Abstract
Objective:
To investigate the association between orthodontic treatment and periodontitis in a nationally representative sample of South Korea.
Materials and Methods:
Data from the Fifth and Sixth Korean National Health and Nutrition Examination Survey (KNHANES V, VI-1, and VI-2), conducted from 2012 to 2014, were used in this study. The final sample size consisted of 14,693 adults aged ≥19 years. Logistic regression analysis was performed to assess the association between orthodontic treatment and periodontitis.
Results:
The orthodontic treatment group exhibited a lower prevalence of periodontitis compared with the nonorthodontic treatment group. The adjusted odds ratios for periodontitis in subjects with a history of orthodontic treatment compared with those with no history of orthodontic treatment were 0.553, 0.614, and 0.624, when adjusted for various confounding variables (P < .0001). The subjects with periodontitis were of higher age, body mass index, waist circumference, and white blood cell counts compared with the subjects without periodontitis regardless of history of orthodontic treatment.
Conclusions:
History of orthodontic treatment was associated with a decreased rate of periodontitis.
Keywords: Orthodontics, Periodontitis, National survey, Cross-sectional study
INTRODUCTION
Orthodontic treatment can improve facial esthetics and mastication through the alignment of teeth. However, dental caries, tooth discoloration, and gingival hyperplasia have been reported as complications of this treatment. It is difficult to maintain oral hygiene because of the presence of orthodontic appliances, bands, and elastics. These conditions may lead to plaque accumulation and changes in the composition and type of oral bacteria.1,2 On the contrary, a systematic review indicated that there is a positive relationship between malocclusion and periodontitis.3 However, it was also reported that there was no reliable evidence suggesting a positive effect of orthodontic treatment on periodontal health.3 Some authors have reported that plaque and bleeding indexes improve after orthodontic treatment.4 Despite this controversy, periodontal disease is not an absolute contraindication for orthodontic treatment. It is possible that properly aligned teeth contribute to the maintenance or improvement of oral health. The confusion or debate over the relationship between orthodontic treatment and periodontitis may be primarily due to a lack of evidence. Moreover, the target population of previous studies did not consist of representative samples.
The current study focused on the association between orthodontic treatment and periodontitis in a nationally representative sample. The hypothesis was that orthodontic treatment may be associated with decreased prevalence of periodontitis, which was tested using multiple regression models. The purpose of this study was to investigate the association between orthodontic treatment and periodontitis in a nationally representative sample of the South Korean population.
MATERIALS AND METHODS
Study Population
Data were obtained from the Fifth and Sixth Korean National Health and Nutrition Examination Survey (KNHANES V, VI-1, and VI-2), conducted from 2012 to 2014. The KNHANES is a nationwide survey of a representative sample of the South Korean population and is conducted by the Korean Center for Disease Control and Prevention. This survey was approved by the Institutional Review Board for Human Subjects of the Korea Center for Disease Control and Prevention. Before the survey, each participant signed an informed consent form. The survey was composed of health interviews, health examinations, and a nutrition survey. Trained interviewers carried out face-to-face interviews with a structured questionnaire. Trained and calibrated examiners inspected the participant's physical status. Physical examinations and blood sampling were performed at a mobile examination center. All data used in the present study are available in public files provided by the Korea Centers for Disease Control and Prevention and the Ministry of Health and Welfare of Korea.
