Fibroblast activation and subsequent collagen deposition in the heart is a one of the most deleterious consequences of hypertension and is an indicator of hypertensive heart disease. It contributes to ventricular stiffness and impaired relaxation and is considered as the main mechanism of heart failure in patients with preserved ejection fraction (HFpEF). The excessive myocardial cross‐linking of collagen type I, a process catalyzed by the enzyme lysyl oxidase increases the insolubility, stiffness, and resistance to degradation of the collagen fibers and contributes to elevated filling pressures in patients with HFpEF. Transvenous myocardial biopsy to assess collagen deposition is considered the gold standard for the diagnoses of myocardial fibrosis. The levels of collagen in the biopsies have been shown to correlate with the degree of heart failure and inversely correlate with the ejection fraction by independent studies.
Considering the invasive nature of the needle, biopsy serum biomarkers have been studied as surrogates for myocardial fibrosis. Collagens are synthesized from procollagen types I and III are secreted from fibroblasts and myofibroblasts as a triple‐helix procollagen precursor containing terminal propeptides. The propeptides are cleaved by procollagen proteinases and released to the bloodstream. The carboxy‐terminal propeptide of procollagen type I (PICP) is one of the propeptides generated during the extracellular conversion of procollagen type I into collagen type I by the enzyme procollagen carboxy‐terminal proteinase (Figure 1). Since PICP is formed in a 1:1 stoichiometric ratio to the collagen type I molecule, its concentrations in blood is a direct indicator of collagen type I synthesis. 1 The synthesized collagen is subsequently degraded by matrix metalloproteinases (MMP) such as MMP‐1, which releases the carboxy‐terminal telopeptide of collagen type I (CITP), a collagen type I degradation‐derived serum peptide into circulation. The higher the cross‐linking of collagen type I fibers, the lower the cleavage of the carboxy‐terminal telopeptide of collagen type I (CITP) by the enzyme MMP‐1. Accordingly, excessive myocardial collagen cross‐linking enhances myocardial collagen's resistance to degradation by MMP‐1 and triggers interstitial accumulation of collagen fibers with impairment of cardiac function. The intensity of collagen cross‐linking can be assessed by the CITP/MMP‐1 ratio, where a higher value is associated with less crosslinked cardiac collagen.
FIGURE 1.
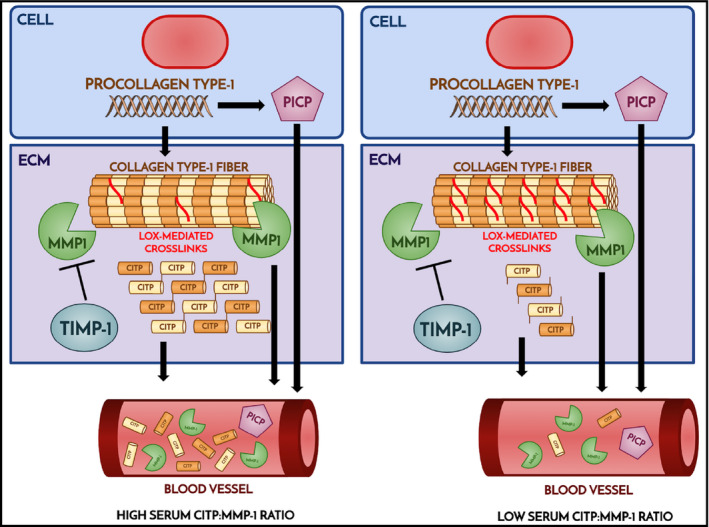
Schematic of collagen synthesis and degradation are shown. Left panel shows high CITP levels generated by MMP1 degradation of collagen with fewer cross‐links compared to higher levels of cross‐link shown on the right. For abbreviations, please refer to the text.
The MMP‐1 is inhibited by tissue inhibitors of metalloproteinase 1 (TIMP‐‐1), which restrict ECM proteolysis and promote ECM deposition.
