A simple and efficient strategy to generate human trophoblast stem cells is reported to aid investigations of early placentation.
Abstract
Human trophoblast stem cells (hTSCs) provide a valuable model to study placental development and function. While primary hTSCs have been derived from embryos/early placenta, and transdifferentiated hTSCs from naïve human pluripotent stem cells (hPSCs), the generation of hTSCs from primed PSCs is problematic. We report the successful generation of TSCs from primed hPSCs and show that BMP4 substantially enhances this process. TSCs derived from primed hPSCs are similar to blastocyst-derived hTSCs in terms of morphology, proliferation, differentiation potential, and gene expression. We define the chromatin accessibility dynamics and histone modifications (H3K4me3/H3K27me3) that specify hPSC-derived TSCs. Consistent with low density of H3K27me3 in primed hPSC-derived hTSCs, we show that knockout of H3K27 methyltransferases (EZH1/2) increases the efficiency of hTSC derivation from primed hPSCs. Efficient derivation of hTSCs from primed hPSCs provides a simple and powerful model to understand human trophoblast development, including the pathogenesis of trophoblast-related disorders, by generating disease-specific hTSCs.
INTRODUCTION
The placenta is an essential organ during pregnancy for fetal development (1). Its major functional components are specialized epithelial cells named trophoblasts that arise from directional differentiation of the trophoblast stem cells (TSCs) (2). In decades after derivation of mouse TSCs in 1998 (3), TSCs have been isolated from rhesus monkey or bovine blastocysts in the presence of fibroblast growth factor 4 (FGF4) and transforming growth factor–β1 (TGF-β1)/activin (4–6). However, for many years, attempts using similar strategies to obtain equivalent cells in humans failed because of their rapid differentiation. Recently, using a specific culturing system containing TGF-β inhibitor and WNT activator (7, 8), human TSCs (hTSCs) were successfully derived from pre- or peri-implantation human embryos. These primary hTSCs provide a powerful tool to investigate normal placenta development at an early stage. However, the use of early human embryos to derive hTSCs faces ethical challenges. Moreover, we and others have been unable to isolate hTSCs from placentas at later stages of pregnancy (8), a period when most trophoblast-related disorders including preeclampsia are initially diagnosed. Hence, analysis of primary early-pregnancy hTSCs are unlikely to aid understanding of underlying pregnancy disease mechanisms, screening for predictive biomarkers, and eventually development of targeted therapies (9).
On the other hand, investigators have begun to use human pluripotent stem cells (hPSCs) including human embryonic stem cells (hESCs) and induced PSCs (hiPSCs) as resources to generate trophoblast lineage. First, a population of terminal trophoblast-like cells have been directly generated from hPSCs in response to a critical factor, bone morphogenetic protein 4 (BMP4) (10–12). Recent studies have also described hTSC-like cells that could be established from human expanded potential stem cells (EPSCs) or naïve PSCs and hold the potential to differentiate toward terminal trophoblast cells (13–16). However, primed PSCs, although a resource for resetting EPSCs and naïve PSCs, do not support hTSC derivation, and moreover, BMP4 is not required as it is in the direct generation of trophoblast-like cells (14).
Because of the difficulties in obtaining naïve PSCs in most laboratories, since 2018, our team has used primed hPSCs as a source to derive long-term cultured hTSCs, before reports of naïve PSCs-derived hTSCs. Despite a low efficiency of TSC generation, by a careful clone-screening process, we successfully obtained hTSC-like cells from primed hPSCs. These cells exhibit a similar phenotype to the blastocyst-derived hTSCs in terms of morphology, proliferation, differentiation potential, and transcriptome profiles. Moreover, we gained evidence that BMP4 plays a critical role in significantly enhancing the efficiency of generating hTSC-like cells from primed PSCs. On the basis of this approach, we further dissected epigenetic patterns of human hPSC-derived TSCs and identified enhancing factors for hTSC derivation from primed hPSCs. Our findings here report an alternative and easier system for hTSC derivation, which provides a powerful tool to explore transitions from embryonic to extraembryonic cell fates, trophoblast development, and associated disease mechanisms.
RESULTS
Derivation of hTSCs from primed hPSCs
Initially, we chose the widely available and well-characterized primed hESC lines (H1 and HN10) as the donor cell source for induction of hTSCs. After maintenance in hESC culture medium for 1 day, both cell lines were then cultured under hTSC medium, containing l-ascorbic acid, epidermal growth factor (EGF), glycogen synthase kinase 3 (GSK3) inhibitor CHIR99021, TGF-β inhibitors A83-01/SB431542, histone deacetylase inhibitor valproic acid (VPA), and rho-associated, coiled-coil-containg protein kinase (ROCK) inhibitor Y27632, to trigger induction (Fig. 1A; see Materials and Methods). Both cell lines appeared to exit pluripotency with loss of colony shape and then gradually underwent complete morphological change in hTSC culture medium (Fig. 1B and fig. S1A). Intriguingly, following continuous culturing under hTSC culture medium for around 7 and 8 days, several hTSC-like colonies with distinct boundaries became visible and enlarged (Fig. 1B, arrows, and fig. S1A). At days 10 to 12, consistent with the morphological changes, both cell lines were shown to be negative for OCT4 expression, a hESC marker, but positive for GATA3, a mononuclear trophoblast marker (fig. S1B) (8). These findings indicate that the OCT4+GATA3− hESCs had converted to OCT4−GATA3+ cell types, although with a low efficiency of generation (2.73% of H1 cells and 23.1% of HN10 cells). We next picked these trophoblast-like colonies on days 10 to 12 for purification and expansion. Starting from passage 2 (P2), all cells grew homogeneously in colonies with similar morphology to the primary hTSblast and hTSCT cell lines established by Okae et al. (8) (Fig. 1B and fig. S1A). Moreover, these hTSC-like cells could be stably maintained for more than 30 passages (Fig. 1C). To exclude the possibility that these hTSC-like cells preexisted in the hESCs, the hTSC-like cells were seeded reversely under hESC culture medium (mTeSR1), which resulted in cell cycle arrest and cell death within 4 days of culture (fig. S1C). These results imply that these hTSC-like OCT4−GATA3+ cells were converted, instead of isolated, from primed hESCs. Thus, we named these cells as primed hPSC-derived hTSCs (hTSPS cells).
Fig. 1. Derivation of hTSPS cells from primed hPSCs.
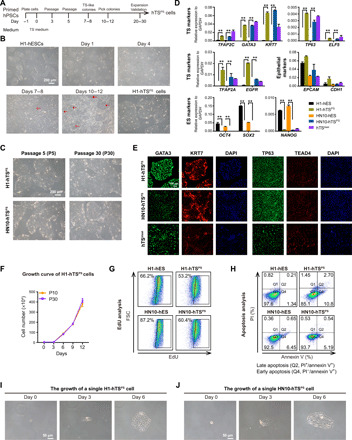
(A) Overview of conversion strategy to generate hTSCs (hTSPS cells) from PSCs (hPSCs). hPSCs were maintained in mTeSR1 medium in monolayer culture. The TS-like colonies appeared at days 7 and 8 and were picked at days 10 to 12 for subculture expansion as purified hTSPS cells (see Materials and Methods). (B) Morphology of converted cells derived from H1 hESCs under TS medium. Scale bar, 200 μm. (C) Morphology of hTSPS cells derived from H1 or HN10 hESCs at early (P5) or late passage (P30). Scale bar, 200 μm. (D) Quantitative real-time polymerase chain reaction (qRT-PCR) expression of TS, epithelial, and ES marker genes in hTSPS cells derived from H1 or HN10 hESCs. H1 and HN10 hESCs serve as negative controls. hTSblast serves as a positive control. **P < 0.01. Error bars represent means ± SD from three independent replicates. Results represent two samples, H1-hTSPS and HN10-TSPS (n = 2). (E) Immunostaining of TS marker genes GATA3/KRT7/TP63/TEAD4 in hTSPS cells derived from H1 or HN10 hESCs. hTSblast serves as a positive control. Scale bar, 100 μm. (F) Growth curve of H1 hTSPS cells at early (P10) or high passage (P30). Error bars represent means ± SD from three independent replicates. Similar results were obtained using another independent cell line HN10-TSPS (fig. S2H). (G) EdU incorporation assay for hTSPS cells and their parent hESCs. (H) Apoptosis assay in hTSPS cells and their parent hESCs. PI and/or annexin V–positive cells were analyzed by fluorescence-activated cell sorting (FACS). The apoptotic cells include late apoptotic cells (PI+/annexin+) in Q2 and early apoptotic cells (PI−/annexin V+) in Q4. (I and J) Phase-contrast images showing growth of a single hTSPS cell. Scale bar, 50 μm. The results shown in (E), (G), and (H) are representative of three independent experiments from two samples, H1-hTSPS and HN10-TSPS (n = 2). See figs. S1 to S3 for more details.
