Abstract
Perchlorate is an important oxidizer used in propellants, pyrotechnics, and as a gas generator in commercial airbags, fireworks, and roadside flares. It is highly water soluble interferes with thyroidal iodide uptake and is an environmental contaminant. By changing the reaction chemistry, 5-aminotetrazole (5-AT) and nitrates replace perchlorate in some propellants. The short term toxicity of 5-AT was evaluated. Using a modified Ames assay, 5-AT was not mutagenic with or without S9 metabolic activation. 5-AT was considered “slightly toxic” with an EC50 of 28.8 mg 5-AT/L for a 15 minute exposure in Aliivibrio fischeri. In the in vitro sodium iodide symporter test, 5-AT did not inhibit the uptake of iodine. In the acute rat oral test, no adverse effects and no mortalities were observed at the limit dose of 2000 mg 5-AT/kg. In the 14-day sub-acute study, there were no clinical signs of toxicity or morbidity up to 623 mg 5-AT/kg-day; the highest dose tested. No differences were observed in hematology, clinical chemistry, organ weight, body weight, food consumption, histopathology, or DNA damage (peripheral blood micronucleus assay) of treatments compared with controls. The No Observed Adverse Effect Level (NOAEL) was 623 mg 5-AT/kg-day, the highest dose in the subacute oral bioassay.
Keywords: Tetrazole, 5-AT, Ames, oral, toxicity, rat, 14-day sub-acute, thyroid
1. Introduction
Finding replacements for perchlorate that will reduce or eliminate the health risks from environmental exposures has been identified as a research goal in the U.S. military and is one of the missions of the Army Environmental Quality Technology (EQT) program (AERTA, 2012; AR 40–5; AR 70–1; AR 200–1; DoDI, 2018). Perchlorate is an effective oxidizing compound that is currently used by the U.S. Army in weapon systems. Perchlorate is known to interfere with iodide uptake in the thyroid gland and is persistent and mobile in the environment. Regulatory authorities, therefore, are developing contaminant levels protective of the general population. Perchlorate salts (e.g., potassium, sodium, ammonium) fully ionize in aqueous solutions. The perchlorate anion has been found in many water sources associated with military installations. Consequently, critical training activities are threatened due to perchlorate contamination. Thus, there is a need to identify a suitable replacement for perchlorate. Commercially, 5-aminotetrazole (5-AT) is used in gas generator formulations for airbags, fireworks, and roadside flares. 5-AT, a compound with high-nitrogen content, derives its energy from a high heat of formation and releases benign nitrogen gas (N2) upon reaction with an oxidizing agent, such as a metal nitrate (Sabatini and Moretti, 2013). A literature review found a single report for 5-AT toxicity, which reported an intraperitoneal (i.p.) LD50 of 2500 mg 5-AT/kg in the mouse, and no established regulatory values for 5-AT have been reported (CIDPL, 2013). For the data presented here, a tiered toxicity testing strategy was used to characterize aquatic toxicity, bacterial mutagenicity, short term mammalian oral toxicity, and peripheral blood DNA damage of 5-AT (American Society for Testing and Materials (ASTM), 2008). Data from these tiered experiments were used to calculate the oral LD50, estimate aquatic toxicity and predict genotoxicity. These results were used to compare the toxicity of 5-AT to other compounds being considered as replacements for energetics (e.g., perchlorate) currently in use.
2. Methods
2.1. Test Compound.
5-Aminotetrazole (5-AT) is a white medium grain crystalline powder. Three lots with corresponding certificates of analyses were acquired from Sigma-Aldrich (St. Louis, MO, USA) for the experiments. For the Ames and Microtox ™ assays, 5-AT anhydrous (97 percent pure, CAS 4418–61-5, Lot # MKBF1104V; Sigma Aldrich, St. Louis, MO, USA) was used. Due to product discontinuation, 5-aminotetrazole monohydrate, 97 percent; (MW 103.08 g/mol; CAS 15454–54-3; product number A80602, lot number STBB9454V;) was used for the animal study. 5-AT Lots STBB9454V and STBG0437V were used for the sodium-iodide symporter inhibition assay (NIS-IA). The monohydrate was preferred due to the instability of the anhydrous 5-AT. 5-AT (85.07 g/mol) constitutes 82.5 percent of the 5-AT·H2O formula weight. All experiments accounted for the percent formula weight of 5-AT and data are reported for 5-AT not 5-AT·H2O. 5-AT monohydrate was water soluble (12 g/L).
2.2. Test Material Vehicles.
The vehicle (solvent) for the Ames, Microtox ™ and NIS-IA assays was DMSO (CAS 67–68-5; Sigma Aldrich, St. Louis MO, USA). The vehicle for the rat dosing studies was food grade Mazola™ corn oil purchased locally.
2.3. Chemical Analysis.
All purity confirmation and concentration verification analysis of the dosing solutions and stability analyses were performed by Army Public Health Center (APHC) Laboratory Sciences Directorate (LAB)-Client Services Division (CSD)-Method Development Section (MDV). Purity and concentration determination of 5-AT in either DMSO or corn oil and stability of 5-AT in corn oil were accomplished by high performance liquid chromatography with ultraviolet detection (LC-UV; Dionex U3000, Bannockburn, IL). The samples were isocratically analyzed using an Agilent Eclipse XDB C-18 column and quantitated at 232 nm. Results were reported in mg/mL. To confirm concentration of dosing solutions, the lowest concentration used in each assay was verified by LC-UV and the concentrations of the higher treatments were determined by back calculation.
2.4. In Vitro Microplate Ames Assay.
A microplate Ames assay (Xenometrics MPF Ames Assay ™, Gewerbestrasse 5 Allschwil, CH-4123 Switzerland) was used for bacterial mutagenicity testing. The test uses the standard Salmonella typhimurium and Escherichia coli strains (E. coli tested as a mixture) (Claxton et al., 2010). The MPF Ames Assay was purchased as a kit that contained all necessary reagents, positive controls for both –S and +S, media and bacterial strains; see Table 1. The assays were conducted in a manner consistent with the standards found in Title 40 Code of Federal Regulations (CFR), Part 792, Good Laboratory Practices.
