Supplemental Digital Content is available in the text.
Keywords: H5N1, AS03, influenza vaccine, children, dose finding
Background:
This phase 2 observer-blind, randomized, multicenter, dose-ranging study evaluated immunogenicity and safety of different formulations of an AS03-adjuvanted H5N1 influenza vaccine in children 6–35 months of age.
Methods:
One hundred eighty-five children randomized into 5 groups [1.9 µg hemagglutinin (HA)/AS03B, 0.9 µg HA/AS03C, 1.9 µg HA/AS03C, 3.75 µg HA/AS03C or 3.75 µg HA/AS03D] were to receive 2 doses administered 21 days apart (primary vaccination). AS03 was classified by amount of DL-α-tocopherol, with AS03B the highest amount. One year later, all subjects were to receive unadjuvanted 3.75 µg HA as antigen challenge. Immunogenicity was assessed 21 days after primary vaccination (day 42) and 7 days after antigen challenge (day 392). Immunogenicity-fever index, based on hemagglutination inhibition and microneutralization antibody titers at day 42 and fever 7 days after each vaccination, was used to guide the selection of an acceptable formulation.
Results:
After primary vaccination, formulations elicited strong homologous immune responses with all subjects’ hemagglutination inhibition titers ≥1:40 post-vaccination. Immunogenicity-fever index based on hemagglutination inhibition and microneutralization assays showed that 1.9 µg HA/AS03B ranked the highest. Antibody levels persisted >4 times above baseline 12 months after primary vaccination with all formulations (day 385). Antibodies increased >4-fold after antigen challenge (day 392/day 385) with 1.9 µg HA/AS03B, 0.9 µg HA/AS03C and 1.9 µg HA/AS03C formulations. Overall per subject, the incidence of fever ranged from 28.6% (3.75 µg HA/AS03D) to 60.5% (1.9 µg HA/AS03B).
Conclusions:
All formulations were highly immunogenic and demonstrated acceptable safety profiles, with the 1.9 µg HA/AS03B providing the most favorable balance of immunogenicity versus reactogenicity for use in children 6–35 months of age.
The H5N1 avian influenza A viruses have been associated with diseases in humans since 1997.1 Transmission of the H5N1 virus from animals to humans has raised concerns about its potential to cause pandemics.2 As a component of pandemic preparedness, several H5N1 influenza vaccine candidates have been developed.2 GSK has produced inactivated split-virion H5N1 influenza vaccines containing the A/Indonesia or A/Vietnam hemagglutinin (HA) antigen, formulated with the AS03 adjuvant system. AS03 is an adjuvant system containing DL-α-tocopherol and squalene in oil-in-water emulsion (AS03B, AS03C and AS03D, respectively, contain 5.93, 2.97 and 1.48 mg of DL-α-tocopherol). Multiple vaccines have been assessed in clinical trials in children and adults including older adults,3–10 including the D-Pan H5N1 (Dresden, Germany) and Q-Pan H5N1 (Québec, Canada) vaccines that use various H5N1 HA antigens and variable doses of AS03. The vaccine program in children includes 4 completed clinical studies using the D-Pan H5N1 or the Q-Pan H5N1 vaccine. When children 6 months to 6 years of age received the D-Pan H5N1 vaccine (1.9 μg HA antigen and AS03B), an increase in reactogenicity, especially fever, after a second (or third) dose was observed when compared with the first dose.9,11 In contrast, another clinical trial in 607 children from 6 months to 17 years of age who received the Q-Pan H5N1 vaccine (1.9 μg HA antigen and AS03B as well) found no increase in fever after the second dose of the vaccine.5 However, the overall incidence of local solicited and unsolicited symptoms 7 days after vaccination increased relative to placebo. As observed previously with adjuvanted vaccines in children of the same age,5 there was an increase in solicited local and general adverse events (AEs) within 7 days post-vaccination in H5N1 vaccine recipients compared with placebo recipients.
The aim of the dose-ranging study was to assess the immunogenicity and reactogenicity and rank different formulations of the Q-Pan H5N1 vaccine to determine whether reactogenicity can be reduced while maintaining adequate immunogenicity in children 6–35 months of age. An immunogenicity-fever index was developed to either confirm that the current pediatric formulation represents an acceptable balance between immunogenicity and reactogenicity or suggest that the adjuvant or antigen dose, or both, could be modified.
MATERIALS AND METHODS
The design of this phase 2 randomized, multicenter, observer-blind, parallel-group study is provided in Supplemental Digital Content 1; http://links.lww.com/INF/E463.
Trial Countries and Participants
The study was conducted in Thailand (2 centers) and Taiwan (5 centers) because children in these countries are at a relatively higher risk of exposure to H5N1 due to a history of domestic epizootic H5N1 disease among poultry.1 Study enrolled 185 healthy male or female children 6–35 months of age at the time of first vaccination. The criteria used to determine eligibility to the trial are provided in the available trial protocol.12
Vaccines, Schedule and Blinding
The Q-Pan H5N1 vaccine used in this study was provided by GSK. Different doses of HA from A/Indonesia/5/2005 (H5N1), clade 2.1.3.2 (0.9, 1.9 or 3.75 µg), were combined with different concentrations of AS03. The formulations were 1.9 µg HA/AS03B (used as the reference in this study based on previous findings), 0.9 µg HA/AS03C, 1.9 µg HA/AS03C, 3.75 µg HA/AS03C and 3.75 µg HA/AS03D. Subjects were equally randomized (1:1:1:1:1; using a minimization procedure to reduce the imbalance of subjects among groups with respect to the center, age group and pre-study seasonal influenza vaccination history) to receive 2 doses of the corresponding vaccine at days 0 and 21 (primary vaccination). Immune memory was evaluated through administration of 1 dose of unadjuvanted vaccine (3.75 µg HA) at day 385 as antigen challenge. All vaccines were administered intramuscularly in the anterolateral thigh.
