Cell therapy has strong promise for treating COVID-19 survivors due to customizability, targetability, and regenerative function.
Abstract
Coronavirus disease 2019 (COVID-19) continues to burden society worldwide. Despite most patients having a mild course, severe presentations have limited treatment options. COVID-19 manifestations extend beyond the lungs and may affect the cardiovascular, nervous, and other organ systems. Current treatments are nonspecific and do not address potential long-term consequences such as pulmonary fibrosis, demyelination, and ischemic organ damage. Cell therapies offer great potential in treating severe COVID-19 presentations due to their customizability and regenerative function. This review summarizes COVID-19 pathogenesis, respective areas where cell therapies have potential, and the ongoing 89 cell therapy trials in COVID-19 as of 1 January 2021.
INTRODUCTION
Coronavirus disease 2019 (COVID-19) continues to strain patients, providers, and health care systems worldwide. Since its discovery, the disease has contributed to approximately 200 million infections and 4 million deaths worldwide. The scientific community has focused vast resources on understanding the virus causing COVID-19, named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), and the pathologies associated with the infection. Enormous effort has been placed to shed light on the mechanisms of viral entry and infection, the interaction between the virus and the host’s immune system, and the mechanisms of injury underlying the common manifestations of the disease.
SARS-CoV-2 initially emerged as a pathogen causing mainly viral pneumonias; however, experience in the proceeding months showed that the disease manifests throughout the body, leading to pathologies of the immune, renal, cardiac, and nervous systems, among others. While most patients have a mild course, over 15% develop severe and critical disease (1), leading to a substantial number of patients requiring prolonged hospitalization with intensive care services and potentially facing subsequent chronic manifestations related to pathological injuries from the disease process. In addition, mortality can be as high as 61.5% in critically ill patients with the disease (2).
As we begin to appreciate the subacute and chronic sequela of COVID-19, it is crucial to focus research efforts on finding therapies that not only dampen the acute damage but also can do so in a targeted manner while restoring physiological function and addressing the long-term sequela of the disease. Cell therapies have the potential to regenerate damaged tissue and tackle the immune system and, hence, are a treatment option with great promise. Here, we provide an overview of the COVID-19 pathogenesis in various organ systems, the overall advantages of cell therapies, potential cell targets and strategies within each organ system, and a summary of current cell therapy studies and trials for COVID-19 as of 1 January 2021.
VIRAL ENTRY AND TROPISM
SARS-CoV-2 first interacts with cells via binding of the viral spike protein to angiotensin-converting enzyme 2 (ACE2) on the cell surface (3, 4). After binding to ACE2, the spike protein is processed by the host transmembrane protease serine 2 (TMPRSS2), priming it for membrane fusion. This is considered to be the primary route of infection in vivo. Alternatively, the virus can be taken up into the cell via endocytosis and the spike protein processed by the endosomal proteases cathepsins B and L (3). After fusion with the cell membrane and release into the cytoplasm, the RNA replication machinery encoded in the first open reading frame of the viral genome is translated, followed by RNA replication and viral protein translation. SARS-CoV-2 co-opts and alters numerous cellular proteins and pathways, many of which are yet to be elucidated (5). It has been indicated that neuropilin 1 (NRP1) has a role in potentiating SARS-CoV-2 entry through the ACE2 pathway (6, 7). Studies from other coronaviruses provided evidence for CD147 and the 78-kDa glucose-regulated protein (GRP78) as putative alternative receptors, but more investigations on how the collective tissue distribution of these factors correlate with viral tropism and disease symptoms are under active investigation (8, 9).
Cellular tropism of SARS-CoV-2 is considered to be largely dictated by the distribution of ACE2. Bulk transcriptomic studies found ACE2 primarily expressed in the lungs, intestinal tract, kidneys, gallbladder, and heart; lower levels of expression were observed in the brain, thyroid, adipose tissue, epididymis, ductus deferens, breast, pancreas, rectum, ovary, esophagus, liver, seminal vesicle, salivary gland, placenta, vagina, lung, appendix, and skeletal muscle (10–12). In the respiratory tract, ACE2 is most highly expressed in nasal epithelial cells, where SARS-CoV-2 is thought to initially infect followed by propagation into the distal alveoli (13). Many organs that express higher levels of ACE2 are not major sites of viral replication, indicating that expression of other host factors, including TMPRSS2, NRP1, and host restriction factors likely contributes to viral tropism (12).
ADVANTAGES AND POTENTIAL FOR CELL THERAPIES
Although most patients infected with SARS-CoV-2 present with mild symptoms (14), a considerable part of the population, including elderly patients and those with underlying comorbidities, have an increased risk of more severe outcomes, including death (15). Current treatment options for severely ill patients, aimed at reducing inflammation during the acute phase of the infection, have their limitations. Medications may be nonspecific for SARS-CoV-2 targets or are repurposed without a clear mechanism of benefit, while others such as remdesivir and tocilizumab may not be readily accessible because of federal allocations or cost barriers (16). In addition, these treatments have not focused on long-term sequela of the disease such as regeneration of damaged tissue structure and function. Cell therapies may thus be a promising class of therapies that could overcome these challenges through their customizability, targetability, scalable manufacturing, and restoration of function.
Cellular therapies have shown success in treating conditions that have otherwise been challenging to manage with mainstream treatment modalities, including, but not limited to, oncologic, neurodegenerative, and immunologic disorders. Cell therapy approaches including, but not limited to, mesenchymal stromal cells (MSCs), induced pluripotent stem cells (iPSCs), and T cells have been widely studied, and their efficacy has led to several U.S. Food and Drug Administration (FDA) approvals of cell therapies including, most famously, axicabtagene ciloleucel (Yescarta) and tisagenlecleucel (Kymriah) (17–20). Extensive safety and efficacy data from cell therapies trials in various indications suggest that cell therapies could play a role in treating patients with COVID-19 as well.
Two potential concerns with cell therapies are immune rejection and tumorigenicity. Immune rejection concerns for allogeneic cell therapy have been discussed in the literature, especially as new cell therapies emerge. MSCs, for example, are considered to be immune suppressive and immune evasive, yet, the standard of treatment using allogeneic MSCs is the addition of immunosuppressive regimens alongside the cell therapy (21, 22). While immunosuppressive therapy may be used to protect the graft, it may not always prevent graft rejection and can come with its own adverse effects. Genome engineering can help address the immune system by tackling both the innate and adaptive immune systems. Potential strategies include knocking out genes responsible for immune system activation, such as major histocompatibility complex I and II (23, 24). These modifications could address both the acute and chronic rejection phases, making the cell grafts more resistant to the host immune system.
Tumorigenicity is an important consideration with cell therapies. The risk of tumorigenicity seems to be greater with MSCs, iPSCs, and human embryonic stem cells (hESCs), and it can present in the form of teratoma or as a true tumor (25–27). This risk can be reduced by increasing the efficiency of differentiation to the target cell type thereby reducing residual pluripotent cells, such as by transcription factor–mediated cell programming or by incorporating suicide genes into cell grafts that can be activated in the rare chance a graft becomes malignant (28–30). Several suicide mechanisms have been described in the literature, including a recent study by Itakura et al. (31) in which iCaspase9 was inserted as a fail-safe system in iPSC cell lines. If these cell lines become cancerous once transplanted in mice, induction of the iCaspase9 with a small molecule showed the formed tumors to rapidly reduce in size (31). These approaches increase the safety profile of cell therapies for clinical applications in patients with COVID-19 and beyond.
COVID-19 PATHOGENESIS AND CELL THERAPY POTENTIAL BY ORGAN SYSTEM
A clear understanding of COVID-19 pathogenesis is necessary to appreciate the potential benefit of cell therapies. Cell therapies provide paramount benefit as potential targeted treatment strategies to address localized damage inflicted by the disease and restore physiological functions (Fig. 1). In 2020, March and April recorded a large initial surge in global COVID-19 cases and deaths, as presented by the World Health Organization. There was a concurrent increase in the numbers of cell therapy–based clinical trials initiated during those 2 months (Fig. 2A). As of 1 January 2021, there are 89 cell therapy–based clinical trials registered on clinicaltrials.gov (Table 1) targeting COVID-19 pathology. Most of the clinical trials are held in the United States and China, 36% and 16%, respectively, with the rest of the clinical trials spread across the globe (Fig. 1B). MSCs constitute the majority cell type used in the cell therapy clinical trials, around 71%, with the rest using cell types such as natural killer (NK) cells, T cells, early apoptotic cells, and others (Fig. 1C). About 88% of the clinical trials are in phases 1 and 2, with one trial in phase 2/3 and one in phase 3 (Fig. 2D). The enrollment in each clinical trial was most frequently 21 to 30 patients but ranged up to 400 depending on the phase of the trial (Fig. 2E). In addition, the variability of patient enrollment numbers could be due to the varying statuses of each clinical trial (Fig. 2F). It is also worth noting that over half of the cell therapy–based clinical trials are sponsored and supported by the industry sector (Fig. 2G), which indicates the pivotal role for industry in accelerating the necessary research to combat COVID-19.
Fig. 1. Summary of COVID-19 pathogenesis and respective potential for cell therapies by organ system.
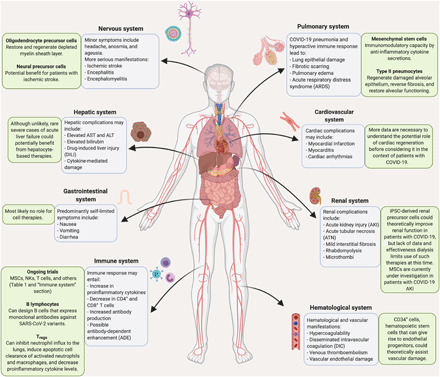
Blue text boxes describe specific pathogenesis for each organ system. Green text boxes describe potential and ongoing cell therapy applications for each organ system. ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Fig. 2. Descriptive summary of the 89 clinical trials using cell therapies for COVID-19.
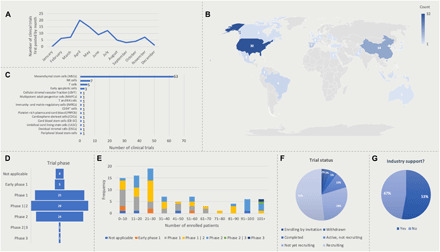
(A) Number of COVID-19 targeting cell therapy clinical trials started in each month of the year 2020. (B) World map showing global distribution of the registered cell therapy clinical trials and their numbers per country. (C) Different cell types used in the cell therapy–based clinical trials and their respective count. (D) Stages of the 89 cell therapy clinical trials registered as of 1 January 2021. (E) Distribution of patient enrollment numbers across the 89 clinical trials. (F) Breakdown of the 89 cell therapy clinical trial statuses. (G) The percentages of cell therapies sponsored and supported by the industry sector.
Table 1. Cell therapy–based clinical trials for COVID-19.