Table 1.
Ames assay bacterial strains and positive control chemicals.
| Bacterial strains Salmonella typhimurium (S.t.) E. coli (E.c.) |
Positive control chemical | |
|---|---|---|
| Without S9 fraction | With S9 fraction | |
| S.t. TA98 | 2-nitrofluorene | 2-aminoanthracene |
| S.t. TA100 | 4-nitroquinoline-N-oxide | |
| S.t. TA1535 | N4-aminocytidine | |
| S.t. TA1537 | 9-aminoacridine | |
| E.c. WP2 uvrA and WP2[pKM101] | 4-nitroquinoline | |
The assay method has been previously described (Williams et al., 2012). Briefly, the day before the assay was performed, the bacterial strains were thawed and expanded overnight in growth medium at 37°C in an orbital shaker at 250 rpm, supplemented with ampicillin per the manufacturer’s protocol. Based on the tested maximal water solubility of 5-AT (10 mg/mL), a 23.8 mg/mL stock solution (25X) of 5-AT in DMSO was prepared on the day of the assay. S. typhimurium and E.coli bacterial suspensions were diluted with incubation medium, distributed in triplicate into 24 well plates (Corning Costar, Fisher Scientific, Pittsburgh, PA, USA) and incubated with either a DMSO vehicle control, the appropriate strain specific positive control (see Table 1) or 6 serial ⅓ log dilutions of the 23.8 mg 5-AT/mL stock solution for 90 minutes at 37°C 250 RPM. Separate preparations containing Aroclor-induced S9 rat liver extracts were prepared and incubated in parallel for either 90 minutes for S. typhimurium or 20 minutes for E. coli. After the timed exposure incubation, the bacterial suspensions were diluted 11-fold with incubation (indicator) medium and 48 – 50 μL aliquots for each suspension/treatment were distributed to 384-well plates (Corning Costar, Fisher Scientific, Pittsburgh, PA, USA) to yield 6 384-well plates (3 -S9 / 3 + S9) for each bacterial strain. The plates were then incubated at 37°C for 48 hours. The indicator medium contains a pH sensitive dye that shifts from purple to yellow in the presence of metabolically active bacteria. After the 48-hour incubation, the wells were scored for reversion (i.e., a mutagenic event) by assessing the color of the medium.
Concurrent with the mutagenicity assay, a separate replicate for each of the strains/treatments (without S9 extract) was incubated to determine 5-AT cytotoxicity after 90 minutes of exposure for the S. typhimurium and mixed E. coli strains. Cytotoxicity was measured using ATP concentration as an endpoint (BacTiter-Glo, Promega, Madison WI, USA). Luminescence was measured using a Synergy HT Multi-detection Microplate Reader and Gen5 software (BioTek Instruments Inc. Winooski, VT, USA). Data were expressed as percent control relative to the vehicle control samples.
2.5. Microtox®Assay.
The Microtox® assay utilizes a manufacturer-prepared diluent (AZF686011), acute reagent of freeze dried Allivibrio fischeri (NRRL-B-11177) (AZF686018) and reconstitution solution (AZF686016), purchased from Modern Water Inc. (New Castle, DE). As recommended, zinc sulfate (ZnSO4·7H2O) was used as a standard or positive control and acquired from Sigma-Aldrich (St. Louis, MO). The assays were carried out in the Microtox Model 500 Analyzer® with MicrotoxOmni™ software. (Microtox Model 500 Analyzer® and MicrotoxOmni™ software are initially registered trademarks of AZUR Environmental and currently owned by Modern Water Inc.). The manufacturer’s protocol with modifications was followed for the 5-AT toxicity test. Three independent replicate assays were performed. For each test day, 100 mg/mL (100X) 5-AT test solutions were prepared in DMSO and then diluted in reconstitution solution for the test. Raw luminescence values were recorded at 5, 15, and 30 minutes by the Microtox analyzer. The EC50 values at 5, 15, and 30 minutes were calculated by the MicrotoxOmni software and further fitted to Hill Function using GraphPad PRISM 5®, a registered trademark of GraphPad Software, Inc. (San Diego, CA, USA).
2.6. Sodium/iodide symporter inhibition assay.
FRTL-5 rat thyroid follicular cells (91030711, Millipore-Sigma, St. Louis, MO) were cultured in Coon’s modified F-12 medium supplemented with 5% FBS (fetal bovine serum) (Gibco, Waltham, MA), 2mM L-glutamine, 10mU/mL TSH (thyroid stimulating hormone), 10μg/mL insulin, 10nM hydrocortisone, 5μg/mL transferrin, 10ng/mL Gly-His-Lys, 10ng/mL somatostatin and penicillin-streptomycin at 37°C and 5% CO2. Cells were grown to 80–90% confluence in T75 flasks (Corning, Corning, NY) before trypsinization with 0.25% trypsin/EDTA (Gibco, Waltham, MA). For the radioactive iodide uptake (RAIU) assay, FRTL-5 cells were seeded into 96-well plates (Corning Costar, Corning, NY) at a density of 3.0× 104 cells per well in 200μL media and incubated 48 hours before experimentation. RAIU assays were conducted as previously described (Hallinger et al., 2017) with minor modifications. All control chemicals were initially solubilized in DMSO at 10 mM: sodium perchlorate (NaClO4; RAIU assay positive control), sodium nitrate (NaNO3; RAIU assay EC80 control), sodium thiocyanate (NaSCN; RAIU assay EC20 control), 2,4-dichlorophenoxyacetic acid (2,4-D; RAIU assay negative control), and 2,3-dichloro-1,4-napthoquinone (DCNQ; cell viability assay positive control for cytotoxicity). On the day of the assay, carrier-free 125I (Perkin-Elmer, Waltham, MA; specific activity: 17.4Ci/mg) was diluted with Hanks’ balanced salt solution (HBSS) supplemented with 10mM HEPES (uptake buffer; pH 7.4) at 10μCi/mL. The assay was initiated by gently washing cells twice with 200μL uptake buffer using a 96-well Tecan HydroSpeed plate washer (Tecan, Männedorf, Switzerland). Uptake buffer (89μL) was then added back to each well. Chemical samples (1uL at 100X) were added to each well to achieve a 1X chemical/1.0% final DMSO concentration with subsequent addition of 10μL of 125I dilution for a final reaction volume of 100μL/well. Cells were incubated at room temperature for 2 hours and then washed twice with 200μL ice-cold uptake buffer. All uptake buffer was aspirated from wells and 100uL 1N NaOH was added to each well for 10 minutes and then transferred to 12×75mm tubes to measure intracellular radioactivity with a Wizard gamma counter (Perkin Elmer, Shelton, CT). RAIU assay raw data, measured in counts per minute (CPM), were normalized per plate with the mean CPM of DMSO control wells and expressed as percent control (% control). Chemicals that elicited an RAIU inhibition greater than 20% of DMSO control were considered active for NIS inhibition {Hallinger, 2017 #22}. Normalized active chemical concentration responses (n=3; 3 biological replicates with the mean of 2 technical replicates for each concentration) were fit using a four-parameter logistic curve model with top and bottom asymptotes constrained to 100 and 0, respectively (GraphPad Prism 6.0, La Jolla, CA).
