Abstract
Objective
To examine screening strategies for identifying problematic sleep in a diverse sample of infants.
Methods
Parents of infants (5–19 months; N = 3,271) presenting for a primary care visit responded to five screening items and the Infant Sleep Questionnaire (ISQ), a validated measure of problematic infant sleep. If parents responded affirmatively to any screening item, primary care providers received a prompt to evaluate. For each of the screening questions, we examined differences in item endorsement and criterion related validity with the ISQ. Using conceptual composites of night waking and sleep difficulty, prevalence, criterion-related validity, and concurrent demographic correlates were analyzed.
Results
Infants were primarily of Black race (50.1%) or Hispanic ethnicity (31.7%), with the majority (63.3%) living in economically distressed communities. Rates of problematic sleep ranged from 7.4%, for a single item assessing parental perception of an infant having a sleep problem, to 74.0%, for a single item assessing night wakings requiring adult intervention. Items assessing sleep difficulty had high (95.0–97.8%) agreement with the ISQ in identifying infants without problematic sleep, but low agreement (24.9–34.0%) in identifying those with problematic sleep. The opposite was true for items assessing night waking, which identified 91.0–94.6% of those with sleep problems but only 31.8–46.9% of those without.
Conclusions
Screening strategies for identifying problematic infant sleep yielded highly variable prevalence rates and associated factors, depending on whether the strategy emphasized parent-perceived sleep difficulty or night wakings. The strategy that is most appropriate will depend on the system’s goals.
Keywords: health disparities and inequities, infancy and early childhood, primary care, sleep
Introduction
Healthy sleep in infants is associated with many positive outcomes for child and family functioning, both concurrently and prospectively. Problematic sleep, however, is associated with parental distress and sleep fragmentation (Meltzer & Mindell, 2007; Mindell et al., 2006). For infants, sleep problems have been linked to lower cognitive, language, and executive functioning scores and elevated irritability and obesity risk (Lam et al., 2003; Miller et al., 2018; Tham et al., 2017). In addition, there is evidence of a disparity in infant sleep, with a higher likelihood of problematic sleep in infants of Black racial heritage, Latinx ethnicity, and for those from economically distressed families (Honaker et al., 2020; Nevarez et al., 2010). As infant sleep disturbances can be effectively treated (Meltzer & Mindell, 2014), it is important to examine screening approaches for identifying infants with problematic sleep.
Screening strategies to identify problematic infant sleep in primary care and other community settings should be feasible, employing as few items as possible (Hoffses et al., 2018). Primary care systems in particular are mandated to routinely screen for a broad range of health, safety, developmental, and social concerns (Hagan et al., 2017), of which infant sleep is only one area. As a result, brevity is often considered one of the most important characteristics of a screening strategy in this setting (Maruish, 2018).
The objective of a screening strategy is typically to identify those who would benefit from further evaluation (i.e., those who screen positive), rather than to diagnose or provide a basis for treatment decisions (Pajek & Stancin, 2018). The utility of screening tools or items can thus be evaluated in regard to their sensitivity (i.e., the likelihood of correctly identifying individuals who require further evaluation) and their specificity (i.e., the likelihood of correctly identifying those who are not in need of further evaluation). Ideally, a measure will have both high sensitivity and specificity in comparison to a criterion measure. As a longer measure of infant sleep may not be feasible for universal screening in busy clinics, it will be important to evaluate how individual screening items perform in relation to a longer validated measure.
When relying on an individual screening item, the specific wording of the item is particularly important, as different terms (e.g., “problem” vs. “concern”) may elicit different parental responses to a screening item. The utility of single screening items for problematic infant sleep has not yet been evaluated to our knowledge. Furthermore, in light of racial/ethnic and socioeconomic sleep disparities, which start in early childhood and persist into adulthood (Nevarez et al., 2010; Williamson & Mindell, 2020), it is critical to include diverse families in studies that will inform screening practices.
In addition to evaluating specific screening items, it important to consider different conceptual approaches for identifying problematic infant sleep. One approach is to assess parent-perceived sleep difficulty. With this approach, infant sleep would be considered problematic only to the extent that it causes impairment, distress, or concern for the family. Prior studies examining parent-perceived infant sleep difficulty have reported relatively consistent prevalence rates, ranging from 21.5% to 26.3% (Honaker et al., 2020; Mindell & Leichman, 2018; Mindell et al., 2010). However, parent-perceived prevalence is rarely evaluated in samples of children from predominantly racial/ethnic minority and/or economically distressed backgrounds.
Parental perceptions of sleep difficulty have been found to reflect several developmental, sociocultural, and sociodemographic factors (Sadeh et al., 2011). For example, parents with concerns about their child’s development are more likely to report sleep concerns (Schwichtenberg et al., 2013), and cultural beliefs also impact parental perception of problematic sleep (Sadeh et al., 2011). Thus, utilizing a parental-perspective screening approach takes the family context, culture, and experience into consideration, and also identifies families who may be more motivated to receive guidance and intervention. An important limitation, however, is that there are gaps in parental knowledge about pediatric sleep (Honaker et al., 2020; Owens & Jones, 2011). As a result, parents may not be aware of negative outcomes associated with some sleep patterns, or may incorrectly perceive certain sleep behaviors as normative or not amenable to intervention.
A related approach is to screen for specific sleep patterns and behaviors that have documented associations with child and/or family impairment. One such sleep pattern is frequent night wakings that require parental involvement throughout the night. Although normative and driven primarily by nutritional need in younger infants (<5–6 months of age), in older infants night wakings requiring parental involvement are more likely related to sleep-onset associations. That is, older infants who associate falling asleep with parental presence are more likely to signal for a parent throughout the night to reinstate that association (e.g., feeding or soothing) to be able to easily return to sleep (Mindell et al., 2009). Night wakings in older infants are linked to shorter sleep duration, less consolidation of sleep, disrupted parental sleep, and parent-reported sleep difficulty (Mindell et al., 2006). In addition, night wakings in older infants can be effectively treated with brief interventions (Meltzer & Mindell, 2014), and are the most frequent infant sleep concern reported by parents (Mindell et al., 2015). Previous studies, however, report a high prevalence of infant night wakings (e.g., Sadeh et al., 2009), including in diverse samples (Honaker et al., 2020). Thus, this screening approach could result in over-identification of problematic sleep.
