Abstract
Multiple sclerosis (MS) is a chronic inflammatory, demyelinating and neurodegenerative disease affecting the central nervous system (CNS), often characterized by the accumulation of irreversible clinical disability over time. During last years, there has been a dramatic evolution in several key concepts of immune pathophysiology of MS and in the treatment of this disease. The demonstration of the strong efficacy and good safety profile of selective B-cell-depleting therapies (such as anti-CD20 monoclonal antibodies) has significantly expanded the therapeutic scenario for both relapsing and progressive MS patients with the identification of a new therapeutic target. The key role of B cells in triggering MS disease has been also pointed out, determining a shift from the traditional view of MS activity as largely being ‘T-cell mediated’ to the notion that MS-related pathological processes involve bi-directional interactions between several immune cell types, including B cells, both in the periphery and in the CNS. This review provides an updated overview of the involvement of B cells in the immune pathophysiology and pathology of MS. We summarize the rationale regarding the use of anti-CD20 therapies and the results of the main randomized controlled trials and observational studies investigating the efficacy and safety profile of rituximab, ocrelizumab, ofatumumab and ublituximab. Suggestions regarding vaccinations and management of MS patients during COVID-19 pandemic with anti-CD20 therapies are also discussed. Finally, therapies under investigation and future perspectives of anti-CD20 therapies are taken into consideration.
Keywords: Multiple sclerosis, Anti-CD20 therapy, Randomized clinical trials, Disease modifying therapy, B cells
Introduction
Multiple sclerosis (MS) is a chronic, inflammatory, demyelinating and neurodegenerative disease affecting the central nervous system (CNS), often leading to the accumulation of irreversible clinical disability.
During recent years, there has been a dramatic evolution in the arsenal of MS treatments.
Among the developed therapies, anti-CD20 monoclonal antibodies mediating B-cell depletion, such as rituximab, ocrelizumab and ofatumumab, have received increasing attention. The demonstration of the strong efficacy and good safety profile of these selective B-cell-depleting therapies [1–3] has significantly contributed to expand the therapeutic scenario to treat MS patients.
An increasing amount of data regarding the use of anti-CD20 therapies has emerged. Accordingly, an updated overview is currently needed to summarize the most relevant findings supporting B-cell involvement in MS immune pathophysiology as well as the main results regarding pharmacology, efficacy and safety of such therapies obtained from the most recent randomized controlled trials (RCTs) and observational studies. Guidelines for the proper timing of vaccinations and recent evidence about the risk of COVID-19 disease in association with anti-CD20 therapies are also discussed. Finally, future perspectives of anti-CD20 therapies and new promising anti-B-cell treatments are described.
Methods
We review the most recent evidence regarding the involvement of B cells in the immune pathophysiology and pathology of MS, the rationale underlying the use of anti-CD20 therapies and the results of the main RCTs and observational studies investigating the efficacy and safety profile of anti-CD20 therapies currently available.
References for this Review were identified through searches of PubMed with the search terms ‘adverse event(s)’, ‘antigen-presenting cell’, ‘atrophy’, ‘B-cell’, ‘blood’, ‘Bruton tyrosine kinase’, ‘CD19’, ‘CD20’, ‘COVID-19’, ‘cerebrospinal fluid’, ‘demyelination’, ‘depleting therapy(ies)’, ‘disability’, disease activity’, ‘disease-modifying’, ‘gadolinium-enhancing lesion(s)’, ‘immunoglobulin’, ‘immunology’, ‘inebilizumab’, ‘infusion reaction(s)’, ‘lymphocyte’, ‘malignancy(ies)’, ‘monoclonal antibody(ies)’, ‘MRI’, ‘multiple sclerosis’, ‘ocrelizumab’, ‘ofatumumab’, ‘outcomes’, ‘overall drug persistence’, ‘pathology’, ‘phenotype(s)’, ‘prediction’, ‘progression’, ‘primary progressive’, ‘randomized controlled trial’, ‘relapse’, ‘relapsing–remitting’, ‘rituximab’, ‘safety’, ‘SARS-CoV2’, ‘secondary progressive’, ‘T-cell’, ‘T2-hyperintense lesion(s)’, ‘tolebrutinib’, ‘treatment’, ‘ublituximab’, ‘vaccination’ from 1 January 1979 to 5 July 2021. Articles were also identified through searches of the authors’ own files. Abstract presented at main congresses in the field were also evaluated. Only papers published in English were reviewed. The final reference list was generated on the basis of originality and relevance to the broad scope of this Review, with a focus on articles published during the past 3 years.
The role of B cells in MS immune pathophysiology
Historically, the classical view of MS immune pathophysiology, based on the convergence of studies from MS patients and experimental models, supposed that bouts of MS inflammatory activities are principally mediated by aberrantly activated and/or dysregulated pro-inflammatory CNS-reactive T effector (Teff) CD4 + cells (including T helper 1 [Th1], interleukin-17 [IL-17]-expressing CD4 [Th17], granulocyte–macrophage colony-stimulating factor [GM-CSF] expressing CD4) and CD8 + T cells (including IL-17-expressing CD8 [Tc17], GM-CSF expressing CD8, and mucosal-associated invariant T-cell receptor [TCR]-expressing CD8+ cells). By trafficking into the CNS, these cells are supposed to cause perivascular demyelination, glial cell activation and neuro-axonal injury [4, 5].
During the last few years, there has been a dramatic evolution in several key concepts of MS immune pathophysiology. One update involves a shift from the traditional view of MS disease activity as largely being ‘T-cell mediated’ to the view that MS relapses involve key bi-directional interactions between several immune cell types, including B cells, both in the periphery and in the CNS [4, 6].
This updated conceptual framework of the cellular immunology underlying MS activity has occurred after the demonstration of the strong efficacy of selective B-cell-depleting therapies (such as anti-CD20 monoclonal antibodies), pointing out the key role of B cells in triggering MS disease activity [1–3, 7].
The original impetus for targeting B cells in MS was based on the long-standing recognition of abnormally produced antibodies in the CNS of patients with MS (e.g., increased immunoglobulin [Ig] synthesis rates, cerebrospinal fluid (CSF)-restricted oligoclonal bands, antibodies bound to myelin fragments within phagocytic cells in the CNS parenchyma, Ig and complement detection in demyelinated lesions) [8–11].
Of note, B cells, plasmablasts and plasma cells are increased in the CSF of MS patients and their number is positively associated with intrathecal inflammation and Ig synthesis [12]. Despite this, the antigenic targets of the aberrant immune cell activation in MS remain incompletely defined and the long-term contribution of autoantibodies is largely unknown. Historically, the focus of investigation has been on myelin proteins, such as myelin basic protein (MBP), proteolipid protein (PLP) and myelin oligodendrocyte glycoprotein (MOG) [6, 13]. However, studies of circulating antibodies in MS patients including those directed against myelin antigens (MBP, MOG) [14] and the inward rectifying potassium channel (Kir) 4.1 [15, 16] have not led to the same pathogenic implications of specific CNS-directed antibodies, as those recognized in other conditions such as anti-aquaporin 4 (AQP4) antibodies in neuromyelitis optica spectrum disorders (NMOSD) or anti-MOG associated disease (MOGAD).
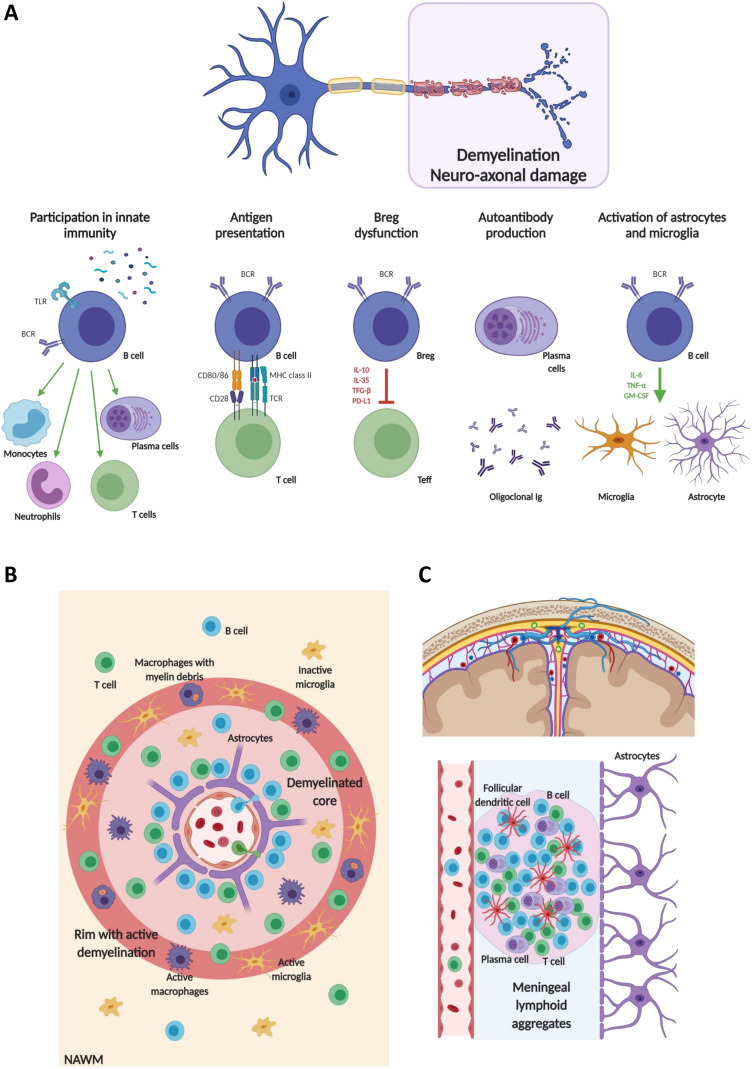
Growing evidence suggests that antibody-independent functions of B cells play key roles in mediating disease activity. B cells have been demonstrated to contribute to cascades of cellular immune interaction in the periphery, to act as antigen presenting cells (APCs) to T cells, thus promoting T-cell activation and proliferation, to interact with APCs to influence antigen trafficking, and to be directly involved in the production of cytokines and chemokines exerting both anti- and pro-inflammatory actions and contributing to oligodendrocyte and neuronal damage (Fig. 1) [4, 6].
Fig. 1.
Summary of the involvement of B cells in the immune pathophysiology and pathology of MS. A Roles of B cells in immunity and disimmunity. In MS, B cells are involved in innate immunity, antigen presentation, production of regulatory and pro-inflammatory cytokines, chemokines and autoantibodies. B, C Overview of the distribution of B cells in the different CNS areas involved in MS pathology. B In an active MS lesion with a central inflamed vein, a demyelinated core, with microglia and macrophages, and a rim of active ongoing demyelination with activated microglia, macrophages with different stages of myelin degradation, and oligodendrocyte injury, the highest density of lymphocytes is seen in the perivascular space of the central vein, with the majority of B cells in the lesion present at this site. C Aggregates of B cells can also be observed in the leptomeninges. This compartmentalized inflammation, characterized by the development of ectopic follicle-like lymphoid aggregates, is mainly driven by B cells, plasma cells, T cells and follicular dendritic cells. Created with biorender.com. See text for further details. BCR B-cell receptor, Breg regulatory B-cell, Ig immunoglobulin, GM-CSF granulocyte–macrophage colony-stimulating factor, IL-6 interleukin 6, IL-10 interleukin 10, IL-35 interleukin 35, MHC class II major histocompatibility complex class II, NAWM normal-appearing white matter, PD-L1 ligand programmed death ligand 1, TCR T-cell receptor, Teff effector T-cell, TFG-β transforming growth factor beta, TLR Toll-like receptor, TNF-α tumor necrosis factor alpha
B cells are well-known efficient APCs, characterized by the expression of class-II major histocompatibility complex (MHC class II), and specialized in capturing soluble and membrane-tethered antigens, with a higher efficiency in presenting antigens and activating T cells than non-B-cell APCs [6, 17]. Due to the possible relevant role of B cells in processing CNS antigens, B cells could promote an increased activation of Teff, thanks to strong B-cell–T-cell interactions mediated by more than 20 co-stimulatory molecule-receptor pairs, with CD80/86 and their T-cell-activating binding partner CD28 being among the best characterized [6]. In addition to expressing co-stimulatory molecules, B cells can also express co-inhibitory molecules involved in downregulating the responses of Teff, such as the programmed death ligand 1 (PD-L1) and its receptor, programmed death 1 (PD-1) [6].
