Abstract
Objective:
To clarify whether low-intensity pulsed ultrasound (LIPUS) exposure has recovery effects on the hypofunctional periodontal ligament (PDL) and interradicular alveolar bone (IRAB).
Materials and Methods:
Twelve-week-old male Sprague-Dawley rats were divided into three groups (n = 5 each): a normal occlusion (C) group, an occlusal hypofunction (H) group, and an occlusal hypofunction group subjected to LIPUS (HL) treatment. Hypofunctional occlusion of the maxillary first molar (M1) of the H and HL groups was induced by the bite-raising technique. Only the HL group was irradiated with LIPUS for 5 days. The IRAB and PDL of M1 were examined by microcomputed tomography (micro-CT) analysis. To quantify mRNA expression of cytokines involved in PDL proliferation and development, real-time reverse transcription quantitative PCR (qRT-PCR) was performed for twist family bHLH transcription factor 1 (Twist1), periostin, and connective tissue growth factor (CTGF) in the PDL samples.
Results:
Micro-CT analysis showed that the PDL volume was decreased in the H group compared with that of the C and HL groups. Both bone volume per tissue volume (BV/TV) of IRAB was decreased in the H group compared with that in the C group. LIPUS exposure restored BV/TV in the IRAB of the HL group. qRT-PCR analysis showed that Twist1, periostin, and CTGF mRNA levels were decreased in the H group and increased in the HL group.
Conclusion:
LIPUS exposure reduced the atrophic changes of alveolar bone by inducing the upregulation of periostin and CTGF expression to promote PDL healing after induction of occlusal hypofunction.
Keywords: LIPUS, Hypofunctional tooth, Periodontal ligament, Orthodontics, Periostin, Ultrasound
INTRODUCTION
Orthodontic treatment is often applied to malocclusions with high canines, an open bite, and impacted teeth. Reduced occlusal stimuli are widely known to induce atrophic changes in the periodontal ligament (PDL) and alveolar bone. When compared with that of functional teeth, the PDL in occlusal hypofunctional teeth is thinner1 and bone formation and density are lower.2,3 Mechanical stress plays an important role in the homeostasis of PDL4 and alveolar bone remodeling.5
The PDL is composed of heterogeneous cell populations such as osteoblasts, cementoblasts, fibroblasts and their progenitor cells,6,7 and extracellular matrix.8 Especially, type I collagen is the predominant collagen in the PDL, and its production is promoted by connective tissue growth factor (CTGF)9 and periostin.10 Periostin is mainly expressed in the periosteum, PDL, and osteoblastic cells on the alveolar bone surface, and it regulates collagen I fibrillogenesis.11 Periostin also mediates tissue remodeling by modifying the constitution of collagen fibers, while CTGF induces collagen synthesis. On the other hand, Twist1 modulates bone morphogenetic protein (BMP)-2-induced periostin expression.12 Periostin and CTGF are involved in the homeostasis of PDL tissues through a TGF-β/Twist1 pathway.13 CTGF enhances protein production of type I and III collagens and periostin in periodontal progenitor cells and promotes mature PDL-like tissue regeneration with dense periostin localization in collagen fiber bundles.9 In addition, occlusal loading promotes the expression of Twist1 and periostin in the rodent PDL.14,15 Loss of occlusal stimuli downregulates both gene and protein levels of periostin and CTGF in the PDL tissue during the atrophic change.16
Low-intensity pulsed ultrasound (LIPUS) irradiation is an orthopedic application of mechanical stimulus to tissues to facilitate bone fracture healing.17 LIPUS accelerates bone fusion with an increase of bone density and bone mineral content.18 LIPUS suppresses adipogenesis and promotes mesenchymal stem cell and preosteoblast differentiation to mature osteoblasts through the MEK-ERK pathway.19 Various genes including Vegf, BMP2, and runt-related transcription factor 2 (Runx2) participate in osteoblast proliferation and in the differentiation and transcription of osteoblasts under LIPUS treatment.20 LIPUS prompts cell proliferation of tendon fibroblasts and synthesis of collagen in cultures treated with collagenase, which injures the connective tissue matrix.21 LIPUS exposure increases CTGF gene and protein expression in cultured gingival cells.22 LIPUS may restore bone volume in the alveolar bone proper of occlusal hypofunctional teeth and reduce atrophic changes of the connective tissue matrix via stimulation of collagen synthesis in PDL fibroblasts.