Next, we validated the trophoblastic criteria and stemness of these hTSPS cells. First, hTSC-related markers (TFAP2C, GATA3, KRT7, TP63, and so on) were up-regulated, whereas hESC-related markers (OCT4, SOX2, and NANOG) were fully suppressed in both H1- and HN10-derived hTSPS cells (referred to as H1-hTSPS and HN10-hTSPS, respectively) similar to the primary hTSblast cell line (Fig. 1, D and E, and fig. S2, A to C). Also, both hTSPS cell lines expressed epithelium-specific genes (EPCAM and CDH1) like their parent hESCs (Fig. 1D). Meanwhile, both hTSPS cell lines did not express three germ layer marker genes, including PAX6/SOX1 (ectoderm), SOX17/FOXA2 (endoderm), and CD31/MYB1 (mesoderm) (fig. S2D). Moreover, in line with the gene expression level (Fig. 1C), the promoter of ELF5, which is largely hypermethylated in the parent hESCs, was hypomethylated in both hTSPS cell lines at P10 (fig. S2E). Furthermore, both hTSPS cell lines did not express the human leukocyte antigen (HLA) class I molecules (such as HLA-ABC) and exhibited up-regulation of microRNAs (miRNAs) on chromosome 19, such as miR517-5p and miR525-3p (fig. S2, F and G). Together, these data indicate that these human TSPS cells, derived from hESCs, have features of true trophoblast (17).
Furthermore, we examined the growth properties of these hTSPS cells. Both the 10th and 30th passage hTSPS cells showed similar growth curves (Fig. 1F and fig. S2H). Like the hESCs, both hTSPS cell lines also showed low apoptosis and high proliferation ability (Fig. 1, G and H) and maintained normal karyotypes (fig. S2I). Individual hTSPS cells were able to survive and expand to form an hTS-like colonies (Fig. 1, I and J). Together, these data suggest that the human TSPS cells show growth features of stem cells (17).
Meanwhile, we also repeated this conversion protocol using primed hiPSC lines derived from urine epithelial cells or term placenta–derived amniotic mesenchymal cells (AMCs). Again, we successfully derived hTSPS cell lines in each case with expression of trophoblast-specific markers and loss of specific iPSC markers (fig. S3, A and C). Together, these results suggest the technical possibility of obtaining hTSCs from primed hPSCs, regardless of their donor cell source.
The human TSPS cells have the capacity to give rise to both extravillous cytotrophoblast and syncytiotrophoblast cells
We next tested the capacity of these hTSPS cells to differentiate toward downstream trophoblast subtypes, including syncytiotrophoblast (STB) and extravillous cytotrophoblast (EVT). Using a strategy similar to that reported in primary hTSblast (Fig. 2A) (8), these hTSPS cells differentiated into HLA-G+ cells displaying EVT morphology (Fig. 2B). Furthermore, quantitative polymerase chain reaction (qPCR) analysis demonstrated the down-regulation of hTSC-specific genes (ELF5, TP63, EPCAM, and TEAD4) and up-regulation of EVT-specific genes (HLA-G, MMP2, and ITAG1) in hTSPS-derived EVT cells (fig. S4A). Moreover, hTSC-derived EVT cells did not express HLA-A and HLA-B (fig. S4B). Immunofluorescent staining further confirmed that these hTSPS cell–derived differentiated cells expressed the EVT marker (HLA-G) in their cell membrane but were negative for the STB markers (SDC1 and CGB) (Fig. 2D and fig. S4, D and E). Conversely, in the presence of forskolin, a cyclic adenosine 3′,5′-monophosphate (cAMP) agonist used to induce syncytialization of trophoblast in vitro, these hTSPS cells demonstrated efficient fusion to form syncytia (Fig. 2C, left, and fig. S4F), secrete human chorionic gonadotropin (Fig. 2C, right), and express STB-specific genes (CGB, CGA, and CSH1) (fig. S4C), and demonstrated positive staining for STB-specific markers (SDC1 and CGB) (Fig. 2D and fig. S4, D and E). Together, our data demonstrate that hTSPS cells, similar to primary hTSC derived from blastocyst, have the potential to give rise to downstream trophoblast subtypes including EVT and STB, which further confirms their comparable properties to primary hTSCs.
Fig. 2. Differentiation of hTSPS cells toward EVT and STB cells.

(A) Schematic diagram of protocols for directed differentiation of human TSPS cells toward EVT and STB cells. (B) Top: Mesenchyme-like morphology of EVT cells derived from hTSPS cells. Scale bar, 100 μm. Bottom: FACS analysis on the expression of HLA-G on the differentiated EVT cells. Primary hTSCs serve as a positive control. Scale bar, 200 μm. (C) Left: Morphology of STB cells derived from the hTSPS cells. Scale bar, 50 μm. Primary hTSCs serve as a positive control. Scale bar, 100 μm. Right: Enzyme-linked immunosorbent assay (ELISA) analysis for hCG secretion of STB cells derived from the hTSPS cells. H1-hTSPS and HN10-hTSPS cells serve as controls. Error bars represent means ± SD from three biological replicates. Results represent two samples, H1-hTSPS and HN10-TSPS (n = 2). **P < 0.01. (D) Immunostaining on KRT7 (trophoblast pan-marker), HLA-G (EVT marker), and SDC1/CGB (STB markers) in the H1-derived hTSPS cells and their differentiated cells. Scale bar, 100 μm. The results shown in (B) and (D) are representative of three independent experiments from two samples, H1-hTSPS and HN10-TSPS (n = 2).
BMP4 enhances human TSPS cell generation from primed PSCs
Despite the successful generation of hTSPS cells from primed PSCs, the efficiency was extremely low, especially in H1 cell lines (only 2.73%) (fig. S1B). Previously, many studies report that BMP4 is a key initiator to form terminated trophoblast subtypes from primed hPSCs (12, 18). Therefore, we investigated whether BMP4 might play a role in the generation of human TSPS cells in our system. We added BMP4 to human TS medium during hTSPS derivation process from primed hPSCs (Fig. 3A). The process of generating human TSPS with or without BMP4 was termed “BMP4 strategy” or “Standard strategy,” respectively, in the following description (Fig. 3A). As expected, the generation efficiency of hTSPS from either primed ES cells or iPSCs was increased in the BMP4 strategy than in the Standard strategy (Fig. 3, B to D). While only a small population of cells gained TS morphology using the Standard strategy, most primed hPSCs in the BMP4 strategy were converted to a TS morphology by day 10 (Fig. 3B). Consistently, the efficiency was elevated from approximately 2 to 20% with respect to the level of KRT7 expression and from around 20 to 90% for GATA3 expression (Fig. 3C). In addition, the expression of OCT4 was more rapidly down-regulated using the BMP4 strategy (Fig. 3C). Likewise, using primed iPSC lines as a source for hTSPS derivation, KRT7 and GATA3 expression was also significantly higher using the BMP4 strategy than the Standard strategy by day 10 (Fig. 3D). These data indicate that BMP4 enhances the efficiency of generating human TSPS cells from primed hPSCs.
Fig. 3. BMP4 promotes human TSPS cell generation from primed hPSCs.
