Abstract
INTRODUCTION:
Past research has focused on risk factors for developing dementia, with increasing recognition of “resilient” people who live to old age with intact cognitive function despite pathologic features of Alzheimer’s disease (AD).
OBJECTIVE:
To evaluate demographic factors, mid-life characteristics, and non-AD neuropathology findings that may be associated with cognitive resilience to AD pathology.
METHODS:
We analyzed data from 276 autopsy cases with intermediate or high levels of AD pathology from the Adult Changes in Thought study. We defined cognitive resilience as having Cognitive Abilities Screening Instrument scores ≥86 within two years of death and no clinical dementia diagnosis; non-resilient people had dementia diagnoses from AD or other causes before death. We compared mid-life characteristics, demographics, and additional neuropathology findings between resilient and non-resilient people. We used multivariable logistic regression to estimate odds ratios (ORs) with 95% confidence intervals (CIs) for being resilient compared to not being resilient adjusting for demographic and neuropathology factors.
RESULTS:
We classified 68 (25%) people as resilient and 208 (75%) as not resilient. A greater proportion of resilient people had a college degree (50%) compared with non-resilient (32%, p=0.01). The odds of being resilient were significantly increased among people with a college education (OR=2.01, 95%CI=1.01–3.99) and significantly reduced among people with additional non-AD neuropathology findings such as hippocampal sclerosis (OR=0.28, 95%CI=0.09–0.89) and microinfarcts (OR=0.34, 95%CI=0.15–0.78).
DISCUSSION:
Increased education and absence of non-AD pathology may be independently associated with cognitive resilience, highlighting the importance of evaluating co-morbid factors in future research on mechanisms of cognitive resilience.
Keywords: dementia, Alzheimer’s disease, cognition, neuropathology, aging, education
INTRODUCTION
Resilience is defined as a person’s toughness or capacity to recover quickly from difficulties.[1] In Alzheimer’s disease (AD) research, cognitive resilience has been described using many different terms including asymptomatic AD, preclinical AD, cognitive reserve, and even “aging well”.[2–8] The terms cognitive and/or brain reserve represent overarching concepts that explain why some older adults maintain cognitive ability despite brain aging or development of brain pathology through either adaptation of cognitive processes (i.e. cognitive reserve) or structural characteristics in the brain (i.e. brain reserve).[7, 9] However, cognitive resilience refers specifically to overcoming substantial development of plaques and tangles in the brain that would normally result in dementia. In other words, some adults may be considered cognitively resilient to the effects of AD pathology and avoid developing dementia due to AD or other causes– but we have very little understanding about how or why some people develop cognitive resilience.
Cognitive resilience to AD pathology may not be rare - prior studies have estimated anywhere from 5–40% of adults with plaques and tangles at levels typically associated with AD, have no symptoms of cognitive decline.[10–19] Most of these studies are small autopsy studies with fewer than 100 people and use varying definitions to describe AD neuropathology in people without a prior clinical diagnosis of AD.[3, 14, 15, 17, 18, 20–22] Definitions of resilience have been proposed based on imaging data,[23, 24] but a standard definition incorporating detailed neuropathology findings is needed.[7] In addition, most of these studies have not assessed demographic characteristics or other risk factors that occurred earlier in life in resilient people, nor have they compared these factors between resilient and non-resilient people, which may help us understand how some people develop neuropathological resilience and avoid dementia due to AD or other causes.
This study aims to provide new information by characterizing a population of older adults from the Adult Changes in Thought (ACT) study who were cognitively resilient to AD pathology. That is, they developed substantial plaques and tangles in their brain while maintaining higher than expected cognitive performance. Our goal was to compare selected mid-life and autopsy characteristics along with additional neuropathology findings among resilient and non-resilient people. Understanding differences in characteristics of older adults who are cognitively resilient to AD pathology, compared to those who are not, may enable us to identify modifiable risk factors, elucidate possible mechanisms of resilience, and, ultimately, advance AD research.
METHODS
Study population
ACT, a longitudinal study of dementia and AD incidence, has been described previously.[25–27] Participants had to be 65 years or older, dementia-free at baseline, and community-dwelling members of Kaiser Permanente Washington, a non-profit integrated healthcare system in Washington state. ACT enrollment includes: the original cohort enrolled between 1994 and 1996 (n=2,581), an expansion cohort enrolled between 2000 and 2003 (n=811), and a continuously enrolled cohort starting in 2004 to maintain a living population without dementia of approximately 2000 people. Study participants provided written informed consent, including a separate consent for brain autopsy, and all study procedures were approved by Institutional Review Boards at Kaiser Permanente Washington and the University of Washington.
In-person follow-up visits occur every two years until the participant dies, develops dementia, or withdraws from the study. The Cognitive Abilities Screening Instrument (CASI) was administered at every ACT study baseline and follow-up visit. The CASI is a 40-item test of global cognitive functioning with scores that range from 0–100; higher scores represent better cognitive functioning.[28] Any participant with a CASI score <86 undergoes a full work-up for dementia, including a clinical examination at the participant’s home and a full neuropsychological battery.[25] A cutoff score of 86 represents 96.5% sensitivity and 92.0% specificity to accurately classify people who need additional evaluation for dementia.[25, 28] Those data, along with medical records information and results of any neuroimaging are reviewed at a consensus conference where standard research criteria are applied to identify cases of dementia (Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria to define dementia[29] and the National Institute of Neurological and Communicative Disorders and Stroke-Alzheimer’s Disease and Related Disorders Association criteria to define possible or probable AD[30]). These criteria have been in place since the beginning of the ACT study. For this analysis, we did not distinguish between various forms of dementia.
The study population included 4,541 ACT participants with at least one follow-up visit, which was the first opportunity for study participants to be diagnosed with all-cause dementia, including AD (Figure 1). Data for this report were further limited to 683 people who died and had complete autopsy data on AD neuropathology outcomes as of December 2017. We excluded people with a CASI <86 and no dementia diagnosis within 2 years of death (N=17); these people could not be considered resilient because of their low CASI score nor could they be considered not-resilient without a formal dementia diagnosis. We also excluded people with final CASI score more than 2 years before death (N=75) because of the potential for unmeasured cognitive decline in that time. Our sample included 591 people (N=318 with clinically diagnosed all-cause dementia, and N=273 with a CASI ≥86 within 2 years of death and no dementia).
Figure 1.