In this study, we aimed to characterize the effects of LIPUS on atrophic changes in the periodontal tissues in response to occlusal hypofunction. We hypothesized that LIPUS causes morphological recovery of the PDL and interradicular alveolar bone (IRAB) by inducing the expression of cytokines associated with fibrogenic signaling. Thus, we analyzed transcriptional levels of periostin, CTGF, and their transcription regulator, Twist1, in the hypofunctional PDL exposed to LIPUS.
MATERIALS AND METHODS
Animal Model
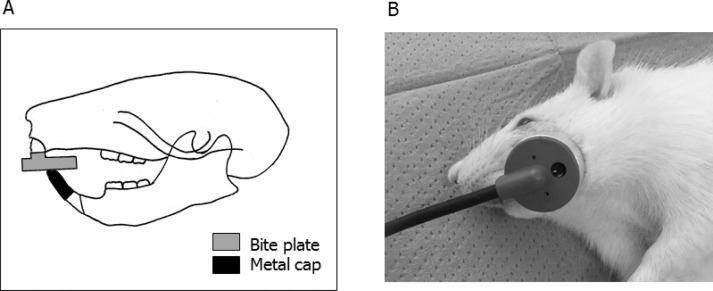
Twelve-week-old male Sprague-Dawley rats were used in this study. The rats were randomly divided into three groups (n = 5 each): a normal occlusion (C) group, an occlusal hypofunction (H) group, and an occlusal hypofunction group irradiated with LIPUS (HL). Hypofunctional occlusion of the maxillary first molar (M1) of the H and HL groups was induced by the bite-raising technique (Figure 1A).23 After a 2-week bite-raising period, LIPUS irradiation was initiated in the HL group. Hairs on the left cheek of the animals in the HL group were shaved frequently to allow for consistent transducer placement. Under general anesthesia, ultrasound gel (Ito, Tokyo, Japan) was placed on the left check overlying the left M1, and LIPUS was applied 20 minutes a day using an Osteotron D2 device (Ito) for 5 days (Figure 1B). Parameters of LIPUS were as follows: ultrasonic frequency, 1.5 MHz; repetition rate, 1.0 kHz; pulse duration, 200 μs; intensity, 30 mW/cm2. These stimulation conditions were used in the ultrasound treatment.24 Both the C and H groups were sham-irradiated. All rats in the C, H, and HL groups were euthanized by CO2 inhalation 8 hours after the final LIPUS irradiation. The maxillae were resected and fixed with 4% formaldehyde. All experimental procedures were approved by the Animal Experimental Committee of Tokyo Medical and Dental University (approval 0170209A).
Figure 1.
(A) Occlusal hypofunctional technique. An anterior metal bite plate and metal cap were attached to the mandibular and maxillary incisors by a light-curing composite resin. (B) LIPUS exposure image. The skin overlying the left M1 region was exposed to LIPUS under general anesthesia.
