Abstract
OBJECTIVES:
Few studies have reported the complications and outcomes of patients with Legionella pneumonia requiring ICU admission. The objective of our study is to report the clinical course, complications, and 30-day mortality of patients with Legionella pneumonia admitted to the critical care units at our medical center over a 10-year period.
DESIGN:
Retrospective observational study.
SETTING:
Tertiary care teaching hospital.
PATIENTS:
All adult (≥ 18 yr old) patients with Legionella pneumonia admitted to the ICUs from January 1, 2010, to December 31, 2019.
INTERVENTIONS:
None.
MEASUREMENTS AND MAIN RESULTS:
A total of 88 patients with Legionella pneumonia were admitted to ICUs over the 10-year period. The majority of infections (n = 80; 90.9%) were community acquired. The median (interquartile range) age of patients was 60 years (51.5–71.0 yr); 58 (66%) were male, and 41 (46.6%) identified their race as Black. The median (interquartile range) Sequential Organ Failure Assessment score at ICU admission was 6 (3–9). The distribution of infections showed seasonal dominance with most cases (86%) occurring in the summer to early fall (May to October). Invasive mechanical ventilation was required in 62 patients (70.5%), septic shock developed in 57 patients (64.8%), and acute respiratory distress syndrome developed in 42 patients (47.7%). A majority of patients developed acute kidney injury (n = 69; 78.4%), with 15 (21.7%) receiving only intermittent hemodialysis and 15 (21.7%) requiring continuous renal replacement therapy. Ten patients required venovenous extracorporeal membrane oxygenation support; eight (80%) survived and were successfully decannulated. Overall 30-day mortality was 26.1% (n = 23). Advanced age, higher Sequential Organ Failure Assessment score at admission, and not receiving Legionella-specific antimicrobial therapy within 24 hours of hospital admission were predictors of 30-day mortality.
CONCLUSIONS:
Patients with Legionella pneumonia may require ICU admission and major organ support. Legionella-targeted antibiotics should be included in the empiric regimen for any patient with severe pneumonia. Outcomes of extracorporeal membrane oxygenation therapy in this population are encouraging.
Keywords: intensive care unit, Legionella, pneumonia, respiratory, septic, shock
Legionnaires’ disease (LD) is a severe, potentially fatal form of pneumonia caused by Legionella species, comprising approximately 1% cases of community-acquired pneumonia (CAP) per recent studies (1, 2). It was first identified in 1976 when an outbreak of pneumonia occurred among the members of the American Legion attending their annual convention (3). The illness spreads through the aspiration and inhalation of infected aerosols from contaminated natural or man-made water supplies (4). The clinical and radiological presentation of LD is nonspecific and can mimic other types of pneumonia (5). Symptoms include fever, cough, chills, dyspnea, and pleuritic chest pain; however, the presence of gastrointestinal (nausea, vomiting, diarrhea, abdominal pain) and/or neurologic (headache, altered mental status) symptoms might raise suspicion that Legionella is the causative organism of the illness (1, 6).
Although the crude national frequency of LD increased by 5.5-fold from 0.42 cases per 100,000 persons in 2000 to 2.29 per 100,000 persons in 2017, the condition is still underdiagnosed and underreported (7). Patients with chronic lung disease, smoking history, age over 50 years, and immunocompromised state (chronic steroid use, solid tumors, hematologic malignancies) are at increased risk of contracting LD (5).
Few studies have reported the complications and outcomes of patients with Legionella pneumonia requiring ICU admission (8–16). Acute respiratory failure, septic shock, acute kidney injury (AKI), and rhabdomyolysis have been described in ICU patients with Legionella. The mortality rate for patients with Legionella pneumonia in the ICU varies between 9.1% and 41.7% (8–13). The aim of this study was to describe our institutional experience and report the characteristics, clinical course, complications, and 30-day mortality of patients with Legionella pneumonia admitted to the critical care units at our medical center over a 10-year period.