Analyses
The total vaccinated cohort (TVC) and the according-to-protocol (ATP) cohort included subjects who received at least 1 dose of vaccine and subjects who received study vaccine dose(s) per protocol, respectively. The adapted ATP cohort presents data for subjects who complied with the protocol and had results available for the relevant assay (see below), for the relevant time intervals. The analysis of safety was performed on the TVC and cell-mediated immunity (CMI) on a subcohort of TVC.
Ethics
All study documents were approved by the independent ethics committees of participating institutions. The study was performed in accordance with Good Clinical Practice guidelines, the Declaration of Helsinki and applicable regulatory requirements. In line with local requirements, written informed consent in relation to the subject’s participation in the study was obtained from the parents/guardians of the subjects. Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
This study is registered with ClinicalTrials.gov (NCT02719743).
Co-primary End Points
Immunogenicity and reactogenicity were measured by the following immunogenicity-fever index (D), which considered antibody responses at 21 days after the second priming dose (day 42) and fever scores (≥38 °C) post-dose 1 and dose 2. An index D at day 42 was computed for each formulation:
D ranged between 0 and 1 (0: not desirable; 1: highly desirable), and the same weight (0.5) was assigned for immunogenicity and reactogenicity (fever) in the index. Two immunogenicity-fever indices were separately calculated using immunogenicity assessments by the hemagglutination inhibition (HI) and microneutralization (MN) assay against the vaccine-homologous virus. Each was used to rank the different dosing regimens as a tool to guide dosing regimen selection (details on the calculations of the index are provided in Supplemental Digital Content 2; http://links.lww.com/INF/E463).
Anamnestic response to the unadjuvanted 3.75 µg HA vaccine measured by HI and MN assay at day 392, 7 days after antigen challenge at day 385, was also estimated (Supplemental Digital Content 2; http://links.lww.com/INF/E463).
Secondary End Points
Secondary end points included assessment of humoral immune response (HI and MN assay) against vaccine-homologous and heterologous strain on days 0, 42, 385 and 392, vaccine-induced CMI responses on days 0, 42, 385 and 392 and reactogenicity and safety of all vaccine formulations.
Immunogenicity Assessment
Humoral Immune Response
Blood samples were collected on days 0, 42, 385 (assessment of antibody persistence) and 392 (assessment of anamnestic immune response) for serologic testing. Assays of HI were performed using an established method, modified for equine rather than avian erythrocytes.13–16
A subject was considered seronegative when its antibody titer was below the cutoff value (<1:10). Parameters for HI included geometric mean titer (GMT; for calculations of GMT, HI titers <10 were given a value of 5), seroprotection rate (SPR; defined as the percentage of subjects with HI titers ≥1:40 post-vaccination), seroconversion rate (SCR; proportion of subjects who have either a prevaccination reciprocal HI titer <10 and a postvaccination reciprocal titer ≥40, or a prevaccination reciprocal HI titer ≥10 and at least a 4-fold increase in postvaccination reciprocal titer against the vaccine virus), and mean geometric increase (MGI; defined as geometric mean of within-subject ratios of postvaccination to prevaccination reciprocal HI titer).
The MN assay was performed using an established method, described elsewhere.13–15 Based on an assay validation study, MN titers <1:28 were considered below the cutoff. For calculations of GMT, MN titers <28 were assigned a value of 14. The MN antibody parameters were GMT and vaccine response rate (VRR, defined as percentage of subjects with a postvaccination reciprocal titer at least 4-fold higher compared with their prevaccination reciprocal titer).
CMI Response
To assess vaccine-induced CMI responses, frequency of H5N1-specific polypositive CD4+ T cells per million CD4+/CD8+ cells expressing at least 2 of the following activation markers (CD40L, interleukin-2, interferon-gamma and tumor necrosis factor-α) was measured on days 0, 42, 385 and 392 using intracellular cytokine staining on whole blood samples.4
Reactogenicity and Safety Assessment
Solicited local and general AEs were recorded for 7 days after each dose, and unsolicited AEs were recorded for 21 days after primary vaccination (doses 1 and 2) and 30 days after antigen challenge dose. Solicited local AEs included pain, redness and swelling. Solicited general AEs included drowsiness, fever (temperature: ≥38 °C), irritability/fussiness and loss of appetite. Incidence of medically attended AEs (MAEs), AE of special interest (AESI), potential immune-mediated disease and serious AEs (SAEs) were recorded throughout the entire study period.
RESULTS
Baseline Characteristics
A total of 185 subjects were enrolled and vaccinated (TVC). Of these, 7 subjects withdrew from the study [withdrawal of consent (n = 3), moved from the study area (n = 2) and lost to follow-up (n = 2)]. Demographic characteristics are provided in Supplemental Digital Content 3; http://links.lww.com/INF/E463.
One hundred seventy-two subjects were included in the ATP cohort for immunogenicity at day 42, 167 subjects were included in the ATP cohort for immunogenicity at days 385 and 392.