Variables and Measurements
Clinical and laboratory data were collected. The education level was classified as high if the respondent had finished high school. Individuals with household incomes <25% of the total equivalized income were classified in the low-income group. Residential location was divided into two groups (rural versus urban). Alcohol intake status was classified into three groups: nondrinkers, mild-to-moderate drinkers (<30.0 g alcohol/d), and heavy drinkers (≥30.0 g alcohol/d). Smoking status was classified into three groups: nonsmokers, ever-smokers (have smoked at least five packs of cigarettes in their whole lives), and current smokers (have smoked at least five packs of cigarettes in their whole lives and still smoke). Occupation was defined as the participant's self-reported economic activity. Regular physical exercise was defined as intense physical activity performed for at least 20 minutes at a time at least three times a week according to the International Physical Activity Questionnaire short form modified for Korea.5
Blood samples were obtained after fasting for at least 8 hours and appropriately processed. Serum levels of triglyceride (TG), high-density lipoprotein (HDL), and fasting blood glucose (FBG) were measured using a Hitachi Automatic Analyzer 7600 (Hitachi, Tokyo, Japan) in 2012 and Hitachi Automatic Analyzer 7600-210 (Hitachi, Tokyo, Japan) in 2013 and 2014. White blood cells (WBCs) were counted using a Sysmex XE-2100D (Sysmex, Kobe, Japan) in 2012, 2013, and 2014. Diabetes was diagnosed when FBG was >126 mg/dL or when the individual was currently using antidiabetic medication. Hypertension was defined as systolic blood pressure >160 mm Hg, diastolic blood pressure >90 mm Hg, or current use of systemic antihypertensive drugs. Waist circumference was measured at the narrowest point between the lower border of the rib cage and the iliac crest. The waist circumference cutoff was defined as ≥90 cm in men and ≥80 cm in women. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. The BMI cutoff was 23 kg/m2 for overweight and 25 kg/m2 for obesity.6 Metabolic syndrome was defined according to the American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement criteria for Asians.7 Specifically, metabolic syndrome was diagnosed when greater than or equal to three of the following criteria were met: (1) waist circumference ≥90 cm in men or ≥80 cm in women, (2) fasting TG ≥150 mg/dL or use of lipid-lowering medication, (3) HDL-C <40 mg/dL in men or <50 mg/dL in women or use of cholesterol-lowering medication, (4) blood pressure ≥130/85 mm Hg or use of antihypertensive medication, and (5) FBG ≥100 mg/dL or current use of antidiabetic medication.
History of orthodontic treatment was assessed by a “yes” or “no” questionnaire.
Periodontitis
Periodontal health was evaluated according to the World Health Organization community periodontal index (CPI).8 Each CPI score ranged from 0 to 4: healthy periodontal conditions (CPI = 0), gingival bleeding (CPI = 1), calculus and bleeding (CPI = 2), a shallow periodontal pocket of 3.5 to <5.5 mm (CPI = 3), or a deep periodontal pocket of ≥5.5 mm (CPI = 4). Periodontal pocket depth was measured at six sites (mesiobuccal, midbuccal, distobuccal, distolingual, midlingual, and mesiolingual sites) of each tooth with a CPI probe. Periodontitis was defined as a CPI value of 3 or 4, indicating that at least one site had a >3.5-mm pocket.9,10
The eight molars and the upper right and lower left central incisors were examined by 30 (2012 KNHANES), 33 (2013 KNHANES), and 21 (2014 KNHANES) trained and calibrated dentists. Training was performed to standardize the survey and minimize errors in the measurement of periodontal probing depths. Each examiner was trained using dental models and simulation of the oral health examination with human subjects. They were also provided field training, reproducing examinations, and periodic calibration. Reliability of examination was confirmed by specialists commissioned by the Ministry of Health and Welfare of Korea. The interexaminer means of the Kappa value were 0.69 in 2012, 0.70 in 2013, and 0.79 in 2014.11–13
Statistical Analyses
All survey analyses included the sampling weights, strata, and clusters because KNHANES is a stratified multistage clustered probability design. All data were expressed as mean ± standard error or percentage. Logistic regression analyses were performed to assess odds ratios (ORs) and 95% confidence intervals (CIs), which were used to determine the association between orthodontic treatment and periodontal disease. Regression analyses were performed in accordance with KNHANES statistical guidelines. ORs and CIs were estimated after adjustment for potential confounders.
Three multiple regression models were used. Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, income, education, alcohol intake, smoking, regular exercise, BMI, and WBC count. Model 3 was adjusted for age, sex, income, education, alcohol intake, smoking, regular exercise, BMI, WBC count, diabetes, hypertension, metabolic syndrome, and tooth brushing times per day.
All data were analyzed using SAS software version 9.2 for Windows (SAS Institute Inc, Cary, NC). Results were considered significantly different when P < .05.
RESULTS
A total of 23,625 subjects participated in the periodontal disease questionnaire and examinations (8057 participants in 2012, 8018 in 2013, and 7550 in 2014). The participation rate was 80.0% in 2012, 79.3% in 2013, and 77.8% in 2014. Participants aged <19 years (5244 participants) and patients with missing values in the health assessment or questionnaires (3689 participants) were excluded from the current analysis. As a result, the final sample size for the present study was 14,693.
