Background:
Without treatment, HIV infection in pregnant women is associated with adverse pregnancy outcomes. We compared adverse pregnancy outcomes among HIV-positive women on antiretroviral therapy (ART) and HIV-negative women who enrolled for antenatal care in selected health facilities in Maseru district, Lesotho.
Methods:
We enrolled a cohort of HIV-positive and HIV-negative women at their first antenatal visit and followed them through delivery. Study data on miscarriage, stillbirth, preterm birth, low birth weight and birth defects were collected through participant interviews and medical record abstraction. We used the Rao-Scott χ2 test and the t test to assess differences in characteristics and outcomes between HIV-positive and HIV-negative women and generalized estimating equations for multivariable analysis.
Results:
A total of 614 HIV-positive and 390 HIV-negative pregnant women were enrolled in the study with delivery information on 571 (93.1%) and 352 (90.3%) respectively. In the delivery cohort, the median age at enrolment was 28 years for HIV-positive women and 23 years for HIV-negative women with median gestational ages of 20 and 21 weeks, respectively. A total of 149 singleton pregnancies had documented adverse pregnancy outcomes; 33 (9.6%) HIV-negative pregnancies and 116 (20.6%) HIV-positive pregnancies. Compared with their HIV-negative counterparts, HIV-positive women were more likely to experience an adverse pregnancy outcome, adjusted odds ratio (AOR) 2.6 [95% confidence interval (CI): 1.71–3.97]; an intrauterine death (miscarriage or stillbirth), AOR 2.64 [95% CI: 1.25–5.49]; or a low birth weight delivery, AOR 1.89 [95% CI: 1.16–3.09].
Conclusion:
Adverse pregnancy outcomes remained 2–3 times higher among HIV-positive women compared with HIV-negative women despite universal ART.
Keywords: HIV, pregnancy, birth outcomes, Lesotho, ART
HIV infection during pregnancy is associated with an increased risk of adverse pregnancy outcomes.1,2 Several studies have shown that untreated maternal HIV infection increases the risk of stillbirth, preterm delivery, low birth weight and small-for-gestational-age infants.3–5 In 2013, the World Health Organization (WHO) recommended the use of antiretroviral therapy (ART) for all pregnant and breastfeeding women for the prevention of mother-to-child HIV transmission (PMTCT)6; and in 2016, adopted the “treat all” approach for HIV treatment and prevention.7
As the “treat all” approach expands across sub-Saharan Africa, increasing numbers of women are conceiving while on ART and additional women are newly diagnosed and initiated on ART when they attend antenatal care (ANC). In settings like Lesotho where HIV prevalence among women of reproductive age is 29.7% and ANC attendance is over 97%, this approach has led to ART coverage of up to 98.5% among pregnant women.8
The benefits of ART for PMTCT are well known; however, the effect of antiretroviral drugs on pregnancy outcomes is not fully known. There is inconclusive evidence on the occurrence of adverse pregnancy outcomes such as stillbirth, preterm delivery, low birth weight or small-for-gestational-age deliveries among women on ART. With increased ART use, some studies have shown declining trends for preterm birth, low birth weight and stillbirths.5,9–11 However, other studies show no reduction in adverse pregnancy outcomes among HIV-positive women on ART compared with HIV-negative women,12,13 and additional studies suggest differences between antiretroviral drugs in risk for adverse pregnancy outcomes.14,15 There is a need to clearly understand the relationship between HIV and adverse pregnancy outcomes in the setting of current WHO guidelines for PMTCT and as new antiretroviral drugs enter widespread use.
The “Integrated Management Team to Improve Maternal-Child Outcomes” (IMPROVE) study was a cluster-randomized prospective cohort study at 12 health facilities to determine the effect of facility-based interventions on PMTCT and maternal child health service delivery and uptake in Lesotho. The IMPROVE intervention included the use of multi-disciplinary team support for pregnant/postpartum women, integrated training on client-centered care and enhanced home visiting early in pregnancy. The IMPROVE study provided the opportunity to compare adverse pregnancy outcomes between HIV-positive women on ART and HIV-negative women receiving ANC in selected health facilities in Maseru District in Lesotho. Maseru is Lesotho’s most populous district with one of the highest HIV prevalence.
MATERIALS AND METHODS
Study Design and Setting
Between July 2016 and February 2017, HIV-positive and HIV-negative pregnant women attending their first ANC visit at 12 health facilities in the Maseru district were enrolled into the study and followed every 3 months until 12–24 months postpartum. Two main hospitals and 10 affiliated health centers serving between 150 and 900 pregnant women annually were randomized (each hospital with its 5 affiliated referral health centers forming a cluster) to receive the IMPROVE intervention or the standard of care. Because there were no significant differences in pregnancy outcome by study arms; for this analysis, we combined data from the entire cohort of women who enrolled in the IMPROVE study and compared adverse pregnancy outcomes experienced by HIV-positive women on ART and their HIV-negative counterparts.
