Abstract
Objectives:
To assess and compare the concentration of leptin in saliva between normal weight and overweight individuals and to evaluate the rate of orthodontic tooth movement.
Materials and Methods:
Thirty female subjects were divided into two groups: I (normal weight) and II (overweight group) based on their body mass index. All subjects underwent fixed appliance therapy requiring upper first premolar extraction and distal movement of the canine. Distal force was applied to the maxillary right canine using active lacebacks. Salivary samples were collected just before force application (T0), 1 hour (T1), and 1 month (T2) after force application. The rate of tooth movement was evaluated over 3 months and was measured on study models.
Results:
At all three time intervals, mean leptin concentration was greater in overweight individuals than normal weight individuals. In both groups at T1, the mean leptin concentration was found to increase significantly compared with the baseline value (T0), but at T2, the leptin concentration declined to values lesser than the baseline values (T0).
Conclusions:
Overweight individuals had greater salivary leptin concentration. There was a positive correlation between salivary leptin concentration and rate of tooth movement in both normal and overweight individuals. The rate of tooth movement is decreased in overweight individuals as compared with normal weight individuals.
Keywords: Salivary leptin levels, Orthodontic tooth movement
INTRODUCTION
Forces from orthodontic appliances induce cells in the periodontal tissues to form biologically active substances such as enzymes and cytokines, which are responsible for bone remodeling.1 During application of orthodontic force, there is a release of proinflammatory cytokines such as interleukin (IL)–1β, IL-2, IL-6, IL-8, and tumor necrosis factor–α.2–6 Leptin is a 16-KDa, nonglycosylated polypeptide hormone produced primarily by adipocytes and in minor quantities by the placenta, stomach, osteoblast, and salivary glands.7–12 There is a strong positive correlation between serum leptin concentration and body mass index (BMI) or fat mass.13–15
Leptin plays a dual role as a hormone and cytokine. As a hormone, it plays a major role as a hypothalamic modulator of food intake, fat storage, and body weight maintenance. As a cytokine, it plays a key role in bone remodeling, resorption, and new bone formation.16 Leptin controls bone metabolism by two different regulatory pathways: a direct stimulatory effect by osteoblastic cell growth and prolonging the life span of human primary osteoblasts, and an indirect suppressive effect through the hypothalamus.17–20 Leptin shares structural and functional similarities with long-chain helical cytokines such as IL-1, IL-6, IL-10, IL-12, and granulocyte stimulating factor, and it also modulates cytokine production from monocytes.21–24
The presence of leptin has been reported in inflamed and healthy gingival tissues.25 Leptin concentration is decreased in gingival crevicular fluid as the adjacent probing depth increases.26 Leptin expression is also seen in pulp samples, and it is found to increase during pulpal inflammation.27 Recent studies evaluated leptin levels in gingival crevicular fluid during orthodontic tooth movement and found that the leptin level is decreased during orthodontic tooth movement.28
Leptin is also expressed in saliva, and there is a strong positive correlation between leptin concentration from saliva and serum samples.11 Analysis of leptin in saliva is noninvasive, and its concentration is high compared with plasma samples.12
Orthodontists are in a unique position to see children and adolescents over a significant period of time. As childhood obesity is a major growing global health problem, more emphasis should be given to treatment planning in obese patients. It has been reported that obese persons have altered growth and development, pubertal development,29 and bone metabolism.30 A recent study stated that treatment duration was slightly greater in the overweight group but attributed that finding to poor patient compliance rather than altered bone metabolism.31
In overweight or obese individuals, increased fat mass increases serum leptin level, which in turn can have an effect on bone remodeling.10,17,18 However, the literature also mentions leptin resistance, a condition developed in obese individuals, characterized by resistance to the effects of leptin due to decreased transport of leptin through the blood-brain barrier and other pathways.32
As orthodontic tooth movement involves the inflammatory process and leptin has cytokine-like effects responsible for bone remodeling,21–24 it is logical to assume that leptin can have an effect on the rate of orthodontic tooth movement, and this would differ between normal weight and overweight individuals.
