Abstract
Objective:
The aim of this study was to develop an experimental orthodontic adhesive and evaluate how adding phosphate invert glass containing niobium pentoxide (PIG-Nb) affected the adhesive's properties.
Material and Methods:
PIG-Nb was added at 1, 2.5, and 5 wt% to experimental adhesive (75 wt% bisphenol A methacrylate [BisGMA], 25 wt% triethylene glycol dimethacrylate [TEGDMA], 5 wt% colloidal silica and photoinitiator system). The adhesives were evaluated for mineral deposition, degree of conversion (DC), softening solvent by Knoop microhardness (KNH) variation, pH changes, and shear bond strength (SBS). One-way analysis of variance (ANOVA) (DC and ΔKHN%), two-way ANOVA (SBS), repeated measures ANOVA (pH), and paired test (KNH1 and KNH2) were used at a significance level of P < .05.
Results:
Adding PIG-Nb to orthodontic adhesives induced deposition on its surface associated with a constant neutral pH. The SBS increased after immersion in artificial saliva, and the PIG-Nb5 exhibited less softening.
Conclusion:
The addition of PIG-Nb into orthodontic adhesives induced mineral deposition. Experimental orthodontic adhesive containing 5% wt of PIG-Nb exhibited increased mineral deposition and suitable properties for orthodontic applications.
Keywords: Mineral deposition, Orthodontic, Adhesive, Niobium, Bioactive glass
INTRODUCTION
White spot lesions (WSL) around brackets are an important issue during orthodontic treatment. Inefficient hygiene in these sites leads to an increase in oral colonization by Streptococcus mutans, which reduces pH and promotes enamel demineralization.1 To reduce decalcification, fluoridated orthodontic adhesives, sealants, and varnishes have been suggested as solutions. However, none of them seem to be effective.2 The development of an orthodontic adhesive that stimulates mineral deposition could act against the development of WSL.3–6
Bioactive glass stimulates mineral deposition by acting as source of calcium and phosphate,5 allowing tissue remineralization. Phosphate glasses are bioactive glasses formed by PO4 tetrahedral chains or rings that increase its chemical stability.7 Due to its high content of phosphate and ability to release ions, these glasses have potential to be applied in remineralization strategies to reduce WSL.5 Phosphate glasses with less than 40 mol% of P2O5 are referred as phosphate invert glasses (PIGs) once their properties are more related to the network modifier ions than on the network former.8 Niobium pentoxide (Nb2O5) can stimulate mineral deposition when in contact with saliva9 and when present in dental adhesives.10 Its addition to PIG increases its chemical durability and mechanical properties, showing no cytotoxicity.11
Due to the potential of Nb2O5 to improve the properties and activity of PIG, the aim of this study was to develop an experimental orthodontic adhesive and evaluate the influence of the addition of PIG-Nb on the adhesive's properties. The null hypothesis was that adding PIG-Nb would not influence the material's properties.
MATERIALS AND METHODS
PIG-Nb Preparation and Characterization
PIGs are prepared by the melt-quenching method using CaCO3, H3PO4, and NbO5 as precursors. Nb2O5 was added in 10 mol%. The precursor mixture was melted at 1400°C in an electric furnace for 30 minutes and quenched at room temperature.12 The PIG-Nb composition was 60% CaO, 30% P2O5, and 10% Nb2O5 molar. Glasses were milled, and the particles were selected by Granutest (Telastem, Sao Paulo, SP, Brazil) with a final mesh size of 74 μm. Surface area was determined by automated gas sorption (NOVA 100 Quantachrome Instruments, Boynton Beach, Florida, USA) through the Brunauer–Emmett–Teller or BET method. Particle size was assessed by laser diffraction (CILAS 1180, Cilas, Orleans, France).
Formulation of Experimental Orthodontic Adhesives
Adhesives were formulated with 75 wt% of bisphenol A glycidyl methacrylate (BisGMA) and 25 wt% of triethylene glycol dimethacrylate (TEGDMA); 1 mol% of camphorquinone, ethyl-4-dimethylamino benzoate (EDAB), and diphenyliodonium hexafluorophosphate (DPIHFP) as the photoinitiator system; and 0.1 wt% of hydroxytoluene butylated (BHT) (Sigma-Aldrich, Darmstadt, Germany). To adjust the viscosity, 5% of colloidal silica (Aerosil 200, Evonik, Essen, Germany) was added. PIG-Nb was added in 1, 2.5, and 5 wt% concentrations; for the control, a group without PIG-Nb was produced.
Artificial Saliva Preparation
Preparation was made as described by Karlinsey et al.9 Reagents (CaCl2, H2O, KH2PO4, KCl, and NaCl) were dissolved in distilled water and buffered with trishydroxymethyl aminomethane to a pH of 9; then, the pH was adjusted to 7.04 using hydrochloric acid.