Search approach: performed 1 January 2021; Clinicaltrials.gov: advanced search; Condition - –OVID; Study Type -Interventi–nal; Intervention/treatment - Cell; of 157 studies, exclude– non–COVID-19 patients (n = 12) and non–cell therapy trials (n = 56); leaving 89 available studies. NCT, national clinical trial.
| Cell type | Title | Status | Phase | Country | NCT number |
|
Mesenchymal
stem cells |
|||||
| Clinical trial of allogeneic mesenchymal cells from umbilical cord tissue in patients with COVID-19 |
Recruiting | Phase 2 | Spain | NCT04366271 | |
| Autologous adipose- derived stem cells (AdMSCs) for COVID-19 |
Not yet recruiting | Phase 2 | United States | NCT04428801 | |
| Treatment of severe COVID-19 pneumonia with allogeneic mesenchymal stromal cells (COVID_MSV) |
Recruiting | Phase 2 | Spain | NCT04361942 | |
| Mesenchymal stem cell infusion for COVID-19 infection |
Recruiting | Phase 2 | Pakistan | NCT04444271 | |
| Mesenchymal stem cell for acute respiratory distress syndrome due for COVID-19 |
Recruiting | Phase 2 | Mexico | NCT04416139 | |
| Safety and feasibility of allogenic MSC in the treatment of COVID-19 |
Not yet recruiting | Phase 1 | Brazil | NCT04467047 | |
| BAttLe against COVID-19 using mesenchYmal stromal cells |
Not yet recruiting | Phase 2 | Spain | NCT04348461 | |
| Mesenchymal stromal cells for the treatment of SARS-CoV-2 induced acute respiratory failure (COVID-19 disease) |
Not yet recruiting | Early phase 1 | United States | NCT04345601 | |
| Cellular immuno- therapy for COVID-19 acute respiratory distress syndrome - Vanguard |
Recruiting | Phase 1 | Canada | NCT04400032 | |
| Cord blood-derived mesenchymal stem cells for the treatment of COVID-19 related acute respiratory distress syndrome |
Recruiting | Phase 1 | United States | NCT04565665 | |
| Safety and efficacy study of allogeneic human dental pulp mesenchymal stem cells to treat severe COVID-19 patients |
Recruiting | Phase 1|phase 2 | China | NCT04336254 | |
| Study of intravenous administration of allogeneic adipose stem cells for COVID-19 |
Not yet recruiting | Phase 1 | United States | NCT04486001 | |
| Safety and efficacy of mesenchymal stem cells in the management of severe COVID-19 pneumonia |
Not yet recruiting | Phase 2 | Colombia | NCT04429763 | |
| NestaCell mesenchymal stem cell to treat patients with severe COVID-19 pneumonia |
Not yet recruiting | Phase 2 | Brazil | NCT04315987 | |
| Use of mesenchymal stem cells in acute respiratory distress syndrome caused by COVID-19 |
Active, not recruiting | Early phase 1 | Mexico | NCT04456361 | |
| Efficacy of infusions of MSC from Wharton jelly in the SARS-Cov-2 (COVID-19) related acute respiratory distress syndrome |
Not yet recruiting | Phase 2 | France | NCT04625738 | |
| hCT-MSCs for COVID19 ARDS |
Recruiting | Phase 1|phase 2 | United States | NCT04399889 | |
| Clinical trial to assess the efficacy of MSC in patients with ARDS due to COVID-19 |
Recruiting | Phase 2 | Spain | NCT04615429 | |
| Treatment of Covid-19 associated pneumonia with allogenic pooled olfactory mucosa- derived mesenchymal stem cells |
Enrolling by invitation | Phase 1|phase 2 | Belarus | NCT04382547 | |
| Clinical trial to assess the safety and efficacy of intravenous administration of allogeneic adult mesenchymal stem cells of expanded adipose tissue in patients with severe pneumonia due to COVID-19 |
Active, not recruiting | Phase 1|phase 2 | Spain | NCT04366323 | |
| Regenerative medicine for COVID-19 and flu-elicited ARDS using longeveron mesenchymal stem cells (LMSCs) (RECOVER) |
Recruiting | Phase 1 | United States | NCT04629105 | |
| An exploratory study of ADR-001 in patients with severe pneumonia caused by SARS-CoV-2 infection |
Not yet recruiting | Phase 1 | Japan | NCT04522986 | |
| Novel coronavirus induced severe pneumonia treated by dental pulp mesenchymal stem cells |
Not yet recruiting | Early phase 1 | China | NCT04302519 | |
| Multiple dosing of mesenchymal stromal cells in patients with ARDS (COVID-19) |
Recruiting | Phase 2 | United States | NCT04466098 | |
| MSC-based therapy in COVID-19-associated acute respiratory distress syndrome |
Recruiting | Phase 1 | Brazil | NCT04525378 | |
| Adipose mesenchymal cells for abatement of SARS-CoV-2 respiratory compromise in COVID-19 disease |
Not yet recruiting | Phase 1 | United States | NCT04352803 | |
| Safety and efficacy of intravenous Wharton’s jelly derived mesenchymal stem cells in acute respiratory distress syndrome due to COVID 19 |
Not yet recruiting | Phase 1|phase 2 | Colombia | NCT04390152 | |
| Efficacy of intravenous infusions of stem cells in the treatment of COVID-19 patients |
Recruiting | Phase 2 | Pakistan | NCT04437823 | |
| Treatment with human umbilical cord- derived mesenchymal stem cells for severe corona virus disease 2019 (COVID-19) |
Completed | Phase 2 | China | NCT04288102 | |
| Therapeutic study to evaluate the safety and efficacy of DW-MSC in COVID-19 patients |
Active, not recruiting | Phase 1 | Indonesia | NCT04535856 | |
| Administration of allogenic UC-MSCs as adjuvant therapy for critically-ill COVID-19 patients |
Recruiting | Phase 1 | Indonesia | NCT04457609 | |
| Clinical research of human mesenchymal stem cells in the treatment of COVID-19 pneumonia |
Recruiting | Phase 1|phase 2 | China | NCT04339660 | |
| Treatment of COVID-19 patients using Wharton’s jelly-mesenchymal stem cells |
Recruiting | Phase 1 | Jordan | NCT04313322 | |
| Study of human umbilical cord mesenchymal stem cells in the treatment of severe COVID-19 |
Not yet recruiting | Not applicable | China | NCT04273646 | |
| Mesenchymal stromal cell therapy for severe Covid-19 infection |
Recruiting | Phase 1|phase 2 | Belgium | NCT04445454 | |
| Bone marrow-derived mesenchymal stem cell treatment for severe patients with coronavirus disease 2019 (COVID-19) |
Not yet recruiting | Phase 1|phase 2 | China | NCT04346368 | |
| A study of cell therapy in COVID-19 subjects with acute kidney injury who are receiving renal replacement therapy |
Recruiting | Phase 1|phase 2 | United States | NCT04445220 | |
| Mesenchymal stem cells in patients diagnosed with COVID-19 |
Recruiting | Phase 1 | Mexico | NCT04611256 | |
| Use of UC-MSCs for COVID-19 patients |
Completed | Phase 1|phase 2 | United States | NCT04355728 | |
| Mesenchymal stem cell treatment for pneumonia patients infected with COVID-19 |
Recruiting | Phase 1 | China | NCT04252118 | |
| Study to evaluate the efficacy and safety of AstroStem-V in treatment of COVID-19 pneumonia |
Not yet recruiting | Phase 1|phase 2 | South Korea | NCT04527224 | |
| A randomized, double-blind, placebo-controlled clinical trial to determine the safety and efficacy of Hope Biosciences allogeneic mesenchymal stem cell therapy (HB-adMSCs) to provide protection against COVID-19 |
Enrolling by invitation | Phase 2 | United States | NCT04348435 | |
| A clinical trial to determine the safety and efficacy of Hope Biosciences autologous mesenchymal stem cell therapy (HB-adMSCs) to provide protection against COVID-19 |
Active, not recruiting | Phase 2 | United States | NCT04349631 | |
| ASC therapy for patients with severe respiratory COVID-19 |
Withdrawn | Phase 1|phase 2 | Denmark | NCT04341610 | |
| Safety and effectiveness of mesenchymal stem cells in the treatment of pneumonia of coronavirus disease 2019 |
Active, not recruiting | Early phase 1 | China | NCT04371601 | |
| Cell therapy using umbilical cord- derived mesenchymal stromal cells in SARS-CoV-2-related ARDS |
Active, not recruiting | Phase 1|phase 2 | France | NCT04333368 | |
| Clinical use of stem cells for the treatment of Covid-19 |
Recruiting | Phase 1|phase 2 | Turkey | NCT04392778 | |
| Mesenchymal stem cells for the treatment of COVID-19 |
Completed | Phase 1 | United States | NCT04573270 | |
| Treatment of coronavirus COVID-19 pneumonia (pathogen SARS-CoV-2) with cryopreserved allogeneic P_MMSCs and UC-MMSCs |
Recruiting | Phase 1|phase 2 | Ukraine | NCT04461925 | |
| Efficacy and safety Study of allogeneic HB-adMSCs for the treatment of COVID-19 |
Active, not recruiting | Phase 2 | United States | NCT04362189 | |
| Use of hUC-MSC product (BX-U001) for the treatment of COVID-19 With ARDS |
Not yet recruiting | Phase 1|phase 2 | United States | NCT04452097 | |
| Mesenchymal stem cells (MSCs) in inflammation- resolution programs of coronavirus disease 2019 (COVID-19) induced acute respiratory distress syndrome (ARDS) |
Not yet recruiting | Phase 2 | Germany | NCT04377334 | |
| Umbilical cord tissue (UC) derived mesenchymal stem cells (MSCs) versus placebo to treat acute pulmonary inflammation due to COVID-19 |
Not yet recruiting | Phase 1 | United States | NCT04490486 | |
| Repair of acute respiratory distress syndrome by stromal cell administration (REALIST) (COVID-19) |
Recruiting | Phase 1|phase 2 | United Kingdom | NCT03042143 | |
| The MEseNchymal coviD-19 trial: A pilot study to investigate early efficacy of MSCs in adults with COVID-19 |
Recruiting | Phase 1|phase 2 | Australia | NCT04537351 | |
| Efficacy and safety evaluation of mesenchymal stem cells for the treatment of patients with respiratory distress due to COVID-19 |
Recruiting | Phase 1|phase 2 | Spain | NCT04390139 | |
| Study of the safety of therapeutic Tx with immunomodulatory MSC in adults with COVID-19 infection requiring mechanical ventilation |
Recruiting | Phase 1 | United States | NCT04397796 | |
| Open-label multicenter study to evaluate the efficacy of PLX-PAD for the treatment of COVID-19 |
Recruiting | Phase 2 | Germany | NCT04614025 | |
| MSCs in COVID-19 ARDS |
Active, not recruiting | Phase 3 | United States | NCT04371393 | |
| Therapy for pneumonia patients iInfected by 2019 novel coronavirus |
Withdrawn | Not applicable | China | NCT04293692 | |
| Umbilical cord(UC)- derived mesenchymal stem cells (MSCs) treatment for the 2019-novel coronavirus (nCOV) pneumonia |
Recruiting | Phase 2 | China | NCT04269525 | |
| ACT-20 in patients with severe COVID-19 pneumonia |
Not yet recruiting | Phase 1|phase 2 | United States | NCT04398303 | |
| Double-blind, multicenter, study to evaluate the efficacy of PLX PAD for the treatment of COVID-19 |
Recruiting | Phase 2 | United States | NCT04389450 | |
| Natural killer (NK) cells | |||||
| Fase I clinical trial on NK cells for COVID-19 |
Not yet recruiting | Phase 1 | Brazil | NCT04634370 | |
| A phase I/II study of universal off-the- shelf NKG2D-ACE2 CAR-NK Cells for therapy of COVID-19 |
Recruiting | Phase 1|phase 2 | China | NCT04324996 | |
| Phase I / II clinical study of immunotherapy based on adoptive cell transfer as a therapeutic alternative for patients with COVID-19 in Colombia |
Not yet recruiting | Phase 1|phase 2 | Colombia | NCT04344548 | |
| NK cells treatment for COVID-19 |
Recruiting | Phase 1 | China | NCT04280224 | |
| Natural killer cell (CYNK-001) infusions in adults with COVID-19 |
Recruiting | Phase 1|phase 2 | United States | NCT04365101 | |
| Study of FT516 for the treatment of COVID-19 in hospitalized patients with hypoxia |
Recruiting | Phase 1 | United States | NCT04363346 | |
| An experiment to evaluate the safety of agenT-797 in COVID-19 patients with severe difficulty breathing |
Recruiting | Phase 1 | United States | NCT04582201 | |
| T cells | |||||
| Part two of novel adoptive cellular therapy with SARS-CoV-2 specific T cells in patients with severe COVID-19 |
Recruiting | Phase 1|phase 2 | Singapore | NCT04457726 | |
| RAPA-501-Allo off-the-shelf therapy of COVID-19 |
Recruiting | Phase 1|phase 2 | United States | NCT04482699 | |
| REgulatory T cell infuSion fOr lung injury due to COVID-19 PnEumonia |
Recruiting | Phase 1 | United States | NCT04468971 | |
| Anti-SARS Cov-2 T cell infusions for COVID 19 |
Recruiting | Phase 1 | United States | NCT04401410 | |
| Viral specific T-cells for treatment of COVID-19 |
Not yet recruiting | Phase 2 | United States | NCT04406064 | |
| Early apoptotic cells | |||||
| Study evaluating safety, tolerability and efficacy of Allocetra-OTS in patients with COVID-19 |
Not yet recruiting | Phase 1 | Israel | NCT04659304 | |
| Allocetra-OTS in COVID-19 |
Active, not recruiting | Phase 1 | Israel | NCT04513470 | |
| Allocetra-OTS in COVID-19, phase II |
Recruiting | Phase 2 | Israel | NCT04590053 | |
| Other | |||||
| Cardiosphere-derived cells |
Intravenous infusion of CAP-1002 in patients with COVID-19 |
Recruiting | Phase 2 | United States | NCT04623671 |
| CD34+ cells | CLBS119 for repair of COVID-19 induced pulmonary damage |
Withdrawn | Phase 1 | United States | NCT04522817 |
| Cellular stromal vascular fraction (cSVF) |
Use of cSVF Via IV deployment for residual lung damage after symptomatic COVID-19 infection |
Recruiting | Early phase 1 | United States | NCT04326036 |
| Cord blood stem cells | Stem cell educator therapy treat the viral inflammation in COVID-19 |
Not yet recruiting | Phase 2 | China | NCT04299152 |
| Decidual stromal cells | Study of decidual stromal cells to treat COVID-19 respiratory failure |
Not yet recruiting | Not applicable | Canada | NCT04451291 |
| Immunity- and matrix-regulatory cells |
Safety and efficacy of CAStem for severe COVID-19 associated with/without ARDS |
Recruiting | Phase 1|phase 2 | China | NCT04331613 |
| Multipotent adult progenitor cells |
MultiStem administration for COVID-19 induced ARDS (MACoVIA) |
Recruiting | Phase 2|phase 3 | United States | NCT04367077 |
| Peripheral blood stem cells |
Study evaluating the safety and efficacy of autologous non-hematopoietic peripheral blood stem cells in COVID-19 |
Completed | Phase 1|phase 2 | United Arab Emirates | NCT04473170 |
| Platelet-rich plasma and cord blood |
Using PRP and cord blood in treatment of Covid -19 |
Recruiting | Not applicable | Egypt | NCT04393415 |
| T and NK cells | Safety infusion of NatuRal KillEr celLs or MEmory T cells as adoptive therapy in COVID-19 pnEumonia or lymphopenia |
Recruiting | Phase 1|phase 2 | Spain | NCT04578210 |
| Umbilical cord lining stem cells (ULSCs) |
Umbilical cord lining stem cells (ULSC) in patients with COVID-19 ARDS |
Recruiting | Phase 1|phase 2 | United States | NCT04494386 |
PULMONARY SYSTEM
Pulmonary symptoms are the mainstay of COVID-19 and may include dry cough, dyspnea, pneumonia, and acute respiratory distress syndrome (ARDS) (32). Bilateral pulmonary infiltrates and ground-glass opacities are seen radiographically in over 70% of hospitalized patients (14). Furthermore, ARDS has shown to be present in over 90% of deceased patients (33). ARDS and the associated alveolar damage are thought to be primarily due to immune-related response (3, 34). Other pulmonary complications may include secondary pulmonary hypertension, hypercoagulability-related pulmonary emboli, and long-lasting fibrosis in patients who do recover from the acute infection (35, 36).