Leptin concentration in women is higher than men because of greater subcutaneous adipose tissue in women than men.13 Thus, the aim of this study was to assess and compare the salivary leptin concentration between normal weight and overweight female individuals and to correlate this with rate of orthodontic tooth movement in both groups.
MATERIALS AND METHODS
Thirty female subjects were divided into two groups: I (control group, age 14–28 years) and group II (overweight group, age 14–30 years). Group I comprised 15 normal weight patients whose BMI range was between 18.5 and 25 kg/m2, and group II comprised 15 overweight patients whose BMI range was between 25 and 30 kg/m2. The BMI range was determined according to the chart devised by the World Health Organization (WHO).33
Inclusion Criteria
-
•
Only female subjects were included in this study
-
•
Patients who were to undergo orthodontic treatment with fixed mechanotherapy requiring upper first premolar extraction and distal movement of the canine
-
•
Clinically healthy periodontium with no radiographic evidence of bone loss
-
•
Individuals with permanent dentition
Exclusion Criteria
-
•
Subjects with any history of systemic disease or taking any medication
-
•
Presence of any salivary gland disorders
-
•
Subjects with smoking and alcohol habits
Informed consent was obtained from all patients, and ethical clearance was obtained from the institutional ethical committee.
Collection and Processing of Saliva
Salivary samples were collected just before orthodontic force application (T0) and 1 hour (T1) and 1 month (T2) after force application. Salivary samples (5 mL) were collected between 9:00 AM and 12:00 PM at least 1 hour after meals. Unstimulated whole saliva was collected by the passive drool method. The subjects were seated upright and leaned their head forward with the test tube placed toward the corner of the mouth to allow saliva to drain into the test tube.34 Collected salivary samples were centrifuged at 15,000 g for 15 minutes at 4°C to remove insoluble material, and the supernatants were stored in 1-mL cryotubes under −80°C, until leptin analysis was accomplished.
Estimation of Leptin Concentration
Leptin concentration in saliva was estimated using a commercially available enzyme-linked immunosorbent assay (Ray Biotech Inc, Germany). Leptin concentration was normalized according to the salivary levels in overweight and normal weight individuals, and the volume for each sample was calculated by dividing the amount of leptin by the volume of the sample (ng/100 μL).
Application of Force and Calculation of Rate of Tooth Movement
The appliances were placed 2 weeks after extraction of premolars in all subjects, and an initial archwire of 014-inch NiTi was used. A distal force was applied to the maxillary right canine using active lacebacks, and the force was standardized by giving an equal number of turns (five turns) for each subject.35,36
An upper alginate impression was made for all subjects before and 3 months after appliance placement, and models were poured using type IV dental stone (Orthokal, Kalabhai Company). The distance from the mesial contact point of the right maxillary canine to that of the second premolar was measured from the study models before and after force application using an electronic digital caliper, and the difference between the two measurements was recorded as the net distal movement of the right maxillary canine over 3 months.
Statistical Analysis
Statistical analysis was carried out using SPSS 16.0 statistical software (SPSS Inc, Chicago, Ill). Student's t-tests were used to compare mean salivary leptin levels with the rate of tooth movement between normal weight and overweight subjects. The Shapiro Wilk's test was used to test the normality of the data, and repeated-measures analysis of variance was used to compare the differences in salivary leptin levels within the groups with adjustment for multiple comparisons. The cumulative mean of the mean salivary leptin concentrations at three time intervals was calculated, and this was correlated to the rate of tooth movement using the Pearson correlation test.
RESULTS
At all three time intervals, the mean leptin concentration was two to three times greater in overweight individuals compared with normal weight individuals. The differences were statistically significant at all three time intervals (P < .01; Table 1; Figure 1).
Table 1.
Mean and Standard Deviation of Salivary Leptin Levels (ng/100 μL) Between Normal Weight and Overweight Individuals
| Group |
n |
Mean |
Standard Deviation |
P Value |
| T0 | <.01 | |||
| Normal | 15 | 322.9289 | 99.83514 | |
| Overweight | 15 | 748.1269 | 303.09050 | |
| T1 | <.01 | |||
| Normal | 15 | 407.1345 | 57.69496 | |
| Overweight | 15 | 895.8558 | 454.45105 | |
| T2 | <.01 | |||
| Normal | 15 | 238.4858 | 106.34972 | |
| Overweight | 15 | 618.4668 | 259.39389 |
Figure 1.