Microcomputed Tomography Analysis
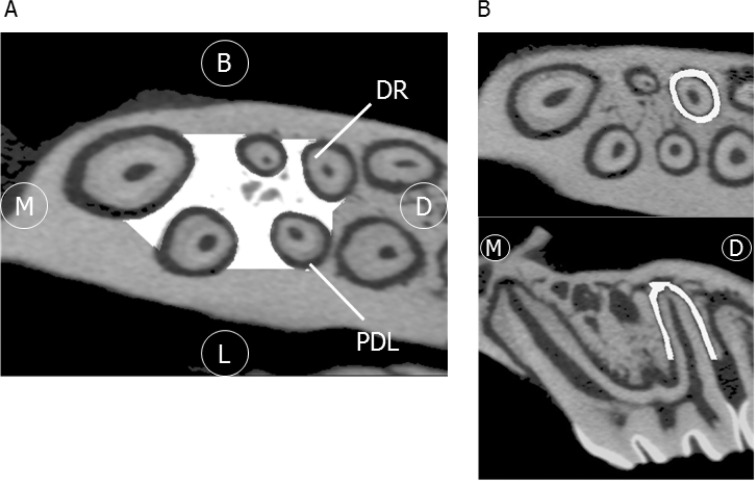
All samples were scanned by a desktop x-ray micro-CT system (SMX-100CT; Shimadzu, Kyoto, Japan) with a scanning resolution of 20-μm intervals. PDL volume of the distobuccal root of M1 was measured as previously described.25 The bone morphology of IRAB was also investigated. The region of interest was from the M1 furcation area to the apex (Figure 2A,B),26 analyzed by using three-dimensional (3D) image-analysis software. (TRI/3D-BON; Ratoc System Engineering, Tokyo, Japan). Bone volume per tissue volume (BV/TV, %) was quantified.
Figure 2.
3D micro-CT images. (A) The region of interest (ROI; white-filled area) is IRAB of M1, horizontal view. (B) ROI is the PDL space. Upper panel, horizontal view; lower panel, sagittal view, Abbreviations: M, mesial; D, distal; B, buccal; L, lingual; DR, dental root; PDL, periodontal ligament.
Real-time Reverse Transcription Quantitative Polymerase Chain Reaction Analysis
The PDL tissue was collected from the distobuccal root of M1. Total RNA was extracted from these tissues using the RecoverAll Total Nucleic Acid Isolation Kit for FFPE (Invitrogen, Carlsbad, Calif) and converted to cDNA using reverse transcription random primers with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, Calif) following the manufacturer's protocol. The mRNA levels of Vegf, Runx2, Twist1, CTGF, and periostin were quantified by real-time RT-polymerase chain reaction (PCR) using Premix Ex Taq (Takara Bio, Otsu, Shiga, Japan) and TaqMan gene expression assays (Applied Biosystems). All primers were obtained commercially (Applied Biosystems), and their composition is proprietary information. Glyceraldehyde-3-phosphate dehydrogenase was used as an internal control.
Statistical Analysis
Data from all groups were evaluated using Tukey's multiple comparisons with a significance level of 5%. Statistical calculations were performed using statistical analysis software (SPSS Statistics for Windows, Version 20.0; IBM, Armonk, NY).
RESULTS
Micro-CT Analysis of Bone Volume in Hypofunctional Teeth
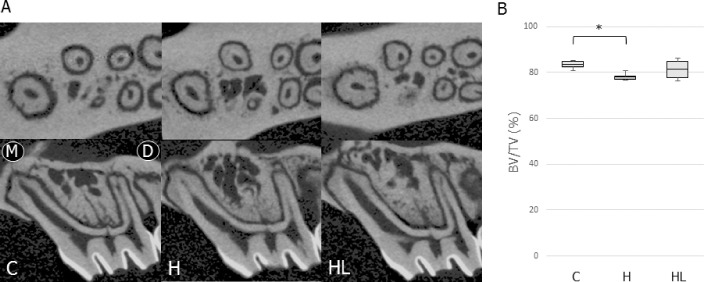
Figure 3A shows the micro-CT images of IRAB around M1 in the experimental groups. The H group showed a significant decrease in BV/TV of the IRAB (0.94-fold vs the C group) (Figure 3B). However, there was no significant difference in BV/TV between the HL and C groups.
Figure 3.
(A) Images of IRAB in the M1 region of the control and experimental groups. Upper panel, horizontal view; lower panel, sagittal view, (B) Comparisons of BV/TV in the alveolar bone of M1 in the control and experimental groups. For this figure and Figures 4–6, the box edges represent the upper and lower quantiles, the middle lines in the boxes represent the mean, and the whiskers represent the maxima and minima. Abbreviations for this figure and Figures 4–6: C, normal occlusion group; H, occlusal hypofunction group; HL, occlusal hypofunction irradiated with LIPUS group. * P < .05.