Sample Preparation
Adhesive discs were prepared and photoactivated with Radii cal (1200 mW/cm2; SDI, Victoria, Australia) for 40 seconds for each group. Specimens of 5 ± 0.05 mm in diameter by 1.05 ± 0.02 mm in thickness were produced.
Mineral Deposition Assay
Samples (n = 3) were immersed in 20 mL of artificial saliva for 7, 14, and 28 days at 37°C. Samples that were not immersed were also used.
Raman Spectroscopy
Samples were analyzed by a Senterra Raman microscope (Bruker Optics, Ettlingen, Germany). An area of 10 × 10 μm2 of each sample was irradiated for 3 seconds during 5 seconds by a 100mW diode laser with 785 nm wavelength on 100 points. Spectra were obtained at 440 to 1800 cm–1 Raman band. Analysis was performed in Opus 6.5 (Bruker Optics, Ettlingen, Germany) by integration of the 960 cm–1 phosphate peak.
Scanning Electron Microscopy (SEM) Energy Dispersive X-Ray Spectroscopy (EDS)
After 28 days, the samples were carbon-sputtered and analyzed by a JSM 5800 microscope (JEOL, Tokyo, Japan) equipped with EDS at 10 kV at different magnifications (95, 1500, and 5500×).
pH Analysis
Samples (n = 3) were immersed in 20 mL of artificial saliva. The pH was measured before immersion and 30 minutes; 1, 2, 3, 4, 5, 6, 18, and 24 hours, and 2, 3, 4, 5, 6, and 7 days after immersion.
Degree of Conversion (DC)
DC was evaluated by FTIR-ATR (Vertex 70, Bruker Optics, Ettlingen, Germany). Light-curing was performed for 40 seconds at a standard distance of 5 mm from the sample. The spectra were obtained before and after polymerization. Data were evaluated with the Opus 6.5 (Bruker Optics, Ettlingen, Germany), and DC was calculated.13
Softening in Solvent
Samples (n = 3) were prepared and polished to determine Knoop microhardness (KNH) values. Three indentations were made (10 g for 5 seconds) in each specimen (HMV 2, Shimadzu, Kyoto, Japan) before (KNH1) and after (KNH2) immersion in ethanol for 2 hours. The percentage of variation of KNH (ΔKHN%) was calculated for each specimen.
Shear Bond Strength Test (SBS)
Ninety-six crowns of bovine incisors were cleaned on the facial surface for 10 seconds, etched with 37% phosphoric acid gel (Atacktec, CaiTECH Indústria LTDA, Sao Jose dos Pinhais, Brazil) for 30 seconds and rinsed with water for 30 seconds. Transbond XT Primer (3M Unitek, St Paul, MN) was applied to the surface. Teeth were randomly divided into four groups, and the experimental orthodontic adhesives were applied to the bracket base and placed on the tooth surface. Maxillary central incisor metal brackets (Roth Max, Morelli, Sorocaba, Brazil) were used. Brackets were placed using 300 g of force, and the adhesive excess was removed. Adhesives were light-cured for 10 seconds for each side of the bracket. Each group was then divided into two subgroups (n = 12): immediate (24 hours) and 28 days of storage in artificial saliva. Specimens were submitted to SBS testing with a universal test machine (EZ-SX, Shimadzu) using a knife-edge chisel with a crosshead speed of 1 mm/min, and the results were recorded in MPa. The Adhesive Remnant Index (ARI) was determined with a stereoscopic microscope (10×), and the ARI14 was recorded to analyze residual adhesive after SBS testing.
Statistical Analysis
Sigma Plot 12.0 (Systat Software Inc, San Jose, California, USA) was used for statistical analysis. The sample size was based on previous studies.15,16 One-way analysis of variance (ANOVA) and Tukey post hoc testing was used for DC and ΔKHN% analysis. SBS was compared by two-way ANOVA and Student-Newman-Keuls multiple comparison test. pH was compared with repeated measures ANOVA. Comparisons of KNH1 and KNH2 values were performed with paired t-tests.