Study Population
Pregnant women attending their first ANC visit with known HIV status (including women tested on the day of screening) who lived within the facility catchment area and were willing to provide informed consent were eligible for enrolment. All HIV-positive women and a randomly selected subset of HIV-negative women (~1 woman/week/facility) were screened for study eligibility and enrolment.
Study Procedures
Trained study nurses screened women for study eligibility and obtained written informed consent before study enrolment. Nurses interviewed participants using structured data collection forms and reviewed medical charts and facility registers to obtain information on demographic, clinical and laboratory data. Data were collected on electronic tablets and uploaded into a web-based data management system for data cleaning and analysis. Women who missed study appointments were followed up by phone and if unavailable, home visits were carried out. Information on delivery outcomes, including birth weights, was obtained by review of patient charts, health facility registers and obstetric records. Information on pregnancy loss or deliveries that took place at home or in nonstudy sites was obtained through phone interviews with the mothers and subsequent review of any discharge notes obtained from the nonstudy site.
In accordance with national HIV treatment guidelines, women attending their first ANC were tested for HIV followed by immediate ART initiation if positive. The ART combination of choice was tenofovir (TDF), lamivudine (3TC) and efavirenz (EFV).16 Tuberculosis (TB) symptom screening was carried out at each visit and daily trimethoprim-sulfamethoxazole was given to women with CD4≤350 cells/mm3 or WHO clinical stage 3 or 4.
Study Outcomes
Gestational age was computed based on dates of the last menstrual period and through obstetric examination of the fundal height. Ultrasound is not routinely available in ANC at primary care health facilities in Lesotho. Preconception ART was defined as ART initiated at least 42 weeks before the date of delivery or at least 30 weeks before the date of a miscarriage. Miscarriage was defined as noninduced pregnancy loss before 28 weeks of gestation; stillbirth was defined as delivery of a baby with no signs of life at or after 28 weeks’ gestation17; and preterm birth was defined as live birth before completion of 37 weeks of gestation.18 We defined low birth weight as an infant weight at birth less than 2500 g19; and birth defects were defined as any structural anomalies detected on neonatal examination at birth.
The primary outcome of interest was a composite outcome defined as any adverse pregnancy event and included any of the following: miscarriage (spontaneous abortion), stillbirth, preterm birth, low birth weight and birth defects. In addition, we evaluated the occurrence of the individual pregnancy outcomes.
Statistical Analysis
We summarized baseline data including participant age, marital status, gestational age, level of education, gravidity and syphilis infection during pregnancy in frequency tables. Analysis of pregnancy outcomes was limited to singleton pregnancies. We used the Rao-Scott χ2 test and the t test to assess differences in characteristics and outcomes between HIV-positive and HIV-negative women, accounting for clustering by study site. Generalized estimating equations were used for multivariable analysis, also accounting for correlation between participants within a site. We present odds ratios adjusted for baseline characteristics identified a priori: maternal age, marital status and education (gravidity was also considered but was highly associated with age and therefore not included in final models). Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
Ethics
The study protocol was reviewed and approved by the Lesotho National Health Research Ethics Committee (FWA00023574), the George Washington University Institutional Review Board (IRB) (FWA00005945) and the Population Council IRB (FWA00000279). All participants provided written informed consent.
