival.” It is noted in the same paper that the resources required for regular follow‐up make recall protocols unfeasible in some areas across the globe and that some patients may stop participating.40 This opinion is not widely held in the literature, as patient education and re‐examination for a typically slow growing malignancy like oral cancer should result in earlier stage diagnosis, significantly improving survival. One challenge leukoplakia presents is its long clinically relevant duration. Taiwan has instituted a nationwide oral cancer screening program, and in a paper describing leukoplakia in this population, Lian et al. recommend a 10‐y surveillance in leukoplakia patients, and 15 y in patients with submucous fibrosis. In a large retrospective study, Silverman et al. describe how transformation of leukoplakia occurred most commonly in the second year of follow‐up (5% rate), but that at least 1% of lesions transformed to cancer annually subsequently, with a mean time to transformation of 8.1 y.41 Given the low but unceasing risk for transformation, the authors recommend that leukoplakia requires “constant observations, regardless of how long it has existed or how benign it appears.” Indeed, we believe these patients should be followed closely.
9. ACTIVE SURVEILLANCE
Active surveillance is an accepted management protocol for cancers and precancers in other fields and may be adopted for otolaryngologic surveillance of oral leukoplakia. Prostate and breast cancer are two examples. More than half of men have histologic evidence of prostate adenocarcinoma on autopsy, but only a small percentage of older men have clinically significant prostate cancer. Using biomarkers (PSA), pathology (Gleason score), staging, and some more investigative variables (RNA assays of relevant genes and prostate MRI), clinicians are able to discriminate clinically important lesions, thus sparing patients with indolent lesions from surgical morbidity and appropriately treating aggressive lesions.42 The treatment of breast cancer is notable for the trend toward more conservative surgical treatment instead of radical mastectomy. Earlier disease is more commonly diagnosed now, which improves overall survival for this malignancy. Imaging and molecular studies (estrogen and progesterone receptor and HER2) have been instrumental in treatment de‐escalation. In a retrospective study of patients who elected to pursue conservative treatment after a diagnosis of ductal carcinoma‐in‐situ (DCIS), Meyerson et al. describe how six of 14 patients were spared any kind of surgery using close follow‐up, imaging, and medications like selective estrogen receptor modulators (SERMs) and aromatase inhibitors.43, 44 Certainly these are not perfect comparisons. Both prostate and breast cancer have effective medical treatments (endocrine deprivation) and useful imaging. Unfortunately, multiple studies in chemoprevention for oral leukoplakia have failed to yield a durable solution.35 There are also no imaging options that are useful for leukoplakia until it transforms to cancer. Visual aids like toluidine blue, veloscope, and autofluorescence may help with screening, but are not widely used.18, 45 Until there are reliable and specific diagnostic aids and treatments for oral premalignancy, the otolaryngologist should manage patients with regular follow‐up and biopsy. We present our protocol for oral premalignancy at the University of Minnesota below.
10. ACTIVE SURVEILLANCE PROGRAM FOR ORAL PREMALIGNANCY AT THE UNIVERSITY OF MINNESOTA
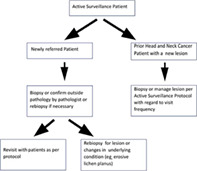
In 2000, the senior author and an oral medicine colleague (Dr. Nelson Rhodus, DMD, MPH) started a joint program at the University of Minnesota Medical school and Dental school for the express purpose of preneoplastic lesion referral and follow‐up (Figure 1). There was a void in this service in the patient catchment of the institution, and these practitioners were employed or contracted to multiple health care systems in the area. Presently, the active surveilliance clinic has performed between 4000 to 8000 outpatient surveillance visits for new referrals and existing patients. We have also completed hundreds of visits for industry or NIH funded clinical trials that they have lead as principal investigators.
FIGURE 1.