Table 1 shows the general characteristics of the subjects in this survey. The prevalence of periodontitis was lower in the orthodontic treatment group compared with the nonorthodontic treatment group (9% vs 44%). The subjects with periodontitis were of higher age, BMI, waist circumference, and WBC count compared with the subjects without periodontitis regardless of orthodontic treatment. In the nonorthodontic treatment group, the subjects with periodontitis exhibited higher rates of diabetes, hypertension, and metabolic syndrome. However, in the orthodontic treatment group, the subjects with periodontitis did not show a higher prevalence of diabetes. The subjects with periodontitis showed higher rates of smokers regardless of orthodontic treatment. In the orthodontic treatment group, household income level was not associated with prevalence of periodontitis, unlike the nonorthodontic treatment group. Frequency of tooth brushing and use of an interdental brush and floss were not associated with periodontitis in the orthodontic treatment group.
Table 1.
Demographic Characteristics of the Study Population (N = 14,693)a
|
|
Orthodontic Treatment (No) |
Orthodontic Treatment (Yes) |
||||
| Nonperiodontitis (n = 9752) |
Periodontitis (n = 4157) |
P
|
Nonperiodontitis (n = 716) |
Periodontitis (n = 68) |
P
|
|
| Age, y (mean ± SD) | 42.96 ± 0.24 | 54.49 ± 0.31 | <.0001 | 30.17 ± 0.38 | 40.07 ± 1.34 | <.0001 |
| Male sex (%) | 46.2 (0.6) | 59.5 (0.8) | <.0001 | 39.1 (2.2) | 54.8 (7.2) | .0373 |
| Education (%) | <.0001 | .0076 | ||||
| Elementary school or less | 12.8 (0.5) | 28.7 (1) | 0.6 (0.3) | 3.8 (1.7) | ||
| Middle school | 8.1 (0.3) | 14.5 (0.7) | 1.1 (0.4) | 3.3 (2) | ||
| High school | 41.7 (0.7) | 34.3 (1) | 44.2 (2.4) | 50.2 (6.9) | ||
| University or higher | 37.4 (0.8) | 22.5 (1) | 54.1 (2.4) | 42.7 (6.7) | ||
| Household income (%) | <.0001 | .3225 | ||||
| <25% | 12.6 (0.6) | 20.8 (1) | 5.9 (1.1) | 5.2 (2.7) | ||
| 25%∼50% | 25.8 (0.8) | 27.1 (1) | 22.5 (2) | 23.3 (5.5) | ||
| 50%∼75% | 30.2 (0.8) | 27 (1) | 32.5 (2.1) | 21.6 (5.4) | ||
| >75% | 31.4 (1) | 25.1 (1.2) | 39.1 (2.4) | 49.9 (7.5) | ||
| Smoking (%) | <.0001 | .0035 | ||||
| Nonsmoker | 63.5 (0.6) | 46.3 (0.9) | 71.9 (2.1) | 47.4 (7.2) | ||
| Ex-smoker | 15.2 (0.4) | 22.6 (0.8) | 9.7 (1.3) | 18.1 (6) | ||
| Current smoker | 21.2 (0.6) | 31 (0.9) | 18.4 (1.8) | 34.5 (7.4) | ||
| Alcohol consumption (%) | <.0001 | .6922 | ||||
| Nondrinker | 23.1 (0.6) | 28.6 (0.9) | 13.2 (1.4) | 12.3 (4.7) | ||
| Mild to moderate-drinker | 68.6 (0.6) | 60.1 (1) | 79.2 (1.7) | 76.6 (6.4) | ||
| Heavy drinker | 8.3 (0.4) | 11.3 (0.6) | 7.5 (1.2) | 11.1 (5.3) | ||
| Place, urban (%) | 84.4 (1.5) | 75.5 (2.3) | <.0001 | 90.8 (1.7) | 71.1 (6.8) | <.0001 |
| Occupation (yes, %) | 62.5 (0.6) | 65.4 (1) | .0093 | 60.1 (2.4) | 67.7 (6.5) | .2994 |
| Frequency of tooth brushing per day (%) | <.0001 | .1791 | ||||
| 1 | 8.4 (0.4) | 15.3 (0.7) | 4.1 (0.9) | 3.8 (2.4) | ||
| 2 | 36.9 (0.6) | 43.3 (0.9) | 27.6 (2) | 39.5 (6.8) | ||
| 3 | 54.8 (0.6) | 41.4 (1) | 68.2 (2) | 56.8 (6.9) | ||
| Use of interdental brush (yes, %) | 23.3 (0.6) | 11.8 (0.6) | <.0001 | 32.6 (2.1) | 29 (6.8) | .624 |
| Use of floss (yes, %) | 19 (0.6) | 18.1 (0.8) | .3658 | 24.1 (1.9) | 28 (6.5) | .5481 |