RESULTS
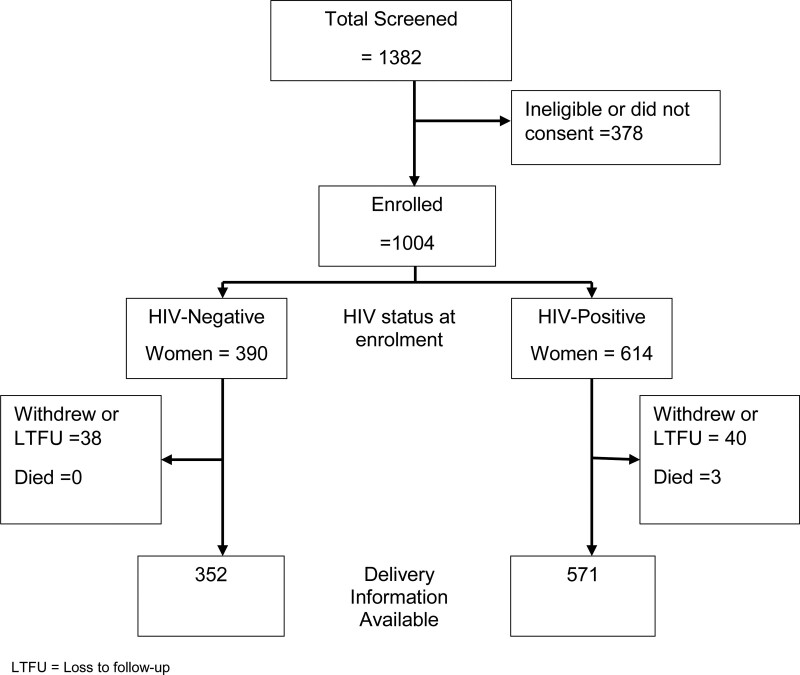
Overall, 1,004 pregnant women were enrolled in the study; 614 were HIV positive and 390 were HIV negative (Fig. 1). Pregnancy outcomes were available for 571 (93.0%) HIV-positive women and 352 (90.3%) HIV-negative women, who constitute the delivery cohort with results presented in this paper. In this cohort, the median estimated gestational age at enrolment was 20 weeks for HIV-positive women and 21 weeks for HIV-negative women. Of the HIV-positive women, 150 (26.3%) tested positive for HIV on their first ANC visit, while the remainder knew their HIV status before attending ANC. Among 558 HIV-positive women screened for TB symptoms, 555 (99.5%) had no symptoms. Of these, 120 (21.6%) initiated TB preventive therapy (TPT) and an additional 145 women had recently completed TPT. Two women received TB treatment. All HIV-positive women received ART with approximately 90% on TDF/3TC/EFV. Other ART regimens mainly included TDF/3TC/nevirapine (NVP), zidovudine (AZT)/3TC/EFV or AZT/3TC/NVP. Of 315 women with a viral load test carried out within the 12-month period preceding delivery, 292 (92.7%) had viral loads <1000 copies/ml, of which 280 (88.9%) had <50 copies/ml. HIV-negative women were younger, had a higher level of education and were more likely to be in their first pregnancy compared with their HIV-positive counterparts (Table 1).
FIGURE 1.
Participant screening and enrolment information.
TABLE 1.
Baseline Characteristics of HIV-negative and HIV-positive Pregnant Women with a Known Delivery Outcome
| Baseline Characteristics | Total (n = 923) | HIV-negative women (n = 352) | HIV-positive women (n = 571) | P |
|---|---|---|---|---|
| Age (years) at first ANC visit (median, range) | 26 (14–48) | 23 (14–42) | 28 (16–48) | <0.0001 |
| Age categories (%) | ||||
| <19 years | 64 (6.9) | 44 (12.5) | 20 (3.5) | <0.0001 |
| 19–24 years | 316 (34.2) | 177 (50.3) | 139 (24.3) | |
| >24 years | 543 (58.8) | 131 (37.2) | 412 (72.2) | |
| Marital status (%) | ||||
| Married or living with partner | 712 (77.1) | 262 (74.4) | 450 (78.8) | 0.063 |
| Not married/living with partner | 211 (22.9) | 90 (25.6) | 121 (21.2) | |
| Education (%) | ||||
| Primary education or less | 293 (31.8) | 84 (23.9) | 209 (36.7) | <0.0001 |
| Any secondary education | 557 (60.4) | 236 (67.0) | 321 (56.3) | |
| Beyond secondary school | 72 (7.8) | 32 (9.1) | 40 (7.0) | |
| Missing | 1 | 0 | 1 | |
| Gravidity | ||||
| First pregnancy | 284 (31.1) | 166 (47.6) | 118 (20.9) | <0.0001 |
| Second pregnancies | 329 (36.0) | 115 (33.0) | 214 (37.9) | |
| Third pregnancies | 173 (18.9) | 47 (13.5) | 126 (22.3) | |
| ≥ 4 pregnancies | 127 (13.9) | 21 (6.0) | 106 (18.8) | |
| Missing | 10 | 3 | 7 | |
| Positive syphilis test during pregnancy (%) | ||||
| Yes | 23 (2.8) | 4 (1.3) | 19 (3.6) | 0.131 |
| No | 807 (97.2) | 300 (98.7) | 507 (96.4) | |
| No test results available | 93 | 48 | 45 | |
There were 17 twin deliveries, 8 from HIV-negative women and 9 from HIV-positive women. Twin deliveries were excluded from the analysis. Overall, the median birth weight was 2.9 kg, (range 0.7–5.2); 2.9 kg (range 0.7–4.4) in HIV-exposed infants and 3.0 kg (range 1.2–5.2) in HIV-unexposed infants. A total of 149 singleton pregnancies were classified as having at least one adverse pregnancy outcome, including 33 (9.6%) HIV-negative pregnancies and 116 (20.6%) HIV-positive pregnancies (Table 2). Forty-eight pregnancies ended in intrauterine death; this included 8 (2.3%) HIV-negative pregnancies (2 miscarriages and 6 stillbirths) and 40 (7.1%) HIV-positive pregnancies (20 miscarriages and 20 stillbirths). Additional adverse outcomes included 53 preterm deliveries among live-born infants; 89 low birth weight infants; and 11 infants with birth defects. Birth defects documented in infants of HIV-negative mothers included 2 infants with cleft lip, 1 infant with congenital heart disease and 1 infant with microcephaly. HIV-positive women delivered 3 infants with club foot, 2 infants with extra digits, 1 infant with hydrocephalus and 1 infant with spina bifida and hydrocephalus.