In MS, B cells are also recognized to have not only an abnormal propensity to produce pro-inflammatory cytokines (interleukin 6 [IL-6], GM-CSF, tumor necrosis factor alpha [TNF-α], and lymphotoxin alpha [LT-α]), but also a deficient capacity to produce regulatory cytokines (such as interleukin 35 [IL-35], and transforming growth factor beta [TFG-β]) [4, 6, 18–22]. Due to such an abnormal cytokine response profile, B cells can induce aberrant pro-inflammatory Th1, Th17 and myeloid cell responses, contributing to the cellular immune cascades involved in disease activity [4, 6, 18–22].
B cells in MS pathology
Pathological studies have consistently shown that B cells significantly contribute to MS pathology in the CNS [23–27]. B-cell infiltrates are significantly higher in MS compared with other inflammatory CNS diseases, especially in patients at early stages of MS and with active lesions.
In early and active focal demyelinating lesions, CD20+ B cells are mainly located focally in the perivascular space of only one or a few larger veins and have pro-inflammatory functions (Fig. 1) [23, 24]. Conversely, a more abundant plasma cell infiltrate can be found in the perivascular space and in the meninges from patients with progressive MS (Fig. 1) [23, 24]. This evidence suggests a gradual differentiation of infiltrating B cells into a stable plasma cell population, showing expression of markers involved in B-cell survival and plasmablast differentiation (CD27 and CD38) [23, 24].
In addition to cascades of the peripheral cellular immune interactions contributing to ‘relapse biology’, there is also an important role for a ‘CNS-compartmentalized’ inflammation that sustains chronic inflammation, demyelination, and neurodegeneration, which can be maintained in the absence of ongoing relapse biology.
This ‘CNS-compartmentalized’ inflammation is characterized by prominent B-cell-rich inflammatory aggregates resembling tertiary lymph follicles that can be found in the meninges of MS patients, mainly within deep cortical sulci, but also in the perivascular spaces (Fig. 1). These inflammatory aggregates in the CNS may provide an environment that fosters B-cell homing, survival and functional activation [28, 29], and, in turn, contribute to degenerative mechanisms [30].
For instance, the extent of meningeal inflammation and the levels of pro-inflammatory cytokines (e.g., interferon gamma [IFN-γ], TNF-α, LT-α, IL-6) in the CSF of MS patients have been associated with the severity of subpial cortical demyelination, promoting also a graded pattern of neuronal loss and microglial activation consistent with a ‘surface-in’ process possibly mediated by one or more toxic substances contained in the CSF [25–27, 31, 32].
Mechanisms of action of anti-CD20 therapies
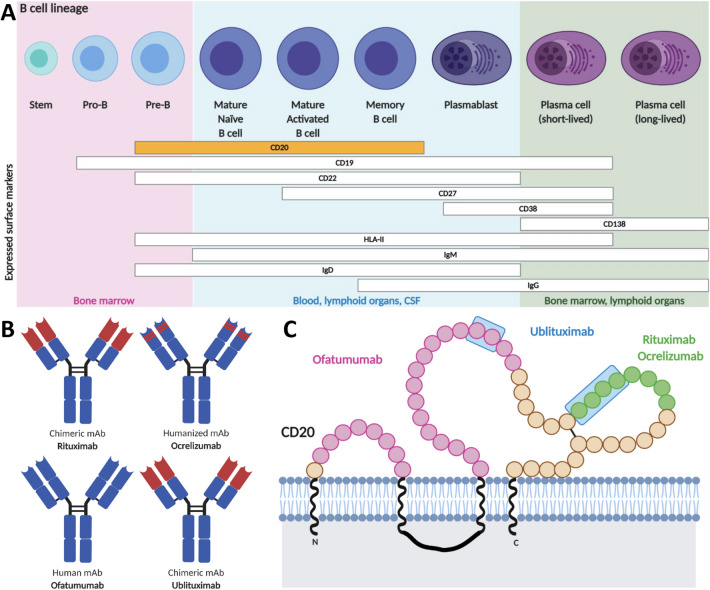
CD20 is a transmembrane, non-glycosylated phosphoprotein of 33–37 kDa that is expressed in tetramers associated with lipid rafts on the surface of cell lineage from pre-B cells to naïve and memory B cells (Fig. 2) [33].
Fig. 2.
B-cell lineage, main anti-CD20 monoclonal antibodies and their targeted CD20 epitopes. A Summary of the B-cell maturation stages, defined according to the expression of specific cell-surface antigens. CD20 is expressed in pre-B cells, mature and memory B cells. Of note, both early and late maturation stages are not depleted since they do not express CD20, thus B-cell repopulation and humoral immune memory are preserved. B Structure of the different anti-CD20 monoclonal antibodies used in MS. C Schematic overview of the different CD20 epitopes recognized by each specific anti-CD20 monoclonal antibody. Created with biorender.com. See text for further details. CSF cerebrospinal fluid, HLA-II human leukocyte antigen II, IgD immunoglobulin D, IgG immunoglobulin G, IgM immunoglobulin M, mAb monoclonal antibody
Monoclonal antibodies directed against specific targets typically deplete targeted cells through at least four possible different mechanisms: (i) antibody-dependent cellular cytotoxicity (ADCC); (ii) complement-dependent cytotoxicity (CDC); (iii) antibody-dependent cellular phagocytosis (ADCP); and (iv) induction of cell apoptosis. Currently available anti-CD20 monoclonal antibodies induce B-cell depletion mainly through ADCC, CDC and ADCP [33].
The infusion of anti-CD20 monoclonal antibodies promotes a depletion of CD20+ B cells within hours, mainly occurring in the liver [34]. Such a depletion reaches the nadir typically after 8 weeks and can be sustained for several weeks to months according to the posology and the features of the specific anti-CD20 monoclonal antibody.
After anti-CD20 therapies, B-cell repopulation starts in bone marrow and spleen, followed by blood, with a different rate between memory B cells and CD19+ cells [35].
Of note, B-cell counts are usually determined using CD19, which largely overlaps with CD20 during B-cell differentiation, because it is less prone to potential interference in presence of anti-CD20 monoclonal antibodies [35].
B-cell reappearance can be defined when CD19+ B cells reach 1% of lymphocyte counts [36]; however, other criteria are also applied, including 2% of CD19+ B cells [36].
Efficacy of anti-CD20 therapies
Rituximab
Rituximab is a chimeric monoclonal antibody that is commonly prescribed in highly active MS patients, although it is not approved from Food and Drug Administration (FDA) nor European Medicines Agency (EMA) for use in MS (Fig. 2).
Its efficacy has been investigated in several studies as summarized in Table 1.
Table 1.
Summary of randomized controlled trials and observational studies assessing rituximab in multiple sclerosis patients
| References | Number and type of patients | Trial design | Clinical findings | MRI findings | Adverse effects |
|---|---|---|---|---|---|
| Bar-Or et al. [72] | 26 RRMS | Phase I, multicenter open label (72 weeks) | ↓ mean ARR from b to week 72 (1.27 → 0.18) |
↓ T2 LV (↓ 119.6 mm3 at week 48, ↓ 272.7 mm3 at week 72) ↓ Gd+ lesions from b to week 72 (1.31 → 0) |
AEs in all patients (77% mild-moderate, 23% severe) No serious AEs 65% with IARs 62% mild-moderate infection-associated events |
| Hauser et al. [1] | 104 RRMS | Phase II, randomized, parallel, double-blind, Pbo-controlled study (HERMES-NCT00097188) (48 weeks) | ↓ ARR at week 24 (0.37 with RTX vs 0.84 Pbo, p = 0.04) |
↓ in T2 LV (–163 mm3 with RTX vs + 436 mm3 with Pbo at week 24, p = 0.008, − 175 mm3 with RTX vs + 418 mm3 with Pbo at week 36, p = 0.004) ↓ Gd+ lesions at weeks 12, 16, 20, 24, and 48 (0.5 with RTX vs 5.5 with Pbo; RR = 91%, p < 0.001) |
More IARs with RTX (87.3% vs 40%) after first infusion, opposite after second infusion (20.3% vs 40%), 7.4% severe, remaining mild–moderate Similar numbers of serious AEs with RTX vs Pbo (13% vs 14.3%) Similar number of infections (69.6% vs 71.4%) |
| Hawker et al. [37] | 439 PPMS | Phase II/III, randomized, double-blind, Pbo-controlled study (OLYMPUS-NCT00087529) (96 weeks) |
Proportion of patients with 3-month CDP not significantly different at week 96 (RTX = 30.2% vs Pbo = 38.5%, p = 0.14) Delayed CDP in patients aged < 51 years at b with RTX |
Less ↑ of T2 LV at week 96 (median increase: + 302.0 mm3 with RTX, + 809.5 mm3 with Pbo, p < 0.001) Similar rate of brain atrophy between groups (− 13.1 cm3 with RTX vs − 14.0 cm3 with Pbo, p = 0.62) Delayed CDP in patients with Gd+ lesions at b with RTX |
16.1% with RTX vs 13.6% Pbo with serious AEs 4.5% with RTX vs < 1% Pbo serious infections Mild–moderate IARs with RTX during the first course, decreased to rates comparable to Pbo with successive courses |
| Naismith et al. [73] | 32 RRMS | Phase II single center (no Pbo-group)—no MRI until week 20 (52 weeks) |
Stable EDSS during follow-up ↑ MSFC improvement (+ 0.039 z-score) |
↓ Gd+ lesions (74% at b vs 26% at week 20, p < 0.0001) No effect on T2 and T1 lesion burden |
No serious AEs 2 withdrawn due to IARs 4 uncomplicated urinary tract infections 1 upper respiratory tract infection |
| Alping et al. [74] | 256 RRMS | Prospective multicenter (at least 1-year follow-up) | ↓ relapses with RTX vs FTY (1.8% vs 17.6%) | ↓ Gd+ lesions in RTX vs FTY (1.4% vs 24.2%) | More AEs with FTY (21%) vs RTX (5%) |
| De Flon et al. [75] | 75 RRMS | Open-label, uncontrolled phase II study (24 weeks) | 5 patients experienced disease activity (2 clinical relapses, 4 MRI activity) |
↓ Gd+ lesions (0.028 at b → 0.036 at 6 months) ↓ new/enlarged T2 lesions (0.28 at b → 0.01 at 12 months) |
Moderate IARs 3 serious AEs (pyelonephritis, influenza) |
| Salzer et al. [52] | 822 MS (557 RRMS, 198 SPMS, 67 PPMS) | Retrospective uncontrolled observational study (mean follow-up 21.8 months) |
Stable EDSS in RRMS, ↑ in SPMS/PPMS ↓ ARR (RRMS = 0.044, SPMS = 0.038, PPMS = 0.015) |
↓ Gd+ lesions (26.2% at b → 4.6% at the last available follow-up) |
IARs in 7.8% 89 AEs grade > 2 in 70 patients (infections) |
| Alcala et al. [76] | 90 MS (31 RRMS, 45 SPMS, 14 PPMS) | Retrospective single-center (follow-up 6 months–5 years) |
↓ 88.4% ARR NEDA-3 at 1 year: all MS = 70% RRMS = 74.2%, PMS = 67% |
↓ Gd+ lesions (2.56 at b → 0.06 at the last available follow-up, p < 0.001) |
18.8% IARs 4 SAE (1 agranulocytosis, 3 thrombotic events, 1 death due to pulmonary embolism) |
| Durozard et al. [77] | 50 RRMS | Nationwide retrospective multicenter (median follow-up 1.1 years) | ↓ ARR (0.8 pre-RTX → 0.18 post-RTX, p < 0.001) | ↓ Gd+ lesions (72% pre-RTX → 8% post-RTX, p < 0.001) |
16 AEs, 10 patients with ≥ 1 AE (mainly infections) 3 SAEs 2 treatment discontinuations due to AE |
| Granqvist et al. [78] | 120 RRMS | Retrospective multicenter (follow-up up to 4 years) | ↓ ARR with RTX vs injectable DMTs (p < 0.01) | ↓ Gd+ lesions with RTX- (1.7%) vs injectable DMTs (12.6%) and DMF (12.8%) |
No serious AEs with RTX Mild AEs more common for injectable DMTs vs RTX |
| Yamout et al. [79] | 59 RRMS, 30 PMS | Retrospective single center (mean follow-up 22.2 months) |
↓ ARR (1.07 → 0.11 RRMS and 0.25 → 0.16 PMS) Stable EDSS in both groups NEDA-3 = 74% at 1 year |
↑ of patients free from new MRI lesions (18.6 → 92.6% in RRMS and 43.3% → 82% in PMS) |
64 AEs with RTX (71.9% of patients), IARs (25.8%) 2 serious AEs (pyoderma gangrenosum, increase in meningioma size) |
| Honce et al. [80] | 55 RRMS | Prospective double-blind single center (mean follow-up 1.5 years) | NEDA-3 = 44.4% with RTX-GA vs 19.2% Pbo-GA | ↓ proportion of patients with new T2 lesions (25.9% RTX-GA vs 61.5% Pbo-GA, p = 0.009) |
↑ IARs in RTX 4 serious AEs in RTX, 5 in Pbo |
| Zecca et al. [81] | 355 MS (188 RRMS, 43 PPMS, 124 SPMS) | Retrospective, uncontrolled, observational study (median treatment 1.9 years) | ↓ ARR vs 1 year before (RRMS = 0.86 → 0.09, p < 0.001; SPMS = 0.34 → 0.06, p < 0.001; PPMS = 0.12 → 0.07, p = 0.45) | At m12, 15.8% had new T2 and/or Gd+ lesions; 4.1% Gd+ lesions and 13.4% new T2 lesions |
23.7% at least 1 IAR 3.1% serious AE 8 patients withdrew 1 death due to mediastinal neoplasm |
AE adverse events, ARR annualized relapse activity, CDP confirmed disability progression, DMTs disease-modifying therapies, EDSS expanded Disability Status Scale, FTY fingolimod, GA glatiramer acetate, Gd+ gadolinium-enhancing, IARs infusion-associated reactions, IFN-β1a interferon-β1a, LV lesion volume, MRI magnetic resonance imaging, MSFC multiple sclerosis functional composite, NEDA-3 no evidence of disease activity 3, Pbo placebo, PMS progressive multiple sclerosis, PPMS primary progressive multiple sclerosis, RR relative reduction, RRMS relapsing–remitting multiple sclerosis, RTX rituximab, SPMS secondary progressive multiple sclerosis
In particular, in a 48-week phase-II RCT (HERMES), 104 relapsing–remitting (RR) MS patients were randomized to receive either a single administration of i.v. rituximab 1000 mg or placebo on days 1 and 15 [1]. Compared with placebo, patients who received rituximab had a lower ARR at week 24 (p = 0.04), not confirmed at week 48 (p = 0.08), and a reduction of T2-hyperintense lesion volume (LV) from baseline to week 24 (p = 0.008) and 36 (p = 0.004). From week 12, rituximab also reduced Gadolinium (Gd)-enhancing lesions (p ≤ 0.003).