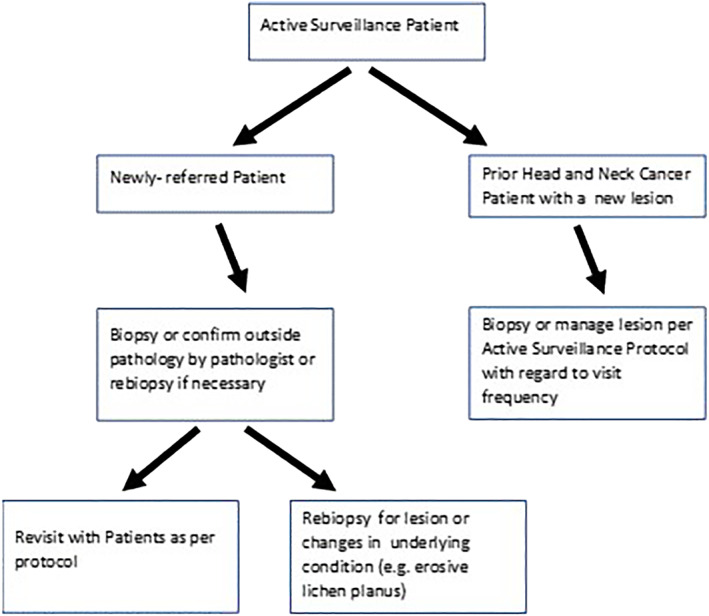
University of Minnesota Head and Neck Cancer Active Surveillance algorithm
The formats for the visit are reasonably standardized. Social history includes recording all forms of tobacco use in the past and present with conversion to pack years if possible. There is a secondary assessment of current and past alcohol use. On review of systems, there is assessment of any conditions that may be associated with oral preneoplastic lesions, such as oral lichen planus, prior bone marrow, or solid tumor transplantation, and Fanconi Anemia, for example. Compete head and neck physical exams are performed and flexible direct laryngoscopy is performed based on signs and symptoms. Photographic documentation is performed at times as well as bidimensional lesion measurements, and characterization of the type of leukoplakia (eg, speckled, erythroplakia).
Patients who come in with biopsy results are stratified into follow‐up protocols. Patients presenting for the first time with oral lichen planus or erosive lichen planus are treated with topical anti‐inflammatory agents and followed up in 6 to 12 wk depending on the severity of condition. Baseline 4 mm punch biopsies of lesions are performed for any lesions that are not identifiable as the most benign lesions (linea alba, friction ridges, aphthous stomatitis, classic oral lichen planus). Patients with outside biopsies have their pathologies reviewed by either head and neck or oral pathologists. If there is microinvasive or invasive cancer in the lesions, there is standard treatment for oral carcinoma offered. For high‐grade lesions (moderate or severe dysplasia) that have been excised or biopsied, there is follow‐up every 3 mo for the first year then less frequently. For low‐grade dysplasia or hyperplasia, there would typically be follow‐up every 6 mo for the first year for lesion monitoring (Table 1). Typically, benign lesions like linea alba and friction ridges are a one‐time visit. At times diagnostic aids like velscope exams or tolonium chloride may be employed to clarify the physical exam as necessary. There is significant patient education about the level of risk of their premalignant lesion. There is counseling for smoking, diet, and alcohol use. Incisional biopsies are performed when needed and this occurs yearly for high grade lesions and less frequently for lower grade lesions. Changes in symptoms or physical character of the lesions will prompt additional biopsies. Participation in available clinical trials is offered. There is phone communication or electronic access for patients in the lesion program to the head and neck tumor nurse as well. It is felt this combination of outpatient visits, specific counseling and education, and participation in clinical trials constitutes a comprehensive active surveillance program for premalignancy. An extension of the program is professional education to regional dental and medical professionals for CME purposes every 24 to 36 mo. Also, individualized visits and lectures are given to local medical and dental groups.
TABLE 1.