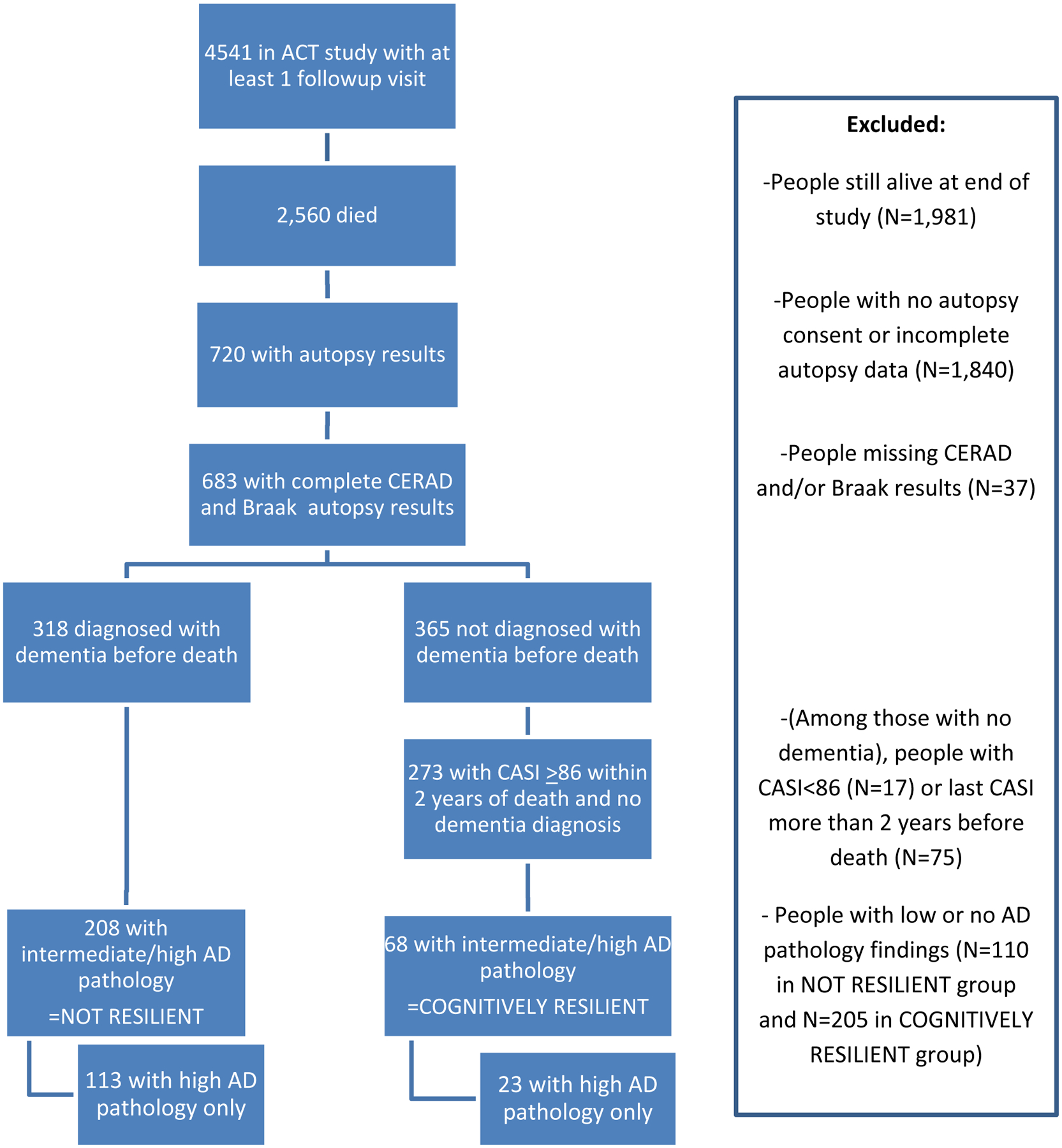
Flow chart of study population. This figure shows the number of people included in the study population, and the number excluded for each reason.
Resilience definition
To develop an operational definition of cognitive resilience to AD pathology, we combined autopsy data on neuritic plaques (assessed by Consortium to Establish a Registry for Alzheimer’s Disease (CERAD) scores as none, sparse, moderate, or frequent) and neurofibrillary tangles (assessed by Braak staging as 0-VI) with each person’s cognitive status at the time of death (Table 1). Using similar methods to Latimer et al.[19] adapted from the National Institute on Aging and Alzheimer’s Association (NIA-AA) guidelines for the neuropathologic assessment of AD pathologic change,[31, 32] we classified anyone with both a CERAD score of moderate or frequent and a Braak stage from III through VI as having intermediate or high neuropathology. Among 591 people eligible for analysis, 276 had intermediate or high AD pathology. These 276 people were “cognitively resilient” if they had no dementia diagnosis and a CASI score ≥86 within 2 years of death (n=68), or “not resilient” if they had a dementia diagnosis before death (n=208). We developed a secondary, more restrictive definition of cognitive resilience limited to 136 people with high AD pathology only (CERAD frequent and Braak stage V/VI). This restricted definition included 23 “cognitively resilient” people with no dementia diagnosis and a CASI score ≥86 within 2 years of death, and 113 “not resilient” people with a dementia diagnosis before death (Table 1). We required all people in the restricted definition to have high AD pathology so that differences between cognitively resilient and non-resilient groups would not be due to underlying differences in AD pathology levels.
Table 1.
Distribution of CERAD and Braak AD neuropathology results to define cognitively resilient and non-resilient populations
| CERAD/BRAAK | 0 | I | II | III | IV | V | VI | AD pathology |
|---|---|---|---|---|---|---|---|---|
| CASI score > 86 within 2 years of death and NO dementia diagnosis (N=273) | ||||||||
| None | 10 | 23 | 26 | 22 | 7 | 0 | 1 | |
| Sparse | 3 | 15 | 22 | 24 | 14 | 4 | 1 | |
| Moderate | 1 | 4 | 13 | 20 | 9 | 8 | 0 | Intermediate or high (N=68)* |
| Frequent | 0 | 1 | 14 | 5 | 3 | 14 | 9 | High (N=23)** |
| Dementia diagnosis before death (N=318) | ||||||||
| None | 5 | 3 | 8 | 6 | 12 | 3 | 3 | |
| Sparse | 1 | 6 | 14 | 14 | 13 | 5 | 8 | |
| Moderate | 0 | 0 | 4 | 11 | 13 | 31 | 20 | Intermediate or high (N=208)† |
| Frequent | 0 | 1 | 4 | 6 | 14 | 45 | 68 | High (N=113) †† |
Cognitively resilient to intermediate or high AD pathology: People with CERAD score of moderate or frequent, Braak stage III-VI, and CASI score ≥86 within 2 years of death and no dementia diagnosis
Cognitively resilient to high AD pathology only: People with CERAD score of frequent, Braak stage V-VI, and CASI score ≥86 within 2 years of death and no dementia diagnosis
Non-resilient to intermediate or high AD pathology: People with CERAD score of moderate or frequent, Braak stage III-VI, and dementia diagnosis before death
Non-resilient to high AD pathology only: People with CERAD score of frequent, Braak stage V-VI, and dementia diagnosis before death
Neuropathology data
Approximately 55% of brains had a post-mortem interval of 8 hours or less; the remainder had post-mortem intervals less than 48 hours. Neuropathology evaluations were performed by a board-certified neuropathologist. Each brain underwent thorough gross evaluation followed by sampling and neuropathological histopathological assessment according to the latest guidelines and as previously described.[33] Specifically, using histochemical and immunohistochemical stains in multiple brain regions, CERAD score and Braak stage were assigned for every case, which were required to be moderate/frequent and III-VI, respectively, for inclusion in this study. Additional neuropathology outcomes were recorded for each case including cortical or deep microinfarcts (2+ vs 0–1), macroscopic cystic infarcts (any vs none), atherosclerosis (moderate/severe vs none/mild), arteriolosclerosis (moderate/severe vs none/mild), amyloid angiopathy (moderate/severe vs none/mild), Lewy bodies in isocortex (any vs none), hippocampal sclerosis (any vs none), and brain weight (measured in grams).