The efficacy of rituximab has been also evaluated in a 96-week phase-II/III RCT of 439 primary progressive (PP) MS patients (OLYMPUS) [37]. Rituximab (two i.v. administrations of 1000 mg 2 weeks apart) was not associated with changes in the proportion of patients developing confirmed disease progression (CDP) (p = 0.14), the primary outcome of the study, nor in brain atrophy rate (p = 0.62). However, it promoted a significant lower increase of T2-hyperintense LV at week 96 (p < 0.001) compared with placebo [37]. In a subgroup analysis, rituximab showed a delayed time to CDP in younger PPMS patients (aged < 51 years) or those with Gd-enhancing lesions at baseline. An additive predictive effect of age and Gd-enhancing lesions at baseline was found, suggesting that B-cell depletion might be effective in PPMS patients who are younger and have higher inflammatory activity [38].
Although further exploration of efficacy has not been carried out in phase-III RCTs, several other observational studies have confirmed a significant reduction of disease activity with rituximab (Table 1).
Few studies have utilized an intrathecal approach to administer rituximab to target compartmentalized inflammation in progressive MS. In an open-label study in eight progressive MS patients with MRI evidence of meningeal inflammation, a significant and sustained reduction in circulating B cells and a transient drop in CSF B cells were observed, but this did not translate in a change in the number or appearance of leptomeningeal enhancement [39].
Ocrelizumab
Ocrelizumab is a humanized anti-CD20 monoclonal antibody (Fig. 2) approved by the FDA (March 2017) and EMA (January 2018), at a dose of 600 mg i.v. twice yearly, as a therapy for the treatment of highly active relapse-onset MS and PPMS with evidence of disease activity.
A first phase-II RCT explored ocrelizumab efficacy in RRMS patients, assigned to either i.v. low (600 mg; n = 55) or high (2000 mg; n = 55) ocrelizumab administrations divided into two doses on days 1 and 15, i.v. placebo (n = 54) or weekly intramuscular (i.m.) INFβ-1a (30 µg; n = 54) (Table 2) [40]. RRMS patients treated with ocrelizumab showed a significantly lower ARR (0.13 in the low- and 0.17 in the high-dose group) compared with placebo (0.64) and INFβ-1a (0.36) group [40]. Change in T2-hyperintense LV did not differ among groups at week 24 (p = 0.2 in the low- and high-dose groups; p = 0.5 in the INFβ-1a-group), whereas the total number of Gd-enhancing lesions was significantly lower in ocrelizumab groups compared with placebo- and INFβ-1a groups (both p < 0.001).
Table 2.
Clinical trials assessing treatment with ocrelizumab in patients with MS
| References | Number of patients | Trial design | Clinical findings | MRI findings | Adverse effects |
|---|---|---|---|---|---|
| Kappos et al. [40] | 220 RRMS | Phase II, randomized, parallel, double-blind, Pbo-controlled study (48 weeks) | ↓ ARR with OCR (low dose = 0.13; high dose = 0.17) vs Pbo (0.64) and INFβ-1a (0.36) | ↓ Gd+ lesions with 600 and 2000 mg OCR (0.6 and 0.2, respectively) vs Pbo (5.5) and INFβ-1a (6.9) |
↑ IARs with OCR vs Pbo (44% vs 9%) No ↑ serious AE Similar rate of infections |
| Hauser et al. [2] |
821 RRMS (OPERA I) 835 RRMS (OPERA II) |
Phase III, randomized, double-blind, active-controlled, parallel group studies (OPERA I and OPERA II) (96 weeks) |
↓ ARR with OCR (46% and 47%, p < 0.001) vs IFN-β1a ↓ proportion of patients with 3-month CDP with OCR (43% and 37%, p < 0.05) |
↓ Gd+ lesions (94% OPERA I; 95% OPERA II, p < 0.001) ↓ new/enlarged T2 lesions (77% OPERA I; 83% OPERA II, p < 0.001) with OCR vs INFβ-1a ↓ percentage of brain volume loss from week 24 to 96 in OPERA I (− 0.57% vs − 0.74%, p = 0.004) but not in OPERA II (− 0.64% vs − 0.75%, p = 0.09) |
↑ IARs 34% OCR vs 10% INFβ-1a or Pbo ↑ infections 59.9% with OCR (vs 54.3% INFβ-1a) in OPERA I, and 60.2% (vs 52.5% with INFβ-1a) in OPERA II → ↑ upper respiratory tract infections with OCR No ↑ serious AE ↑ neoplasm OCR (0.4%) vs INF-β-1a (0.2%) |
| Montalban et al. [2] | 732 PPMS | Phase III, double-blind, randomized, Pbo-controlled, parallel group study (ORATORIO) (120 weeks) | ↓ proportion of patients with 3-month CDP (32.9% OCR vs 39.3% Pbo, p = 0.03) and 6-month CDP (29.6% vs 35.7%, p = 0.04) |
↓ 34% T2 lesions from b to week 120 (mean change, − 3.4% with OCR vs 7.4% with Pbo, p < 0.001) ↓ loss of brain volume (− 0.90 with OCR vs − 1.09 with Pbo, p = 0.02) |
↑ IARs with OCR (40%) vs Pbo (26%) ↑ infections with OCR (71.4%) vs Pbo (69.9%) → ↑ upper respiratory tract infections with OCR No ↑ SAE ↑ neoplasm with OCR (2.3%) vs Pbo (0.8%) |
| Turner et al. [46] | 1656 RRMS | Phase III, randomized, double-blind, active-controlled, parallel group studies (pooled OPERA I and OPERA II) (96 weeks) | ↓ ARR and NEDA-3 re-baselined at week 24 in patients aged < 40 years or with ≥ 1 Gd+ lesion at b with OCR | ↓ Gd+ lesions in patients aged < 40 years or with ≥ 1 Gd+ lesion at b with OCR | – |
| Ellwardt et al. [82] | 210 MS (155 RRMS/SPMS, 55 PPMS) | Retrospective, single-center (median follow-up 200 days) | 13% of patients experienced a relapse and 5% experienced a 12-week CDP | – |
22% AE, 9% IARs Minor infections (8%) and 2 cases of a prolonged herpes labialis 1 case of toxic drug-induced hepatopathy |
| Hauser et al. [3] | 702 RRMS | Open-label extension, phase-III trials (OPERA I and OPERA II) |
↓ proportion of patients with 6 months CDP (16.1% with OCR/OCR vs 21.3% with IFN-β-1a/OCR at y5, p = 0.014) NEDA-3 = 65.4% with OCR/OCR vs 55.1% with IFN-β-1a/OCR (p < 0.001) |
Sustained suppression of new brain MRI lesion activity from years 3 to 5. ↓whole brain volume loss at 5 years vs b in those starting OCR earlier (OCR/OCR = − 1.87% vs IFN-β-1a/OCR = − 2.15%; p < 0.01) | AE consistent with past reports and no new safety signals emerged with prolonged treatment |
| Hartung et al. [44] | 678 RRMS | Open-label, prospective, single-arm, phase-IIIb study (ENSEMBLE) |
92.8% of patients free from clinical disease activity NEDA-3 = 84.8% after 1 year of treatment |
91.3% of patients free from MRI disease activity | AE consistent with past reports |
| Wiendl et al. [45] | 680 RRMS | Phase-IIIb study (CASTING) |
80.4% of patients free from clinical disease activity NEDA-3 = 74.8% after 2 years of treatment |
91.5% of patients free from MRI disease activity | AE consistent with past reports |
| Wolinsky et al. [47] | 732 PPMS | Open-label extension, phase-III trial (ORATORIO) (144 weeks) | ↓ proportion of patients with 24 weeks CDP (EDSS: 51.7% vs 64.8%, p = 0.002; 9-hole peg test: 30.6% vs 43.1% p = 0.003) with OCR vs Pbo |
↓ T2 LV (0.4% vs 13.0%, p < 0.0001) ↓ T1 LV (36.7% vs 60.9%, p < 0.001) with OCR vs Pbo ↓ rates of whole brain (− 3.1% vs − 3.4%; p = 0.13) and cortical gray matter atrophy (− 2.5% vs − 2.6; p = 0.38) from b to week 144 |
AE consistent with past reports |
AE adverse events, ARR annualized relapse activity, CDP confirmed disability progression, DMTs disease-modifying therapies, EDSS expanded Disability Status Scale, Gd+ gadolinium-enhancing, IARs infusion-associated reactions, IFN-β1a interferon-β1a, LV lesion volume, NEDA-3 no evidence of disease activity 3, OCR ocrelizumab, Pbo placebo, PPMS primary progressive multiple sclerosis, RRMS relapsing–remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis
At week 24, patients initially treated with placebo, 600 mg ocrelizumab and i.m. INFβ-1a received 600 mg of ocrelizumab, whereas the 2000 mg ocrelizumab group received 1000 mg of ocrelizumab. An interim analysis of the open-label extension (OLE) phase showed a maintained low ARR in ocrelizumab groups (0.06 vs 0.07 in the placebo/ocrelizumab group and 0.07 in the INFβ-1a/ocrelizumab group) over 96–144 weeks [41].