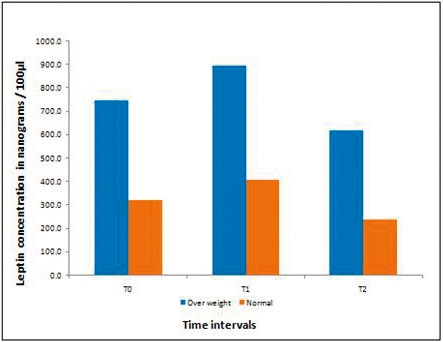
Mean salivary leptin concentration between normal weight and overweight individuals.
In both groups after force application (T1), the mean leptin concentration was found to increase significantly compared with the baseline value (T0). One month later at T2, the levels decreased to less than the baseline value (T0). The differences were statistically significant within the groups (Table 2).
Table 2.
Repeated-Measures Analysis of Variance Test Comparing Leptin Concentrations at Different Time Intervals in Group I (Normal Weight) and II (Overweight)
| Time Interval |
Comparison Within Group |
P Value |
|
| Normal Weight |
Overweight |
||
| T0 | T1 | .013* | .024* |
| T2 | .001* | <.001* | |
| T1 | T2 | <.001* | .001* |
The P value is significant at the .05 level.
The mean rate of orthodontic tooth movement was less in the overweight group compared with the normal weight group, and the difference was statistically significant (P = .01; Table 3). The rate of tooth movement was correlated to the mean leptin concentration (ng/100 μL) between T0, T1, and T2 in both groups (normal weight = 809.55, overweight = 1850.13). The results showed that there was a positive correlation of salivary leptin concentration and rate of tooth movement in both groups. The correlation was significant in the overweight group (P = .01) and the normal weight group (P = .03; Table 4).
Table 3.
Comparison of Rate of Tooth Movement (mm/3 months) Between Normal Weight and Overweight Individuals
| Group |
n |
Rate of Tooth Movement |
||
| Mean |
Standard Deviation |
P Value |
||
| Normal | 15 | 1.99 | 0.218 | .01 |
| Overweight | 15 | 1.25 | 0.349 | |
Table 4.
Pearson Correlation Test in Groups I (Normal Weight) and II (Overweight)
|
|
Mean Leptin Concentration Between T0, T1, T2 |
Rate of Tooth Movement |
| Mean leptin concentration between T0, T1, T2 | ||
| Overweight | ||
| Pearson correlation | 1 | .598 |
| Significance | .019* | |
| Normal weight | ||
| Pearson correlation | 1 | .558 |
| Significance | .031* | |
| Rate of tooth movement | ||
| Overweight | ||
| Pearson correlation | .598 | 1 |
| Significance | .019* | |
| Normal weight | ||
| Pearson correlation | .558 | 1 |
| Significance | .031* | |
The correlation is significant at the .05 level.
DISCUSSION
Leptin, a polypeptide hormone produced primarily by adipocytes, was originally found to be related to obesity and later proven to have a dual role as a hormone and cytokine.7–12,16 This study sought to assess the concentration differences of leptin in saliva between normal weight and overweight individuals and its correlation with orthodontic tooth movement. An association was found in this study between salivary leptin levels, BMI, and orthodontic tooth movement.
In overweight or obese individuals, increased fat mass results in increased serum leptin levels, which could have a strong influence on bone metabolism when compared with normal weight individuals.15 This might have further effects on the rate of orthodontic tooth movement in those individuals.
BMI is reflective of body fat mass and has a strong positive correlation with serum leptin levels.13–15 In this study, individuals were categorized into two groups (normal weight and overweight) based on their BMI according to the chart devised by the WHO.33 Leptin concentrations in women are higher than in men because of greater subcutaneous adipose tissue in women than men.13 Therefore, to reduce any gender-related bias, only female subjects were included in this study.