MATERIALS AND METHODS
Study Design
We performed a retrospective observational study of all adult (≥ 18 yr old) patients with the diagnosis of Legionella pneumonia admitted to the critical care units of the three hospitals comprising the Montefiore Healthcare System (Bronx, NY). The diagnosis of Legionella pneumonia was made by positive urinary antigen test, positive respiratory culture, or both for Legionella species in ICU patients admitted for evaluation of pneumonia. Electronic health records for the 10-year period from January 1, 2010, to December 31, 2019, were retrospectively analyzed incorporating Looking Glass Clinical Analytics (Streamline Health, Atlanta, GA) to identify the target population. The study was approved by the Institutional Review Board (IRB) of the Albert Einstein College of Medicine (IRB number 2018-9728), and waiver of informed consent was granted due to minimal risk. Data regarding patient demographics, baseline characteristics, laboratory values, hospital course, complications, and outcomes were collected for enrolled subjects.
Study Definitions
Community-acquired cases were defined as patients with Legionella pneumonia diagnosed at or within 48 hours of hospital admission and were not hospitalized or residing in a healthcare facility for greater than or equal to 14 days prior to the onset of symptoms (17).
Nosocomial cases were defined as patients with Legionella pneumonia that developed infection 48 hours or more after hospital admission, was not incubating at the time of hospital admission, and patients were not hospitalized or residing in a healthcare facility for greater than or equal to 14 days prior to the onset of symptoms (18).
Statistical Analysis
Continuous variables were reported as mean and sd or median and interquartile range (IQR), whereas categorical variables were reported as counts and percentages. Associations between continuous/categorical variables and 30-day mortality were tested via Student t test, Wilcoxon Mann-Whitney U test, or chi-square/Fisher exact test, as appropriate. An exploratory multivariable model for the probability of 30-day survival was selected after exhaustive search of the variable space consisting of patient characteristics, admission laboratory values, and hospital treatment. The final model presented is representative of other similarly ranked models (based on score statistic). All analyses were performed using SAS software, Version 9.4 (SAS Institute, Cary, NC). A two-sided p value of 0.05 or less was considered statistically significant.
RESULTS
Patient Characteristics
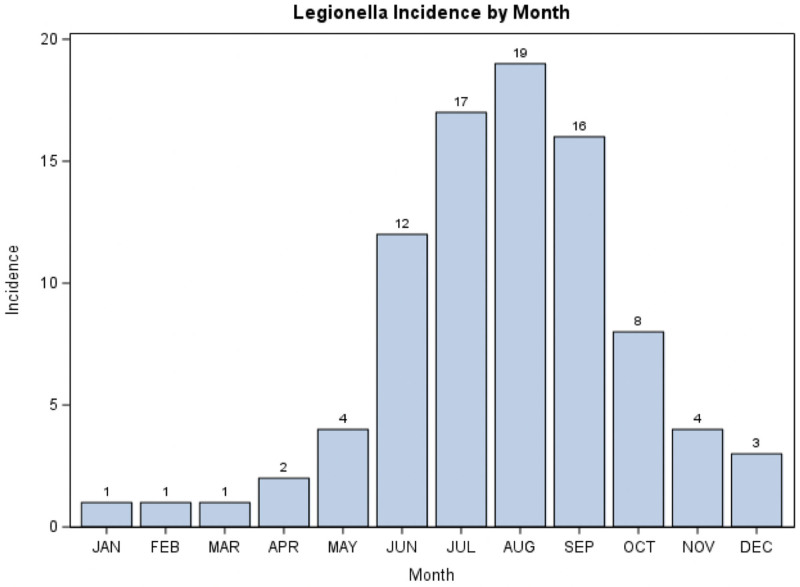
A total of 88 patients with Legionella pneumonia were admitted to critical care units at our institution over the 10-year period evaluated. The majority of infections (n = 80; 90.9%) were community acquired; eight (9.1%) were nosocomial. The median (IQR) age of patients was 60 years (51.5–71.0 yr); 58 (66%) were male, and 41 (46.6%) identified their race as Black. The median (IQR) Sequential Organ Failure Assessment (SOFA) score on presentation was 6 (3–9) and was significantly higher in 30-day nonsurvivors than survivors (p = 0.002). Diabetes mellitus (n = 30; 34.1%) and chronic kidney disease (n = 22; 25%) were the major comorbid conditions in our study cohort. Forty patients (45.5%) had a history of current or prior smoking. The distribution of infections showed seasonal dominance with most cases (86%) occurring in the summer to early fall (May to October) (Fig. 1). Most of the diagnoses were made by positive urine Legionella antigen testing in 82 (93%); however, six patients had positive respiratory culture results with negative (n = 4) or no (n = 2) urine antigen results. Three of four patients with negative urine Legionella antigen but positive culture results did not survive. Positive urine antigen determination is limited to Legionella pneumophila serogroup 1. Twenty-nine of 35 positive respiratory culture results were L. pneumophila serogroup 1, one was serogroup 6, and five were of unreported/unidentified serogroup. Table 1 lists the baseline characteristics of our patients.