Immunogenicity
Immunogenicity-fever Index
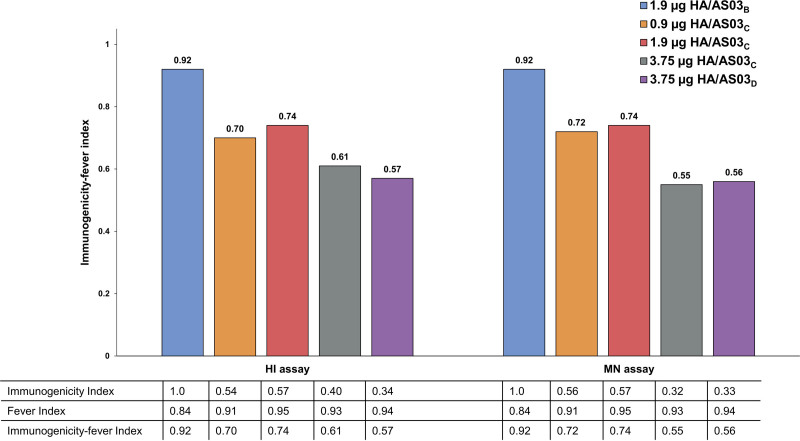
The immunogenicity-fever index based on HI and MN assays showed that all formulations were immunogenic and that the reference formulation (1.9 µg HA/AS03B) ranked the highest of all formulations (Fig. 1).
FIGURE 1.
Immunogenicity index, fever index and immunogenicity-fever index for H5N1 HI and MN antibodies at day 42 (ATP cohort for immunogenicity). AS03 is an adjuvant system containing DL-α-tocopherol and squalene in oil-in-water emulsion (AS03B containing 5.93 mg DL-α-tocopherol, AS03C containing 2.97 mg DL-α-tocopherol, and AS03D containing 1.48 mg DL-α-tocopherol).
Humoral Immune Response
At day 42, the trend for the highest antibody GMT versus the homologous H5N1 virus was observed with the 1.9 µg HA/AS03B formulation although all formulations elicited a strong homologous H5N1 immune response by HI assay [SCR = 100%, SPR = 100% and MGI range = 101.0 (3.75 µg HA/AS03D)–219.5 (1.9 µg HA/AS03B)]. At day 42, nonoverlapping confidence intervals (CIs) for the HI GMTs were observed with the 1.9 µg HA/AS03B and 3.75 µg HA/AS03C or 3.75 µg HA/AS03D formulations. The trend for the highest immune response was observed with the 1.9 µg HA/AS03B formulation versus the 0.90 µg HA/AS03C and 1.90 µg HA/AS03C formulations. The trend for the highest antibody level with the 1.9 µg HA/AS03B formulation lasted through day 385 although antibody response by HI assay was sustained with SPR >70%, SCR >40% and MGIDay 385/Day 0 >4 for all formulations. The 1.9 µg HA/AS03B formulation had the highest immune responses among all formulations with an SPR of 97.1% (95% CI 84.7–99.9), SCR of 97.1% (95% CI 84.7–99.9) and MGIDay 385/Day 0 of 19.2 (95% CI 14.8–24.9) (Table 1). The highest HI antibody titers were observed against the influenza A/duck/Bangladesh/19097/2013 (clade 2.3.2.1a) H5N1 isolate, the clade of which is the closest to the vaccine strain, followed by the A/Vietnam/1194/2004 (clade 1; H5N1) isolate. The 1.9 µg HA/AS03B formulation showed a trend for the highest cross-reactivity against 3 heterologous strains versus other formulations (Table 2). Results for immune response assessment by MN assay showed a similar pattern to those observed with the HI assay. Neutralizing antibody parameters (GMT and VRR) peaked at day 42 and persisted well above baseline with all formulations at day 385. Relative to day 0, VRR was 100% for all formulations at day 392 (Supplemental Digital Content 4; http://links.lww.com/INF/E463).
TABLE 1.
Summary of Vaccine Homologous HI Antibody Parameters at Days 0, 42, 385 and 392 (Adapted ATP Cohort for Immunogenicity)
| ≥10 1/DIL | SPR | GMT | SCR | MGI | ||||
|---|---|---|---|---|---|---|---|---|
| Formulation | N | n (%; 95% CI) | n (%; 95% CI) | Value (95% CI) | N′ | n′ (%; 95% CI) | Value (95% CI) | |
| 1.9 µg HA/AS03B | Day 0 | 36 | 1 (2.8; 0.1–14.5) | 0 (0.0; 0.0–9.7) | 5.1 (4.9–5.3) | - | - | - |
| Day 42 | 36 | 36 (100; 90.3–100) | 36 (100; 90.3–100) | 1118.6 (884.4–1414.9) | 36 | 36 (100; 90.3–100) | 219.5 (172.6–279.0) | |