TABLE 2.
Adverse Pregnancy Outcomes Among HIV-negative and HIV-positive Women, With and Without Syphilis (Excluding Twin Pregnancies)
| Adverse Pregnancy Outcome | Total | All Cohort Women | Excluding Women with Syphilis | ||||
|---|---|---|---|---|---|---|---|
| HIV-negative Women | HIV-positive Women | P | HIV-negative Women | HIV-positive Women | P | ||
| Pregnancy outcomes | N = 906 | N = 334 | N = 562 | N = 340 | N = 543 | ||
| Composite: any adverse outcome (intrauterine death, preterm, low birth weight, birth defect) | 149/906 | 33/344 (9.6%) | 116/562 (20.6%) | <0.0001 | 33/340 (9.7%) | 110/543 (20.3%) | <0.0001 |
| Intrauterine death | 48/905 | 8/344 (2.3%) | 40/561 (7.1%) | 0.002 | 8/340 (2.4%) | 38/543 (7.0%) | 0.002 |
| Delivery outcomes (excluding miscarriage) | N = 884 | N = 342 | N = 542 | N = 338 | N = 523 | ||
| Any birth defect | 11/882 | 4/342 (1.2%) | 7/540 (1.3%) | 0.871 | 4/338 (1.2%) | 7/523 (1.3%) | 0.842 |
| Preterm delivery (<37 week) | 53/879 | 13/342 (3.8%) | 40/537 (7.4%) | 0.046 | 13/338 (3.9%) | 37/519 (7.1%) | 0.108 |
| Low birth weight (<2500 g) | 89/803 | 25/317 (7.9%) | 64/486 (13.2%) | 0.010 | 25/313 (8.0%) | 61/469 (13.0%) | 0.016 |
| Term low birth weight (≥37 weeks and <2500 g) | 61/764 | 16/305 (5.3%) | 45/459 (9.8%) | 0.031 | 16/301 (5.3%) | 44/445 (9.9%) | 0.028 |
Compared with their HIV-negative counterparts, a higher proportion of HIV-positive women experienced an intrauterine death (miscarriage or stillbirth), 7.1% versus 2.3% (P = 0.002), low birth weight delivery, 13.2% versus 7.9% (P = 0.010) and preterm delivery, 7.4% versus 3.8% (P = 0.046) in the bivariate analysis (Table 2). No significant difference was seen in the occurrence of birth defects. In a multivariable model that adjusted for maternal age, marital status, level of education and study site, the odds of having any adverse pregnancy outcome remained significantly higher in HIV-positive women compared with HIV-negative women [adjusted odds ratio (AOR) = 2.60, 95% CI: 1.71–3.97] (Table 3). The odds of intrauterine death and low birth weight delivery, including term low birth weight, also remained significantly higher in HIV-positive compared with HIV-negative women.
TABLE 3.
Adjusted Odds of Adverse Pregnancy Outcome for HIV-positive Versus HIV-negative Pregnant Women (Excluding Twin Pregnancies)
| Adverse Pregnancy Outcome | N | Adjusted Odds Ratio (95% CI) |
|---|---|---|
| Composite: any adverse pregnancy outcome | 905 | 2.60 (1.71–3.97) |
| Intrauterine death (miscarriage or stillbirth) | 904 | 2.62 (1.25–5.49) |
| Preterm* | 854 | 1.41 (0.64–3.09) |
| Low birth weight | 802 | 1.89 (1.16–3.09) |
| Term low birth weight | 763 | 2.31 (1.15–4.63) |
*Live born, excluding stillbirths
CI indicates confidence interval.