Figure 1.
Legionella frequency by month (2010–2019).
TABLE 1.
Baseline Characteristics of Patients
| Variable | Total (N = 88) | Survivor at 30 d (N = 65) | Nonsurvivor at 30 d (N = 23) | p a |
|---|---|---|---|---|
| Age, median (IQR) | 60 (51.5–71.0) | 58 (51–66) | 68 (60–82) | 0.001 |
| Sex, n (%) | ||||
| Female | 30 (34.1) | 22 (33.8) | 8 (34.8) | 1.0 |
| Male | 58 (65.9) | 43 (66.2) | 15 (65.2) | |
| Race, n (%) | 0.79 | |||
| Black/African-American | 41 (46.6) | 32 (49.2) | 9 (39.1) | |
| White | 18 (20.5) | 12 (18.5) | 6 (26.1) | |
| Other | 29 (33.0) | 21 (32.4) | 8 (34.7) | |
| Sequential Organ Failure Assessment score at admission, median (IQR) | 6 (3–9) | 5 (3–7) | 9 (5–10) | 0.002 |
| Admission origin, n (%) | 0.15 | |||
| Floor | 39 (44.3) | 32 (49.2) | 7 (30.4) | |
| Emergency department | 49 (55.7) | 33 (50.8) | 16 (69.6) | |
| Chronic obstructive pulmonary disease, n (%) | 14 (15.9) | 10 (15.4) | 4 (17.4) | 1.0 |
| Diabetes mellitus, n (%) | 30 (34.1) | 19 (29.2) | 11 (47.8) | 0.13 |
| Chronic kidney disease, n (%) | 22 (25.0) | 14 (21.5) | 8 (34.8) | 0.26 |
| Chronic liver failure, n (%) | 4 (4.5) | 2 (3.1) | 2 (8.7) | 0.28 |
| Neoplasm, n (%) | 13 (14.8) | 9 (13.8) | 4 (17.4) | 0.74 |
| Immunocompromised, n (%) | 22 (25.0) | 17 (26.2) | 5 (21.7) | 0.78 |
| HIVb, n (%) | 6 | 5 (7.7) | 1 (4.4) | 0.46 |
| Alcoholism, n (%) | 14 (15.9) | 11 (16.9) | 3 (13.0) | 1.0 |
| Smoking (current/past), n (%) | 40 (45.5) | 33 (50.8) | 7 (30.4) | 0.14 |
| Respiratory symptoms | 75 (78.3) | 57 (87.7) | 18 (78.3) | 0.27 |
| Gastro-intestinal symptoms | 51 (58.0) | 9 (39.1) | 42 (64.6) | 0.05 |
| Neurologic symptoms | 55 (62.5) | 16 (69.6) | 39 (60.0) | 0.42 |
| Diagnosis, n (%) | ||||
| Urine antigen +/respiratory culture + | 29 (33.0) | 22 (33.9) | 7 (30.4) | 1.0 |
| Urine antigen –/respiratory culture + | 4 (4.6) | 1 (1.5) | 3 (13.0) | 0.05 |
| Urine antigen +/respiratory culture – | 29 (33.0) | 21 (32.3) | 8 (34.8) | 1.0 |
| No antigen/respiratory culture + | 2 (2.3) | 1 (1.5) | 1 (4.4) | 0.46 |
| Antigen +/no respiratory culture | 24 (27.3) | 20 (30.8) | 4 (17.4) | 0.28 |
| Legionella pneumophila serogroup, n (%) | 0.08 | |||
| Serogroup 1 | 29 (33.0) | 19 (29.2) | 10 (43.5) | |
| Serogroup 6 | 1 (1.1) | 0 (0.0) | 1 (4.4) | |
| Unknown | 58 (65.9) | 46 (70.8) | 12 (52.2) |
IQR = interquartile range, n = available sample size.
aFisher exact test (categorical), two-sample t test (continuous, normal), or Wilcoxon Mann-Whitney U test (continuous, nonnormal).
bn = 10 nonsurvivors and 25 survivors are missing HIV status data.