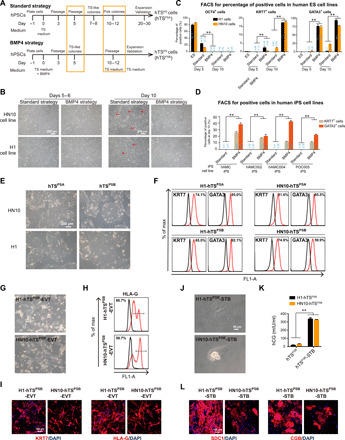
(A) Overview of derivation strategy for hTSCs (hTSPS cells) from primed PSCs (hPSCs) in the absence (Standard strategy) or presence of BMP4 (BMP4 strategy). (B) Morphology of converted cells from H1 or HN10 hESCs under Standard or BMP4 strategies at days 5 and 6 and day 10. Scale bar, 200 μm. (C) FACS analysis of OCT4+, KRT7+, and GATA3+ cells during conversion under Standard or BMP4 strategies at ES state, day 5, and day 10 from H1 and HN10 hESC lines, respectively. **P < 0.01. Error bars represent means ± SD from three independent replicates. Results represent two samples, H1 and HN10 cell lines (n = 2). (D) FACS analysis of KRT7+ and GATA3+ cells during hTSPS derivation under Standard or BMP4 strategies at day 10 from four iPSC lines (n = 4). **P < 0.01. Error bars represent means ± SD from three independent replicates. (E and F) Morphology/FACS analysis of KRT7+ and GATA3+ cells of hTSPS cells derived from H1 or HN10 hESCs under Standard (hTSPSA) or BMP4 strategy (hTSPSB). Scale bar, 200 μm. (G and H) Morphology/FACS analysis of HLA-G+ EVT cells derived from human TSPS cells under BMP4 strategy (hTSPSB). Scale bar, 100 μm. (I) Immunostaining of trophoblast marker KRT7 and EVT marker HLA-G in EVT cells. Scale bar, 100 μm. (J and K) Morphology/ELISA analysis for hCG secretion by STB cells derived from human TSPS cells under BMP4 strategy (hTSPSB). Error bars represent means ± SD from three independent replicates. Results represent two samples, H1-hTSPSB and HN10-TSPSB (n = 2). Scale bar, 50 μm. (L) Immunostaining of STB markers SDC1/CGB in STB cells. Scale bar, 100 μm. Results shown in (F), (H), (I), and (L) are representative of three independent experiments from two samples, H1-hTSPSB and HN10-TSPSB (n = 2). See figs. S5 and S6 for details.
Using the BMP4 strategy, as mentioned above, most cells demonstrated hTSPS derivation in morphology. We therefore directly changed the medium to be TS medium without exogenous BMP4 at day 10 without the need for colony picking. (Fig. 3A). For ease of description, hTSPS cells harvested using the Standard strategy or BMP4 strategy were named as hTSPSA and hTSPSB cells, respectively (Fig. 3, A and E, and fig. S6A). We next characterized the hTSPSB cells. Similar to hTSPSA cells, hTSPSB cells also exhibited typical TS morphology and highly expressed TS markers but lacked expression of pluripotent markers (Fig. 3, E and F, and figs. S5, A to C, and S6, A to C). In addition, the promoter of ELF5 was hypomethylated and the HLA class I molecules were not expressed in these hTSPSB cells similar to hTSPSA cells at P10 (fig. S5, D and E). Furthermore, the hTSPSB cells exhibited low apoptosis, high proliferation ability, and colony formation from single cells as with hTSPSA cells (fig. S5, F to H). These data indicate that hTSCs generated under TS medium containing BMP4 also have the trophoblastic features and stem cell properties. To assess the role of BMP4 in the maintenance of hTSCs, we added BMP4 inhibitors LDN193189 and Dorsomophin into TS medium in the absence of exogenous BMP4 to culture our hTSPS cells, respectively. We found the hTSPS cells treated with two BMP4 inhibitors for 5 days were significantly reduced (fig. S6D). Our data indicate that endogenous BMP4 is important in the maintenance of hTSCs. Moreover, hTSPSB cells were able to differentiate toward both EVT (hTSPSB-EVT) and STB trophoblast subtypes (hTSPSB-STB) as evidenced by typical EVT/STB morphology and the expression of specific EVT/STB markers similar to hTSPSA cells (Fig. 3, G to L, and fig. S5, I and J). Together, we demonstrate a key role of BMP4 in promoting human TS generation from primed PSCs.
Human TSPS cells share a similar transcriptome profile with primary blastocyst–derived hTSCs
To further confirm the complete transition of hTSPS cells, including hTSPSA and hTSPSB from primed hESCs, we performed whole-genome analysis to compare the RNA transcriptome profiles among hTSPS cells at P20, parent hESCs, and the primary blastocyst–derived hTSCs. Pearson correlation analysis and cluster analysis revealed a close relationship between hTSPS cells and hTSblast compared with the parent hESCs (Fig. 4A and fig. S7, A and B). Selected trophoblast-specific genes were confirmed to be expressed in all hTSPS cell lines and hTSblast but not hESCs (Fig. 4B and fig. S7B), which indicated a complete segregation from the hESCs that express only ES-specific genes (Fig. 4B and fig. S7B). Next, the full gene expression profiles from hTSPS cells and hESCs were subjected to unsupervised cluster analysis that identified discrete segregation between the hESCs and hTSPS cells (Fig. 4C and fig. S7C). Gene Ontology (GO) term analysis of the up-regulated genes in the hTSPS cells showed relationships with placental development, female pregnancy, etc. (Fig. 4C). Conversely, the down-regulated genes were related to stem cell maintenance and somatic stem cell population maintenance (Fig. 4C). Moreover, enrichment of the top 50 and top 20 differentially expressed genes in the hTSPS cell subset further highlighted gene expression profiles for the activation of placenta development and inhibition of pluripotency-related maintenance in hTSPS cells (fig. S7, D and E). Together, these data demonstrate that the transcriptome profiles of human TSPS cells are similar to primary blastocyst–derived hTSCs and fully distinct from their parent hESCs.
Fig. 4. RNA sequence–based transcriptome profiles and ATAC sequence–based chromatin accessibility of hTSPS cells.
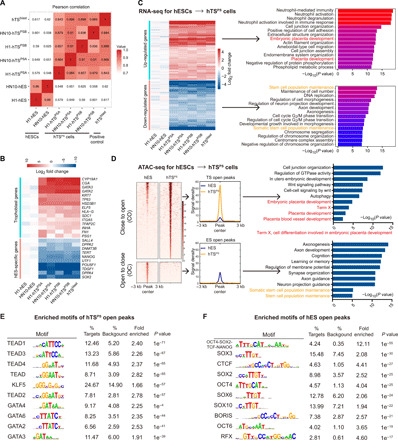
(A) Pearson rank correlation analysis on the whole-genome transcriptome of hTSPS cells at P20 and their parent hESCs, and primary hTSC line, hTSblast. hTSPS cells (n = 4, four samples) include H1-hTSPSA, H1-hTSPSB, HN10- hTSPSA, and HN10-hTSPSB cells. hESCs (n = 2, two samples) include H1-hESCs and HN10-hESCs. hTSblast (n = 1, one sample) serves as a positive control. (B) Heatmap on the selected trophoblast genes and hES-specific genes in indicated cells. (C) Left: Heatmap of up- or down-regulated genes in hTSPS cells compared with their parent hESCs. Right: GO analysis for up-regulated or down-regulated genes in hTSPS cells. hTSblast serves as a positive control. (D) Left: Heatmap and signal intensity of ATAC-seq data for differential accessible chromatin regions between hTSPS cells (n = 2, two biological replicates) at P20 and hESCs (n = 2, two biological replicates). CO, close-to-open, indicates regions with increased accessibility in hTSPS cells. OC, open-to-close, indicates regions with reduced accessibility in hTSPS cells. Right: GO analysis for the indicated regions. (E and F) Enriched motifs of hTSPS cell-open regions (CO) or hESC-open regions (OC) described in (D).
Human TSPS cells exhibit more open chromatin accessibility of essential genes for placenta development
The developmental stages of mammalian embryogenesis are orchestrated by both genetic and epigenetic events (19). However, the epigenetic signatures of hTSCs such as chromatin accessibility and H3K4me3/H3K27me3 enrichment are as yet unknown (20, 21). Furthermore, the dynamic changes of epigenetic patterns during derivation of hTSPS cells from primed hPSCs remain poorly understood. Therefore, we used our hTSPS cells as a model to explore these epigenetic patterns and their dynamic changes during the conversion process.