Cell viability assays were conducted in parallel with the RAIU assays. FRTL-5 cells were seeded and grown and washed with uptake buffer in 96-well solid white assay plates (Corning, Corning, NY) as described for RAIU assay. Uptake buffer (99uL) was then added back to each well with the addition of 1μL of 100X chemical samples. Cells were incubated at room temperature for 2 hours and Cell Titer-Glo (Promega, Fitchburg, WI) reagent (100μL) was then added to each well and plates were shaken for 5 minutes at 700rpm. Plates were incubated at room temperature for 10 minutes and then read on a FLUOstar Omega plate reader (BMG Labtech, Cary, NC) using an endpoint luminescence protocol (top read) to measure relative light units (RLU) indicative of ATP concentration. Assays were replicated in three independent runs containing different passages of the cells (i.e., 3 independent biological replicates). Cell viability raw data, measured in RLU, were normalized per plate with the mean RLU of DMSO control wells and expressed as percent % control. Chemicals that produced a 20% reduction in cell viability relative to DMSO control were considered cytotoxic and subjected to the same curve fitting as RAIU data.
2.7. Animals.
Testing was conducted in compliance with DOD and Federal statutes and regulations relating to animals and experiments involving animals and adhered to principles stated in the Guide for the Care and Use of Laboratory Animals, Institute of Laboratory Animal Resources, Commission on Life Sciences, National Research Council, National Academy Press, Washington D.C. 2011. The studies reported herein were performed in animal facilities fully accredited by AAALAC International.
All studies were conducted using young adult Sprague-Dawley Crl:CD(SD) rats obtained from Charles River Laboratories (Wilmington, MA, USA). Female rats were used for the acute study and at the time of their arrival the animals were 43–50 days old. Male and female rats were used for the 14-day study and at the time of their arrival the animals were 38–42 days old. Rats were pair-housed (same sex) in suspended polycarbonate boxes with Diamond Dry Bedding (product number 7070C; Diamond Star Products, WI, USA). The APHC Quality Systems Office audited critical phases of this study. For histopathology, tissues were preserved, packaged and shipped to Battelle Columbus, OH for processing, slide preparation, staining and histopathological evaluation. Tissues and slides were returned to the APHC for archiving.
2.8. Acute Toxicity Test.
The sequential stagewise probit (SSWP) method was used to estimate the oral median lethal dose (ASTM, 2010; Feder et al., 1991). The SSWP method potentially reduces the number of animals used for the LD50 test by first testing one animal per dose across the dose range and then subsequently testing additional animals at doses that bracket the LD50. If no deaths are observed in the first round, the method defaults to a “limit test” where the limit dose is tested with 3 additional animals in the second round. The published i.p. LD50 (2500 mg/kg), the lower bound of GHS category 5 (2000 mg/kg) and guidance from Schlede et al {Schlede, 2005 #27} were considered in setting the initial 4-dose test. The dosing strategy for the test is described in Table 2. The use of a single animal per dose group in the first round to gauge mortality is justified considering that mortality is verifiable (>99%) when additional animals are tested in subsequent rounds of the test {Schlede, 2005 #27}. The animals were fasted overnight prior to dosing and fasted weights for each animal were obtained. The doses were prepared for each animal based on individual fasted body weight. The vehicle was corn oil for both stages. The dose volumes were 90 percent of the maximum of 10 mL/kg body weight. Following the administration of 5-AT by gavage, the rats were observed daily for 14 days. The surviving animals were euthanized 14 days after dosing and submitted for gross pathological examination.
Table 2.
SSWP acute oral toxicity study design.
| Dose 5-AT mg/kg | Sequential Stage-wise Probit | |
|---|---|---|
| Stage One # animals | Stage Two # animals | |
| 61 | 1 | -- |
| 195 | 1 | -- |
| 625 | 1 | -- |
| 2000 * | 1 | 3** |
limit dose based on mouse i.p. LD50 = 2500 mg/kg (CIDPL, 2013) and lower bound of GHS Category 5 range (OECD, 2002).
No deaths in 4/4 animals tested at limit dose (stage 1 and 2 combined).
2.9. Sub-acute Toxicity Test.
The dose levels for the subacute study were based on the lack of clinical signs in the acute phase at the highest dose tested – 2000 mg 5-AT/kg and and the i.p. LD50 was 2500 mg 5-AT/kg. The two values were averaged and used to calculate the nominal highest dose for the subacute study: 700 mg/kg-day (= 1/2 log 2250 mg/kg). Sprague-Dawley rats (54 male and 42 female; approximately 40 days old upon arrival) were used for this phase of the study. The animals were sorted into 7 treatment groups (six 5-AT treatments and one vehicle control) of six male and six female rats each based on body weight such that the weight range was evenly distributed between the groups. An additional 12 male rats were used as positive and negative controls for the micronucleus assay. These rats were housed concurrently with animals from the subacute study.