To maintain hemostasis, human body also synthesizes antifibrotic proteins. N‐acetyl‐seryl‐aspartyl‐lysyl‐proline (Ac‐SDKP) is an endogenous anti‐fibrotic and anti‐inflammatory tetra‐peptide generated from the N‐terminal sequence of thymosin β4 (Tβ4) by the action of propyl oligopeptidases. Ac‐SDKP inhibits human mesangial cell, renal, and cardiac fibroblast proliferation. In addition, it has been shown to inhibit collagen deposition in mouse cardiac fibroblasts. Ac‐SDKP is degraded by angiotensin‐converting enzyme (ACE). ACE has N‐terminal and C‐terminal catalytic domains. The in vivo studies have suggested that N‐terminal catalytic domain has relatively lower catalytic effects compared to C‐terminal. The affinity of ACE inhibitors for the ACE catalytic domains is structure dependent. Captopril, the first clinically used ACE inhibitor has greater affinity for the N‐terminal domain compared to the C‐terminal domain, while lisinopril has much greater affinity for the C‐terminal domain. Ac‐SDKP is hydrolyzed only by the N‐terminal catalytic ACE domain and is released by ACE inhibitors, and hence, their levels are also used to detect adherence to the medication. Overall, the activation of renin‐angiotensin‐aldosterone (RAAS) system in hypertension is a major inducer of fibroblast activation and collagen deposition. The role of TGFβ and CTGF as the downstream signal mediator of RAAS axis in the pathogenesis of myocardial fibrosis has been extensively studied in the animal models. 2
PICP has been shown to be the most sensitive and specific marker of increased collagen deposition. 3 Blood PICP level (AKA as PIP) has been previously investigated in small studies of hypertensive patients with left ventricular hypertrophy. 4 In a study of 65 hypertensives patients with left ventricular hypertrophy with and without heart failure, the levels of PIP measured by radioimmunoassay in serum samples from the coronary sinus correlated with the amount of collagen detected by the tissue biopsies and was higher in hypertensives vs. normotensives and those with heart failure and hypertension compared with non‐heart failure hypertensives. The amount of collagen in the biopsates inversely correlated with the ejection fraction and directly correlated with both coronary and peripheral PIP in all hypertensives.
CITP has been shown to be a useful marker of collagen turnover. Its increased levels are associated with myocardial infarction, incident heart failure, hospitalization for heart failure, cardiovascular, and all‐cause mortality and have been shown to have additive prognostic value comparable to NT‐proBNP levels. PICP/CITP ratio reflects the balance between type I collagen synthesis and degradation.
In the study published in this issue of the Journal of Clinical Hypertension by Romero et al, 5 the levels of pro‐ and anti‐fibrotic markers in hypertensive African American patients with LVH after one year of intensive treatment with ACE inhibitors vs. other antihypertensive medications are compared. The investigators performed a post hoc analysis of a randomized controlled trial in which hypertensive African American patients with LVH and vitamin D deficiency have been randomized to receive intensive antihypertensive therapy plus vitamin D supplementation or placebo. The subjects were African American men and women aged 30 to 74 years who presented at the emergency departments affiliated with Wayne State University in Detroit with systolic blood pressure > 160 mmHg on two measurements and had evidence for LVH on CMR imaging. The average of five seated blood pressure measurements was used. A combination therapy of diuretic plus Lisinopril vs. calcium channel antagonist aimed at achieving systolic blood pressure <130 mm Hg was used. After screening, a total of 353 patients 113 patients with LVH were identified. 66 patients (mean age of 46.2 ± 8) had detectible lisinopril levels (lisinopril group) in plasma by LC/MS analysis at the one‐year follow‐up. Except for age the characteristics between cases and controls were similar. No difference was observed in the number and intensity of antihypertensive medications prescribed in each group. The plasma levels of PICP, MMP‐1, TIMP‐1, CITP, and Ac‐SDKP peptides were measured. Patients with detectable lisinopril had lower blood pressure and higher anti‐fibrotic markers MMP‐1 and Ac‐SDKP compared to those with undetectable lisinopril levels (all p<0.05). In a model adjusted for systolic blood pressure, MMP‐1/TIMP‐1 (p = 0.02) and Ac‐SDKP (p < 0.001) levels correlated with lisinopril levels. The authors conclude that ACE inhibitors increase anti‐fibrotic biomarkers in hypertensive African Americans with LVH and may offer added benefit over other agents in such patients in preventing cardiac fibrosis.