When considering sleep problems, parents are often the first reporter of problems but coendorsement by primary care providers (PCPs) is a critical second step toward receiving recommendations and intervention (as needed). To date, our understanding of this second step in diverse families is limited and likely reflects elements of the PCP’s training, family characteristics, and available community resources.
To inform primary care screening efforts for problematic sleep in diverse infants, the present study was organized around three aims. For our first aim, we evaluated the performance of five individual parent-report screening questions, examining their prevalence (% endorsement), concordance (across items), and criterion-related validity (relative to a validated measure of problematic infant sleep). We hypothesized that parents would be most likely to report night wakings, followed by a sleep “concern,” and least likely to report a sleep “problem,” as the latter term might suggest a greater severity of sleep disruption. Given the item composition of the validated measure (Infant Sleep Questionnaire; ISQ; Morrell, 1999), we predicted that sleep “problem” reports would most closely align with ISQ scores.
For our second aim, we grouped questions by concept (night waking or sleep difficulty) and evaluated prevalence, concordance, and criterion-related validity. Additionally, common demographic features of this sample were explored as concurrent correlates of each conceptual composite. We hypothesized that parents would be more likely to perceive a sleep difficulty in older infants as well as in infants with other developmental concerns. Given higher rates of problematic sleep in children from racial/ethnic minority and economically distressed backgrounds, we further hypothesized higher likelihood of parent-perceived difficulty in these groups.
For our final aim, we examined problematic infant sleep endorsed by PCPs in response to a positive parental screen. As PCP endorsement of a sleep problem was only possible in this dataset given a positive parent-report screen, only descriptive and concurrent correlates were assessed. We hypothesized that PCPs would be more likely to coendorse sleep problems in children who were older, had a developmental concern, were from racial/ethnic minority families, and/or economically distressed backgrounds.
Materials and Methods
Participants and Procedures
Participants were older infants and younger toddlers (5–19 months), hereafter referred to as infants, and their primary caregivers, hereafter referred to as parents. This age range was selected in collaboration with PCPs to target a typical age group in which sleep guidance is often provided. Infants were seen at one of five primary care clinics in the Eskenazi healthcare system located in Indianapolis, Indiana. As part of standard clinical care, parents completed screening questionnaires before each visit, delivered via a novel computer decision support system (Child Health Improvement through Computer Automation [CHICA]). Participant demographics are summarized in Table I.
Table I.
Family Demographic Information
| Variable | N (%) |
|---|---|
| Child sex (% female) | 1,597 (48.8) |
| Child Age (months) | 11.7 (4.7)a |
| Insurance coverage (% Medicaid) | 2,866 (87.6) |
| Primary language (% English) | 2,356 (72.6)b |
| Developmental concerns (% yes) | 448 (13.7) |
| Child race/ethnicityc | |
| White non-Hispanic | 247 (7.6) |
| Black non-Hispanic | 1,640 (50.1) |
| White Hispanic | 1,037 (31.7) |
| Other | 347 (10.6) |
| Distressed Community Index mean | 69.6 (27.0)a |
| Distressed Community Index rankc | |
| Prosperous (<20) | 148 (7.6) |
| Comfortable (21–40) | 309 (9.5) |
| Mid-tier (41–60) | 640 (19.6) |
| At-risk (61–80) | 433 (13.3) |
| Distressed (>80) | 1,629 (50.0) |
Means and SDs (in parentheses) are reported. All percentages are out of the total sample (3,271), unless otherwise noted.
The total number of participants for whom there was information about language spoken in the home was 3,244.
The total number of participants for whom there was information about race/ethnicity and Distressed Community Index was 3,259.
The CHICA system has been described in greater detail elsewhere (e.g., Anand et al., 2018). Briefly, CHICA provides preventive care and chronic disease management decision support based on clinical guidelines encoded in Arden Syntax rules. Once a child is registered for a medical encounter, CHICA produces a set of tailored screening items that contains 20 health risk questions (in English and Spanish) for the parent to complete. These questions are selected using a unique prioritization scheme that incorporates the child’s age at the time of the visit, previous patient information contained in the database, and the likelihood and seriousness of each health risk. Screening is completed via an electronic tablet in the waiting room before the medical encounter. Once completed, information captured via parental report is transmitted to CHICA, and the collected data are integrated into the patient’s electronic health record (EHR). A tab within the EHR is then generated for the provider to use during the patient encounter, which includes up to six prioritized prompts highlighting recommended areas for the provider to address during that visit. After the provider responds to these prompts, the data are integrated with the information already in the patient’s EHR.
