Abstract
Objective:
To see whether there is an advantage to using a self-limiting phosphoric acid etchant versus a traditional 34% phosphoric acid etchant for bonding by measuring the depth of etch at multiple time intervals.
Materials and Methods:
A total of 25 bovine teeth were mounted and etched on the facial surface with two different etchants: standard 34% phosphoric acid and a self-limiting 35% phosphoric acid etchant at varied time intervals of 15, 30, 60, 90, and 120 seconds. Teeth were scanned using a three-dimensional laser confocal scanning microscope prior to etching and scanned again after etching to determine the depth of enamel etched compared to the baseline enamel surface prior to etching.
Results:
The 34% phosphoric acid etchant etched significantly deeper than the self-limiting etch. Etch times exceeding 30 seconds also etched significantly deeper for both types of etchant.
Conclusion:
The etch depth of the self-limiting etchant was consistently less than the standard etchant. Both types of etchant etched deeper after 30 seconds, but the depth of etch at 120 seconds was not different than at 60 seconds, indicating that both etchants are somewhat self-limiting in depth. Therefore, there is no advantage to using the self-limiting etchant.
Keywords: Acid etch, Self-limiting etch, Enamel
INTRODUCTION
Buonocore1 and Silverstone et al.2 led the profession in acid etching and bonding of resin to etched enamel surfaces. These developments allowed orthodontists to bond brackets reliably and apply forces to move teeth without banding every tooth or having brackets debond.
Buonocore reported that 85% phosphoric acid etchant significantly increased the adhesion of acrylic resin to enamel.1 Since then, a variety of etchants have been used with different concentrations and etching times. These include hydrofluoric acid, citric acid, hydrochloric acid, maleic acid, nitric acid, and phosphoric acid in a variety of concentrations. Suggested etching times can vary from 15 to 60 seconds.3 In vitro, phosphoric acid has been the most effective in promoting enamel adhesion to dental materials.4
Silverstone5 investigated different acid solutions for their effect on human enamel in vitro. An unbuffered solution of 30% phosphoric acid produced the most favorable conditions.5 Two distinct surface changes were noted after etching. First, a shallow layer of enamel was removed along with plaque and surface and subsurface cuticles. Second, the remaining enamel surface was rendered porous by the acid solution. It is this porous region that the resin can penetrate and micromechanically bond with the enamel.5
Acid etching removes approximately 10 μm of enamel and creates a morphologically porous layer between 5 to 50 μm deep.6 Silverstone reported three different types of etch patterns on enamel surfaces after acid etching. Type 1 had preferential dissolution of enamel cores; type 2 had preferential dissolution of enamel prism peripheries; and type 3 dissolution typically occurred randomly. Type 1 and type 2 patterns were preferred to retain adhesives on enamel surface by micromechanical interlocking.5 Development of the acid-etching technique was based on the type 1 and/or type 2 patterns by optimizing the types, concentration, and etching duration of the acid etchant.
Etching enamel with 30% to 50% phosphoric etch for 60 seconds was accepted as the preferred method in the early 1980s.7 It has since been shown that etching for 15 to 20 seconds is equally effective.8 Certain situations may dictate a variation in etching times. Deciduous teeth require 120 seconds of etching to achieve the same etching pattern as permanent teeth because of a lower mineral content and higher internal pore volume.5,9
Overetching is thought to occur beyond 60 seconds, resulting in compromised tooth structure and bond strength.10 Confocal microscopy provides a way to measure depth of etch, and an ideal depth of etch is gauged to be 5 to 50 μm.11
Numerous studies have measured etching depth in either dentin or enamel. Scanning electron microscopy, contact profilometer, and the noncontact profilometer have been used.12–14 A recent development in depth measurement is confocal laser scanning microscopy (CLSM), which combines laser scanning with traditional visible light microscopy, producing a detailed three-dimensional image of the surface. This has been shown to be a reliable method for measuring enamel erosion.15
In 2006, Opal® Etch (Ultradent, South Jordan, UT), a 35% phosphoric acid gel, was introduced and advertised to prevent overetching. There is no peer-reviewed research to support this observation. The purpose of this study was to evaluate the self-limiting etch of Opal® Etch against a traditional 34% phosphoric acid etchant (Dentsply Caulk, Milford, Del) at 15, 30, 60, 90, and 120 seconds by measuring the depth of etch with confocal microscopy. An average depth of etch was measured as well as the deepest single point of etch.