Covariates
Demographic characteristics including age, sex, education level, and self-reported race and ethnicity were collected at study entry. Self-reported comorbidities collected at baseline included hypertension, diabetes, stroke, cerebrovascular disease [including stroke, transient ischemic attack, or carotid endarterectomy procedure], coronary heart disease [including myocardial infarction, angina, coronary artery bypass graft, or angioplasty], and congestive heart failure. Additional self-reported risk factors included smoking history, regular exercise (15 minutes or more at least 3 times per week), and level of difficulty with activities of daily living (walking around the house, getting out of a bed/chair, feeding oneself, dressing oneself, bathing/showering oneself, and getting to or using the toilet). We calculated body mass index (BMI) from measured height and weight at the baseline study visit. Head circumference was measured at the baseline study visit through year 2001 (head circumference data were missing on 24 people). We classified participants as APOE ε4 positive if they had one or two copies of the ε4 allele as previously described.[25]
Analyses
We compared distributions of demographics, risk factors for dementia, comorbidities, and neuropathology outcomes between cognitively resilient and non-resilient groups (using both operational definitions) using chi-squared tests for categorical variables and T-tests or Wilcoxon rank-sum tests of the median for continuous variables. We graphically compared the number of neuropathology findings between cognitively resilient and non-resilient groups. We considered p-values <0.05 statistically significant.
We used logistic regression to estimate the odds of being cognitively resilient (using the intermediate or high AD pathology definition) associated with selected demographic and neuropathology characteristics. We calculated odds ratios (ORs) and 95% confidence intervals (CIs) using logistic regression. We ran individual logistic models for each demographic and neuropathology characteristic adjusted for age at baseline, age at death, and having a college degree. Then we ran one large multivariable model that included all demographic and neuropathology findings. We selected characteristics based on whether they were statistically significantly (p-value<0.05) associated with resilience in univariate analyses (college degree, CERAD score, Braak stage, microinfarcts, hippocampal sclerosis, and brain weight). We added Lewy Body Disease to the regression model because it has been associated with resilience in other publications.[10, 19, 34] We adjusted for age at baseline and age at death because age is an important potential confounder of the association between other factors and resilience. We evaluated whether to include macroscopic cystic infarcts, which were missing for 79 study participants. Adjusting for macroscopic infarcts did not change our results; therefore, we have not included them in the final models. The final adjusted model included data for 258 study participants. All analyses were conducted in Stata15 (StataCorp, College Station, TX).
We conducted a sensitivity analysis limiting the population to 231 people who lived to at least 85 years or older. This sensitivity analysis helped address whether our results were impacted by cognitively resilient people potentially dying at younger ages, before they had adequate time to develop signs of dementia. This analysis only included an unadjusted comparison of baseline and neuropathology characteristics following the same methods as the main analysis. We conducted a separate sensitivity analysis limiting the cognitively resilient population to 32 people who had a CASI score ≥86 and no dementia diagnosis within one year of death. This analysis helped address the potential for misclassification of study subjects who may have experienced unmeasured cognitive decline between one and two years before death. This analysis included an unadjusted comparison of baseline and neuropathology characteristics and multivariable logistic regression analyses following the same methods as the main analysis. In both of these sensitivity analyses, we included people who were cognitively resilient to intermediate or high AD pathology; we did not conduct these analyses among people cognitively resilient to high AD pathology only because of very small sample sizes.
We know from prior analyses using ACT neuropathology data that the people who consent to autopsy are somewhat different than people who participate in the overall ACT study and have a slightly higher incidence of dementia or family history of dementia.[35, 36] We conducted a sensitivity analysis following the methods of Haneuse et al. to understand how our results might be impacted by selection bias.[36] Briefly, we generated inverse probability weights from a selection model predicting the probability of being autopsied among those that died based on age at death, age at baseline, dementia status, gender, education, race, and ACT cohort. We then re-estimated our logistic regression model for cognitive resilience incorporating the weights from the selection model.
RESULTS
Among 276 people with intermediate or high AD pathology, 68 (24.6%) were classified as cognitively resilient to AD pathology and 208 (75.4%) were classified as not-resilient (Table 1). When limiting to people with high AD pathology only, 23 (16.9%) were classified as cognitively resilient and 113 (83.1%) as not-resilient. The distributions of age at enrollment in the ACT study, gender, self-reported race, most baseline comorbid conditions, smoking status, BMI, head circumference, self-reported exercise, activities or daily living, and APOE ε4 allele status did not differ between cognitively resilient and not-resilient groups regardless of the operational definition used (Table 2). More people in the cognitively resilient group had a college degree compared to the non-resilient group (50% vs 32%, p=0.01 when using the intermediate/high definition).
Table 2.
Baseline characteristics of people with and without cognitive resilience to AD pathology
| Intermediate or high AD pathology | High AD pathology only | |||||
|---|---|---|---|---|---|---|
| Not resilient N (%‡) N=208* |
Cognitively Resilient N (%‡) N=68† |
p-value (chi2) | Not resilient N (%‡) N=113** |
Cognitively Resilient N (%‡) N=23†† |
p-value (chi2) | |
| ACT cohort: | ||||||
| Original | 139 (66.8) | 47 (69.1) | 0.48 | 74 (65.5) | 14 (60.9) | 0.22 |
| Expansion | 49 (23.6) | 12 (17.7) | 29 (25.7) | 4 (17.4) | ||
| Replacement | 20 (9.6) | 9 (13.2) | 10 (8.9) | 5 (21.7) | ||
| Baseline median age (IQR) | 77 (73–82) | 78 (73–84) | 0.68 | 77 (73–81) | 78 (73–85) | 0.49 |
| Median years between baseline and death (IQR) | 13 (9–16) | 10 (6–14) | 0.07 | 13 (10–17) | 12 (6–18) | 0.82 |
| Sex: | ||||||