RESULTS
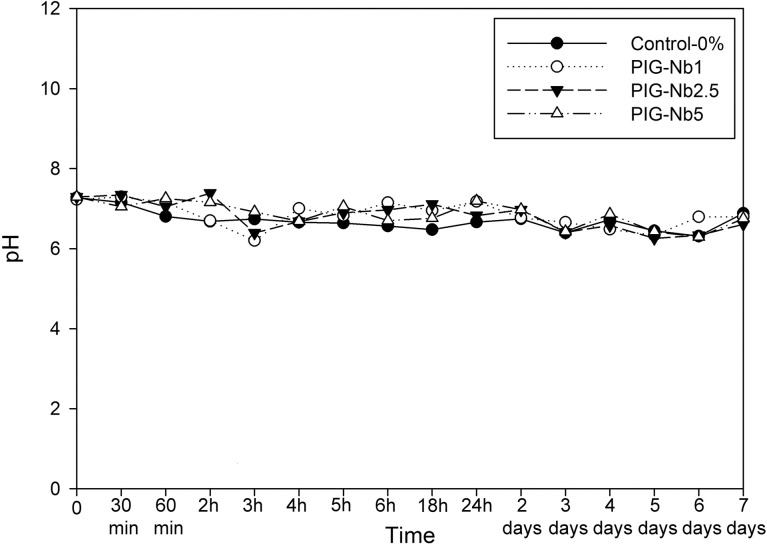
The PIG-Nb specific surface area was 3.16 m2/g, and the mean particle size was 67.55 μm. The initial pH in artificial saliva was 7.04, varying from 6.29 to 7.29 in the groups with no statistical differences (Figure 1). While no phosphate content was observed on the surface of the control-0% groups (Figure 2), phosphate was observed in PIG-Nb groups after 14 and 28 days of immersion. The intensity of the phosphate peaks increased from the PIG-Nb1 to PIG-Nb5. After 28 days, Raman spectra of mineral deposits displayed peaks related to octacalcium phosphate (OCP) in 1010 and 955 cm–1 peaks (Figure 3).
Figure 1.
The pH changes in artificial saliva with immersed samples as a function of time.
Figure 2.
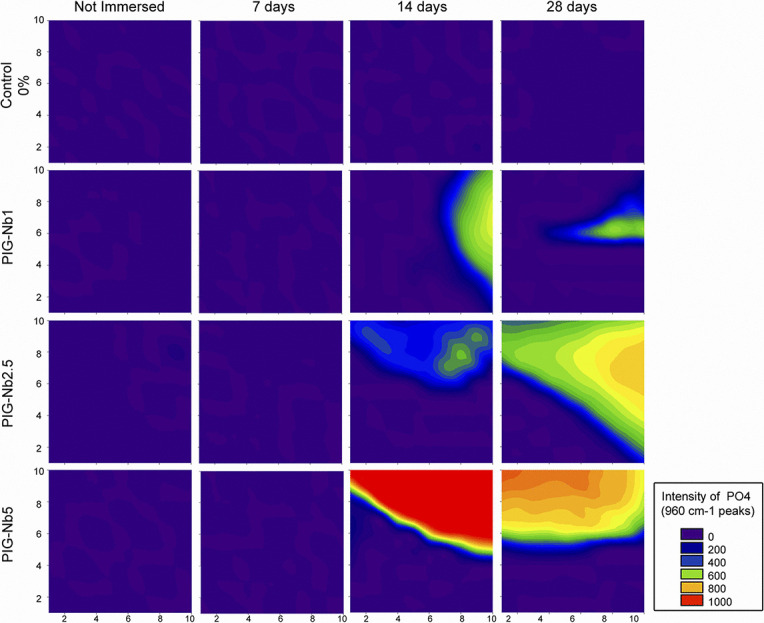
Raman map of phosphate deposition. The intensity is given by the integration of 960 cm−1 peaks.
Figure 3.
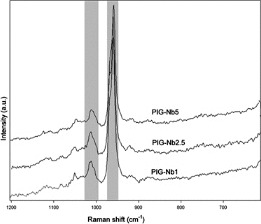
Raman spectrums of mineral deposits on adhesives after the 28-day immersion. Absorbance band with 1010 and 955 cm–1 peaks related to OCP are highlighted.