We investigated the transposase-accessible chromatin using sequencing (ATAC-seq) assay and identified a cluster of accessible chromatin regions that significantly differed between hTSPS cells at P20 and parent hESCs (Fig. 4D and fig. S8A). Intriguingly, more genes with “open” chromatin accessibility than those with “closed” chromatin accessibility were found in the hTSPS cell (Fig. 4D and fig. S8B). These “hTS-specific open” genes were up-regulated (fig. S8, B and C) and were related to placental development or the WNT signaling pathway (Fig. 4D), whereas the “hESC-specific open” genes related to stem cell maintenance were down-regulated (Fig. 4D and fig. S8, B and C). Consistently, more open chromatin regions were verified in widely recognized trophoblast development–related genes (ELF5, CDX2, GATA3, EGFR, TFAP2C, PGF, etc.) in hTSPS cells, while hESC-related genes (SOX2, NANOG, PRDM14, LEFTY1, etc.) only displayed more open chromatin in the hESCs (fig. S8D). These data may support the maintenance and development potential of hTSPS cells. We further examined enriched binding motifs of known transcription factors (TFs) in these accessible chromatin regions. The top 10 enriched TF motifs in hTSPS cell-open regions have been reported as essential regulators in placentation, such as TEAD4 (P < 0.001), GATA3 (P < 0.001), and GATA2 (P < 0.001) (Fig. 4E) (22). In contrast, enriched motifs in hES-open regions were related to pluripotency of ESCs, such as OCT4-SOX2-TCF-NANOG (P < 0.001), OCT4 (P < 0.001), and SOX2 (P < 0.001) (Fig. 4F). Together, these data demonstrate that chromatin accessibility of essential genes for placenta development and TS maintenance are in an open state in hTSCs derived from primed hPSCs.
Distinct H3K4me3 and H3K27me3 patterns between human TSPS cells and ESCs
Previously, we have described the patterns of active histone modification H3K4me3 and repressive histone modification H3K27me3 in hESCs, showing that the vast majority of H3K27me3 colocalized on genes modified with H3K4me3 (21). These commodified genes displayed low expression levels and were enriched for developmental function pathways (21). However, the patterns and dynamic changes of these histone methylations during the generation of hTSPS cells from hESCs are so far unknown. We therefore performed chromatin immunoprecipitation sequencing (ChIP-seq) for genome-wide mapping of H3K4me3 and H3K27me3 in hTSPS cells at P20 and their parent hESCs. Overall, the H3K4me3 intensities were similar in both cell types (Fig. 5A), but the H3K27me3 intensity was much lower in hTSPS cells than in hESCs (Fig. 5B). Furthermore, on the basis of the locations of H3K4me3 and H3K27me3, we categorized all genes into H3K4me3-only, H3K27me3-only, bivalent domains (colocalized with both H3K4me3 and H3K27me3), and nonmarked (fig. S9A). Notably, those genes with bivalent domains in hESCs were associated with embryonic development of multiple organs, as reported (fig. S9B) (21). Of significance, almost half of genes with bivalent domains in hESCs lost repressive H3K27me3 and only enriched active H3K4me3 in hTSPS cells (Fig. 5C). We further identified that the genes with bivalent domains in hESCs but H3K4me3-only in hTSPS cells were related to placenta development and WNT signaling pathways (Fig. 5, C to E). These genes exhibited more accessible chromatin and up-regulated expression levels in hTSPS cells compared with hESCs (Fig. 5F). For example, critical genes for placenta development and TS maintenance, including GATA3, GATA2, KRT7, and EGFR, and WNT signaling pathway genes showed loss of H3K27me3 upon conversion from hESCs to hTSPS cells (fig. S9C). In contrast, hESC-related genes, such as SOX2, NANOG, PRDM14, and TDGF1, lost active H3K4me3 and gained repressive H3K27me3 upon conversion from hESCs to hTSPS cells (fig. S9C). Consolidation of these data showed that the patterns of histone modifications H3K4me3 and H3K27me3 are distinctly different between hESCs and hTSPS cells and highlight the differential activity of loss of H3K27me3 activity in this hTSPS cell culture system.
Fig. 5. Dissection of histone modification H3K4me3/H3K27me3 pattern in specification of hTSPS cells.
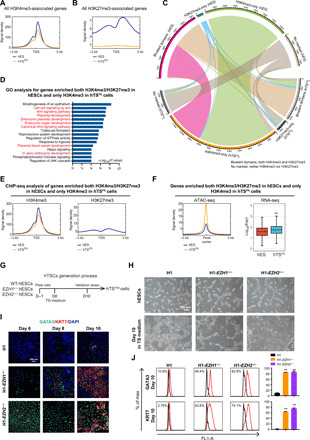
(A) Signal intensity of ChIP-seq data in H3K4me3-associated genes in hTSPS cells at P20 and hESCs. (B) Signal intensity of ChIP-seq data in H3K27me3-associated genes in hTSPS cells and hESCs. ChIP-seq experiments for H3K4me3 and H3K27me3 in hTSPS cells and hESCs are one replicate (n = 1), respectively. (C) Dynamic changes of H3K4me3- and H3K27me3-located genes in hTSPS cells and hESCs. (D) GO analysis for genes enriched with both H3K4me3 and H3K27me3 in hESCs but only H3K4me3 in hTSPS cells. (E) Signal density of H3K4me3 (left) and H3K27me3 (right) enrichment for genes described in (D) in hTSPS cells and hESCs. (F) ATAC-seq (left) and RNA-seq (right) analysis for genes described in (D) in hTSPS cells and hESCs. **P < 0.01. (G) Overview of derivation strategy for hTSPS cells from indicated primed ESCs (WT, EZH1−/−, and EZH2−/− hESCs) under the defined culture medium without BMP4 (Standard strategy). (H) Morphology of converted cells from WT, EZH1−/−, and EZH2−/− hESCs under Standard strategy at day 10. Scale bar, 200 μm. (I) Immunostaining on the TS marker genes GATA3/KRT7 in converted cells from WT, EZH1−/−, and EZH2−/− hESCs under Standard strategy at days 6, 8, and 10, respectively. The result is representative of three biological experiments. Scale bar, 100 μm. (J) FACS analysis on percentage of the KRT7+ and GATA3+ cells during the hTSPS derivation process under Standard strategy in WT, EZH1−/−, and EZH2−/− cells at day 10. **P < 0.01. Error bars represent means ± SD from three biological replicates (n = 3).
Loss of H3K27 methyltransferases enhances derivation of hTSPS cells
As we observed lower H3K27me3 enrichment in hTSPS cells, we hypothesized that loss of H3K27me3 methyltransferases EZH2 or EZH1 might promote the process for derivation of hTSPS cells from primed hPSCs. Thus, we used our previously established EZH2- and EZH1-knockout primed hESC lines (referred to as H1-EZH2−/− and H1-EZH1−/−, respectively) as donor cells for generation of TSPS cells under the Standard strategy (Fig. 5G) (23). On day 10, comparing with scattered hTSC-like colonies generated in the wild-type (WT) hESCs, most cells of both knockout cell lines had transited to an hTSC-like cell morphology (Fig. 5H). In addition, trophoblast-related genes/markers were significantly up-regulated in H1-EZH2−/− and H1-EZH1−/− cells compared with the WT H1 cells at days 8 and 10 during the hTSPS derivation process (Fig. 5, I and J, and fig. S9, D and E). These data suggest that H3K27me3 methyltransferases EZH2 and EZH1 are a critical obstacle for hTSPS derivation from primed hPSCs to hTSPS cells.
DISCUSSION
In this study, we report a successful derivation of hTSCs (named as hTSPS cells) from primed hPSCs, including primed hESC lines and hiPSCs from diverse tissue sources (urine epithelial cells and term placental amniotic membrane–derived mesenchymal cells). We further revealed that BMP4 could enhance this process with a significantly higher efficiency. hTSPS cells derived from primed hPSCs share identical features with primary blastocyst–derived hTSCs, including morphological characteristics, gene expression profiles, and capacity for differentiation toward ETV and STB cells (8). Moreover, the dynamic changes in epigenetic patterns during conversion of hESCs to hTSPS cells highlighted the greater chromatin accessibility and loss of histone modifications H3K27me3 for genes associated with placenta development.