| Professional oral examination within 1 year (yes, %) | 26.8 (0.6) | 26.5 (0.9) | .8123 | 38.7 (2.1) | 27.4 (6.5) | .1257 |
| Self-reported oral health status (%) | <.0001 | <.0001 | ||||
| Good | 14.9 (0.5) | 10.4 (0.5) | 21 (1.9) | 11.8 (4.1) | ||
| Moderate | 44.9 (0.7) | 32.6 (0.9) | 47.9 (2.2) | 29.2 (5.9) | ||
| Bad | 40.3 (0.7) | 57 (1) | 31.1 (2) | 59 (6.6) | ||
| White blood cell count (×109/L)b | 5.84 (5.79–5.88) | 6.2 (6.13–6.27) | <.0001 | 5.92 (5.78–6.06) | 6.29 (5.96–6.65) | .0421 |
| Body mass index, kg/m2 (mean ± SD) | 23.63 ± 0.05 | 24.37 ± 0.07 | <.0001 | 22 ± 0.17 | 23.92 ± 0.46 | <.0001 |
| Waist circumference, cm (mean ± SD) | 80.03 ± 0.16 | 83.72 ± 0.21 | <.0001 | 75.2 ± 0.48 | 81.64 ± 1.23 | <.0001 |
| Number of remaining teeth | 25.83 ± 0.06 | 23.85 ± 0.11 | <.0001 | 25.97 ± 0.09 | 24.96 ± 0.38 | .0093 |
| Regular exercise within a week (yes, %) | 40.3 (0.7) | 35.7 (0.9) | <.0001 | 41.8 (2.2) | 48 (6.9) | .3628 |
| Diabetes (yes, %) | 6.1 (0.3) | 16.8 (0.7) | <.0001 | 1.3 (0.5) | 4 (2.5) | .0994 |
| Hypertension (yes, %) | 20.7 (0.6) | 38.9 (1) | <.0001 | 4.3 (0.8) | 15.2 (4.6) | .0002 |
| Metabolic syndrome (yes, %) | 22.8 (0.6) | 40.1 (1) | <.0001 | 7.4 (1.1) | 28.6 (6.7) | <.0001 |
Values are presented as the mean ± standard error for continuous variables or as the proportion (standard error) for categorical variables. Ex-smokers have smoked at least five packs of cigarettes in their whole lives; current smokers have smoked at least five packs of cigarettes in their whole lives and smoke currently; mild-to-moderate drinkers drink <30.0 g alcohol/d; heavy drinkers drink ≥30.0 g alcohol/d.
Log transformation was performed to obtain P values, and the value represents the geometric mean (95% confidence interval).
Table 2 shows the associations between oral hygiene habits and orthodontic treatment. The orthodontic treatment group showed lower rates of chewing and speaking difficulties and higher rates of having had a professional oral examination within 1 year compared with the nonorthodontic treatment group. The subjects with orthodontic treatment exhibited more frequent tooth brushing and felt that their oral health status was above normal.
Table 2.
Association Between Oral Hygiene Habits and Orthodontic Treatmenta
|
|
Orthodontic T |
||
| No (n = 13,752) |
Yes (n = 782) |
P
|
|
| Daily frequency of tooth brushing | <.0001 | ||
| ≤1 | 10.3 (0.3) | 4.1 (0.8) | |
| 2 | 38.6 (0.5) | 28.6 (1.8) | |
| ≥3 | 51.1 (0.6) | 67.3 (1.9) | |
| Professional oral examination within the past year (yes, %) | 26.7 (0.5) | 37.8 (2.0) | <.0001 |
| Chewing difficulty (yes, %) | <.0001 | ||
| Discomfort | 20.8 (0.4) | 9.9 (1.2) | |
| Minor problem | 16.1 (0.4) | 15.2 (1.6) | |
| No problem | 63.1(0.5) | 75.0 (1.9) | |
| Speaking difficulty (yes, %) | .0003 | ||
| Discomfort | 8.1 (0.3) | 3.7 (0.8) | |
| Minor problem | 10.4 (0.3) | 9.4 (1.2) | |
| No problem | 81.4 (0.4) | 86.9 (1.4) | |
| Self-reported oral health status | <.0001 | ||
| Good | 13.6 (0.4) | 20.3 (1.8) | |
| Moderate | 41.5 (0.6) | 46.4 (2.1) | |
| Bad | 44.9 (0.6) | 33.3 (2.0) | |
Values are presented as the mean ± standard error for continuous variables or as the proportion (standard error) for categorical variables.