Leptin expression has been detected in salivary glands, and a positive correlation between serum and salivary leptin concentration has been shown.11 In this study, unstimulated saliva was collected for leptin estimation as stimulated salivary secretion can increase the salivary flow rate and leptin concentration up to threefold.11,12 Salivary leptin, like plasma leptin, displays circadian variations, and therefore, the collection of salivary samples was accomplished only in the morning between 9:00 AM and 12:00 PM.12 Salivary samples were collected 1 hour after dietary intake to eliminate the influence of recent food intake on salivary leptin level concentration, although Schrauwen et al.37 concluded that there was a poor correlation between salivary leptin concentration and recent food intake.
In this study, active lacebacks were used to distalize the maxillary canine as they produced a light continuous force, and the force applied was standardized by giving an equal number of turns (five) for all subjects.35,36 At all three time intervals of the study, salivary leptin concentrations were higher in overweight individuals when compared with normal weight individuals. These results were consistent with previous studies, which suggested that serum leptin levels were higher in overweight individuals as compared with normal weight individuals.13–15
After force application (T1), the mean leptin concentration showed an initial increase but then declined at T2 to a level even lower than the baseline value (T0) both in normal weight and overweight individuals. However, this rise and fall in the leptin concentration during one cycle of orthodontic force application was contrary to results obtained by Dilsiz et al.,28 who showed a decrease in leptin concentration 1 hour after force application. Studies have demonstrated that, after force application, several proinflammatory cytokines tend to increase in the first 24 hours. Later, the system stabilizes and reaches a new physiological homeostasis until the next activation.3,4 These observations were reflected in the results of this study. A significant increase of salivary leptin concentration 1 hour after force application was seen at T1, demonstrating leptin as an inflammatory marker,16 and at T2, the leptin level decreased. Tuncer et al.5 also showed that cytokine levels fluctuated with a 30-day cycle when continuous orthodontic force was applied. This could also explain the decrease observed in the leptin concentration at the T2 time interval.
Since the leptin concentration varied at different time intervals, an average (cumulative mean) of the leptin concentration at different time intervals for each group was correlated to the corresponding rate of tooth movement. The results revealed that there was a strong positive correlation of mean leptin concentration with the rate of tooth movement in both the overweight group and the normal weight group. The correlation was stronger in the overweight group when compared with the group with normal weight. In addition, the mean rate of tooth movement was lesser in the overweight group (1.25 mm) than in the normal weight group (1.99 mm; P = .01).
Although previous studies have shown that leptin has both bone stimulatory and inhibitory effects,17,19,20 in this study, a significant decrease in the rate of tooth movement in the overweight group compared with the normal weight group was found. This can be attributed to leptin's bone stimulatory effects being through direct peripheral action, which could be more prominent than its inhibitory effects. Leptin's bone inhibitory effects are indirect through the hypothalamus. The bone stimulatory effects increase bone mass, which, in turn, could delay orthodontic tooth movement.31 This action of leptin is also supported by the increased bone mineral density observed in obese individuals as measured using dual energy x-ray absorptiometry.38
Another factor to be considered is possible leptin resistance in obese individuals. Although Leptin's central action is to inhibit bone formation, leptin resistance is characterized by decreased effects of leptin, primarily mediated through the central nervous system, because of diminished blood to brain transport of leptin. In turn, the centrally mediated bone suppressive action of leptin is diminished. This could further explain the increased bone deposition seen in obese individuals. Increased bone deposition usually develops in patients who are classified as obese with a BMI greater than 30. In this study, overweight patients with a BMI between 25 and 30 were included.39 Hence, the influence of leptin resistance on the subjects in this study is debatable.
In this study, the leptin concentration was assessed for one orthodontic cycle, and the rate of tooth movement was measured for 3 months. Evaluation of the rate of tooth movement over a longer period, and correlating that with leptin levels at each time point, could contribute more information. Further studies at the molecular level during orthodontic tooth movement are necessary to determine the exact biological role of leptin in orthodontic tooth movement.
CONCLUSIONS
-
•
Overweight individuals had greater mean salivary leptin concentrations compared with normal weight individuals.
-
•
Overweight individuals had a significantly lesser rate of tooth movement compared with the normal weight group.
-
•
There was a strong positive correlation between the rate of tooth movement and mean salivary leptin concentration, suggesting an important role of leptin in controlling the rate of tooth movement in individuals with different body masses.