In the subset of women with at least one syphilis test result during pregnancy, the adjusted odds of adverse pregnancy outcomes remained significantly higher in HIV-positive women even after the addition of syphilis infection to the model. With syphilis in the model, the AOR was 2.58 (95% CI: 1.63–4.07) for any adverse pregnancy outcome; 2.69 (95% CI: 1.54–4.70) for intrauterine death; 1.34 (95% CI: 0.43–4.15) for preterm and 1.78 (95% CI: 1.07–2.98) for low birth weight. Syphilis infection itself was also significantly associated with any adverse pregnancy outcome (P = 0.040) and preterm (P = 0.001), but not with intrauterine death or low birth weight.
Initiation of ART was pre-conception in 285 women, during pregnancy in 267 women and 10 women had an unknown time for ART initiation. No significant differences in adverse pregnancy outcomes were observed by the timing of ART initiation (Table 4).
TABLE 4.
Adverse Pregnancy Outcomes Among Women who Started ART Preconception Versus During Pregnancy
| Adverse Pregnancy Outcomes | Pre-conception | During Pregnancy | Adjusted Odds Ratio (95% CI) |
|---|---|---|---|
| Composite: any adverse pregnancy outcome | 58/285 (20.4%) | 53/267 (19.9%) | 1.08 (0.69–1.69) |
| Intrauterine death (miscarriage or stillbirth) | 20/285 (7.0%) | 15/266 (5.6%) | 1.17 (0.64–2.14) |
| Preterm* | 20/274 (7.3%) | 20/258 (7.8%) | 1.41 (0.95–2.09) |
| Low birth weight | 35/241 (14.5%) | 29/241 (12.0%) | 1.49 (0.77–2.89) |
| Term low birth weight | 24/228 (10.5%) | 21/227 (9.3%) | 1.48 (0.68–3.21) |
*Live born, excluding stillbirth.
CI indicates confidence interval.
DISCUSSION
In this study, we observed that adverse pregnancy outcomes remained 2–3 times higher among HIV-positive women compared with HIV-negative women despite universal ART. The odds of having any adverse pregnancy outcome were more than 2 times higher among HIV-positive women compared with HIV-negative women. Specifically, intrauterine death (miscarriage and stillbirth) and low birth weight were significantly higher, while preterm deliveries and infants with birth defects were not. Background adverse pregnancy rates in Lesotho stratified by HIV status have not been previously described; UNICEF data indicates the rate of stillbirth in the overall population as 20/1,000 total births (2%), which is similar to that observed in the HIV-negative cohort in this study.20
Our findings add to the growing body of evidence that HIV-positive women continue to experience more adverse pregnancy outcomes than their HIV-negative counterparts despite receipt of ART. Recent findings from studies carried out in South Africa, Botswana and the United Kingdom similarly demonstrated higher adverse pregnancy outcomes in HIV-positive women than HIV-negative women, even when ART was initiated at preconception.21–23 In contrast, a study in Malawi did not observe significant differences in preterm delivery, low birth weight and small for gestational age between HIV-positive on ART and HIV-negative women; however, they did not assess the intrauterine fetal loss.11 The Malawi authors noted that rates of adverse pregnancy outcomes were high regardless of HIV status. Our study, which similarly did not find differences by HIV status in preterm delivery, had much lower rates of preterm delivery (7.4% in HIV-positive and 3.8% in HIV-negative women) than observed in the Malawi study (10.6% in HIV-positive and 9.5% HIV-negative women). In contrast, the rate of low birth weight among term neonates in the Malawi study (3.8% in HIV-positive and 2.0% in HIV-negative women) was 2-3 times lower than observed in our study (9.8% in HIV-positive and 5.3% in HIV-negative women), which may have limited the power of the Malawi study to detect the significant difference observed in our study.
Comparison of stillbirths in our study with other published studies is complicated by the different definitions used for stillbirth. We defined stillbirth as any baby born with no signs of life at or after 28 weeks gestation as recommended by WHO.17 Some researchers have used a lower gestational threshold of 20 weeks,5 while others have used 24 weeks.21 Despite these differences in definition, our findings on intrauterine death are in agreement with other studies from both resource-limited and resource-rich countries.5,24 Similar to our study, researchers from the United Kingdom/Ireland found that the stillbirth rate in HIV-positive women remained higher than the general population, despite nearly half of their HIV-positive women being on ART before conception.23 A study in Botswana demonstrated high rates of adverse outcomes, particularly stillbirth, in HIV-positive women co-infected with syphilis compared with HIV-positive and HIV-negative women without syphilis.22 In our study, after controlling for syphilis coinfection, intrauterine fetal death remained higher compared with a similar cohort of HIV-negative women.