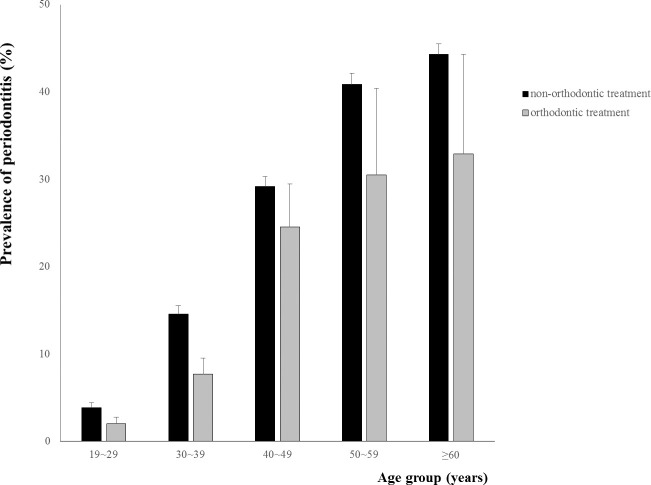
Generally, the orthodontic treatment group showed a lower prevalence of periodontitis compared with the nonorthodontic treatment group in all age groups (Figure 1). The orthodontically treated adults may have come from a higher socioeconomic status to begin with, which would have influenced the results as this is a significant factor in adult periodontal health. However, considering confounding factors including income and education level, the orthodontic treatment group also showed lower prevalence of periodontitis compared with the nonorthodontic treatment group (Table 3). The adjusted ORs were 0.553 (model 1), 0.614 (model 2), and 0.624 (model 3) in the orthodontic treatment group (P < .0001).
Figure 1.
Prevalence of periodontitis by age groups between patients with and without a history of orthodontic treatment.
Table 3.
Adjusted Odds Ratios (95% Confidence Interval) for Periodontitis According to Orthodontic Treatmenta
|
|
Model 1 |
Model 2 |
Model 3 |
| Orthodontic treatment | 0.553 (0.414–0.74) | 0.614 (0.452–0.836) | 0.624 (0.455–0.854) |
| P < .0001 | P < .0001 | P < .0001 |
Model 1 was adjusted for age and sex. Model 2 was adjusted for age, sex, income, education, alcohol intake, smoking, regular exercise, body mass index, and white blood cell counts. Model 3 was adjusted for age, sex, income, education, alcohol intake, smoking, regular exercise, body mass index, white blood cell counts, diabetes, hypertension, metabolic syndrome, and daily frequency of tooth brushing.
DISCUSSION
This study investigated the association between orthodontic treatment and periodontitis in a nationally representative sample of the Korean population. The results of this study indicated that orthodontic treatment is associated with a decreased prevalence of periodontitis. Periodontitis was less prevalent in the orthodontic treatment group. To the best of our knowledge, this is the first study to examine the relationship between orthodontic treatment and periodontitis in the general population.
The effect of orthodontic treatment on prevalence of periodontitis has been debated. Recently, the importance of periodontal health has increased as the number of adult orthodontic patients has increased. The relationship between orthodontic treatment and periodontitis has been widely studied. Many clinical studies have reported that plaque accumulation and gingivitis increased during orthodontic treatment.14–16 The composition and types of oral bacteria were altered as a result of orthodontic treatment.1,17,18 Recent animal studies suggested that orthodontic tooth movement had a synergistic effect on periodontitis by increasing the presence of IL-1β and TNF-α.19
DNA probe analysis revealed that periodontal pathogens increased after 6 months of orthodontic treatment but decreased to the level of pretreatment after 12 months of therapy.20 In that study, the authors hypothesized that the fixed appliances may provide some advantages for periodontal pathogens only during the initial stage of orthodontic treatment.20 One long-term study showed that pocket depths were not significantly different between subjects with a history of orthodontic treatment and a nonorthodontic control group.21 This study also showed that the gingival index was higher in the control group. Another population-based longitudinal study found that periodontal attachment loss and gingival recession was not significantly different between the orthodontic treatment group and nonorthodontic treatment group.22 The current study found that periodontitis tended to be less prevalent in the orthodontic treatment group.
Some interesting results were found in the present study. Patients with periodontitis exhibited higher WBC counts, increased BMI, and a greater incidence of diabetes, hypertension, and metabolic syndrome than subjects without periodontitis. These findings were similar to those of previous studies.23,24 These health states may also be related to socioeconomic status. The relationship between periodontitis and socioeconomic position such as education and income has been reported since the 1960s.25 Periodontitis was less common in the subjects with higher levels of education and income.25,26 However, household income was not associated with periodontitis in the orthodontic treatment group in this study. This may be due to a relatively lower proportion in the lowest income group in the orthodontic treatment group because of the high cost of orthodontic treatment.
