Abstract
The present study examined the effects of aging and CSF biomarkers of Alzheimer’s disease (AD) on the ability to control production of unexpected words in connected speech elicited by reading aloud. Fifty-two cognitively healthy participants aged 66–86 read aloud 6 paragraphs with 10 malapropisms including 5 on content words (e.g., “window cartons” which elicited autocorrect errors to “window curtains”), and 5 on function words (e.g., “thus concept” which elicited autocorrections to “this concept”), and completed a battery of neuropsychological tests including a standardized Stroop task. Reading aloud elicited more autocorrect errors on function than content words, but these were equally correlated with age and Aβ1–42 levels. The ability to stop autocorrect errors declined in aging, and with lower (more AD-like) levels of Aβ1–42, and multiplicatively so, such that autocorrect errors were highest in the oldest-old with the lowest Aβ1–42 levels. Critically, aging effects were significant even when controlling statistically for Aβ1–42. Finally, both autocorrect and Stroop errors were correlated with Aβ1–42, but only autocorrect errors captured unique variance in predicting Aβ1–42 levels. Reading aloud requires simultaneous planning and monitoring of upcoming speech. These results suggest that healthy aging leads to decline in the ability to intermittently monitor for and detect conflict during speech planning, and that subtle cognitive changes in preclinical AD magnify this aging deficit.
Keywords: monitoring, reading aloud, aging, amyloid, rare conflict
Normal aging involves decline in some cognitive abilities including, most notably, processing speed and explicit memory (for reviews see Salthouse, 2010; 2012; Lezak, 2012), while other aspects of cognition improve with age (e.g., vocabulary knowledge; Keuleers, Stevens, Mandera, & Brysbaert, 2015; Salthouse, 2009; Verhaeghen, 2003). Language processing is thought to be relatively less affected by aging than other cognitive abilities, but identifying such changes (Kavé & Goral, 2017), and indeed aging effects on cognition in general, is challenging. Age-related changes vary across seemingly quite related language tasks in ways that are not fully understood. For example, a wealth of studies consistently show that older adults have difficulty retrieving proper names (subjective complaints of difficulty with proper name retrieval are practically a card-carrying feature of both healthy aging and dementia; Burke, MacKay, Worthley, & Wade, 1991). By contrast, aging-related changes in object-name retrieval are subtle, and on some measures (e.g., tip-of-the-tongue states, TOTs), appear not to reflect cognitive decline, but rather increased vocabulary knowledge (which in turn leads to increased ability to at least partially retrieve very difficult target words, i.e., have TOTs; Dahlgren, 1998; Gollan & Brown, 2006; but see Shafto, Burke, Stanatakis, Tam & Tyler, 2007; Huijbers et al., 2017).
Another challenge for identifying aging-related language processing deficits is that older adults may approach the same task with different priorities, perspective and strategies from those of younger participants (Wingfield & Grossman, 2006), and bring many more years of experience to any given task (Ramscar, Hendrix, Shaoul, Milin, & Baayen, 2014; Stanovich, West, & Harrison, 1995). This experience could lead to greater automaticity, a factor that might affect some of the most widely studied psycholinguistic effects. For example, it is well known that expected sentence completions are processed more quickly than unexpected completions (e.g., Rayner, Slattery, Drieghe, & Liversedge, 2011); in some studies, older adults have produced larger predictability effects than young adults (e.g., in word skipping, Rayner, Reichle, Stroud, Williams, & Pollatsek, 2006; or gaze durations and regression-path times in silent reading Zhao et al., 2019). Such effects could reflect greater reliance on context (Steen-Baker et al., 2017) or greater predictive processing (Choi, Lowder, Ferreira, Swaab, & Henderson, 2017) with aging (increased difficulty recovering from unexpected completions is also possible; see discussion in Zhao et al., 2019). Other results, however, suggest reduced predictive processing with aging. For example, older participants exhibited smaller transitional probability effects than younger participants on spoken word durations in reading aloud (in cross-sectional comparisons in Moers, Meyer, & Janse, 2017; although in the same study young-old and older-old exhibited equal effects), and appeared to use context relatively less than younger participants to predict upcoming words in ERP measures of silent reading (Federmeier & Kutas, 2005; Federmeier, Kutas, & Schul, 2010).
A third challenge in identifying aging-related decline in language is that some aspects of linguistic processing may rely on nonlinguistic processes that decline in aging. For example, speaking requires message formulation, lexical and sound selection, planning of syntactic structures, and monitoring to prevent planned speech errors before they are produced overtly (Levelt, 1989; Levelt, Roelofs, & Meyer, 1999). Monitoring requires detection of conflict between simultaneously active alternative candidate representations, and selection of intended words over their competitors (Nozari, Dell, & Schwartz, 2011; Nozari & Novick, 2017). These processes may rely on domain-general mechanisms of conflict resolution that involve brain regions important for resolving nonlinguistic conflict (e.g., Gauvin, De Baene, Brass, & Hartsuiker, 2016) and that decline with aging (West, 1996; Raz, 2008; Reuter-Lorenz & Park, 2010). On this view, production of speech errors might be expected to increase with aging given that executive control declines with aging (Hasher & Zacks, 1988; Mayr, Spieler, & Kliegl, 2001, but see Verhaeghen, 2011). Supporting this prediction, one recent study showed that older adults produced more speech errors than young adults, especially function word substitution errors, implicating aging-related deficits in attention-demanding monitoring (Gollan & Goldrick, 2019).
A fourth challenge that makes it difficult to identify the consequences of normal aging on language processing is that it is often not feasible in many research studies (due to limited time and resources) to separate normal aging effects from those due to neurodegeneration and other pathological changes of Alzheimer’s disease (AD). Pathology associated with AD can accumulate in the brain long before frank symptoms of dementia are apparent. Brain autopsy studies reveal substantial AD pathology in a nontrivial number of aging individuals who did not seem to exhibit cognitive decline before death (DeTure & Dickson, 2019; Montine et al., 2012; Rodrigue, Kennedy, & Park, 2009). As a consequence, the very definition of “healthy aging” is a matter of some debate, and some of the effects attributed to aging might instead reflect the cognitive consequences of unidentified pre-clinical AD (Weintraub, Wickland, & Salmon, 2012). In recent years, considerable research has been devoted to characterizing this prodromal or pre-clinical phase of AD in relation to the presence of biomarkers that can be detected with imaging or in cerebrospinal fluid (CSF) long before the onset of cognitive decline. PET imaging studies have shown accumulation of Amyloid β (Aβ) 12–42 (the constituent protein of AD neuritic plaques), and Tau (the constituent protein of neurofibrillary tangles) in cognitively normal individuals who go on to develop AD dementia (Perrin et al. 2009; Sperling et al. 2009; Jack et al. 2010). Studies of CSF show high levels of Tau (both phosphorylated and total Tau) and low levels of Aβ1–42 (indicative of an accumulation of amyloid deposition in the brain and lack of clearance into CSF) beginning in pre-clinical AD (Galasko et al., 1998). With these biomarkers, it is now possible to identify older individuals with or without pre-clinical or prodromal AD and differentiate effects of disease from effects of aging on language processing (Ahmed et al., 2013; Venneri et al., 2018) and other aspects of cognition (e.g., Farrell et al., 2017; Rodriguez et al., 2009; 2012; McDonough & Popp, 2020) from effects of aging.