ICU Course and Complications
Invasive mechanical ventilation (IMV) was required in 62 patients (70.5%), septic shock developed in 57 patients (64.8%), and acute respiratory distress syndrome (ARDS) developed in 42 patients (47.7%). Of the 62 patients needing IMV, 26 (41.9%) initially received a trial of noninvasive ventilatory (NIV) support (high-flow nasal cannula or bilevel positive airway pressure ventilation) and failed. The median (IQR) duration of NIV support was 1 day (1–2 d), with the duration of the trial determined by the clinical judgment of the treatment team. A majority of patients developed AKI (n = 69; 78.4%), with 15 (21.7%) receiving only intermittent hemodialysis and 15 (21.7%) requiring continuous renal replacement therapy. Need for IMV, septic shock, and ARDS was significantly higher in nonsurvivors than survivors (p = 0.0001, 0.0002, 0.02, respectively). Ten patients required venovenous extracorporeal membrane oxygenation (VV-ECMO) support; eight (80%) survived and were successfully decannulated. Table 2 describes the ICU complications and outcomes; Table 3 provides characteristics of patients with Legionella on extracorporeal membrane oxygenation (ECMO); Table 4 shows selected laboratory data.
TABLE 2.
ICU Complications and Outcomes
| Variable | Total (N = 88) | Survivor at 30 d (N = 65) | Nonsurvivor at 30 d (N = 23) | p a |
|---|---|---|---|---|
| Invasive mechanical ventilation, n (%) | 62 (70.5) | 39 (60.0) | 23 (100) | 0.0001 |
| Noninvasive ventilation only (high-flow nasal cannula, bilevel positive airway pressure), n (%) | 18 (20.5%) | 18 (27.7) | 0 (0.0) | 0.001 |
| Trial of noninvasive ventilation before intubation, n (%) | 26/62 (41.9) | 15/39 (62.9) | 11/23 (37.1) | 0.60 |
| Septic shock, n (%) | 57 (64.8) | 35 (53.8) | 22 (95.7) | 0.0002 |
| Acute respiratory distress syndrome, n (%) | 42 (47.7) | 26 (40.0) | 16 (69.6) | 0.02 |
| Acute kidney injury, n (%) | 69 (78.4) | 50 (76.9) | 19 (82.6) | 0.77 |
| Required intermittent hemodialysis | 15 (21.7) | 13 (26.0) | 2 (10.5) | 0.21 |
| Required continuous renal replacement therapy | 15 (21.7) | 8 (16.0) | 7 (36.8) | 0.10 |
| Rhabdomyolysis, n (%) | 37 (42.0) | 28 (43.1) | 9 (39.1) | 0.81 |
| Acute liver failure, n (%) | 15 (17.0) | 6 (9.2) | 9 (39.1) | 0.003 |
| Bacteremia/fungemia, n (%) | 9 (10.2) | 4 (6.2) | 5 (21.7) | |
| Pleural effusion, n (%) | 52 (59.1) | 36 (55.4) | 16 (69.6) | 0.32 |
| Arrhythmias, n (%) | 28 (31.8) | 16 (24.6) | 12 (52.2) | 0.02 |
| Chest radiograph infiltrate, n (%) | 1.0 | |||
| Unilateral | 42 (47.7) | 34 (52.3) | 12 (52.2) | |
| Bilateral | 46 (52.3) | 31 (47.7) | 11 (47.8) | |
| Antibiotics received, n (%) | 0.03 | |||
| Fluoroquinolone | 58 (65.9) | 48 (73.8) | 10 (43.5) | |
| Macrolide | 19 (21.6) | 11 (16.9) | 8 (34.8) | |
| Both | 11 (12.5) | 6 (9.2) | 5 (21.7) | |
| Legionella-specific antibiotic, n (%) | 0.005 | |||
| Within first 24 hr of admission | 57 (64.8) | 48 (73.8) | 9 (39.1) | |
| After 24 hr of admission | 31 (35.2) | 17 (16.2) | 14 (60.9) | |
| Pneumonia type, n (%) | 0.03 | |||
| Community acquired | 80 (90.9) | 62 (95.4) | 18 (78.3) | |
| Nosocomial | 8 (9.1) | 3 (4.6) | 5 (21.7) | |
| Extracorporeal membrane oxygenation, n (%) | 10 (11) | 8 (12) | 2 (9) | 1.0 |
| Tracheostomy, n (%) | 7 (8.0) | 6 (9.2) | 1 (4.3) | 0.67 |
| Hospital days, median (IQR)b | 8.0 (4.0–18.0) | 20 (12–30) | 8 (4–18) | 0.0001 |
| ICU days, median (IQR)b | 7.5 (4.0–13.5) | 10 (4–14) | 6 (2–10) | 0.06 |
| Ventilator days (n = 62)b | 4.0 (2.0–10.0) | 10 (7–17) | 4 (2–10) | 0.002 |
IQR = interquartile range, n = available sample size.