Some preclinical data suggest that patients with COVID-19 may benefit from cell therapies, particularly using MSCs in models of viral and inflammatory lung damage (37). For instance, MSCs were found to reduce the impairment of alveolar fluid clearance caused by influenza A H5N1 infection in vitro and mitigate lung injury in vivo (38). Another study showed that MSC treatment reduces influenza H9N2–induced acute lung injury in mice and reduces pulmonary inflammation (39). In another study, MSCs were shown to promote macrophages to become anti-inflammatory and take on a phagocytic phenotype through extracellular vesicles, thereby ameliorating lung injury in mice (40).
Several studies have described promising treatment of pneumonia and ARDS in critically ill patients with COVID-19 using cell therapies. In China, Liang et al. (41) reported treatment of one patient with severe COVID-19 unresponsive to steroid medications, after three successive injections of 5 × 107 human umbilical cord MSCs at days 1, 4, and 7 of treatment initiation. The patient’s pulmonary lesions had begun to resolve by day 7 after the first MSC injection. Tang et al. (42) reported treatment with allogeneic menstrual blood–derived MSCs of two patients with COVID-19 with ARDS. Treatment involved three successive injections of 1 × 106 MSCs/kg of body weight at days 1, 2, and 4 of treatment initiation. Both patients were discharged from the hospital. Leng et al. (43) reported a pilot study where they transplanted a single dose of 1 × 106 MSCs/kg of body weight in seven patients with mild, severe, and critical COVID-19, with three patients on the placebo arm. Results from the study showed overall safety of the treatment, with two severe patients recovering and being discharged within 10 days of treatment. In Spain, Sanchez-Guijo et al. (44) treated 10 patients under mechanical invasive intubation with either one, two, or three doses of 1 × 106 adipose-derived MSCs/kg of body weight. Seven of the 13 patients were extubated approximately 7 days after initiation of treatment. Furthermore, the authors observed that patients who received cell therapy earlier in their disease course had better outcomes. These open label–uncontrolled administrations are important as they demonstrate apparent safety with no obvious adverse events.
Various MSC-based strategies are assessing treatment of patients with COVID-19 with pulmonary symptoms, especially pneumonia and ARDS. One phase 1/2a randomized double-blind trial (NCT04355728) assessed administration of two infusions of 1 × 107 umbilical cord–derived MSCs for COVID-19 ARDS, showing improved 28-day survival following therapy (91% in treatment group, n = 12 versus 42% in control, n = 12) (45). Another phase 3 study comparing administration of two injections of 2 × 106 MSCs/kg of body weight and standard of care compared to placebo injection and standard of care in patients with COVID-19 with moderate to severe ARDS failed to meet the primary end point of 43% reduction in mortality in an interim analysis (NCT04371393). Thus, further investigation is necessary to determine whether MSC-based therapy could improve COVID-related lung injury.
COVID-19–related lung fibrosis has been characterized by fibroblast proliferation, airspace obliteration, and microhoneycombing, which is thought to persist in patients who survive the acute infection (46). This pattern of fibrotic change may be similar to that of idiopathic pulmonary fibrosis (IPF) (36), and prior cell therapy studies in IPF may shed light on potential avenues for cell therapy applications in patients with COVID-19. IPF is a progressive disease of unknown etiology that leads to fibrosis of the lungs and is the primary cause of more than half of all lung transplants worldwide (47). Cell therapies using type II pneumocytes (PTIIs), which are progenitors of the lung alveolar epithelium, have shown efficacy in preclinical animal models of IPF by regenerating lung epithelium, releasing surfactant, and reversing pulmonary fibrosis (48, 49). A phase 1 clinical study also showed that targeted intratracheal delivery of PTIIs showed safety and clinical stability at 12-month follow-up of 16 patients with moderate to severe IPF (50). In addition to PTIIs, MSCs have also been used in IPF. A recent randomized trial of patients with IPF treated with two doses of 2 × 108 allogenic bone marrow MSCs every 3 months for 1 year showed safety and improved respiratory function when compared to control participants (51). This suggests that even patients with COVID-19 with residual chronic fibrosis may benefit from cell-based therapies in the future, although further data are necessary to support this conclusion. Ultimately, cell therapies that can reverse fibrotic changes or supplement normal pneumocyte function could address potential chronic pulmonary effects from COVID-19.
IMMUNE SYSTEM
The host’s immune response toward SARS-CoV-2 has been studied carefully since the outbreak, with many potential mechanisms of interaction being elucidated on the basis of similarities of the virus to SARS-CoV. Most patients with COVID-19 mount antibody responses to SARS-CoV-2, which vary in magnitude and potency (52). Neutralizing antibodies appear to target the receptor binding domain of the spike proteins (52, 53). Patients with high immunoglobulin M (IgM) and immunoglobulin G (IgG) titers have a worse prognosis (54), which could be correlated with high viral load but could also indicate a harmful robust immune response through antibody-dependent enhancement (ADE). ADE is a phenomenon that has been observed in several viruses, including SARS-CoV, where viral-specific antibodies promote viral entry into immune cells expressing Fc receptors (55), such as monocytes, macrophages, and B cells, leading to enhanced amplification of the virus. Implications of ADE in COVID-19 have been discussed in greater detail by Eroshenko et al. (56). With regard to T cells, several studies have compared leukocyte profiles between patients with mild and severe manifestations of the disease and showed decreased T cell count in both CD4+ and CD8+ populations, more commonly in intensive care unit (ICU) patients but highly prevalent in non-ICU patients as well (57). Lower levels of CD4+ T helper cells and CD8+ cytotoxic T cells likely hinder the ability of the immune system to neutralize and kill viral-infected cells.
In addition, a marked increase of proinflammatory cytokines such as interleukin-1 (IL-1), IL-6, tumor necrosis factor–α (TNF-α), and interferon-γ (IFN-γ) has been observed in patients with severe COVID-19 (57, 58). In these cases, SARS-CoV-2 immune evasion leads to a robust viral replication and a delayed and dysregulated IFN-γ response, resulting in recruitment and accumulation of inflammatory macrophages and neutrophils (58, 59). Further IFN-γ activation by these cells leads to additional cytokine and chemokine signals [IFN-γ, TNF-α, C-C motif chemokine ligand (CCL)2, CCL7, and CCL12] that enhance infiltration and activation of monocytes and neutrophils, further exacerbating the inflammatory response and inducing high cytokine levels, a phenomenon referred to as cytokine storm, which has been linked to more severe manifestations of COVID-19 (60).
Several immune-based cell strategies can be proposed for targeting different pathologies of COVID-19. Several NK cell therapies for COVID-19 are under investigation (Table 1). NK cells are activated and recruited at the site of infection in response to IL-12 and IL-18 signals. They control viral replication using perforin and granzyme granules and induce Fas ligand– or TNF-a–related apoptosis-inducing ligand–mediated apoptosis in infected cells (61). Cell therapies involving NK cells and chimeric antigen receptor (CAR) NK cells have shown clinical safety and efficacy in numerous oncological indications (62), and they may have a role in treating various infectious pathologies as well (63). As NK cells recognize viral infected cells by identifying up-regulated stress markers and down-regulated inhibitory ligands, exogenous administration of NK cell–based therapies could thus assist in identifying SARS-CoV-2–infected cells and promote viral clearance (64). A phase 1 study is assessing the efficacy and safety of CYNK-001 cells, which are allogeneic, off-the-shelf, and cryopreserved NK cells derived from CD34+ human placental stem cells, in 14 adult patients with mild to moderate COVID-19 (NCT04365101). In another phase 1 study, FT516 cells, which are allogeneic, off-the-shelf, and cryopreserved NK cells derived from iPSCs, are being tested for efficacy and safety in 12 adult patients with COVID-19 who are hospitalized and fulfill requirements for hypoxia (NCT04363346). With regard to CAR NK cells, a phase 1/2 study in China is using off-the-shelf NKG2D-ACE2 CAR NK cells to target viral infected cells while also secreting IL-15 as a superagonist and granulocyte-macrophage colony-stimulating factor neutralizing single-chain variable fragment to reduce the likelihood of cytokine release syndrome (NCT04324996). Intravenous infusion of 1 × 108 cells/kg of body weight will be administered weekly in patients with COVID-19, and the study is currently recruiting patients.
Given that immune system overactivation is a significant factor in pathologies of COVID-19, another potential strategy could involve CD4+CD25+Foxp3+ regulatory T cells (Tregs). Tregs function by secreting anti-inflammatory cytokines IL-10 and transforming growth factor–β (TGF-β) as well as by contact-dependent signaling, and have been shown to inhibit the influx of neutrophils to the lung, induce apoptotic cell clearance of activated neutrophils and macrophages, and decrease proinflammatory cytokine levels (65, 66). Moreover, they can inhibit excessive innate immune responses via induction of secondary immunosuppressive neutrophils that generate anti-inflammatory cytokines and via enzymes indoleamine 2,3-dioxygenase and heme oxygenase-1, which further inhibit cellular proliferation (66). The safety and feasibility of Tregs has been clinically evaluated over the past decade, showing tolerability and clinical improvement especially in the setting of solid-organ transplantation and autoimmune disease (67). Hence, the immunosuppressive role of Tregs may be beneficial in quelling the cytokine storm in patients with COVID-19. Potential strategies may include using polyclonal expanded Tregs versus engineered antigen-specific Treg approaches. Polyclonal Tregs offer a more generalized immunosuppressive strategy, which may be similar to current immunosuppressive medications. Polyclonal Tregs have been clinically evaluated with promising results in type 1 diabetes and other autoimmune diseases (68), but they have not been clinically tested in immune overactivation in viral infections. A concern with this therapy would be the exacerbation of acute infection by excessive quelling of the host immune response to SARS-CoV-2. Engineered antigen-specific Tregs could help localize immunosuppressive effects (65), but this could also facilitate enhanced viral replication. Overall, Treg therapies could aid in suppressing the overactive immune system in patients with COVID-19 (69), but generalizing early safety data from clinical trials of autoimmune and transplant patients toward patients with COVID-19 would need careful evaluation. Two phase 1 clinical trials, which are not yet recruiting, are aiming to test the efficacy and safety of allogeneic, off-the-shelf, and cryopreserved Treg cell infusions in patients with COVID-19 with moderate to severe ARDS (NCT04468971) or intubated and mechanically ventilated (NCT04482699).