Some of the most striking evidence of cognitive decline years before AD dementia onset involved detailed linguistic analysis of spontaneous speech and written texts. The first study of this kind examined autobiographical essays written by nuns upon entry to the convent decades before they developed dementia, and found reduced idea density and grammatical complexity (Snowdon et al., 1996; Riley et al., 2005). Similar lexical and syntactic changes in written texts were found in case studies of British writers when comparing novels they wrote many years before developing dementia to those they wrote closer to the time of diagnosis (Le et al., 2011). A more recent case study suggested that similar effects may be found in spontaneous speech by comparing non-scripted news conferences delivered by Ronald Reagan (who was diagnosed with AD 6 years after he left office) to George H.W. Bush (who remained cognitively healthy for 23 years after he left office before dying at the age of 94). This study revealed a reduction in the number of unique words produced over time, and an increase in conversational fillers and non-specific nouns over time for Reagan but not for Bush (Berisha, Wang, LaCross, & Liss, 2015).
A question that arises given this evidence is if it might be possible to design language tasks that could reveal similar processes in individual cognitive assessments at a single point in time (perhaps still involving complex forms of language but without requiring detailed linguistic analysis, and without availability of data from years before disease onset). Two recent studies demonstrate some promise along these lines using reading aloud of full paragraphs to elicit connected speech in cognitively healthy aging bilinguals vs. in bilinguals with AD. Participants in these studies read aloud paragraphs written mostly in one language but with some switches into their other language; bilinguals with AD were more likely than controls to spontaneously translate language-switched words to avoid switching languages in their speech (Gollan, Stasenko, Li, & Salmon, 2017; Gollan, Li, Stasenko, Salmon, 2020).
In the present study we attempted to adapt the reading-aloud task to examine aging effects on speech production in cognitively normal older monolinguals in relation to biomarkers of AD to distinguish effects of normal aging from pre-clinical AD. To elicit errors, participants read aloud short paragraphs in which a small number of words were replaced with incorrectly written words or malapropisms, i.e., an incorrect word that is similar in sound to the intended word, often resulting in a nonsensical and unusual utterance. For example, Every day in the early morning hours the sunlight would barge through the window carton, waking Doug up. Participants were instructed to read aloud and to avoid correcting the incorrectly written words (e.g., to avoid saying window curtains instead of window cartons). Of interest was a) whether the task would elicit automatic correction of the malapropisms, b) if autocorrection would increase with aging, and c) if the aging-related increase in autocorrect errors would be modulated by evidence of preclinical AD determined by CSF biomarkers. Aging might be expected to increase autocorrection errors exclusively because of greater accumulated life-time language experience that leads to stronger activation of expected completions, a normal consequence of healthy aging. However, if autocorrect errors also increase in association with an increased accumulation of amyloid or Tau in the brain, it might suggest that decline in monitoring and executive control processes needed to stop autocorrection are affected by healthy aging and magnified by unidentified pre-clinical AD. In previous studies of reading aloud in bilinguals with AD (Gollan et al., 2017;2020), and healthy aging monolinguals reading paragraphs without malapropisms (Gollan & Goldrick, 2019), function words elicited more errors than content words, a result we attributed to the difficulty of monitoring function words (Staub, Dodge, & Cohen, 2019). Thus, we hypothesized that errors on function word targets (e.g., saying “with” instead of “within” in “She’d try to be careful but she’d always end up within food on her clothes.”) might be more sensitive to aging and/or AD-related CSF biomarker levels than content word errors (e.g., saying “evolve” instead of “involve” in “As this technology continues to involve and I continue to get older…”).
To obtain further insight as to the mechanisms possibly underlying autocorrect errors, we examined relationships with errors produced in the Color Word Interference Test (CWIT; a subtest of the D-KEFS Delis-Kaplan Executive Function System; Delis, Kaplan, & Kramer, 2001), a standardized version of the classic Stroop task. Of particular interest were errors on the CWIT Inhibition subtest (in which participants are shown a display with 50 color words all printed in incongruent ink color, and are asked to name the ink color; e.g., say “red” in response to the word green printed in red ink), and the Inhibition-Switching subtest (which is like the Inhibition subtest but participants must switch to word-reading on a random half of responses as indicated by a box surrounding the word). Though the autocorrect task requires participants to read-aloud while the Stroop requires them to avoid reading aloud, both tasks require stopping an automatic tendency (to produce expected completions in the read-aloud task, or to read written words in the CWIT) to instead complete a less automatic process (to produce malapropisms, or name ink-color). Previous studies demonstrated sensitivity of Stroop errors to AD, pre-clinical AD, and Aβ1–42 levels using a trial-by-trial computer-administered version of the Stroop task (Balota et al., 2010; Bélanger, Belleville, & Gauthier, 2010; Ducheck et al., 2013; Hutchison, Balota, & Duchek, 2010). For comparison we also examined performance on measures of global cognitive function (Mattis Dementia Rating Scale; DRS) and verbal list learning (California Verbal Learning Test II) that are sensitive to early AD (Lange et al., 2002; Monsch et al., 1995; Woods, Delis, Scott, Kramer, & Holdnack, 2006).
Method
Participants
Participants were individuals classified as cognitively normal (English-speaking) monolinguals who were healthy control participants from the UCSD Shiley-Marcos Alzheimer’s Disease Research Center (ADRC) longitudinal study. Participants in the study are evaluated each year with detailed clinical and medical history, medical, neurological and neuropsychological assessments, and laboratory tests (completed under IRB-approved protocols as previously described; Galasko et al., 1994). Table 1 shows demographics for 52 participants who were included in the analyses reported below (these participants met our inclusion criteria of being classified as cognitively normal and having biomarker data available). A power analysis showed that 46 participants would be sufficient to detect a correlation of r = 0.4 vs (0), with an alpha of 0.05 and power of 0.8. One participant was missing APOE e4 data, and another participant was missing CWIT data.
Table 1:
Participant demographics and test scores
| Variable | n | % | |
|---|---|---|---|
| Number of participants | 52 | ||
| Female | 31 | 60 | |
| 0 APOE ε4 alleles | 35 | 69 | |
| 1 APOE ε4 allele | 16 | 31 | |
| 2 APOE ε4 alleles | 0 | 0 | |
| M | SD | Range | |
| Age | 75.6 | 4.8 | 66.6–86.2 |
| Years of Education | 16.5 | 2.3 | 12–20 |
| Mini-Mental State Exam (/30) | 29.3 | 1.1 | 25–30 |
| Dementia Rating Scale Total (/144) | 140.0 | 2.6 | 134–144 |
| CVLT Immediate Recall Trials 1–5 (/75) | 49.4 | 10.5 | 25–68 |
| CWIT Inhibition Response Time (ms) | 62.5 | 13.6 | 41–108 |
| CWIT Inhibition Switching Response Time (ms) | 67.4 | 17.7 | 42–133 |
| CWIT Inhibition Errors (/50) | 0.3 | 0.9 | 0–5 |
| CWIT Inhibition Switching Errors (/50) | 0.6 | 0.8 | 0–5 |
| Total Errors (/60) | 9.4 | 5.9 | 1–29 |
| Content Errors (/30) | 3.0 | 2.5 | 0–12 |
| Function Errors (/30) | 6.3 | 3.8 | 1–17 |
| Paragraph Reading Time (s) | 78.2 | 12.5 | 57.6–117.97 |
M = Mean, SD = Standard Deviation, APOE = Apolipoprotein E, CVLT = California Verbal Learning Test, CWIT = Color Word Interference Test
Materials
Participants read aloud 6 paragraphs (180 words on average, SD=17), each with 5 malapropism substitutions (i.e., target words) on function words and 5 substitutions on content words. Content targets tended to preserve part of speech between target and its contextually appropriate pair (e.g., nodded/needed, arch/ache, figure/ finger, valley/value, cram/cream, stooped/stopped), while function targets did not (e.g., this/his, or/our, had/and, around/about, wherever/whenever, what/with). The full set of paragraphs along with information on measure reliability is presented in the Supplemental Materials (including the correlation between errors produced in each individual paragraph with the average number of errors produced across all paragraphs). The paragraph that elicited error rates most highly correlated to average error rates across the full set of paragraphs is shown as an example below. Target words are underlined for illustration purposes (words were not underlined in materials shown to participants; font and spacing were also different). Each paragraph was presented in its entirety on an individual 9.5 × 11” paper with 0.8” margins, in black ink, and Times New Roman font size 18. Though we did not have information on test-retest reliability, Cronbach’s Alpha calculated on error rates with 2 sets of 3 paragraphs using a split-half approach (randomly selected; α=.784), and with each of the 6 paragraphs treated as a separate item (α=.780) were comparable and in the Acceptable to Good range (for these calculations we included all participants tested on the read-aloud task at the ADRC, including a small number of patients with MCI or AD, and many controls who did not have CSF data available for analysis, n=134).