The development of treatments for placenta-related diseases has progressed slowly mainly due to the lack of appropriate human cellular models to understand the underlying molecular impairment of trophoblast development at very early stages (9). The recent derivation of primary hTSCs provides a powerful tool to explore normal early placental development (8), while the induction of hTSCs from naïve PSCs and EPSCs promises greater opportunity to reveal pathological development within the trophoblast lineage (13–16). Unfortunately, primed PSCs, if not reset to a naïve state, are unable to be used directly as a source for generating hTSCs (14). Here, we report a successful derivation of hTSCs from primed hPSCs. During the process of conversion in our system, the primed hPSCs underwent complete transition in the first week; however, starting from days 7 to 8, other clones with TS morphology appear. Therefore, continuous culturing after complete differentiation of hPSCs might be a critical step to induce hTSCs from primed PSCs. We found an extremely low efficiency in obtaining hTSCs, especially in H1 with only 2.73%, which might lead to rare TSC clones being missed during clone picking. In addition, differences in initial cell density, cell culture matrix, and cell dissociation solutions used in our system compared to previous studies might contribute to our ability to derive hTSCs from primed PSCs.
Previously, BMP4 has been regarded as an indispensable factor to drive the differentiation of primed hPSCs into terminated trophoblast subtypes. However, in recent studies using naïve hPSCs to derive hTSCs, exogenous BMP4 is not required, which is consistent with our derivation of hTSCs from primed PSCs. Our findings further highlight the important role of BMP4 in enhancing the efficiency of this transition, where virtually all cells underwent transition at day 7 without the need for a clone-picking procedure. Furthermore, we found that endogenous BMP4, not exogenous BMP4, is required for the maintenance of hTSCs (Fig. 3 and fig. S6D). Recently, BMP4 has been reported to promote differentiation of epiblast-like cells into amnion rather than trophoblast cells (24). Thus, to exclude the existence of amnion derived from extra-embryonic mesoderm (Exe-Mes) and primordial germ cells (PGCs) in our system, we showed that the expression level of Exe-Mes genes VIM/C8ORF4/IGFBP3, etc., amniotic ectoderm marker BMP4/ISL1, and PGC marker genes NANOG/PDPN/BAMBI/SOX17/EOMES, etc., in our hTSPS cells is similar to that of primary hTSCs derived from blastocyst from Okae et al. (fig. S10, A to D) (24–28). However, Exe-Mes VIM/C8ORF4/IGFBP3, etc., in our hTSPS cells were lower than in AMCs derived from Exe-Mes, and the expression level of some PGC genes NANOG/PDPN/BAMBI in our hTSPS cells, primary hTSCs, and AMCs was significantly lower than that in hESCs (fig. S10, A and C). In addition, we also provided evidence that the efficiency of hTSC generation could be greatly improved by knocking out H3K27 methyltransferases EZH1/2. We have previously revealed that the promoter of BMP4 is enriched with H3K27me3 in hESCs and deletion of PRC2, including EZH1/2, induces up-regulation of BMP4 in hESCs (21, 23). We also found that the expression of EZH1 and EZH2 was down-regulated following BMP4 treatment during the transition process (fig. S9F). These data suggest that BMP4 may interact with these epigenetic factors through direct and indirect mechanisms to enhance derivation of hTSCs from primed hPSCs.
We believe that the genomic and epigenetic data associated with hTSPS cell specification presented here could provide the basis for more extensive future investigations. We not only revealed the chromatin accessibility dynamics and histone H3K4me3/H3K27me3 modification patterns of known placenta development genes but also identified a set of previously unknown TFs (Figs. 4 and 5 and fig. S10E), such as TFs related to hippo signaling, notch signaling, heart trabecula formation, and blood vessel endothelial cell migration (fig. S10E). These TFs might participate in the maintenance and specification of hTSPS cells. However, their precise roles in specifying hTSPS cells need to be further validated.
In conclusion, we have established an effective and simple system to obtain hTSCs from primed hESCs and hiPSCs. This protocol expands the source of hTSCs and holds promise of providing a useful tool to investigate mechanisms regulating placental development and understanding the pathogenesis of trophoblast-related disorders, such as miscarriage, preeclampsia, and fetal growth restriction.
MATERIALS AND METHODS
Cell culture
hESC lines H1 (Wi Cell) and HN10 (29) and their knockout cell lines H1-EZH1−/− (23) and H1-EZH2−/− (23) were maintained on Matrigel (Corning)–coated plates in mTeSR1 (STEMCELL Technologies). hiPSC lines UE005-iPS (30), hAMC-iPS (10), hAMC002-iPS (10), and hAMC004-iPS (10) were also cultured on the Matrigel (Corning)–coated plates in mTeSR1 (STEMCELL Technologies). hESCs and iPSCs were passaged every 3 days at a 1:3 ratio, and fresh medium was changed every day. Cells were maintained at 37°C and 5% CO2.
hTSC lines hTSblast and hTSCT (provided by Okae’s laboratory) and hTSC lines (hTSPS) derived from hPSCs were maintained on the Matrigel-coated plates in TS medium [Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco) supplemented with 0.3% bovine serum albumin (BSA) (Sigma-Aldrich), 1% Insulin, Transferrin, Selenium, Ethanolamine Solution (ITS-X) (Gibco), 0.1 mM β-mercaptoethanol (Invitrogen), 0.5% penicillin-streptomycin (HyClone), 0.2% fetal bovine serum (Gibco), l-ascorbic acid (1.5 μg/ml; Sigma-Aldrich), EGF (50 ng/ml; PeproTech), 2 μM CHIR99021 (Selleck), 0.5 μM A83-01 (Selleck), 1 μM SB431542 (Selleck), 0.8 mM VPA (MedChemExpress), and 5 μM Y27632 (Selleck)]. These TS and TSPS cells were passaged every 3 days at a 1:3 ratio, and the culture medium was changed every day. When passaged, these cells were dissociated with TrypLE Express (Gibco) for 10 to 15 min at 37°C. These cells were maintained at 37°C and 5% CO2. For cryopreservation, TS cells were suspended in Cell Banker 2 and stored in a deep freezer at −80°C and subsequently liquid nitrogen.
Derivation of hTSCs from primed hPSCs
After many attempts and modification of the hTSPS derivation system, we were successful in establishing the conversion system for generation of hTSCs (hTSPS cells) from hPSCs, including primed hESCs and hiPSCs (Fig. 1A). In detail, hPSCs (4 × 105 per well) were seeded on the Matrigel-coated six-well plates and cultured in mTeSR1 medium. The following day (day 0), the culture medium was replaced with TS medium (see the previous section) for hTSPS derivation. After three more days (day 3), the converted cells grew to about 90 to 100% confluence and were then passaged at a 1:3 ratio with 0.5 mM EDTA and cultured on the Matrigel-coated six-well plate in TS medium. After 2 days (day 5), the converted cells were passaged at a 1:3 ratio with 0.5 mM EDTA and cultured on Matrigel-coated plate in TS medium continuously for a week. After approximately 7 to 8 days (days 7 and 8) of conversion, hTS-like colonies appeared and enlarged. After about 10 to 12 days (days 10 to 12), these TS colonies were picked for purifying and expanding on the Matrigel-coated 24-well plate in TS medium and the mixed non-TS like cells were scraped off. Thereafter, the purified hTSC-like colonies were routinely passaged every 3 days at a 1:3 ratio. The purified hTSC-like colonies were named human TSPS cells in the later description.
For the BMP4 strategy, briefly, primed hPSCs (4 × 105 per well) were cultured in mTeSR1 medium on the Matrigel-coated six-well plate. The next day (day 0), TS medium plus BMP4 (10 ng/ml; R&D Systems) were used to induce hTSPS derivation. Three days later (day 3), the converted cells were passaged at a 1:3 ratio with 0.5 mM EDTA and cultured on the Matrigel-coated six-well plate in TS medium plus BMP4 (10 ng/ml). After a further 2 days (day 5), converted cells were passaged and cultured on Matrigel-coated plates in TS medium for 5 days. Then, at days 10 to 12, these hTSC-like cells under BMP4 strategy were not picked but were cultured and expanded in TS medium for further validation in TS medium. Thereafter, the hTSC cells were routinely passaged every 3 days at a 1:3 ratio.