| Male | 75 (36.1) | 28 (41.2) | 0.45 | 43 (38.1) | 7 (30.4) | 0.49 |
| Female | 133 (63.9) | 40 (58.8) | 70 (62.0) | 16 (69.6) | ||
| Median years of education (IQR) | 14 (12–16) | 16 (12–17) | 0.06 | 14 (12–16) | 16 (13–17) | 0.06 |
| College degree: | ||||||
| No | 141 (67.8) | 34 (50.0) | 0.01 | 81 (71.7) | 10 (43.5) | 0.01 |
| Yes | 67 (32.2) | 34 (50.0) | 32 (28.3) | 13 (56.5) | ||
| Race: | ||||||
| Non-Hispanic | 196 (94.2) | 63 (92.7) | 0.64 | 106 (93.8) | 21 (91.3) | 0.65 |
| white Non-white |
12 (5.8) | 5 (7.4) | 7 (6.2) | 2 (8.7) | ||
| Hypertension | 71 (34.5) | 25 (37.3) | 0.67 | 38 (33.6) | 10 (43.5) | 0.37 |
| Diabetes | 19 (9.1) | 6 (9.0) | 0.97 | 7 (6.2) | 1 (4.6) | 1.0 |
| Stroke | 4 (1.9) | 3 (4.4) | 0.26 | 2 (1.8) | 2 (8.7) | 0.13 |
| Cerebrovascular disease | 19 (9.2) | 6 (8.8) | 0.92 | 11 (9.8) | 2 (8.7) | 0.87 |
| Coronary heart disease | 32 (15.5) | 9 (13.2) | 0.66 | 17 (15.2) | 1 (4.4) | 0.31 |
| Congestive heart failure | 2 (1.0) | 2 (3.0) | 0.23 | 2 (1.8) | 0 (0) | 0.53 |
| Smoking: | ||||||
| Never | 113 (54.3) | 33 (48.5) | 0.70 | 57 (50.4) | 12 (52.2) | 0.79 |
| Past | 86 (41.4) | 32 (47.1) | 50 (44.3) | 11 (47.8) | ||
| Current | 9 (4.3) | 3 (4.4) | 6 (5.3) | 0 (0) | ||
| BMI (kg/m2) | ||||||
| <25 | 84 (41.0) | 23 (35.4) | 0.69 | 44 (39.6) | 9 (39.1) | 0.96 |
| 25–29.9 | 81 (39.5) | 27 (41.5) | 43 (38.7) | 10 (43.5) | ||
| 30+ | 40 (19.5) | 15 (23.1) | 24 (21.6) | 4 (17.4) | ||
| Median head circumference cm (IQR) | 56.2 (55.0–58.0) | 56.7 (55.2–58.4) | 0.12 | 56.2 (55.0–58.0) | 56.6 (55.8–58.2) | 0.33 |
| Regular exercise (≥15 min 3× week) | 151 (73.0) | 49 (72.1) | 0.89 | 81 (72.3) | 18 (78.3) | 0.56 |
| Number of difficult activities of daily living | ||||||
| 0 | 159 (76.8) | 51 (75.0) | 0.72 | 89 (78.8) | 18 (78.3) | 0.71 |
| 1 | 29 (14.0) | 12 (17.7) | 14 (12.4) | 4 (17.4) | ||
| 2+ | 19 (9.2) | 5 (7.4) | 10 (8.9) | 1 (4.4) | ||
| APOE ε4 allele | 79 (39.3) | 24 (35.8) | 0.61 | 49 (44.6) | 9 (39.1) | 0.63 |
People diagnosed with dementia before death, and a CERAD score of intermediate or frequent AND a BRAAK stage of III-VI
People diagnosed with dementia before death, and a CERAD score of frequent AND a BRAAK stage of V-VI
People with a CASI score ≥86 within 2 years of death and no dementia diagnosis, and a CERAD score of intermediate or frequent AND a BRAAK stage of III-VI
People with a CASI score ≥86 within 2 years of death and no dementia diagnosis, and a CERAD score of frequent AND a BRAAK stage of V-VI
Percent calculated among people with non-missing covariate data.
As selected, everyone in the analytic sample had intermediate or high AD pathology; however, the distributions of CERAD levels and Braak stages differed between cognitively resilient and non-resilient groups (Table 3). Among people with intermediate/high pathology, the cognitively resilient group had a lower proportion of people with frequent CERAD scores (45.6%) and Braak stages V or VI (45.6%) compared to the not-resilient group (64.0% and 78.8%, respectively). Smaller proportions of people in the cognitively resilient group had 2+ microinfarcts (17.9%), any macroscopic cystic infarcts (25.0%), or hippocampal sclerosis (6.0%) compared with the non-resilient group (35.1%, 44.0%, and 23.0%, respectively). The cognitively resilient group had a higher average brain weight at death (1235 gm) compared to the not-resilient group (1165 gm). There were no differences in atherosclerosis or arteriolosclerosis between groups. Results were similar when limiting the analysis to people with high AD pathology only (Tables 2 and 3), cognitively resilient people with CASI scores ≥86 within one year before death (Supplementary tables 2 and 3), and people who lived to at least 85 years or older (Supplementary tables 5 and 6). Minor differences included a loss of statistical significance in the distribution of college education between cognitively resilient and non-resilient groups, likely as a result of a decline in sample size.
Table 3.
Neuropathology characteristics of people with and without cognitive resilience to AD pathology
| Intermediate or high AD pathology | High AD pathology only | |||||
|---|---|---|---|---|---|---|
| Not resilient N (%‡) N=208* |
Cognitively Resilient N (%‡) N=68† |
p-value (chi2) | Not resilient N (%‡) N=113** |
Cognitively Resilient N (%‡) N=23†† |
p-value (chi2) | |
| Median age at death (IQR) | 91 (88–95) | 89 (84–94) | 0.26 | 91 (87–94) | 90 (87–96) | 0.49 |
| Median years between dementia diagnosis and death (IQR) | 5.2 (3.2–7.5) | N/A | N/A | 5.5 (3.9–7.6) | N/A | N/A |
| Median years between last CASI score ≥86 and death (IQR) | N/A | 1.0 (0.5–1.6) | N/A | N/A | 1.0 (0.3–1.6) | N/A |
| CERAD | ||||||
| Intermediate | 75 (36.1) | 37 (54.4) | 0.01 | 0 | 0 | N/A |
| Frequent | 133 (64.0) | 31 (45.6) | 113 (100) | 23 (100) | ||
| Braak | ||||||
| III | 17 (8.2) | 25 (36.8) | <0.001 | 0 | 0 | 0.06 |
| IV | 27 (13.0) | 12 (17.7) | 0 | 0 | ||
| V | 76 (36.5) | 22 (32.4) | 45 (39.8) | 14 (60.9) | ||
| VI | 88 (42.3) | 9 (13.2) | 68 (60.2) | 9 (39.1) | ||
| Atherosclerosis | ||||||
| None/mild | 45 (21.7) | 16 (24.2) | 0.67 | 23 (20.5) | 7 (30.4) | 0.30 |
| Moderate/severe | 162 (78.3) | 50 (75.8) | 89 (79.5) | 16 (69.6) | ||
| Arteriolosclerosis | ||||||
| None/mild | 28 (15.3) | 10 (18.9) | 0.53 | 14 (14.0) | 6 (30.0) | 0.08 |
| Moderate/severe | 155 (84.7) | 43 (81.1) | 86 (86.0) | 14 (70.0) | ||
| Macroscopic cystic infarcts | ||||||
| None | 84 (56.0) | 36 (75.0) | 0.01 | 50 (60.2) | 10 (76.9) | 0.36 |
| Any | 66 (44.0) | 11 (25.0) | 33 (39.8) | 3 (23.1) | ||
| Cortical or deep microinfarcts | ||||||
| 0–1 | 135 (64.9) | 55 (82.1) | 0.01 | 77 (68.1) | 20 (90.9) | 0.03 |
| 2+ | 73 (35.1) | 12 (17.9) | 36 (31.9) | 2 (9.1) | ||
| Hippocampal sclerosis | ||||||
| No | 157 (77.0) | 63 (94.0) | 0.01 | 84 (75.0) | 20 (87.0) | 0.28 |
| Yes | 47 (23.0) | 4 (6.0) | 28 (25.0) | 3 (13.0) | ||
| Amyloid angiopathy | ||||||
| None/mild | 152 (73.8) | 53 (77.9) | 0.49 | 78 (69.6) | 12 (52.2) | 0.11 |
| Moderate/severe | 54 (26.2) | 14 (22.1) | 34 (30.4) | 11 (47.8) | ||
| Lewy bodies in isocortex | ||||||
| No | 184 (89.8) | 64 (95.5) | 0.15 | 101 (90.2) | 22 (95.7) | 0.69 |
| Yes | 21 (10.2) | 3 (3.0) | 11 (9.8) | 1 (4.4) | ||
| Brain weight mean, gm (SD) | 1165 (136) | 1235 (140) | <0.001 | 1155 (137) | 1253 (136) | 0.002 |
People diagnosed with dementia before death, and a CERAD score of intermediate or frequent AND a BRAAK stage of III-VI
People diagnosed with dementia before death, and a CERAD score of frequent AND a BRAAK stage of V-VI
People with a CASI score ≥86 within 2 years of death and no dementia diagnosis, and a CERAD score of intermediate or frequent AND a BRAAK stage of III-VI
People with a CASI score ≥86 within 2 years of death and no dementia diagnosis, and a CERAD score of frequent AND a BRAAK stage of V-VI
Percent calculated among people with non-missing neuropathology data.