MATERIALS AND METHODS
Power analysis was calculated based on a pilot study and determined that five samples per test group provided sufficient strength in this study. A sample size of five teeth per group would provide 80% power to detect an effect size of 0.23 (approximately 0.46 standard deviation difference) among means for the main factor of etch material, and a small effect size of 0.29 (or approximately a 0.58 standard deviation difference) among means for the main factor of time and for the interaction term when testing with a two-factor analysis of variance (ANOVA) at the alpha level of 0.05 using NCSS PASS 2002 statistical software (NCSS, LLC, Kaysville, UT).
A total of 100 bovine maxillary incisors were gathered for the study with the intent of using only 25 good specimens (Animal Technologies, Inc., Tyler, Tex). Teeth with defects, extensive craze lines, cracks, or chips were excluded. A total of 25 teeth were selected and stored in 0.5% chloramine-T solution until their use in the study. The teeth were sectioned by removing the root at the CEJ from the crown using a high speed air-turbine handpiece (MASTERtorque M8900L; KaVo Dental, Charlotte, N.C.) and a double-sided serrated diamond disk (NTI #D365-220; Kerr Corporation, Orange, Calif).
Each tooth was placed facial surface down on packing tape, sticky side up to prevent dislodging. A 1.25″ circular jig (Buehler, Lake Bluff, Ill) was placed around the tooth and orthodontic stone (Whipmix, Louisville, Ky) was poured into the jig and allowed to set. Mounted samples were polished (Ecomet 3; Buehler). Six jig samples at a time were polished with a g-force of 7.1, water, and 10 pounds pressure using 400-grit silicon carbide paper (Carbimet; Buehler) for 10 minutes, 600 grit for 10 minutes, 800 grit for 10 minutes, and 1000 grit for 20 minutes to achieve a smooth, even surface for testing. Samples had top and bottom surfaces parallel with each other and a smooth facial surface. It was verified that no dentin was exposed. Two etchant groups (representing the two types of etchant) were subdivided into five subgroups (representing the five different etching times) of five specimens each. To minimize variation between tooth samples, the same tooth was used for both the Dentsply and Opal® Etch samples. The polished facial surface was divided into two square samples roughly 2 mm × 2 mm using blue adhesive tape (3M Company, St. Paul, Minn).
Specimens were scanned using a three-dimensional laser confocal scanning microscope (VK-X250/X260K; Keyence, Osaka, Japan) prior to etching to provide a baseline to compare the postetch scan. Scanning parameters included an initial 10× magnification to capture the entire polished surface for ease of identifying landmarks followed by 20× magnification. The sample was etched in the first square with Dentsply etchant for the designated amount of time, rinsed with filtered water for 10 seconds, and blotted dry (Kimtech; Kimberly Clark, Dallas, Tex). The neighboring square of enamel was etched with Opal® Etch for the same amount of time followed by the same rinsing and drying regimen. After etching both squares, a postscan was completed in exactly the same manner as the initial scan.
Each subgroup consisting of five samples was etched with phosphoric acid using both Dentsply and Opal® Etch for the subgroup's designated time (15, 30, 60, 90, and 120 seconds). Samples were measured with a three-dimensional laser confocal scanning microscope before and immediately after etching to compare the two images to calculate the depth of etch. Measurements were recorded at six different lines throughout the etched area for each Dentsply and Opal® Etch. The results were collected after matching the prescan to the postscan with the MultiFileAnalyzer (Keyence). The following two different measurements were obtained: average depth of the entire etched surface (width × depth) and maximum depth (single deepest point). Scanning electron microscopic images (Field Emission SEM, Zeiss Sigma VP 1400; Carl Zeiss AG, Oberkochen, Germany) were made of selected specimens at various etching times.
A two-way ANOVA and Tukey's post hoc test were performed to evaluate the two independent variables of etchant type (two levels) and time (five levels) at α = .05. A one-way ANOVA and Tukey's post hoc test was selected to determine if there was a significant difference based on time of etching between the two etchants. A Bonferroni correction was applied because of multiple comparisons between time groups and etchant materials (α = .05).