University of Minnesota Active Surveillance biopsy timeline guidelines
| Type of lesion | Follow‐up frequency | Length of follow‐up | Biopsy frequency |
|---|---|---|---|
| By Histologic Grade | |||
| Hyperkeratosis/no dysplasia | Every 6 mo | 1–2 y | Only if lesion changes |
| Mild dysplasia | Every 6–12 mo | 5 y | Rebiopsy every 2 years to confirm dysplasia level |
| Moderate dysplasia | Every 3 mo | 5 y | Rebiopsy in 12‐18 months |
| Severe dysplasia | Every 3 mo | 5 y | Rebiopsy in 3–9 months unless change |
| Severe dysplasia/CIS | Every 3 mo | 5 y | Confirm histology on referral biopsy; ascertain CIS vs severe dysplasia by standardized criteria |
| By Condition | |||
| Erosive lichen planus/difficult to control lichen planus with any dysplasia history | Every 3–6 mo | 5 y | Rebiopsy based on grade of dysplasia as above, or for change in symptoms or capacity to medically control the condition |
| Proliferative verrucous leukoplakia | Every 3–6 mo | 5 y | Rebiopsy based on grade of dysplasia as above, or for change in symptoms or capacity to medically control the condition. Aggressive screening if any change in jaw/dentition |
From the program's outset, a key component of our Active Surveillance program has been to foster translational research. We offer our large available patient cohort the opportunity to participate in chemoprevention trials and advance head and neck cancer prevention science. We first designed highly successful minimally invasive collection techniques for oral fluids and tissues. For oral fluids, we collect whole unstimulated saliva for 5 min in 50 mL polypropylene conical tubes, which we immediately catalog and freeze at −80°C. Next, we have patients rinse their mouths with 10 mL of normal saline for 30 s which we freeze at −80°C. In tissues, we often perform biopsies of both lesions and normal appearing mucosa. To minimize crush artifact, we use 3 and 4 mm Baker's punch biopsies to cut through the mucosa into the submucosa. After removal, lesions are bisected on filter paper (eg, Whatman). One half of each “en face” portion of the lesion goes into formalin. After paraffin embedding, we achieve 20 to 40 four micron sections for pathologic evaluation and immunohistochemistry. The other half of the lesion goes into either OCT freezing medium or RNAlater (ThermoFisher, Grand Island, NY) for gene expression studies. Specimens collected this way can be used for pathology evaluation, immunohistochemistry studies, and high‐throughput biology studies including genomics (work flow depicted in Figure 2). We have been able to reliably utilize these techniques in multiple clinical trials. Notably, from our biopsy techniques we get high RNA integrity to the extent where we have 100% usable RNA from 58/58 specimens as well as highly usable specimens from salivary fluids (Table 2).
FIGURE 2.
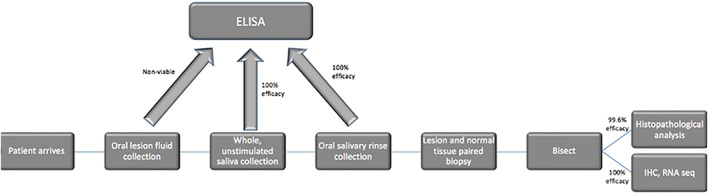
Active surveillance translational research workflow
TABLE 2.
High diagnostic yield rate of oral rinse and biopsy techniques
| Four interventional or natural history studies | ELISA performed/whole unstimulated saliva collected | ELISA performed/salivary rinse performed | Histological diagnosis/biopsies performed | Average number tissue sections | RNA sequenced/biopsy collected |
|---|---|---|---|---|---|
| Total | 42/42 | 42/42 | 238/239 | 43 ± 11 (SEM) | 58/58 |
| % usable specimens | 100% | 100% | 99.6% | Not applicable | 100% |
We have been overall principal investigators or site principal investigators for the majority of oral cancer peer review funded prevention trials offered by the National Cancer Institute's Division of Cancer Prevention (NCI/DCP) as well as industry prevention or surveillance trials (Table 3).
TABLE 3.