Odds of being cognitively resilient among people with a college degree were twice that of those without a college degree, after adjustment for additional neuropathology characteristics (OR=2.01, 95%CI=1.01–3.99, Table 4). Having a Braak stage V or VI, 2+ cortical or deep microinfarcts, and hippocampal sclerosis were each independently associated with significantly reduced odds of being cognitively resilient compared to people without those findings. Results were similar when limiting the cognitively resilient group to 32 people with CASI scores ≥86 within one year before death (Supplementary table 4). When graphically looking at the distribution of multiple pathology findings by group (Figures 2a and 2b), 60.3% of the cognitively resilient group had 0–1 findings compared with 25.0% of the non-resilient group. However, 6 (8.8%) cognitively resilient people had 3 or more neuropathology findings indicating substantial neuropathology burden, while 13 (6.3%) non-resilient people had no findings other than intermediate CERAD scores or Braak stage III or IV.
Table 4.
Odds of being cognitively resilient to intermediate or high AD pathology versus not adjusted for demographic characteristics and non-AD neuropathology findings
| Demographic-adjusted* OR (95% CI) | p-value | Demographic and non-AD neuropathology adjusted** OR (95% CI) | p-value | |
|---|---|---|---|---|
| College degree | ||||
| No | 1.0 | 1.0 | ||
| Yes | 2.19 (1.23, 3.88) | 0.01 | 2.01 (1.01, 3.99) | 0.047 |
| CERAD | ||||
| Intermediate | 1.0 | 1.0 | ||
| Frequent | 0.53 (0.30, 0.95) | 0.03 | 0.82 (0.40, 1.70) | 0.60 |
| Braak | ||||
| III | 1.0 | 1.0 | ||
| IV | 0.37 (0.12, 0.96) | 0.04 | 0.50 (0.17, 1.49) | 0.21 |
| V | 0.25 (0.11, 0.57) | 0.001 | 0.38 (0.15, 0.99) | 0.048 |
| VI | 0.08 (0.03, 0.21) | <0.001 | 0.12 (0.04, 0.32) | <0.001 |
| Cortical or deep microinfarcts | ||||
| 0–1 | 1.0 | 1.0 | ||
| 2+ | 0.42 (0.21, 0.85) | 0.02 | 0.34 (0.15, 0.78) | 0.01 |
| Hippocampal sclerosis | ||||
| No | 1.0 | 1.0 | ||
| Yes | 0.23 (0.08, 0.69) | 0.01 | 0.28 (0.09, 0.89) | 0.03 |
| Lewy bodies in isocortex | ||||
| No | 1.0 | 1.0 | ||
| Yes | 0.36 (0.10, 1.31) | 0.12 | 0.35 (0.09, 1.44) | 0.15 |
| Brain weight (per gm) | 1.003 (1.001–1.006) | 0.004 | 1.004 (1.001, 1.007) | 0.004 |
adjusted for age at baseline, age at death, and college degree.
adjusted for age at baseline, age at death, college degree, CERAD, Braak, microinfarcts, hippocampal sclerosis, Lewy body disease, and brain weight. Model includes 258 observations with non-missing data.
Figure 2a and 2b.
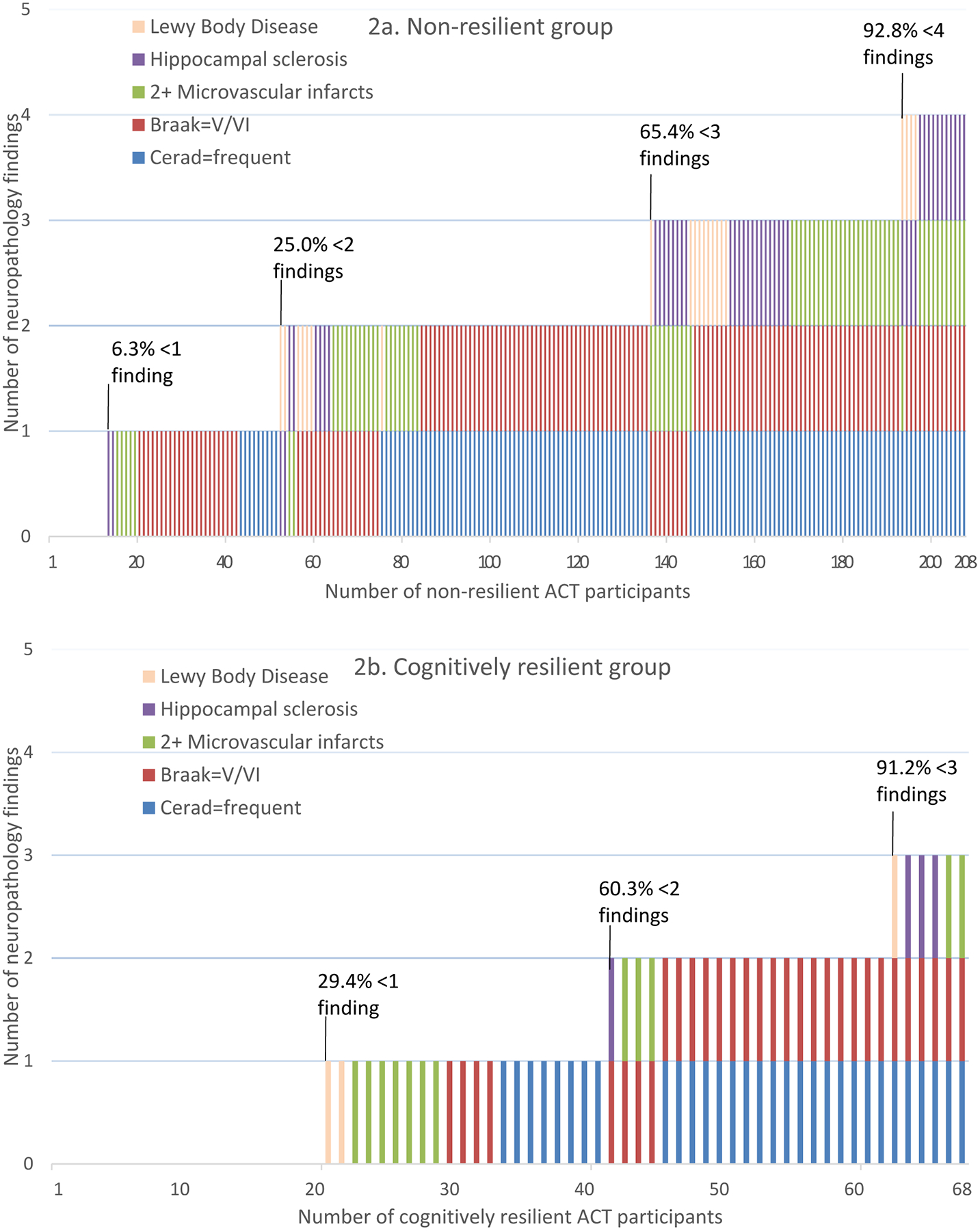
Distribution of multiple neuropathology characteristics across ACT study participants, stratified by cognitively resilient and non-resilient groups. This figure shows the distribution of five neuropathology findings (Lewy Body Disease, hippocampal sclerosis, 2+ microvascular infarcts, Braak stage V/VI, and frequent CERAD score) by number of non-resilient (2a) and cognitively resilient (2b) study participants. The figure shows the combinations of various neuropathology findings and the proportions of non-resilient (2a) and cognitively resilient (2b) groups that have <1, <2, <3 or <4 findings. People with no findings have Braak stage III/IV and intermediate CERAD score.