RESULTS
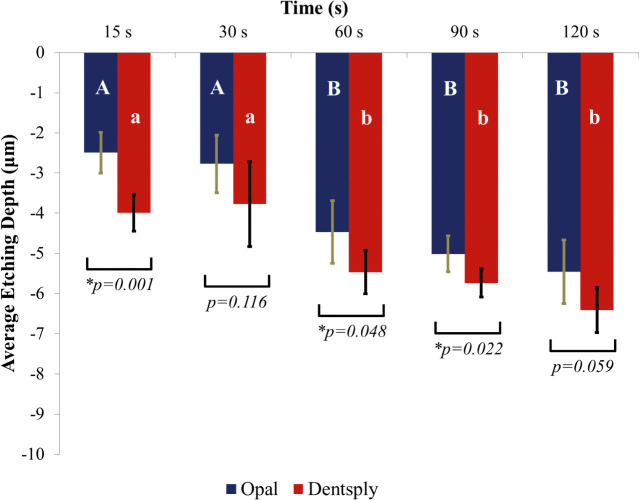
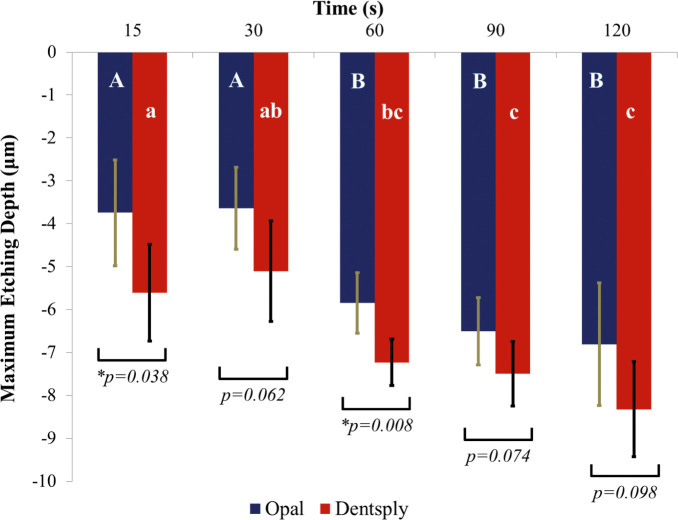
Mean etching depth and the single deepest point of etching depth in microns were recorded for each specimen and averaged. Standard deviations were also calculated. A graph depicting the average depth of both of the etchants at five different time points is illustrated in Figure 1. A graph depicting the single deepest point of etching of both of the etchants at five different time points is illustrated in Figure 2. For the average etching depth, a two-factor ANOVA revealed significant differences for both of the main effects: etchant type and elapsed etching time (P < .05). There was no interaction between the main effects (P = 0.752). Similarly, for the single deepest point of etch a two-factor ANOVA revealed significant differences for both main effects (P < .05). There was no interaction between the main effects (P = .918).
Figure 1.
Average etching depth in microns of Opal Etch and Dentsply 34% phosphoric acid etch at increasing etch duration in seconds. Bars indicate standard deviations.
Figure 2.
Single deepest point of etch depth in microns of Opal Etch and Dentsply 34% phosphoric acid etch at increasing etch duration in seconds. Bars indicate standard deviations.
Post hoc tests (Tukey's honest significant difference) were performed for Dentsply and Opal® Etch separately. For average etching depth, the 15-second and 30-second specimens for Opal® Etch were not significantly different from each other, but the 60-second, 90-second, and 120-second specimens were all significantly different from the shorter etch times. The 60-second, 90-second, and 120-second groups were not statistically different from each other. The same result was seen for the groups in the single deepest point of etch. Tukey's honest significant difference revealed the same differences for the Dentsply control etchant.
The data demonstrated that the average etching depth and the single deepest point of Dentsply Etch would etch deeper than Opal® Etch and that as time progressed past 30 seconds, the depth of etch would also increase. However, the increase in etching depth did not statistically increase any more after 30 seconds than at 120 seconds.