Trials at the University of Minnesota
| Type of trial | Agent | Cohort | Primary outcome | Clinical Trials.gov identifier |
|---|---|---|---|---|
| Chemoprevention | Celecoxib | Preneoplasia | Leukoplakia Reduction | Not listed |
| Exam adjunct | Tolonium Chloride | Post resection | Recurrence detection | Not listed |
| Phase IIA | Pioglitazone | Preneoplasia | Leukoplakia Reduction | NCT00099021 |
| Phase IIB | Pioglitazone | Preneoplasia | Leukoplakia Reduction | NCT00951379 |
| Phase IIa | Metformin | Preneoplasia | Leukoplakia Reduction | NCT02581137 |
| Window of opportunity | Actoplus Met XR (pioglitazone metformin) | Preresection oral cancer | Cell proliferation marker reduction (Ki 67) | NCT02917629 |
A second important component of any Active Surveillance program for advanced precancers and people at continued risk for more cancers (second primaries) would be a spectrum of available risk reductions. Our program has offered a number of items, but there is no question that we aspire to a more comprehensive portfolio than simply examinations, counseling, and biopsies. First, Minnesota received the first large tobacco settlement in 1998 ($6B) that continues to pay out funding that supports tobacco reduction programs. Therefore, we counsel our patients to seek out available tobacco cessations (a primary prevention) for their high risk condition if they are smokers. These are fortunately readily available. We ask patients to counsel family members and friends as well.
Third, although there are not proven diet or diet supplement preventions for oral cancer prevention, we do promote reducing alcohol intake while maintaining dietary balance. For patients with erosive lichen planus and similar conditions, we actively seek dietary triggers to the condition for their amelioration. These include avoidance of alcohol (including mouthwash), detergents in toothpaste, and foodstuffs (citrus, tomato, chocolate, and caffeine among others). Patients will often query if there are items in the diet that can be used as cancer prevention agents. In this case, we will discuss specifically items that have some evidence base in oral cancer prevention. These will include green tea polyphenols as teas or supplements, berries (including raspberry, blueberry supplementation), and topical curcumin.10, 36, 39, 46 At times our patients will ask for dietary recommendations, and we will simply fall back to simple nutritional recommendations including diets enriched in brassicas family members including broccoli, kale, and brussels sprouts.47 There are some other topicals we will recommend to reduce inflammation and these include fluorinated steroid ointments, Tacrolimus, and Acutane ointment.48, 49, 50
11. SUMMARY AND CONCLUSIONS
Leukoplakia is an insidious disease. It may not resolve after smoking and alcohol cessation and frequently recurs after excision. It may remain clinically stable for many years before transforming into aggressive cancer. There is some evidence that the revolution of immunotherapy in head and neck SCC may eventually reach its less‐threatening precursor, oral leukoplakia.51, 52, 53 Several authors have shown that leukoplakia express markers characteristic for immune evasion and that are now targeted in head and neck cancer. However, this work is in its infancy. For now, the otolaryngologist must remain vigilant in monitoring patients with leukoplakia. Serial examination over extended periods of time will allow clinicians to prevent development of advanced lesions and thus give the best chance of improved survival.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Archibald H, Buryska S, Ondrey FG. An active surveillance program in oral preneoplasia and translational oncology benefit. Laryngoscope Investigative Otolaryngology. 2021;6(4):764-772. 10.1002/lio2.612