Low birth weight is known to increase neonatal morbidity and mortality, and hence it is of concern that we observed higher rates of low birth weight among HIV-positive compared with HIV-negative women, even among the subset of term low birth weight infants. There are mixed findings related to the association between treated maternal HIV infection and low birth weight.13,21,24 In one of the largest observational studies of birth outcomes in HIV-positive and HIV-negative women in the Botswana Tsepamo birth surveillance study, there were no differences in pregnancy outcomes between HIV-positive women receiving EFV and dolutegravir (DTG)-based ART regimens. However, similar to our study, the rate of adverse pregnancy outcomes, including small for gestational age, were higher among HIV-positive women receiving EFV or DTG compared with HIV-negative women.25
The proportions of women who experienced preterm birth were not significantly different between the HIV-positive and HIV-negative groups. Data on the association of ART with preterm birth are conflicting, and some studies suggest that ART regimens may differ in their association with preterm birth.11,26–31 Most of the HIV-positive women in our cohort were on a combination of TDF, 3TC and EFV, which has appeared to have a lower rate of adverse pregnancy outcomes than other ART regimens in a study in Botswana.25
The lack of association of maternal ART with birth defects seen in our study is consistent with findings from other studies, as well as the Antiretroviral Pregnancy Registry, that confirm the safety of EFV-based ART regimens as used in our cohort of women.21,32–34 The lack of association between this regimen and birth defects is reassuring; however, as countries move away from EFV to DTG-based regimens, there is a need for continued pharmacovigilance of pregnant women receiving the newer ART regimens for adverse outcomes.35
The underlying etiology for the discrepancy in birth outcomes between HIV-positive and HIV-negative women is unclear. One study evaluated the prevalence of placental growth disorders in HIV-positive pregnant women on ART, reporting a higher prevalence of such disorders compared with international reference values, noting that restriction of placental growth was most common.36 Another study has suggested that altered angiogenesis may be a mechanism underlying adverse outcomes in women with HIV receiving ART.37
We noted that the timing of ART initiation did not have any influence on the occurrence of adverse events. While a meta-analysis in 2017 suggested preconception ART was associated with higher rates of adverse outcomes, this report included older studies using less optimal ART regimens.38 Other studies using ART regimens recommended over the past 5 years such as the EFV-based ART regimen used in Lesotho have, similar to our study, not found timing of ART to be associated with adverse outcome39–41
Our study had several strengths. We prospectively followed up women and collected primary data in a public healthcare setting that implemented the WHO guidelines for HIV treatment and PMTCT. The data presented here may, therefore, be relevant to the many settings where the same WHO guidelines have been adopted. In addition, the quality of data collected was high, with only a few missing values for some of the variables. Nevertheless, interpretation of our findings should take into consideration the following limitations. Gestational age was determined using the date of the last menstrual period and fundal height measurements. Although these are established ways of determining gestational age, they are not as precise as ultrasound measurement. In addition, there may have been some underreporting of adverse pregnancy outcomes since women were enrolled at different time points when they came to facilities for their first ANC. Some women may have experienced adverse events at home and never visited the clinics for their ANC, or those lost to follow-up before delivery may have experienced an intrauterine death. Lastly, a priori computation of power to assess differences in pregnancy outcomes among women who started ART preconception versus during pregnancy was not carried out.
CONCLUSIONS
Our findings indicate that despite optimization of maternal health through ART, pregnancy outcomes among HIV-positive women remain worse than those among HIV-negative women residing in the same area. Whether this is due to the effects of the underlying HIV infection, the antiretroviral drugs or both is not clear. Further research is needed to examine the reasons for this persistent discrepancy to enable interventions to improve outcomes among HIV-positive women and their children.
ACKNOWLEDGMENTS
We acknowledge the study participants for their cooperation, time and effort, and the support obtained from the study health facilities, the Maseru District Management Team (DHMT) and the Lesotho Ministry of Health. We thank the USAID for funding this study, specifically Dr. Justine Mirembe and Mr. Ian Membe from USAID/Lesotho and Anouk Amzel from USAID/Washington who were instrumental in the development and funding of this study. We also are indebted to the SOAR leadership team at the Population Council for their continued input and support.
Footnotes
The authors have no conflicts of interest to disclose.
This study was funded by the United States Agency for International Development (USAID) through the Project SOAR (Supporting Operational AIDS Research); award no. AID-OAA-A-14-00060. The contents included here are; however, the responsibility of the authors and do not necessarily represent the official views of USAID.