It was in elementary school that I came into contact with my first computer. It was an Apple computer then was introduced as a tool to play games, and taught math, writing, rearing, geography and history. I remember sitting there amazed at this box what engaged with me in a way I had never expired before. It was so much fun! This machine has evolved exponentially since then, now merging with web and phone technologies. This has allowed us to have more information at the touch of a figure than we’ve even experienced. The world has never felt smaller now that we can learn first-hand about all parts of the whirl through information, pictures, videos, maps, individual personal accounts, had stories. As this technology continues to involve and I continue to get older, however, I can tell it is getting harder to keep up with there latest trends. It’s all moving so quickly.
(Computer paragraph, r=.784 with total errors)
Standard protocols were followed for assessment of the AD biomarker including preanalytical preparation and storage of CSF and plasma (Vanderstichele et al., 2012; Xiao et al., 2017). In brief, CSF (20–30 mL) was collected by lumbar puncture in the early morning after overnight fasting. Samples were processed, aliquoted into 500 μL fractions in polypropylene microtubes, snap frozen and stored at −80°C until assayed. Levels of Aβ1–42 and total Tau in CSF were analyzed using ELISA assays (ADx, Ghent, Belgium; (Sutphen et al., 2015)). CSF samples were run in duplicate, blind to diagnosis, in randomized order on each ELISA plate.
Design and Procedure
Participants completed the read-aloud task at the end of a comprehensive neuropsychological test battery (which included the DRS, the CVLT, and the standardized Stroop/CWIT subtests and several other behavioral tests). Instructions were to read aloud as accurately as possible at a comfortable pace, and participants were warned they might notice some words were “not written correctly” but to try to read them exactly as they were written. Approximately one third of participants commented on the malapropisms and were given an additional prompt: “Try to read exactly what you see written without reacting to those.” If participants laughed while reading the psychometrist responded with “Try to stop laughing as soon as you can and keep reading.” Autocorrect errors were marked on a coding sheet during testing and speech was recorded for later verification. Partially produced errors were not counted, but fully produced and then self-corrected errors were included.
Results:
Table 2 shows exploratory correlations between total errors (and paragraph reading times) and predictors of potential interest including age, Aβ1–42, Tau, and other demographic and neuropsychological test data. Autocorrect errors were positively correlated with age (r = .34, p=.01), and negatively correlated with CSF Aβ1–42 levels (r=−.36, p=.008). In other words, those with lower (more AD-like) Aβ1–42 concentrations produced more autocorrect errors. Similar analysis of CSF Tau revealed no significant relationships with behavioral measures. Target function words elicited significantly more speech errors than target content words (t(51)=9.15, p<.001; see Table 1, but age (rs = −.32 and −.31, ps≤.03) and Aβ1–42 (rs = −.38 and −.36, ps≤.01) were similarly correlated with the two error types. The only other variable that was significantly correlated with Aβ1–42 was errors produced on the Inhibition subtest of the CWIT (r = −.28 p = .045).
Table 2:
Pearson bivariate correlations between demographic, biomarker, and behavioral test scores (n=52).
| Variable | Total Autocorrect Errors | Paragraph Reading Time | Age | Aβ1–42 | Tau | Education | DRS | CVLT Trials 1–5 | CWIT-I Inhibition RT | CWIT Inhibition Switching RT |
|---|---|---|---|---|---|---|---|---|---|---|
| Total Errors | 1 | |||||||||
| Reading Time | 0.36** | 1 | ||||||||
| Age | 0.34* | 0.15 | 1 | |||||||
| Aβ1–42a | −0.36** | −0.02 | −0.25 | 1 | ||||||
| Tau | 0.06 | −0.12 | 0.19 | −0.04 | 1 | |||||
| Education | 0.02 | −0.10 | −0.14 | −0.04 | 0.13 | 1 | ||||
| DRSa | −0.28* | −0.10 | −0.05 | 0.02 | 0.16 | −0.10 | 1 | |||
| CVLTa Trials 1–5 | −0.30* | 0.00 | 0.08 | 0.12 | 0.26 | −0.16 | 0.45*** | 1 | ||
| CWITa -Inhibition RT | 0.47*** | 0.42** | 0.27 | −0.10 | 0.11 | 0.14 | −0.24 | −0.35* | 1 | |
| CWITa -Inhibition Switching RT | 0.47*** | 0.40** | 0.26 | −0.21 | 0.02 | −0.03 | −0.39** | −0.34* | 0.63*** | 1 |
| CWITa -Inhibition Errors | 0.30* | 0.01 | 0.17 | −0.28* | 0.21 | 0.12 | −0.18 | −0.05 | 0.22 | 0.02 |
| CWITa -Inhibition Switching Errors | −0.08 | −0.06 | −0.08 | −0.14 | −0.06 | 0.11 | −0.13 | −0.15 | −0.05 | 0.09 |
p < .05.
p < .01.
p < .001
Aβ1–42 = Amyloid β, DRS = Dementia Rating Scale, CVLT = California Verbal Learning Test, CWIT = Color Word Interference Test, RT = Response Time
An item-level logistic mixed effects model was used to assess the joint effects of age, Aβ1–42, and their interaction for predicting autocorrect errors after controlling for sex, and education; the results of this analysis are shown in Figure 1. The model (Table 3) revealed significant effects of age (β=−0.05± 0.02; p=.03), Aβ1–42 (β =−3.56± 1.55; p=.02), and their interaction (β =0.05± 0.02; p=.01), in addition to male gender (β =−0.46± 0.21; p=.03). Older participants with more AD-like levels of Aβ1–42, and especially individuals with both risk factors, produced the most errors. In addition, men produced more errors than women, and including the presence of an APOE e4 allele (a known genetic risk factor for AD) in the model as a covariate did not alter the pattern of significance (though the allele effect was significant in the opposite of the expected direction). These effects are shown Supplemental Materials which also show the results of an additional model which replaced age with CWIT Inhibition subtest RTs as a predictor (this variable was most highly correlated with autocorrect errors in Table 2). The results of this model revealed significant effects of CWIT RT (β =−0.03± 0.01; p=.001) and male gender (β =−0.46± 0.20; p=.02), but no significant effect of Aβ1–42 (β =−0.11± 0.62; p=.86), and the interaction between CWIT and Aβ1–42 (β =0.01± 0.01; p=.44) was also not significant. Thus, age could not simply be replaced by a measure of executive control to obtain the same result. The relationship between age and autocorrect errors, and the modulation of the aging effect by the AD biomarker, was a unique interaction between these two predictors.
Figure 1.