3D Measurement of the PDL Space in Micro-CT Images
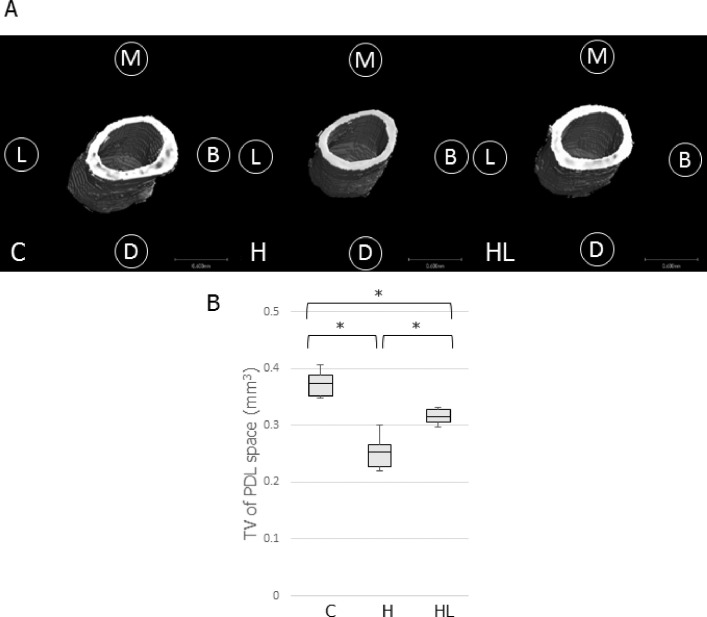
The reconstructed 3D images of the PDL space are shown in Figure 4A. The samples in the H groups displayed a thinner PDL space than that in the C group. The H group showed a smaller PDL volume than that of the C group (0.68-fold vs the C group). LIPUS irradiation significantly increased the PDL volume in the HL group compared with that in the H group (1.24-fold vs the H group), although the PDL volume in the HL group was lower than that of the C group (0.84-fold vs the C group; Figure 4B).
Figure 4.
(A), 3D image of the PDL space constructed by micro-CT analysis software. (B), Comparison of the tissue volume around the distobuccal root of M1. * P < .05.
qRT-PCR Analysis of Osteoblast Differentiation Factors in the PDL
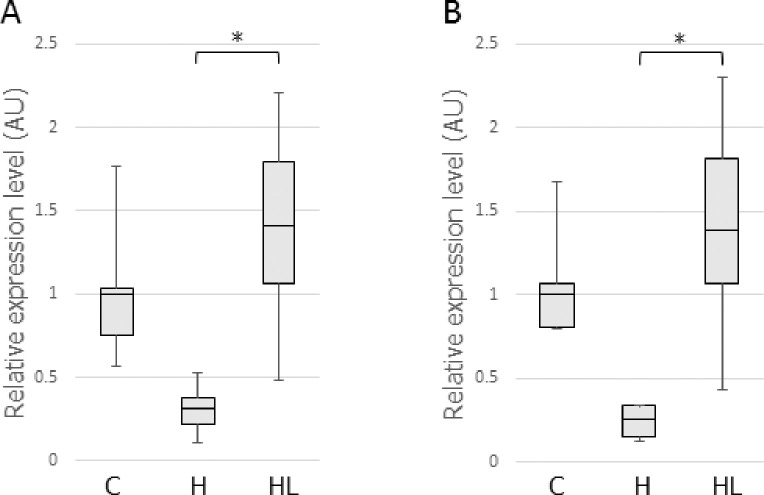
Vegf and Runx2 are involved in osteoblast differentiation and in the alteration of bone microstructure. Vegf and Runx2 mRNA levels in the PDL, which can affect IRAB, were therefore evaluated. The qRT-PCR showed that LIPUS treatment significantly increased Vegf (4.48-fold vs the H group) and Runx2 (5.45-fold vs the H group) mRNA levels in the HL group compared with those in the H group (Figure 5A,B).