Three stock solutions were prepared at the nominal concentrations 80, 20 and 5 mg 5-AT/mL corn oil. The stability of 5-AT in corn oil was confirmed via LC-UV over a 19-day period prior to the 14-day study. 5-AT was sufficiently stable so that the stock solutions were used for the duration of the 14-day study. Samples from these stock solutions (top, middle and bottom of the container) were taken and analyzed to verify homogeneity of the concentrations prior to the first day of dosing. The doses were delivered daily for 14 days to each rat via gavage. The volume of the dosing solution delivered to each rat was calculated from the body weight of the corresponding animal. Stock solutions were sufficiently concentrated so that the dose volume did not exceed 10 mL/kg body weight. The nominal dose groups were 700, 350, 175, 88, 44, 22, and 0 mg 5-AT/kg body weight. Rats were pair housed (same sex) for the duration of the 14-day study. Body weights were recorded on days 0 (first dosing day), 3, 7, 13 (last dosing day), and 14 (terminal body weights-fasted). Dosing volumes for each animal were adjusted based on body weights taken on days 0, 3, and 7 so that the rats continued to receive the intended dose. Food consumption based on change in feed container weights was monitored weekly. Water was provided ad libitum and intake was not monitored. Animals were observed daily for clinical signs of toxicity, morbidity and mortality.
2.10. Necropsy.
Approximately 24 hours after the 14th dose, each rat was anesthetized via CO2 gas. Blood was collected via cardiac puncture for clinical chemistry and hematology analyses, and rats were immediately euthanized via terminal CO2 asphyxiation. The brain, heart, kidneys, liver, spleen, epididymides and gonads (i.e., testes or ovaries/uterus) were removed and weighed and gross necropsies were completed. The following parameters, by test group, were analyzed and compared to the controls: (a) body weight; (b) weight gain; (c) food consumption; (d) absolute organ weight; (e) organ-to-body weight ratio; and (f) organ-to-brain weight ratio. Collected organs were preserved in 10% buffered formalin.
2.11. Hematology/Clinical Chemistry.
Blood aliquots were placed in EDTA, serum-gel, and sodium citrate microtubes for analysis of hematology, clinical chemistry, and hemostasis, respectively. Hematology analyses [white blood cell count (WBC K/mL), WBC differential (K/mL and percent for neutrophils (NEU-%N)), lymphocytes (LYM-%L), monocytes (MONO-%M), eosinophils (EOS-%E), basophils (BASO-%B)), red blood cell count (RBC M/mL), hemoglobin (HGB g/dL), hematocrit (HCT %), mean cell volume (MCV fL), mean cell hemoglobin (MCH pg), mean cell hemoglobin concentration (MCHC g/dL), RBC distribution width (RDW %), platelets (PLT K/mL), mean platelet volume (MPV fL)] were performed with a Cell-Dyne 3700 Hematology Analyzer (Abbott Laboratories, Abbott Park, Illinois 60064). Clinical chemistry samples were allowed to clot at room temperature, centrifuged, and the serum analyzed on VetTest 8008 and VetLyte Analyzers (IDEXX Laboratories, Inc., Westbrook, ME) for the following parameters: albumin (ALB g/dL), alkaline phosphatase (ALK P U/L), alanine aminotransferase (ALT U/L), aspartate aminotransferase (AST U/L), blood urea nitrogen (BUN mg/dL), calcium (Ca mg/dL), cholesterol (CHOL mg/dL), creatinine (CREA mg/dL), glucose (GLU mg/dL), globulin (GLOB g/dL), phosphorus (PHOS), total bilirubin (TBIL mg/dL), total protein (TP g/dL), sodium (Na mmol/L), potassium (K mmol/L), and chlorine (Cl mmol/L). Samples for prothrombin time were centrifuged for collection of plasma and analyzed on a BFT-II analyzer (Siemens Health Care Diagnostics, Inc., Tarrytown, NY).
2.12. Micronucleus Assay.
The micronucleus (MN) assay (or, MNA)-positive and negative control male rats were housed, maintained and necropsied with the animals from the subacute study. The positive control rats were dosed twice with 200 mg/kg ethylmethyl sulfonate (EMS), a known DNA damaging agent (CAS 62–50-0, Sigma M0880, Lot # BCBK5968V) 48 and 24 hours prior to euthanasia. The negative control rats were untreated and served as a negative control for both the EMS and the vehicle (corn oil) treated rats. Saphenous blood from the MNA control rats and male rats from the three highest dose groups with no mortalities was collected the day of necropsy. Blood collected in support of the MNA was processed according to the MicroFlow Basic Kit ® (Litron Laboratories, Rochester NY, USA) instructions. The prepared samples were then shipped to Litron Laboratories for analysis. The experimental method used by Litron is summarized as follows. On the day of analysis, the fixed blood samples were thawed and then washed with physiological buffer containing 1% FBS. Cells were isolated by centrifugation, and the cell pellets were stored at 2 °C to 10 °C until staining. An aliquot (20 μL) of each washed blood sample was added to 80 μL of a solution containing 1 mg/mL RNase, 10 μL/mL reticulocyte (RET) specific anti-CD71-FITC, and 5 μL/mL platelet specific anti-CD61-PE. The samples were incubated in the staining solution for 30 ± 10 minutes at 2°C to 10°C and 30 ± 10 minutes at room temperature. After incubation, the cells were kept at 2°C to 10°C until analysis. A propidium iodide (PI) solution (2 mL ± 0.5 mL) was added to each sample immediately before flow cytometric analysis to stain all DNA, including MN in the cells. Each blood sample was analyzed by high-speed flow cytometry (488 nm) using CellQuest software, version 5.2 (Becton Dickinson, San Jose, CA). Platelets were gated out of the analysis and 20,000 RETs (CD71+) were evaluated for the presence of MN. Normochromatic erythrocytes (NCEs), MN-NCEs, RETs and MN-RETs were counted for each sample.