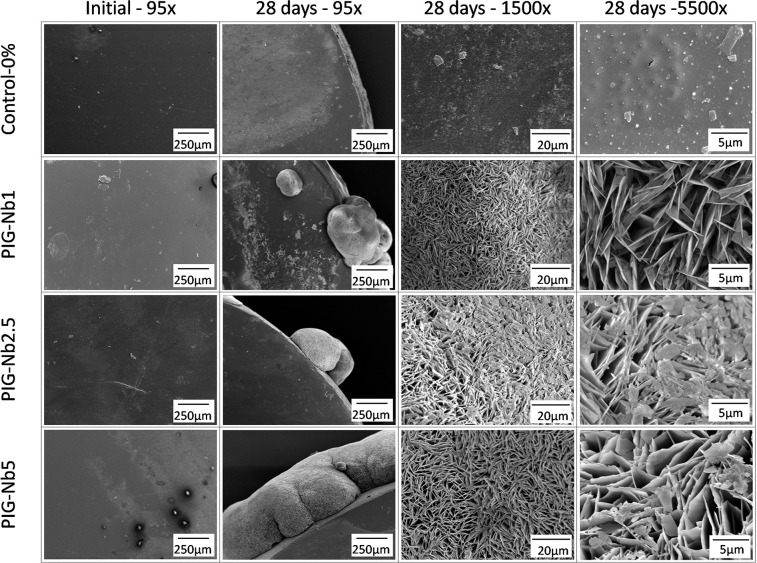
Upon SEM analysis, all PIG-Nb groups displayed mineral deposits on their surfaces. At the 5500× magnification, images denoted plate-like crystals with the sharp-edged structures of these mineral deposits (Figure 4). Surface composition of the samples was assessed by SEM-EDS (Table 1). Before immersion, all samples presented a similar composition, mostly of carbon and silicon; the control-0% group presented no niobium as the niobium percentage increased in the groups. After 28 days of immersion, a great amount of calcium was observed in all PIG-Nb groups. Phosphorus was present in all groups but in different amounts.
Figure 4.
SEM images of sample surfaces not immersed and after 28 days of immersion in artificial saliva at 95×, 1500×, and 5500× magnifications.
Table 1.
Surface composition of Samples Assessed by Scanning Electron Microscopy–Energy Dispersive X-Ray Spectroscopya
| Groups |
Initial |
28 Days Immersion |
||||||
| % C |
% Si |
% Nb |
%P |
%Ca |
%Nb |
%C |
%O |
|
| Control-0% | 97.74 | 2.26 | 0 | 1.84 | 3.23 | 0 | 94.8 | 0.12 |
| PIG-Nb1 | 69.18 | 1.44 | 29.38 | 30.44 | 33.99 | 16.81 | 11.71 | 7.05 |
| PIG-Nb2.5 | 60.34 | 1.35 | 38.31 | 13.29 | 79.48 | 6.09 | 0.30 | 0.84 |
| PIG-Nb5 | 54.68 | 1.68 | 43.64 | 5.97 | 76.04 | 16.58 | 0.70 | 0.71 |
C indicates carbon; Si, silicon; Nb, niobium; P, phosphorus; Ca, calcium; O, oxygen; PIG-Nb indicated adhesives containing niobium pentoxide.
The FTIR analysis showed a significant difference between the control-0% and all PIG-Nb groups (P < .001) (Table 2). For the control-0% group, a DC of 57.1% was found, while the PIG-Nb1, PIG-Nb2.5, and PIG-Nb5 groups presented 52.3%, 52.0%, and 51.9% respectively.
Table 2.
Degree of Conversion and Softening Solvent Results for Experimental Orthodontic Adhesives Containing Different Concentrations of PIG-Nba–c
| Groups |
%DC | KHN1 | KHN2 | ΔKHN% |
| (Mean ± SD) |
(Mean ± SD) |
(Mean ± SD) |
(Mean ± SD) |
|
| Control-0% | 57.05 ± 0.08Y | 17.43 ± 0.99YZ,y | 4.46 ± 0.53z | 74.50 ± 1.64Y |
| PIG-Nb1 | 52.32 ± 0.80Z | 14.71 ± 2.02Z,y | 4.06 ± 0.67z | 72.33 ± 3.58Y |
| PIG-Nb2.5 | 51.95 ± 0.51Z | 19.47 ± 0.21Y,y | 5.68 ± 0.69z | 70.84 ± 3.47YZ |
| PIG-Nb5 | 51.87 ± 0.93Z | 18.30 ± 1.96YZ,y | 6.47 ± 0.58z | 64.61 ± 1.25Z |
DC indicates degree of conversion; KNH, Knoop microhardness; PIG-Nb indicates experimental adhesives containing niobium pentoxide; SD, standard deviation.
KNH values before (KHN1) and after solvent immersion (KHN2) and the variation of Knoop hardness values (ΔKHN%).
Different uppercase letters indicate statistically significant difference in the column and different lowercase letters indicate statistically significant differences in the row. (P<0.05)
Table 3.
Shear Bond Strength of Metallic Brackets Bonded to Bovine Teeth With Experimental Adhesives, Immediate and 28 Days After Immersion in Artificial Salivaa
| Groupsb |
Immediate |
28 Days |
| Control-0% | 18.30 ± 3.74Yy | 16.03 ± 1.17Yy |
| PIG-Nb1 | 13.38 ± 3.52Zy | 19.68 ± 1.23Yz |
| PIG-Nb2.5 | 13.78 ± 3.31 Zy | 17.45 ± 1.17 Yz |
| PIG-Nb5 | 14.15 ± 2.53 Zy | 18.96 ± 1.12 Yz |
Different uppercase letters indicate statistically significant differences in the column, and different lowercase letters indicate statistically significant differences in the row.