REFERENCES
- 1.Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006;129:469e.1–32. doi: 10.1016/j.ajodo.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Alhashimi N, Frithiof L, Brudvik P, Bakhiet M. Orthodontic tooth movement and de novo synthesis of proinflammatory cytokines. Am J Orthod Dentofacial Orthop. 2001;119:307–312. doi: 10.1067/mod.2001.110809. [DOI] [PubMed] [Google Scholar]
- 3.Lee KJ, Park YC, Yu HS, Choi SH, Yoo YJ. Effects of continuous and interrupted orthodontic force on interleukin-1β and prostaglandin E2 production in gingival crevicular fluid. Am J Orthod Dentofacial Orthop. 2004;125:168–167. doi: 10.1016/j.ajodo.2003.03.006. [DOI] [PubMed] [Google Scholar]
- 4.Grieve WG, Johnson GK, Moore RN, Reinhardt RA, DuBois LM. Prostaglandin E (PGE) and interleukin-1β (IL-1β) levels in gingival crevicular fluid during human orthodontic tooth movement. Am J Orthod Dentofacial Orthop. 1994;105:369–374. doi: 10.1016/s0889-5406(94)70131-8. [DOI] [PubMed] [Google Scholar]
- 5.Tuncer BB, Ozmeriç N, Tuncer C, et al. Levels of interleukin-8 during tooth movement. Angle Orthod. 2005;75:631–636. doi: 10.1043/0003-3219(2005)75[631:LOIDTM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 6.Başaran G, Ozer T, Kaya FA, Hamamci O. Interleukins 2, 6, and 8 levels in human gingival sulcus during orthodontic treatment. Am J Orthod Dentofacial Orthop. 2006;130:7.e1–7.e6. doi: 10.1016/j.ajodo.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 8.Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 9.Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 10.Reseland JE, Syversen U, Bakke I, et al. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J Bone Miner Res. 2001;16:1426–1433. doi: 10.1359/jbmr.2001.16.8.1426. [DOI] [PubMed] [Google Scholar]
- 11.Groschl M, Rauh M, Wagner R, et al. Identification of leptin in human saliva. J Clin Endocrinol Metab. 2001;86:5234–5239. doi: 10.1210/jcem.86.11.7998. [DOI] [PubMed] [Google Scholar]
- 12.Randeva HS, Karteris E, Lewandowski KC, Sailesh S, O'Hare P, Hillhouse EW. Circadian rhythmicity of salivary leptin in healthy subjects. Mol Genet Metab. 2003;78:229–235. doi: 10.1016/s1096-7192(03)00004-0. [DOI] [PubMed] [Google Scholar]
- 13.Paul RF, Hassan M, Nazar HS, Gillani S, Afzal N, Qayyum I. Effect of body mass index on serum leptin levels. J Ayub Med Coll Abbottabad. 2011;23(3):40–43. [PubMed] [Google Scholar]
- 14.Sinha MK, Opentanova I, Ohannesian JP, et al. Evidence of free and bound leptin in human circulation: studies in lean and obese subjects and during short-term fasting. J Clin Invest. 1996;98:1277–1282. doi: 10.1172/JCI118913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Considine RV, Sinha MK, Heiman ML, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996;334:292–295. doi: 10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 16.Lago R, Gomez R, Lago F, Gomez-Reino J, Gualillo O. Leptin beyond body weight regulation: current concepts concerning its role in immune function and inflammation. Cell Immunol. 2008;252(1–2):139–145. doi: 10.1016/j.cellimm.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 17.Thomas T. Leptin: a potential mediator for protective effects of fat mass on bone tissue. Joint Bone Spine. 2003;70:18–21. doi: 10.1016/s1297-319x(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 18.Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporosis Int. 2008;19:905–912. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- 19.Steppan CM, Crawford DT, Chidsey-Frink KL, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul Pept. 2000;92(1–3):73–78. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- 20.Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–120. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- 21.Fantuzzi G, Faggioni R. Leptin in the regulation of immunity, inflammation and hematopoiesis. J Leukoc Biol. 2000;68:437–446. [PubMed] [Google Scholar]