2.13. Statistical Analysis.
Descriptive statistics and statistical analyses were performed using SigmaPlot for Windows Version 12.3, (© Systat Software, Inc. 2011). One-factor analysis of variance (ANOVA) was used for data collected at the end of the study. The available parametric statistical tests included Dunnett’s, Holm-Sidak and Bonferroni and the most rigorous test was chosen for each analysis. A non-parametric test was conducted for data that were not normally distributed or of equal variance (i.e., Kruskal-Wallis rank sum analyses and subsequent Dunn’s test).
3. Results
3.1. Analytical Chemistry.
The stability of 5-AT at room temperature was confirmed for a 19-day period, which justified the usage of stock solutions for the duration of the 14-day repeated dose test. Stability was not measured beyond 19 days. For the 14-day study, the nominal concentrations of the dosing solutions were 5, 20, and 80 mg 5-AT/mL corn oil. The measured concentrations were 4.98, 20.2, and 71.2 mg 5-AT/mL corn oil. The dose groups were calculated to be 0, 22, 44, 89, 177, 313, and 623 mg 5-AT/kg body weight-day. Doses and concentrations account for the contribution of water weight in the formulation and are reported as mg anhydrous 5-AT.
3.2. Ames Assay.
5-AT cytotoxicity and mutagenicity was tested in S. typhimurium strains TA98, 100, 1535 and 1537 and E. coli strains WP2 uvrA and WP2[pKM101] (as a mixture) in the presence and absence of rat liver microsomal fraction (S9) for metabolic competency. The concentrations tested were 0, 2.3, 7.7, 25.8, 85.9, 286, and 952 μg/mL. Without S9, 5-AT was cytotoxic to strain TA98 and the E. coli mix at 952 μg/mL see Figure 1. Cytotoxicity was not observed in any of the tester strains in the presence of S9 or in any of the mutagenicity positive control test conditions (data not shown). In both S9-/S9+ test conditions, 5-AT was not mutagenic up to and including the highest concentration tested.
Figure 1.
Mean cytotoxicity of 5-AT observed at 952 μg/mL in TA98 and E.coli mix without S9. Viability was assessed using an ATP luminescent assay. Cytotoxicity was measured as a decrease in ATP. Viability below 80% was considered to represent cytotoxicity. Error bars represent SEMs.
3.3. Microtox ™ Assay.
Toxicity to A. fischeri from 5-AT exposure was measured at 5, 15, and 30 minutes. Table 3 presents the data (EC50 mean ± 95%CI) collected from four individual experiments. Using the aquatic toxicity criteria of the U.S. Environmental Protection Agency (US EPA), Organization for Economic Cooperation and Development (OECD), and Global Harmonized System (GHS) to categorize the potential ecotoxicity (Table 3), 5-AT is considered “Slightly Toxic” by US EPA, potentially harmful to aquatic life by OECD standards, and Acute Category III by GHS standards.
Table 3.
Microtox aquatic toxicity and risk assessment.
| Compound | Microtox EC50 (mg/L) [95%CI] | USEPA Hazard Categories1 | OECD Hazard Classes2 | GHS Acute Aquatic Toxicity3 | ||
|---|---|---|---|---|---|---|
| 5 min | 15 min* | 30 min | ||||
| 5-AT | 25.91 [13.17–50.96] | 28.79 [18.10–45.80] | 43.95 [32.31–59.79] | Slightly Toxic | Acute Toxicity III (harmful to aquatic life) | Acute Category III |
The value of EC50 at 15 min was used for the hazard categorization determinations.
=(USEPA, 2017);
=(Organization for Economic Cooperation and Development (OECD), 2001);
=(UNCED, 2005)
3.4. Sodium/iodide symporter inhibition.
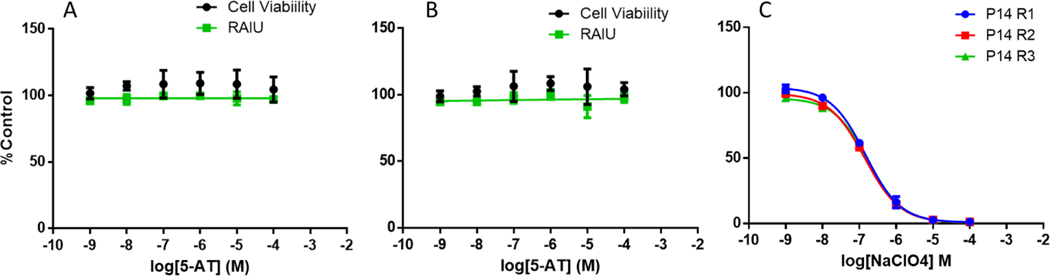
Results for 5-AT were compared to those obtained for NaClO4 tested on the same plates for each test of the RAIU and cell viability assays. No statistically-significant changes in NIS-mediated uptake of iodide or cell viability were observed for either of the two samples of 5-AT when tested from 0.001 to 100μM (Figure 2).
Figure 2.
Sodium-iodide symporter inhibition and cell viability for 5-AT and NaClO4 compared to control treatment. A. 5-AT (lot # STBG0437V) cell viability (closed circle) and RAIU (closed square). B. 5-AT (lot # STBB9454V) cell viability (closed circle) and RAIU (closed square). Graphs A and B show mean +/− SEM (N=3 independent experiments); each concentration was tested in technical duplicate for each biological replicate. C. RAIU for NaClO4 tested concurrently with each 5-AT test plate (mean +/− SEM; N=3).
3.5. Acute Oral Toxicity.
No animals died at any of the doses for the first stage; thus, the limit dose toxicity test (at 2000 mg 5-AT/kg body weight) was used. Three additional animals were dosed at 2000 mg 5-AT/kg body weight. No clinical signs of distress or discomfort were observed in the dosed animals. For the acute toxicity LD50 test, no animals died from compound toxicity at the highest dose tested, 2000 mg 5-AT/kg body weight for the limit-dose procedure. Thus, the LD50 is greater than the limit dose of 2000 mg 5-AT/kg body wt. Without lethality, the slope and confidence interval (CI) for the dose response curve were not defined.