Figure 5.
mRNA expression of Vegf (A) and Runx2 (B) in the PDL. Abbreviation for this figure and Figure 6: AU, arbitrary unit. * P < .05.
qRT-PCR Analysis of Collagen Fibrillogenesis-related Factors in the PDL
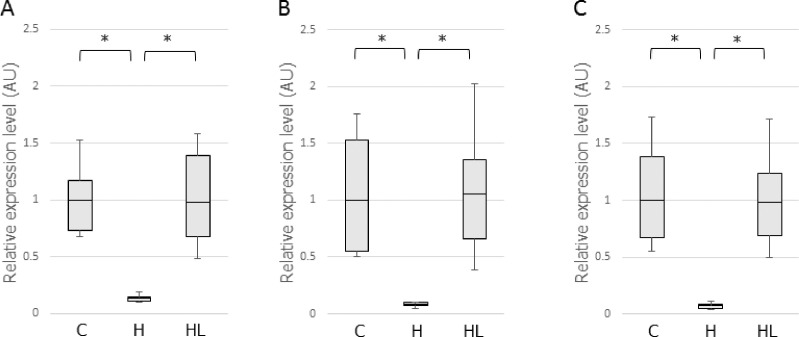
The qRT-PCR showed that occlusal hypofunction significantly reduced mRNA expression of Twist1, CTGF, and periostin in the PDL of the H group (Figure 6A,B,C). LIPUS irradiation upregulated Twist1 (7.43-fold vs the H group), periostin (13.63-fold vs the H group), and CTGF (12.91-fold vs the H group) expression in the HL group, and their transcriptional levels in the HL group were not significantly different from that in the C group.
Figure 6.
mRNA expression of Twist1 (A), CTGF (B), and periostin (C) in the PDL. * P < .05.
DISCUSSION
Hypofunctional periodontal tissue exhibits atrophic changes such as narrowing of the periodontal space, degradation of the periodontal fiber arrangement, and cessation of blood vessel formation.27,28 LIPUS serves as a potential noninvasive therapeutic procedure for bone fracture healing, and a previous study demonstrated that LIPUS exposure accelerates orthodontic tooth movement.29 In this study, it was shown that LIPUS exposure reduced the atrophic change of PDL structure and upregulated periostin and CTGF transcriptional levels to promote PDL healing after occlusal hypofunction induction.
Runx2 is the first marker of premature osteoblasts in early osteoblastic differentiation and is a transcriptional component of the BMP2/Smad/Runx2 pathway.30 In this study, there was no significant difference in BV/TV between the H and HL groups in the IRAB of M1 after 5 days of LIPUS irradiation, although Runx2 gene expression was dramatically higher (5.45-fold change) in the PDL of the HL group than that of the H group. Xue et al.29 demonstrated that LIPUS upregulates BMP2 gene expression through Runx2 in the human PDL cells and that BMP2 protein level increases gradually from day 5 of culture by daily LIPUS treatment. LIPUS-stimulated BMP2 expression peaks at day 7 in cultured PDL cells and remains nearly constant through day 14. However, the LIPUS irradiation period of 5 days may have been too short to induce structural recovery of the alveolar bone from atrophy, according to other animal studies.31,32
Periostin is a matricellular protein that maintains the homeostasis and integrity of the PDL.15 Its expression is transcriptionally upregulated by Twist1 and CTGF. In this study, periostin gene expression as well as Twist1 and CTGF mRNA levels were decreased in the H group; these factors were upregulated in the HL group by LIPUS treatment. Oshima et al.33 revealed that periostin is expressed in undifferentiated MC3T3-E1 preosteoblasts, but not in differentiated MC3T3-E1 osteoblasts. Periostin, a specific marker of preosteoblasts, enhances cell adhesion and spreading of MC3T3-E1 osteoblasts.34 Twist1 binds to the promoter region of periostin to activate periostin expression in undifferentiated osteoblasts.33 The Twist box interacts with the Runx2 DNA binding domain under Twist1 overexpression, which results in the inhibition of osteoblast differentiation.35
Periostin also induces dermal fibroblast proliferation,36 whereas LIPUS prompts tendon fibroblast proliferation.21 In view of the increase in periodontal ligament volume and upregulation of periostin, LIPUS is considered to promote cell proliferation primarily or secondarily in the hypofunctional PDL.