A two-sided p-value of 0.05 or less was considered significant and indicated in bold.
aFisher exact test (categorical), two-sample t test (continuous, normal), or Wilcoxon Mann-Whitney U test (continuous, nonnormal).
bIn nonsurvivors, summarizes the length of time before death (if prior to discharge or extubation).
TABLE 3.
Legionella Extracorporeal Membrane Oxygenation Outcomes (All Patients Received Venovenous Extracorporeal Membrane Oxygenation)
| Patient ID | Age (yr) | Sex | Sequential Organ Failure Assessment Score | Ventilator Days | ICU LOS (d) | Hospital LOS (d) | Extracorporeal Membrane Oxygenation Days | Survival |
|---|---|---|---|---|---|---|---|---|
| 1 | 56 | Female | 6 | 9 | 10 | 14 | 2 | Yes |
| 2 | 36 | Male | 6 | 30 | 37 | 40 | 10 | Yes |
| 3 | 41 | Female | 9 | 12 | 11 | 33 | 9 | Yes |
| 4 | 64 | Female | 6 | 7 | 7 | 35 | 5 | No |
| 5 | 59 | Male | 12 | 9 | 18 | 20 | 8 | Yes |
| 6 | 39 | Female | 8 | 16 | 15 | 19 | 2 | No |
| 7 | 53 | Male | 10 | 8 | 6 | 30 | 5 | Yes |
| 8 | 44 | Male | 12 | 14 | 13 | 21 | 7 | Yes |
| 9 | 48 | Female | 17 | 7 | 16 | 58 | 10 | Yes |
| 10 | 58 | Male | 11 | 2 | 11 | 12 | 9 | Yes |
LOS = length of stay.
Adapted with permission from Naqvi et al (19).
TABLE 4.
Laboratory Results
| Laboratory Test | Total (N = 88) | Survivor at 30 d (N = 65) | Nonsurvivor at 30 d (N = 23) | p a |
|---|---|---|---|---|
| Peak creatine phosphokinase, median (IQR) (U/L) | 1,214.5 (248.5–3,768.0) | 1,304 (267–5,574) | 683 (205–2,705) | 0.34 |
| WBC at admission, mean (sd) (K/µL) | 15.1 (6.6) | 15.3 (5.8) | 14.8 (8.6) | 0.67 |
| Sodium at admission, mean (sd) (mEq/L) | 133.8 (5.6) | 133.5 (6.1) | 134.6 (4.2) | 0.48 |
| Blood urea nitrogen, median (IQR) (mg/dL) | 30 (19–56) | 26.5 (18–47.5) | 48 (27–74) | 0.01 |
| Creatinine, median (IQR) (mg/dL) | 1.9 (1.2–3.9) | 1.7 (1.2–3.3) | 2.4 (1.5–4.6) | 0.20 |
| Alanine transaminase, median (IQR) (U/L) | 35 (19–77) | 41 (22.5–86.5) | 23 (12–34) | 0.01 |
| Aspartate transaminase, median (IQR) (U/L) | 68 (34–126) | 83 (37–136) | 39 (33–90) | 0.16 |
| Total bilirubin, median (IQR) (mg/dL) | 0.8 (0.5–1.4) | 0.8 (0.5–1.2) | 0.9 (0.5–1.7) | 0.35 |
IQR = interquartile range.
A two-sided p-value of 0.05 or less was considered significant and indicated in bold.
aFisher exact test (categorical), two-sample t test (continuous, normal), or Wilcoxon Mann-Whitney U test (continuous, nonnormal).