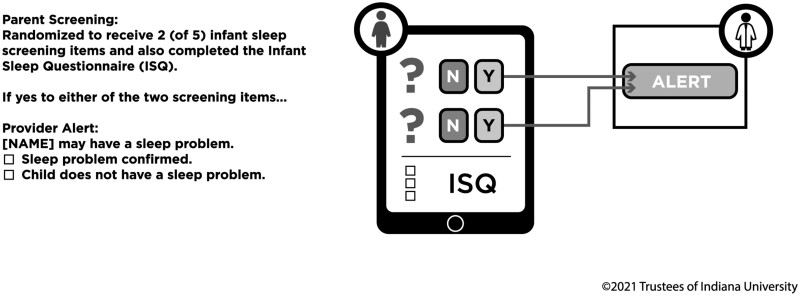
Most CHICA content is organized into modules that are focused on a particular health area, with a specific module including screening items, provider prompts, and decision rules to guide screening and prompts. A CHICA Infant Sleep module (see Figure 1) was designed as a clinical tool to support PCPs in assessing infant night wakings and parent-perceived sleep difficulty, and thus identify families who might benefit from sleep guidance. In this module, parents of infants received two of five items (detailed below) in a pseudo-randomized order. Parents were equally likely to receive any two of the five items, with the exception of three pairs of items that were deemed too similar and might provide redundant information (detailed below). Randomization was achieved by creating a set of possible pairs of items. A pseudorandom number generator in CHICA was then used to select a pair of items at the time that the screening form was generated. All parents also completed the ISQ. For parents who answered “yes” to any of the individual screening items (e.g., endorsed night wakings or a sleep difficulty), PCPs received a prompt in the child’s EHR during that visit. The prompt was generated based on the individual screening items and was independent of the ISQ score. PCPs did not have access to the ISQ score. This version of CHICA Infant Sleep was considered preliminary or exploratory, with the intent to refine the module for long-term clinical use. That is, clinical output from the module was ultimately used to design a revised CHICA Infant Sleep module, which was launched in June of 2018. The five screening items and the content of both versions of the CHICA Infant Sleep module were developed by the authors, who have more than 50 years of combined experience in the assessment and management of infant sleep disruption, including in primary care settings. All module content was reviewed by several providers and staff from each of the five clinics, and feedback was incorporated into the module by the authors. All materials were professionally translated into Spanish language and reviewed by bilingual PCPs and clinic staff prior to implementation.
Figure 1.
CHICA Infant Sleep module
Data were extracted for all patients whose parents responded to at least one item within the CHICA Infant Sleep module between February 2016 and July 2017. Of age-eligible children (N = 3,520) seen in one of the five primary care clinics, 92.9% of parents completed at least one screening item and thus were included in this study, resulting in a final sample of 3,271 families. Reasons for not completing the screening items were not assessed but likely included: (a) late arrival to the appointment or (b) module items not generated by the prioritization scheme. Because CHICA is a part of standard clinical care in these clinics, this study was approved by the Institutional Review Board of Indiana University with a waiver of informed consent.
Measures
Parent-Reported Sleep Difficulty
Participating parents may have responded to one of three items regarding parental perceptions of problematic sleep, specifically: (a) Do you think [CHILD NAME]’s sleep is a problem? (b) Do you think [CHILD NAME] has a sleep problem? and (c) Do you have any concerns about [CHILD NAME]’s sleep? Per the pseudo-randomization scheme, parents could not receive both items (a) and (b) at the same visit, due to concerns that similarity between the two items might be confusing to parents and/or suggest an error in the screening instrument. All three items were included to evaluate how the items performed and which one(s) could be included in an updated version of the module. Individual items were examined in Aim 1. Aims 2 and 3 used a conceptual composite of sleep difficulty, which reflected a “yes” response to any one of these three items. In total, 3,117 caregivers (95.3% of the sample) responded to at least one item about sleep problems or concerns.
Night Wakings
The CHICA Infant Sleep module included two individual items assessing night wakings: (a) Does [CHILD NAME] often wake up one or more times in the night? or (b) Does [CHILD NAME] often wake up one or more times in the night and does an adult go to [HIM/HER]? Based on the pseudo-randomization scheme, parents could receive one or zero of these items, but not both, as they were deemed too similar. The first item was more concise and direct, but could elicit parental reports of normative infant night wakings (i.e., a brief awakening between sleep cycles followed by a quick return to sleep) that were not problematic. The individual items were examined in Aim 1. A night wakings composite was used in Aim 2, reflecting whether night wakings were endorsed at the conceptual level, regardless of phrasing used. In total, 2,561 caregivers (78.3% of the sample) responded to a night waking item.
Infant Sleep Questionnaire
All parents completed the ISQ (Morrell, 1999). The ISQ is a validated clinical and research tool to assess infant sleep difficulties. Respondents are asked to report on their child’s sleep over the past month. Items assess parental perception of a sleep difficulty and three common areas of infant sleep disruption, specifically difficulty settling to sleep, night wakings, and taking a child to the parental bed in response to settling difficulty and/or night wakings. The ISQ consists of 10 items, 6 of which contribute to a total score that can range from 0 to 38. A score of 12 or higher is designated as the cutoff for problematic sleep. The ISQ has moderate test–retest reliability (kappa = .76), high sensitivity (89.5%), and high specificity (93.4%). The ISQ was originally validated in infants 12–18 months (Morrell, 1999) though has subsequently been used in infants ranging from birth to 24 months (e.g., Scher et al., 2008; Teti et al., 2010). The original validation sample included families from a range of socioeconomic backgrounds; racial/ethnic background was not reported. The measure was professionally translated into Spanish language and reviewed by bilingual primary care team members for accuracy; however, the Spanish language version has not been validated.
PCP-Endorsed Sleep Problem
A prompt for the PCP was generated in the medical record for any “yes” response to an individual parent-report screening item. The prompt read “[CHILD NAME] may have a sleep problem.” The PCP could then check one of two options: (a) sleep problem confirmed; or (b) child does not have a sleep problem. This prompt was generated for 1,887 PCP-infant dyads (57.7% of the sample). Of the 1,887 PCP prompts generated, PCPs responded to 1,190 (63.1%). This response rate is slightly higher than the average response rate for CHICA prompts, which averages around 50% (Bauer et al., 2016).
Distressed Communities Index
To account for socioeconomic differences in the sample, we used the Distressed Communities Index (DCI), which was created by the Economic Innovation Group (www.eig.org/dci). The DCI reflects seven community-level variables drawn from the 2012–2016 US Census Bureau’s American Community Survey 5-Year Estimates and Business Patterns Datasets. The DCI can range from 0 to 100, with higher scores reflecting more economic distress. Communities are grouped into quintiles, ranging from prosperous (DCI < 20) to distressed (DCI > 80). Zip codes were used to identify the DCI for each participant. The DCI was analyzed in regression models as a continuous variable, ranging from 0 to 100.