3.6. Subacute Oral Toxicity.
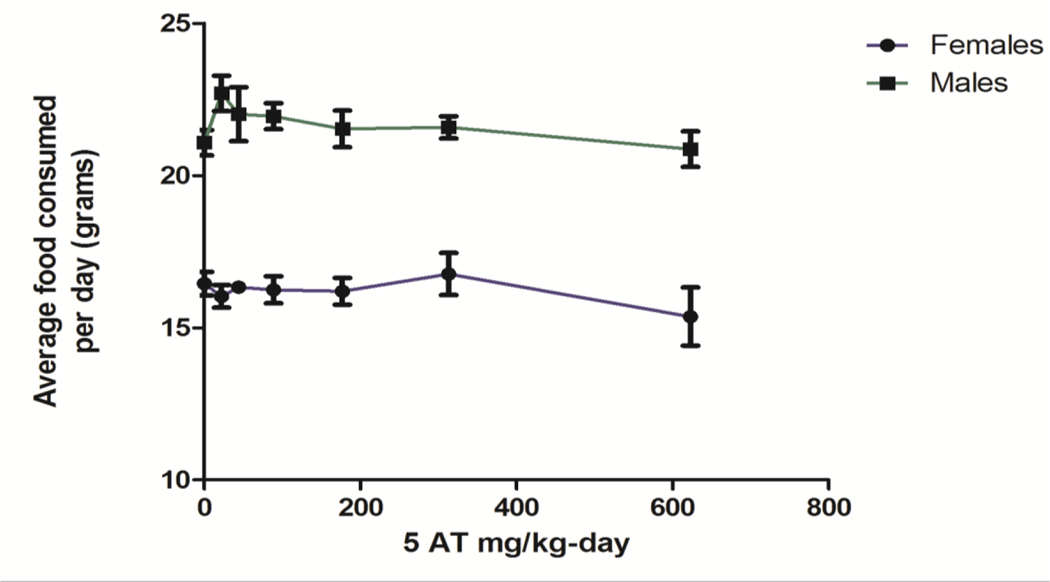
No clinical signs of toxicity were noted in any of the dose groups (0, 22, 44, 89, 177, 313, and 623 mg 5-AT/kg body weight-day) for the duration of the 14-day dosing period. All animals survived until their scheduled necropsy day. No observations were considered compound-related. To account for paired housing, the consumption per individual rat was estimated by calculating percent consumption using the individual body weights of the cage-pair (Figure 3). Males consumed more food per day than females (proportional to their increased weight); however, there were no statistically significant differences in food consumption due to 5-AT treatment. The body weight of each study animal was obtained on days 0, 3, 7, 13 and 14 of the study. The animals in this study were young and were expected to gain weight during the study. Although there were expected differences between males and females, there were no dose-related differences in body weights. The weights of the heart, kidneys, epididymides, liver, spleen, thymus, and gonads were collected at necropsy. No differences in absolute brain weight, absolute organ weight or organ weight/brain weight ratios were found between the treatment groups. Summary tables for weight gain, organ weights and organ weight ratios are provided in SD Tables 1-5. The summary clinical chemistry and hematology data are provided in SD Tables 6 and 7. Several hematology and clinical chemistry parameters exhibited differences between sexes that were expected and not treatment related (see SD Table 8). BUN in female rats was different in the 177 and 313 mg/kg-day dose groups. However, BUN in the highest dose group was not different from the controls. Without a dose-response increase in BUN between the 313 and 623 mg/kg-day dose groups and only a minor increase in BUN in the 313 mg/kg-day group compared to the control dose group, the finding was considered not to be biologically relevant. No significant dose-related differences among the treatment groups were found in either the hematology or clinical chemistries.
Figure 3.
Mean food consumption in female and male rats dosed with 5-AT for 14 days. Food containers were weighed and estimated food consumed per rat was calculated as a percent of the body weight for each member of the pair. Error bars represent SEMs.
The tiered testing program approach extends to the histopathological examination of selected organs from the vehicle and highest dose groups, followed by reviewing intermediate dose groups based on findings in the high dose group. Histopathology was assessed from the major blood conditioning organs (liver, spleen, and kidney) and the heart. Formalin-fixed liver, kidney, spleen and heart tissues for the corn oil vehicle and highest dose (623 mg 5-AT/kg-day) were embedded in paraffin, sectioned (4 μm), stained with hematoxylin and eosin, and subjected to histopathological examination. The slides were examined by the pathologist without prior knowledge of the dose group identity. In both the vehicle and high dose samples a correlation between the necropsy finding of mottled and/or pale liver and the histopathological finding of minimal cytoplasmic vacuolization was observed. As this observation occurred in both the untreated and treated groups, it was not considered biologically meaningful. There were no statistically significant microscopic findings related to exposure of 5-AT in the liver, kidney, spleen or heart in the highest 5-AT treatment group.
3.7. Micronucleus Assay.
Male rats from the 5-AT study (three highest dose groups and the vehicle control) were tested for DNA damage in their peripheral blood. The EMS positive control group had a significantly higher percent MN-RET (p≤0.001) compared to the negative and 5-AT treated groups. There were no significant differences between the negative and 5-AT treated groups (p=0.785). The percent RET was reduced in the EMS-treated controls compared to the negative, vehicle and 5-AT treated groups (p≤0.001) while the negative control group had an increased percent RET compared to the EMS treated, vehicle and 5-AT treatment groups (0.027≤p≥0.001); see Table 4.
Table 4.
Peripheral blood micronucleus assay results for male rats exposed to 5-AT.
| Controls | 5-AT mg/kg-day | ||||||
|---|---|---|---|---|---|---|---|
| untreated | EMS | 0 | 177 | 313 | 623 | ||
| %-RET | Mean | 3.842 a | 1.15 c | 2.577 b | 2.795 b | 2.927 b | 2.933 b |
| S.D.* | 0.646 | 0.26 | 0.476 | 0.673 | 0.457 | 0.344 | |
| %MN-RET | Mean | 0.118 d | 1.053 e | 0.1 d | 0.09 d | 0.105 d | 0.118 d |
| S.D.* | 0.026 | 0.204 | 0.017 | 0.021 | 0.042 | 0.042 | |
N= 6; % RET:
- statistically significant difference from b and c;
- no statistical difference between dose groups;
-statistically significant difference from a and b;
% MN-RET:
- no statistically significant difference between groups;
-statistically significant difference (p<0.001) from d.