Two identical, phase-III RCTs, including 821 (OPERA I) and 835 (OPERA II) RRMS patients [2], further demonstrated ocrelizumab efficacy (Table 2). Patients were randomized (1:1) to i.v. ocrelizumab 600 mg every 24 weeks or subcutaneous (s.c.) IFNβ-1a 44 μg three times per week over 96 weeks. Compared with IFNβ-1a, ocrelizumab showed a higher reduction of ARR (p < 0.001), a lower prevalence of 12-week CDP (p < 0.05), and lower numbers of new/enlarged T2-hyperintense and Gd-enhancing lesions (p < 0.001) [2]. The proportion of RRMS patients with 12-week confirmed disability improvement was also higher with ocrelizumab compared with IFNβ-1a, being significant in the OPERA I (p = 0.01) but not in the OPERA II (p = 0.40) [2]. Percentage of brain volume loss from week 24 to 96 was significantly lower in ocrelizumab- vs IFN-β-1a-group in the OPERA I (p = 0.004), but not in the OPERA II (p = 0.09) [2].
In the 3-year follow-up OLE [42], the cumulative proportion of patients with 24-week CDP was lower in patients who initiated ocrelizumab earlier vs those initially receiving IFNβ-1a (p = 0.014). ARR did not differ between RRMS patients continuing ocrelizumab and those receiving ocrelizumab after switching from IFNβ-1a (p ≥ 0.70); both groups attained an almost complete and sustained suppression of brain MRI lesion activity.
The proportion of RRMS patients with no evidence of disease activity 3 (NEDA-3) (i.e., no relapses, no disability progression, no new/enlarged T2-hyperintense or Gd-enhancing lesions) was also significantly higher in ocrelizumab groups compared with the INFβ-1a groups both in OPERA I and OPERA II (p < 0.001) [43]. In the OLE period, the proportion of patients with NEDA-3 was 65.4% in patients continuously treated with ocrelizumab compared with 55.1% in those switching from IFNβ-1a to ocrelizumab (p < 0.001, relative difference = 19%).
Some preliminary results of the open-label, prospective, single-arm, phase-IIIb ENSEMBLE study showed that early RRMS (disease duration ≤ 3 years) treated with ocrelizumab had a high NEDA-3 rate (84.8%) after 1 year of treatment [44]. Similarly, a high 2-year NEDA-3 rate (74.9%) was detected in the primary analysis of the phase-IIIb CASTING trial, which evaluated ocrelizumab efficacy in RRMS patients with prior suboptimal response to one or two disease-modifying therapies (DMTs) (Table 2) [45].
Ocrelizumab has been the first DMT showing significant effects in PPMS, as demonstrated in the phase-III RCT (ORATORIO), which evaluated 732 PPMS patients receiving either i.v. ocrelizumab (600 mg) (n = 488) or placebo (n = 244) every 24 weeks for at least 120 weeks [2]. Ocrelizumab reached the primary study endpoint since it was associated with a significant reduction of 12-week CDP (p = 0.03) compared with placebo, further confirmed with the 24-week CDP (p = 0.04) [2]. The prevalence of worsening on timed 25-foot walk test (25-FWT) was also significantly reduced with ocrelizumab (p = 0.04) [2]. Brain T2-hyperintense LV decreased with ocrelizumab and increased with placebo from baseline to week 120 (p < 0.001), and the rate of brain atrophy was significantly lower with ocrelizumab compared with placebo (p = 0.02) [2].
Similarly to rituximab, a post hoc analysis (pooled OPERA studies) suggested that patients who were younger (aged < 40 years) and with baseline disease activity (≥ 1 Gd-enhancing lesions) had a greater treatment benefit with ocrelizumab, relative to IFNβ-1a, than patients who were older and with inactive disease [46].
An interim report from ORATORIO OLE has shown consistent and sustained treatment-associated benefit in multiple measures of CDP and a good safety profile over 6.5 years [47]. Mean percentage changes of brain T2-hyperintense (p < 0.001) and T1-hypointense (p < 0.001) LVs were lower in patients who initiated ocrelizumab early than in those initially receiving placebo from baseline to week 168 [47]. No difference in the rates of whole brain (p = 0.13) and cortical gray matter atrophy (p = 0.38) from baseline to week 144 was found between the two groups.
Some ongoing RCTs and observational studies are investigating additional aspects, including long-term effectiveness and safety of ocrelizumab and of treatment switch from natalizumab or rituximab to ocrelizumab [48].
Ofatumumab
Ofatumumab is the first fully human anti-CD20 monoclonal antibody, with a 20 mg s.c. monthly dosing regimen (Fig. 2), which has been approved by FDA (August 2020) and EMA (March 2021) for the treatment of active relapsing MS forms.
A phase-II RCT evaluated 38 RRMS patients who received two i.v. infusions of ofatumumab 100, 300, or 700 mg or placebo 2 weeks apart (Table 3) [49]. After 24 weeks, ofatumumab promoted an almost completed suppression of brain new/enlarging T2-hyperintense and Gd-enhancing lesions (> 99%).
Table 3.
Clinical trials assessing treatment with ofatumumab in patients with MS
| References | Number of patients | Trial design | Clinical findings | MRI findings | Adverse effects |
|---|---|---|---|---|---|
| Sorensen et al. [49] | 38 RRMS | Phase II, double-blind, randomized, Pbo-controlled study (48 weeks) | Lower proportion of patients with relapse(s) with OFT vs Pbo (19% vs 25%) |
↓ new/expanding T2 lesions at week 24 (p < 0.001) ↓ new/total number of Gd+ lesions at week 24 (p < 0.001) |
Mostly mild-to-moderate severity AEs 2 patients discontinued for grade-2 (pruritic rash, bronchospasm, cough) and grade-3 (pharyngeal edema, erythema, pruritus) AEs |
| Bar-Or et al. [50] | 232 RRMS | Phase II, double-blind, randomized, Pbo-controlled study (MIRROR) (48 weeks) | Stable EDSS in 79% of patients at week 12 and 24 | ↓ 65% new Gd+ lesions at week 12 (p < 0.001) |
↑ IARs in OFT (52%) vs Pbo (15%) Equivalent infections with OFT and Pbo |
| Hauser et al. [3] | 946 (ASCLEPIOS I) and 936 (ASCLEPIOS II) RRMS | Phase III, double-blind, double-dummy, active-controlled studies (ASCLEPIOS I and ASCLEPIOS II) (30 months) |
↓ ARR (ASCLEPIOS I 0.11 OFT vs 0.22 TER, p < 0.001; ASCLEPIOS II 0.10 OFT vs 0.25 TER, p < 0.001) ↓ proportion of patients with 3- and 6-month CDP (10.9% and 8.1% OFT vs 15.0% and 12.0% TER) |
↓ Gd+ lesions with OFT vs TER (97.5% ASCLEPIOS I, 93.8% ASCLEPIOS II) ↓ new/enlarging T2 lesions (82% ASCLEPIOS I, 84.5% ASCLEPIOS II) No differences in the annualized rate of brain atrophy OFT vs TER (− 0.28% vs − 0.35% in ASCLEPIOS I; − 0.29% vs − 0.35% ASCLEPIOS II) |
Equivalent IARs with OFT (20.2%) vs TER (15%) Equivalent infections with OFT (51.6%) vs TER (52.7%) Equivalent % of neoplasm with OFT (0.5%) vs TER (0.4%) |
AE adverse events, ARR annualized relapse activity, CDP confirmed disability progression, EDSS expanded Disability Status Scale, Gd+ gadolinium-enhancing, IARs infusion-associated reactions, OFT ofatumumab, Pbo placebo, RRMS relapsing–remitting multiple sclerosis, TER teriflunomide
Another phase-II RCT, the MIRROR study, randomized 232 RRMS patients [50] into placebo, ofatumumab 3, 30 or 60 mg every 12 weeks, or ofatumumab 60 mg every 4 weeks. All patients were treated for 24 weeks and followed up until B-cell repletion. Overall, 26 RRMS patients had a relapse during the first 12 weeks, 11 (42%) of whom during the first 4 weeks. Over 24 weeks, 17 (25%) RRMS patients relapsed in the placebo group compared with three to ten RRMS patients (9–22%) in the ofatumumab groups. Most patients (79%) had unchanged EDSS scores at weeks 12 and 24 [50]. With all ofatumumab regimens, the mean cumulative number of Gd-enhancing lesions was reduced by 65% from baseline to week 12 (p < 0.001), with reductions ≥ 90% for each dose ≥ 30 mg (p < 0.002) [50].
Two recent phase-III RCTs (ASCLEPIOS I and II) in RRMS patients compared the efficacy and safety of ofatumumab (20 mg, s.c. administration every 4 weeks) (465 and 481 RRMS patients) with oral teriflunomide 14 mg daily (462 and 474 patients) [3]. Ofatumumab groups showed a significant lower ARR compared with teriflunomide groups (p < 0.001). In pooled analysis, ofatumumab significantly reduced the risk of 12-week CDP by 34.4% and 32.5% at 3 and 6 months (p = 0.002 and p = 0.012), whereas percentages of patients with 24-week disability improvement were not significantly different between treatment groups (p = 0.09) (Table 3).
Ofatumumab promoted also a more effective suppression of MRI activity compared with teriflunomide, with a significant reduction of new/enlarging T2-hyperintense (ASCLEPIOS I = 82%; ASCLEPIOS II = 84.5%) and Gd-enhancing lesions (ASCLEPIOS I = 97.5%; ASCLEPIOS II = 93.8%). The annualized brain atrophy rate did not differ significantly between the ofatumumab- and teriflunomide groups (− 0.28% vs − 0.35% in ASCLEPIOS I; − 0.29% vs − 0.35% ASCLEPIOS II).
Safety and tolerability of anti-CD20 therapies
Infusion-associated adverse events
Treatment infusion-associated reactions (IARs) are of mild-to-moderate severity. The most common symptoms are fever, headache, rash, nausea, throat irritation, hypotension, and itching. There are no studies comparing the safety profile of different anti-CD20 therapies; evaluating IARs across studies is challenging given different premedication regimens [51].
In a phase-II RCT, IARs were disclosed in 78.3% of RRMS patients treated with rituximab compared with 40.0% in the placebo group [1].
In the OPERA I and II studies, IARs were reported in 34% of the RRMS patients treated with ocrelizumab compared with 10% of those treated with INFβ-1a or placebo, whereas the prevalence was 40% with ocrelizumab versus 26% with placebo in PPMS from the ORATORIO trial [2]. In the OPERA I/II and ORATORIO OLE studies [42, 47], IARs incidence was consistent with past reports.
In the MIRROR study, a similar percentage of IARs was reported for ofatumumab (41–66% according to the regimen used vs 15% for placebo) [50]. In ASCLEPIOS I/II, IARs occurred in 20.2% in the ofatumumab group and 15.0% in the teriflunomide group [3].
Hypogammaglobulinemia
Although CD20 is not expressed on plasmablasts and plasma cells (Fig. 2), anti-CD20-depleting therapies have been shown to reduce Ig levels. Rituximab has some impact on serum IgM and IgG levels [52], and hypogammaglobulinemia can be found during long-time treatment [53]. In RCTs, ocrelizumab reduced the total serum Ig levels to some extent with greatest impact on IgM [2]. However, there was no association between low IgM levels and serious infections. In the MIRROR study, only two out of 232 (1%) RRMS patients treated with ofatumumab developed decreased IgG levels, leading to study termination [50]. In ASCLEPIOS I and II, no association was observed between a decrease in Ig levels and the incidence of serious/non-serious infections in ofatumumab-treated patients who experienced infections within 1 month prior and until 1 month after a reduction in Ig levels below the lower lymphocyte number [3].