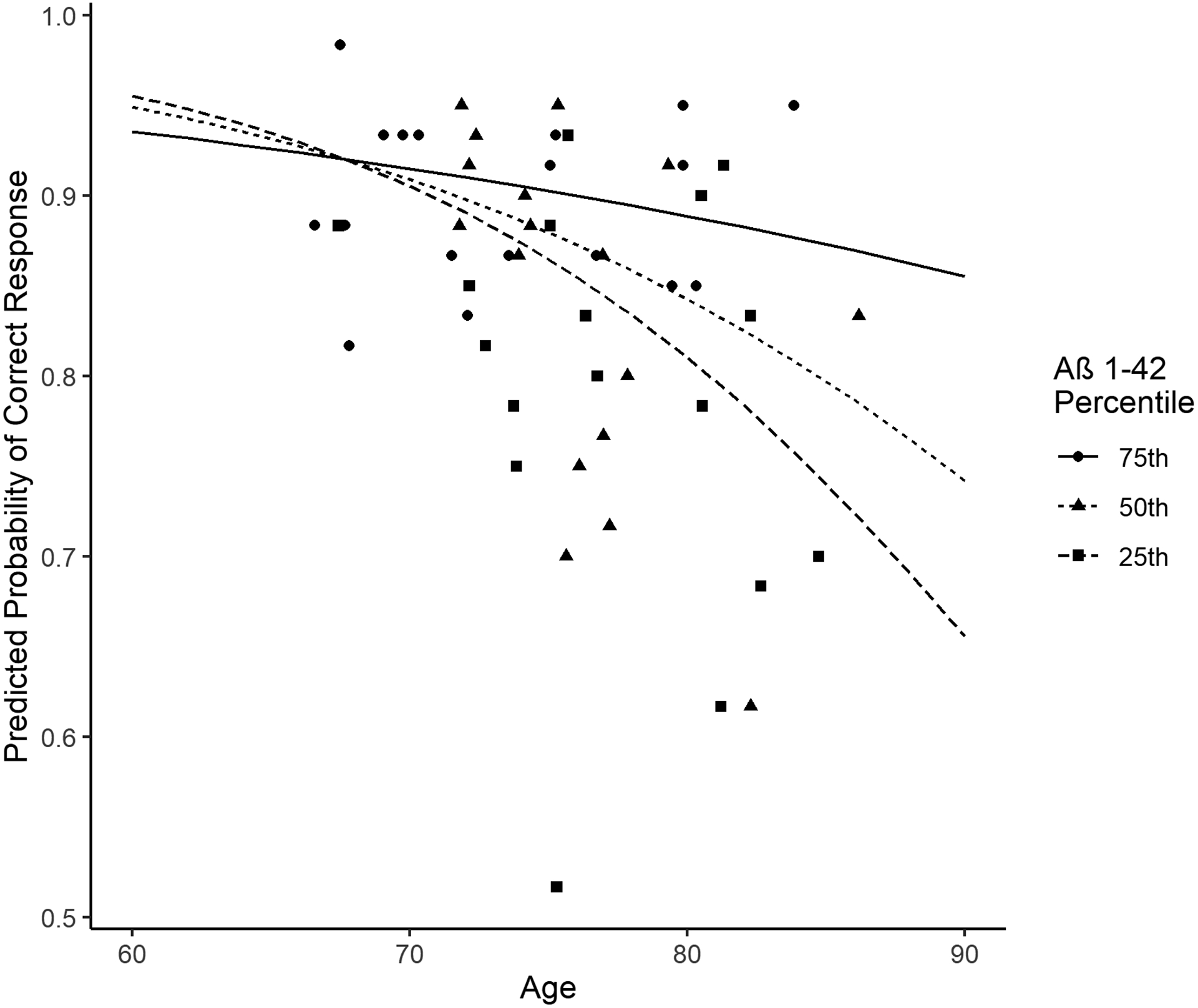
Aβ1–42 modifies the relationship between age and autocorrect errors. Predictions of the probability of the correct response are plotted for the 25th, 50th, and 75th percentile of Aβ1–42 values. Individual points represent participants within each tertile, the lines represent the median for each tertile.
Table 3.
The fixed effects of the item-level mixed-effects logistic regression for the target words in the Autocorrect task. Random effects of participant and word were included. Amyloid levels were z-transformed.
| term | Beta | Standard Error | z statistic | p value |
|---|---|---|---|---|
| (Intercept) | 6.66 | 2.06 | 3.24 | 0.001 |
| AB1–42 (z-score) | −3.56 | 1.56 | −2.29 | 0.02 |
| Age | −0.05 | 0.02 | −2.21 | 0.03 |
| Sex=Male | −0.46 | 0.21 | −2.18 | 0.03 |
| Education | −0.02 | 0.05 | −0.52 | 0.61 |
| AB1–42:age | 0.05 | 0.02 | 2.50 | 0.01 |
Having found significant correlations between Aβ1–42 and two behavioral measures (i.e., autocorrect errors and CWIT inhibition errors), we conducted a linear regression to determine which measure best predicts Aβ1–42 after controlling for demographic variables (i.e., age, education, sex, and the presence of an APOE e4 allele) since this could have implications for screening for AD before measuring biomarkers, and diagnosis of AD in settings where biomarker data are not available. This model demonstrated significant relationships between Aβ1–42 levels and speech errors (summing function and content errors, β=−0.06± 0.03; p=.03) and presence of an APOE e4 allele (β =−.626± .277; p=.03), but not between Aβ1–42 levels and the CWIT inhibition measure (p = .24).
For all participants CSF was drawn before behavioral testing; the average interval between CSF data collection and behavioral testing was 3.4 years (±1.9 SD; range 0–7 years). The results remained unchanged if the participant pool was limited to those whose CSF collection was within 3.5 years of testing (n = 30; for whom the average interval was 1.9±0.9 years, range 0–3.5 years). It should be noted that CSF biomarkers change slowly in cognitively normal individuals; e.g., there was no significant change in serial CSF samples obtained at 2 year intervals in an aging study by Lleó et al., (2019) subjects were clinically normal at the time of CSF collection and cognitive testing.
Discussion
Reading aloud paragraphs with a small number of words that were replaced with words similar in form to the expected targets (malapropisms) elicited autocorrect errors at a higher rate in older-old than in younger-old participants, and at a higher rate in participants with lower (more AD-like) levels of CSF Aβ1–42 (see Table 2). However, these relationships were qualified by an interaction which revealed a multiplicatively higher rate of autocorrect errors in the oldest-old participants who also had more AD-like (i.e., low) Aβ1–42 levels (see Figure 1). Though function words elicited significantly higher error rates than content words, these were about equally correlated with age and Aβ1–42 (see Tables 1 and 2). Importantly, the autocorrect task elicited a few handfuls of errors in each participant (see Table 1), even though the vast majority of words, including hundreds of nontarget words and most target words (M=84.4%, SD=9.8% of the 60 targets), were produced without error. Finally, we did not focus on spontaneous self-corrections (these were not significantly correlated with age, or Aβ1–42; rs≤.17), but participants did self-correct a non-trivial number of the errors they produced (M=22.3%, SD=20.0). Thus, participants had a clear understanding of the task.
The aging effects we observed on autocorrect errors replicate and extend findings we reported in another reading-aloud study in which, after correcting for the aging-related increase in vocabulary knowledge, older adults produced more speech errors and fewer self-corrections than young adults (Gollan & Goldrick, 2019). Thus, aging appears to increase production of errors in reading aloud both in comparison to younger participants and when looking at age as a continuous variable within older adults. The fact that the main effect of age remained significant in the present study even after controlling for Aβ1–42 levels (and the interaction between age and Aβ1–42; see Table 3), suggests that aging per se, not the effects of pre-clinical AD, leads to changes in linguistic processing needed to support reading aloud.