Developmental Concern
This variable was assessed using the Developmental Surveillance and Screening CHICA module (Carroll et al., 2014) to identify the contribution of developmental concerns to the identification of sleep problems by parents and PCPs. In this module, parents completed the Ages and Stages Questionnaire during the 9-month visit, with a positive screen generating a PCP prompt. At other visits (i.e., at 4, 6, 12, and 18 months), parents were asked if their infant or toddler was engaging in a specific age-expected developmental task in three domains (language, gross motor skills, and social) with any “no” response also generating a provider prompt. A PCP-endorsed developmental concern in response to either of the prompts above was coded as a developmental concern.
Analysis Plan
The first aim examined the individual parent-report screenings items by considering their prevalence (% endorsement), concordance (with cross-tabulation and X2 analyses), and validity (relative to the established ISQ). Our second aim examined both parent-reported night wakings and sleep difficulty at the conceptual level by analyzing the composite indexes of night wakings (across the two relevant items) and sleep difficulty (across the three relevant items). For this aim, we again considered the prevalence (% endorsement) and validity (relative to the established ISQ) of each composite. We also tested common correlates of night wakings and sleep difficulty at the composite level to determine which factors (namely race/ethnicity, economic distress, language spoken in the home, developmental status, age, and sex) were significantly associated with parental endorsement of night wakings and parental perception of sleep difficulty. To test these common correlates, we conducted separate binary logistic regressions for each outcome (night wakings composite and sleep difficulty composite) using the six predictors listed above. Based on expert recommendations and conceptual differences between the items, posthoc analyses were also completed at the item-level for the two night wakings items separately. The third aim examined the prevalence (% endorsement) and common correlates (testing the six predictors listed above in a third binary logistic regression) of PCP-endorsed sleep problems. Analyses were performed using SAS v9.4 (SAS Institute, Cary, NC), and all analytic assumptions were verified.
Results
Prevalence, Concordance, and Criterion Related Validity for Screening Items
Prevalence rates of the individual screening items and their concordance with ISQ scores (<12 and ≥12) are presented in Table II. The percentages of endorsement for the three items that assessed parent-perceived sleep problems or concerns were similar (7.4%, 8.4%, and 11.6%), with higher endorsement rates for sleep “concerns” compared with “problems” (χ2 = 15.20, p < .001). Of the three items that pertained to parental perception of sleep problems or concerns, the two that operated most similarly were the two that included the term “problem.” These did not significantly differ from each other (χ2 = 0.79, p = .37). Interestingly, parents were more likely to report night wakings requiring parental intervention (74.0%) compared with night wakings in general (62.8%), although theoretically the phrasing of the first item is more specific and thus would be expected to have lower endorsement than the second item. These items significantly differed from each other (χ2 = 37.04, p < .001).
Table II.
Descriptive Statistics of Individual Screening Items and Composites With the Infant Sleep Questionnaire (ISQ)
| Item/composite | PQ (N) | PQ answered “yes”% (N) | Answered PQ and ISQ (N) | Answered PQ and had ISQ > 12 (N) | Concordance between PQ “yes” and ISQ score >12 % (N) | Answered PQ had ISQ < 12 (N) | Concordance between PQ “no” and ISQ score <12 % (N) |
|---|---|---|---|---|---|---|---|
| Does [CHILD NAME] often wake up one or more times in the night? | 1,313 | 62.8 (825) | 1,110 | 268 | 91.0 (244) | 842 | 46.9 (395) |
| Does [CHILD NAME] often wake up one or more times in the night and does an adult go to [HIM/HER]? | 1,258 | 74.0 (931) | 1,062 | 239 | 94.6 (226) | 823 | 31.8 (262) |
| Night waking conceptual composite | 2,561 | 68.5 (1,755) | 2,162 | 506 | 92.9 (470) | 1,656 | 39.2 (649) |
| Do you think [CHILD NAME]’s sleep is a problem? | 1,123 | 8.4 (94) | 948 | 218 | 29.4 (64) | 730 | 97.8 (714) |
| Do you think [CHILD NAME] has a sleep problem? | 1,184 | 7.4 (88) | 978 | 213 | 24.9 (53) | 765 | 97.8 (748) |
| Do you have any concerns about [CHILD NAME]’s sleep? | 1,439 | 11.6 (167) | 1,201 | 294 | 34.0 (100) | 907 | 95.0 (862) |
| Sleep difficulty conceptual composite | 3,117 | 9.4 (293) | 2,614 | 607 | 29.8 (181) | 2,007 | 96.6 (1,938) |
Note. PQ = parent question or conceptual composite (generated from parent questions). The percentage agreement with ISQ score ≥12 is associated with the measure’s sensitivity as it reflects how sensitively the item detects children who screen positive on the validated tool. The percentage of nonagreement was calculated by dividing the number of parents who responded “no” to that item by the total number of parents with an ISQ score <12. This estimate is associated with specificity as it reflects how well the item correctly avoids misidentification of children with negative screens.
To test the validity and utility of the five screening items, we examined their concordance with the ISQ. The night waking items tended to perform well for identifying infants with an elevated (≥12) ISQ score (91.0% and 94.6%), but poorly for excluding infants with a normal (<12) ISQ score [46.9% and 31.8%]). In contrast, the sleep problem screening items identified only a small proportion of infants with an elevated ISQ score (range 24.9–34.0%), but differentiated most infants with a normal ISQ score (range 95.0–97.8%).
Prevalence, Criterion Related Validity, and Common Correlates of Conceptual Composites
Table II also contains the prevalence rates at the composite level for parent-reported sleep difficulty and night wakings. These composites were formed based on conceptual distinctions between the night waking items and the sleep problems/concerns items, even though, as noted above, these items within categories did operate differently (e.g., the item about sleep concerns was more likely to be endorsed than the two items about sleep problems). Consistent with the item-level information described above, night waking was endorsed by the majority of parents (68.5%), but sleep difficulty was endorsed by a smaller proportion of parents (9.4%). The sleep difficulty composite identified only about a third of the infants with an elevated ISQ score (29.8%) but differentiated most infants with a normal ISQ score (96.6%), consistent with the performance of the three individual items assessing parental perception of sleep problems/concerns. In contrast, the night wakings composite performed well at identifying children who screened positive for further sleep problem evaluation based on the validated ISQ (92.9%), yet excluded only 39.2% of children with a negative screen with the validated ISQ.