Besides Tregs, other T cell therapies are being evaluated for COVID-19 (Table 1). Viral-specific T cells are currently under investigation in three trials, and they are using viral-specific T cells from healthy donors who have mounted an appropriate response to the SARS-CoV-2 (NCT04457726, NCT04406064, and NCT04401410). A better understanding of effective targets could aid in the development of engineered T cells from more accessible and scalable sources than previously infected healthy donors. In addition, a phase 1/2 trial evaluating the use of RAPA-501, a hybrid T helper 2/Treg phenotype, aims to suppress immune overactivation in a T cell receptor–independent manner (NCT04482699). Engineered T cells, particularly CAR T therapies, have shown promise in the treatment of immune system overactivation in diseases such as pemphigus vulgaris, type 1 diabetes, and lupus (70), and targeted T cell therapies could play a role in treating COVID-19 immune overactivation and facilitating viral clearance. Recent single-cell sequencing studies of patients with COVID-19 have shown an increase in monocytes, macrophages, and clonally expanded CD8+ T cells, which may contribute to the cytokine storm seen in severe cases (71, 72). This provides a rationale to direct cell therapies such as CAR T/NK cells to target these enriched populations with the goal of reducing the excess cell population, and potentially decreasing the severity of the cytokine storm. In addition, B lymphocytes could theoretically be engineered to recombinantly express humanized monoclonal antibodies with neutralizing anti–SARS-CoV-2 activity. However, convalescent plasma or monoclonal antibodies likely have similar benefits without the increased complexity of a cell therapy–based modality (73).
In addition to their role in targeting COVID-19–related lung damage, MSCs are also an intriguing target for immune-based cell therapy because of their immunomodulatory capacities. In the lung, MSCs mediate immune homeostasis by TNF-α– and IL-1–induced up-regulation of anti-inflammatory cytokines such as protein TNF-stimulated gene 6, IL-10, TGF-β, prostaglandin E2, and nitric oxide (74, 75). Moreover, by modulating overactivation of the immune system, MSCs have shown efficacy for the treatment of immune-related conditions such as steroid-refractory graft-versus-host disease and systemic lupus erythematosus (76, 77). Hence, MSC therapy may play a role in suppressing COVID-19–associated immune activation and cytokine storm. Several recent studies have reported decreases in inflammatory marker levels after treatment with MSCs that correlated with clinical improvement (41–44). Moreover, ongoing clinical trials are assessing the immunomodulatory capabilities of MSCs in patients with COVID-19 (NCT04348435, NCT04377334, and NCT04397796). Another phase 1 clinical trial is assessing the efficacy and safety of allogeneic umbilical cord blood–derived MSCs as adjuvant therapy in patients receiving oseltamivir and azithromycin (NCT04457609). Dosing for MSC trials varies widely between 5 × 105 and 1 × 107 cells/kg or 2 × 107 and 2 × 108 cells per dose with the number of doses ranging from one to four. Cell sourcing for MSC trials includes the umbilical cord, placenta, adipose tissue, intra-aortic tissue, olfactory mucosa, and the dental pulp (78). More detailed reviews on mechanisms of MSC immunomodulation and potential benefits in COVID-19 have been previously explored (75, 78–89).
NEUROLOGICAL
Neurological manifestations are a significant consideration in patients with COVID-19 and are reported in 57.4% of confirmed cases (90). Presenting symptoms range from headache, anosmia, and ageusia to more serious manifestations such as ischemic stroke, encephalitis, and encephalomyelitis (91). The innate immune response is likely responsible for symptoms such as headache and encephalitis through uncontrolled cytokine release. However, symptoms such as anosmia, encephalomyelitis, and stroke suggest potential viral invasion of the central nervous system (CNS). Proposed mechanisms of CNS viral access include retrograde axonal transport through vagal afferents peripherally (92) or via direct CNS invasion, as studies have shown ACE2 receptors to be expressed in several regions of the brain, especially in oligodendrocytes and astrocytes (93). The symptoms of anosmia and ageusia were initially suggestive of CNS invasion, especially as SARS-COV studies had shown that the virus could enter the brain through the olfactory nerve within days of infection, causing inflammation and demyelination (94). However, analysis of human RNA sequencing and single-cell sequencing data showed that ACE2 and TMPRSS2 are not expressed in olfactory sensory nerves but instead in olfactory epithelium (95). Acute cerebral ischemic events have been reported in patients with COVID-19, especially in younger patients without typical risks of cerebrovascular disease (96, 97). These manifestations are likely due to an overall prothrombotic state, potential down-regulation of ACE2, which causes an overall loss of neuroprotection, and hyperinflammatory cytokine release. In addition, there has been an increasing number of reports of Guillain-Barre syndrome and its variants, transverse myelitis, and other demyelinating conditions in affected patients, some with multifocal lesions in the brain and spine (98). The presence of demyelination has also been present in autopsy studies (98). The etiology of these lesions is likely immune-related, potentially because of a delayed immune reaction.
To date, there have been no reports of cell-based clinical trials addressing neurologic manifestations in patients with COVID-19. However, the high incidence of neurologic manifestations coupled with increasing reports of demyelinating disease and ischemic stroke in affected patients may require treatment options that focus on long-term deficits, which can potentially be addressed via cell therapy. Regarding demyelination, oligodendrocyte precursor cells (OPCs) have been explored in the setting of spinal cord injury and have showed safety, tolerability, cell engraftment, and improved motor function at 12-month follow-up in patients (NCT02302157). In addition, human iPSC (hiPSC)–derived OPCs were shown to remyelinate denuded axons in nonhuman primates with experimental autoimmune encephalomyelitis (EAE), a common animal model for multiple sclerosis (99). As COVID-19–related demyelination is likely due to immune-mediated myelin damage, successful applications of OPCs in other demyelinating animal models such as EAE suggest a potential benefit of OPCs in COVID-19–related refractory demyelination.
Patients with COVID-19 who suffered acute ischemic strokes, especially those with persistent deficits after mechanical thrombectomy or thrombolytic therapy, could also be a target of cell therapy. The long-term outcomes of patients suffering strokes, most of whom are younger and suffer large vessel occlusions, could be devastating. The prospect of stem cell therapies in stroke has expanded, with several concluded and ongoing clinical trials using bone marrow–derived stem cells and neural stem cells (100). MASTERS-2 (NCT03545607) and TREASURE (NCT02961504) are ongoing phase 3 clinical trials assessing treatment outcomes after intravenous administration of bone marrow–derived adult progenitor stem cells in patients suffering from ischemic stroke in the acute setting. Hence, this subpopulation of patients with COVID-19 may benefit from neuroregenerative cell therapies in the future.
CARDIAC SYSTEM
Cardiac manifestations, such as elevated troponin levels and myocardial ischemic infarctions, are commonly seen in patients with COVID-19, particularly in severe presentations (101). Myocardial injury was found in 22% of hospitalized patients and nearly 60% of deceased patients (4, 14). Moreover, cardiac arrhythmias were shown to be present in 44% of patients with COVID-19 in the ICU (102). Although cardiac cells express high levels of ACE2 (11), it remains unclear whether these cases constitute direct or indirect injury. One study on hiPSC-derived cardiomyocytes from patients with COVID-19 suggests viral invasion and cytopathic features in cardiac tissue (103). As cell therapies are designed, one potential way to mitigate the risk of SARS-CoV-2 viral entry of the treatment may be to genetically modulate viral entry proteins within the cell therapy itself. Indirect injury could be due to systemic hypoxia, secondary pulmonary hypertension, arrhythmia due to metabolic derangements, and cytokine storm damage (104).
Early cell therapy trials in acute myocardial infarct have largely focused on bone marrow mononuclear cells (BMMNCs), and early studies such as BOOST (105) and TOPCARE-MI (106) showed improvements in infarct size and left ventricular ejection fraction. Subsequent trials such as BOOST-2 (107) and TIME (108) showed no clinical benefit, however, questioning the role of BMMNCs in acute myocardial infarction. Preclinical data using a combination of cardiopoietic stem cells and MSCs have been promising and are under investigation in human trials (NCT02501811) (109). Further, Menasché et al. (110) showed that hESC-derived cardiac progenitors given to six patients with ischemic left ventricular dysfunction showed clinical improvement in systolic function without new tumors or arrhythmias. Clinical applications of iPSC-derived cardiomyocytes are also being explored (111). These advances in cell-based cardiac therapy can potentially be exploited for patients suffering from COVID-19–related cardiac ischemia. In addition, a recent clinical study used cardiosphere-derived cells, which are cardiac progenitor cells, to assess treatment of severe pulmonary manifestations in six patients with COVID-19. Four of the six patients were discharged from the hospital, while the remaining two were in stable conditions at the time the study was published (112). A phase 2 trial further assessing the efficacy of these cardiosphere-derived cells is currently under investigation (NCT04623671).
GASTROINTESTINAL SYSTEM
Gastrointestinal manifestations occur in 5 to 10% of COVID-19 cases; however, symptoms have been mild and self-limited to nausea, diarrhea, and vomiting, despite ACE2 and TMPRSS2 being coexpressed in the small and large intestines and SARS-CoV-2 being detected in fecal samples of infected patients, suggesting direct viral invasion of enterocytes (113). This suggests that chronic intestinal sequela is unlikely to occur, negating the need for advanced treatments such as cell therapy. Hepatic involvement also appears to be frequent. Elevations in alanine aminotransferase and aspartate aminotransferase have been reported in up to a third of patients (114). ACE2 expression has been identified in cholangiocytes (115, 116); however, histopathological examinations have yet to show direct viral inclusions in the liver (117). Other possibilities for hepatic injury may include immune-mediated damage, systemic hypoxia secondary to lung damage, and drug-induced liver injury (118). Stem cell–derived hepatic cells have been studied in the setting of acute and chronic liver failure. Patients have received cell therapies through the portal vein or splenic artery with improvement in serological markers such as prothrombin time or severity of hepatic encephalopathy (119). Although hepatocyte-based therapies have largely been considered a bridge to transplantation rather than a curative therapy itself, rare cases of patients with COVID-19 with acute liver failure may benefit from hepatocyte-based therapies (120).
RENAL SYSTEM
Renal manifestations are frequent and range from mild proteinuria to severe injury requiring renal replacement therapy (121). Pei et al. (122) showed that 75% of patients with COVID-19 presenting with pneumonia were found to have an abnormal urine dipstick. Moreover, the presence of acute kidney injury (AKI) was associated with increased mortality, and only 46% of those patients who developed an AKI showed complete resolution after 12 days of follow-up. Over 80% of AKIs were intrinsic, with the remainder being secondary to rhabdomyolysis; there were no cases of prerenal AKI (122). Pathological studies have demonstrated acute tubular necrosis, presence of microthrombi, and mild interstitial fibrosis; however, no evidence of lymphocytic infiltrate in affected patients was found (123). While direct viral invasion is possible as ACE2 expression is present in tubular epithelium and podocytes, secondary mechanisms appear more relevant in inducing renal damage, which may include systemic hypoxia, rhabdomyolysis, cytokine-mediated damage, microemboli due to hypercoagulability, and cardiorenal congestion from right heart strain (121).
Cellular therapies for kidney disease are currently being explored and may benefit patients with COVID-19 suffering from permanent kidney injury. For example, preclinical studies using iPSC-derived renal precursor cells have shown the ability for these cells to engraft into damaged tubules and improve renal function (124). In addition, Swaminathan et al. (125) conducted a phase 2 trial using intra-aortic allogenic MSCs in the setting of postcardiac surgery–related AKI. However, the results showed no significant improvement in time to recover from AKI, dialysis use, or 30-day mortality. A phase 1 clinical trial, which is not yet recruiting, is aiming to assess the efficacy and safety of allogeneic MSCs infused via continuous renal replacement therapy (CRRT) in patients with COVID-19 with AKI undergoing CRRT (NCT04445220). Patients will be divided into three arms: low dose (2.5 × 107 cells), high dose (7.5 × 107 cells), and control. These studies could shed light on a possible role for cell therapies for the treatment of COVID-related renal damage.