| Day 385 | 34 | 34 (100; 89.7–100) | 33 (97.1; 84.7–99.9) | 98.1 (76.7–125.4) | 34 | 33 (97.1; 84.7–99.9) | 19.2 (14.8–24.9) | |
| Day 392 | 34 | 34 (100; 89.7–100) | 34 (100; 89.7–100) | 476.2 (348.4–650.9) | 34 | 34 (100; 89.7–100) | 93.3 (67.9–128.3) | |
| 0.9 µg HA/AS03C | Day 0 | 33 | 1 (3.0; 0.1–15.8) | 0 (0.0; 0.0–10.6) | 5.1 (4.9–5.3) | - | - | - |
| Day 42 | 33 | 33 (100; 89.4–100) | 33 (100; 89.4–100) | 858.8 (659.2–1118.8) | 33 | 33 (100; 89.4–100) | 168.2 (127.4–222.0) | |
| Day 385 | 33 | 33 (100; 89.4–100) | 26 (78.8; 61.1–91.0) | 61.5 (47.1–80.3) | 33 | 26 (78.8; 61.1–91.0) | 12.0 (9.2–15.8) | |
| Day 392 | 33 | 33 (100; 89.4–100) | 33 (100; 89.4–100) | 407.6 (315.0–527.4) | 33 | 33 (100; 89.4–100) | 79.8 (61.5–103.5) | |
| 1.9 µg HA/AS03C | Day 0 | 37 | 1 (2.7; 0.1–14.2) | 0 (0.0; 0.0–9.5) | 5.1 (4.9–5.4) | - | - | - |
| Day 42 | 37 | 37 (100; 90.5–100) | 37 (100; 90.5–100) | 913.6 (672.6–1241.1) | 37 | 37 (100; 90.5–100) | 177.7 (131.5–240.1) | |
| Day 385 | 37 | 37 (100; 90.5–100) | 28 (75.7; 58.8–88.2) | 72.1 (51.6–100.7) | 37 | 28 (75.7; 58.8–88.2) | 14.0 (10.0–19.7) | |
| Day 392 | 37 | 37 (100; 90.5–100) | 37 (100; 90.5–100) | 305.4 (227.1–410.6) | 37 | 37 (100; 90.5–100) | 59.4 (44.5–79.4) | |
| 3.75 µg HA/AS03C | Day 0 | 31 | 0 (0.0; 0.0–11.2) | 0 (0.0; 0.0–11.2) | 5.0 (5.0–5.0) | - | - | - |
| Day 42 | 31 | 31 (100; 88.8–100) | 31 (100; 88.8–100) | 640.0 (488.3–839.0) | 31 | 31 (100; 88.8–100) | 128.0 (97.7–167.8) | |
| Day 385 | 31 | 31 (100; 88.8–100) | 28 (90.3; 74.2–98.0) | 79.1 (59.2–105.6) | 31 | 28 (90.3; 74.2–98.0) | 15.8 (11.8–21.1) | |
| Day 392 | 31 | 31 (100; 88.8–100) | 31 (100; 88.8–100) | 286.2 (216.0–379.1) | 31 | 31 (100; 88.8–100) | 57.2 (43.2–75.8) | |
| 3.75 µg HA/AS03D | Day 0 | 35 | 3 (8.6; 1.8–23.1) | 0 (0.0; 0.0–10.0) | 5.6 (4.9–6.5) | - | - | - |
| Day 42 | 35 | 35 (100; 90.0–100) | 35 (100; 90.0–100) | 568.4 (442.7–729.8) | 35 | 35 (100; 90.0–100) | 101.0 (74.8–136.4) | |
| Day 385 | 32 | 32 (100; 89.1–100) | 23 (71.9; 53.3–86.3) | 59.6 (45.0–79.0) | 32 | 21 (65.6; 46.8–81.4) | 10.5 (7.6–14.5) | |
| Day 392 | 32 | 32 (100; 89.1–100) | 32 (100; 89.1–100) | 201.0 (156.1–258.7) | 32 | 32 (100; 89.1–100) | 35.3 (25.9–48.1) | |
AS03 indicates adjuvant system containing DL-α-tocopherol and squalene in oil-in-water emulsion (AS03B containing 5.93 mg DL-α-tocopherol, AS03C containing 2.97 mg DL-α-tocopherol, and AS03D containing 1.48 mg DL-α-tocopherol); DIL, dilution; N, number of subjects with results available for seropositivity rates, SPR and GMT; N', number of subjects with both pre and post results available for SCR and MGI; n, number of subjects meeting the SPR; n', number of seroconverted subjects.
MGI is defined as geometric mean of within-subject ratios of post-vaccination reciprocal HI titer to pre-vaccination reciprocal HI titer. SPR is defined as the percentage of subjects with HI titers ≥1:40 post-vaccination. SCR for initially seronegative subjects is defined as percentage of subjects with HI titers ≥1:40 post-vaccination and for initially seropositive subjects it is defined as percentage of subjects with ≥4-fold post-vaccination increase in HI titer from pre-vaccination antibody..
TABLE 2.