To examine common correlates of parent-reported night wakings and sleep difficulty, we first used the conceptual composites (Table III). At this level, infants who were younger and of black race were more likely to have parent-reported night wakings. Children who were classified as having a potential developmental delay were more likely to be perceived by their parent as having a sleep difficulty. Next, we examined correlates of parent-reported night wakings for the two separate night waking items—with and without parental intervention (Supplementary Table 1). Of note, regardless of item used, black race and younger child age were still significantly predictive of parent-reported night wakings, replicating the composite-level finding. The only difference that emerged from this item-level analysis was that language spoken in the home was significantly related to parent-reported night wakings (without parental intervention), such that more wakings were endorsed in Spanish-speaking families.
Table III.
Binary Logistic Regressions for Correlates of Parent-Reported Night Wakings and Sleep Difficulty and PCP Endorsement of Sleep Problem
| Outcome | Parameter | Est | s | Wald χ2 | Pr > χ2 | Point estimates |
|---|---|---|---|---|---|---|
| Parent-reported night wakings conceptual compositea | Black Race | 0.46 | 0.11 | 16.56 | .00 | 1.59 |
| DCI | 0.00 | 0.00 | 1.67 | .20 | 1.00 | |
| Language | −0.19 | 0.13 | 2.27 | .13 | 0.83 | |
| Age | −0.14 | 0.01 | 195.72 | .00 | 0.87 | |
| Dev Con | 0.14 | 0.13 | 1.16 | .28 | 1.15 | |
| Sex | −0.17 | 0.01 | 3.73 | .05 | 0.84 | |
| Parent-reported sleep difficulty conceptual composite | Black Race | −0.19 | 0.16 | 1.48 | .23 | 0.82 |
| DCI | 0.00 | 0.00 | 0.24 | .62 | 1.00 | |
| Language | −0.01 | 0.18 | 0.00 | .96 | 0.99 | |
| Age | 0.02 | 0.01 | 1.18 | .28 | 1.02 | |
| Dev Con | 1.01 | 0.15 | 47.85 | .00 | 2.74 | |
| Sex | −0.01 | 0.13 | 0.00 | .97 | 0.08 | |
| PCP-endorsed sleep problem | Black Race | −0.46 | 0.20 | 5.31 | .02 | 0.64 |
| DCI | −001 | 0.00 | 4.40 | .04 | 0.99 | |
| Language | −0.25 | 0.20 | 1.55 | .21 | 0.78 | |
| Age | 0.09 | 0.02 | 26.44 | .00 | 1.09 | |
| Dev Con | 0.04 | 0.21 | 0.03 | .86 | 1.04 | |
| Sex | −0.07 | 0.15 | 0.20 | 0.65 | 0.94 |
Note. Bolded parameters are statistically significant. Est = beta estimates; s = standard error; PCP = primary care provider; DCI = Distressed Communities Index where higher scores reflect more economic distress; Language = primary language spoken in the home, coded as 1 = English, 0 = Spanish; Age = age of the target child in months at the time of assessment; Dev Con = target child had a developmental concern, such as speech delay, where 1 = concern and 0 = no concern; Sex = sex of the target child; 1 = female and 0 = male.
Item-level analysis presented in Supplementary Table I.
Sleep Problem Coendorsement and Common Correlates of PCP Reports
PCPs endorsed a sleep problem for 21.5% of the infants for whom they responded. PCPs were more likely to endorse a sleep problem when the infants were older, white, or came from more advantaged backgrounds (see Table III).
Discussion
Our findings suggest that parent endorsement of problematic infant sleep is heavily influenced by the way in which the construct is assessed. Rates of problematic sleep in a large primary care sample were lowest (7.4%) when parents were asked to endorse a “problem” and highest (74.0%) when asked about night wakings with parental assistance. This high degree of variability presents a challenge in identifying infants with problematic sleep in primary care and other community settings.
Parent-Endorsed Sleep Difficulty
Sleep concerns or problems were reported broadly by only 8.4–11.6% of parents, a lower prevalence than previously reported (21.5–26.3%; Honaker et al., 2020; Mindell & Leichman, 2018; Mindell et al., 2010). One important difference in this study was the sample, which consisted predominantly of parents from racial/ethnic minority backgrounds (7.6% White non-Hispanic), with fewer economic resources (87.6% with Medicaid insurance), and living in at-risk or economically distressed communities (63.3%). Families with more sociodemographic risk factors may have lower educational attainment, which has been associated with less knowledge about infant development more broadly (Bornstein et al., 2010), as well as less familiarity with strategies to optimize infant sleep (Honaker et al., 2020). Moreover, lower income is associated with more life stressors (Evans & Kim, 2013), which could lead to families prioritizing concerns that are more pressing and/or immediate than their infant’s sleep. In addition, the construct of sleep difficulty perception for sleep in young children has been identified as highly variable across cultures. In a survey of parental concerns about infant sleep across 17 countries, Mindell et al. (2010) reported rates by country ranging from 11% to 76%, with a mean estimate of 26.3% in countries identified as predominantly Caucasian (e.g., United States, Australia, United Kingdom). Cultural differences might also contribute to different rates of sleep difficulty perception amongst US ethnic/minority families, though this has not yet been assessed.
In examining specific screening items assessing parental perception of sleep problems/concerns, the three items performed comparably, with a higher prevalence when assessing a sleep “concern” (11.6%) versus a sleep “problem” (7.4–8.4%), which was consistent with our hypotheses. When compared to the ISQ measure, none of these items performed well in identifying problematic sleep, with agreement rates ranging from 24.9% to 34.0%. That is, relying on one question to identify problematic infant sleep would likely be ineffective.