Periostin is involved in bone-related mechanical stress signaling and in fibrillogenesis during tissue regeneration.13 An animal study of orthodontic tooth movement demonstrated that the amount of tooth movement and rate of mineral deposition were significantly reduced with a decrease of TRAP-positive cells in the PDL of null-periostin mice compared with that in wild-type mice.37 Occlusal hypofunction decreases the expression of periostin and Twist1 in PDL cells in vivo.38 Taken together with previous studies, the results of the current study suggest that LIPUS stimulation as well as occlusal stimuli induce periostin expression through upregulation of Twist1 and CTGF transcriptional levels and prevent ossification and atrophy in the PDL. Further investigations using the Twist1 antagonist are necessary to reveal the mechanisms of the LIPUS effect through Twist1 and periostin in the PDL.
CONCLUSIONS
-
•
LIPUS exposure alleviated atrophic changes of the PDL and IRAB after induction of occlusal hypofunction.
-
•
Twist1, CTGF, and periostin mRNA levels were decreased in the PDL under occlusal hypofunction.
-
•
LIPUS exposure upregulated the transcriptional levels of Twist1, CTGF, and periostin in the hypofunctional PDL tissue as well as did Vegf and Runx2.
-
•
The results suggest LIPUS application as a potential therapeutic approach to promote periodontal healing and bone regeneration in occlusal hypofunctional teeth.
ACKNOWLEDGMENTS
This research was supported by Grants-in-Aid for Scientific Research to RU (15K20582) and SK (26463089) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
REFERENCES
- 1.Motokawa M, Terao A, Karadeniz EI, et al. Effects of long-term occlusal hypofunction and its recovery on the morphogenesis of molar roots and the periodontium in rats. Angle Orthod. 2013;83:597–604. doi: 10.2319/081812-661.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimizu Y, Hosomichi J, Kaneko S, Shibutani N, Ono T. Effect of sympathetic nervous activity on alveolar bone loss induced by occlusal hypofunction in rats. Arch Oral Biol. 2011;56:1404–1411. doi: 10.1016/j.archoralbio.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Shimomoto Y, Chung CJ, Iwasaki-Hayashi Y, Muramoto T, Soma K. Effects of occlusal stimuli on alveolar/jaw bone formation. J Dent Res. 2007;86:47–51. doi: 10.1177/154405910708600107. [DOI] [PubMed] [Google Scholar]
- 4.Termsuknirandorn S, Hosomichi J, Soma K. Occlusal stimuli influence on the expression of IGF-1 and the IGF-1 receptor in the rat periodontal ligament. Angle Orthod. 2008;78:610–616. doi: 10.2319/0003-3219(2008)078[0610:OSIOTE]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Huiskes R, Ruimerman R, van Lenthe GH, Janssen JD. Effects of mechanical forces on maintenance and adaptation of form in trabecular bone. Nature. 2000;405:704–706. doi: 10.1038/35015116. [DOI] [PubMed] [Google Scholar]
- 6.Lallier TE, Spencer A. Use of microarrays to find novel regulators of periodontal ligament fibroblast differentiation. Cell Tissue Res. 2007;327:93–109. doi: 10.1007/s00441-006-0282-5. [DOI] [PubMed] [Google Scholar]
- 7.Lekic P, Rojas J, Birek C, Tenenbaum H, McCulloch CA. Phenotypic comparison of periodontal ligament cells in vivo and in vitro. J Periodontal Res. 2001;36:71–79. doi: 10.1034/j.1600-0765.2001.360202.x. [DOI] [PubMed] [Google Scholar]