Summary of Vaccine Heterologous HI Antibody Parameters (SPR, GMT, SCR and MGI) at Day 42 (Adapted ATP Cohort for Immunogenicity)
| N | ≥10 1/DIL | SPR | GMT | N′ | SCR | MGI | ||
|---|---|---|---|---|---|---|---|---|
| Antibody | Formulation | n (%; 95% CI) | n (%; 95% CI) | Value (%; 95% CI) | n (%; 95% CI) | Value (%; 95% CI) | ||
| Influenza A/duck/Bangladesh/19097/2013 H5N1 HI (clade 2.3.2.1a) | 1.9 µg HA/AS03B | 32 | 32 (100; 89.1–100) | 32 (100; 89.1–100) | 167.1 (128.4–217.3) | 30 | 30 (100; 88.4–100) | 31.3 (23.5–41.6) |
| 0.9 µg HA/AS03C | 27 | 27 (100; 87.2–100) | 25 (92.6; 75.7–99.1) | 135.3 (97.6–187.5) | 24 | 22 (91.7; 73.0–99.0) | 22.9 (17.2–30.6) | |
| 1.9 µg HA/AS03C | 36 | 36 (100; 90.3–100) | 35 (97.2; 85.5–99.9) | 109.9 (81.2–148.7) | 34 | 33 (97.1; 84.7–99.9) | 20.4 (14.9–28.1) | |
| 3.75 µg HA/AS03C | 31 | 31 (100; 88.8–100) | 28 (90.3; 74.2–98.0) | 91.5 (70.2–119.1) | 25 | 23 (92.0; 74.0–99.0) | 18.9 (14.0–25.5) | |
| 3.75 µg HA/AS03D | 35 | 35 (100; 90.0–100) | 31 (88.6; 73.3–96.8) | 78.5 (58.5–105.3) | 30 | 25 (83.3; 65.3–94.4) | 12.6 (9.0–17.6) | |
| Influenza A/Vietnam/1194/2004 H5N1 HI (clade 1) | 1.9 µg HA/AS03B | 32 | 32 (100; 89.1–100) | 32 (100; 89.1–100) | 128.9 (103.1–161.2) | 30 | 30 (100; 88.4–100) | 24.3 (19.0–31.0) |
| 0.9 µg HA/AS03C | 27 | 27 (100; 87.2–100) | 25 (92.6; 75.7–99.1) | 104.7 (79.0–138.6) | 24 | 22 (91.7; 73.0–99.0) | 20.1 (15.2–26.7) | |
| 1.9 µg HA/AS03C | 36 | 36 (100; 90.3–100) | 31 (86.1; 70.5–95.3) | 88.9 (63.2–125.0) | 34 | 29 (85.3; 68.9–95.0) | 16.7 (11.7–23.8) | |
| 3.75 µg HA/AS03C | 31 | 31 (100; 88.8–100) | 26 (83.9; 66.3–94.5) | 77.2 (59.7–99.9) | 25 | 21 (84.0; 63.9–95.5) | 15.1 (11.1–20.6) | |
| 3.75 µg HA/AS03D | 35 | 35 (100; 90.0–100) | 29 (82.9; 66.4–93.4) | 69.0 (51.7–92.0) | 30 | 24 (80.0; 61.4–92.3) | 11.4 (8.4–15.6) | |
| Influenza A/gyrfalcon/Washington/41088-6/2014 H5N8 HI (clade 2.3.4.4) | 1.9 µg HA/AS03B | 32 | 32 (100; 89.1–100) | 19 (59.4; 40.6–76.3) | 35.8 (27. 9–46.0) | 30 | 17 (56.7; 37.4–74.5) | 6.7 (5.0–9.0) |
| 0.9 µg HA/AS03C | 27 | 27 (100; 87.2–100) | 10 (37.0; 19.4–57.6) | 27.8 (21.1–36.7) | 24 | 9 (37.5; 18.8–59.4) | 5.5 (4.0–7.4) | |
| 1.9 µg HA/AS03C | 36 | 33 (91.7; 77.5–98.2) | 11 (30.6; 16.3–48.1) | 22.8 (17.2–30.3) | 34 | 10 (29.4; 15.1–47.5) | 4.3 (3.2–5.8) | |
| 3.75 µg HA/AS03C | 31 | 30 (96.8; 83.3–99.9) | 7 (22.6; 9.6–41.1) | 21.6 (16.7–27.8) | 25 | 5 (20.0; 6.8–40.7) | 4.1 (3.0–5.6) | |
| 3.75 µg HA/AS03D | 35 | 32 (91.4; 76.9–98.2) | 9 (25.7; 12.5–43.3) | 19.9 (15.4–25.8) | 30 | 8 (26.7; 12.3–45.9) | 3.5 (2. 7–4.6) |
AS03 indicates adjuvant system containing DL-α-tocopherol and squalene in oil-in-water emulsion (AS03B containing 5.93 mg DL-α-tocopherol, AS03C containing 2.97 mg DL-α-tocopherol, and AS03D containing 1.48 mg DL-α-tocopherol); DIL, dilution; N, number of subjects with results available for seropositivity rates, SPR and GMT computation; N', number of subjects with both pre and post results available for SCR and MGI computation; n, number of subjects meeting the SPR; n', number of seroconverted subjects.
MGI is defined as geometric mean of within-subject ratios of post-vaccination reciprocal HI titer to pre-vaccination reciprocal HI titer. SPR is defined as the percentage of subjects with HI titers ≥1:40 post-vaccination. SCR for initially seronegative subjects is defined as percentage of subjects with HI titers ≥1:40 post-vaccination and for initially seropositive subjects it is defined as percentage of subjects with ≥4-fold post-vaccination increase in HI titer from pre-vaccination antibody.
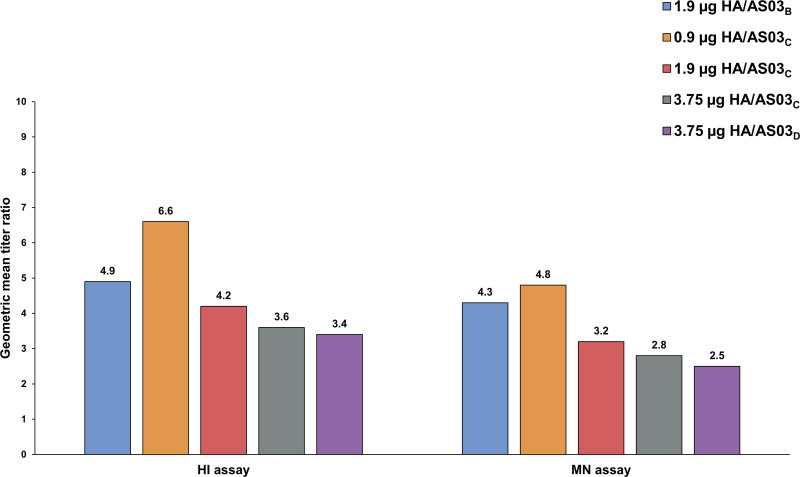
At day 392, a trend for the highest immune response with the 1.9 µg HA/AS03B formulation was observed in anamnestic response with GMT 476.2 (95% CI 348.4–650.9), although strong anamnestic responses were observed for all formulations. The highest MGIDay 392/Day 385 for HI antibodies was observed in 0.9 µg HA/AS03C formulation (6.6) followed by 1.9 µg HA/AS03B (4.9), which is because the GMT at day 385 in 0.9 µg HA/AS03C was lower than 1.9 µg HA/AS03B (Fig. 2).