Night Wakings
More than two-thirds of parents reported that their infant had regular night wakings. This estimate may reflect a normative infant sleep pattern rather a sleep difficulty, in which case guidance would not be warranted. Indeed, in the youngest infants in the sample (e.g., 5–9 months) a single awakening could be attributed to nutritional need. Supporting this premise, within our sample, younger age was a predictor of parent-reported night wakings, both at the conceptual composite-level and at the item-levels. Based on established developmental change in physiological needs (i.e., older infants do not require feedings during the night), night waking in older infants could be an intervention target. Indeed, we found that PCPs were more likely to endorse problematic sleep in older infants.
Black race was another predictor of higher night waking endorsement rates, regardless of method of assessment (composite or item level). Higher rates of night wakings are associated with worse outcomes both concurrently (e.g., parental sleep and functioning) and prospectively (e.g., risk for future sleep problems), and thus could be linked with broader disparities. Unfortunately, most studies on the efficacy of infant behavioral sleep intervention (BSI) have included few Black infants and families (Schwichtenberg et al., 2019). To address this disparity, there is a need for targeted inclusion of Black families in both assessment and intervention studies of infant sleep.
Two screening items about night wakings identified most (91.0–94.6%) infants with an elevated score on a validated measure of problematic sleep, but also identified many infants (31.8–46.9%) without an elevated score. Surprisingly, parents endorsed night wakings requiring parental involvement at a higher rate (74.0%) than night wakings more generally (62.8%). An implication of this pattern is that asking about night wakings more generally (without specifying parental involvement) does not appear to result in an overestimate of the target behavior (i.e., night wakings involving parental involvement), and may indicate that parental involvement in infant night wakings may not be a significant factor in their identification of night wakings.
PCP-Endorsed Problematic Infant Sleep
PCPs endorsed a sleep problem in 21.5% of the infants they evaluated. This rate likely represents an overestimate of the true prevalence of PCP-perceived problematic infant sleep; in this sample, PCPs received a prompt to evaluate infant sleep only when parents responded affirmatively to at least one of the individual screening items (57.7% of the overall sample). Presumably, those families for whom PCPs did not receive a prompt were less likely to have an infant with problematic sleep. Although a prevalence of 21.5% is comparable with epidemiological estimates of problematic infant sleep (20–30%; Mindell et al., 2006, 2010), it is far higher than estimates found in primary care documentation of problematic sleep (<1%; Charles et al., 2011) or insomnia diagnosis (<1%; Meltzer et al., 2010) in young children. As a robust literature demonstrates higher rates of problem identification in systems that utilize computer decision support (Garg et al., 2005), it is likely that PCP identification of problematic infant sleep in the current study was enhanced through the use of the CHICA Infant Sleep module.
Notably, PCPs were less likely to identify sleep problems in children of black race and in families living in more distressed communities, despite previous studies suggesting higher rates of problematic sleep in these groups (Nevarez et al., 2010; Williamson & Mindell, 2020). This may reflect a health disparity in care for income and race. Alternatively, other areas of health and/or psychosocial need may have been deemed more pressing to address for children from racial/ethnic minority backgrounds and children living in more distressed communities. It is also worth noting that different factors predicted identification of a sleep problem by parents compared with PCPs. Parents were more likely to indicate a sleep difficulty within the context of developmental concerns, whereas PCPs were more likely to endorse a sleep problem when the infants were older, of white race, or came from more advantaged backgrounds. Future research aimed at advancing understanding of parental conceptualizations of sleep issues and potential biases by PCPs related to when sleep is problematic is warranted.
Identifying Families for BSI
Parent-reported sleep difficulty and night wakings each provide valuable information that might be used to identify families who could benefit from sleep guidance and intervention in community settings, such as primary care. The study objective was not to identify a “best” approach to screening for problematic infant sleep, but to provide outcome data that health systems can use to inform the development of a system tailored to meet their goals.
Night waking frequency identified a large number of infants (two-third in our sample), including many whose parents did not describe these night wakings as a difficulty and thus might not be expected to initiate a discussion about their infant’s sleep. If the goal is to identify opportunities for sleep guidance and intervention, asking directly about night wakings is an advantageous approach, particularly as some parents may not be aware of BSI interventions that can effectively reduce night wakings in infants. In a survey of US mothers of infants (6–18 months), one study reported 39% of mothers were not familiar with infant BSI (Honaker et al., 2020). Thus, screening for parent-reported night wakings offers an opportunity for PCPs to evaluate infants and, where needed and desired, offer BSI that could mitigate later sleep disruption and the affiliated cascade of negative health, educational, and familial correlates.
One limitation to relying on parent-reported night wakings as a screening strategy is that other sleep-related parental concerns (e.g., naps, settling problems) are not identified. Further, this approach is more labor-intensive for PCPs, as many infants will be identified who require additional evaluation, and, if appropriate, intervention. Conversely, screening for parent-perceived sleep difficulty is an approach that may be more inclusive of varied sleep problems, but identifies fewer infants. If resources are less available, screening for parent-perceived difficulty is a more selective approach, and might identify those families who are most in need of BSI and most motivated to apply it.
Where feasible, using screening items assessing both night wakings and parental sleep concerns might be ideal, as each approach has value in identifying infants with problematic sleep. Parental endorsement of either sleep difficulty or night waking could prompt providers to further evaluate and determine whether intervention is warranted. However, in large primary care settings, devoting more than one screening item to infant sleep may limit implementation feasibility, particular if the use of multiple items resulted in more positive screens requiring further PCP evaluation. In sum, the two screening approaches performed very differently, and a preferred approach can be identified only when considering the goals and resources in the target setting.
The real-world application of study findings has informed clinical practice, and each of the issues noted above were considered. Specifically, data from this study were presented to clinic providers, staff, and leadership in order to develop a revised CHICA Infant Sleep screening protocol (see Supplementary Figure 1 for a description of the process and resulting module).