Plaque control habits such as frequency of tooth brushing and the use of an interdental brush and floss were not associated with prevalence of periodontitis in the orthodontic treatment group, unlike in the nonorthodontic treatment group. It may be suggested that the subjects with periodontitis in the orthodontic treatment group try to maintain good oral hygiene. It was also found that the orthodontic treatment group tended to have a lower prevalence of periodontitis compared with the nonorthodontic treatment group in all age groups. This suggests that orthodontic treatment had a preventive effect on periodontitis. According to these results, there are several possible explanations for the association between orthodontic treatment and periodontitis.
First, the lower prevalence of periodontitis in the orthodontic treatment group may be associated with tooth alignment. In fact, it has been observed that crowded or tipped teeth are difficult to clean. Orthodontic treatment may help patients clean their teeth and facilitate oral health care. Previous studies have shown that the plaque and gingival indexes of crowded teeth were significantly higher than that of noncrowded teeth.27,28 Also, the number of periodontal pathogens in crowded teeth was found to be greater than the number in noncrowded teeth.29 Orthodontic uprighting of tipped teeth may also eliminate plaque-retentive areas and reduce periodontal pocket depths on mesial surfaces.30–32
Second, occlusal conditions may have contributed to the lower prevalence of periodontitis in the orthodontic treatment group in this study. Traumatic occlusion has been considered as one of the detrimental factors in periodontal disease. A population-based cross-sectional study showed that nonworking side contacts were associated with increased attachment loss.31 Some retrospective studies indicated that occlusal discrepancies between initial contact and centric occlusion, centric relation, and working and balancing contacts are associated with periodontal disease.33,34 Teeth with occlusal discrepancies exhibited deeper periodontal pockets but did not increase in probing depth after occlusal treatment.34
Third, it may be supposed that improved plaque control habits and periodontal therapy before orthodontic treatment may provide positive long-term effects on the management of periodontal health. Recently, orthodontists have become more interested in periodontal disease and maintenance because of the increasing number of adult orthodontic patients. Regular oral health checkups during orthodontic treatment may be helpful for motivating patients and create more interest in maintaining periodontal health.
The main strength of the present study was that it included a nationally representative sample of Koreans with sufficient power for investigation of an association between orthodontic treatment and periodontitis. Also, statistical adjustments were performed to minimize the effects of confounding factors such as socioeconomic status and periodontal plaque control methods. To the best of our knowledge, this is the first study to examine the association between orthodontic treatment and periodontitis in the general population.
Limitations
The results should be interpreted with some caution because there are some limitations. First, it is difficult to generalize these results on a global scale because this health survey was conducted in only one Asian country and a relatively small number of people were included in the orthodontic treatment group. Second, as in other cross-sectional studies, it is not possible to explain the cause-and-effect relationship between orthodontic treatment and reduction in the prevalence of periodontitis. Future well-controlled longitudinal studies are needed to clarify the causal relationships between orthodontic treatment and reducing periodontitis. Third, in the present study, periodontal health was evaluated according to the CPI. This index has some limitations, although it has been widely used to evaluate the periodontal health and treatment needs of populations.35 This index is based on the assumption that periodontal disease progresses hierarchically. Thus, the teeth with scores of 3 or 4 are assumed to have calculus or bleeding.35 However, all teeth with deep periodontal pockets do not have calculus.
CONCLUSIONS
-
•
Orthodontic treatment is associated with a decreased prevalence of periodontitis.
-
•
Future well-controlled longitudinal studies are needed to clarify the causal relationships between orthodontic treatment and reduced prevalence of periodontitis.
ACKNOWLEDGMENT
The authors have stated explicitly that there are no conflicts of interest in connection with this article. The authors thank the Korea Centers for Disease Control and Prevention for providing the data.