Differentiation of human TSPS cells toward EVT and STB cells
Protocols of EVT and STB cell differentiation from hTSCs were performed similarly as previously described (8). For EVT differentiation, the human TSPS cells were dissociated with TrypLE Express for 10 to 15 min at 37°C, seeded at 4 × 105 cells per well on the Matrigel-coated six-well plate, and cultured in EVT medium [DMEM/F12 (Gibco) supplemented with 0.3% BSA (Sigma-Aldrich), 1% ITS-X (Gibco), 0.1 mM β-mercaptoethanol (Invitrogen), 0.5% penicillin-streptomycin (HyClone), 7.5 μM A83-01 (Selleck), 20 μM Y27632 (Selleck), 0.2 μM WNT-C59 (Selleck), 1 μM XAV939 (Selleck), 0.5 μM PD0325901 (Selleck), 0.5 μM thiazovivin (Selleck), 2% Matrigel (Corning), NRG1 (100 ng/ml; Cell Signaling Technology), and 4% KnockOut Serum Replacement (Gibco)] for 3 days. After 3 days, the EVT medium was replaced with the medium without WNT-C59, XAV939, PD0325901, thiazovivin, and NRG1, and Matrigel was added to a final concentration of 0.5% for the next 3 days. At days 6 to 8, these EVT cells were analyzed.
For STB differentiation, human TSPS cells were dissociated with TrypLE Express for 10 to 15 min at 37°C and then seeded at 1 × 105 cells per well on Matrigel-coated six-well plates and cultured in STB medium [DMEM/F12 (Gibco) supplemented with 0.3% BSA (Sigma-Aldrich), 1% ITS-X (Gibco), 0.1 mM β-mercaptoethanol (Invitrogen), 0.5% penicillin-streptomycin (HyClone), 2 μM forskolin (Selleck), 5 μM Y27632 (Selleck), 4% KnockOut Serum Replacement (Gibco), EGF (50 ng/ml; PeproTech), and cAMP (Sigma-Aldrich)] for 3 days. After 3 days, the fresh STB medium was changed for the next 3 days. At day 6, STB cells were analyzed.
Quantitative real-time PCR
Total RNA was extracted from cells with TRIzol (Invitrogen) and reverse-transcribed with oligo dT (Takara) and RT-ACE (Toyobo) to generate complementary DNA (cDNA). Then, quantitative real-time PCR (qRT-PCR) was performed with ChamQ SYBR qPCR Master Mix (Vazyme) and CFX96 qRT-PCR machine (Bio-Rad). Human GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used to normalize qRT-PCR of these samples.
For miRNA, total RNA of cells was reverse-transcribed using specific stem-loop primers (31). The qRT-PCR for miRNA detection was carried out with ChamQ SYBR qPCR Master Mix (Vazyme) and a CFX96 qRT-PCR machine (Bio-Rad). U6 small nucleolar RNA was used for normalization.
All reactions were analyzed with three repeats. All primer sequences were listed in table S1.
Flow cytometry analysis
Samples were dissociated to single cells with Accutase (Sigma-Aldrich) and then fixed using fixation buffer (BD Biosciences) at room temperature for 20 min. After washing in phosphate-buffered saline (PBS), fixed cells were permeabilized in perm/wash buffer (BD Biosciences) at 4°C for about 10 min. Cells were then incubated with corresponding primary antibodies and corresponding isotype controls at 37°C for 30 min. After washing, samples were incubated with secondary antibodies at 37°C for 30 min and washed twice, and the cells were resuspended in PBS and analyzed with Accuri C6 PLUS (BD Biosciences). Detailed information of these antibodies was listed in table S2.
Immunostaining assay
Cells were fixed in 4% paraformaldehyde at room temperature for 20 min. After washing with PBS, cells were permeabilized with 0.3% Triton X-100 (Sigma-Aldrich) and 10% goat serum in PBS and incubated with the primary antibodies at 4°C for 12 hours. Cells were then washed with PBS and incubated with secondary antibodies at room temperature for 1 hour. After washing with PBS, cells were stained with 4′,6-diamidino-2-phenylindole (Sigma-Aldrich) at room temperature for 5 min. Images of immunostained samples were taken with a laser scanning confocal microscope (LSM 800, Carl Zeiss). The detailed information of these antibodies was listed in table S2.
DNA methylation analysis for ELF5 promoter
Genomic DNA was extracted with TIANamp Genomic DNA Kit (Tiangen) and used to analyze methylation of the ELF5 promoter. Following this, bisulfite treatment was performed using EpiTect Bisulfite Kits (QIAGEN) following the manufacturer’s recommendations. The processed DNA was then used for PCR analysis of the ELF5 promoter. PCR products were cloned into PMD-18T (Takara), and eight randomly selected clones were sequenced by Sanger sequencing. PCR primers for ELF5 promoter are listed in table S1.
Apoptosis and EdU analysis
Apoptosis analysis was performed using an Annexin V-FITC/PI Cell Apoptosis Detection kit (Keygen) following the manufacturer’s recommendations. Human TSPS and ES cells were dissociated into single cells using TrypLE Express. After washing with PBS, 1 × 105 cells were incubated with binding buffer, annexin V–FITC (fluorescein isothiocyanate), and PI (propidium iodide) at room temperature for 5 to 15 min. Samples were subsequently analyzed on Accuri C6 PLUS.
The 5-Ethynyl-2′-deoxyuridine (EdU) assay was performed using the Click-iT EdU Pacific Blue Flow Cytometry Assay Kit (Invitrogen) following the manufacturer’s recommendations. A total of 1 × 106 human TSPS and ES cells were plated onto a six-well plate in proper medium with 10 μM EdU or without EdU as a negative control for 3 hours. Cells were then dissociated and fixed in fixation buffer at room temperature for 20 min. Cells were then permeabilized in perm/wash buffer at 4°C for 10 to 15 min, washed in PBS, and incubated in PBS with CuSO4, fluorescent dye picolylazide, and reaction buffer additive at room temperature for 1 hour. Samples were then analyzed on Accuri C6 PLUS.
Enzyme-linked immunosorbent assay for hCG
hTSPS cells (1 × 105 per well) were seeded to differentiate into STB cells as described above. The supernatants were collected at day 6. As controls, hTSPS cells (1 × 105 per well) were seeded and cultured in TS medium. After day 2, the supernatants were collected and stored at −80°C. The level of secreted human chorionic gonadotropin (hCG) was measured using an hCG ELISA kit (Calbiotech).
RNA sequencing, rank correlation, and heatmap analysis
hTSPS cells and hESCs were lysed with TRIzol (Invitrogen), and total RNA was extracted following the manufacturer’s recommendations. Sequencing libraries were generated with the TruSeq RNA Sample Preparation Kit (Illumina) following the manufacturer’s recommendations. The libraries for these samples were run on a NextSeq system with the NextSeq 500 Mid Output Kit (Illumina).
All RNA sequencing (RNA-seq) data were analyzed as described previously (23). In brief, reads were aligned to human genome (UCSC hg38) using HISAT2 (v2.0.4). Gene expression was counted using samtools (v1.3.1) and htseq-count (v0.6.0), filtered by a threshold of at least 20 averaged raw read counts among samples, and normalized by GC-count and Gene-length using EDASeq (v2.12.0). Differential expression was then performed using DESeq (v1.18.1), and genes with P < 0.05 and fold change > 2 were considered as significant difference. Correlation plot and heatmap analysis were performed using ggplot2 (v2.2.1) and pheatmap (v1.0.10), respectively. GO analysis was performed using clusterProfiler (v3.6.0).
ATAC sequencing
ATAC-seq experiments and data analysis of hTSPS and hESCs were performed as described previously (20, 32). Briefly, 5 × 104 cells from each sample were processed and used to generate DNA libraries with TruePrep DNA Library Prep Kit V2 for Illumina (Vazyme) following the manufacturer’s recommendations. The DNA libraries were run on NextSeq 500 (Illumina). Adaptors were cut from reads using cutadapt (v1.13), and reads were aligned to human genome (UCSC hg38) using bowtie2 (v2.2.5). Samtools (v1.3.1) and Picard Tools (v1.90) was used to remove duplicates. MACS2 callpeak and bdgcmp were used to pile up signals. Differential open peaks were called using MACS2 bdgdiff (33). Signal density heatmaps and profiles were plotted using deeptools (v2.4.2). Motifs were found using homer. Peaks were annotated with gene annotation using ChIPpeakAnno (v3.12.7). GO analysis was performed using clusterProfiler (v3.6.0).