Infections and COVID-19 interaction
Before starting treatment, the screening for latent infections should be performed. Reactivation of tuberculosis, hepatitis, and human immunodeficiency virus has been reported in patients treated with anti-CD20 therapies [54].
Although the incidence of infections in the placebo (71.4%) and treated (69.7%) groups in the first phase-II RCT of rituximab in RRMS was similar [1], rituximab was associated with an increased incidence of urinary tract infections and sinusitis.
In RCTs with ocrelizumab, the percentage of patients reporting any infection was 59.9% (vs 54.3% in patients treated with INFβ-1a) in OPERA I, 60.2% (vs 52.5% in patients treated with IFNB-1a) in OPERA II and 71.4% in ORATORIO (vs 69.9% in the placebo group). A slightly increased incidence of upper respiratory tract infections was observed in patients treated with ocrelizumab compared with placebo or INF-β-1a [2]. No increased risk of serious infections was reported in any of these studies.
No cases of progressive multifocal leukoencephalopathy (PML) were reported in RCTs with ocrelizumab, whereas ten cases of PML (as of June 2021) have been described in post-marketing surveillance, of which eight were carry-over cases from prior DMTs [55].
According to data from the phase-II/III RCTs, ofatumumab was not associated with an increased risk of infection-related adverse events (AE) compared with placebo and teriflunomide [3, 50].
Recently, the COVID-19 pandemic raised some concerns about immunosuppression in MS, leading to treatment delay or cessation. Some publications have suggested that anti-CD20 therapies in a 6-month schedule (i.e., rituximab and ocrelizumab) may be associated with an increased risk of severe COVID-19 disease and need for hospitalization [56], not confirmed by a recent study [57]. MS patients appear to respond to SARS-CoV2 in a similar way to the general population and high disability or a progressive disease course represent the most relevant risk factors for a severe COVID-19 disease in MS. Moreover, innate immune response, and, probably, anti-viral CD8 T-cell responses play a major role in eliminating the SARS-CoV2 before significant antibody responses have developed, thus B cells do not appear to be an absolute requirement for recovery. Duration of exposure might play a role, as suggested by the North American Registry, which disclosed an increased risk of hospitalization in patients treated with rituximab, but not in those treated with ocrelizumab [58].
Malignancies
Immunosuppressive drugs could influence immunological tumor surveillance; therefore, long-term data are required to exclude an increased rate of malignancies. In MS patients, rituximab was not associated with an increased neoplastic risk over the long term compared with the general population [59].
In the OPERA I and OPERA II trials, four patients (0.7%) treated with ocrelizumab developed malignancies compared with two patients (0.2%) treated with INF-β-1a [2]. In the ORATORIO trial, 11 patients receiving ocrelizumab (2.3%) developed malignancies (4 breast cancer) compared with two patients (0.8%) in the placebo group [2]. The percentage of patients developing breast cancer in the ocrelizumab-treated group was similar to those expected from epidemiological studies and the incidence decreased during the extension studies [51].
In ASCLEPIOS I/II trials, no unexpected imbalance in the rates of malignancies was observed in ofatumumab-treated patients [3].
Vaccination
Patients should complete any required vaccinations at least 6 weeks prior to treatment initiation. Live attenuated or live vaccines are not recommended during treatment and until B-cell recovery, and at least 6 months after the last administration of rituximab [60].
A recent phase-IIIb RCT (VELOCE) [60] provided class-II evidence that peripherally B-cell-depleted ocrelizumab recipients mounted humoral responses to clinically relevant vaccines and the neo-antigen, KLH, although attenuated. For non-live/inactivated vaccines, such as seasonal influenza vaccines, it is recommended to vaccinate patients treated with ocrelizumab since a protective humoral response can be expected, even if attenuated [60]. An expert consensus recently suggested that patients planned to receive ocrelizumab should be vaccinated at least 6 weeks before the first administration, whereas in those already receiving ocrelizumab, vaccinations should be administered at least 3 months after the last infusion [61].
Therapies currently under investigation and future perspectives
Few studies have investigated ublituximab, a third-generation glycol-engineered chimeric anti-CD20 antibody promoting B-cell depletion through ADCC (Table 4). In a phase-II study of 48 RRMS patients [62], ublituximab promoted a complete B-cell depletion (> 99%) within 4 weeks. The ARR was 0.07, 93% of the patients were relapse-free at week 48, and no patients demonstrated CDP. T2-hyperintense LV decreased by 8% at week 24 (p = 0.004) and 10% at week 48 (p = 0.016), whereas the mean number of Gd-enhancing lesions was reduced from 3.8 at baseline to 0 at week 24 (p = 0.003) and maintained at week 48 (p < 0.001). The most common AEs were IARs that were all grade 1 or 2; no severe AEs were reported. No serious or opportunistic infections and no liver disease occurred. Ublituximab is currently being tested against teriflunomide in two fully enrolled phase-III studies (ULTIMATE I and II) in patients with RRMS (ClinicalTrials.gov identifier: NCT03277261 and NCT03277248).
Table 4.
Overview of the most relevant B-cell-targeting treatments currently under investigation in multiple sclerosis patients
| Target | Name and posology | References | Number of patients | Trial design | Efficacy | Safety |
|---|---|---|---|---|---|---|
| CD20 | Ublituximab (150 mg on day 1, 450 or 600 mg on day 15 and 24 weeks) | NCT02738775 [83] | 48 RRMS | Phase II (48 weeks), Pbo-controlled |
ARR 0.07 93% of the patients were relapse free at 48 weeks No patients demonstrated CDP ↓ T2 LV by 8% at 24 weeks (p = 0.004) and 10% at 48 weeks (p = 0.016) ↓ Gd+ lesions (3.8 at b vs 0 at 24 weeks, p = 0.003) |
The most common AEs were IARs that were all grade 1 or 2 No SAE No serious or opportunistic infections and no liver disease |
| CD19 | Inebilizumab (MEDI-551) (2 i.v. doses, days 1 and 15: 30, 100 or 600 mg; or single s.c. dose on day 1: 60 or 300 mg) | NCT01585766 [65] | 28 RRMS | Phase I (24 weeks) |
No relapses and no median EDSS score changes at 24 weeks ↓ new/newly enlarging T2-hyperitense (0.4 vs 2.4) ↓ Gd+ lesions (0.1 vs 1.3) ↑ proportion of patients free from new inflammatory activity (75% vs 43%) |
IARs occurring in 7 out of 21 (33.3%) RRMS patients and with upper respiratory tract and urinary tract infections, pyrexia and increased blood pressure |
| BTKi | Evobrutinib (25 mg daily, 75 mg daily or 75 mg twice daily) | NCT02975349 [69] |
228 RRMS 33 SPMS |
Phase II, double-blind, randomized, Pbo or DMF (24 weeks) |
75 mg of evobrutinib once daily: ↓ Gd+ lesions vs Pbo at 12 and 24 weeks (1.69 vs 3.85, lesion rate ratio 0.30, p = 0.005) 75 mg of evobrutinib twice daily (1.15, lesion rate ratio 0.44, p = 0.06) No difference in the ARR, relapse-free status or CDP at any dose |
Most common AEs: nasopharyngitis and asymptomatic ↑ of aminotransferase levels |
| Tolebrutinib (5 mg, 15 mg, 30 mg, 60 mg) | NCT03889639 [70] | 130 RRMS | Phase II, double-blind, Pbo (16 weeks) |
↓ new/newly enlarging T2-hyperitense lesions vs Pbo at 12 weeks (2.12 with Pbo, 1.90 with 5 mg, 1.32 with 15 mg, 1.30 with 30 mg, 0.23 with 60 mg) ↓ Gd+ lesions vs Pbo at 12 weeks (1.03 with Pbo, 1.39 with 5 mg, 0.77 with 15 mg, 0.76 with 30 mg, 0.13 with 60 mg) |
– | |
| Tolebrutinib (5 mg, 15 mg, 30 mg, 60 mg) | NCT03889639 [71] | 61 RRMS | Phase II, double-blind, Pbo (16 weeks) |
↓ new/newly enlarging T2-hyperitense lesions vs Pbo at 12 weeks (1.44 with Pbo, 1.09 with 5 mg, 0.89 with 15 mg, 0.75 with 30 mg, 0.15 with 60 mg) ↓ Gd+ lesions vs Pbo at 12 weeks (0.89 with Pbo, 0.82 with 5 mg, 0.5 with 15 mg, 0.38 with 30 mg, 0.08 with 60 mg) |
– |
AE adverse events, BTKi Bruton’s tyrosine kinase inhibitors, CDP confirmed disability progression, DMF dymethil fumarate, EDSS expanded Disability Status Scale, Gd+ gadolinium-enhancing, IARs infusion-associated reactions, Pbo placebo, RRMS relapsing–remitting multiple sclerosis, SPMS secondary progressive multiple sclerosis
Given the central role of B cells in MS pathogenesis, new therapeutic strategies directed against B-cell targets are currently under investigations (Table 4). CD19, a member of Ig superfamily, is involved in signal transduction following B-cell-receptor activation and represents one of the main regulator of B-cell activation and humoral immunity [63]. CD19 is more broadly expressed on cells of B lineage since, in contrast to CD20, it is also expressed on pro-B cells, plasmablasts and Ig-producing plasma cells (Fig. 2) [63]. Anti-CD19 therapies produce a more long-lasting B-cell depletion and a marked decline in Ig concentrations [63].
Among the different anti-CD19 monoclonal antibodies, inebilizumab (MEDI-551) is a glycoengineered, afucosylated anti-CD19 antibody having high affinity to FcyRIIA and causing B-cell depletion through ADCC [64]. A 24-week phase-I RCT (ClinicalTrials.gov study identifier: NCT01585766) investigated pharmacological properties and safety of inebilizumab in RRMS patients compared with placebo [65]. Inebilizumab promoted a complete B-cell depletion with all doses (2 i.v. doses, days 1 and 15: 30, 100 or 600 mg; or single s.c. dose on day 1: 60 or 300 mg), with IAR occurring in seven out of 21 (33.3%) RRMS patients and with upper respiratory tract and urinary tract infections, pyrexia and increased blood pressure as the most common AEs. At week 24, neither relapses nor median EDSS score changes occurred in RRMS patients receiving inebilizumab. Moreover, inebilizumab promoted trend in reductions in new/newly enlarging T2-hyperitense (0.4 vs 2.4) and Gd-enhancing lesions (0.1 vs 1.3), with a higher proportion of patients free from new inflammatory activity (75% vs 43%) [65].
Bruton’s tyrosine kinase (BTK) is a cytoplasmic kinase expressed on cells of the hematopoietic lineage, except for T cells, natural killer (NK) cells and plasma cells, and contributes to signal transductions from B-cell receptor (BCR) to the PI3K, MAPK and NF-κB pathways, thus regulating B-cell survival, activation, proliferation, and differentiation to plasma cells [66, 67].
Several BTK inhibitors have been proposed for the treatment of hematological malignancies and dysimmune disorders [66, 68]. A recent 24-week phase-II RCT (ClinicalTrials.gov study identifier: NCT02975349) [69] compared oral evobrutinib at different doses (25 mg daily, 75 mg daily or 75 mg twice daily) with placebo or dimethyl fumarate in patients with RRMS or active SPMS. MS patients who received 75 mg of evobrutinib once daily had significantly fewer Gd-enhancing lesions compared with placebo at weeks 12 through 24 (1.69 vs 3.85, lesion rate ratio 0.30, p = 0.005), whereas only a trend for patients who received 75 mg of evobrutinib twice daily was found (1.15, lesion rate ratio 0.44, p = 0.06) [69]. No significant difference in the ARR, relapse-free status or disability progression at any dose occurred [69]. The most common AEs were nasopharyngitis and asymptomatic increase of aminotransferase levels.