REFERENCES
- 1.Petti S, Barbato E, Simonetti DAA. Effect of orthodontic therapy with fixed and removable appliances on oral microbiota: a six-month longitudinal study. New Microbiol. 1997;20:55–62. [PubMed] [Google Scholar]
- 2.Lee SM, Yoo SY, Kim H, et al. Prevalence of putative periodontopathogens in subgingival dental plaques from gingivitis lesions in Korean orthodontic patients. J Microbiol. 2005;43:260. [PubMed] [Google Scholar]
- 3.Bollen A-M. Effects of malocclusions and orthodontics on periodontal health: evidence from a systematic review. J Dent Educ. 2008;72:912–918. [PubMed] [Google Scholar]
- 4.Speer C, Pelz K, Hopfenmüller W, Holtgrave E-A. Investigations on the influencing of the subgingival microflora in chronic periodontitis. J Orof Orthop. 2004;65:34–47. doi: 10.1007/s00056-004-0333-z. [DOI] [PubMed] [Google Scholar]
- 5.Oh JY, Yang YJ, Kim BS, Kang JH. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. J Korean Acad Fam Med. 2007;28:532–541. [Google Scholar]
- 6.Kim MK, Lee W-Y, Kang J-H, et al. 2014 clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab. 2014;29:405–409. doi: 10.3803/EnM.2014.29.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chun YH, Kim HR, Han K, Park Y-G, Song HJ, Na K-S. Total cholesterol and lipoprotein composition are associated with dry eye disease in Korean women. Lipids Health Dis. 2013;12:1. doi: 10.1186/1476-511X-12-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Oral health surveys: basic methods. 2016 Available at: http://apps.who.int/iris/bitstream/10665/41905/1/9241544937.pdf Accessed July 20,
- 9.Lee JB, Yi HY, Bae KH. The association between periodontitis and dyslipidemia based on the Fourth Korea National Health and Nutrition Examination Survey. J Clin Periodontol. 2013;40:437–442. doi: 10.1111/jcpe.12095. [DOI] [PubMed] [Google Scholar]
- 10.Lim SG, Han K, Kim HA, et al. Association between insulin resistance and periodontitis in Korean adults. J Clin Periodontol. 2014;41:121–130. doi: 10.1111/jcpe.12196. [DOI] [PubMed] [Google Scholar]
- 11.Korea Center for Disease Control and Prevention. Standardization for Oral Health Survey in KNHANES. 20122016 Available at: http://cdc.go.kr/CDC/mobile/info/CdcKrInfo0201.jsp?menuIds=HOME001-MNU1154-MNU0004-MNU1889&fid= 28&q_type=&q_value=&cid=20598&pageNum=1 Accessed October 18,
- 12.Korea Center for Disease Control and Prevention. Standardization for Oral Health Survey in KNHANES. 20132016 Available at: http://cdc.go.kr/CDC/mobile/info/CdcKrInfo0201.jsp?menuIds=HOME001-MNU1154-MNU0004-MNU1889&fid= 28&q_type=&q_value=&cid=25569&pageNum=1 Accessed October 18,
- 13.Korea Center for Disease Control and Prevention. Standardization for Oral Health Survey in KNHANES. 20142016 Available at: https://knhanes.cdc.go.kr/knhanes/index.do Accessed October 18,
- 14.Alexander SA. Effects of orthodontic attachments on the gingival health of permanent second molars. Am J Orthod Dentofacial Orthop. 1991;100:337–340. doi: 10.1016/0889-5406(91)70071-4. [DOI] [PubMed] [Google Scholar]
- 15.Glans R, Larsson E, Øgaard B. Longitudinal changes in gingival condition in crowded and noncrowded dentitions subjected to fixed orthodontic treatment. Am J Orthod Dentofacial Orthop. 2003;124:679–682. doi: 10.1016/j.ajodo.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 16.Boyd RL, Baumrind S. Periodontal considerations in the use of bonds or bands on molars in adolescents and adults. Angle Orthod. 1992;62:117–126. doi: 10.1043/0003-3219(1992)062<0117:PCITUO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Huser MC, Baehni PC, Lang R. Effects of orthodontic bands on microbiologic and clinical parameters. Am J Orthod Dentofacial Orthop. 1990;97:213–218. doi: 10.1016/S0889-5406(05)80054-X. [DOI] [PubMed] [Google Scholar]
- 18.Ristic M, Svabic MV, Sasic M, Zelic O. Clinical and microbiological effects of fixed orthodontic appliances on periodontal tissues in adolescents. Orthod Craniof Res. 2007;10:187–195. doi: 10.1111/j.1601-6343.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 19.Boas Nogueira AV, Chaves de Souza JA, Kim YJ, Damião de Sousa-Neto M, Chan Cirelli C, Cirelli JA. Orthodontic force increases interleukin-1β and tumor necrosis factor-α expression and alveolar bone loss in periodontitis. J Periodontol. 2013;84:1319–1326. doi: 10.1902/jop.2012.120510. [DOI] [PubMed] [Google Scholar]