ChIP sequencing
ChIP experiments and data analysis of hTSPS and hESCs were performed as previously described (20, 32). Briefly, 1 × 107 cells were cross-linked in 1% formaldehyde with rotation at room temperature for 10 min. Then, 0.125 M glycine was used to terminate cross-linked reactions with rotation at room temperature for 5 min. After washing in cold PBS, cells were sonicated to obtain 200– to 500–base pair fragments in 1% SDS lysis buffer with 1 mM phenylmethylsulfonyl fluoride and protease inhibitor cocktail. The sonicated samples were then diluted with ChIP dilution buffer and subsequently incubated with magnetic beads [Dynabeads protein A and G (1:1)] (Invitrogen) and 5 μg of H3K27me3 antibody (Millipore, 17-622) or H3K4me3 antibody (Abcam, ab8580) with rotation overnight at 4°C. Antibody-bound complexes were washed consecutively with rotation for 5 min with low-salt wash buffer [0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM tris-HCl (pH 8.0), and 150 mM NaCl], high-salt wash buffer [0.1% SDS, 1% Triton X-100, 2 mM EDTA, 20 mM tris-HCl (pH 8.0), and 500 mM NaCl], LiCl wash buffer [0.25 M LiCl, 1% IGEPAL-CA630, 1% deoxycholic acid (sodium salt), 1 mM EDTA, 10 mM tris-HCl (pH 8.0)], and TE buffer [10 mM tris-HCl (pH 8.0) and 1 mM EDTA]. Washed complexes were reverse–cross-linked and purified to obtain ChIPed DNA for ChIP-seq. About 10 ng of ChIPed DNA and corresponding input DNA were measured with Qubit Fluorometer (Invitrogen) and used to generate DNA libraries using the ChIP-seq Sample Prep Kit (Illumina). DNA libraries were subsequently run on NexSeq 500.
For ChIP analysis, the sequenced reads were base-called and demultiplexed using standard Illumina software. Reads were then aligned to the human genome (UCSC hg38) using Bowtie2 (34). Reads mapped to identical positions with the same orientation in the genome were collapsed into one read. The regions of H3K27me3 or H3K4me3 enrichment (peaks) were called using the SICER software package (35), with the input genomic DNA as a background control (parameters: W = 200; G = 600; false discovery rate = 0.01 cutoff applied). Binding profiles and heatmaps were generated using ngsplot (36) and deeptools (37). GO analysis was performed using clusterProfiler (v3.6.0).
Statistical analyses
In general, results were presented as means ± SD calculated using Microsoft Excel and GraphPad Prism with at least three independent repeats. Unpaired two-tailed Student’s t tests were used to determine the significance level between samples. P < 0.05 was considered statistically significant in the data. No samples were excluded from any analysis.
Acknowledgments
We thank H. Okae and T. Arima (Department of Informative Genetics, Tohoku University Graduate School of Medicine, Senai, Japan) for providing the primary human trophoblast stem cell lines and their experience to help our experiments. We thank all staff in the Department of Obstetrics and Gynecology, Nanfang Hospital of Southern Medical University (especially C. Chen, M. Zhong, and L. Huang), laboratory members in G.P.’s laboratory of GIBH, and S.J.L. laboratory in LTRI for help and support. Funding: This work was supported by the National Natural Science Foundation of China (81801457, 31801220, and 81471464); the Natural Science Foundation of Guangdong Province, China (2020A1515010139); CHIR grant (FDN-143262 to S.J.L.); China Postdoctoral Science Foundation Funded Project (2019 M652953); Science and Technology Program of Guangzhou (201904010462); the State Key Development Program for Basic Research of China (2012CB966502); and Presidential Foundation of Nanfang Hospital (2016C043). Author contributions: Y.W., Y.S., S.J.L., and G.P. initiated and designed the project. Y.W. and Y.S. wrote the manuscript. Y.W. and Y.S. performed most experiments and analyzed data results. L.M. performed immunostaining and ELISA for hCG. Y. Zhang performed ATAC-seq. T.W. and S.L. analyzed the RNA-seq, ChIP-seq, and ATAC-seq data. Y. Zhao performed ChIP-seq. L.X. performed RNA-seq. X.Z. and F.S. performed FACS and RT-qPCR. Y.M., Z.W., C.C., W.L., and Y.Y. gave suggestions about experiments. C.D. and K.L. helped edit the manuscript. A.N. supervised the design and structure of the manuscript and reviewed and helped revise this manuscript. S.J.L. supervised the experiments and design and revision of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: The RNA-seq, ATAC-seq, and ChIP-seq data have been deposited in the Gene Expression Omnibus database under the accession code GSE135696. All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. No custom computer code was used in this study.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/33/eabf4416/DC1
REFERENCES AND NOTES
- 1.Kaiser J., Reproductive biology. Gearing up for a closer look at the human placenta. Science 344, 1073 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Douglas G. C., VandeVoort C. A., Kumar P., Chang T.-C., Golos T. G., Trophoblast stem cells: Models for investigating trophectoderm differentiation and placental development. Endocr. Rev. 30, 228–240 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanaka S., Kunath T., Hadjantonakis A.-K., Nagy A., Rossant J., Promotion of trophoblast stem cell proliferation by FGF4. Science 282, 2072–2075 (1998). [DOI] [PubMed] [Google Scholar]
- 4.Vandevoort C. A., Thirkill T. L., Douglas G. C., Blastocyst-derived trophoblast stem cells from the rhesus monkey. Stem Cells Dev. 16, 779–788 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Matsumoto S., Porter C. J., Ogasawara N., Iwatani C., Tsuchiya H., Seita Y., Chang Y.-W., Okamoto I., Saitou M., Ema M., Perkins T. J., Stanford W. L., Tanaka S., Establishment of macaque trophoblast stem cell lines derived from cynomolgus monkey blastocysts. Sci. Rep. 10, 6827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmidt J. K., Keding L. T., Block L. N., Wiepz G. J., Koenig M. R., Meyer M. G., Dusek B. M., Kroner K. M., Bertogliat M. J., Kallio A. R., Mean K. D., Golos T. G., Placenta-derived macaque trophoblast stem cells: Differentiation to syncytiotrophoblasts and extravillous trophoblasts reveals phenotypic reprogramming. Sci. Rep. 10, 19159 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hemberger M., Hanna C. W., Dean W., Mechanisms of early placental development in mouse and humans. Nat. Rev. Genet. 21, 27–43 (2020). [DOI] [PubMed] [Google Scholar]
- 8.Okae H., Toh H., Sato T., Hiura H., Takahashi S., Shirane K., Kabayama Y., Suyama M., Sasaki H., Arima T., Derivation of human trophoblast stem cells. Cell Stem Cell 22, 50–63.e6 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Phipps E. A., Thadhani R., Benzing T., Karumanchi S. A., Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 15, 275–289 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wei Y., Zhou X., Huang W., Long P., Xiao L., Zhang T., Zhong M., Pan G., Ma Y., Yu Y., Generation of trophoblast-like cells from the amnion in vitro: A novel cellular model for trophoblast development. Placenta 51, 28–37 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Amita M., Adachi K., Alexenko A. P., Sinha S., Schust D. J., Schulz L. C., Roberts R. M., Ezashi T., Complete and unidirectional conversion of human embryonic stem cells to trophoblast by BMP4. Proc. Natl. Acad. Sci. U.S.A. 110, E1212–E1221 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Horii M., Li Y., Wakeland A. K., Pizzo D. P., Nelson K. K., Sabatini K., Laurent L. C., Liu Y., Parast M. M., Human pluripotent stem cells as a model of trophoblast differentiation in both normal development and disease. Proc. Natl. Acad. Sci. U.S.A. 113, E3882–E3891 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cinkornpumin J. K., Kwon S. Y., Guo Y., Hossain I., Sirois J., Russett C. S., Tseng H.