HEMATOLOGICAL SYSTEM
Hematological and vascular sequela, especially hypercoagulability and disseminated intravascular coagulation (DIC), are serious manifestations of SARS-CoV-2 (126). The hypercoagulable state increases the risk of venous thromboembolism, which can lead to ischemic stroke and multisystem organ failure via microemboli (127). Rates of venous thromboembolism in critically ill patients with COVID-19 have been estimated to be as high as 31% (128). Moreover, Tang et al. (129) reported that 70% of deceased patients met criteria for DIC. The hypercoagulable state may be related to stimulated production of antiphospholipid antibodies and complement activation, vascular endothelial damage, and prolonged immobility in the ICU (130). Although the hypercoagulable state is likely due to a variety of factors, endothelial disruption is one potential cause that may contribute to multisystem end-organ damage in COVID-19 (131). CD34+ cells, hematopoietic stem cells that can give rise to endothelial progenitors and restore vasculature, have been approved for an investigational new drug by the FDA to assess their efficacy and safety for lung damage repair. CD34+ cells are thought to promote vascular regeneration to counter ischemic damage and have shown efficacy and safety in trials evaluating their potential in cardiac and critical limb ischemia (132). Cord blood CD34+ cells also showed protective effects on acute lung injury induced by lipopolysaccharide challenge in mice, similar to another study that showed that peripheral blood CD34+ cells attenuated acute lung injury induced by oleic acid in rats (133, 134). Hence, therapy with CD34+ cells could prove feasible for promoting vascular growth in the lungs of patients with COVID-19 suffering from significant pulmonary damage (NCT04522817).
FUTURE DIRECTIONS AND LIMITATIONS
Overall, cell therapies show great promise in several diseases, and data from other studies suggest that certain cell therapies may be applicable in particular pathogenesis aspects of COVID-19. Specific factors such as dosing of the cells, route of administration, allogenic versus autologous cells, role of immunosuppressive therapy, tolerance of treatment in elderly patients, role of extracellular vesicles, and readouts of effectiveness need to be better delineated. As an example, risk for severe illness with COVID-19 increases with age. There are lessons to be learned about recipient age from studies using hematopoietic stem cell transplantation (HSCT) or MSCs. For instance, HSCT studies have shown that patient age is correlated with transplant-related morbidity and mortality, but improvements such as the use of cytokines and less toxic or reduced conditioning have allowed older patients to receive these therapies. In the context of MSCs, a study conducted to evaluate patient age on the efficacy of MSC cell therapy in ischemic cardiomyopathy showed that older patients did not have an impaired response. Although these studies are not directly translatable to other cell types or patients with COVID-19, they nevertheless represent a starting point for future investigation (135–140). Cell dosing and number of injections should be tailored to patient-specific responses and tolerance of treatment. Route of administration should be localized as much as possible to reduce the risk of unintentional side effects in distant organs while maximizing efficacy at the infected organ system. Disseminated coronavirus involving multiple organ systems, for example, may benefit from intravenous infusion of cell therapy to allow treatment to reach multiple infected organs. Various routes of administration have been previously explored for respiratory and pulmonary diseases including intravenous, intratracheal instillation, inhalation, aerosolization, and nebulizers. Intratracheal instillation could be advantageous, as it provides highly precise, local delivery to the respiratory tract using a small dose; however, instillation is highly nonphysiological and may result in inconsistent and heterogeneous deposition focused on the upper airways (141). Five clinical trials for lung cell therapies have used aerosolization as the route of administration (NCT04313647, NCT04473170, NCT04389385, NCT04491240, and NCT04276987). This route of administration may be preferred because of the potentially broader distribution of cells in the lung while reducing the probability of cell damage and loss (141).
Another interesting avenue to consider is the use of a combination of various cell therapies. MSCs, for example, have been studied for their synergistic effects with other cell types, including pulmonary endothelial cells and epithelial cells (142). For instance, MSCs were shown to stimulate endothelial progenitors in patients with heart failure and preserve endothelial integrity after hemorrhagic shock (143, 144). These findings could support investigation of both cell types as a combination cell therapy.
From a scalability standpoint, allogenic or off-the-shelf–based therapies that are either human leukocyte antigen (HLA)–matched or do not have HLAs present would be favored over autologous cells. HLA matching or depletion may also reduce the need for immunosuppression. Clinical trial readouts should include COVID-19–related outcomes and organ function related to the cell therapy being administered. Last, the idea of leveraging the field of synthetic biology to further adapt engineered cell lines should also be considered. For example, cell therapies that modulate expression of viral entry proteins, decrease residual potentially tumorigenic pluripotent cells, or adopt genome-scale mammalian translational recoding to confer viral resistance could be of keen advantage (145, 146).
Acknowledgments
Funding: The Blavatnik Biomedical Accelerator at Harvard University has provided funding for this article. Author contributions: M.M.Z., E.L., A.H.M.N., P.K., and G.M.C. conceived the idea and wrote the manuscript. K.S., K.K., L.R.-M., H.G., A.H., E.A., and S.L. wrote the manuscript. Competing interests: Full disclosure of G.M.C. is available on http://arep.med.harvard.edu/gmc/tech.html. A.H.M.N., P.K., and G.M.C. are cofounders and have equity in GC Therapeutics Inc. No reagents or funding from GC Therapeutics Inc. was used in this study. The authors declare that they have no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper. Additional data related to this paper may be requested from the authors.
REFERENCES AND NOTES
- 1.Wu Z., McGoogan J. M., Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: Summary of a report of 72314 cases from the Chinese Center for Disease Control and Prevention. JAMA 323, 1239–1242 (2020). [DOI] [PubMed] [Google Scholar]
- 2.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M., Yu T., Wang Y., Pan S., Zou X., Yuan S., Shang Y., Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 8, 475–481 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T. S., Herrler G., Wu N. H., Nitsche A., Muller M. A., Drosten C., Pohlmann S., SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280.e8 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou P., Yang X. L., Wang X. G., Hu B., Zhang L., Zhang W., Si H. R., Zhu Y., Li B., Huang C. L., Chen H. D., Chen J., Luo Y., Guo H., Jiang R. D., Liu M. Q., Chen Y., Shen X. R., Wang X., Zheng X. S., Zhao K., Chen Q. J., Deng F., Liu L. L., Yan B., Zhan F. X., Wang Y. Y., Xiao G. F., Shi Z. L., A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 579, 270–273 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gordon D. E., Jang G. M., Bouhaddou M., Xu J., Obernier K., White K. M., O’Meara M. J., Rezelj V. V., Guo J. Z., Swaney D. L., Tummino T. A., Huettenhain R., Kaake R. M., Richards A. L., Tutuncuoglu B., Foussard H., Batra J., Haas K., Modak M., Kim M., Haas P., Polacco B. J., Braberg H., Fabius J. M., Eckhardt M., Soucheray M., Bennett M. J., Cakir M., McGregor M. J., Li Q., Meyer B., Roesch F., Vallet T., Kain A. M., Miorin L., Moreno E., Naing Z. Z. C., Zhou Y., Peng S., Shi Y., Zhang Z., Shen W., Kirby I. T., Melnyk J. E., Chorba J. S., Lou K., Dai S. A., Barrio-Hernandez I., Memon D., Hernandez-Armenta C., Lyu J., Mathy C. J. P., Perica T., Pilla K. B., Ganesan S. J., Saltzberg D. J., Rakesh R., Liu X., Rosenthal S. B., Calviello L., Venkataramanan S., Liboy-Lugo J., Lin Y., Huang X. P., Liu Y., Wankowicz S. A., Bohn M., Safari M., Ugur F. S., Koh C., Savar N. S., Tran Q. D., Shengjuler D., Fletcher S. J., O’Neal M. C., Cai Y., Chang J. C. J., Broadhurst D. J., Klippsten S., Sharp P. P., Wenzell N. A., Kuzuoglu D., Wang H. Y., Trenker R., Young J. M., Cavero D. A., Hiatt J., Roth T. L., Rathore U., Subramanian A., Noack J., Hubert M., Stroud R. M., Frankel A. D., Rosenberg O. S., Verba K. A., Agard D. A., Ott M., Emerman M., Jura N., von Zastrow M., Verdin E., Ashworth A., Schwartz O., d’Enfert C., Mukherjee S., Jacobson M., Malik H. S., Fujimori D. G., Ideker T., Craik C. S., Floor S. N., Fraser J. S., Gross J. D., Sali A., Roth B. L., Ruggero D., Taunton J., Kortemme T., Beltrao P., Vignuzzi M., Garcia-Sastre A., Shokat K. M., Shoichet B. K., Krogan N. J., A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantuti-Castelvetri L., Ojha R., Pedro L. D., Djannatian M., Franz J., Kuivanen S., van der Meer F., Kallio K., Kaya T., Anastasina M., Smura T., Levanov L., Szirovicza L., Tobi A., Kallio-Kokko H., Osterlund P., Joensuu M., Meunier F. A., Butcher S. J., Winkler M. S., Mollenhauer B., Helenius A., Gokce O., Teesalu T., Hepojoki J., Vapalahti O., Stadelmann C., Balistreri G., Simons M., Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science 370, 856–860 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daly J. L., Simonetti B., Klein K., Chen K. E., Williamson M. K., Anton-Plagaro C., Shoemark D. K., Simon-Gracia L., Bauer M., Hollandi R., Greber U. F., Horvath P., Sessions R. B., Helenius A., Hiscox J. A., Teesalu T., Matthews D. A., Davidson A. D., Collins B. M., Cullen P. J., Yamauchi Y., Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science 370, 861–865 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soni S., Jiang Y., Tesfaigzi Y., Hornick J. L., Cataltepe S., Comparative analysis of ACE2 protein expression in rodent, non-human primate, and human respiratory tract at baseline and after injury: A conundrum for COVID-19 pathogenesis. PLOS ONE 16, e0247510 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masre S. F., Jufri N. F., Ibrahim F. W., Abdul Raub S. H., Classical and alternative receptors for SARS-CoV-2 therapeutic strategy. Rev. Med. Virol. e2207 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keen J. C., Moore H. M., The Genotype-Tissue Expression (GTEx) Project: Linking clinical data with molecular analysis to advance personalized medicine. J. Pers. Med. 5, 22–29 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Uhlén M., Fagerberg L., Hallström B. M., Lindskog C., Oksvold P., Mardinoglu A., Sivertsson A., Kampf C., Sjostedt E., Asplund A., Olsson I., Edlund K., Lundberg E., Navani S., Szigyarto C. A., Odeberg J., Djureinovic D., Takanen J. O., Hober S., Alm T., Edqvist P. H., Berling H., Tegel H., Mulder J., Rockberg J., Nilsson P., Schwenk J. M., Hamsten M., von Feilitzen K., Forsberg M., Persson L., Johansson F., Zwahlen M., von Heijne G., Nielsen J., Pontén F., Proteomics. Tissue-based map of the human proteome. Science 347, 1260419 (2015). [DOI] [PubMed] [Google Scholar]
- 12.Singh M., Bansal V., Feschotte C., A single-cell RNA expression map of human coronavirus entry factors. Cell Rep. 32, 108175 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hou Y. J., Okuda K., Edwards C. E., Martinez D. R., Asakura T., Dinnon K. H. III, Kato T., Lee R. E., Yount B. L., Mascenik T. M., Chen G., Olivier K. N., Ghio A., Tse L. V., Leist S. R., Gralinski L. E., Schafer A., Dang H., Gilmore R., Nakano S., Sun L., Fulcher M. L., Livraghi-Butrico A., Nicely N. I., Cameron M., Cameron C., Kelvin D. J., de Silva A., Margolis D. M., Markmann A., Bartelt L., Zumwalt R., Martinez F. J., Salvatore S. P., Borczuk A., Tata P. R., Sontake V., Kimple A., Jaspers I., O’Neal W. K., Randell S. H., Boucher R. C., Baric R. S., SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182, 429–446.e14 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B., Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395, 1054–1062 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imam Z., Odish F., Gill I., O’Connor D., Armstrong J., Vanood A., Ibironke O., Hanna A., Ranski A., Halalau A., Older age and comorbidity are independent mortality predictors in a large cohort of 1305 COVID-19 patients in Michigan, United States. J. Intern. Med. 288, 469–476 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta N., Mazer-Amirshahi M., Alkindi N., Pourmand A., Pharmacotherapy in COVID-19; A narrative review for emergency providers. Am. J. Emerg. Med. 38, 1488–1493 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feins S., Kong W., Williams E. F., Milone M. C., Fraietta J. A., An introduction to chimeric antigen receptor (CAR) T-cell immunotherapy for human cancer. Am. J. Hematol. 94, S3–S9 (2019). [DOI] [PubMed] [Google Scholar]