BIBLIOGRAPHY
- 1.Bray F, Ferlay J, Soerjomataram R, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 2.Canning M, Guo G, Yu M, et al. Heterogeneity of the head and neck squamous cell carcinoma immune landscape and its impact on immunotherapy. Front Cell Dev Biol. 2019;7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asma S.The GATS atlas: global adult tobacco survey. (2015). [DOI] [PMC free article] [PubMed]
- 4.Slaughter DP, Southwick HW, Smejkal W. ‘Field cancerization’ in oral stratified squamous epithelium. Clinical implications of multicentric origin. Cancer. 1953;6:963‐968. [DOI] [PubMed] [Google Scholar]
- 5.Winawer S, Fletcher R, Rex D, et al. Colorectal cancer screening and surveillance: clinical guidelines and rationale—update based on new evidence. Gastroenterology. 2003;124:544‐560. [DOI] [PubMed] [Google Scholar]
- 6.Lauby‐Secretan B, Scoccianti C, Loomis D, et al. Breast‐cancer screening — viewpoint of the IARC working group. N Engl J Med. 2015;372:2353‐2358. [DOI] [PubMed] [Google Scholar]
- 7.Wolf AMD, Elizabeth TH, Church TR, et al. Colorectal cancer screening for average‐risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250‐281. [DOI] [PubMed] [Google Scholar]
- 8.Ahn HS, Welch HG. South Korea's thyroid‐cancer ‘epidemic’—turning the tide. N Engl J Med. 2015;373:2389‐2390. [DOI] [PubMed] [Google Scholar]
- 9.Arnaoutakis D, Bishop J, Westra W, Califano JA. Recurrence patterns and management of oral cavity premalignant lesions. Oral Oncol. 2013;49:814‐817. [DOI] [PubMed] [Google Scholar]
- 10.Lodi G, Franchini R, Warnakulasurya S, et al. Interventions for treating oral leukoplakia to prevent oral cancer. Cochrane Database Syst Rev. 2016;7:CD001829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Lung Screening Trial Research Team , Aberli DR, Adams AM, et al. Reduced lung‐cancer mortality with low‐dose computed tomographic screening. N Engl J Med. 2011;365:395‐409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Villa A, Sonis S. Oral leukoplakia remains a challenging condition. Oral Dis. 2018;24:179‐183. [DOI] [PubMed] [Google Scholar]
- 13.Wils LJ, Poell JB, Evren I, et al. Incorporation of differentiated dysplasia improves prediction of oral leukoplakia at increased risk of malignant progression. Mod Pathol. 2020;33:1033‐1040. 10.1038/s41379-019-0444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Villa A, Celentalo A, Glurich I, et al. World workshop on Oral medicine VII: prognostic biomarkers in oral leukoplakia: a systematic review of longitudinal studies. Oral Dis. 2019;25 (Suppl 1:64‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Waal I, van der Waal I. Oral leukoplakia: present views on diagnosis, management, communication with patients, and research. Curr Oral Health Rep. 2019;6:9‐13. [Google Scholar]
- 16.Schepman KP, van der Meij EH, Smeele LE, van der Waal I. Malignant transformation of oral leukoplakia: a follow‐up study of a hospital‐based population of 166 patients with oral leukoplakia from The Netherlands. Oral Oncol. 1998;34:270‐275. [PubMed] [Google Scholar]
- 17.Noto Z, Tomihara K, Furukawa K, Noguchi M. Dyskeratosis congenita associated with leukoplakia of the tongue. Int J Oral Maxillofac Surg. 2016;45:760‐763. [DOI] [PubMed] [Google Scholar]
- 18.Villa A, Woo SB. Leukoplakia‐a diagnostic and management algorithm. J Oral Maxillofac Surg. 2017;75:723‐734. [DOI] [PubMed] [Google Scholar]