Antibiotic Therapy and Outcomes
Legionella-specific antimicrobial therapy in our subjects included fluoroquinolones (n = 58; 65.9%, levofloxacin or moxifloxacin), macrolides (n = 19; 21.6%, azithromycin), and a combination of both (n = 11; 12.5%). Thirty-one patients (35.2%) did not receive Legionella-specific microbial coverage within the first 24 hours of hospital admission. Median (IQR) ventilator days was 4 (2–10), ICU length of stay (LOS) was 7.5 days (4–13.5 d), and hospital LOS was 8 days (4–18 d). In all cases, duration was significantly shorter in nonsurvivors than survivors.
Overall 30-day mortality was 26.1% (n = 23). Advanced age, higher SOFA score at admission, and not receiving Legionella-specific antimicrobial therapy within 24 hours of hospital admission were predictors of 30-day mortality in multivariate analysis (Table 5). Receiving a fluoroquinolone (singularly or in combination with a macrolide) was associated with higher odds of 30-day survival; however, this was not statistically significant.
TABLE 5.
Multivariate Model for the Probability of Survival at 30 Days
| Effect | OR | 95% Wald Confidence Limits | p | |
|---|---|---|---|---|
| Age | 0.936 | 0.892 | 0.982 | 0.007 |
| Sequential Organ Failure Assessment score at admission | 0.752 | 0.624 | 0.906 | 0.003 |
| Antibiotics within 24 hr of admission | 3.619 | 1.064 | 12.306 | 0.04 |
| Fluoroquinolone received (reference: macrolide only) | 3.012 | 0.763 | 11.900 | 0.12 |
OR = odds ratio.
The C-statistic associated with this model is 0.83. Advanced age and higher SOFA scores are strongly predictive of 30-d postadmission mortality. Antibiotics received within 24 hr are marginally associated with 30-d survival. Receiving fluoroquinolone (singularly or in combination with macrolide) was associated with the higher odds of 30-d survival; however, this was not statistically significant.
DISCUSSION
To our knowledge, this is the largest single-center retrospective observational study describing the characteristics, clinical course, complications, and outcomes of critically ill patients with Legionella pneumonia admitted to an ICU. A majority of the patients in our cohort developed multiple organ failure and septic shock requiring organ support. There is a high reported frequency of severe acute respiratory failure necessitating IMV in the ICU setting, between 54.5% and 82.3% according to published reports (11–13, 15). Sixty-two patients (70.5%) in our study required invasive mechanical ventilatory support; most developed ARDS and multifocal pneumonias. Interestingly, we also report a high failure rate of NIV support (high-flow nasal cannula or bilevel positive airway pressure ventilation) in this population as 42% of our mechanically ventilated patients had a failed trial of NIV prior to intubation. Overall, 59% of patients (26/44) who received NIV progressed to requirement of IMV. Patients with severe respiratory failure should be considered for ECMO support as the reported outcomes of this therapeutic intervention in patients with severe Legionella are very encouraging with survival rates of up to 78.1% (19). Our 10-year institutional experience yielded a survival rate of 80% for patients requiring VV-ECMO.
We observed a high (78%) frequency of AKI in this population. Multiple previous reports have studied the association of Legionella pneumonia, rhabdomyolysis, and renal failure (20, 21–24). The reported frequency of AKI in Legionella pneumonia ranges from 13% to 15%; etiology is probably multifactorial: rhabdomyolysis, hypovolemia due to gastrointestinal fluid losses, sepsis, and possible direct injury by the Legionella organism itself (21, 22). Presence of AKI with Legionella pneumonia has previously been associated with poor prognosis and higher mortality (23, 24). In a prospective study of 84 patients with Legionella in the ICU, el-Ebiary (11) reported serum creatinine greater than or equal to 1.8 mg/dL as a poor prognostic factor. One of the earliest studies in 17 critically ill patients with Legionella pneumonia reported the frequency of AKI to be 47% (8/17), seven of eight required renal replacement therapy, and all except one returned to normal renal function by a mean of 3 weeks (12).