- 18.June C. H., O’Connor R. S., Kawalekar O. U., Ghassemi S., Milone M. C., CAR T cell immunotherapy for human cancer. Science 359, 1361–1365 (2018). [DOI] [PubMed] [Google Scholar]
- 19.Parekkadan B., Milwid J. M., Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 12, 87–117 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh V. K., Kalsan M., Kumar N., Saini A., Chandra R., Induced pluripotent stem cells: Applications in regenerative medicine, disease modeling, and drug discovery. Front. Cell Dev. Biol. 3, 2 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ankrum J. A., Ong J. F., Karp J. M., Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 32, 252–260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ryan J. M., Barry F. P., Murphy J. M., Mahon B. P., Mesenchymal stem cells avoid allogeneic rejection. J. Inflamm. 2, 8 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deuse T., Hu X., Gravina A., Wang D., Tediashvili G., De C., Thayer W. O., Wahl A., Garcia J. V., Reichenspurner H., Davis M. M., Lanier L. L., Schrepfer S., Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37, 252–258 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han X., Wang M., Duan S., Franco P. J., Kenty J. H., Hedrick P., Xia Y., Allen A., Ferreira L. M. R., Strominger J. L., Melton D. A., Meissner T. B., Cowan C. A., Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl. Acad. Sci. U.S.A. 116, 10441–10446 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barkholt L., Flory E., Jekerle V., Lucas-Samuel S., Ahnert P., Bisset L., Buscher D., Fibbe W., Foussat A., Kwa M., Lantz O., Maciulaitis R., Palomaki T., Schneider C. K., Sensebe L., Tachdjian G., Tarte K., Tosca L., Salmikangas P., Risk of tumorigenicity in mesenchymal stromal cell-based therapies--bridging scientific observations and regulatory viewpoints. Cytotherapy 15, 753–759 (2013). [DOI] [PubMed] [Google Scholar]
- 26.Ben-David U., Benvenisty N., The tumorigenicity of human embryonic and induced pluripotent stem cells. Nat. Rev. Cancer 11, 268–277 (2011). [DOI] [PubMed] [Google Scholar]
- 27.Sato Y., Bando H., Di Piazza M., Gowing G., Herberts C., Jackman S., Leoni G., Libertini S., MacLachlan T., McBlane J. W., Pereira Mouries L., Sharpe M., Shingleton W., Surmacz-Cordle B., Yamamoto K., van der Laan J. W., Tumorigenicity assessment of cell therapy products: The need for global consensus and points to consider. Cytotherapy 21, 1095–1111 (2019). [DOI] [PubMed] [Google Scholar]
- 28.Martin U., Therapeutic application of pluripotent stem cells: Challenges and risks. Front. Med. 4, 229 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang K., Lin R. Z., Hong X., Ng A. H., Lee C. N., Neumeyer J., Wang G., Wang X., Ma M., Pu W. T., Church G. M., Melero-Martin J. M., Robust differentiation of human pluripotent stem cells into endothelial cells via temporal modulation of ETV2 with modified mRNA. Sci. Adv. 6, eaba7606 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Busskamp V., Lewis N. E., Guye P., Ng A. H., Shipman S. L., Byrne S. M., Sanjana N. E., Murn J., Li Y., Li S., Stadler M., Weiss R., Church G. M., Rapid neurogenesis through transcriptional activation in human stem cells. Mol. Syst. Biol. 10, 760 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Itakura G., Kawabata S., Ando M., Nishiyama Y., Sugai K., Ozaki M., Iida T., Ookubo T., Kojima K., Kashiwagi R., Yasutake K., Nakauchi H., Miyoshi H., Nagoshi N., Kohyama J., Iwanami A., Matsumoto M., Nakamura M., Okano H., Fail-safe system against potential tumorigenicity after transplantation of iPSC derivatives. Stem Cell Rep. 8, 673–684 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B., Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395, 497–506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang D., Lian X., Song F., Ma H., Lian Z., Liang Y., Qin T., Chen W., Wang S., Clinical features of severe patients infected with 2019 novel coronavirus: A systematic review and meta-analysis. Ann. Transl. Med. 8, 576 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang W., Imaging changes of severe COVID-19 pneumonia in advanced stage. Intensive Care Med. 46, 841–843 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGonagle D., O’Donnell J. S., Sharif K., Emery P., Bridgewood C., Immune mechanisms of pulmonary intravascular coagulopathy in COVID-19 pneumonia. Lancet Rheumatol. 2, e437–e445 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.George P. M., Wells A. U., Jenkins R. G., Pulmonary fibrosis and COVID-19: The potential role for antifibrotic therapy. Lancet Respir. Med. 8, 807–815 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harrell C. R., Sadikot R., Pascual J., Fellabaum C., Jankovic M. G., Jovicic N., Djonov V., Arsenijevic N., Volarevic V., Mesenchymal stem cell-based therapy of inflammatory lung diseases: Current understanding and future perspectives. Stem Cells Int. 2019, 4236973 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan M. C., Kuok D. I., Leung C. Y., Hui K. P., Valkenburg S. A., Lau E. H., Nicholls J. M., Fang X., Guan Y., Lee J. W., Chan R. W., Webster R. G., Matthay M. A., Peiris J. S., Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc. Natl. Acad. Sci. U.S.A. 113, 3621–3626 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li Y., Xu J., Shi W., Chen C., Shao Y., Zhu L., Lu W., Han X., Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res. Ther. 7, 159 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison T. J., Jackson M. V., Cunningham E. K., Kissenpfennig A., McAuley D. F., O’Kane C. M., Krasnodembskaya A. D., Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am. J. Respir. Crit. Care Med. 196, 1275–1286 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang B., Chen J., Li T., Wu H., Yang W., Li Y., Li J., Yu C., Nie F., Ma Z., Yang M., Xiao M., Nie P., Gao Y., Qian C., Hu M., Clinical remission of a critically ill COVID-19 patient treated by human umbilical cord mesenchymal stem cells: A case report. Medicine 99, e21429 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang L., Jiang Y., Zhu M., Chen L., Zhou X., Zhou C., Ye P., Chen X., Wang B., Xu Z., Zhang Q., Xu X., Gao H., Wu X., Li D., Jiang W., Qu J., Xiang C., Li L., Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front. Med. 14, 664–673 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Leng Z., Zhu R., Hou W., Feng Y., Yang Y., Han Q., Shan G., Meng F., Du D., Wang S., Fan J., Wang W., Deng L., Shi H., Li H., Hu Z., Zhang F., Gao J., Liu H., Li X., Zhao Y., Yin K., He X., Gao Z., Wang Y., Yang B., Jin R., Stambler I., Lim L. W., Su H., Moskalev A., Cano A., Chakrabarti S., Min K. J., Ellison-Hughes G., Caruso C., Jin K., Zhao R. C., Transplantation of ACE2− mesenchymal stem cells improves the outcome of patients with COVID-19 pneumonia. Aging Dis. 11, 216–228 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sanchez-Guijo F., García-Arranz M., López-Parra M., Monedero P., Mata-Martínez C., Santos A., Sagredo V., Álvarez-Avello J.-M., Guerrero J. E., Pérez-Calvo C., Sánchez-Hernández M.-V., Del-Pozo J. L., Andreu E. J., Fernández-Santos M.-E., Soria-Juan B., Hernández-Blasco L. M., Andreu E., Sempere J. M., Zapata A. G., Moraleda J. M., Soria B., Fernández-Avilés F., García-Olmo D., Prósper F., Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine 25, 100454 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lanzoni G., Linetsky E., Correa D., Cayetano S. M., Alvarez R. A., Kouroupis D., Alvarez Gil A., Poggioli R., Ruiz P., Marttos A. C., Hirani K., Bell C. A., Kusack H., Rafkin L., Baidal D., Pastewski A., Gawri K., Lenero C., Mantero A. M. A., Metalonis S. W., Wang X., Roque L., Masters B., Kenyon N. S., Ginzburg E., Xu X., Tan J., Caplan A. I., Glassberg M. K., Alejandro R., Ricordi C., Umbilical cord mesenchymal stem cells for COVID-19 acute respiratory distress syndrome: A double-blind, phase 1/2a, randomized controlled trial. Stem Cells Transl. Med. 10, 660–673 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Grillo F., Barisione E., Ball L., Mastracci L., Fiocca R., Lung fibrosis: An undervalued finding in COVID-19 pathological series. Lancet Infect. Dis. 21, e72 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Laporta Hernandez R., Aguilar Perez M., Lazaro Carrasco M. T., Ussetti Gil P., Lung transplantation in idiopathic pulmonary fibrosis. Med. Sci. 6, 68 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serrano-Mollar A., Nacher M., Gay-Jordi G., Closa D., Xaubet A., Bulbena O., Intratracheal transplantation of alveolar type II cells reverses bleomycin-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 176, 1261–1268 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Wang D., Morales J. E., Calame D. G., Alcorn J. L., Wetsel R. A., Transplantation of human embryonic stem cell-derived alveolar epithelial type II cells abrogates acute lung injury in mice. Mol. Ther. 18, 625–634 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Serrano-Mollar A., Gay-Jordi G., Guillamat-Prats R., Closa D., Hernandez-Gonzalez F., Marin P., Burgos F., Martorell J., Sanchez M., Arguis P., Soy D., Bayas J. M., Ramirez J., Tetley T. D., Molins L., de la Bellacasa J. P., Rodriguez-Villar C., Rovira I., Fibla J. J., Xaubet A.; Pneumocyte Study Group , Safety and tolerability of alveolar type II cell transplantation in idiopathic pulmonary fibrosis. Chest 150, 533–543 (2016). [DOI] [PubMed] [Google Scholar]
- 51.Averyanov A., Koroleva I., Konoplyannikov M., Revkova V., Lesnyak V., Kalsin V., Danilevskaya O., Nikitin A., Sotnikova A., Kotova S., Baklaushev V., First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl. Med. 9, 6–16 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Robbiani D. F., Gaebler C., Muecksch F., Lorenzi J. C. C., Wang Z., Cho A., Agudelo M., Barnes C. O., Gazumyan A., Finkin S., Hagglof T., Oliveira T. Y., Viant C., Hurley A., Hoffmann H. H., Millard K. G., Kost R. G., Cipolla M., Gordon K., Bianchini F., Chen S. T., Ramos V., Patel R., Dizon J., Shimeliovich I., Mendoza P., Hartweger H., Nogueira L., Pack M., Horowitz J., Schmidt F., Weisblum Y., Michailidis E., Ashbrook A. W., Waltari E., Pak J. E., Huey-Tubman K. E., Koranda N., Hoffman P. R., West A. P. Jr., Rice C. M., Hatziioannou T., Bjorkman P. J., Bieniasz P. D., Caskey M., Nussenzweig M. C., Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature 584, 437–442 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poh C. M., Carissimo G., Wang B., Amrun S. N., Lee C. Y., Chee R. S., Fong S. W., Yeo N. K., Lee W. H., Torres-Ruesta A., Leo Y. S., Chen M. I., Tan S. Y., Chai L. Y. A., Kalimuddin S., Kheng S. S. G., Thien S. Y., Young B. E., Lye D. C., Hanson B. J., Wang C. I., Renia L., Ng L. F. P., Two linear epitopes on the SARS-CoV-2 spike protein that elicit neutralising antibodies in COVID-19 patients. Nat. Commun. 11, 2806 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y., Wang X., Yuan J., Li T., Li J., Qian S., Hong C., Wang F., Liu Y., Wang Z., He Q., Li Z., He B., Zhang T., Fu Y., Ge S., Liu L., Zhang J., Xia N., Zhang Z., Antibody responses to SARS-CoV-2 in patients with novel coronavirus disease 2019. Clin. Infect. Dis. 71, 2027–2034 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwasaki A., Yang Y., The potential danger of suboptimal antibody responses in COVID-19. Nat. Rev. Immunol. 20, 339–341 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Eroshenko N., Gill T., Keaveney M. K., Church G. M., Trevejo J. M., Rajaniemi H., Implications of antibody-dependent enhancement of infection for SARS-CoV-2 countermeasures. Nat. Biotechnol. 38, 789–791 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., Yuan Z., Feng Z., Zhang Y., Wu Y., Chen Y., Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19). Front. Immunol. 11, 827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mu J., Xu J., Zhang L., Shu T., Wu D., Huang M., Ren Y., Li X., Geng Q., Xu Y., Qiu Y., Zhou X., SARS-CoV-2-encoded nucleocapsid protein acts as a viral suppressor of RNA interference in cells. Sci. China Life Sci. 63, 1413–1416 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lu X., Pan J., Tao J., Guo D., SARS-CoV nucleocapsid protein antagonizes IFN-β response by targeting initial step of IFN-β induction pathway, and its C-terminal region is critical for the antagonism. Virus Genes 42, 37–45 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Channappanavar R., Perlman S., Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 39, 529–539 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prager I., Watzl C., Mechanisms of natural killer cell-mediated cellular cytotoxicity. J. Leukoc. Biol. 105, 1319–1329 (2019). [DOI] [PubMed] [Google Scholar]