- 19.Kuribayashi Y, Vedtofe P, Riebel J, et al. Long‐term outcome of non‐surgical treatment in patients with oral leukoplakia. Oral Oncol. 2015;51:1020‐1025. [DOI] [PubMed] [Google Scholar]
- 20.Castagnola P, Malacarne D, Scaruffi P, et al. Chromosomal aberrations and aneuploidy in oral potentially malignant lesions: distinctive features for tongue. BMC Cancer. 2011;11:445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang L, Cheung J, Lam WL, et al. Increased genetic damage in oral leukoplakia from high risk sites: potential impact on staging and clinical management. Cancer. 2001;91:2148‐2155. [DOI] [PubMed] [Google Scholar]
- 22.Holmstrup P, Dabelsteen E. Oral leukoplakia‐to treat or not to treat. Oral Dis. 2016;22:494‐497. [DOI] [PubMed] [Google Scholar]
- 23.Holmstrup P, Vedtofte P, Reibel J, Stoltze K. Long‐term treatment outcome of oral premalignant lesions. Oral Oncol. 2006;42:461‐474. [DOI] [PubMed] [Google Scholar]
- 24.Lydiatt DD. Cancer of the oral cavity and medical malpractice. Laryngoscope. 2002;112:816‐819. [DOI] [PubMed] [Google Scholar]
- 25.Lee J‐J, Hung HC, Cheng SJ, et al. Factors associated with underdiagnosis from incisional biopsy of oral leukoplakic lesions. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;104:217‐225. [DOI] [PubMed] [Google Scholar]
- 26.Mehrotra R, Mishra S, Singh M, Singh M. The efficacy of oral brush biopsy with computer‐assisted analysis in identifying precancerous and cancerous lesions. Head Neck Oncol. 2011;3:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawaguchi H, AKE N, Papadimitrakopoulou V, et al. Podoplanin: a novel marker for oral cancer risk in patients with oral premalignancy. J Clin Oncol. 2008;26:354‐360. [DOI] [PubMed] [Google Scholar]
- 28.Bagan JV, Mata‐Riog M, Cartio‐Gimeno J, et al. Epidermal growth factor receptor copy number in potentially malignant oral disorders and oral squamous cell carcinoma: a short communication and preliminary study. J Oral Pathol Med. 2012;41:662‐666. [DOI] [PubMed] [Google Scholar]
- 29.Massarelli E, Brown E, Tran NK, et al. Loss of E‐cadherin and p27 expression is associated with head and neck squamous tumorigenesis. Cancer. 2005;103:952‐959. [DOI] [PubMed] [Google Scholar]
- 30.Li Y, Wang B, Zheng S, He Y. Photodynamic therapy in the treatment of oral leukoplakia: a systematic review. Photodiagnosis Photodyn Ther. 2019;25:17‐22. [DOI] [PubMed] [Google Scholar]
- 31.Yang Y, Li YX, Yang X, et al. Progress risk assessment of oral premalignant lesions with saliva miRNA analysis. BMC Cancer. 2013;13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maimaiti A, Abudoukeremu K, Tie L, Pan Y, Li X. MicroRNA expression profiling and functional annotation analysis of their targets associated with the malignant transformation of oral leukoplakia. Gene. 2015;558:271‐277. [DOI] [PubMed] [Google Scholar]
- 33.Hong WK, Lippman SM, Itri LM, et al. Prevention of second primary tumors with isotretinoin in squamous cell carcinoma of the head and neck. Am J Otolaryngol. 1991;12:177. [DOI] [PubMed] [Google Scholar]
- 34.Khuri FR, Lee JJ, Lippman SM, et al. Randomized phase III trial of low‐dose isotretinoin for prevention of second primary tumors in stage I and II head and neck cancer patients. JNCI. 2006;98:441‐450. [DOI] [PubMed] [Google Scholar]
- 35.Chau L, Jabara JT, Lai W, et al. Topical agents for oral cancer chemoprevention: a systematic review of the literature. Oral Oncol. 2017;67:153‐159. [DOI] [PubMed] [Google Scholar]
- 36.Han C, Ding H, Casto B, Stoner GD, D'Ambrosio SM. Inhibition of the growth of premalignant and malignant human oral cell lines by extracts and components of black raspberries. Nutr Cancer. 2005;51:207‐217. [DOI] [PubMed] [Google Scholar]