-W., Okae H., Arima T., Duchaine T. F., Liu W., Pastor W. A., Naive human embryonic stem cells can give rise to cells with a trophoblast-like transcriptome and methylome. Stem Cell Rep. 15, 198–213 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dong C., Beltcheva M., Gontarz P., Zhang B., Popli P., Fischer L. A., Khan S. A., Park K.-m., Yoon E.-J., Xing X., Kommagani R., Wang T., Solnica-Krezel L., Theunissen T. W., Derivation of trophoblast stem cells from naïve human pluripotent stem cells. eLife 9, e52504 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao X., Nowak-Imialek M., Chen X., Chen D., Herrmann D., Ruan D., Chen A. C. H., Eckersley-Maslin M. A., Ahmad S., Lee Y. L., Kobayashi T., Ryan D., Zhong J., Zhu J., Wu J., Lan G., Petkov S., Yang J., Antunes L., Campos L. S., Fu B., Wang S., Yong Y., Wang X., Xue S.-G., Ge L., Liu Z., Huang Y., Nie T., Li P., Wu D., Pei D., Zhang Y., Lu L., Yang F., Kimber S. J., Reik W., Zou X., Shang Z., Lai L., Surani A., Tam P. P. L., Ahmed A., Yeung W. S. B., Teichmann S. A., Niemann H., Liu P., Establishment of porcine and human expanded potential stem cells. Nat. Cell Biol. 21, 687–699 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Z., Kurosawa O., Iwata H., Establishment of human trophoblast stem cells from human induced pluripotent stem cell-derived cystic cells under micromesh culture. Stem Cell Res. Ther. 10, 245 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee C. Q. E., Gardner L., Turco M., Zhao N., Murray M. J., Coleman N., Rossant J., Hemberger M., Moffett A., What is trophoblast? A combination of criteria define human first-trimester trophoblast. Stem Cell Rep. 6, 257–272 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu R.-H., Chen X., Li D. S., Li R., Addicks G. C., Glennon C., Zwaka T. P., Thomson J. A., BMP4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 20, 1261–1264 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Yu J., Vodyanik M. A., Smuga-Otto K., Antosiewicz-Bourget J., Frane J. L., Tian S., Nie J., Jonsdottir G. A., Ruotti V., Stewart R., Slukvin I. I., Thomson J. A., Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920 (2007). [DOI] [PubMed] [Google Scholar]
- 20.Shan Y., Zhang Y., Zhao Y., Wang T., Zhang J., Yao J., Ma N., Liang Z., Huang W., Huang K., Zhang T., Su Z., Chen Q., Zhu Y., Wu C., Zhou T., Sun W., Wei Y., Zhang C., Li C., Su S., Liao B., Zhong M., Zhong X., Nie J., Pei D., Pan G., JMJD3 and UTX determine fidelity and lineage specification of human neural progenitor cells. Nat. Commun. 11, 382 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pan G., Tian S., Nie J., Yang C., Ruotti V., Wei H., Jonsdottir G. A., Stewart R., Thomson J. A., Whole-genome analysis of histone H3 lysine 4 and lysine 27 methylation in human embryonic stem cells. Cell Stem Cell 1, 299–312 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Krendl C., Shaposhnikov D., Rishko V., Ori C., Ziegenhain C., Sass S., Simon L., Müller N. S., Straub T., Brooks K. E., Chavez S. L., Enard W., Theis F. J., Drukker M., GATA2/3-TFAP2A/C transcription factor network couples human pluripotent stem cell differentiation to trophectoderm with repression of pluripotency. Proc. Natl. Acad. Sci. U.S.A. 114, E9579–E9588 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shan Y., Liang Z., Xing Q., Zhang T., Wang B., Tian S., Huang W., Zhang Y., Yao J., Zhu Y., Huang K., Liu Y., Wang X., Chen Q., Zhang J., Shang B., Li S., Shi X., Liao B., Zhang C., Lai K., Zhong X., Shu X., Wang J., Yao H., Chen J., Pei D., Pan G., PRC2 specifies ectoderm lineages and maintains pluripotency in primed but not naïve ESCs. Nat. Commun. 8, 672 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng Y., Xue X., Shao Y., Wang S., Esfahani S. N., Li Z., Muncie J. M., Lakins J. N., Weaver V. M., Gumucio D. L., Fu J., Controlled modelling of human epiblast and amnion development using stem cells. Nature 573, 421–425 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gaengel K., Genové G., Armulik A., Betsholtz C., Endothelial-mural cell signaling in vascular development and angiogenesis. Arterioscler. Thromb. Vasc. Biol. 29, 630–638 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Saykali B., Mathiah N., Nahaboo W., Racu M.-L., Hammou L., Defrance M., Migeotte I., Distinct mesoderm migration phenotypes in extra-embryonic and embryonic regions of the early mouse embryo. eLife 8, e42434 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.S. Chhabra, A. Warmflash, BMP-treated human embryonic stem cells transcriptionally resemble amnion cells in the monkey embryo. bioRxiv 2021.01.21.427650 [Preprint]. 22 January 2021. https://doi.org/10.1101/2021.01.21.427650. [DOI] [PMC free article] [PubMed]
- 28.R. Yang, A. Goedel, Y. Kang, C. Si, C. Chu, Y. Zheng, Z. Chen, P. J. Gruber, Y. Xiao, C. Zhou, N. Witman, C.-Y. Leung, Y. Chen, J. Fu, W. Ji, F. Lanner, Y. Niu, K. Chien, Amnion signals are essential for mesoderm formation in primates. bioRxiv 2020.05.28.118703 [Preprint]. 5 January 2021. https://doi.org/10.1101/2020.05.28.118703.
- 29.Huang K., Zhu Y., Ma Y., Zhao B., Fan N., Li Y., Song H., Chu S., Ouyang Z., Zhang Q., Xing Q., Lai C., Li N., Zhang T., Gu J., Kang B., Shan Y., Lai K., Huang W., Mai Y., Wang Q., Li J., Lin A., Zhang Y., Zhong X., Liao B., Lai L., Chen J., Pei D., Pan G., BMI1 enables interspecies chimerism with human pluripotent stem cells. Nat. Commun. 9, 4649 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xue Y., Cai X., Wang L., Liao B., Zhang H., Shan Y., Chen Q., Zhou T., Li X., Hou J., Chen S., Luo R., Qin D., Pei D., Pan G., Generating a non-integrating human induced pluripotent stem cell bank from urine-derived cells. PLOS ONE 8, e70573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liao B., Bao X., Liu L., Feng S., Zovoilis A., Liu W., Xue Y., Cai J., Guo X., Qin B., Zhang R., Wu J., Lai L., Teng M., Niu L., Zhang B., Esteban M. A., Pei D., MicroRNA cluster 302-367 enhances somatic cell reprogramming by accelerating a mesenchymal-to-epithelial transition. J. Biol. Chem. 286, 17359–17364 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang T., Huang K., Zhu Y., Wang T., Shan Y., Long B., Li Y., Chen Q., Wang P., Zhao S., Li D., Wu C., Kang B., Gu J., Mai Y., Wang Q., Li J., Zhang Y., Liang Z., Guo L., Wu F., Su S., Wang J., Gao M., Zhong X., Liao B., Chen J., Zhang X., Shu X., Pei D., Nie J., Pan G., Vitamin C-dependent lysine demethylase 6 (KDM6)-mediated demethylation promotes a chromatin state that supports the endothelial-to-hematopoietic transition. J. Biol. Chem. 294, 13657–13670 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denny S. K., Yang D., Chuang C.-H., Brady J. J., Lim J. S., Grüner B. M., Chiou S.-H., Schep A. N., Baral J., Hamard C., Antoine M., Wislez M., Kong C. S., Connolly A. J., Park K.-S., Sage J., Greenleaf W. J., Winslow M. M., Nfib promotes metastasis through a widespread increase in chromatin accessibility. Cell 166, 328–342 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langmead B., Salzberg S. L., Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zang C., Schones D. E., Zeng C., Cui K., Zhao K., Peng W., A clustering approach for identification of enriched domains from histone modification ChIP-Seq data. Bioinformatics 25, 1952–1958 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen L., Shao N., Liu X., Nestler E., ngs.plot: Quick mining and visualization of next-generation sequencing data by integrating genomic databases. BMC Genomics 15, 284 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramírez F., Dündar F., Diehl S., Grüning B. A., Manke T., deepTools: A flexible platform for exploring deep-sequencing data. Nucleic Acids Res. 42, W187–W191 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/7/33/eabf4416/DC1