Limitations
Several limitations in this study deserve note. The CHICA Infant Sleep system was designed to provide (and inform) clinical care, rather than as a research tool. Therefore, some of the assessment strategies were interdependent, limiting analyses that required independence. For example, the PCP sleep prompt was dependent on parental endorsement of “yes” on one or more items. Thus, the PCP sleep problem endorsement rate, independent of parent endorsement, could not be ascertained. In addition, items were pseudo-randomized such that parents completed different combinations of items. However, not all items were randomized or interdependent; the validated sleep measure was independent from both the screening items and the PCP prompt. Although the ISQ measure had strong psychometric properties, the validation study likely did not include families from US ethnic/minority backgrounds, nor was it validated in Spanish language. Our sample included children from predominantly black or Hispanic racial/ethnic backgrounds, as well as children from predominantly from at-risk or distressed communities, limiting generalizability to a more representative United States or international population. However, including diverse and under-studied groups can also be considered a strength of the study. Finally, our study included parent-reported sleep data, but did not assess parent sleep knowledge or perspectives on sleep screening and guidance.
Summary and Next Steps
In light of the prevalence and negative impact of problematic infant sleep, the identification of sleep disturbances that are amenable to guidance and intervention is necessary. Yet, assessment of problematic infant sleep is highly complex and dependent on a variety of factors. This study assessed different strategies to identify problematic infant sleep, providing data that can be used by healthcare and other community-based systems to develop a screening strategy that meets their goals. Future studies should incorporate parent perspectives on screening and intervention for infant sleep, and should also evaluate the impact of screening strategies on more downstream outcomes, such as family participation in intervention and intervention outcomes.
Supplementary Data
Supplementary data can be found at: https://academic.oup.com/jpepsy.
Supplementary Material
Acknowledgments
The authors would like to thank the dedicated providers and staff at the five participating Eskenazi Health clinic sites, as well as all of the participating children and families. We further wish to acknowledge the technical expertise and efforts of the individual members of the Child Health Informatics and Research Development Lab (CHIRDL) team, which provides programming and technical support for CHICA, and the Pediatric Research Network (PReSNet) at Indiana University School of Medicine for regulatory support.
Funding
This study was funded, in part, with support by Indiana University Health, with assistance from the Indiana Clinical and Translational Sciences Institute funded by a grant from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award. Dr. Honaker’s time was supported by Grant Number UL1TR002529 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award and the Indiana University School of Medicine, and also by the National Heart, Lung, and Blood Institute of the National Institutes of Health under Award Number K23HL150299. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflicts of interest: Dr. Downs is a co-developer of CHICA and a cofounder of Digital Health Solutions, LLC, which makes the software available commercially.
References
- Anand V., Carroll A. E., Biondich P. G., Dugan T. M., Downs S. M. (2018). Pediatric decision support using adapted Arden Syntax. Artificial Intelligence in Medicine, 92, 15–23. 10.1016/j.artmed.2015.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein M. H., Cote L. R., Haynes O. M., Hahn C. S., Park Y. (2010). Parenting knowledge: Experiential and sociodemographic factors in European American mothers of young children. Developmental Psychology, 46(6), 1677–1693. 10.1037/a0020677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CarrollA. E., , BauerN. S., , DuganT. M., , AnandV., , SahaC., & , Downs S. M. (2014). Use of a Computerized Decision Aid for Developmental Surveillance and Screening. JAMA Pediatrics, 168(9), 815, –821.https://doi.org/ 10.1001/jamapediatrics.2014.464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer N. S., Carroll A. E., Saha C., Downs S. M. (2016). Experience with decision support system and comfort with topic predict clinicians' responses to alerts and reminders. Journal of the American Medical Informatics Association, 23(e1), e125–e130. 10.1093/jamia/ocv148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles J., Harrison C. M., Britt H. (2011). Management of children’s psychological problems in general practice 1970–1971, 1990–1991 and 2008–2009. Australian & New Zealand Journal of Psychiatry, 45(11), 976–984. 10.3109/00048674.2011.610743 [DOI] [PubMed] [Google Scholar]
- Evans G. W., Kim P. (2013). Childhood poverty, chronic stress, self-regulation, and coping. Child Development Perspectives, 7(1), 43–48. 10.1111/cdep.12013 [DOI] [Google Scholar]
- Garg A. X., Adhikari N. K., McDonald H., Rosas-Arellano M. P., Devereaux P. J., Beyene Sam J., Haynes R. B. (2005). Effects of computerized clinical decision support systems on practitioner performance and patient outcomes: A systematic review. JAMA, 293(10), 1223–1238. [DOI] [PubMed] [Google Scholar]
- Hagan J. F., Shaw J. S., Duncan P. M. (2017). Bright futures: Guidelines for health supervision of infants, children, and adolescents: Pocket guide (4th edn). American Academy of Pediatrics. [Google Scholar]
- Hoffses K. W., Honaker S., Riley A. W., Meadows T. J. (2018). Screening and assessment of sleep disturbance. In Maruish M. (Ed.), Handbook of pediatric psychological screening and assessment in primary care (pp. 358–380). Routledge. [Google Scholar]