- 20.Thornberg MJ, Riolo CS, Bayirli B, Riolo ML, Van Tubergen EA, Kulbersh R. Periodontal pathogen levels in adolescents before, during, and after fixed orthodontic appliance therapy. Am J Orthod Dentofacial Orthop. 2009;135:95–98. doi: 10.1016/j.ajodo.2007.02.057. [DOI] [PubMed] [Google Scholar]
- 21.Sadowsky C, BeGole EA. Long-term effects of orthodontic treatment on periodontal health. Am J Orthod. 1981;80:156–172. doi: 10.1016/0002-9416(81)90216-5. [DOI] [PubMed] [Google Scholar]
- 22.Thomson W. Orthodontic treatment outcomes in the long term: findings from a longitudinal study of New Zealanders. Angle Orthod. 2002;72:449–455. doi: 10.1043/0003-3219(2002)072<0449:OTOITL>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 23.Christan C, Dietrich T, Hägewald S, Kage A, Bernimoulin JP. White blood cell count in generalized aggressive periodontitis after non-surgical therapy. J Clin Periodontol. 2002;29:201–206. doi: 10.1034/j.1600-051x.2002.290303.x. [DOI] [PubMed] [Google Scholar]
- 24.Hussain Bokhari SA, Khan AA, Tatakis DN, Azhar M, Hanif M, Izhar M. Non-surgical periodontal therapy lowers serum inflammatory markers: a pilot study. J Periodontol. 80:1574–1580. doi: 10.1902/jop.2009.090001. 200.9; [DOI] [PubMed] [Google Scholar]
- 25.Borrell LN, Crawford ND. Socioeconomic position indicators and periodontitis: examining the evidence. Periodontol 2000. 2012;58:69–83. doi: 10.1111/j.1600-0757.2011.00416.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carasol M, Llodra JC, Fernández-Meseguer A, et al. Periodontal conditions among employed adults in Spain. J Clin Periodontol. 2016;43:548–556. doi: 10.1111/jcpe.12558. [DOI] [PubMed] [Google Scholar]
- 27.Alexander A, Tipnis A. The effect of irregularity of teeth and the degree of overbite and overjet on the gingival health: a study of 400 subjects. Br Dent J. 1970;128:539–544. doi: 10.1038/sj.bdj.4802496. [DOI] [PubMed] [Google Scholar]
- 28.El-Mangoury NH, Gaafar SM, Mostafa YA. Mandibular anterior crowding and periodontal disease. Angle Orthod. 1987;57:33–38. doi: 10.1043/0003-3219(1987)057<0033:MACAPD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 29.Lindhe J, Svanberg G. Influence of trauma from occlusion on progression of experimental periodontitis in the beagle dog. J Clin Periodontol. 1974;1:3–14. doi: 10.1111/j.1600-051x.1974.tb01234.x. [DOI] [PubMed] [Google Scholar]
- 30.Kraal JH, Digiancinto JJ, Dail RA, Lemmerman K, Peden JW. Periodontal conditions in patients after molar uprighting. J Prosthet Dent. 1980;43:156–162. doi: 10.1016/0022-3913(80)90179-1. [DOI] [PubMed] [Google Scholar]
- 31.Bernhardt O, Gesch D, Look JO, et al. The influence of dynamic occlusal interferences on probing depth and attachment level: results of the Study of Health in Pomerania (SHIP) J Periodontol. 2006;77:506–516. doi: 10.1902/jop.2006.050167. [DOI] [PubMed] [Google Scholar]
- 32.Bagga DK. Adult orthodontics versus adolescent orthodontics: an overview. J Oral Health Comm Dent. 2010;4:42–47. [Google Scholar]
- 33.Nunn ME, Harrel SK. The effect of occlusal discrepancies on periodontitis. I. Relationship of initial occlusal discrepancies to initial clinical parameters. J Periodontol. 2001;72:485–494. doi: 10.1902/jop.2001.72.4.485. [DOI] [PubMed] [Google Scholar]
- 34.Harrel SK, Nunn ME. The effect of occlusal discrepancies on periodontitis. II. Relationship of occlusal treatment to the progression of periodontal disease. J Periodontol. 2001;72:495–505. doi: 10.1902/jop.2001.72.4.495. [DOI] [PubMed] [Google Scholar]
- 35.Benigeri M, Brodeur JM, Payette M, Charbonneau A, Ismaïl AI. Community periodontal index of treatment needs and prevalence of periodontal conditions. J Clin Periodontol. 2000;27:308–312. doi: 10.1034/j.1600-051x.2000.027005308.x. [DOI] [PubMed] [Google Scholar]