- 8.Feng L, Zhang Y, Kou X, et al. Cadherin-11 modulates cell morphology and collagen synthesis in periodontal ligament cells under mechanical stress. Angle Orthod. 2017;87(2):193–199. doi: 10.2319/020716-107.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dangaria SJ, Ito Y, Walker C, Druzinsky R, Luan X, Diekwisch TGH. Extracellular matrix-mediated differentiation of periodontal progenitor cells. Differentiation. 2009;78:79–90. doi: 10.1016/j.diff.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wen W, Chau E, Jackson-Boeters L, Elliott C, Daley TD, Hamilton DW. TGF-ß1 and FAK regulate periostin expression in PDL fibroblasts. J Dent Res. 2010;89:1439–1443. doi: 10.1177/0022034510378684. [DOI] [PubMed] [Google Scholar]
- 11.Norris RA, Damon B, Mironov V, et al. Periostin regulates collagen fibrillogenesis and the biomechanical properties of connective tissues. J Cell Biochem. 2012;101:695–711. doi: 10.1002/jcb.21224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu Q, Tong S, Zhao X, et al. Periostin mediates TGF-β-induced epithelial mesenchymal transition in prostate cancer cells. Cell Physiol Biochem. 2015;36:799–809. doi: 10.1159/000430139. [DOI] [PubMed] [Google Scholar]
- 13.Kudo A. Periostin in fibrillogenesis for tissue regeneration: periostin actions inside and outside the cell. Cell Mol Life Sci. 2011;68:3201–3207. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mendoza A, Cecilia E. Periostin and Twist mRNA expression in the hypofunctional mouse periodonta1 ligament. Shikoku Dent Res. 2005;18:1–10. [Google Scholar]
- 15.Rios HF, Ma D, Xie Y, et al. Periostin is essential for the integrity and function of the periodontal ligament during occlusal loading in mice. J Periodontol. 2008;79:1480–1490. doi: 10.1902/jop.2008.070624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi JW, Arai C, Ishikawa M, Shimoda S, Nakamura Y. Fiber system degradation, and periostin and connective tissue growth factor level reduction, in the periodontal ligament of teeth in the absence of masticatory load. J Periodontal Res. 2011;46:513–521. doi: 10.1111/j.1600-0765.2011.01351.x. [DOI] [PubMed] [Google Scholar]
- 17.Erdoǧan Ö, Esen E, Üstün Y, et al. Effects of low-intensity pulsed ultrasound on healing of mandibular fractures: an experimental study in rabbits. J Oral Maxillofac Surg. 2006;64:180–188. doi: 10.1016/j.joms.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 18.Azuma Y, Ito M, Harada Y, Takagi H, Ohta T, Jingushi S. Low-intensity pulsed ultrasound accelerates rat femoral fracture healing by acting on the various cellular reactions in the fracture callus. J Bone Miner Res. 2001;16:671–680. doi: 10.1359/jbmr.2001.16.4.671. [DOI] [PubMed] [Google Scholar]
- 19.Kusuyama J, Bandow K, Shamoto M, Kakimoto K, Ohnishi T, Matsuguchi T. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J Biol Chem. 2014;289:10330–10344. doi: 10.1074/jbc.M113.546382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin T-C, Wang C-J, Yang KD, Wang F-S, Sun Y-C. Shockwaves enhance the osteogenetic gene expression in marrow stromal cells from hips with osteonecrosis. Chang Gung Med J. 2011;34:367–374. [PubMed] [Google Scholar]
- 21.Ramirez A, Schwane J, McFarland C, Starcher B. The effect of ultrasound on collagen synthesis and fibroblast proliferation in vitro. Med Sci Sport Exerc. 1997;29:326–332. doi: 10.1097/00005768-199703000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Shiraishi R, Masaki C, Toshinaga A, et al. The effects of low-intensity pulsed ultrasound exposure on gingival cells. J Periodontol. 2011;82:1498–1503. doi: 10.1902/jop.2011.100627. [DOI] [PubMed] [Google Scholar]
- 23.Suhr E, Warita H, Iida J, Soma K. The effects of occlusal hypofunction and its recovery on the periodontal tissues of the rat molar: ED1 immunohistochemical study. Orthod Waves. 2002;61:165–172. [Google Scholar]