- Honaker S. M., Mindell J. A., Slaven J. E., Schwichtenberg A. J. (2020). Implementation of infant behavioral sleep intervention in a diverse sample of mothers. Behavioral Sleep Medicine, 1–15. 10.1080/15402002.2020.1817745 [DOI] [PubMed] [Google Scholar]
- Lam P., Hiscock H., Wake M. (2003). Outcomes of infant sleep problems: A longitudinal study of sleep, behavior, and maternal well-being. Pediatrics, 111(3), e203–e207. [DOI] [PubMed] [Google Scholar]
- Maruish M. E. (2018). Selection of psychological measures and associated administration, scoring, and reporting technology for use in pediatric primary care settings. In Maruish M. E. (Ed.) Handbook of pediatric psychological screening and assessment in primary care (pp. 71–92). Routledge. [Google Scholar]
- Meltzer L. J., Mindell J. A. (2007). Relationship between child sleep disturbances and maternal sleep, mood, and parenting stress: A pilot study. Journal of Family Psychology, 21(1), 67–73. 10.1037/0893-3200.21.1.67 [DOI] [PubMed] [Google Scholar]
- Meltzer L. J., Mindell J. A. (2014). Systematic review and meta-analysis of behavioral interventions for pediatric insomnia. Journal of Pediatric Psychology, 39(8), 932–948. 10.1093/jpepsy/jsu041 [DOI] [PubMed] [Google Scholar]
- Meltzer L. J., Johnson C., Crosette J., Ramos M., Mindell J. A. (2010). Prevalence of diagnosed sleep disorders in pediatric primary care practices. Pediatrics, 125(6), e1410–e1418. 10.1542/peds.2009-2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M. A., Kruisbrink M., Wallace J., Ji C., Cappuccio F. P. (2018). Sleep duration and incidence of obesity in infants, children, and adolescents: A systematic review and meta-analysis of prospective studies. Sleep, 41(4), zsy018. 10.1093/sleep/zsy018 [DOI] [PubMed] [Google Scholar]
- Mindell J. A., Leichman E. S. (2018). Parental goals for infant and toddler sleep. Sleep, 41(Suppl_1), A284–A285. [Google Scholar]
- Mindell J. A., Kuhn B., Lewin D. S., Meltzer L. J., Sadeh A.; American Academy of Sleep Medicine (2006). Behavioral treatment of bedtime problems and night wakings in infants and young children. Sleep, 29(10), 1263–1276. 10.1093/sleep/29.10.1263 [DOI] [PubMed] [Google Scholar]
- Mindell J. A., Leichman E. S., Puzino K., Walters R., Bhullar B. (2015). Parental concerns about infant and toddler sleep assessed by a mobile app. Behavioral Sleep Medicine, 13(5), 359–374. 10.1080/15402002.2014.905475 [DOI] [PubMed] [Google Scholar]
- Mindell J. A., Meltzer L. J., Carskadon M. A., Chervin R. D. (2009). Developmental aspects of sleep hygiene: Findings from the 2004 National Sleep Foundation Sleep in America Poll. Sleep Medicine, 10(7), 771–779. 10.1016/j.sleep.2008.07.016 [DOI] [PubMed] [Google Scholar]
- Mindell J. A., Sadeh A., Wiegand B., How T. H., Goh D. Y. (2010). Cross-cultural differences in infant and toddler sleep. Sleep Medicine, 11(3), 274–280. 10.1016/j.sleep.2009.04.012 [DOI] [PubMed] [Google Scholar]
- Morrell J. M. B. (1999). The Infant Sleep Questionnaire: A new tool to assess infant sleep problems for clinical and research purposes. Child and Adolescent Mental Health, 4(1), 20–26. 10.1111/1475-3588.00246 [DOI] [Google Scholar]
- Nevarez M. D., Rifas-Shiman S. L., Kleinman K. P., Gillman M. W., Taveras E. M. (2010). Associations of early life risk factors with infant sleep duration. Academic Pediatrics, 10(3), 187–193. 10.1016/j.acap.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens J. A., Jones C. (2011). Parental knowledge of healthy sleep in young children: Results of a primary care clinic survey. Journal of Developmental and Behavioral Pediatrics, 32(6), 447–453. 10.1097/DBP.0b013e31821bd20b [DOI] [PubMed] [Google Scholar]
- Pajek J. A., Stancin T. (2018). An overview of integrated pediatric primary care. In Maruish M. E. (Ed.) Handbook of pediatric psychological screening and assessment in primary care (pp. 57–70). Routledge. [Google Scholar]
- Sadeh A., Mindell J. A., Luedtke K., Wiegand B. (2009). Sleep and sleep ecology in the first 3 years: A web-based study. Journal of Sleep Research, 18(1), 60–73. 10.1111/j.1365-2869.2008.00699.x [DOI] [PubMed] [Google Scholar]
- Sadeh A., Mindell J., Rivera L. (2011). My child has a sleep problem : A cross-cultural comparison of parental definitions. Sleep Medicine, 12(5), 478–482. 10.1016/j.sleep.2010.10.008 [DOI] [PubMed] [Google Scholar]
- ScherA., , TseL., , HayesV. E., & , Tardif M. (2008). Sleep Difficulties in Infants at Risk for Developmental Delays: A Longitudinal Study. Journal of Pediatric Psychology, 33(4), 396–405. 10.1093/jpepsy/jsn013 [DOI] [PubMed] [Google Scholar]
- Schwichtenberg A. J., Abel E. A., Keys E., Honaker S. M. (2019). Diversity in pediatric behavioral sleep intervention studies. Sleep Medicine Reviews, 47, 103–111. 10.1016/j.smrv.2019.07.004 [DOI] [PubMed] [Google Scholar]
- Schwichtenberg A. J., Young G. S., Hutman T., Iosif A. M., Sigman M., Rogers S. J., Ozonoff S. (2013). Behavior and sleep problems in children with a family history of autism. Autism Research , 6(3), 169–176. 10.1002/aur.1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- TetiD. M., , KimB.-R., , MayerG., & , Countermine M. (2010). Maternal emotional availability at bedtime predicts infant sleep quality. Journal of Family Psychology, 24(3), 307–315. 10.1037/a0019306 [DOI] [PubMed] [Google Scholar]
- Tham E. K., Schneider N., Broekman B. F. (2017). Infant sleep and its relation with cognition and growth: A narrative review. Nature and Science of Sleep, 9, 135–149. 10.2147/NSS.S125992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson A. A., Mindell J. A. (2020). Cumulative socio-demographic risk factors and sleep outcomes in early childhood. Sleep, 43(3), zsz233. 10.1093/sleep/zsz233 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.