- 22.Agrawal S, Gollapudi SH, Gupta S. Leptin activates human B cells to secrete TNF-α, IL-6, and IL-10 via JAK2/STAT3 and p38MAPK/ERK1/2 signaling pathway. J Clin Immunol. 2011;31:472–478. doi: 10.1007/s10875-010-9507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meyers JA, McTiernan A, Ulrich CM. Leptin and immune function: integrating the evidence. Nutr Res. 2005;25:791–803. [Google Scholar]
- 24.Faggioni R, Fantuzzi G, Fuller J, Dinarello CA, Feingold KR, Grunfeld C. IL-1β mediates leptin induction during inflammation. Am J Physiol. 1998;274:R204–R208. doi: 10.1152/ajpregu.1998.274.1.R204. [DOI] [PubMed] [Google Scholar]
- 25.Johnson RB, Serio FG. Leptin within healthy and diseased human gingiva. J Periodont. 2001;72:1254–1257. doi: 10.1902/jop.2000.72.9.1254. [DOI] [PubMed] [Google Scholar]
- 26.Karthikeyan BV, Pradeep AR. Leptin levels in gingival crevicular fluid in periodontal health and disease. J Periodontal Res. 2007;42:300–304. doi: 10.1111/j.1600-0765.2006.00948.x. [DOI] [PubMed] [Google Scholar]
- 27.Martin-Gonzalez J, Sanchez-Jimenez F, Perez-Perez A, Carmona-Fernandez A, Sanchez-Margalet V, Segura-Egea JJ. Leptin expression in healthy and inflamed human dental pulp. Int Endod J. 2013;46:442–448. doi: 10.1111/iej.12009. [DOI] [PubMed] [Google Scholar]
- 28.Dilsiz A, Kilic N, Aydin T, Ates N, Zihni M, Bulut C. Leptin levels in gingival crevicular fluid during orthodontic tooth movement. Angle Orthod. 2010;80:504–508. doi: 10.2319/072109-402.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shalitin S, Phillip M. Role of obesity and leptin in the pubertal process and pubertal growth- a review. Int J Obesity Relat Metab Disord. 2003;27:869–874. doi: 10.1038/sj.ijo.0802328. [DOI] [PubMed] [Google Scholar]
- 30.Neeley WW, Gonzales DA. Obesity in adolescence: Implications in orthodontic treatment. Am J Orthod Dentofacial Orthop. 2007;131:581–588. doi: 10.1016/j.ajodo.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 31.Von Bremen J, Wagner J, Ruf S. Correlation between body mass index and orthodontic treatment outcome. Angle Orthod. 2013;83:371–375. doi: 10.2319/070612-555.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 34.Thanakun S, Watanabe H, Thaweboon S, Izumi Y. An effective technique for the processing of saliva for the analysis of leptin and adiponectin. Peptides. 2013;47:60–65. doi: 10.1016/j.peptides.2013.06.010. [DOI] [PubMed] [Google Scholar]
- 35.Sueri MY, Turk T. Effectiveness of laceback ligatures on maxillary canine retraction. Angle Orthod. 2006;76:1010–1014. doi: 10.2319/100605-351. [DOI] [PubMed] [Google Scholar]
- 36.Khambay BS, McHugh S, Millett DT. Magnitude and reproducibility of forces generated by clinicians during laceback placement. J Orthod. 2006;33:270–275. doi: 10.1179/146531205225021762. [DOI] [PubMed] [Google Scholar]
- 37.Schrauwen P, van Marken Lichtenbelt WD, Westerterp KR, Saris WHM. Effect of diet composition on leptin concentration in lean subjects. Metabolism. 1997;46:420–424. doi: 10.1016/s0026-0495(97)90059-7. [DOI] [PubMed] [Google Scholar]
- 38.Javed F, Yu W, Thornton J, Colt E. Effect of fat on measurement of bone mineral density. Int J Body Compos Res. 2009;7:37. [PMC free article] [PubMed] [Google Scholar]
- 39.Myers MG, Leibel RL, Seeley R, Schwartz MW. Obesity and leptin resistance: distinguishing cause from effect. Trends Endocrinol Metab. 2010;21:643–651. doi: 10.1016/j.tem.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]