DISCUSSION
Overetching the tooth surface has been shown to lead to increased bond failures.10 Etching for more than 60 seconds, termed overetching, significantly decreases bond strength.16 A self-limiting etch would therefore be clinically beneficial to the orthodontist. The possibility of being able to etch an entire arch at one time without the risk of more frequent bond failures from overetching is an attractive concept. Etching an entire arch could exceed 60 seconds after all teeth in the arch have been etched. Overetching can occur when the acid is too strong or is left on the tooth for too long, resulting in collapsed enamel prisms that are not as amenable to the penetration of resin adhesives.
The etching of tooth enamel by phosphoric acid is itself somewhat self-limiting because of the high calcium content in enamel. Unless the etchant is constantly agitated, the calcium that is freed from the enamel hydroxyapatite surface while etching serves as a buffer.17 The viscous acid gel, held together mostly by glycerin, sits on top of the enamel. Demineralized surface calcium functions as a buffer to resist changes in hydrogen ion concentration. There is a gradient in the solubility of tooth enamel with depth.18 Therefore, restorative applications of dental adhesives require shorter etching times than orthodontic applications. Excessive etching times of 120 seconds change the pattern of the enamel surface to a point where resin infiltration and entanglement is not as profound as the etch patterns achieved at shorter times.2 If the enamel surface is etched with a viscous gel and not disturbed, the etching depth is limited by this buffering action.18
There are several unknown characteristics regarding the samples used in this study such as age, diet, and environment of the subjects, resulting in differing orientations of enamel prisms with differing reactions to acid conditioning.6 For this reason, each tooth was used to test both etch materials to minimize the variation in the enamel rod orientation. The mid-labial surface was used in the current study, so the enamel rods were likely positioned perpendicular to the surface. Every effort was made to ensure that all parameters of the study were constant to evaluate the interaction between the two independent variables of etchant type (two levels) and time (five levels).
CLSM was used for measuring enamel loss from acid etching via the surface profile. It has been shown that CLSM is as reliable as other methods (contact profilometer and noncontact profilometer) for use in erosion measurement.15 The reason why CLSM was selected for this study was that it does not require fine sectioning and eliminates the cumbersome procedures of scanning electron microscopy.
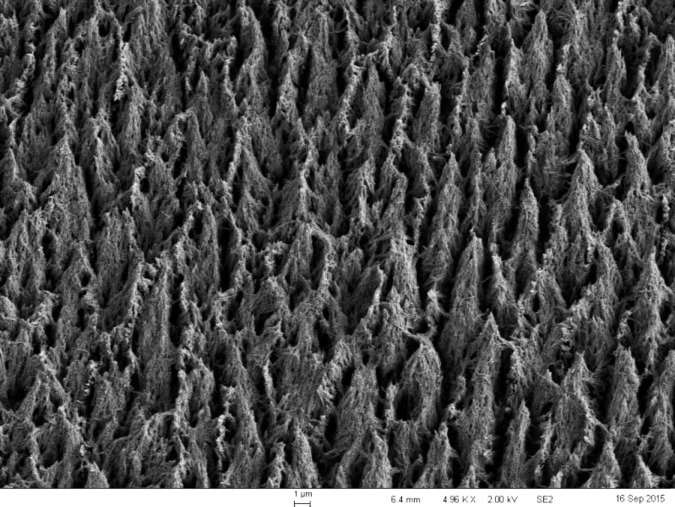
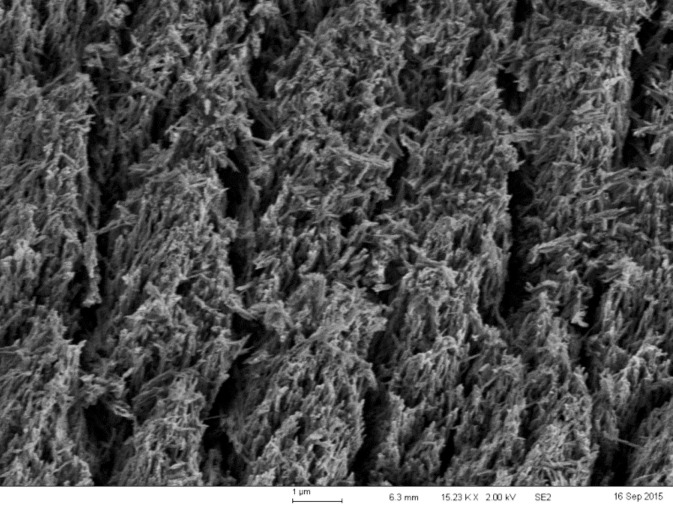
The data demonstrated that, at all etching times, the control etchant etched deeper than Opal® Etch. This difference was statistically significant, but it is questionable whether the difference is clinically relevant. Because the enamel thickness of most anterior teeth at the midfacial aspect exceeds 1000 μm,11 the difference of about 1.5 μm between the two etching methods is very small. The study also determined that, for both the control etchant and the self-limiting etchant, etching times of 15 seconds and 30 seconds were not statistically different from each other, but all etching times exceeding 30 seconds resulted