To evaluate selection bias, we conducted an analysis using inverse probability weights to account for selection into the autopsy sample. The results were not appreciably different from our main analysis (Supplementary Table 1).
DISCUSSION
Cognitive resilience to AD pathology describes a phenomenon where people develop substantial neuritic plaques and neurofibrillary tangles while maintaining higher than expected levels of cognition during life.[7, 9] Our definition of cognitive resilience required people to be not demented, have a CASI score ≥86 within 2 years of death, and have intermediate or high AD pathology defined based on an adaptation of NIA-AA guidelines. Our results suggest a combination of higher education level and fewer non-AD pathology characteristics may be associated with cognitive resilience. They may also simply delay the onset of symptoms of cognitive decline in an elderly population.
A higher proportion of the cognitively resilient group had a college education than the non-resilient population. Prior studies have shown that a higher education level is associated with a reduced risk of dementia;[37–40] therefore, it makes sense that higher education would also be associated with resilience. There may be a component of learning that mitigates the impact of neuropathologic degeneration on cognitive function, or increases the threshold beyond which neuropathologic changes would have an impact on function. In fact, several studies have shown that people with more education tend to be able to withstand neuropathologic changes for a longer period of time before they develop dementia compared to those with lower education levels.[7, 9, 35, 41–44] Higher education level may also increase a person’s ability to perform on tests, thereby hiding their decline, which could imply that high CASI scores may not detect cognitive dysfunction in highly educated individuals.[45]
A somewhat smaller proportion of the cognitively resilient group had ≥1 APOE ε4 allele, though the difference between resilient and non-resilient groups was not statistically significant. This result might seem counter-intuitive in that APOE is one of the strongest genetic risk factors for dementia and, as such, might have been expected to be related to cognitive resilience. However, it’s possible that the APOE ε4 allele is a better marker of neuropathologic changes – not necessarily cognitive decline, resulting in a higher proportion of the ε4 allele in the analytic sample compared to the base ACT population, but little difference between cognitively resilient and non-resilient groups.
The cognitively resilient group in our analyses had higher average brain weights at death compared to the non-resilient group. Preventing brain atrophy could be a critical component to overcoming neuropathologic degeneration. Previous work has demonstrated that higher education level is associated with heavier brain weight, suggesting that individual components that may contribute to cognitive resilience are not necessarily independent of one another.[41] However, if more neuritic plaques and neurofibrillary tangles lead directly to brain atrophy, the lower brain weight result among the non-resilient group may simply reflect upstream processes that lead to dementia. Our adjusted model showed higher brain weight was significantly associated with cognitive resilience, even after adjusting for other neuropathology findings. Head circumference may reflect an individual’s brain reserve by representing an upper bound to brain size since it does not change over time,[46] but we did not see a relationship between head circumference and cognitive resilience in our data.
To account for comorbid neurodegenerative processes in a quantitative manner, we developed a logistic regression model to estimate the odds of being cognitively resilient while controlling for multiple demographic and neuropathologic characteristics. College education, higher brain weight, and absence of Braak stage V or VI, microinfarcts, and hippocampal sclerosis were statistically significant and independently associated with resilience. After adjusting for other characteristics, CERAD level was no longer an independent predictor of cognitive resilience. Our investigations were focused on dementia, and did not consider pathways or cascades such as the amyloid (represented by CERAD) then tau (represented by Braak) hypothesis of the Jack model[47, 48]. Prior studies have shown that having multiple neuropathology findings is more common among people with dementia or AD than people without.[12, 19, 34, 49–51] In our study, 27 (40%) people classified as cognitively resilient had two or more of these neuropathological findings, a pattern that occurred among 75% of the non-resilient group with clinically diagnosed dementia. The range of neuropathology in cognitively resilient participants highlights the impact of co-morbid pathologies on dementia, points to the potential inadequacy of current neuropathologic criteria to adequately classify disease burden, and directs us to a subset of participants with true cognitive resilience to high pathologic burden. It is possible that cognitive resilience simply reflects a lower risk of non-AD neuropathology findings. Larger samples are needed to study the characteristics of these unique individuals to begin to clarify the mechanisms that enabled them to overcome a substantial neuropathology burden.
Currently, our operational resilience definition only categorizes people as cognitively resilient or not after they die because it relies on neuropathology findings. This dichotomous definition of cognitive resilience may not reflect processes that occur throughout aging to a different degree. A continuous scale of resilience or multiple levels of resilience may be a more clinically useful way to approach this phenomenon. If a person lives at least to age 85 or 90 without any signs or symptoms of cognitive decline, that person might also be considered resilient regardless of neuropathology outcomes. Definitions that incorporate additional genetic, imaging, and other data will help us better understand the phenotype of resilience before death.[23, 24, 52]
The potential for selection bias cannot be ignored in our study or other studies of resilience. Our investigations suggested that our final analytic sample with intermediate or high AD pathology and known clinical dementia status may have been healthier overall and lived to older ages compared with the samples from which it was drawn. ACT study participants consent separately for autopsy, which likely causes some participation bias in this study sample compared to our entire population-based cohort. However, our sensitivity analyses showed that our analyses were not substantially affected by selection bias in the sample that consented for autopsy.
Our study has several additional limitations. We may have some misclassification in our cognitive resilience definition, particularly in people who might have had undetected cognitive decline before their death. It is possible that some participants experienced cognitive decline and dementia between their final ACT study visit and the time that they died. In this study, such a person would be misclassified as cognitively resilient. We deliberately excluded participants with more than two years since their last assessment and conducted a sensitivity analysis limiting the cognitively resilient group to people with CASI score ≥86 one year before death to address this possibility; but rapid cognitive decline after the last study visit must be considered. Experiencing such decline until just before death may reflect a compression of cognitive morbidity,[53] which could be considered another form of resilience. There is an additional group of individuals excluded from these analyses: people who did not develop neuropathology and did not have cognitive impairment. There may be important differences in people who are resistant to neuropathology compared to those who are resilient. Subsequent research will be necessary to tease apart the mechanisms of resistance. We did not examine changes in comorbidities in late-life in this report, but evaluating later-life comorbidities and their potential association with or impact on cognitive resilience is an important area of future research. Finally, our sample size limits the extent to which we could draw conclusions about less common risk factors and the definition of cognitive resilience that relied on high AD pathology alone.
Strengths of our study include the use of a population-based, community sample and availability of a relatively large number of persons at old age without clinical evidence of dementia before death. We evaluated mid-life demographics and characteristics collected well in advance of any dementia diagnoses or symptoms of cognitive decline. We also used dementia outcomes made by consensus diagnoses and neuropathology findings using carefully calibrated, blinded, and harmonized neuropathology protocols to develop our cognitive resilience definition. Our breadth of neuropathology results, in addition to CERAD and Braak, provide an important opportunity to study additional brain characteristics of cognitively resilient and non-resilient people. These results enabled us to identify a small group of people with impressively high levels of neuropathological findings who nevertheless remained free of dementia into very late life.