PIG-Nb indicates experimental adhesives containing niobium pentoxide. (P<0.05)
Softening solvent results are shown in Table 2. For PIG-Nb groups, KNH1 measures were similar to those of the control group (P > .05). After 2 hours of immersion in ethanol, all groups displayed a reduction in KNH2 (P < .05). PIG-Nb5 showed a significant reduction in the ΔKHN% compared with the control-0% and PIG-Nb1 groups (P < .05).
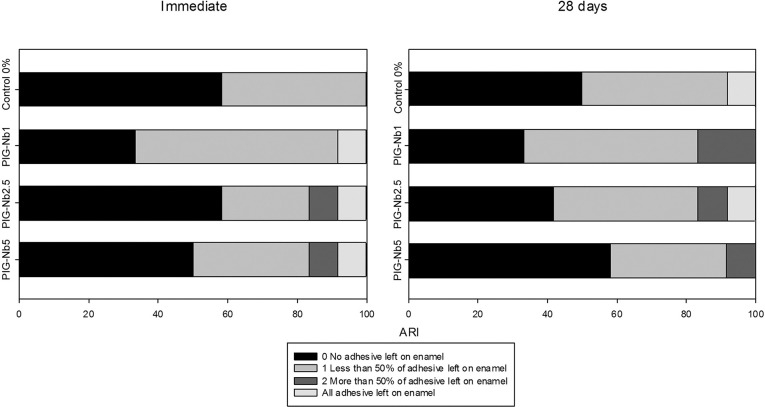
Immediate SBS evaluation for the control-0% group displayed higher values (18.3 MPa) than the PIG-Nb groups (PIG-Nb1: 13.4 MPa; PIG-Nb2.5: 13.8 MPa; PIG-Nb5: 14.1 MPa) (P < .05). In the 28 day analysis, there was no statistically significant difference between the control-0% and PIG-Nb groups. When comparing the SBS values from the immediate and 28-day analyeis, no significant change was observed in the control-0% group (16.03 MPa). However, all PIG-Nb groups showed higher SBS values after the immersion (19.7 MPa; 17.4 MPa; 19.0 MPa, respectively). The ARI scores for the immediate and 28-day immersion in artificial saliva were mainly 0 and 1 for all groups (Figure 5).
Figure 5.
ARI scores of debonded interfaces after immediate and 28-day SBS tests.
DISCUSSION
Subsurface enamel demineralization is clinically detectable as a WSL and represents the first stage of caries development.17 A source of minerals or the induction of mineral deposition on these sites could inhibit demineralization or stimulate remineralization. Therefore, an orthodontic adhesive exhibiting these characteristics could minimize or avoid WSL development. This study showed that orthodontic adhesive containing PIG-Nb can induce mineral deposition in artificial saliva.
PIGs have captured attention in biomedical fields due to their ability to release ions in aqueous media with a neutral constant pH,18 as was observed in this study in which the pH remained close to seven throughout the time periods observed. This constant pH indicated that the mineral deposits were induced by the biomaterial and not by artifacts of precipitation caused by pH change.19 PIGs are known to deposit apatite on their surfaces when immersed in simulated body fluid.20 Adding Nb2O5 can improve this ability and promote crystal growth and mineralization of the surrounding tissues.8 The resulting PIG-Nb exhibited a regular surface area of 3.16 m2/g, which is related to increased surface reactivity leading to higher mineral deposition rates.21
PIGs are glasses with a low content of phosphate (<40% mol)5 and an increased content of calcium, which decreases material solubility.18 The PIG-Nb prepared in this study contained 30% mol phosphate and 60% mol calcium, leading to a less soluble glass. In neutral solutions, addition of Nb2O5 increases PIG's solubility, inducing dramatic changes in glass structure, lowering its dissolution. Higher chemical stability may help mineral deposition because, in the acidic pH promoted by biofilm formation in WSL, these glasses will exhibit lower dissolution rates and longer mineral deposition periods.