- 62.Liu E., Marin D., Banerjee P., Macapinlac H. A., Thompson P., Basar R., Nassif Kerbauy L., Overman B., Thall P., Kaplan M., Nandivada V., Kaur I., Nunez Cortes A., Cao K., Daher M., Hosing C., Cohen E. N., Kebriaei P., Mehta R., Neelapu S., Nieto Y., Wang M., Wierda W., Keating M., Champlin R., Shpall E. J., Rezvani K., Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382, 545–553 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Schmidt S., Tramsen L., Rais B., Ullrich E., Lehrnbecher T., Natural killer cells as a therapeutic tool for infectious diseases - current status and future perspectives. Oncotarget 9, 20891–20907 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Market M., Angka L., Martel A. B., Bastin D., Olanubi O., Tennakoon G., Boucher D. M., Ng J., Ardolino M., Auer R. C., Flattening the COVID-19 curve with natural killer cell based immunotherapies. Front. Immunol. 11, 1512 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ferreira L. M. R., Muller Y. D., Bluestone J. A., Tang Q., Next-generation regulatory T cell therapy. Nat. Rev. Drug Discov. 18, 749–769 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okeke E. B., Uzonna J. E., The pivotal role of regulatory T cells in the regulation of innate immune cells. Front. Immunol. 10, 680 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Romano M., Fanelli G., Albany C. J., Giganti G., Lombardi G., Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front. Immunol. 10, 43 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang X., Olsen N., Zheng S. G., The progress and prospect of regulatory T cells in autoimmune diseases. J. Autoimmun. 111, 102461 (2020). [DOI] [PubMed] [Google Scholar]
- 69.Stephen-Victor E., Das M., Karnam A., Pitard B., Gautier J. F., Bayry J., Potential of regulatory T cell-based therapies in the management of severe COVID-19. Eur. Respir. J. 56, 2002182 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ellebrecht C. T., Bhoj V. G., Nace A., Choi E. J., Mao X., Cho M. J., Di Zenzo G., Lanzavecchia A., Seykora J. T., Cotsarelis G., Milone M. C., Payne A. S., Reengineering chimeric antigen receptor T cells for targeted therapy of autoimmune disease. Science 353, 179–184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liao M., Liu Y., Yuan J., Wen Y., Xu G., Zhao J., Cheng L., Li J., Wang X., Wang F., Liu L., Amit I., Zhang S., Zhang Z., Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 26, 842–844 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Ren X., Wen W., Fan X., Hou W., Su B., Cai P., Li J., Liu Y., Tang F., Zhang F., Yang Y., He J., Ma W., He J., Wang P., Cao Q., Chen F., Chen Y., Cheng X., Deng G., Deng X., Ding W., Feng Y., Gan R., Guo C., Guo W., He S., Jiang C., Liang J., Li Y. M., Lin J., Ling Y., Liu H., Liu J., Liu N., Liu S. Q., Luo M., Ma Q., Song Q., Sun W., Wang G., Wang F., Wang Y., Wen X., Wu Q., Xu G., Xie X., Xiong X., Xing X., Xu H., Yin C., Yu D., Yu K., Yuan J., Zhang B., Zhang P., Zhang T., Zhao J., Zhao P., Zhou J., Zhou W., Zhong S., Zhong X., Zhang S., Zhu L., Zhu P., Zou B., Zou J., Zuo Z., Bai F., Huang X., Zhou P., Jiang Q., Huang Z., Bei J. X., Wei L., Bian X. W., Liu X., Cheng T., Li X., Zhao P., Wang F. S., Wang H., Su B., Zhang Z., Qu K., Wang X., Chen J., Jin R., Zhang Z., COVID-19 immune features revealed by a large-scale single-cell transcriptome atlas. Cell 184, 1895–1913.e19 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marovich M., Mascola J. R., Cohen M. S., Monoclonal antibodies for prevention and treatment of COVID-19. JAMA 324, 131–132 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Wang M., Yuan Q., Xie L., Mesenchymal stem cell-based immunomodulation: Properties and clinical application. Stem Cells Int. 2018, 3057624 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Durand N., Mallea J., Zubair A. C., Insights into the use of mesenchymal stem cells in COVID-19 mediated acute respiratory failure. NPJ Regen. Med. 5, 17 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Le Blanc K., Frassoni F., Ball L., Locatelli F., Roelofs H., Lewis I., Lanino E., Sundberg B., Bernardo M. E., Remberger M., Dini G., Egeler R. M., Bacigalupo A., Fibbe W., Ringdén O., Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 371, 1579–1586 (2008). [DOI] [PubMed] [Google Scholar]
- 77.Yuan X., Qin X., Wang D., Zhang Z., Tang X., Gao X., Chen W., Sun L., Mesenchymal stem cell therapy induces FLT3L and CD1c+ dendritic cells in systemic lupus erythematosus patients. Nat. Commun. 10, 2498 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sadeghi S., Soudi S., Shafiee A., Hashemi S. M., Mesenchymal stem cell therapies for COVID-19: Current status and mechanism of action. Life Sci. 262, 118493 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Canham M. A., Campbell J. D. M., Mountford J. C., The use of mesenchymal stromal cells in the treatment of coronavirus disease 2019. J. Transl. Med. 18, 359 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Coelho A., Alvites R. D., Branquinho M. V., Guerreiro S. G., Mauricio A. C., Mesenchymal Stem Cells (MSCs) as a potential therapeutic strategy in COVID-19 patients: Literature research. Front. Cell Dev. Biol. 8, 602647 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kavianpour M., Saleh M., Verdi J., The role of mesenchymal stromal cells in immune modulation of COVID-19: Focus on cytokine storm. Stem Cell Res. Ther. 11, 404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Basiri A., Pazhouhnia Z., Beheshtizadeh N., Hoseinpour M., Saghazadeh A., Rezaei N., Regenerative medicine in COVID-19 treatment: Real opportunities and range of promises. Stem Cell Rev. Rep. 17, 163–175 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Choudhery M. S., Harris D. T., Stem cell therapy for COVID-19: Possibilities and challenges. Cell Biol. Int. 44, 2182–2191 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Golchin A., Cell-based therapy for severe COVID-19 patients: Clinical trials and cost-utility. Stem Cell Rev. Rep. 17, 56–62 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Golchin A., Seyedjafari E., Ardeshirylajimi A., Mesenchymal stem cell therapy for COVID-19: Present or future. Stem Cell Rev. Rep. 16, 427–433 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rajarshi K., Chatterjee A., Ray S., Combating COVID-19 with mesenchymal stem cell therapy. Biotechnol. Rep. (Amst.) 26, e00467 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Riedel R. N., Perez-Perez A., Sanchez-Margalet V., Varone C. L., Maymo J. L., Stem cells and COVID-19: Are the human amniotic cells a new hope for therapies against the SARS-CoV-2 virus? Stem Cell Res. Ther. 12, 155 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang S. H., Shetty A. K., Jin K., Chunhua Zhao R., Combating COVID-19 with mesenchymal stem/stromal cell therapy: Promise and challenges. Front. Cell Dev. Biol. 8, 627414 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yen B. L., Yen M. L., Wang L. T., Liu K. J., Sytwu H. K., Current status of mesenchymal stem cell therapy for immune/inflammatory lung disorders: Gleaning insights for possible use in COVID-19. Stem Cells Transl. Med. 9, 1163–1173 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Romero-Sanchez C. M., Diaz-Maroto I., Fernandez-Diaz E., Sanchez-Larsen A., Layos-Romero A., Garcia-Garcia J., Gonzalez E., Redondo-Penas I., Perona-Moratalla A. B., Del Valle-Perez J. A., Gracia-Gil J., Rojas-Bartolome L., Feria-Vilar I., Monteagudo M., Palao M., Palazon-Garcia E., Alcahut-Rodriguez C., Sopelana-Garay D., Moreno Y., Ahmad J., Segura T., Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology 95, e1060–e1070 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ellul M. A., Benjamin L., Singh B., Lant S., Michael B. D., Easton A., Kneen R., Defres S., Sejvar J., Solomon T., Neurological associations of COVID-19. Lancet Neurol. 19, 767–783 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Das G., Mukherjee N., Ghosh S., Neurological insights of COVID-19 pandemic. ACS Chem. Nerosci. 11, 1206–1209 (2020). [DOI] [PubMed] [Google Scholar]
- 93.Chen R., Wang K., Yu J., Chen Z., Wen C., Xu Z., The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front. Neurol. 11, 573095 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bohmwald K., Galvez N. M. S., Rios M., Kalergis A. M., Neurologic alterations due to respiratory virus infections. Front. Cell. Neurosci. 12, 386 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Brann D. H., Tsukahara T., Weinreb C., Lipovsek M., Van den Berge K., Gong B., Chance R., Macaulay I. C., Chou H.-J., Fletcher R. B., Das D., Street K., de Bezieux H. R., Choi Y.-G., Risso D., Dudoit S., Purdom E., Mill J., Hachem R. A., Matsunami H., Logan D. W., Goldstein B. J., Grubb M. S., Ngai J., Datta S. R., Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 6, eabc5801 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Beyrouti R., Adams M. E., Benjamin L., Cohen H., Farmer S. F., Goh Y. Y., Humphries F., Jager H. R., Losseff N. A., Perry R. J., Shah S., Simister R. J., Turner D., Chandratheva A., Werring D. J., Characteristics of ischaemic stroke associated with COVID-19. J. Neurol. Neurosurg. Psychiatry 91, 889–891 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Sweid A., Hammoud B., Weinberg J. H., Oneissi M., Raz E., Shapiro M., DePrince M., Tjoumakaris S., Gooch M. R., Herial N. A., Zarzour H., Romo V., Rosenwasser R. H., Jabbour P., Letter: Thrombotic neurovascular disease in COVID-19 patients. Neurosurgery 87, E400–E406 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zanin L., Saraceno G., Panciani P. P., Renisi G., Signorini L., Migliorati K., Fontanella M. M., SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. 162, 1491–1494 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Thiruvalluvan A., Czepiel M., Kap Y. A., Mantingh-Otter I., Vainchtein I., Kuipers J., Bijlard M., Baron W., Giepmans B., Brück W., ‘t Hart B. A., Boddeke E., Copray S., Survival and functionality of human induced pluripotent stem cell-derived oligodendrocytes in a nonhuman primate model for multiple sclerosis. Stem Cells Transl. Med. 5, 1550–1561 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Krause M., Phan T. G., Ma H., Sobey C. G., Lim R., Cell-based therapies for stroke: Are we there yet? Front. Neurol. 10, 656 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kang Y., Chen T., Mui D., Ferrari V., Jagasia D., Scherrer-Crosbie M., Chen Y., Han Y., Cardiovascular manifestations and treatment considerations in covid-19. Heart 106, 1132–1141 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z., Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 323, 1061–1069 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Perez-Bermejo J. A., Kang S., Rockwood S. J., Simoneau C. R., Joy D. A., Silva A. C., Ramadoss G. N., Flanigan W. R., Fozouni P., Li H., Chen P. Y., Nakamura K., Whitman J. D., Hanson P. J., McManus B. M., Ott M., Conklin B. R., McDevitt T. C., SARS-CoV-2 infection of human iPSC-derived cardiac cells reflects cytopathic features in hearts of patients with COVID-19. Sci. Transl. Med. 13, eabf7872 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Geng Y. J., Wei Z. Y., Qian H. Y., Huang J., Lodato R., Castriotta R. J., Pathophysiological characteristics and therapeutic approaches for pulmonary injury and cardiovascular complications of coronavirus disease 2019. Cardiovasc. Pathol. 47, 107228 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Meyer G. P., Wollert K. C., Lotz J., Steffens J., Lippolt P., Fichtner S., Hecker H., Schaefer A., Arseniev L., Hertenstein B., Ganser A., Drexler H., Intracoronary bone marrow cell transfer after myocardial infarction: Eighteen months’ follow-up data from the randomized, controlled BOOST (BOne marrOw transfer to enhance ST-elevation infarct regeneration) trial. Circulation 113, 1287–1294 (2006). [DOI] [PubMed] [Google Scholar]