in a deeper etch. For 60, 90, and 120 seconds, the etch was statistically significantly deeper than at 15 or 30 seconds. Furthermore, there was no statistical difference in the average depth of the etch or the single deepest point of the etch among the 60-, 90-, or 120-second groups. This validates the use of a 15- or 30-second etch time for enamel bonding. Etch times longer than 30 seconds can cause deleterious effects to enamel adhesion as the desirable etch pattern changes into a collapsed layer that is not as amenable to enamel adhesion. The problem with very long etching times is not the depth of the etch, but the change in etch patterns that allow for micromechanical bonding.2 Scanning electron micrograph images were made from selected specimens. The images of the etch patterns for Opal® Etch and Dentsply etch were not discernably different. Figure 3 shows the enamel etch pattern at 30 seconds for the control etchant at 5000× magnification. Figure 4 is the same image magnified to 15,000×. These images clearly demonstrate a preferential dissolution of the enamel prism cores resulting in a “honeycomb” appearance and a type 1 etch. Figure 5 shows the etched enamel at 120 seconds of etch duration at 5,000× magnification, and Figure 6 is an image at 15,000× magnification. At the extended etch time, it appears that the type 1 pattern barely exists because further dissolution of the enamel prism peripheries has occurred. There appears to be less space for a fluid resin to flow, hence less micromechanical interlocking and lower bond strength.19 Figure 7 is Opal® Etch at 120 seconds of etch time. The etch pattern is very similar to that of the control, with further dissolution of prism cores. Both etchants shared the characteristic that they will not significantly etch any deeper past etch times of 60 seconds, so it can be said that both products are somewhat self-limiting in depth. Scanning electron micrograph images suggest that prolonged etching affects the etch pattern and that the depth of etch is clinically less important to adhesion. Currently, all gel acid etchants will not continue etching more deeply into enamel as etch time goes beyond 60 seconds. If the profession desires an acid etchant that maintains a type I etch pattern past 60 seconds, that etchant has not yet been developed.
Figure 3.
A 30-second 34% phosphoric acid etch of enamel at 5000× magnification displaying a classic type I etch pattern, with differential dissolution of prism cores leaving a honeycomb appearance.
Figure 4.
Same image as Figure 3 at 15,000× magnification.
Figure 5.
A 120-second 34% phosphoric acid etch of enamel at 5000× magnification displaying a type I etch pattern but with collapsing peripheries.
Figure 6.
Same image as Figure 5 at 15,000× magnification.
Figure 7.
A 120-second acid etch with Opal Etch at 15,000× magnification displaying a barely discernable type I etch pattern with collapsing prisms.
CONCLUSIONS
-
•
There were significant differences in etching depth as a result of both the etchant type and etching duration.
-
•
In all instances, Opal® Etch etched less deeply than the control etchant.
-
•
Etching times exceeding 30 seconds resulted in etching depths significantly deeper than the depth recorded at 15 and 30 seconds. After 60 seconds of etching, neither product etched any more deeply with etch times of 90 or 120 seconds. Therefore, there is no clinical advantage to using the self-limiting etchant.