Additional work will be needed to determine if aging effects found in these two read-aloud studies reflect the same underlying cognitive mechanism. While reading aloud obviously requires reading, and typically semantic processing, it very likely also engages mechanisms of speech production (e.g., Gollan & Goldrick, 2018; Moers, Meyer, & Janse, 2017; see also Macdonald & Thornton, 2009). Though it can be difficult to determine if aging effects reflect cognitive decline or the benefits of increased experience (or both), the results of the present study seem incompatible with studies that found reduced predictive processing in aging, and more in line with findings that aging leads to greater reliance on context or increased predictive processing, because if older adults predicted the expected completions less than young adults, then they should have produced fewer not more autocorrect errors. However, declines in monitoring processes could also explain the aging-related increase in errors (either assuming no change in reliance on context and prediction in aging, or assuming that monitoring deficits are powerful enough to offset any such changes). Distinguishing between these alternatives would require independent measures of reliance on context and prediction.
The proposal that monitoring processes are critical for preventing errors in reading aloud fits with two aspects of our other recent study (Gollan & Goldrick, 2019) in which older adults spontaneously self-corrected their errors less often than young adults, and function words, that are particularly difficult to monitor (Schotter, Li, & Gollan, 2019; Staub et al., 2019), elicited the strongest aging effects. It should be noted, however, that in the present study, function word targets elicited more errors than content word targets, but function and content word errors were equally correlated with aging (and with Aβ1–42 levels). Additionally, older adults are able to compensate for aging-related decline in conditions that elicit heightened attention to planning of speech (e.g., Gollan & Goldrick, 2019), and in related work with bilinguals reading aloud mixed-language paragraphs, bilinguals produced more errors with language switches on function rather than content words, but function words did not elicit especially strong aging effects (Gollan & Goldrick, 2016). Thus, what might be most critical for observing aging effects, is to effectively “sneak up on the system” so that what is impaired or reduced in aging is not the ability to resolve conflict once it is identified, but rather the ability to initiate conflict resolution when it is very seldom needed.
The results of the present study provided some limited evidence of reduced ability to resolve conflict in aging, and that this was important for avoiding production of autocorrect errors. Specifically, autocorrect errors were more strongly correlated with completion times on the Inhibition subtest of the standardized Stroop test (the CWIT) than with any of the demographic variables or other behavioral test scores. This might not seem surprising given that both tasks require suppressing a prepotent response; i.e., in the CWIT, the automatic tendency to read words must be suppressed to instead produce color names, while in the autocorrect task there is top-down pressure to produce expected completions that must be suppressed to instead read and produce the nonsensical word substitution. However, while conflict resolution appeared to play an important role in preventing autocorrect errors, the CWIT was not robustly correlated with age or Aβ1–42 (see Table 2; and also appeared to reflect a different underlying mechanism than aging effects given the lack of an interaction with Aβ1–42). When autocorrect errors and CWIT inhibition scores were simultaneously entered into a model predicting Aβ1–42 levels, only autocorrect errors captured unique variance1.
Two previous studies showed that errors produced on incongruent trials of a computer-administered Stroop test were sensitive to prodromal AD, either with (Hutchison et al., 2010) or without (Balota et al., 2010) a switching component in the task. Stroop errors were the most powerful predictor of future cognitive decline when pitted against 18 other commonly administered cognitive tests (i.e., of visual and verbal memory, language, attention, executive functions, and cognitive/functional status). Thus, is surprising that CWIT errors were not the strongest predictor of Aβ1–42 levels in the present study. Several differences between the standardized CWIT and the experimental versions of the Stroop might explain the different pattern of results found across studies. Most notably, the Inhibition subtest of the CWIT has exclusively incongruent trials (e.g., the word green written in red ink), whereas the experimental versions of the Stroop included over 50% neutral trials (e.g., the word legal written in red ink; Hutchison et al., 2010) or 33% neutral and 33% congruent trials (e.g., the word green written in green ink; Balota et al., 2010; see also Bélanger, et al., 2010). When the proportion of conflict generating trials is low, heightened monitoring is needed to identify where and when there is a need for conflict resolution (e.g., Bugg, Jacoby, & Toth, 2008; Costa, Hernández, Costa-Faidella, & Sebastián-Gallés, 2009). The autocorrect task takes an extreme step in this direction since the vast majority of words (about 95%) were not substituted words so the need for conflict resolution was relatively rare. Thus, the paragraph-reading test may be most sensitive precisely because the majority of words could be produced relatively automatically and without careful monitoring, making it difficult to initiate monitoring when the need did arise. By contrast all trials on the Inhibition subtest of the CWIT are incongruent trials, thus this task requires the constant application of conflict resolution (with no need to monitor when it should be engaged)2.
Reading aloud is a relatively naturalistic task that elicits fluent connected speech with experimental control over the content of speech that can be used to study language production and monitoring processes. The results of the present study suggest that a brief and relatively simple assessment of autocorrect errors in reading aloud may be useful for identifying pre-clinical AD. While age remains the single most powerful predictor of AD and future cognitive decline (Hebert et al., 2010; Hebert, Weuve, Scherr, & Evans, 2013), we suggested that what might be most affected by aging, and also most critical for predicting future cognitive decline, is the ability to monitor for conflict and quickly initiate conflict resolution when it is needed only very rarely. Because speech is produced quickly, on average about 150 words per minute (Levelt, 1989), reading aloud makes it possible to include experimental manipulations in which conflict occurs only very rarely, while still providing sufficient data for analysis (very rare in the present study was about 5%, while in previous studies “rare” was three times as frequent 15%; e.g., Behforuzi, Feng, Billig, Ryan, Tusch, Holcomb, Mohammed, & Daffner, 2019). Follow up studies are needed to validate prediction of eventual conversion to dementia3, and to identify which types of experimental manipulations might be most sensitive and reliable for distinguishing the effects of healthy aging from dementia (e.g., proportions of switch targets in bilinguals, or proportion malapropisms in monolinguals). Importantly, the aging effects we observed were independent of the effects of pre-clinical AD. We attributed theses effects to internal monitoring of planned speech, but additional studies will be needed to pinpoint possible roles of predictive processing, reliance on context during reading, and ability to resolve conflict (perhaps via inhibitory control), as possibly affected cognitive mechanisms that might be important for controlling speech production in reading aloud.
Supplementary Material
Acknowledgments
The authors thank Vic Ferreira and Shota Momma for helpful discussion, Rosa Montoya, Mayra Murillo, and Tiffany Ho for composition of the paragraphs and error coding, and Cecilia Salcedo Borrego, Brandon Pulido, and Amanda Rodriguez for assistance with data collection. The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article. This research was supported by grants from the National Institute on Deafness and Other Communication Disorders (011492), and by a P50 (AG05131) and a P30 (AG062429) from the National Institute of Aging to the University of California. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the NIH. Some of the research presented here was previously presented at a Cognitive Science colloquium series at Northwestern University. Data are publicly available at https://osf.io/fjxk8/
Footnotes
Note that Tau, another AD biomarker, was not a powerful predictor of Aβ1–42 levels, but this is not surprising in light of our focus on aging participants classified as cognitively normal and the hypothesis that dysfunction in the Aβ pathway precedes aggregation of Tau (Jack & Holtzman, 2013; Guhra et al., 2016).