The ability of orthodontic adhesives containing PIG-Nb to induce mineral deposition was evaluated by Raman spectroscopy and SEM-EDS. After 14 and 28 days, deposits of minerals on PIG-Nb groups were observed through the 960 cm1 Raman peak used as a reference for phosphate.9.16 SEM-EDS analysis after 28 days showed that the mineral deposits were highly selective for calcium and phosphorus (Table 1). Since the artificial saliva used in this study was constructed based on the inorganic content of human stimulated saliva, and it is a supersaturated medium for mineral deposition, it is likely that calcium and phosphorus are the major components in these deposits.9 The plate-like crystal structures observed indicate that the mineral deposits were OCP. This was consistent with previous literature,22 and there was presence of characteristic OCP Raman bands of 1010 cm–1 and 955 cm1 peaks (Figure 3) in all PIG-Nb samples immersed for 14 and 28 days.19 OCP is considered the precursor of hydroxyapatite and seems to play an important role in the enamel mineralization process.23 OCP conversion into hydroxyapatite has been observed in vivo, due to its dissolution by hydrolysis.24 The supersaturation of calcium and phosphate ions could also lead to accelerated deposition of calculus.25 However, the possible benefits of remineralization of WSLs could surpass the disadvantages of having dental calculus.
The percentage of carbon double bond (C=C) conversion analyzed by FTIR spectroscopy allows the assessment of the DC of resins. In this study, the DC of the PIG-Nb groups was lower than the DC of the control-0% group. Unreacted monomers increase degradation,26 reduce mechanical properties,27 and raise the risk of toxicity28 due to leaching of free monomers.27 DC of light-cured composites depends on several factors, including the monomer's structure and filler characteristics. DC values observed in this study may have been due to limited mobility of reactive groups caused by the rapid formation of a cross-linked polymeric network.29 Similar values were found for commercial orthodontic adhesives.29 Orthodontic adhesives were formulated with 75% wt of BISGMA, a viscous monomer that may have a great impact on the mobility. Also, PIG-Nb particles may have increased the viscosity of the adhesive30 and produced light diffraction into the polymer matrix, resulting in a reduced DC.
Although no statistical difference was found between groups in terms of KNH1, all groups underwent a reduction in the KNH2, evincing degradation of the polymer network. Softening is related to organic solvent sorption, reduction in secondary molecular interactions, and increase in leaching of uncured monomers.26 Microhardness can be influenced by reduction in DC. However, the number of C=C converted bonds does not completely describe characteristics of the polymer network. Secondary interactions and cross-linking density might influence KNH values and were not taken into consideration in the DC results.31 Although lower DC was found for the PIG-Nb-containing groups, the PIG-Nb5 group exhibited a significant lower ΔKHN% compared with the other groups due to its higher filler content. Reduction in the amount of the organic matrix and increased PIG-Nb particles, which are less prone to degradation,18 may result in lower variation of the ΔKHN% and increased softening resistance.
Lower DC may result in inferior physical-mechanical properties and clinical performance, such as poor bond strength.29 In this study, the addition of filler reduced immediate SBS results with an increase in the bond strength after 28 days of immersion in artificial saliva. It could be hypothesized that mineral deposits after immersion increased SBS values for PIG-Nb containing adhesives. In this study, values for bond strength varied from 13.4 to 19.7 MPa, and only one enamel fracture was observed in the immersed PIG-Nb1 group. Great variation regarding the threshold of bond strength associated with enamel fracture has been reported, and in vitro bond strength data should be interpreted with caution as many variables are involved.32
Enamel subsurface demineralization occurs due to a loss of calcium and phosphate during local ion concentration imbalances.33 The critical pH for enamel dissolution is inversely related to the content of calcium and phosphate available in the oral environment.34 As long as the surface of the lesion remains undisturbed and the environment adjacent to the WSL is supersaturated, calcium and phosphate can penetrate and remineralize the subsurface.26 Some clinical evidence suggests that WSL can be remineralized using bioactive glasses.5 The experimental orthodontic adhesives developed in this study can, in this way, act as a source of calcium and phosphate for susceptible sites around brackets, preventing mineral loss and promoting mineral deposition in WSL. Although promising results were found, further studies are needed to assess enamel remineralization by adding PIG-Nb to orthodontic adhesives.
CONCLUSION
The addition of PIG-Nb to orthodontic adhesives induced mineral deposition. Experimental orthodontic adhesive containing 5% wt of PIG-Nb exhibited increased mineral deposition and suitable properties for orthodontic applications.