The findings by Romero et. al 5 are consistent with the results from numerous prior studies. It is true that the studied population is once again small, and the concept is not novel. Yet, the study has a major significance in that it concerns the effect of ACE inhibitors on markers of cardiac fibrosis in the understudied black population. ACE inhibitors were initially avoided as an initial therapeutic option in the treatment of hypertension in African Americans due to their poor blood pressure lowering efficacy. In addition, ACE inhibitors like captopril were shown to be less effective compared to high doses of calcium antagonists in African Americans. However, the high prevalence of comorbid conditions such as hypertensive heart disease made treatment with RAAS inhibitors more compelling despite lower circulating renin levels and reduced efficacy. Many clinical trials have shown significant benefit of ACE inhibitors, especially in those with hypertension and cardiovascular diseases. Improved cardiovascular outcomes have been found with the combination of ACE inhibitors or angiotensin II receptor blocker and a calcium channel blocker over a thiazide‐type diuretic Benazepril plus amlodipine or hydrochlorothiazide for hypertension in high‐risk patients. 6 It has become apparent that most black patients will need at least 2 agents for blood pressure control as shown in the BARBER‐2 trial 7 and given the cardiovascular benefit of ACE inhibitors a combination of calcium channel blocker/thiazide‐like diuretics and an ACE inhibitor should be considered for an optimal blood pressure control in this high‐risk population. Future investigations will determine whether the induction of antifibrotic markers by ACE inhibitors have clinical relevance such as reducing myocardial fibrosis.
CONFLICT OF INTEREST
None.
Acknowledgements
This study was supported by the NIH grant RHL135767A to Arya Mani.
Mani K, Mani A. The significance of plasma collagen degradation products as biomarkers for advanced hypertensive heart disease. J Clin Hypertens. 2021;23:1017–1019. 10.1111/jch.14205
REFERENCES
- 1. Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403‐34. [DOI] [PubMed] [Google Scholar]
- 2. Lang C, Sauter M, Szalay G, et al. Connective tissue growth factor: a crucial cytokine‐mediating cardiac fibrosis in ongoing enterovirus myocarditis. J Mol Med (Berl). 2008;86(1):49‐60. [DOI] [PubMed] [Google Scholar]
- 3. Lopez B, Gonzalez A, Ravassa S, et al. Circulating Biomarkers of Myocardial Fibrosis: The Need for a Reappraisal. J Am Coll Cardiol. 2015;65(22):2449‐56. [DOI] [PubMed] [Google Scholar]
- 4. Querejeta R, Lopez B, Gonzalez A, et al. Increased collagen type I synthesis in patients with heart failure of hypertensive origin: relation to myocardial fibrosis. Circulation. 2004;110(10):1263‐8. [DOI] [PubMed] [Google Scholar]
- 5. Romero CASM, Wasinski B, Reed B, et al. Angiotensin‐Converting Enzyme Inhibitors Increase Anti‐Fibrotic Biomarkers in African Americans with Left Ventricular Hypertrophy. J Clin Hypertens. 2021;23(5):1008‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Officers A, Coordinators for the ACRGTA, and Lipid‐Lowering Treatment to Prevent Heart Attack T . Major outcomes in high‐risk hypertensive patients randomized to angiotensin‐converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid‐Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288(23):2981–97. [DOI] [PubMed] [Google Scholar]
- 7. Victor RG, Lynch K, Li N, et al. A Cluster‐Randomized Trial of Blood‐Pressure Reduction in Black Barbershops. N Engl J Med. 2018;378(14):1291‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]