In a 16-week phase-IIb study, 130 patients with RRMS were randomized to receive either placebo or tolebrutinib at different doses (5, 15, 30, and 60 mg) [70]. At week 12, patients treated with tolebrutinib had significantly lower mean number of new/enlarging T2-hyperintense lesions compared with placebo (1.90 with 5 mg, 1.32 with 15 mg, 1.30 with 30 mg, 0.23 with 60 mg vs 2.12 with placebo). Tolebrutinib also reduced the mean number of Gd-enhancing lesions (1.39 with 5 mg, 0.77 with 15 mg, 0.76 with 30 mg, 0.13 with 60 mg vs 1.03 with placebo).
Recently, in a subgroup analysis including 61 patients with highly active disease [71] (i.e., one relapse in the year prior to screening and one or more Gd-enhancing lesion on MRI within 6 months prior to screening or nine or more T2-hyperintense lesions at baseline, or two or more relapses in the year prior to screening), at week 12, tolebrutinib reduced the mean number of new/enlarging T2-hyperintense lesions compared with placebo (1.09 with 5 mg, 0.89 with 15 mg, 0.75 with 30 mg, and 0.15 with 60 mg vs 1.44 with placebo). Tolebrutinib also promoted a reduction in the mean number of new Gd-enhancing lesions compared with placebo (0.82 with 5 mg, 0.50 with 15 mg, 0.38 with 30 mg, and 0.08 with 60 mg vs 0.89).
Several other BTK inhibitors are under investigation (see Ref. [66] for a comprehensive review), possibly representing a new therapeutic opportunity for MS patients in the near future although several aspects regarding their mechanism of action, efficacy and safety still need to be fully investigated.
Conclusions
The introduction of anti-CD20 therapies in the MS scenario confirmed the central role of B cells in MS pathogenesis and established a new therapeutic approach for both relapsing and progressive MS patients.
In RRMS, currently approved anti-CD20 monoclonal antibodies (rituximab, ocrelizumab, and ofatumumab) consistently lead to a dramatic reduction of clinical relapses and MRI disease activity together with a significant limitation of disability worsening and brain atrophy progression.
Interestingly, ocrelizumab has been the first effective DMT in delaying disability progression in PPMS. However, efficacy and benefits in PPMS appear partial and more limited compared with those observed in RRMS. These findings suggest that anti-CD20 therapies exert a strong anti-inflammatory activity and that their beneficial neuroprotective effects could be mainly secondary to the prevention of further inflammatory disease activity. The more limited effect in PPMS could be also due to an inefficient depletion of B cells within a CNS-compartmentalized inflammation, possibly due to a limited permeability of anti-CD20 monoclonal antibodies across the blood–brain barrier. This aspect should be better investigated in future studies which should include reliable clinical, laboratory and MRI biomarkers to identify and monitor MS progression.
Data from RCTs, their OLE and from observational studies suggest that anti-CD20 therapies have a favorable short- and medium-term safety profile, with IARs, upper respiratory tract and urinary infections being the most common side effects, although the risk of malignancies should be carefully monitored over long-term.
Several aspects regarding treatment with anti-CD20 therapies deserve further investigations.
Future studies should define how to optimize anti-CD20 therapy administration regimens in terms of dosing, timing and duration of the treatment. Finally, post-marketing and observational studies should be conducted to shed light on beneficial effects and risks over longer term and to better explore the risk of infections and malignancies, especially in older MS patients.
Acknowledgements
None.
Author contributions
MM: drafting/revising the manuscript, study concept, acquisition and interpretation of the data; PP: drafting/revising the manuscript, study concept, acquisition and interpretation of the data; MF: drafting/revising the manuscript, study concept, acquisition and interpretation of the data; MAR: drafting/revising the manuscript, study concept, acquisition and interpretation of the data.
Funding
None.
Declarations
Conflicts of interest
M. Margoni reports grants and personal fees from Sanofi Genzyme, Merck Serono, Novartis and Almirall. She was awarded a MAGNIMS-ECTRIMS fellowship in 2020. P. Preziosa received speaker honoraria from Biogen Idec, Novartis, Merck Serono and ExceMED. He is supported by a senior research fellowship FISM—Fondazione Italiana Sclerosi Multipla—cod. 2019/BS/009 and financed or co-financed with the ‘5 per mille’ public funding. M. Filippi is Editor-in-Chief of the Journal of Neurology and Associate Editor of Radiology, Human Brain Mapping and Neurological Sciences, received compensation for consulting services and/or speaking activities from Almiral, Alexion, Bayer, Biogen, Celgene, Eli Lilly, Genzyme, Merck-Serono, Novartis, Roche, Sanofi, Takeda, and Teva Pharmaceutical Industries, and receives research support from Biogen Idec, Merck-Serono, Novartis, Roche, Teva Pharmaceutical Industries, Italian Ministry of Health, Fondazione Italiana Sclerosi Multipla, and ARiSLA (Fondazione Italiana di Ricerca per la SLA). M.A. Rocca received speaker honoraria from Bayer, Biogen, Bristol Myers Squibb, Celgene, Genzyme, Merck Serono, Novartis, Roche, and Teva, and receives research support from the MS Society of Canada and Fondazione Italiana Sclerosi Multipla.
References
- 1.Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH, HT Group B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med. 2008;358(7):676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- 2.Montalban X, Hauser SL, Kappos L, Arnold DL, Bar-Or A, Comi G, de Seze J, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Rammohan KW, Selmaj K, Traboulsee A, Sauter A, Masterman D, Fontoura P, Belachew S, Garren H, Mairon N, Chin P, Wolinsky JS, OC Investigators Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med. 2017;376(3):209–220. doi: 10.1056/NEJMoa1606468. [DOI] [PubMed] [Google Scholar]
- 3.Hauser SL, Bar-Or A, Cohen JA, Comi G, Correale J, Coyle PK, Cross AH, de Seze J, Leppert D, Montalban X, Selmaj K, Wiendl H, Kerloeguen C, Willi R, Li B, Kakarieka A, Tomic D, Goodyear A, Pingili R, Haring DA, Ramanathan K, Merschhemke M, Kappos L, Asclepios I, AIT Groups Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546–557. doi: 10.1056/NEJMoa1917246. [DOI] [PubMed] [Google Scholar]
- 4.Baecher-Allan C, Kaskow BJ, Weiner HL. Multiple sclerosis: mechanisms and immunotherapy. Neuron. 2018;97(4):742–768. doi: 10.1016/j.neuron.2018.01.021. [DOI] [PubMed] [Google Scholar]
- 5.Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–558. doi: 10.1038/nri3871. [DOI] [PubMed] [Google Scholar]
- 6.Li R, Patterson KR, Bar-Or A. Reassessing B cell contributions in multiple sclerosis. Nat Immunol. 2018;19(7):696–707. doi: 10.1038/s41590-018-0135-x. [DOI] [PubMed] [Google Scholar]
- 7.Hauser SL, Bar-Or A, Comi G, Giovannoni G, Hartung HP, Hemmer B, Lublin F, Montalban X, Rammohan KW, Selmaj K, Traboulsee A, Wolinsky JS, Arnold DL, Klingelschmitt G, Masterman D, Fontoura P, Belachew S, Chin P, Mairon N, Garren H, Kappos L, Opera I, OIC Investigators Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234. doi: 10.1056/NEJMoa1601277. [DOI] [PubMed] [Google Scholar]
- 8.Storch MK, Piddlesden S, Haltia M, Iivanainen M, Morgan P, Lassmann H. Multiple sclerosis: in situ evidence for antibody- and complement-mediated demyelination. Ann Neurol. 1998;43(4):465–471. doi: 10.1002/ana.410430409. [DOI] [PubMed] [Google Scholar]
- 9.Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5(2):170–175. doi: 10.1038/5532. [DOI] [PubMed] [Google Scholar]
- 10.Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47(6):707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 11.Obermeier B, Mentele R, Malotka J, Kellermann J, Kumpfel T, Wekerle H, Lottspeich F, Hohlfeld R, Dornmair K. Matching of oligoclonal immunoglobulin transcriptomes and proteomes of cerebrospinal fluid in multiple sclerosis. Nat Med. 2008;14(6):688–693. doi: 10.1038/nm1714. [DOI] [PubMed] [Google Scholar]
- 12.Cepok S, Rosche B, Grummel V, Vogel F, Zhou D, Sayn J, Sommer N, Hartung HP, Hemmer B. Short-lived plasma blasts are the main B cell effector subset during the course of multiple sclerosis. Brain. 2005;128(Pt 7):1667–1676. doi: 10.1093/brain/awh486. [DOI] [PubMed] [Google Scholar]
- 13.Bar-Or A. The immunology of multiple sclerosis. Semin Neurol. 2008;28(1):29–45. doi: 10.1055/s-2007-1019124. [DOI] [PubMed] [Google Scholar]
- 14.Kuhle J, Pohl C, Mehling M, Edan G, Freedman MS, Hartung HP, Polman CH, Miller DH, Montalban X, Barkhof F, Bauer L, Dahms S, Lindberg R, Kappos L, Sandbrink R. Lack of association between antimyelin antibodies and progression to multiple sclerosis. N Engl J Med. 2007;356(4):371–378. doi: 10.1056/NEJMoa063602. [DOI] [PubMed] [Google Scholar]
- 15.Brickshawana A, Hinson SR, Romero MF, Lucchinetti CF, Guo Y, Buttmann M, McKeon A, Pittock SJ, Chang MH, Chen AP, Kryzer TJ, Fryer JP, Jenkins SM, Cabre P, Lennon VA. Investigation of the KIR4.1 potassium channel as a putative antigen in patients with multiple sclerosis: a comparative study. Lancet Neurol. 2014;13(8):795–806. doi: 10.1016/S1474-4422(14)70141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Srivastava R, Aslam M, Kalluri SR, Schirmer L, Buck D, Tackenberg B, Rothhammer V, Chan A, Gold R, Berthele A, Bennett JL, Korn T, Hemmer B. Potassium channel KIR4.1 as an immune target in multiple sclerosis. N Engl J Med. 2012;367(2):115–123. doi: 10.1056/NEJMoa1110740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodriguez-Pinto D. B cells as antigen presenting cells. Cell Immunol. 2005;238(2):67–75. doi: 10.1016/j.cellimm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Bar-Or A, Fawaz L, Fan B, Darlington PJ, Rieger A, Ghorayeb C, Calabresi PA, Waubant E, Hauser SL, Zhang J, Smith CH. Abnormal B-cell cytokine responses a trigger of T-cell-mediated disease in MS? Ann Neurol. 2010;67(4):452–461. doi: 10.1002/ana.21939. [DOI] [PubMed] [Google Scholar]
- 19.Li R, Rezk A, Miyazaki Y, Hilgenberg E, Touil H, Shen P, Moore CS, Michel L, Althekair F, Rajasekharan S, Gommerman JL, Prat A, Fillatreau S, Bar-Or A, Canadian BciMST Proinflammatory GM-CSF-producing B cells in multiple sclerosis and B cell depletion therapy. Sci Transl Med. 2015;7(310):310ra166. doi: 10.1126/scitranslmed.aab4176. [DOI] [PubMed] [Google Scholar]