REFERENCES
- 1.Millett DT, Nunn JH, Welbury RR, Gordon PH. Decalcification in relation to brackets bonded with glass ionomer cement or a resin adhesive. Angle Orthod. 1999;69:65–70. doi: 10.1043/0003-3219(1999)069<0065:DIRTBB>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 2.Gange P. The evolution of bonding in orthodontics. Am J Orthod Dentofac Orthop. 2015;147(suppl):S56–S63. doi: 10.1016/j.ajodo.2015.01.011. doi: 10.1016/j.ajodo.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 3.Altmann AS, Collares FM, Leitune VC, Arthur RA, Takimi AS, Samuel SM. In vitro antibacterial and remineralizing effect of adhesive containing triazine and niobium pentoxide phosphate inverted glass. Clin Oral Invest. 2017;21:93–103. doi: 10.1007/s00784-016-1754-y. doi: 10.1007/s00784-016-1754. [DOI] [PubMed] [Google Scholar]
- 4.Abuna G, Feitosa VP, Correr AB, et al. Bonding performance of experimental bioactive/biomimetic self-etch adhesives doped with calcium-phosphate fillers and biomimetic analogs of phosphoproteins. J Dent. 2016;52:79–86. doi: 10.1016/j.jdent.2016.07.016. doi: 10.1016/j.jdent.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 5.Manfred L, Covell DA, Crowe JJ, et al. A novel biomimetic orthodontic bonding agent helps prevent white spot lesions adjacent to brackets. Angle Orthod. 2013;83:97–103. doi: 10.2319/110811-689.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milly H, Festy F, Watson TF, Thompson I, Banerjee A. Enamel white spot lesions can remineralise using bio-active glass and polyacrylic acid-modified bio-active glass powders. J Dent. 2014;42:158–166. doi: 10.1016/j.jdent.2013.11.012. doi: 10.1016/j.jdent.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Bih L, Azrour M, Manoun B, Graça MPF, Valente MA. Raman spectroscopy, x-ray, SEM, and DTA analysis of alkali-phosphate glasses containing and Nb2O5. J Spectrosc. 2012;2013:e123519. doi: 10.1155/2013/123519. [Google Scholar]
- 8.Walter G, Vogel J, Hoppe U, Hartmann P. The structure of CaO–Na2O–MgO–P2O5 invert glass. J Non Cryst Solids. 2001;296:212–223. doi: 10.1016/S0022-3093(01)00912-7. [Google Scholar]
- 9.Karlinsey RL, Hara AT, Yi K, Duhn CW. Bioactivity of novel self-assembled crystalline Nb2O5 microstructures in simulated and human salivas. Biomed Mater Bristol Engl. 2006;1:16–23. doi: 10.1088/1748-6041/1/1/003. doi: 10.1088/1748-6041/1/1/003. [DOI] [PubMed] [Google Scholar]
- 10.Collares FM, Portella FF, Fraga GC da S et al. Mineral deposition at dental adhesive resin containing niobium pentoxide. Appl Adhes Sci. 2014;2:22. doi: 10.1186/s40563-014-0022-0. [Google Scholar]
- 11.Chenu S, Lebullenger R, Rocherullé J. Microwave synthesis and properties of NaPO3–SnO–Nb2O5 glasses. J Mater Sci. 2012;47:4632–4639. doi: 10.1007/s10853-012-6328-z. [Google Scholar]
- 12.Obata A, Takahashi Y, Miyajima T, Ueda K, Narushima T, Kasuga T. Effects of niobium ions released from calcium phosphate invert glasses containing Nb2O5 on osteoblast-like cell functions. ACS Appl Mater Interfaces. 2012;4:5684–5690. doi: 10.1021/am301614a. doi: 10.1021/am301614a. [DOI] [PubMed] [Google Scholar]
- 13.Collares FM, Portella FF, Leitune VCB, et al. Discrepancies in degree of conversion measurements by FTIR. Braz Oral Res. 2014;28:9–15. doi: 10.1590/S1806-83242013000600002. [DOI] [PubMed] [Google Scholar]
- 14.Artun J, Bergland S. Clinical trials with crystal growth conditioning as an alternative to acid-etch enamel pretreatment. Am J Orthod. 1984;85:333–340. doi: 10.1016/0002-9416(84)90190-8. [DOI] [PubMed] [Google Scholar]
- 15.Altmann ASP, Collares FM, Ogliari FA, Samuel SMW. Effect of methacrylated-based antibacterial monomer on orthodontic adhesive system properties. Am J Orthod Dentofac Orthop. 2015;147(suppl):S82–S87. doi: 10.1016/j.ajodo.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Leitune VCB, Collares FM, Trommer RM, Andrioli DG, Bergmann CP, Samuel SMW. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J Dent. 2013;41:321–327. doi: 10.1016/j.jdent.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Enaia M, Bock N, Ruf S. White-spot lesions during multibracket appliance treatment: a challenge for clinical excellence. Am J Orthod Dentofac Orthop. 2011;140:e17–e24. doi: 10.1016/j.ajodo.2010.12.016. doi: 10.1016/j.ajodo.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 18.Knowles JC, Franks K, Abrahams I. Investigation of the solubility and ion release in the glass system K2O-Na2O-CaO-P2O5. Biomaterials. 2001;22:3091–3096. doi: 10.1016/s0142-9612(01)00057-6. [DOI] [PubMed] [Google Scholar]