FIGURE 2.
Mean geometric increase for vaccine homologous hemagglutinin and microneutralizing antibodies at day 392 versus day 385 (adapted ATP cohort for immunogenicity). AS03 is an adjuvant system containing DL-α-tocopherol and squalene in oil-in-water emulsion (AS03B containing 5.93 mg DL-α-tocopherol, AS03C containing 2.97 mg DL-α-tocopherol, and AS03D containing 1.48 mg DL-α-tocopherol).
CMI Response
In terms of CMI, a trend for the highest CD4+ T-cell responses was observed at day 42 for the 1.9 µg HA/AS03B formulation as shown by the frequency of polypositive CD4+ T cells. For all formulations, polypositive CD4+ T-cell responses were higher than baseline at all time points (Supplemental Digital Content 5; http://links.lww.com/INF/E463). No vaccine-specific CD8+ T-cell response was detected with any of the formulations.
Reactogenicity and Safety
Solicited Injection-site and General AEs
Pain was the most frequently reported solicited injection-site AE for all formulations after primary vaccination; it was mild in nature and lasted for 1–2 days. Redness was not reported for any subject, and swelling was reported for only 1 subject of the 3.75 µg HA/AS03C formulation. One subject (of the 1.9 µg HA/AS03B formulation) experienced an injection-site pain that led to withdrawal from the study.
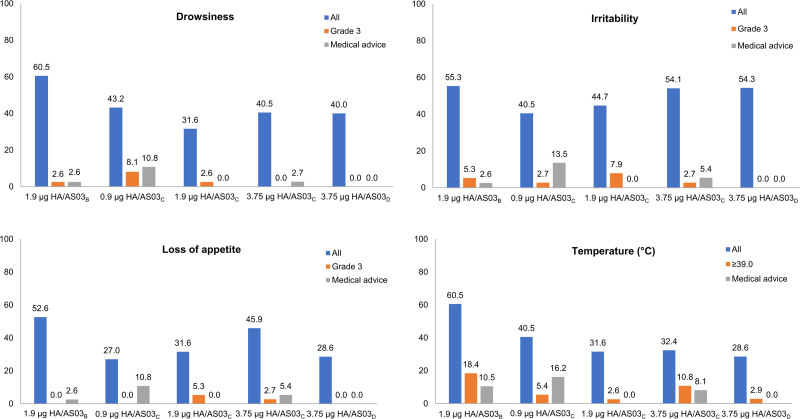
The highest incidence of solicited general AEs was observed with the 1.9 µg HA/AS03B formulation. The overall per-subject incidence of fever 7 days after each dose tended to be the highest with the 1.9 µg HA/AS03B formulation (60.5%) versus other formulations (28.6%–40.5%). Grade 3 fever (fever: ≥39.0 °C) was reported in 18.4% of subjects of 1.9 µg HA/AS03B formulation, and 2.6%–10.8% of the remaining formulations. Medical advice following fever was sought by 10.5%, 16.2% and 8.1% subjects with the 1.9 µg HA/AS03B, 0.9 µg HA/AS03C and 3.75 µg HA/AS03C formulations. The mean duration of fever was less than 3 days across formulations (Fig. 3; Supplemental Digital Content 6; http://links.lww.com/INF/E463).
FIGURE 3.
Overall per-subject incidence of general adverse events (AEs; %) during the 7-day (days 0–6) postvaccination period across the primary dose series (TVC).* *Detailed results are provided in Supplemental Digital Content 6; http://links.lww.com/INF/E463. AS03 is an adjuvant system containing DL-α-tocopherol and squalene in oil-in-water emulsion (AS03B containing 5.93 mg DL-α-tocopherol, AS03C containing 2.97 mg DL-α-tocopherol, and AS03D containing 1.48 mg DL-α-tocopherol).
Unsolicited AE
At least 1 unsolicited AE was reported during the 21-day post-primary vaccination period with all formulations [54.1% (0.9 µg HA/AS03C) to 68.4% (1.9 µg HA/AS03C)]. For all formulations, except 1.9 µg HA/AS03C, upper respiratory tract infection was the most commonly reported unsolicited AE, followed by nasopharyngitis. A total of 7.9%, 10.5%, 8.1% and 11.4% of subjects with 1.9 µg HA/AS03B, 1.9 µg HA/AS03C, 3.75 µg HA/AS03C and 3.75 µg HA/AS03D formulations, reported at least 1 unsolicited AE with causal relationship to vaccination. At least 1 grade 3 unsolicited AE was reported within the 21-day postvaccination period with all formulations [2.6% (1.9 µg HA/AS03C) to 14.3% (3.75 µg HA/AS03D)]. None of the grade 3 AEs were assessed to be causally related to the study vaccination. At least 1 unsolicited AE was reported within the 30-day postantigen challenge period with all formulations [23.5% (0.9 µg HA/AS03C) to 54.3% (3.75 µg HA/AS03C)].