In conclusion, our results show that cognitive resilience to AD pathology was associated with higher education levels and heavier brain weights, and fewer neuropathology findings, such as Braak stage V/VI, microinfarcts, and hippocampal sclerosis. To our knowledge, this is one of the first studies to develop an operational definition of cognitive resilience based on neuropathology findings and evaluate that definition in a population-based cohort. Our work extends prior research by including multiple demographic and neuropathology characteristics in a single regression model, allowing us to start to understand comorbid processes that may enable or hinder cognitive resilience. In addition, our sensitivity analyses allowed us to tease apart our definition of cognitive resilience and understand how our results were affected by time, neuropathology levels, and age. However, this study alone cannot completely tease out mechanisms of cognitive resilience or conclude whether cognitive resilience is simply a reflection of lower neuropathology burden. More work in larger, population-based samples is needed to understand why some people develop substantial plaques and tangles as well as other forms of neuropathology without exhibiting dementia during their lifetime.
Supplementary Material
Acknowledgments/conflicts/funding sources:
This work was supported by the National Institute on Aging at the National Institutes of Health (grant U01AG006781).
Footnotes
Conflicts of interest/disclosure statement: Eric Larson and Dirk Keene receive royalties from UpToDate. All other authors have no conflict of interest to report.
References
- [1].English Oxford Dictionary, https://en.oxforddictionaries.com/definition/resilience,
- [2].Driscoll I, Troncoso J (2011) Asymptomatic Alzheimer’s disease: a prodrome or a state of resilience? Curr Alzheimer Res 8, 330–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR (2000) “Preclinical” AD revisited: neuropathology of cognitively normal older adults. Neurology 55, 370–376. [DOI] [PubMed] [Google Scholar]
- [4].Medaglia JD, Pasqualetti F, Hamilton RH, Thompson-Schill SL, Bassett DS (2017) Brain and cognitive reserve: Translation via network control theory. Neurosci Biobehav Rev 75, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].O’Brien RJ, Resnick SM, Zonderman AB, Ferrucci L, Crain BJ, Pletnikova O, Rudow G, Iacono D, Riudavets MA, Driscoll I, Price DL, Martin LJ, Troncoso JC (2009) Neuropathologic studies of the Baltimore Longitudinal Study of Aging (BLSA). J Alzheimers Dis 18, 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Woods NF, Rillamas-Sun E, Cochrane BB, La Croix AZ, Seeman TE, Tindle HA, Zaslavsky O, Bird CE, Johnson KC, Manson JE, Ockene JK, Seguin RA, Wallace RB (2016) Aging Well: Observations From the Women’s Health Initiative Study. J Gerontol A Biol Sci Med Sci 71 Suppl 1, S3–S12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Arenaza-Urquijo EM, Vemuri P (2018) Resistance vs resilience to Alzheimer disease: Clarifying terminology for preclinical studies. Neurology 90, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hadley EC, Kuchel GA, Newman AB, Workshop S, Participants (2017) Report: NIA Workshop on Measures of Physiologic Resiliencies in Human Aging. J Gerontol A Biol Sci Med Sci 72, 980–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stern Y, Arenaza-Urquijo EM, Bartres-Faz D, Belleville S, Cantilon M, Chetelat G, Ewers M, Franzmeier N, Kempermann G, Kremen WS, Okonkwo O, Scarmeas N, Soldan A, Udeh-Momoh C, Valenzuela M, Vemuri P, Vuoksimaa E, Reserve R, Protective Factors PIAED, Conceptual Frameworks W (2018) Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimers Dement. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sonnen JA, Santa Cruz K, Hemmy LS, Woltjer R, Leverenz JB, Montine KS, Jack CR, Kaye J, Lim K, Larson EB, White L, Montine TJ (2011) Ecology of the aging human brain. Arch Neurol 68, 1049–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].White L (2009) Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis 18, 713–725. [DOI] [PubMed] [Google Scholar]
- [12].Schneider JA, Arvanitakis Z, Bang W, Bennett DA (2007) Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69, 2197–2204. [DOI] [PubMed] [Google Scholar]
- [13].Schneider JA, Aggarwal NT, Barnes L, Boyle P, Bennett DA (2009) The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis 18, 691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Besser LM, Alosco ML, Ramirez Gomez L, Zhou XH, McKee AC, Stern RA, Gunstad J, Schneider JA, Chui H, Kukull WA (2016) Late-Life Vascular Risk Factors and Alzheimer Disease Neuropathology in Individuals with Normal Cognition. J Neuropathol Exp Neurol 75, 955–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, Wilson RS (2006) Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 66, 1837–1844. [DOI] [PubMed] [Google Scholar]
- [16].Negash S, Bennett DA, Wilson RS, Schneider JA, Arnold SE (2011) Cognition and neuropathology in aging: multidimensional perspectives from the Rush Religious Orders Study and Rush Memory And Aging Project. Curr Alzheimer Res 8, 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Price JL, McKeel DW Jr., Buckles VD, Roe CM, Xiong C, Grundman M, Hansen LA, Petersen RC, Parisi JE, Dickson DW, Smith CD, Davis DG, Schmitt FA, Markesbery WR, Kaye J, Kurlan R, Hulette C, Kurland BF, Higdon R, Kukull W, Morris JC (2009) Neuropathology of nondemented aging: presumptive evidence for preclinical Alzheimer disease. Neurobiol Aging 30, 1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Knopman DS, Parisi JE, Salviati A, Floriach-Robert M, Boeve BF, Ivnik RJ, Smith GE, Dickson DW, Johnson KA, Petersen LE, McDonald WC, Braak H, Petersen RC (2003) Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol 62, 1087–1095. [DOI] [PubMed] [Google Scholar]
- [19].Latimer CS, Keene CD, Flanagan ME, Hemmy LS, Lim KO, White LR, Montine KS, Montine TJ (2017) Resistance to Alzheimer Disease Neuropathologic Changes and Apparent Cognitive Resilience in the Nun and Honolulu-Asia Aging Studies. J Neuropathol Exp Neurol 76, 458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Bennett DA, Wilson RS, Boyle PA, Buchman AS, Schneider JA (2012) Relation of neuropathology to cognition in persons without cognitive impairment. Ann Neurol 72, 599–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Boyle PA, Yu L, Wilson RS, Schneider JA, Bennett DA (2013) Relation of neuropathology with cognitive decline among older persons without dementia. Front Aging Neurosci 5, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].SantaCruz KS, Sonnen JA, Pezhouh MK, Desrosiers MF, Nelson PT, Tyas SL (2011) Alzheimer disease pathology in subjects without dementia in 2 studies of aging: the Nun Study and the Adult Changes in Thought Study. J Neuropathol Exp Neurol 70, 832–840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hohman TJ, McLaren DG, Mormino EC, Gifford KA, Libon DJ, Jefferson AL, Alzheimer’s Disease Neuroimaging I (2016) Asymptomatic Alzheimer disease: Defining resilience. Neurology 87, 2443–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mukherjee S, Kim S, Gibbons LE, Nho K, Risacher SL, Glymour MM, Habeck C, Lee GJ, Mormino E, Ertekin-Taner N, Montine TJ, Decarli C, Saykin AJ, Crane PK, Alzheimer’s Disease Neuroimaging I (2012) Genetic architecture of resilience of executive functioning. Brain Imaging Behav 6, 621–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kukull WA, Higdon R, Bowen JD, McCormick WC, Teri L, Schellenberg GD, van Belle G, Jolley L, Larson EB (2002) Dementia and Alzheimer disease incidence: a prospective cohort study. Arch Neurol 59, 1737–1746. [DOI] [PubMed] [Google Scholar]