REFERENCES
- 1.Brocklehurst P, French R. The association between maternal HIV infection and perinatal outcome: a systematic review of the literature and meta-analysis. Br J Obstet Gynaecol. 1998;105:836–848. [DOI] [PubMed] [Google Scholar]
- 2.Habib NA, Daltveit AK, Bergsjø P, et al. Maternal HIV status and pregnancy outcomes in northeastern Tanzania: a registry-based study. BJOG. 2008;115:616–624. [DOI] [PubMed] [Google Scholar]
- 3.Wedi CO, Kirtley S, Hopewell S, et al. Perinatal outcomes associated with maternal HIV infection: a systematic review and meta-analysis. Lancet HIV. 2016;3:e33–e48. [DOI] [PubMed] [Google Scholar]
- 4.Yang M, Wang Y, Chen Y, et al. Impact of maternal HIV infection on pregnancy outcomes in southwestern China – a hospital registry based study. Epidemiol Infect. 2019;147:e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Liu J, Tan D, et al. Maternal HIV infection and risk of adverse pregnancy outcomes in Hunan province, China: a prospective cohort study. Medicine (Baltimore). 2020;99:e19213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO. Consolidated Guidelines on the Use of Antiretroviral Drugs for Treating and Preventing HIV Infection: Recommendations for a Public Health Approach. June 2013. Available at: https://www.who.int/hiv/pub/guidelines/arv2013/download/en/. Accessed May 08, 2020. [PubMed]
- 7.WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: Recommendations for a public health approach. Second edition. 2016. Available at: https://www.who.int/hiv/pub/arv/arv-2016/en/. Accessed May 8, 2020. [PubMed]
- 8.Health LMo. Lesotho Population-Based HIV Impact Assessment 2016-2017. Maseru, Lesotho: Ministry of Health; 2019. [Google Scholar]
- 9.Schulte J, Dominguez K, Sukalac T, et al.; Pediatric Spectrum of HIV Disease Consortium. Declines in low birth weight and preterm birth among infants who were born to HIV-infected women during an era of increased use of maternal antiretroviral drugs: pediatric spectrum of HIV disease, 1989-2004. Pediatrics. 2007;119:e900–e906. [DOI] [PubMed] [Google Scholar]
- 10.Moodley T, Moodley D, Sebitloane M, et al. Improved pregnancy outcomes with increasing antiretroviral coverage in South Africa. BMC Pregnancy Childbirth. 2016;16:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dadabhai S, Gadama L, Chamanga R, et al. Pregnancy outcomes in the era of universal antiretroviral treatment in sub-Saharan Africa (POISE Study). J Acquir Immune Defic Syndr. 2019;80:7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rempis EM, Schnack A, Decker S, et al. Option B+ for prevention of vertical HIV transmission has no influence on adverse birth outcomes in a cross-sectional cohort in Western Uganda. BMC Pregnancy Childbirth. 2017;17:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malaba TR, Phillips T, Le Roux S, et al. Antiretroviral therapy use during pregnancy and adverse birth outcomes in South African women. Int J Epidemiol. 2017;46:1678–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowler MG, Qin M, Fiscus SA, et al.; IMPAACT 1077BF/1077FF PROMISE Study Team. Benefits and risks of antiretroviral therapy for perinatal HIV prevention. N Engl J Med. 2016;375:1726–1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veroniki AA, Antony J, Straus SE, et al. Comparative safety and effectiveness of perinatal antiretroviral therapies for HIV-infected women and their children: systematic review and network meta-analysis including different study designs. PLoS One. 2018;13:e0198447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Government of Lesotho. National Guidelines on the use of Antiretroviral Therapy for HIV Prevention and Treatment. 5th Edition. 2016. Available at: https://www.childrenandaids.org/sites/default/files/2017-04/Lesotho_ART-Guidelines_2016.pdf. Accessed May 19, 2020.
- 17.WHO. Maternal, newborn, child and adolescent health: Stillbirths 2020. Available at: https://www.who.int/maternal_child_adolescent/epidemiology/stillbirth/en/. Accessed May 20, 2020.
- 18.WHO. New global estimates on preterm birth published: Ensuring high-quality healthcare for women and girls essential in prevention of preterm births 2018. Available at: https://www.who.int/reproductivehealth/global-estimates-preterm-birth/en/. Accessed May 20, 2020.
- 19.WHO. Global Nutrition Targets 2025: Low Birth Weight Policy Brief 2014. Available at: https://apps.who.int/iris/bitstream/handle/10665/149020/WHO_NMH_NHD_14.5_eng.pdf?ua=1. Accessed May 20, 2020.
- 20.UNICEF. Maternal and Newborn Health Disparities. Lesotho 2016. Available at: https://data.unicef.org/resources/maternal-newborn-health-disparities-country-profiles/. Accessed February 25, 2021.