- 19.Crane NJ, Popescu V, Morris MD, Steenhuis P, Ignelzi MA. Raman spectroscopic evidence for octacalcium phosphate and other transient mineral species deposited during intramembranous mineralization. Bone. 2006;39:434–442. doi: 10.1016/j.bone.2006.02.059. [DOI] [PubMed] [Google Scholar]
- 20.Lee S, Obata A, Kasuga T. Ion releasing abilities of phosphate invert glasses containing MgO, CaO or SrO in tris buffer solution. Bioceram Dev Appl. 2013 May;1:1–3. [Google Scholar]
- 21.Sepulveda P, Jones JR, Hench LL. Characterization of melt-derived 45S5 and sol-gel-derived 58S bioactive glasses. J Biomed Mater Res. 2001;58:734–740. doi: 10.1002/jbm.10026. [DOI] [PubMed] [Google Scholar]
- 22.Lin X, de Groot K, Wang D, Hu Q, Wismeijer D, Liu Y. A review paper on biomimetic calcium phosphate coatings. Open Biomed Eng J. 2015;9:56–64. doi: 10.2174/1874120701509010056. doi: 10.2174/1874120701509010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown WE, Eidelman N, Tomazic B. Octacalcium phosphate as a precursor in biomineral formation. Adv Dent Res. 1987;1:306–313. doi: 10.1177/08959374870010022201. doi: 10.1177/08959374870010022201. [DOI] [PubMed] [Google Scholar]
- 24.Ito N, Kamitakahara M, Yoshimura M, Ioku K. Importance of nucleation in transformation of octacalcium phosphate to hydroxyapatite. Mater Sci Eng C Mater Biol Appl. 2014;40:121–126. doi: 10.1016/j.msec.2014.03.034. doi: 10.1016/j.msec.2014.03.034. [DOI] [PubMed] [Google Scholar]
- 25.Hoyer I, Gaengler P, Bimberg R. In vivo remineralization of human enamel and dental calculus formation. J Dent Res. 1984;63:1136–1139. doi: 10.1177/00220345840630090801. [DOI] [PubMed] [Google Scholar]
- 26.Witzel MF, Calheiros FC, Gonçalves F, Kawano Y, Braga RR. Influence of photoactivation method on conversion, mechanical properties, degradation in ethanol and contraction stress of resin-based materials. J Dent. 2005;33:773–779. doi: 10.1016/j.jdent.2005.02.005. doi: 10.1016/j.jdent.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Santerre JP, Shajii L, Leung BW. Relation of dental composite formulations to their degradation and the release of hydrolyzed polymeric-resin-derived products. Crit Rev Oral Biol Med. 2001;12:136–151. doi: 10.1177/10454411010120020401. [DOI] [PubMed] [Google Scholar]
- 28.Lovell LG, Newman SM, Bowman CN. The effects of light intensity, temperature, and comonomer composition on the polymerization behavior of dimethacrylate dental resins. J Dent Res. 1999;78:1469–1476. doi: 10.1177/00220345990780081301. doi: 10.1177/00220345990780081301. [DOI] [PubMed] [Google Scholar]
- 29.Çörekçi B, Malkoç S, Öztürk B, Gündüz B, Toy E. Polymerization capacity of orthodontic composites analyzed by Fourier transform infrared spectroscopy. Am J Orthod Dentofac Orthop. 2011;139:e299–e304. doi: 10.1016/j.ajodo.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 30.Lee J-H, Um C-M, Lee I. Rheological properties of resin composites according to variations in monomer and filler composition. Dent Mater. 2006;22:515–526. doi: 10.1016/j.dental.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 31.Fronza BM, Rueggeberg FA, Braga RR, et al. Monomer conversion, microhardness, internal marginal adaptation, and shrinkage stress of bulk-fill resin composites. Dent Mater. 2015;31:1542–1551. doi: 10.1016/j.dental.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Scougall-Vilchis RJ, Ohashi S, Yamamoto K. Effects of 6 self-etching primers on shear bond strength of orthodontic brackets. Am J Orthod Dentofac Orthop. 2009;135:424.e1–e7. doi: 10.1016/j.ajodo.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Brown ML, Davis HB, Tufekci E, Crowe JJ, Covell DA, Mitchell JC. Ion release from a novel orthodontic resin bonding agent for the reduction and/or prevention of white spot lesions. An in vitro study. Angle Orthod. 2011;81:1014–1020. doi: 10.2319/120710-708.1. doi: 10.2319/120710-708.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dawes C. What is the critical pH and why does a tooth dissolve in acid? J Can Dent Assoc. 2003;69:722–724. [PubMed] [Google Scholar]