- 24.Gebauer GP, Lin SS, Beam HA, Vieira P, Parsons JR. Low-intensity pulsed ultrasound increases the fracture callus strength in diabetic BB Wistar rats but does not affect cellular proliferation. J Orthop Res. 2002;20:587–592. doi: 10.1016/S0736-0266(01)00136-X. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu Y, Hosomichi J, Nakamura S, Ono T. Micro-computed tomography analysis of changes in the periodontal ligament and alveolar bone proper induced by occlusal hypofunction of rat molars. Korean J Orthod. 2014;44:263–267. doi: 10.4041/kjod.2014.44.5.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu Y, Ishida T, Hosomichi J, Kaneko S, Hatano K, Ono T. Soft diet causes greater alveolar osteopenia in the mandible than in the maxilla. Arch Oral Biol. 2013;58:907–911. doi: 10.1016/j.archoralbio.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka A, Iida J, Soma K. Effect of hypofunction on the microvasculature in the periodontal ligament of the rat molar. Orthod Waves. 1998;57:180–188. [Google Scholar]
- 28.Kaneko S, Ohashi K, Soma K, Yanagishita M. Occlusal hypofunction causes changes of proteoglycan content in the rat periodontal ligament. J Periodontal Res. 2001;36:9–17. doi: 10.1034/j.1600-0765.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 29.Xue H, Zheng J, Cui Z, et al. Low-intensity pulsed ultrasound accelerates tooth movement via activation of the BMP-2 signaling pathway. PLoS One. 2013;8:e68926. doi: 10.1371/journal.pone.0068926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208–216. doi: 10.1016/j.molmed.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 31.Kang KL, Kim EC, Park JB, Heo JS, Choi Y. High-frequency, low-intensity pulsed ultrasound enhances alveolar bone healing of extraction sockets in rats: a pilot study. Ultrasound Med Biol. 2016;42:493–502. doi: 10.1016/j.ultrasmedbio.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Chai Z, Zhang Y, Deng F, Wang Z, Song J. Influence of low-intensity pulsed ultrasound on osteogenic tissue regeneration in a periodontal injury model: x-ray image alterations assessed by micro-computed tomography. Ultrasonics. 2014;54:1581–1584. doi: 10.1016/j.ultras.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 33.Oshima A, Tanabe H, Yan T, Lowe GN, Glackin CA, Kudo A. A novel mechanism for the regulation of osteoblast differentiation: transcription of periostin, a member of the fasciclin I family, is regulated by the bHLH transcription factor, Twist. J Cell Biochem. 2002;86:792–804. doi: 10.1002/jcb.10272. [DOI] [PubMed] [Google Scholar]
- 34.Horiuchi K, Amizuka N, Takeshita S, et al. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 35.Lee M, Lowe G, Strong D, Wergedal J, Glackin C. TWIST a basic helix-loop-helix transcription factor, can regulate the human osteogenic lineage. J Cell Biochem. 1999;75:566–577. doi: 10.1002/(sici)1097-4644(19991215)75:4<566::aid-jcb3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 36.Ontsuka K, Kotobuki Y, Shiraishi H, et al. Periostin, a matricellular protein, accelerates cutaneous wound repair by activating dermal fibroblasts. Exp Dermatol. 2012;21:331–336. doi: 10.1111/j.1600-0625.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- 37.Rangiani A, Jing Y, Ren Y, Yadav S, Taylor R, Feng JQ. Critical roles of periostin in the process of orthodontic tooth movement. Eur J Orthod. 2016;38(4):373–378. doi: 10.1093/ejo/cjv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Afanador E, Yokozeki M, Oba Y, et al. Messenger RNA expression of periostin and Twist transiently decrease by occlusal hypofunction in mouse periodontal ligament. Arch Oral Biol. 2005;50:1023–1031. doi: 10.1016/j.archoralbio.2005.04.002. [DOI] [PubMed] [Google Scholar]