- 20.Duddy M, Niino M, Adatia F, Hebert S, Freedman M, Atkins H, Kim HJ, Bar-Or A. Distinct effector cytokine profiles of memory and naive human B cell subsets and implication in multiple sclerosis. J Immunol. 2007;178(10):6092–6099. doi: 10.4049/jimmunol.178.10.6092. [DOI] [PubMed] [Google Scholar]
- 21.Barr TA, Shen P, Brown S, Lampropoulou V, Roch T, Lawrie S, Fan B, O’Connor RA, Anderton SM, Bar-Or A, Fillatreau S, Gray D. B cell depletion therapy ameliorates autoimmune disease through ablation of IL-6-producing B cells. J Exp Med. 2012;209(5):1001–1010. doi: 10.1084/jem.20111675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Rezk A, Healy LM, Muirhead G, Prat A, Gommerman JL, Bar-Or A, MCBciM Team Cytokine-defined B cell responses as therapeutic targets in multiple sclerosis. Front Immunol. 2015;6:626. doi: 10.3389/fimmu.2015.00626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frischer JM, Bramow S, Dal-Bianco A, Lucchinetti CF, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Lassmann H. The relation between inflammation and neurodegeneration in multiple sclerosis brains. Brain. 2009;132(Pt 5):1175–1189. doi: 10.1093/brain/awp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machado-Santos J, Saji E, Troscher AR, Paunovic M, Liblau R, Gabriely G, Bien CG, Bauer J, Lassmann H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain. 2018;141(7):2066–2082. doi: 10.1093/brain/awy151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Magliozzi R, Howell O, Vora A, Serafini B, Nicholas R, Puopolo M, Reynolds R, Aloisi F. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 2007;130(Pt 4):1089–1104. doi: 10.1093/brain/awm038. [DOI] [PubMed] [Google Scholar]
- 26.Magliozzi R, Howell OW, Nicholas R, Cruciani C, Castellaro M, Romualdi C, Rossi S, Pitteri M, Benedetti MD, Gajofatto A, Pizzini FB, Montemezzi S, Rasia S, Capra R, Bertoldo A, Facchiano F, Monaco S, Reynolds R, Calabrese M. Inflammatory intrathecal profiles and cortical damage in multiple sclerosis. Ann Neurol. 2018;83(4):739–755. doi: 10.1002/ana.25197. [DOI] [PubMed] [Google Scholar]
- 27.Magliozzi R, Serafini B, Rosicarelli B, Chiappetta G, Veroni C, Reynolds R, Aloisi F. B-cell enrichment and Epstein-Barr virus infection in inflammatory cortical lesions in secondary progressive multiple sclerosis. J Neuropathol Exp Neurol. 2013;72(1):29–41. doi: 10.1097/NEN.0b013e31827bfc62. [DOI] [PubMed] [Google Scholar]
- 28.Meinl E, Krumbholz M, Hohlfeld R. B lineage cells in the inflammatory central nervous system environment: migration, maintenance, local antibody production, and therapeutic modulation. Ann Neurol. 2006;59(6):880–892. doi: 10.1002/ana.20890. [DOI] [PubMed] [Google Scholar]
- 29.Touil H, Kobert A, Lebeurrier N, Rieger A, Saikali P, Lambert C, Fawaz L, Moore CS, Prat A, Gommerman J, Antel JP, Itoyama Y, Nakashima I, Bar-Or A, Canadian BCTiMS Human central nervous system astrocytes support survival and activation of B cells: implications for MS pathogenesis. J Neuroinflammation. 2018;15(1):114. doi: 10.1186/s12974-018-1136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahad DH, Trapp BD, Lassmann H. Pathological mechanisms in progressive multiple sclerosis. Lancet Neurol. 2015;14(2):183–193. doi: 10.1016/S1474-4422(14)70256-X. [DOI] [PubMed] [Google Scholar]
- 31.Magliozzi R, Howell OW, Reeves C, Roncaroli F, Nicholas R, Serafini B, Aloisi F, Reynolds R. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann Neurol. 2010;68(4):477–493. doi: 10.1002/ana.22230. [DOI] [PubMed] [Google Scholar]
- 32.Lucchinetti CF, Popescu BF, Bunyan RF, Moll NM, Roemer SF, Lassmann H, Bruck W, Parisi JE, Scheithauer BW, Giannini C, Weigand SD, Mandrekar J, Ransohoff RM. Inflammatory cortical demyelination in early multiple sclerosis. N Engl J Med. 2011;365(23):2188–2197. doi: 10.1056/NEJMoa1100648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyer S, Evers M, Jansen JHM, Buijs J, Broek B, Reitsma SE, Moerer P, Amini M, Kretschmer A, Ten Broeke T, den Hartog MT, Rijke M, Klein C, Valerius T, Boross P, Leusen JHW. New insights in type I and II CD20 antibody mechanisms-of-action with a panel of novel CD20 antibodies. Br J Haematol. 2018;180(6):808–820. doi: 10.1111/bjh.15132. [DOI] [PubMed] [Google Scholar]
- 34.Montalvao F, Garcia Z, Celli S, Breart B, Deguine J, Van Rooijen N, Bousso P. The mechanism of anti-CD20-mediated B cell depletion revealed by intravital imaging. J Clin Invest. 2013;123(12):5098–5103. doi: 10.1172/JCI70972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cencioni MT, Mattoscio M, Magliozzi R, Bar-Or A, Muraro PA. B cells in multiple sclerosis—from targeted depletion to immune reconstitution therapies. Nat Rev Neurol. 2021 doi: 10.1038/s41582-021-00498-5. [DOI] [PubMed] [Google Scholar]
- 36.Yang CS, Yang L, Li T, Zhang DQ, Jin WN, Li MS, Su N, Zhangning N, Liu Q, Shao ZH, Yu C, Shi FD. Responsiveness to reduced dosage of rituximab in Chinese patients with neuromyelitis optica. Neurology. 2013;81(8):710–713. doi: 10.1212/WNL.0b013e3182a1aac7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hawker K, O’Connor P, Freedman MS, Calabresi PA, Antel J, Simon J, Hauser S, Waubant E, Vollmer T, Panitch H, Zhang J, Chin P, Smith CH, OT Group Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol. 2009;66(4):460–471. doi: 10.1002/ana.21867. [DOI] [PubMed] [Google Scholar]
- 38.Ingle GT, Sastre-Garriga J, Miller DH, Thompson AJ. Is inflammation important in early PPMS? A longitudinal MRI study. J Neurol Neurosurg Psychiatry. 2005;76(9):1255–1258. doi: 10.1136/jnnp.2004.036590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhargava P, Wicken C, Smith MD, Strowd RE, Cortese I, Reich DS, Calabresi PA, Mowry EM. Trial of intrathecal rituximab in progressive multiple sclerosis patients with evidence of leptomeningeal contrast enhancement. Mult Scler Relat Disord. 2019;30:136–140. doi: 10.1016/j.msard.2019.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kappos L, Li D, Calabresi PA, O’Connor P, Bar-Or A, Barkhof F, Yin M, Leppert D, Glanzman R, Tinbergen J, Hauser SL. Ocrelizumab in relapsing-remitting multiple sclerosis: a phase 2, randomised, placebo-controlled, multicentre trial. Lancet. 2011;378(9805):1779–1787. doi: 10.1016/S0140-6736(11)61649-8. [DOI] [PubMed] [Google Scholar]
- 41.Baker D, Pryce G, James LK, Marta M, Schmierer K. The ocrelizumab phase II extension trial suggests the potential to improve the risk: benefit balance in multiple sclerosis. Mult Scler Relat Disord. 2020;44:102279. doi: 10.1016/j.msard.2020.102279. [DOI] [PubMed] [Google Scholar]
- 42.Hauser SL, Kappos L, Arnold DL, Bar-Or A, Brochet B, Naismith RT, Traboulsee A, Wolinsky JS, Belachew S, Koendgen H, Levesque V, Manfrini M, Model F, Hubeaux S, Mehta L, Montalban X. Five years of ocrelizumab in relapsing multiple sclerosis: OPERA studies open-label extension. Neurology. 2020;95(13):e1854–e1867. doi: 10.1212/WNL.0000000000010376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Havrdova E, Arnold DL, Bar-Or A, Comi G, Hartung HP, Kappos L, Lublin F, Selmaj K, Traboulsee A, Belachew S, Bennett I, Buffels R, Garren H, Han J, Julian L, Napieralski J, Hauser SL, Giovannoni G. No evidence of disease activity (NEDA) analysis by epochs in patients with relapsing multiple sclerosis treated with ocrelizumab vs interferon beta-1a. Mult Scler J Exp Transl Clin. 2018;4(1):2055217318760642. doi: 10.1177/2055217318760642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hartung HP, Berger T, Bermel R, Brochet B, Holmøy T, Karabudak R, Killestein J, Nos C, Patti F, Ross AP, Vollmer T, Wuerfel J, Buffels R, Kuenzel T, Freedman M (2020) Ocrelizumab phase IIIb efficacy: 1-year NEDA rates (with MRI re-baselining) from the ENSAMBLE study in early-stage relapsing-remitting MS patients. In: ECTRIMS, vol S3, pp 225–659, Poster P0220. https://library.msvirtual2020.org/. Accessed 5 Jul 2021
- 45.Wiendl H, Comi G, Oreja-Guevara C, Van Wijmeersch BAS, Buffels RJW, Kadner R, Kuenzel T, Vermersch P (2020) Ocrelizumab phase IIIb efficacy from CASTING: 2-year NEDA (MRI re-baselined) subgroup rates in RRMS patients with a suboptimal response to prior DMTs. In: ECTRIMS, 2020, vol S3, pp 224–659, Poster P0219. https://library.msvirtual2020.org/. Accessed 5 Jul 2021
- 46.Turner B, Cree BAC, Kappos L, Montalban X, Papeix C, Wolinsky JS, Buffels R, Fiore D, Garren H, Han J, Hauser SL. Ocrelizumab efficacy in subgroups of patients with relapsing multiple sclerosis. J Neurol. 2019;266(5):1182–1193. doi: 10.1007/s00415-019-09248-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wolinsky JS, Arnold DL, Brochet B, Hartung HP, Montalban X, Naismith RT, Manfrini M, Overell J, Koendgen H, Sauter A, Bennett I, Hubeaux S, Kappos L, Hauser SL. Long-term follow-up from the ORATORIO trial of ocrelizumab for primary progressive multiple sclerosis: a post-hoc analysis from the ongoing open-label extension of the randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2020;19(12):998–1009. doi: 10.1016/S1474-4422(20)30342-2. [DOI] [PubMed] [Google Scholar]
- 48.Mancinelli CR, Scarpazza C, Cordioli C, De Rossi N, Rasia S, Turrini MV, Capra R. Switching to ocrelizumab in RRMS patients at risk of PML previously treated with extended interval dosing of natalizumab. Mult Scler. 2021;27(5):790–794. doi: 10.1177/1352458520946017. [DOI] [PubMed] [Google Scholar]
- 49.Sorensen PS, Lisby S, Grove R, Derosier F, Shackelford S, Havrdova E, Drulovic J, Filippi M. Safety and efficacy of ofatumumab in relapsing-remitting multiple sclerosis: a phase 2 study. Neurology. 2014;82(7):573–581. doi: 10.1212/WNL.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 50.Bar-Or A, Grove RA, Austin DJ, Tolson JM, VanMeter SA, Lewis EW, Derosier FJ, Lopez MC, Kavanagh ST, Miller AE, Sorensen PS. Subcutaneous ofatumumab in patients with relapsing-remitting multiple sclerosis: the MIRROR Study. Neurology. 2018;90(20):e1805–e1814. doi: 10.1212/WNL.0000000000005516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gelfand JM, Cree BAC, Hauser SL. Ocrelizumab and other CD20(+) B-cell-depleting therapies in multiple sclerosis. Neurotherapeutics. 2017;14(4):835–841. doi: 10.1007/s13311-017-0557-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Salzer J, Svenningsson R, Alping P, Novakova L, Bjorck A, Fink K, Islam-Jakobsson P, Malmestrom C, Axelsson M, Vagberg M, Sundstrom P, Lycke J, Piehl F, Svenningsson A. Rituximab in multiple sclerosis: a retrospective observational study on safety and efficacy. Neurology. 2016;87(20):2074–2081. doi: 10.1212/WNL.0000000000003331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ineichen BV, Moridi T, Granberg T, Piehl F. Rituximab treatment for multiple sclerosis. Mult Scler. 2020;26(2):137–152. doi: 10.1177/1352458519858604. [DOI] [PubMed] [Google Scholar]