Another question is why the Inhibition subtest but not the Inhibition-Switching subtest of the CWIT appeared sensitive to Aβ1–42 levels. In previous studies the Inhibition-Switching subtest appeared to be more sensitive to prodromal AD (although with reading times, not errors as the predictor of future cognitive decline; (Clark et al., 2012; Fine et al., 2008). Possibly relevant here is that the Inhibition-Switching subtest is not consistently more difficult than the Inhibition subtest (Berg, Swan, Banks, & Miller, 2016; Lippa & Davis, 2010). But difficulty alone does not reveal the critical underlying cognitive mechanisms. In this respect, monitoring processes may be more affected than inhibitory control or switching by aging and pre-clinical AD.
In our analyses we interpreted Aβ1–42 as a continuous variable in association with behavioral test performance (for a similar approach see Allison et al., 2019; Farrell et al., 2017; Soldan, Pettigrew, Moghekar, Albert, & BIOCARD Research Team, 2016; ), while other studies instead use established cutoffs to define pathology, an approach that will ultimately be needed to distinguish patients from controls (Galasko, Smirnov, Salmon, De With, Vanbrabant, Jacobs, Vanmechelen, Vanderstichele, Xu, Xiao, & Worley, in press; Hansson et al., 2018). It should be noted, however, that cutoffs for Aβ1–42 have been calibrated against amyloid PET or developed through ROC analysis and it is possible that there may be earlier subtle, but relevant, changes in Aβ1–42 detectable in CSF but not by amyloid PET; therefore there is a rationale for analysis of CSF Aβ1–42 as a continuous variable.
References
- Allison SL, Rodebaugh TL, Johnston C, Fagan AM, Morris JC, & Head D (2019). Developing a spatial navigation screening tool sensitive to the preclinical Alzheimer disease dontinuum. Archives of Clinical Neuropsychology, 34(7), 1138–1155. 10.1093/arclin/acz019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balota DA, Tse C-S, Hutchison KA, Spieler DH, Duchek JM, & Morris JC (2010). Predicting conversion to dementia of the Alzheimer type in a healthy control sample: The power of errors in Stroop color naming. Psychology and Aging, 25(1), 208–218. doi: 10.1037/a0017474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behforuzi H, Feng NC, Billig AR, Ryan E, Tusch ES, Holcomb PJ, Mohammed AH & Daffner KR (2019). Markers of Novelty Processing in Older Adults Are Stable and Reliable. Frontiers: Aging Neuroscience, 11:165. doi: 10.3389/fnagi.2019.00165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bélanger S, Belleville S, Gauthier S (2010). Inhibition impairments in Alzheimer’s disease, mild cognitive impairment and healthy aging: effect of congruency proportion in a Stroop task. Neuropsychologia, 48(2), 581–590. doi: 10.1016/j.neuropsychologia.2009.10.021. [DOI] [PubMed] [Google Scholar]
- Berg J-L, Swan NM, Banks SJ, & Miller JB (2016). Atypical performance patterns on Delis-Kaplan Executive Functioning System Color-Word Interference Test: Cognitive switching and learning ability in older adults. Journal of Clinical and Experimental Neuropsychology, 38(7), 745–751. 10.1080/13803395.2016.1161734 [DOI] [PubMed] [Google Scholar]
- Berisha V, Wang S, LaCross A, & Liss J (2015). Tracking discourse complexity preceding Alzheimer’s disease diagnosis: A case study comparing the press conferences of presidents Ronald Reagan and George Herbert Walker Bush. Journal of Alzheimer’s Disease, 45, 959–963. Doi: 10.3233/JAD-142763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugg JM, Jacoby LL, & Toth JP (2008). Multiple levels of control in the Stroop task. Memory & Cognition, 36(8), 1484–1494. 10.3758/MC.36.8.1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DM, MacKay DG, Worthley JS, & Wade E (1991). On the tip of the tongue: What causes word finding failures in young and older adults? Journal of Memory and Language, 30(5), 542–579. 10.1016/0749-596X(91)90026-G [DOI] [Google Scholar]
- Choi W, Lowder MW, Ferreira F, Swaab TY, & Henderson JM (2017). Effects of word predictability and preview lexicality on eye movements during reading: A comparison between young and older adults. Psychology and Aging, 32(3), 232–242. 10.1037/pag0000160 [DOI] [PubMed] [Google Scholar]
- Clark LR, Schiehser DM, Weissberger GH, Salmon DP, Delis DC, & Bondi MW (2012). Specific measures of executive function predict cognitive decline in older adults. Journal of the International Neuropsychological Society, 18(1), 118–127. 10.1017/S1355617711001524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Hernández M, Costa-Faidella J, & Sebastián-Gallés N (2009). On the bilingual advantage in conflict processing: Now you see it, now you don’t. Cognition, 113(2), 135–149. 10.1016/j.cognition.2009.08.001 [DOI] [PubMed] [Google Scholar]
- Dahlgren DJ (1998). Impact of knowledge and age on tip-of-the tongue rates. Experimental Aging Research, 24, 139–153. 10.1080/036107398244283 [DOI] [PubMed] [Google Scholar]
- DeTure MA, Dickson DW (2019). The neuropathological diagnosis of Alzheimer’s disease. Molecular Neurodegeneration, 14, 32. 10.1186/s13024-019-0333-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell ME, Kennedy KM, & Rodrigue KM, Wig. G, Bischof G, Rieck JR, Chen X Festini SB, Devous MD, & Park DC (2017). Association of longitudinal cognitive decline with amyloid burden in middle-aged and older adults: Evidence for a dose-response relationship. JAMA Neurology, 74, 830–838. doi: 10.1001/jamaneurol.2017.0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier KD, & Kutas M (2005). Aging in context: Age-related changes in context use during language comprehension. Psychophysiology, 42(2), 133–141. 10.1111/j.1469-8986.2005.00274.x [DOI] [PubMed] [Google Scholar]
- Federmeier KD, Kutas M, & Schul R (2010). Age-related and individual differences in the use of prediction during language comprehension. Brain and Language, 115(3), 149–161. 10.1016/j.bandl.2010.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fine EM, Delis DC, Wetter SR, Jacobson MW, Jak AJ, McDonald CR, Braga JC, Thal LJ, Salmon DP, & Bondi MW (2008). Cognitive discrepancies versus APOE genotype as predictors of cognitive decline in normal-functioning elderly individuals: A longitudinal study. The American Journal of Geriatric Psychiatry, 16(5), 366–374. 10.1097/JGP.0b013e3181629957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galasko D, Chang L, Motter R, Clark CM, Kaye J, Knopman D, Thomas R, Kholodenko D, Lieberburg I Miller B Basherad R, Kertiles L, Boss MA & Seubert P (1998). High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Archives of Neurology, 55(7), 937–945. DOI: 10.1001/archneur.55.7.937 [DOI] [PubMed] [Google Scholar]
- Galasko D, Hansen LA, Katzman R, Wiederholt W, Masliah E, Terry R, Hill LR, Lessin P, & Thal LJ (1994). Clinical-neuropathological correlations in Alzheimer’s disease and related dementias. Archives of Neurology, 51(9), 888–895. doi: 10.1001/archneur.1994.00540210060013 [DOI] [PubMed] [Google Scholar]
- Galasko D, Smirnov D, Salmon DP, De With N, Vanbrabant J, Jacobs D, Vanmechelen E, Vanderstichele H, Xu D, Xiao M, & Worley P (2019). Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict decline in MCI and AD. Alzheimer’s and Dementia: Translational Research and Clinical Interventions, 5, 871–882. 10.1016/j.trci.2019.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauvin HS, De Baene W, Brass M, & Hartsuiker RJ (2016). Conflict monitoring in speech processing: An fMRI study of error detection in speech production and perception. NeuroImage, 126, 96–105. 10.1016/j.neuroimage.2015.11.037 [DOI] [PubMed] [Google Scholar]