- 21.Santosa WB, Staines-Urias E, Tshivuila-Matala COO, et al. Perinatal outcomes associated with maternal HIV and antiretroviral therapy in pregnancies with accurate gestational age in South Africa. AIDS. 2019;33:1623–1633. [DOI] [PubMed] [Google Scholar]
- 22.Shava E, Moyo S, Zash R, et al. Brief report: high rates of adverse birth outcomes in HIV and syphilis coinfected women in Botswana. J Acquir Immune Defic Syndr. 2019;81:e135–e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Favarato G, Townsend CL, Peters H, et al. Stillbirth in women living with HIV delivering in the United Kingdom and Ireland: 2007-2015. J Acquir Immune Defic Syndr. 2019;82:9–16. [DOI] [PubMed] [Google Scholar]
- 24.González R, Rupérez M, Sevene E, et al. Effects of HIV infection on maternal and neonatal health in southern Mozambique: a prospective cohort study after a decade of antiretroviral drugs roll out. PLoS One. 2017;12:e0178134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zash R, Jacobson DL, Diseko M, et al. Comparative safety of dolutegravir-based or efavirenz-based antiretroviral treatment started during pregnancy in Botswana: an observational study. Lancet Glob Health. 2018;6:e804–e810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Venkatesh KK, Farhad M, Fenton T, et al. Association between HIV antiretroviral therapy and preterm birth based on antenatal ultrasound gestational age determination: a comparative analysis. AIDS. 2019;33:2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Short CE, Taylor GP. Antiretroviral therapy and preterm birth in HIV-infected women. Expert Rev Anti Infect Ther. 2014;12:293–306. [DOI] [PubMed] [Google Scholar]
- 28.Sebikari D, Farhad M, Fenton T, et al. Risk factors for adverse birth outcomes in the PROMISE 1077BF/1077FF trial. J Acquir Immune Defic Syndr. 2019;81:521–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siemieniuk RA, Foroutan F, Mirza R, et al. Antiretroviral therapy for pregnant women living with HIV or hepatitis B: a systematic review and meta-analysis. BMJ Open. 2017;7:e019022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zash R, Jacobson DL, Diseko M, et al. Comparative safety of antiretroviral treatment regimens in pregnancy. JAMA Pediatr. 2017;171:e172222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caniglia EC, Zash R, Jacobson DL, et al. Emulating a target trial of antiretroviral therapy regimens started before conception and risk of adverse birth outcomes. AIDS. 2018;32:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ford N, Mofenson L, Shubber Z, et al. Safety of efavirenz in the first trimester of pregnancy: an updated systematic review and meta-analysis. AIDS. 2014;28(suppl 2):S123–S131. [DOI] [PubMed] [Google Scholar]
- 33.Martinez de Tejada B, Gayet-Ageron A, Winterfeld U, et al. Birth defects after exposure to efavirenz-based antiretroviral therapy at conception/first trimester of pregnancy: a multicohort analysis. J Acquir Immune Defic Syndr. 2019;80:316–324. [DOI] [PubMed] [Google Scholar]
- 34.Antiretrovral Pregnancy Registry Steering Committee. Antiretroviral Pregnancy Registry Interim Report for 1 January 1989 through 31 January 2020. Available at: http://www.apregistry.com/. Accessed September 12, 2020.
- 35.Mofenson LM, Pozniak AL, Wambui J, et al. Optimizing responses to drug safety signals in pregnancy: the example of dolutegravir and neural tube defects. J Int AIDS Soc. 2019;22:e25352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dos Reis HLB, Boldrini NAT, Rangel AFR, et al. Placental growth disorders and perinatal adverse outcomes in Brazilian HIV-infected pregnant women. PLoS One. 2020;15:e0231938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Conroy AL, McDonald CR, Gamble JL, et al. Altered angiogenesis as a common mechanism underlying preterm birth, small for gestational age, and stillbirth in women living with HIV. Am J Obstet Gynecol. 2017;217:684.e1–684.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uthman OA, Nachega JB, Anderson J, et al. Timing of initiation of antiretroviral therapy and adverse pregnancy outcomes: a systematic review and meta-analysis. Lancet HIV. 2017;4:e21–e30. [DOI] [PubMed] [Google Scholar]
- 39.Msukwa MT, Keiser O, Jahn A, et al. Timing of combination antiretroviral therapy (cART) initiation is not associated with stillbirth among HIV-infected pregnant women in Malawi. Trop Med Int Health. 2019;24:727–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stoner MCD, Cole SR, Price J, et al. Timing of initiation of antiretroviral therapy and risk of preterm birth in studies of HIV-infected pregnant women: the role of selection bias. Epidemiology. 2018;29:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zash R, Rough K, Jacobson DL, et al. Effect of gestational age at tenofovir-emtricitabine-efavirenz initiation on adverse birth outcomes in Botswana. J Pediatric Infect Dis Soc. 2018;7:e148–e151. [DOI] [PMC free article] [PubMed] [Google Scholar]