- 37.William WN Jr, Papadimitrakopoulou V, Lee JJ, et al. Erlotinib and the risk of Oral cancer: the Erlotinib prevention of Oral cancer (EPOC) randomized clinical trial. JAMA Oncol. 2016;2:209‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gupta B, Bray F, Kumar N, Johnson NW. Associations between oral hygiene habits, diet, tobacco and alcohol and risk of oral cancer: a case–control study from India. Cancer Epidemiol. 2017;51:7‐14. [DOI] [PubMed] [Google Scholar]
- 39.Srinivasan P, Sabitha KE, Shyamaladevi CS. Modulatory efficacy of green tea polyphenols on glycoconjugates and immunological markers in 4‐Nitroquinoline 1‐oxide‐induced oral carcinogenesis—a therapeutic approach. Chem Biol Interact. 2006;162:149‐156. [DOI] [PubMed] [Google Scholar]
- 40.van der Waal I. Knowledge about oral leukoplakia for use at different levels of expertise, including patients. Oral Dis. 2018;24:174‐178. [DOI] [PubMed] [Google Scholar]
- 41.Silverman S Jr, Gorsky M, Lozada F. Oral leukoplakia and malignant transformation. A follow‐up study of 257 patients. Cancer. 1984;53:563‐568. [DOI] [PubMed] [Google Scholar]
- 42.Pomerantz M. Active surveillance. Surg Pathol Clin. 2015;8:581‐585. [DOI] [PubMed] [Google Scholar]
- 43.Mattsson U, Jontell M, Holmstrup P. Oral lichen planus and malignant transformation: is a recall of patients justified? Crit Rev Oral Biol Med. 2002;13:390‐396. [DOI] [PubMed] [Google Scholar]
- 44.Meyerson AF, Lessing J, Itakura K, et al. Outcome of long term active surveillance for estrogen receptor‐positive ductal carcinoma in situ. Breast. 2011;20:529‐533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heintzelman DL, Urtinger U, Guchs H, et al. Optimal excitation wavelengths for in vivo detection of Oral neoplasia using fluorescence spectroscopy. Photochem Photobiol. 2000;72:103‐113. [DOI] [PubMed] [Google Scholar]
- 46.Zlotogorski A, Dayan A, Dayan D, et al. Nutraceuticals as new treatment approaches for oral cancer – I: curcumin. Oral Oncol. 2013;49:187‐191. [DOI] [PubMed] [Google Scholar]
- 47.Verhoeven DT, Goldbohm RA, van Poppel G, Verhagen H, van den Brandt PA. Epidemiological studies on brassica vegetables and cancer risk. Cancer Epidemiol Biomarkers Prev. 1996;5:733‐748. [PubMed] [Google Scholar]
- 48.Byrd JA, Davis MDP, Bruce AJ, Drage LA, Rogers RS 3rd.Response of oral lichen planus to topical tacrolimus in 37 patients. Arch Dermatol. 2004;140:1508‐1512. [DOI] [PubMed] [Google Scholar]
- 49.Lavanya N, Jayanthi P, Rao UK, Ranganathan K. Oral lichen planus: an update on pathogenesis and treatment. J Oral Maxillofac Pathol. 2011;15:127‐132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giustina TA. Topical application of isotretinoin gel improves oral lichen planus. A double‐blind study. Arch Dermatol. 1986;122:534‐536. [PubMed] [Google Scholar]
- 51.Yagyuu T, Hatakeyama K, Kurihara M, et al. Programmed death ligand 1 (PD‐L1) expression and tumor microenvironment: implications for patients with oral precancerous lesions. Oral Oncol. 2017;68:36‐43. [DOI] [PubMed] [Google Scholar]
- 52.Chen X‐J, Zhang X‐Q, Tang M‐X, Zhou G. PD‐l1 conjugated all‐trans retinoic acid nanoparticles for targeted treatment of oral dysplasia and oral squamous cell carcinoma. Oral Surg, Oral Med, Oral Pathol Oral Radiol. 2020;129:e198‐e199. [Google Scholar]
- 53.Stasikowska‐Kanicka O, Wągrowska‐Danilewicz M, Danilewicz M. CD8+ and CD163+ infiltrating cells and PD‐L1 immunoexpression in oral leukoplakia and oral carcinoma. APMIS. 2018;126:732‐738. [DOI] [PubMed] [Google Scholar]