- 54.Mok CC. Rituximab for the treatment of rheumatoid arthritis: an update. Drug Des Dev Ther. 2013;8:87–100. doi: 10.2147/DDDT.S41645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Patel A, Sul J, Gordon ML, Steinklein J, Sanguinetti S, Pramanik B, Purohit D, Haroutunian V, Williamson A, Koralnik I, Harel A. Progressive multifocal leukoencephalopathy in a patient with progressive multiple sclerosis treated with ocrelizumab monotherapy. JAMA Neurol. 2021 doi: 10.1001/jamaneurol.2021.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sormani MP, De Rossi N, Schiavetti I, Carmisciano L, Cordioli C, Moiola L, Radaelli M, Immovilli P, Capobianco M, Trojano M, Zaratin P, Tedeschi G, Comi G, Battaglia MA, Patti F, Salvetti M, Musc-19 Study Group Disease-modifying therapies and coronavirus disease 2019 severity in multiple sclerosis. Ann Neurol. 2021;89(4):780–789. doi: 10.1002/ana.26028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reder AT, Centonze D, Naylor ML, Nagpal A, Rajbhandari R, Altincatal A, Kim M, Berdofe A, Radhakrishnan M, Jung E, Sandrock AW, Smirnakis K, Popescu C, de Moor C. COVID-19 in patients with multiple sclerosis: associations with disease-modifying therapies. CNS Drugs. 2021;35(3):317–330. doi: 10.1007/s40263-021-00804-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salter A, Fox RJ, Newsome SD, Halper J, Li DKB, Kanellis P, Costello K, Bebo B, Rammohan K, Cutter GR, Cross AH. Outcomes and risk factors associated with SARS-CoV-2 infection in a north American registry of patients with multiple sclerosis. JAMA Neurol. 2021 doi: 10.1001/jamaneurol.2021.0688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alping P, Askling J, Burman J, Fink K, Fogdell-Hahn A, Gunnarsson M, Hillert J, Langer-Gould A, Lycke J, Nilsson P, Salzer J, Svenningsson A, Vrethem M, Olsson T, Piehl F, Frisell T. Cancer risk for fingolimod, natalizumab, and rituximab in multiple sclerosis patients. Ann Neurol. 2020;87(5):688–699. doi: 10.1002/ana.25701. [DOI] [PubMed] [Google Scholar]
- 60.Bar-Or A, Calkwood JC, Chognot C, Evershed J, Fox EJ, Herman A, Manfrini M, McNamara J, Robertson DS, Stokmaier D, Wendt JK, Winthrop KL, Traboulsee A. Effect of ocrelizumab on vaccine responses in patients with multiple sclerosis: the VELOCE Study. Neurology. 2020;95(14):e1999–e2008. doi: 10.1212/WNL.0000000000010380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Centonze D, Rocca MA, Gasperini C, Kappos L, Hartung HP, Magyari M, Oreja-Guevara C, Trojano M, Wiendl H, Filippi M. Disease-modifying therapies and SARS-CoV-2 vaccination in multiple sclerosis: an expert consensus. J Neurol. 2021 doi: 10.1007/s00415-021-10545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fox E, Lovett-Racke AE, Gormley M, Liu Y, Petracca M, Cocozza S, Shubin R, Wray S, Weiss MS, Bosco JA, Power SA, Mok K, Inglese M. A phase 2 multicenter study of ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody, in patients with relapsing forms of multiple sclerosis. Mult Scler. 2020 doi: 10.1177/1352458520918375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tedder TF. CD19: a promising B cell target for rheumatoid arthritis. Nat Rev Rheumatol. 2009;5(10):572–577. doi: 10.1038/nrrheum.2009.184. [DOI] [PubMed] [Google Scholar]
- 64.Chen D, Gallagher S, Monson NL, Herbst R, Wang Y. Inebilizumab, a B cell-depleting anti-CD19 antibody for the treatment of autoimmune neurological diseases: insights from preclinical studies. J Clin Med. 2016 doi: 10.3390/jcm5120107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Agius MA, Klodowska-Duda G, Maciejowski M, Potemkowski A, Li J, Patra K, Wesley J, Madani S, Barron G, Katz E, Flor A. Safety and tolerability of inebilizumab (MEDI-551), an anti-CD19 monoclonal antibody, in patients with relapsing forms of multiple sclerosis: results from a phase 1 randomised, placebo-controlled, escalating intravenous and subcutaneous dose study. Mult Scler. 2019;25(2):235–245. doi: 10.1177/1352458517740641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Contentti EC, Correale J. Bruton’s tyrosine kinase inhibitors: a promising emerging treatment option for multiple sclerosis. Expert Opin Emerg Drugs. 2020;25(4):377–381. doi: 10.1080/14728214.2020.1822817. [DOI] [PubMed] [Google Scholar]
- 67.Torke S, Weber MS. Inhibition of Bruton s tyrosine kinase as a novel therapeutic approach in multiple sclerosis. Expert Opin Investig Drugs. 2020;29(10):1143–1150. doi: 10.1080/13543784.2020.1807934. [DOI] [PubMed] [Google Scholar]
- 68.Liang C, Tian D, Ren X, Ding S, Jia M, Xin M, Thareja S. The development of Bruton’s tyrosine kinase (BTK) inhibitors from 2012 to 2017: a mini-review. Eur J Med Chem. 2018;151:315–326. doi: 10.1016/j.ejmech.2018.03.062. [DOI] [PubMed] [Google Scholar]
- 69.Montalban X, Arnold DL, Weber MS, Staikov I, Piasecka-Stryczynska K, Willmer J, Martin EC, Dangond F, Syed S, Wolinsky JS, Evobrutinib Phase 2 Study Group Placebo-controlled trial of an oral BTK inhibitor in multiple sclerosis. N Engl J Med. 2019;380(25):2406–2417. doi: 10.1056/NEJMoa1901981. [DOI] [PubMed] [Google Scholar]
- 70.Reich DS, Honeycutt WD, Laganke C, Laplaud D-A, Montalban X, Robertson D, Uitdehaag BMJ, Wray S, Wynn D, Matta A, Turner T, Wallstrom E, Zhang X, Traboulsee A. Efficacy and safety outcomes in patients with relapsing forms of MS treated with the CNS-penetrating BTK inhibitor SAR442168: results from the phase 2b trial. Eur J Neurol. 2020;27(Suppl. 1):1–102. [Google Scholar]
- 71.Syed S, Yonkers N, LaGanke C, Honeycutt WD, Traboulsee A, Wynn D, Wray S, Wallstroem E, Dukovic D, Turner T. Efficacy and safety of tolebrutinib in patients with highly active relapsing MS: subgroup analysis of the phase 2b study (2260) Neurology. 2021;96(15 Supplement):2260. [Google Scholar]
- 72.Bar-Or A, Calabresi PA, Arnold D, Markowitz C, Shafer S, Kasper LH, Waubant E, Gazda S, Fox RJ, Panzara M, Sarkar N, Agarwal S, Smith CH. Rituximab in relapsing-remitting multiple sclerosis: a 72-week, open-label, phase I trial. Ann Neurol. 2008;63(3):395–400. doi: 10.1002/ana.21363. [DOI] [PubMed] [Google Scholar]
- 73.Naismith RT, Piccio L, Lyons JA, Lauber J, Tutlam NT, Parks BJ, Trinkaus K, Song SK, Cross AH. Rituximab add-on therapy for breakthrough relapsing multiple sclerosis: a 52-week phase II trial. Neurology. 2010;74(23):1860–1867. doi: 10.1212/WNL.0b013e3181e24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alping P, Frisell T, Novakova L, Islam-Jakobsson P, Salzer J, Bjorck A, Axelsson M, Malmestrom C, Fink K, Lycke J, Svenningsson A, Piehl F. Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol. 2016;79(6):950–958. doi: 10.1002/ana.24651. [DOI] [PubMed] [Google Scholar]
- 75.de Flon P, Gunnarsson M, Laurell K, Soderstrom L, Birgander R, Lindqvist T, Krauss W, Dring A, Bergman J, Sundstrom P, Svenningsson A. Reduced inflammation in relapsing-remitting multiple sclerosis after therapy switch to rituximab. Neurology. 2016;87(2):141–147. doi: 10.1212/WNL.0000000000002832. [DOI] [PubMed] [Google Scholar]
- 76.Alcala C, Gascon F, Perez-Miralles F, Gil-Perotin S, Navarre A, Bosca I, Coret F, Casanova B. Efficacy and safety of rituximab in relapsing and progressive multiple sclerosis: a hospital-based study. J Neurol. 2018;265(7):1690–1697. doi: 10.1007/s00415-018-8899-3. [DOI] [PubMed] [Google Scholar]
- 77.Durozard P, Maarouf A, Boutiere C, Ruet A, Brochet B, Vukusic S, Carra-Dalliere C, Labauge P, Mathey G, Debouverie M, Papeix C, Maillart E, Lubetzki C, Bensa C, Gout O, Giannesini C, Stankoff B, Ciron J, Brassat D, Pelletier J, Rico Lamy A, Audoin B. Efficacy of rituximab in refractory RRMS. Mult Scler. 2019;25(6):828–836. doi: 10.1177/1352458518772748. [DOI] [PubMed] [Google Scholar]
- 78.Granqvist M, Boremalm M, Poorghobad A, Svenningsson A, Salzer J, Frisell T, Piehl F. Comparative effectiveness of rituximab and other initial treatment choices for multiple sclerosis. JAMA Neurol. 2018;75(3):320–327. doi: 10.1001/jamaneurol.2017.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yamout BI, El-Ayoubi NK, Nicolas J, El Kouzi Y, Khoury SJ, Zeineddine MM. Safety and efficacy of rituximab in multiple sclerosis: a retrospective observational study. J Immunol Res. 2018;2018:9084759. doi: 10.1155/2018/9084759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Honce JM, Nair KV, Sillau S, Valdez B, Miravalle A, Alvarez E, Schreiner T, Corboy JR, Vollmer TL. Rituximab vs placebo induction prior to glatiramer acetate monotherapy in multiple sclerosis. Neurology. 2019;92(7):e723–e732. doi: 10.1212/WNL.0000000000006916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zecca C, Bovis F, Novi G, Capobianco M, Lanzillo R, Frau J, Repice AM, Hakiki B, Realmuto S, Bonavita S, Curti E, Brambilla L, Mataluni G, Cavalla P, Di Sapio A, Signoriello E, Barone S, Maniscalco GT, Maietta I, Maraffi I, Boffa G, Malucchi S, Nozzolillo A, Coghe G, Mechi C, Salemi G, Gallo A, Sacco R, Cellerino M, Malentacchi M, De Angelis M, Lorefice L, Magnani E, Prestipino E, Sperli F, Morra VB, Fenu G, Barilaro A, Abbadessa G, Signori A, Granella F, Amato MP, Uccelli A, Gobbi C, Sormani MP. Treatment of multiple sclerosis with rituximab: a multicentric Italian-Swiss experience. Mult Scler. 2020;26(12):1519–1531. doi: 10.1177/1352458519872889. [DOI] [PubMed] [Google Scholar]
- 82.Ellwardt E, Rolfes L, Klein J, Pape K, Ruck T, Wiendl H, Schroeter M, Zipp F, Meuth SG, Warnke C, Bittner S. Ocrelizumab initiation in patients with MS: a multicenter observational study. Neurol Neuroimmunol Neuroinflammation. 2020 doi: 10.1212/NXI.0000000000000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fox E, Lovett-Racke AE, Gormley M, Liu Y, Petracca M, Cocozza S, Shubin R, Wray S, Weiss MS, Bosco JA, Power SA, Mok K, Inglese M. A phase 2 multicenter study of ublituximab, a novel glycoengineered anti-CD20 monoclonal antibody, in patients with relapsing forms of multiple sclerosis. Mult Scler. 2021;27(3):420–429. doi: 10.1177/1352458520918375. [DOI] [PMC free article] [PubMed] [Google Scholar]