The vehicle control and 5-AT treatment groups were not significantly different from each other (0.982≤p≥0.736). Taken together the data indicate that 5-AT was not genotoxic to the hematopoietic cells in male rats. The decrease in %-RET between the untreated controls and all of the 5-AT treatment groups (including the vehicle treated control) suggests a generalized low-level stress response.
4. Discussion
The tetrazole 5-AT, along with perchlorate, has been used extensively in airbag inflators as a fuel to generate inflation gases (Lund and Blau, 1996). Both the anhydrous and monohydrate forms of 5-AT have been used in these compositions. In vitro toxicity experiments and an acute and subacute oral toxicity study exposing rats to 5-AT were conducted. The Ames and Microtox assays were used to estimate genotoxicity and aquatic toxicity, respectively. Following in vitro testing, the short term animal toxicity studies were used to determine acute toxicity as well as understand effects from short-term repeated dose exposures.
5-AT was not toxic at the limit dose of 2000 mg/kg body weight suggesting low potential for acute toxicity. No toxicity or morbidity was observed in the 14-day repeated dose subacute study at any of the doses tested including the highest treatment of 623 mg/kg-day. Thus, the 14-day NOAEL was 623 mg 5-AT/kg-day and the LOAEL was not identified. 5-AT did not stimulate the production of micronuclei in rat peripheral blood during the 14-day bioassay. The micronuclei assay measures DNA damage in reticulocytes and may identify compounds that are genotoxic to bone marrow. These in vivo data were consistent with the lack of mutagenicity observed in the Ames assay. Combined, these data suggest 5-AT is not genotoxic; however, carcinogenicity was not evaluated in this study. 5-AT was considered slightly toxic to fish by EPA standards in the Microtox assay. The lack of adverse effects in the mammalian acute and subacute tests indicated that 5-AT has low to no toxicity in rats over a short exposure time frame at the exposure levels tested. The toxicity data reported here pertain only to 5-AT, as combustion and decomposition products of 5-AT were not evaluated. 5-AT is being considered as a replacement for perchlorate, a very water soluble compound known to disrupt the thyroid system by competing with iodide at the sodium iodide symporter (Fisher et al., 2012; Liu et al., 2008; York et al., 2001). 5-AT did not inhibit the uptake of iodide up to 100 μM 5-AT; the highest concentration tested. In the NIS assay, perchlorate serves as the positive control for the NIS (IC50=0.154 μM). In a 14-day rat oral toxicity study, the NOAEL for ammonium perchlorate (NH4ClO4) was 3.66 mg/kg-day based on thyroid-to-body weight ratio {Eck, 2015 #26}. Subchronic rat oral toxicity tests of NH4ClO4 in drinking water have identified the NOAEL as 1.0 mg/kg-day based on changes in thyroid stimulating hormone (TSH) (Siglin et al., 2000). In this study, male and female rats were provided perchlorate in drinking water (0, 0.01, 0.05, 0.2, 1.0, and 10 mg/kg-day). The study design included sampling thyroid functional biomarkers and collecting thyroid weights after 14, 90 and 120 days (90 days+30 day washout) treatment. A reduction in triiodothyronine (T3) was the most sensitive endpoint in male rats and a statistically significant dose dependent decrease in serum T3 was observed starting at the 0.01 mg/kg-day dose group. A statistically significant increase in thyroid weights was measured in the highest male dose group (10 mg/kg-day) at 14 days. In contrast, thyroid weights and biomarkers in perchlorate treated female rats were not significantly different from the control female rats at 14 days. At the 90-day sampling, males and females both had statistically significant differences in thyroid weights and biomarkers (TSH, T4 and T3). No other organ weights were significantly different from control animals. The changes in thyroid weight at 10 mg/kg-day in perchlorate treated rats compared to no organ weight changes at 623 mg 5-AT/kg-day coupled with no 5-AT inhibition of the NIS suggests 5-AT is a less toxic compound than perchlorate. The tiered assessment strategy for 5-AT is designed to prevent a “regrettable substitution” wherein a chemical with no toxicity information is used to replace a chemical with known toxicity. For this interim assessment, 5-AT should be considered a viable replacement for perchlorate as the concerns of perchlorate (solubility and thyroid toxicity) are not evident in 5-AT. Further testing of 5-AT in chronic tests, to include evaluating thyroid and reproduction/development endpoints, is necessary for a full evaluation of 5-AT.
Supplementary Material
SD-Table 1. Male and female body weights during the 14 day study.
SD-Table 2. 14-day study male absolute organ weights.
SD-Table 3. 14-day study female absolute organ weights. The number of animals equals six (N=6) unless otherwise indicated.
SD-Table 4. 14-day study male organ weights normalized to brain weight.
SD-Table 5. 14-day study female organ weights normalized to brain weight. The number of animals equals six (N=6) unless otherwise indicated.
SD-Table 6. 14-day 5-AT subacute study male and female summary hematology data.
SD-Table 7. 14-day 5-AT subacute study male and female summary clinical chemistry data.
SD-Table 8. 2-way ANOVA of 14-day 5-AT study male (M) and female (F) hematology and clinical chemistry data for gender and treatment effects; no treatment effects identified.
Acknowledgements
The authors would like to acknowledge the support and encouragement provided to this effort by Mr. Erik Hangeland, director (emeritus) of the U.S. Army Research, Development and Engineering Command, Environmental Acquisition and Logistics Sustainment Program. We also thank Dr. John LaScala of the Environmental Quality Technology Program, Pollution Prevention Team. This work was funded by U.S. Army Environmental Quality Technology Program, Pollution Prevention, Ordnance Environmental Program. Military Interdepartmental Purchase Request (MIPR) No. 10322305. Several APHC personnel contributed to the completion of this study through care of the animals, participation in animal dosing, data collection and necropsy; these include Rebecca Kilby, Robert Sunderland, Dr. Larry Williams, Dr. Wil McCain, Dr. Emily Lent, Alicia Shiflett, Allison Narizzano, Lee Crouse, Terry Hanna, Martha Thompson, and Mark Way.