Occurrence of SAEs and Unsolicited AEs
SAEs were reported for 29 subjects (15.7%). Across formulations, the incidence ranged from 10.8% to 26.3%. None of the SAEs were fatal or related to the study vaccination, and all resolved before the end of the study. The percentage of subjects who experienced at least 1 unsolicited AE with MAE or AESI during the study period are listed in Supplemental Digital Content 7; http://links.lww.com/INF/E463. Two subjects reported unsolicited AE needing medical attention with causal relationship to study vaccination with MAE. Three of the AESI were reported to have a causal relationship to vaccination. One subject of the 1.9 µg HA/AS03C formulation reported Kawasaki disease, a potential immune-mediated disease assessed by the investigator as not related to the study vaccine, and the subject recovered before the end of the study. No safety signal was identified.
Supplemental Digital Content 8; http://links.lww.com/INF/E463 elaborates on study findings in a form that could be shared with patients by healthcare professionals.
DISCUSSION
The aim of this phase 2 randomized, multicenter, observer-blind, dose-ranging study was to assess the immunogenicity and reactogenicity and rank different formulations of the Q-Pan H5N1 vaccine to determine whether reactogenicity can be reduced while maintaining adequate immunogenicity in children 6 to 35 months of age. Results showed that vaccination with a 2-dose primary series of Q-Pan H5N1 presented acceptable safety profiles and elicited a robust and persistent immune response across all formulations.
The development of a pandemic vaccine suitable for young children requires an objective assessment of several independent parameters with respect to dose selection (eg, risk-benefit profile, quality and persistence of the immune response, broad cross-reactivity and heterologous boostability, logistics).The immunogenicity against the vaccine-homologous H5N1 strain and the risk-benefit profile of the vaccine formulations are important determinants to guide the selection of an optimal formulation for pandemic or pre-pandemic settings. To support dose selection in this study, we developed an immunogenicity-fever index that considers both fever and immunogenicity equally. The 1.9 μg HA/AS03B formulation ranked the highest on the immunogenicity-fever index. Although this formulation had the highest incidence of solicited general symptoms, including grade 3 fever (≥39.0 °C: 18.4%), the outcomes were considered acceptable in light of the potent immunogenicity observed with this formulation.
The quality and persistence of the immune response after priming are other parameters that should be duly considered in the selection of a vaccine for pre-pandemic and pandemic use because a durable immune response is desirable to protect individuals during the pandemic due to the possibility of occurrence of a second or third wave, months after the first wave.2,17 In this study, at day 385, ie, 1 year after primary vaccination, antibody responses were sustained with SPR >70% and SCR >40% for all formulations, which is reflective of persistence. In addition, the quality of priming was confirmed through the anamnestic responses observed at day 392, 7 days after an antigen challenge with unadjuvanted H5N1 vaccine. Results across formulations are in line with results from a different phase 2/3 study (children, 6 months to 17 years of age), in which about 95% of subjects were seropositive for vaccine-homologous antibodies for at least 1 year.5
Cross-reactivity and heterologous boostability are important attributes in a pre-pandemic vaccine because the specific pandemic strain cannot be accurately predicted. Broad cross-reactivity of pre-pandemic vaccine may provide broader protection to a population compared with a vaccine with a narrow spectrum of cross-reactivity. In this study, the 1.9 μg HA/AS03B vaccine showed a trend for higher cross-reactivity against heterologous strains compared with other formulations.
Logistical considerations are also critical in selecting a recommended reduced vaccine dose for children. Vaccine production, distribution and reconstitution (in case of adjuvanted vaccines) should be as simple as possible. To minimize the use of resources in such an environment, a multi-vial adjuvant-antigen formulation is desirable. A pediatric formulation containing a fraction of the adult dose will allow for this approach. This will also minimize the potential for vaccination errors in pandemic settings. The 1.9 μg HA/AS03B formulation contains precisely half of the licensed adult dose (the 3.75 μg HA/AS03A vaccine, containing 11.86 mg DL-α-tocopherol), thereby facilitating deployment during a time of global and national time and resource constraints.
There are a few study limitations. First, the immunogenicity-fever index did not include other indicators of reactogenicity such as drowsiness and loss of appetite. However, fever was chosen as a proxy for reactogenicity because body temperature is objective compared with other measures of systemic reactogenicity. Other limitations include the small sample size, and the absence of a placebo control to assess safety of the vaccine could limit the generalizability of results from this trial.
CONCLUSION
All formulations were immunogenic and demonstrated acceptable safety profiles. In this study, the 1.9 µg HA/AS03B provided the most favorable balance of immunogenicity versus reactogenicity for use in children 6–35 months of age.
ACKNOWLEDGMENTS
The authors thank the study participants and their families, the clinical study site personnel and GSK study team who contributed to the trial conduct. The authors would like to thank Business & Decision Life Sciences platform for writing support, editorial assistance and manuscript coordination, on behalf of GSK. Thibaud André, Benjamin Lemaire and Aurélie Roth coordinated manuscript development and editorial support. Amrita Ostawal (Arete Communication UG, Berlin, Germany) provided medical writing support. All authors participated in the design or implementation or analysis, and interpretation of the study and the development of this manuscript. All authors had full access to the data and gave final approval before submission.
Supplementary Material
Footnotes
The trial registration number was ClinicalTrials.gov NCT02719743.