- [26].Crane PK, Walker R, Hubbard RA, Li G, Nathan DM, Zheng H, Haneuse S, Craft S, Montine TJ, Kahn SE, McCormick W, McCurry SM, Bowen JD, Larson EB (2013) Glucose levels and risk of dementia. N Engl J Med 369, 540–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Larson EB, Wang L, Bowen JD, McCormick WC, Teri L, Crane P, Kukull W (2006) Exercise is associated with reduced risk for incident dementia among persons 65 years of age and older. Ann Intern Med 144, 73–81. [DOI] [PubMed] [Google Scholar]
- [28].Teng EL, Hasegawa K, Homma A, Imai Y, Larson E, Graves A, Sugimoto K, Yamaguchi T, Sasaki H, Chiu D, et al. (1994) The Cognitive Abilities Screening Instrument (CASI): a practical test for cross-cultural epidemiological studies of dementia. Int Psychogeriatr 6, 45–58; discussion 62. [DOI] [PubMed] [Google Scholar]
- [29].(1994) Diagnostic and Statistical Manual of Mental Disorders. 4th ed., American Psychiatric Association, Washington, D.C. [Google Scholar]
- [30].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM (1984) Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 34, 939–944. [DOI] [PubMed] [Google Scholar]
- [31].Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement. 8, 1–13. doi: 10.1016/j.jalz.2011.1010.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT, National Institute on A, Alzheimer’s A (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Sonnen JA, Larson EB, Crane PK, Haneuse S, Li G, Schellenberg GD, Craft S, Leverenz JB, Montine TJ (2007) Pathological correlates of dementia in a longitudinal, population-based sample of aging. Ann Neurol 62, 406–413. [DOI] [PubMed] [Google Scholar]
- [34].Cholerton B, Larson EB, Baker LD, Craft S, Crane PK, Millard SP, Sonnen JA, Montine TJ (2013) Neuropathologic correlates of cognition in a population-based sample. J Alzheimers Dis 36, 699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bennett DA, Arnold SE, Valenzuela MJ, Brayne C, Schneider JA (2014) Cognitive and social lifestyle: links with neuropathology and cognition in late life. Acta Neuropathol 127, 137–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Haneuse S, Schildcrout J, Crane P, Sonnen J, Breitner J, Larson E (2009) Adjustment for selection bias in observational studies with application to the analysis of autopsy data. Neuroepidemiology 32, 229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Marden JR, Tchetgen Tchetgen EJ, Kawachi I, Glymour MM (2017) Contribution of Socioeconomic Status at 3 Life-Course Periods to Late-Life Memory Function and Decline: Early and Late Predictors of Dementia Risk. Am J Epidemiol 186, 805–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Nguyen TT, Tchetgen Tchetgen EJ, Kawachi I, Gilman SE, Walter S, Liu SY, Manly JJ, Glymour MM (2016) Instrumental variable approaches to identifying the causal effect of educational attainment on dementia risk. Ann Epidemiol 26, 71–76 e71–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Xu W, Tan L, Wang HF, Tan MS, Tan L, Li JQ, Zhao QF, Yu JT (2016) Education and Risk of Dementia: Dose-Response Meta-Analysis of Prospective Cohort Studies. Mol Neurobiol 53, 3113–3123. [DOI] [PubMed] [Google Scholar]
- [40].Tom SE, Hubbard RA, Crane PK, Haneuse SJ, Bowen J, McCormick WC, McCurry S, Larson EB (2015) Characterization of dementia and Alzheimer’s disease in an older population: updated incidence and life expectancy with and without dementia. Am J Public Health 105, 408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Members ECC, Brayne C, Ince PG, Keage HA, McKeith IG, Matthews FE, Polvikoski T, Sulkava R (2010) Education, the brain and dementia: neuroprotection or compensation? Brain 133, 2210–2216. [DOI] [PubMed] [Google Scholar]
- [42].Rapp SR, Espeland MA, Manson JE, Resnick SM, Bryan NR, Smoller S, Coker LH, Phillips LS, Stefanick ML, Sarto GE, Women’s Health Initiative Memory S (2013) Educational attainment, MRI changes, and cognitive function in older postmenopausal women from the Women’s Health Initiative Memory Study. Int J Psychiatry Med 46, 121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].van Loenhoud AC, Wink AM, Groot C, Verfaillie SCJ, Twisk J, Barkhof F, van Berckel B, Scheltens P, van der Flier WM, Ossenkoppele R (2017) A neuroimaging approach to capture cognitive reserve: Application to Alzheimer’s disease. Hum Brain Mapp 38, 4703–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Reed BR, Mungas D, Farias ST, Harvey D, Beckett L, Widaman K, Hinton L, DeCarli C (2010) Measuring cognitive reserve based on the decomposition of episodic memory variance. Brain 133, 2196–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Koepsell TD, Kurland BF, Harel O, Johnson EA, Zhou XH, Kukull WA (2008) Education, cognitive function, and severity of neuropathology in Alzheimer disease. Neurology 70, 1732–1739. [DOI] [PubMed] [Google Scholar]
- [46].Borenstein Graves A, Mortimer JA, Bowen JD, McCormick WC, McCurry SM, Schellenberg GD, Larson EB (2001) Head circumference and incident Alzheimer’s disease: modification by apolipoprotein E. Neurology. 57, 1453–1460. [DOI] [PubMed] [Google Scholar]
- [47].Jack CR Jr., Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J, Liu E, Molinuevo JL, Montine T, Phelps C, Rankin KP, Rowe CC, Scheltens P, Siemers E, Snyder HM, Sperling R, Contributors (2018) NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement 14, 535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, Trojanowski JQ (2010) Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol 9, 119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Azarpazhooh MR, Avan A, Cipriano LE, Munoz DG, Sposato LA, Hachinski V (2017) Concomitant vascular and neurodegenerative pathologies double the risk of dementia. Alzheimers Dement. [DOI] [PubMed] [Google Scholar]
- [50].Dowling NM, Tomaszewski Farias S, Reed BR, Sonnen JA, Strauss ME, Schneider JA, Bennett DA, Mungas D (2011) Neuropathological associates of multiple cognitive functions in two community-based cohorts of older adults. J Int Neuropsychol Soc 17, 602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Nagy Z, Esiri MM, Jobst KA, Morris JH, King EM, McDonald B, Joachim C, Litchfield S, Barnetson L, Smith AD (1997) The effects of additional pathology on the cognitive deficit in Alzheimer disease. J Neuropathol Exp Neurol 56, 165–170. [DOI] [PubMed] [Google Scholar]
- [52].Mukherjee S, Kim S, Ramanan VK, Gibbons LE, Nho K, Glymour MM, Ertekin-Taner N, Montine TJ, Saykin AJ, Crane PK, Alzheimer’s Disease Neuroimaging I (2014) Gene-based GWAS and biological pathway analysis of the resilience of executive functioning. Brain Imaging Behav 8, 110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, Rosen AB (2008) Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimers Dement 4, 134–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