- Gollan& Goldrick TH (2019). Aging deficits in naturalistic speech production and monitoring revealed through reading aloud. Psychology and Aging, 34(1), 25–42. doi: 10.1037/pag0000296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, & Goldrick M (2016). Grammatical constraints on language switching: Language control is not just executive control. Journal of Memory and Language, 90, 177–199. 10.1016/j.jml.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Stasenko A, Li C, & Salmon DP (2017). Bilingual language intrusions and other speech errors in Alzheimer’s disease. Brain and Cognition, 118, 27–44. 10.1016/j.bandc.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gollan TH, Li C Stasenko A Salmon DP (2020). Intact reversed language-dominance but not cognate effects in reading aloud of language switches in bilingual Alzheimer’s disease. Neuropsychology, 34(1), 88–106. doi: 10.1037/neu0000592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guhra M, Thomas C, Boedeker S, Kreisel S, Driessen M, Beblo T, Ohrmann P, & Toepper M (2016). Linking CSF and cognition in Alzheimer’s disease: Reanalysis of clinical data. Experimental Gerontology, 73, 107–113. 10.1016/j.exger.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Hasher L, & Zacks RT(1988). Working memory, comprehension, and aging: A review and a new view, in The Psychology of Learning and Motivation, Vol.22, ed. Bower GH (New York, NY: Academic Press; ), 193–225. doi: 10.1016/S0079-7421(08)60041-9 [DOI] [Google Scholar]
- Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, Lifke v., Corradini V, Eichenlaub U, Batrla R, Buck K, Zink K, Rabe C, Blennow K, & Shaw LM, and the Alzheimer’s Disease Neuroimaging Initiative4, for the Swedish BioFINDER study group (2018). CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 14(11), 1470–1481. 10.1016/j.jalz.2018.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Bienias JL, Aggarwal NT, Wilson RS, Bennett DA, Shah RC, & Evans DA (2010). Change in risk of Alzheimer disease over time. Neurology, 75(9), 786–791. 10.1212/WNL.0b013e3181f0754f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert Liesi E., Weuve J, Scherr PA, & Evans DA (2013). Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology, 80(19), 1778–1783. 10.1212/WNL.0b013e31828726f5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huijbers W, Papp KV, LaPoint M, Wigman SE, Dagley A, Hedden T, Rentz DM, Schultz AP & Sperling RA (2017). Age-related increases in tip-of-the-tongue are distinct from decreases in remembering names: a functional MRI study. Cerebral Cortex, 27, 4339–4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KA, Balota DA, & Duchek JM (2010). The Utility of Stroop Task Switching as a Marker for Early Stage Alzheimer’s Disease. Psychology and Aging, 25(3), 545–559. 10.1037/a0018498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, & Holtzman DM (2013). Biomarker modeling of Alzheimer’s disease. Neuron, 80(6), 1347–1358. 10.1016/j.neuron.2013.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Knopman DS, Jagust WJ, Shaw LM, Aisen PS, Weiner MW, Petersen RC, & Trojanowski JQ (2010). Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurology, 9(1), 119–128. doi: 10.1016/S1474-4422(09)70299-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavé G, & Goral M (2017). Do age-related word retrieval difficulties appear (or disappear) in connected speech? Aging, Neuropsychology, and Cognition, 24(5), 508–527. 10.1080/13825585.2016.1226249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keuleers E, Stevens M, Mandera P, & Brysbaert M (2015). Word knowledge in the crowd: Measuring vocabulary size and word prevalence in a massive online experiment. Quarterly Journal of Experimental Psychology, 68(8), 1665–1692. 10.1080/17470218.2015.1022560 [DOI] [PubMed] [Google Scholar]
- Lange KL, Bondi MW, Salmon DP, Galasko D, Delis DC, Thomas RG, & Thal LJ (2002). Decline in verbal memory during preclinical Alzheimer’s disease: Examination of the effect of APOE genotype. Journal of the International Neuropsychological Society, 8(7), 943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le X, Lancashire I, Hirst G, & Jokel R (2011). Longitudinal detection of dementia through lexical and syntactic changes in writing: A case study of three British novelists. Literary and Linguistic Computing, 26(4), 435–461. 10.1093/llc/fqr013 [DOI] [Google Scholar]
- Levelt WJM (1989), Speaking, The MIT Press, Cambridge, MA. [Google Scholar]
- Levelt WJM, Roelofs A, & Meyer AS (1999). A theory of lexical access in speech production. Behavioral and Brain Sciences, 22, 1–75. DOI: 10.1017/s0140525x99001776 [DOI] [PubMed] [Google Scholar]
- Lezak M, Howieson D, Bigler E, & Tranel D (2012). Neuropsychological Assessment. 5. New York: Oxford University Press. [Google Scholar]
- Lippa SM, & Davis RN (2010). Inhibition/Switching Is not Necessarily Harder than Inhibition: An Analysis of the D-KEFS Color-Word Interference Test. Archives of Clinical Neuropsychology, 25(2), 146–152. 10.1093/arclin/acq001 [DOI] [PubMed] [Google Scholar]
- Lleó A, Alcolea D, Martínez-Lage P, Scheltens P, Parnetti L, Poirier J, Simonsen AH, Verbeek MM, Rosa-Neto P, Slot RER, Tainta M, Izaguirre A, Reijs BLR, Farotti L, Tsolaki M, Vandenbergue R, Freund-Levi Y, Verhey FRJ, Clarimón J, Fortea J, Frolich L, Santana I, Molinuevo JL, Lehmann S, Visser PJ, Teunissen CE, Zetterberg H, & Blennow K (2019). Longitudinal cerebrospinal fluid biomarker trajectories along the Alzheimer’s disease continuum in the BIOMARKAPD study. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 15(6), 742–753. 10.1016/j.jalz.2019.01.015 [DOI] [PubMed] [Google Scholar]
- Moers C, Meyer A, & Janse E (2017). Effects of Word Frequency and Transitional Probability on Word Reading Durations of Younger and Older Speakers. Language and Speech, 60(2), 289–317. 10.1177/0023830916649215 [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, & Mirra SS et al. (2012). National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathologica, 123(1), 1–11. doi: 10.1007/s00401-011-0910-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozari N, Dell GS, & Schwartz MF (2011). Is comprehension necessary for error detection? A conflict-based account of monitoring in speech production. Cognitive Psychology, 63(1), 1–33. 10.1016/j.cogpsych.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozari N, & Novick J (2017). Monitoring and Control in Language Production. Current Directions in Psychological Science, 26(5), 403–410. 10.1177/0963721417702419 [DOI] [Google Scholar]
- Perrin RJ, Fagan AM, & Holtzman DM (2009). Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature, 461(7266), 916–922. doi: 10.1038/nature08538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramscar M, Hendrix P, Shaoul C, Milin P, & Baayen H (2014). The myth of cognitive decline: Non-linear dynamics of lifelong learning. Topics in Cognitive Science, 6(1), 5–42. 10.1111/tops.12078 [DOI] [PubMed] [Google Scholar]
- Rayner K, Reichle ED, Stroud MJ, Williams CC, & Pollatsek A (2006). The effect of word frequency, word predictability, and font difficulty on the eye movements of young and older readers. Psychology and Aging, 21(3), 448–465. 10.1037/0882-7974.21.3.448 [DOI] [PubMed] [Google Scholar]