- 106.Leistner D. M., Fischer-Rasokat U., Honold J., Seeger F. H., Schachinger V., Lehmann R., Martin H., Burck I., Urbich C., Dimmeler S., Zeiher A. M., Assmus B., Transplantation of progenitor cells and regeneration enhancement in acute myocardial infarction (TOPCARE-AMI): Final 5-year results suggest long-term safety and efficacy. Clin. Res. Cardiol. 100, 925–934 (2011). [DOI] [PubMed] [Google Scholar]
- 107.Wollert K. C., Meyer G. P., Muller-Ehmsen J., Tschope C., Bonarjee V., Larsen A. I., May A. E., Empen K., Chorianopoulos E., Tebbe U., Waltenberger J., Mahrholdt H., Ritter B., Pirr J., Fischer D., Korf-Klingebiel M., Arseniev L., Heuft H. G., Brinchmann J. E., Messinger D., Hertenstein B., Ganser A., Katus H. A., Felix S. B., Gawaz M. P., Dickstein K., Schultheiss H. P., Ladage D., Greulich S., Bauersachs J., Intracoronary autologous bone marrow cell transfer after myocardial infarction: The BOOST-2 randomised placebo-controlled clinical trial. Eur. Heart J. 38, 2936–2943 (2017). [DOI] [PubMed] [Google Scholar]
- 108.Traverse J. H., Henry T. D., Pepine C. J., Willerson J. T., Zhao D. X., Ellis S. G., Forder J. R., Anderson R. D., Hatzopoulos A. K., Penn M. S., Perin E. C., Chambers J., Baran K. W., Raveendran G., Lambert C., Lerman A., Simon D. I., Vaughan D. E., Lai D., Gee A. P., Taylor D. A., Cogle C. R., Thomas J. D., Olson R. E., Bowman S., Francescon J., Geither C., Handberg E., Kappenman C., Westbrook L., Piller L. B., Simpson L. M., Baraniuk S., Loghin C., Aguilar D., Richman S., Zierold C., Spoon D. B., Bettencourt J., Sayre S. L., Vojvodic R. W., Skarlatos S. I., Gordon D. J., Ebert R. F., Kwak M., Moye L. A., Simari R. D.; Cardiovascular Cell Therapy Research Network (CCTRN) , Effect of the use and timing of bone marrow mononuclear cell delivery on left ventricular function after acute myocardial infarction: The TIME randomized trial. JAMA 308, 2380–2389 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bolli R., Hare J. M., March K. L., Pepine C. J., Willerson J. T., Perin E. C., Yang P. C., Henry T. D., Traverse J. H., Mitrani R. D., Khan A., Hernandez-Schulman I., Taylor D. A., DiFede D. L., Lima J. A. C., Chugh A., Loughran J., Vojvodic R. W., Sayre S. L., Bettencourt J., Cohen M., Moye L., Ebert R. F., Simari R. D.; Cardiovascular Cell Therapy Research Network (CCTRN) , Rationale and design of the CONCERT-HF trial (Combination of Mesenchymal and c-kit+ Cardiac Stem Cells As Regenerative Therapy for Heart Failure). Circ. Res. 122, 1703–1715 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Menasché P., Vanneaux V., Hagege A., Bel A., Cholley B., Parouchev A., Cacciapuoti I., Al-Daccak R., Benhamouda N., Blons H., Agbulut O., Tosca L., Trouvin J. H., Fabreguettes J. R., Bellamy V., Charron D., Tartour E., Tachdjian G., Desnos M., Larghero J., Transplantation of human embryonic stem cell-derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J. Am. Coll. Cardiol. 71, 429–438 (2018). [DOI] [PubMed] [Google Scholar]
- 111.Cyranoski D., ‘Reprogrammed’ stem cells approved to mend human hearts for the first time. Nature 557, 619–620 (2018). [DOI] [PubMed] [Google Scholar]
- 112.Singh S., Chakravarty T., Chen P., Akhmerov A., Falk J., Friedman O., Zaman T., Ebinger J. E., Gheorghiu M., Marban L., Marban E., Makkar R. R., Allogeneic cardiosphere-derived cells (CAP-1002) in critically ill COVID-19 patients: Compassionate-use case series. Basic Res. Cardiol. 115, 36 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ng S. C., Tilg H., COVID-19 and the gastrointestinal tract: More than meets the eye. Gut 69, 973–974 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L., Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 395, 507–513 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang C., Shi L., Wang F. S., Liver injury in COVID-19: Management and challenges. Lancet Gastroenterol. Hepatol. 5, 428–430 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.X. Chai, L. Hu, Y. Zhang, W. Han, Z. Lu, A. Ke, J. Zhou, G. Shi, N. Fang, J. Fan, J. Cai, J. Fan, F. Lan, Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bioRxiv 2020.2002.2003.931766. [Preprint]. 4 February 2020. 10.1101/2020.02.03.931766. [DOI]
- 117.Wong S. H., Lui R. N., Sung J. J., Covid-19 and the digestive system. J. Gastroenterol. Hepatol. 35, 744–748 (2020). [DOI] [PubMed] [Google Scholar]
- 118.Feng G., Zheng K. I., Yan Q.-Q., Rios R. S., Targher G., Byrne C. D., Poucke S. V., Liu W.-Y., Zheng M.-H., COVID-19 and liver dysfunction: Current insights and emergent therapeutic strategies. J. Clin. Transl. Hepatol. 8, 18–24 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huebert R. C., Rakela J., Cellular therapy for liver disease. Mayo Clin. Proc. 89, 414–424 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Strom S. C., Fisher R. A., Thompson M. T., Sanyal A. J., Cole P. E., Ham J. M., Posner M. P., Hepatocyte transplantation as a bridge to orthotopic liver transplantation in terminal liver failure. Transplantation 63, 559–569 (1997). [DOI] [PubMed] [Google Scholar]
- 121.Ronco C., Reis T., Husain-Syed F., Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 8, 738–742 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pei G., Zhang Z., Peng J., Liu L., Zhang C., Yu C., Ma Z., Huang Y., Liu W., Yao Y., Zeng R., Xu G., Renal involvement and early prognosis in patients with COVID-19 pneumonia. J. Am. Soc. Nephrol. 31, 1157–1165 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.B. Diao, C. Wang, R. Wang, Z. Feng, J. Zhang, H. Yang, Y. Tan, H. Wang, C. Wang, L. Liu, Y. Liu, Y. Liu, G. Wang, Z. Yuan, X. Hou, L. Ren, Y. Wu, Y. Chen, Human kidney is a target for novel severe acute respiratory syndrome coronavirus 2 infection. 12, 2506 (2020). [DOI] [PMC free article] [PubMed]
- 124.Rota C., Morigi M., Imberti B., Stem cell therapies in kidney diseases: Progress and challenges. Int. J. Mol. Sci. 20, 2790 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Swaminathan M., Stafford-Smith M., Chertow G. M., Warnock D. G., Paragamian V., Brenner R. M., Lellouche F., Fox-Robichaud A., Atta M. G., Melby S., Mehta R. L., Wald R., Verma S., Mazer C. D.; ACT-AKI investigators , Allogeneic mesenchymal stem cells for treatment of AKI after cardiac surgery. J. Am. Soc. Nephrol. 29, 260–267 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Terpos E., Ntanasis-Stathopoulos I., Elalamy I., Kastritis E., Sergentanis T. N., Politou M., Psaltopoulou T., Gerotziafas G., Dimopoulos M. A., Hematological findings and complications of COVID-19. Am. J. Hematol. 95, 834–847 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oxley T. J., Mocco J., Majidi S., Kellner C. P., Shoirah H., Singh I. P., De Leacy R. A., Shigematsu T., Ladner T. R., Yaeger K. A., Skliut M., Weinberger J., Dangayach N. S., Bederson J. B., Tuhrim S., Fifi J. T., Large-vessel stroke as a presenting feature of Covid-19 in the young. N. Engl. J. Med. 382, e60 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Klok F. A., Kruip M. J. H. A., van der Meer N. J. M., Arbous M. S., Gommers D. A. M. P. J., Kant K. M., Kaptein F. H. J., van Paassen J., Stals M. A. M., Huisman M. V., Endeman H., Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb. Res. 191, 145–147 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang N., Li D., Wang X., Sun Z., Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 18, 844–847 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Connors J. M., Levy J. H., COVID-19 and its implications for thrombosis and anticoagulation. Blood 135, 2033–2040 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Varga Z., Flammer A. J., Steiger P., Haberecker M., Andermatt R., Zinkernagel A. S., Mehra M. R., Schuepbach R. A., Ruschitzka F., Moch H., Endothelial cell infection and endotheliitis in COVID-19. Lancet 395, 1417–1418 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sietsema W. K., Kawamoto A., Takagi H., Losordo D. W., Autologous CD34+ cell therapy for ischemic tissue repair. Circ. J. 83, 1422–1430 (2019). [DOI] [PubMed] [Google Scholar]
- 133.Abd-Allah S. H., Shalaby S. M., Abd-Elbary E., Saleh A. A., El-Magd M. A., Human peripheral blood CD34+ cells attenuate oleic acid-induced acute lung injury in rats. Cytotherapy 17, 443–453 (2015). [DOI] [PubMed] [Google Scholar]
- 134.Huang X., Sun K., Zhao Y. D., Vogel S. M., Song Y., Mahmud N., Zhao Y. Y., Human CD34+ progenitor cells freshly isolated from umbilical cord blood attenuate inflammatory lung injury following LPS challenge. PLOS ONE 9, e88814 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hassanpour M., Rezaie J., Nouri M., Panahi Y., The role of extracellular vesicles in COVID-19 virus infection. Infect. Genet. Evol. 85, 104422 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rezakhani L., Kelishadrokhi A. F., Soleimanizadeh A., Rahmati S., Mesenchymal stem cell (MSC)-derived exosomes as a cell-free therapy for patients Infected with COVID-19: Real opportunities and range of promises. Chem. Phys. Lipids 234, 105009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pironti G., Andersson D. C., Lund L. H., Mechanistic and therapeutic implications of extracellular vesicles as a potential link between Covid-19 and cardiovascular disease manifestations. Front. Cell Dev. Biol. 9, 640723 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Pocsfalvi G., Mammadova R., Ramos Juarez A. P., Bokka R., Trepiccione F., Capasso G., COVID-19 and extracellular vesicles: An intriguing interplay. Kidney Blood Press. Res. 45, 661–670 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Popplewell L. L., Forman S. J., Is there an upper age limit for bone marrow transplantation? Bone Marrow Transplant. 29, 277–284 (2002). [DOI] [PubMed] [Google Scholar]
- 140.Golpanian S., El-Khorazaty J., Mendizabal A., DiFede D. L., Suncion V. Y., Karantalis V., Fishman J. E., Ghersin E., Balkan W., Hare J. M., Effect of aging on human mesenchymal stem cell therapy in ischemic cardiomyopathy patients. J. Am. Coll. Cardiol. 65, 125–132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Brave H., MacLoughlin R., State of the art review of cell therapy in the treatment of lung disease, and the potential for aerosol delivery. Int. J. Mol. Sci. 21, 6435 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mahendiratta S., Bansal S., Sarma P., Kumar H., Choudhary G., Kumar S., Prakash A., Sehgal R., Medhi B., Stem cell therapy in COVID-19: Pooled evidence from SARS-CoV-2, SARS-CoV, MERS-CoV and ARDS: A systematic review. Biomed. Pharmacother. 137, 111300 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pati S., Gerber M. H., Menge T. D., Wataha K. A., Zhao Y., Baumgartner J. A., Zhao J., Letourneau P. A., Huby M. P., Baer L. A., Salsbury J. R., Kozar R. A., Wade C. E., Walker P. A., Dash P. K., Cox C. S. Jr., Doursout M. F., Holcomb J. B., Bone marrow derived mesenchymal stem cells inhibit inflammation and preserve vascular endothelial integrity in the lungs after hemorrhagic shock. PLOS ONE 6, e25171 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Premer C., Blum A., Bellio M. A., Schulman I. H., Hurwitz B. E., Parker M., Dermarkarian C. R., DiFede D. L., Balkan W., Khan A., Hare J. M., Allogeneic mesenchymal stem cells restore endothelial function in heart failure by stimulating endothelial progenitor cells. EBioMedicine 2, 467–475 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boeke J. D., Church G., Hessel A., Kelley N. J., Arkin A., Cai Y., Carlson R., Chakravarti A., Cornish V. W., Holt L., Isaacs F. J., Kuiken T., Lajoie M., Lessor T., Lunshof J., Maurano M. T., Mitchell L. A., Rine J., Rosser S., Sanjana N. E., Silver P. A., Valle D., Wang H., Way J. C., Yang L., The Genome Project-write. Science 353, 126–127 (2016). [DOI] [PubMed] [Google Scholar]
- 146.Ng A. H. M., Khoshakhlagh P., Rojo Arias J. E., Pasquini G., Wang K., Swiersy A., Shipman S. L., Appleton E., Kiaee K., Kohman R. E., Vernet A., Dysart M., Leeper K., Saylor W., Huang J. Y., Graveline A., Taipale J., Hill D. E., Vidal M., Melero-Martin J. M., Busskamp V., Church G. M., A comprehensive library of human transcription factors for cell fate engineering. Nat. Biotechnol. 39, 510–519 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]