Funding: U.S. Army Environmental Quality Technology Program, Pollution Prevention, Ordnance Environmental Program. Military Interdepartmental Purchase Request (MIPR) No. 10322305. The work was conducted as part of the authors’ normal course of employment. The funding source did not contribute to the study design, collection, analysis, or interpretation of the data. The funding source did not participate in writing or publishing the manuscript.
Footnotes
Disclaimer: The views, opinions, and/or findings contained in this report are those of the author(s) and should not be construed as official Department of the Army position, policy, or decision, unless so designated by other official documentation. Citations of commercial organizations or trade names in this report do not constitute an official Department of the Army endorsement or approval of the products or services of these organizations.
References
- AERTA, Army Environmental Requirements and Technology Assessments: Requirement PP-3–02-05. Compliant Ordnance Lifecycle for Readiness of the Transformation and Objective Forces. U.S. Army Environmental Command, Aberdeen Proving Ground, MD, 2012. [Google Scholar]
- American Society for Testing and Materials (ASTM), Standard Guide for Assessing the Environmental and Human Heath Impacts of New Energetic Materials. Water and Environmental Technology, Biological Effects and Environmental Fate, Biotechnology. ASTM International, Conshohocken, PA, USA, 2008. [Google Scholar]
- AR 40–5, Preventive Medicine, 25 May 2007.
- AR 70–1, Army Aquisition Policy, 31 December 2003.
- AR 200–1, Environmental Protection and Enhancement, 31 December 2007.
- ASTM, E1163–10: Standard Test Method for Estimating Acute Oral Toxicity in Rats. ASTM International, West Conshohocken, PA USA, 2010. [Google Scholar]
- CIDPL, ChemIDplus Lite. U.S. National Library of Medicine, Bethesda, MD, 2013. [Google Scholar]
- Claxton LD, et al. , 2010. The Salmonella mutagenicity assay: the stethoscope of genetic toxicology for the 21st century. Environmental Health Perspectives. 118, 1515–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DoDI, Department of Defense Instruction 4715.23, Integrated Recycling and Solid Waste Management. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/471523.pdf?ver=2018-11-13-140624-277, 2018.
- Feder PI, et al. , 1991. Stagewise, group sequential experimental designs for quantal responses. one-sample and two-sample comparisons. Neurosci Biobehav Rev. 15, 129–33. [DOI] [PubMed] [Google Scholar]
- Fisher J, et al. , 2012. Extrapolation of hypothalamic-pituitary-thyroid axis perturbations and associated toxicity in rodents to humans: case study with perchlorate. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 30, 81–105. [DOI] [PubMed] [Google Scholar]
- Hallinger DR, et al. , 2017. Development of a screening approach to detect thyroid disrupting chemicals that inhibit the human sodium iodide symporter (NIS). Toxicol In Vitro. 40, 66–78. [DOI] [PubMed] [Google Scholar]
- Liu F, et al. , 2008. Arsenate and perchlorate toxicity, growth effects, and thyroid histopathology in hypothyroid zebrafish Danio rerio. Chemosphere. 71, 1369–76. [DOI] [PubMed] [Google Scholar]
- Lund GK, Blau RJ, Anhydrous 5-aminotetrazole gas generant compositions and methods of preparation. In: USPTO, (Ed.). Thiokol Corporation, 1996. [Google Scholar]
- OECD, 2002. Test No. 420: Acute Oral Toxicity - Fixed Dose Procedure. [Google Scholar]
- Organization for Economic Cooperation and Development (OECD), Guidance document on the use of the harmonised system for the classification of chemicals which are hazardous for the aquatic environment. In: EN/JM/MONO(2001)8, (Ed.), 2001. [Google Scholar]
- Sabatini JJ, Moretti JD, 2013. High-nitrogen-based pyrotechnics: perchlorate-free red- and green-light illuminants based on 5-aminotetrazole. Chemistry. 19, 12839–45. [DOI] [PubMed] [Google Scholar]
- Siglin JC, et al. , 2000. A 90-Day Drinking Water Toxicity Study in Rats of the Environmental Contaminant Ammonium Perchlorate. Toxicological Sciences. 57, 61–74. [DOI] [PubMed] [Google Scholar]
- UNCED, The Globally Harmonized System of Classification and Labelling of Chemicals (GHS). United Nations Conference on Environment and Development, 2005. [Google Scholar]
- USEPA, Technical Overview of Ecological Risk Assessment, Analysis phase: Ecological effects characterization, ecotoxicity categories for terrestrial and aquatic organisms. Office of Prevention, Pesticides, and Toxic Substances, U.S. Environmental Protection Agency, 2017. [Google Scholar]
- Williams LR, et al. , 2012. Toxicologic characterization of a novel explosive, guanidinium 3,4-dinitropyrazolate (GDNP), in female rats and Ames mutagenicity assay. Int J Toxicol. 31, 441–53. [DOI] [PubMed] [Google Scholar]
- York RG, et al. , 2001. Two-generation reproduction study of ammonium perchlorate in drinking water in rats evaluates thyroid toxicity. Int J Toxicol. 20, 183–97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SD-Table 1. Male and female body weights during the 14 day study.
SD-Table 2. 14-day study male absolute organ weights.
SD-Table 3. 14-day study female absolute organ weights. The number of animals equals six (N=6) unless otherwise indicated.
SD-Table 4. 14-day study male organ weights normalized to brain weight.
SD-Table 5. 14-day study female organ weights normalized to brain weight. The number of animals equals six (N=6) unless otherwise indicated.
SD-Table 6. 14-day 5-AT subacute study male and female summary hematology data.
SD-Table 7. 14-day 5-AT subacute study male and female summary clinical chemistry data.
SD-Table 8. 2-way ANOVA of 14-day 5-AT study male (M) and female (F) hematology and clinical chemistry data for gender and treatment effects; no treatment effects identified.