J.H.K., M.D., J.D., D.F. and B.S. are employees of the GSK group of companies. W.W., D.W.V., B.I. and A.S. were employees of the GSK group of companies during the conduct of the study. J.H.K., B.S., M.D., J.D., W.W. and A.S. hold shares from the GSK group of companies. T.P., N.-C.C., K.S. and L.-M.H. received funds from the GSK group of companies through their institution for the conduct of the study. L.-M.H. received personal fees from the GSK group of companies for educational lectures. J.H.K., M.D., T.P., N.-C.C., K.S., L.-M.H., J.D., D.F., B.S., W.W., D.W.V., B.I. and A.S. declare no other financial and nonfinancial relationships and activities. K.-P.H., P.-Y.C. and C.-H.C. declare no financial and nonfinancial relationships and activities and no conflicts of interest.
Wayne Woo, MSc is currently at Biostatistics Department, Novavax, Gaithersburg, Maryland; David W. Vaughn, MD is currently at the Bill & Melinda Gates Foundation, Seattle, Washington; and Anne Schuind, MD is currently at Center for Vaccine Innovation and Access, PATH, Washington, District of Columbia.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.pidj.com).
REFERENCES
- 1.World Health Organization. H5N1 highly pathogenic avian influenza: timeline of major events. 2014. Available at: http://www.who.int/influenza/human_animal_interface/H5N1_avian_influenza_update20141204.pdf. Accessed July 1, 2019.
- 2.de Vries RD, Herfst S, Richard M. Avian influenza a virus pandemic preparedness and vaccine development. Vaccines (Basel). 2018;6:E46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chu DW, Hwang SJ, Lim FS, et al. ; H5N1 Flu Study Group for Hong Kong, Singapore, Taiwan and Thailand. Immunogenicity and tolerability of an AS03(A)-adjuvanted prepandemic influenza vaccine: a phase III study in a large population of Asian adults. Vaccine. 2009;27:7428–7435. [DOI] [PubMed] [Google Scholar]
- 4.Díez-Domingo J, Garcés-Sanchez M, Baldó JM, et al. Immunogenicity and Safety of H5N1 A/Vietnam/1194/2004 (Clade 1) AS03-adjuvanted prepandemic candidate influenza vaccines in children aged 3 to 9 years: a phase II, randomized, open, controlled study. Pediatr Infect Dis J. 2010;29:e35–e46. [DOI] [PubMed] [Google Scholar]
- 5.Kosalaraksa P, Jeanfreau R, Frenette L, et al. AS03B-adjuvanted H5N1 influenza vaccine in children 6 months through 17 years of age: a phase 2/3 randomized, placebo-controlled, observer-blinded trial. J Infect Dis. 2015;211:801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langley JM, Frenette L, Ferguson L, et al. Safety and cross-reactive immunogenicity of candidate AS03-adjuvanted prepandemic H5N1 influenza vaccines: a randomized controlled phase ½ trial in adults. J Infect Dis. 2010;201:1644–1653. [DOI] [PubMed] [Google Scholar]
- 7.Langley JM, Risi G, Caldwell M, et al. Dose-sparing H5N1 A/Indonesia/05/2005 pre-pandemic influenza vaccine in adults and elderly adults: a phase III, placebo-controlled, randomized study. J Infect Dis. 2011;203:1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leroux-Roels I, Borkowski A, Vanwolleghem T, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–589. [DOI] [PubMed] [Google Scholar]
- 9.Nolan T, Izurieta P, Lee BW, et al. Heterologous prime-boost vaccination using an AS03B-adjuvanted influenza A(H5N1) vaccine in infants and children<3 years of age. J Infect Dis. 2014;210:1800–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vaughn DW, Seifert H, Hepburn A, et al. Safety of AS03-adjuvanted inactivated split virion A(H1N1)pdm09 and H5N1 influenza virus vaccines administered to adults: pooled analysis of 28 clinical trials. Hum Vaccin Immunother. 2014;10:2942–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izurieta P, Uy-Aragon MJ, Dramé M, et al. Assessment of prime-boost vaccination using an AS03B-adjuvanted Influenza A (H5N1) vaccine: a randomized trial in children of three to less than eighteen years of age. Pediatr Infect Dis J. 2016;35:e35–e47. [DOI] [PubMed] [Google Scholar]
- 12.GSK Study Register. NCT02719743. Available at: https://s3.amazonaws.com/ctr-gsk-7381/116938/1d7d0395-0280-4168-bc18-1a015aa5038d/1dbecf6f-fed4-40fb-8a10-d47be2a4146d/116938_Prot_000-v1.pdf. Accessed March 3, 2019.
- 13.Hehme N, Engelmann H, Kuenzel W, et al. Immunogenicity of a monovalent, aluminum-adjuvanted influenza whole virus vaccine for pandemic use. Virus Res. 2004;103:163–171. [DOI] [PubMed] [Google Scholar]
- 14.Kendal A, Pereira M, Skehel J. Concepts and procedures for laboratory-based influenza surveillance. (distributed by the viral disease unit, W.H.O., Geneva, or the WHO collaborating center for influenza, centers for disease control. U.S. Department of Health and Human Services;1982. [Google Scholar]
- 15.Rowe T, Abernathy RA, Hu-Primmer J, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stephenson I, Wood JM, Nicholson KG, et al. Detection of anti-H5 responses in human sera by HI using horse erythrocytes following MF59-adjuvanted influenza A/Duck/Singapore/97 vaccine. Virus Res. 2004;103:91–95. [DOI] [PubMed] [Google Scholar]
- 17.Jennings LC, Monto AS, Chan PK, et al. Stockpiling prepandemic influenza vaccines: a new cornerstone of pandemic preparedness plans. Lancet Infect Dis. 2008;8:650–658. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.