REFERENCES
- 1.Buonocore MG. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J Dent Res. 1955;34:849–853. doi: 10.1177/00220345550340060801. [DOI] [PubMed] [Google Scholar]
- 2.Silverstone LM, Saxton CA, Dogon IL, Fejerskov O. Variation in the pattern of acid etching of human dental enamel examined by scanning electron microscopy. Caries Res. 1975;9:373–387. doi: 10.1159/000260179. [DOI] [PubMed] [Google Scholar]
- 3.Perdigao J. An Ultra-Morphological Study of Human Dentine Exposed to Adhesive Systems [thesis] Leuven, Belgium: Catholic University of Leuven; 1995. [Google Scholar]
- 4.Gwinnett AJ. Histologic changes in human enamel following treatment with acidic adhesive conditioning agents. Arch Oral Biol. 1971;16:731–788. doi: 10.1016/0003-9969(71)90118-x. [DOI] [PubMed] [Google Scholar]
- 5.Silverstone LM, Dogon IL, editors. Proc Int Symp Acid Etch Tech. The Acid Etch Technique. St. Paul, Minn: North Central Publishing; 1975. The acid etch technique: in vitro studies with special reference to the enamel surface and the enamel-resin interface; pp. 13–39. [Google Scholar]
- 6.Lopes GC, Thys DG, Klaus P, Oliviera GM, Widmer N. Enamel acid etching: a review. Compend Contin Educ Dent. 2007;28:18–24. [PubMed] [Google Scholar]
- 7.Zhu JJ, Tang AT, Matinlinna JP, Hägg U. Acid etching of human enamel in clinical applications: a systematic review. J Prosthet Dent. 2014;112:122–135. doi: 10.1016/j.prosdent.2013.08.024. [DOI] [PubMed] [Google Scholar]
- 8.Gwinnet AJ. Oper Dent. 1992;(suppl. 5):10–17. [PubMed] [Google Scholar]
- 9.Angmar B, Carlstrom D, Glas JE. Studies of the ultrastructure of dental enamel. IV. The mineralization of normal human enamel. J Ultrastruct Res. 1963;8:12–23. doi: 10.1016/s0022-5320(63)80017-9. [DOI] [PubMed] [Google Scholar]
- 10.Wang W, Lu T. Bond strength with various etching times on young permanent teeth. Am J Orthod Dentofacial Orthop. 1991;100:72–79. doi: 10.1016/0889-5406(91)70052-X. [DOI] [PubMed] [Google Scholar]
- 11.Fruits TJ, Khajotia SS, Nicholson JW. Biologic considerations. In: TJ Hilton, Ferracane JL, Broome JC., editors. Summitt's Fundamentals of Operative Dentistry: A Contemporary Approach 4th ed. Chicago, Ill: Quintessence Publishing Co; 2013. pp. 7–26. Inc.; [Google Scholar]
- 12.Barkmeier WW, Erickson RL, Kimmes NS, Latta MA, Wilwerding TM. Effect of enamel etching time on roughness and bond strength. Oper Dent. 2009;34:217–222. doi: 10.2341/08-72. [DOI] [PubMed] [Google Scholar]
- 13.Reis AF, Giannini M, Kavaguchi A, Soares CJ, Line SR. Comparison of microtensile bond strength to enamel and dentin of human, bovine, and porcine teeth. J Adhes Dent. 2004;6:117–121. [PubMed] [Google Scholar]
- 14.Legler LR, Retief DH, Bradley EL. Effects of phosphoric acid concentration and etch duration on enamel depth of etch: an in vitro study. Am J Orthod Dentofacial Orthop. 1990;98:154–160. doi: 10.1016/0889-5406(90)70009-2. [DOI] [PubMed] [Google Scholar]
- 15.Paepegaey AM, Barker ML, Bartlett DW, et al. Measuring enamel erosion: a comparative study of contact profilometry, non-contact profilometry and confocal laser scanning microscopy. Dent Mater. 2013;29:1265–1272. doi: 10.1016/j.dental.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Barkmeier WW, Shaffer SE, Gwinnet AJ. Effects of 15 vs. 60 second enamel acid conditioning on adhesion and morphology. Oper Dent. 1986;11:111–116. [PubMed] [Google Scholar]
- 17.Driessens FCM, Verbeeck RMH. Biominerals. Boca Raton, Fla: CRC Press; 1990. [Google Scholar]
- 18.Driessens FCM. Mineral aspects of dentistry. In: Myers HM, editor. Monographs in Oral Science, Vol. 10. Basel, Switzerland: Karger; 1982. pp. 139–152. [PubMed] [Google Scholar]
- 19.Carstensen W. The effects of different phosphoric acid concentrations on surface enamel. Angle Orthod. 1992;62:51–58. doi: 10.1043/0003-3219(1992)062<0051:TEODPA>2.0.CO;2. [DOI] [PubMed] [Google Scholar]