- Rayner K, Slattery TJ, Drieghe D, & Liversedge SP (2011). Eye movements and word skipping during reading: Effects of word length and predictability. Journal of Experimental Psychology. Human Perception and Performance, 37(2), 514–528. 10.1037/a0020990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N (2008). Aging of the brain and its impact on cognitive performance: Integration of structural and functional findings. In Craik FIM & Salthouse TA (Eds.), Handbook of aging and cognition-II (pp. 1–90). Mahwah, NJ: Erlbaum. [Google Scholar]
- Reuter-Lorenz PA, & Park DC (2010). Human neuroscience and the aging mind: a new look at old problems. Journal of Gerontology: Psychological Sciences, 65B(4), 405–415. doi: 10.1093/geronb/gbq035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley KP, Snowdon DA, Desrosiers MF, & Markesbery WR (2005). Early life linguistic ability, late life cognitive function, and neuropathology: Findings from the Nun Study. Neurobiology of Aging, 26(3), 341–347. 10.1016/j.neurobiolaging.2004.06.019 [DOI] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy K., Devous MD, Rieck JR, Hebrank AC, Diaz Arrastia R Mathews D, Park DC (2012). β-Amyloid burden in healthy aging. Neurology, 78(6), 387–395. doi: 10.1212/WNL.0b013e318245d295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigue KM, Kennedy KM & Park DC Beta-Amyloid Deposition and the Aging Brain. (2009). Neuropsychology Review, 19(4), 436 (2009). doi: 10.1007/s11065-009-9118-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA (2009). Decomposing age correlations on neuropsychological and cognitive variables. Journal of the International Neuropsychological Society, 15(5), 650–661. 10.1017/S1355617709990385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse TA. (2010). Selective review of cognitive aging. Journal of the International Neuropsychological Society, 16(5), 754–60. doi: 10.1017/S1355617710000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T (2012). Consequences of age-related cognitive declines. Annual Review of Psychology, 63, 201–226. doi: 10.1146/annurev-psych-120710-100328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schotter ER, Li C, & Gollan TH (2019). What reading aloud reveals about speaking: Regressive saccades implicate a failure to monitor, not inattention, in the prevalence of intrusion errors on function words. Quarterly Journal of Experimental Psychology, 72(8), 2032–2045. 10.1177/1747021818819480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafto MA, Burke DM, Stamatakis EA, Tam PP, & Tyler LK (2007). On the tip-of-the-tongue: neural correlates of increased word-finding failures in normal aging. Journal of Cognitive Neuroscience, 19(12), 2060–2070. DOI: 10.1162/jocn.2007.19.12.2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snowdon DA, Kemper SJ, Mortimer JA, Greiner LH, Wekstein DR, & Markesbery WR (1996). Linguistic ability in early life and cognitive function and Alzheimer’s disease in late life: Findings from the Nun Study. JAMA, 275(7), 528–532. doi: 10.1001/jama.1996.03530310034029 [DOI] [PubMed] [Google Scholar]
- Soldan A, Pettigrew C, Moghekar A, Albert M, & BIOCARD Research Team. (2016). Computerized Cognitive Tests Are Associated with Biomarkers of Alzheimer’s Disease in Cognitively Normal Individuals 10 Years Prior. Journal of the International Neuropsychological Society, 22(10), 968–977. 10.1017/S1355617716000722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RA, Laviolette PS, O’Keefe K, O’Brien J, Rentz DM, Pihlajamaki M, Marshall G, Hyman BT, Selkoe DJ, & Hedden T, Buckner RL, Becker JA, & Johnson KA (2009). Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron, 63(2), 178–188. doi: 10.1016/j.neuron.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanovich KE, West RF, & Harrison MR (1995). Knowledge growth and maintenance across the life span: The role of print exposure. Developmental Psychology, 31(5), 811–826. 10.1037/0012-1649.31.5.811 [DOI] [Google Scholar]
- Staub A, Dodge S, & Cohen AL (2019). Failure to detect function word repetitions and omissions in reading: Are eye movements to blame? Psychonomic Bulletin & Review, 26(1), 340–346. 10.3758/s13423-018-1492-z [DOI] [PubMed] [Google Scholar]
- Steen-Baker AA, Ng S, Payne BR, Anderson CJ, Federmeier KD, & Stine-Morrow EAL (2017). The effects of context on processing words during sentence reading among adults varying in age and literacy skill. Psychology and Aging, 32(5), 460–472. 10.1037/pag0000184 [DOI] [PubMed] [Google Scholar]
- Sutphen CL, Jasielec MS, Shah AR, Macy EM, Xiong C, Vlassenko AG, … Fagan AM (2015). Longitudinal Cerebrospinal Fluid Biomarker Changes in Preclinical Alzheimer Disease During Middle Age. JAMA Neurology, 72(9), 1029–1042. 10.1001/jamaneurol.2015.1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderstichele H, Bibl M, Engelborghs S, Le Bastard N, Lewczuk P, Molinuevo JL, Parnetti L, Perret-Liaudet A, Shaw LM, Teunissen C, Wouters D, & Blennow K (2012). Standardization of preanalytical aspects of cerebrospinal fluid biomarker testing for Alzheimer’s disease diagnosis: A consensus paper from the Alzheimer’s Biomarkers Standardization Initiative. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 8(1), 65–73. 10.1016/j.jalz.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Venneri A, Jahn-Carta C, Marco MD, Quaranta D, & Marra C (2018) Diagnostic and prognostic role of semantic processing in preclinical Alzheimer’s disease. Biomarkers in Medicine, 12(6), 637–651. 10.2217/bmm-2017-0324 [DOI] [PubMed] [Google Scholar]
- Verhaeghen P (2003). Aging and vocabulary score: A meta-analysis. Psychology and Aging, 18(2), 332–339. 10.1037/0882-7974.18.2.332 [DOI] [PubMed] [Google Scholar]
- Verhaeghen P (2011). Aging and Executive Control: Reports of a Demise Greatly Exaggerated. Current Directions in Psychological Science, 20(3), 174–180. 10.1177/0963721411408772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West RL (1996). An application of prefrontal cortex function theory to cognitive aging. Psychological Bulletin, 120(2), 272–292. DOI: 10.1037/0033-2909.120.2.272 [DOI] [PubMed] [Google Scholar]
- Wingfield A, & Grossman M (2006). Language and the aging brain: Patterns of neural compensation revealed by functional brain imaging. Journal of Neurophysiology, 96(6), 2830–2839. 10.1152/jn.00628.2006 [DOI] [PubMed] [Google Scholar]
- Woods SP, Delis DC, Scott JC, Kramer JH, & Holdnack JA (2006). The California Verbal Learning Test--second edition: Test-retest reliability, practice effects, and reliable change indices for the standard and alternate forms. Archives of Clinical Neuropsychology, 21(5), 413–420. 10.1016/j.acn.2006.06.002 [DOI] [PubMed] [Google Scholar]
- Xiao M-F, Xu D, Craig MT, Pelkey KA, Chien C-C, Shi Y, Zhang J, Resnick S, Pletnikova O, Salmon DP, Brewer J, Edland S, Wegiel J, Tycko B, Savonenko A, Reeves RH, Toncoso JC, McBain CJ, Galasko D, Worley PF (2017). NPTX2 and cognitive dysfunction in Alzheimer’s Disease. ELife, 6. 10.7554/eLife.23798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Li L, Chang M, Xu Q, Zhang K, Wang J, & Paterson KB (2019). Older adults make greater use of word predictability in Chinese reading. Psychology and Aging, 34(6), 780–790. 10.1037/pag0000382 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