Our observed 30-day mortality was 26.1%. Advanced age, higher SOFA scores, and a delay in Legionella-specific antibiotic therapy were predictors of 30-day mortality. A prospective study of 84 critically ill patients with Legionella observed over a 9-year period reported a crude mortality rate of 30%; the authors showed that Acute Physiology and Chronic Health Evaluation 2 score greater than 15 at admission, serum sodium level less than or equal to 136 mEq/L, presence of diabetes mellitus or cardiac disease, septic shock, mechanical ventilation, and acute renal failure were associated with poor outcomes (11). They reported no differences in mortality between community-acquired and nosocomial groups. A Dutch study of 17 patients with Legionella in the ICU reported a low mortality rate of 17.6% (12). This is probably due to underdiagnosis of Legionella in that study as the diagnosis was made using a serological test which took weeks to result. Other series have reported a mortality rate ranging from 9.1% to as high as 41.7% for patients with Legionella in the ICU (8, 9, 10, 13, 15). A recent report from China describes the outcomes of 34 patients with Legionella pneumonia in the ICU (10). Mortality in that study was 29%, and predictors of mortality also included higher SOFA score at admission and delay in initiation of Legionella-targeted treatment from the time of onset of pneumonia. Falcone et al (9), in a retrospective study on 116 patients with Legionella pneumonia in Italy, found that receipt of macrolide or levofloxacin therapy within 24 hours of hospital admission was protective against clinical deterioration and need for ICU admission. A few other observational studies also suggest higher mortality in patients with Legionella pneumonia when there is a delay in initiation of Legionella-targeted therapy (9, 10, 16, 25–28). We report similar findings from our study as patients who received a Legionella-specific antibiotic regimen within the first 24 hours of presentation had higher 30-day survival compared with those who received appropriate antibiotics only after 24 hours. We observed improved survival with fluoroquinolones compared with macrolides, but this trend did not reach statistical significance.
The Infectious Disease Society of America and American Thoracic Society guidelines recommend against routine urine antigen testing for Legionella except in severe CAP patients or when associated with a Legionella outbreak or recent travel (29). The guidelines also recommend empiric use of either a fluoroquinolone or macrolide for the treatment of CAP in the inpatient setting to provide Legionella coverage (29). A recent retrospective analysis of a large healthcare database revealed that only 27% of ICU patients with pneumonia were tested for Legionella and 77% of inpatients received empiric Legionella therapy (30).
Gershengorn et al (31) and Garcia-Vidal et al (32) found no differences in hospital mortality with the use of azithromycin alone or fluoroquinolone alone for the treatment of Legionella pneumonia. Combination therapy with fluoroquinolone and macrolide antibiotics also did not improve outcomes (33), although Rello et al (13) reported lower mortality with combination therapy in patients with Legionella with severe CAP and shock.
Of note, six patients in our study with Legionella pneumonia were diagnosed by positive respiratory culture results but had negative or no urine antigen tests. Hospitals often diagnose Legionella with a urine antigen test which is highly sensitive for serogroup 1 but can fail to identify other serogroups, as well as other species of Legionella such as L. longbeachae. Sputum culture for Legionella pneumonia has a range of reported sensitivities from less than 10% to 80%, but its specificity approaches 100% (34, 35). It is imperative to send sputum, endotracheal aspirate, or bronchoalveolar lavage samples for Legionella culture in addition to urine antigen testing to avoid missing the diagnosis.
A major strength of our study is that we describe the largest single-center retrospective ICU experience of Legionella pneumonia patients, observed over a 10-year period. Our study has a few limitations that should be acknowledged. First, it is a single-center study. Second, it is retrospective in nature, and hence, some data elements may not be captured accurately. Third, Legionella pneumonia cases could have been missed if appropriate diagnostics tests were not sent by the treating physicians. Fourth, our study presents a tertiary care referral center experience; the clinical features and mortality observed in our cohort of Legionella patients likely present a bias toward the sickest of these patients and may not be representative of the Legionella cases as a whole.
CONCLUSIONS
Patients with Legionella pneumonia may require ICU admission and major organ support with a frequent requirement of IMV and vasopressor therapy. There is a high failure rate of NIV. Incidence of AKI is extremely high (78%). We observed a 30-day mortality of 26.1%, similar to prior studies. Advanced age, higher SOFA scores at ICU admission, and failure to initiate Legionella-specific antibiotic therapy are associated with worse outcomes. Legionella respiratory culture should be routinely sent as part of the initial evaluation of suspected Legionella pneumonia in patients with severe pneumonia. Legionella-targeted antibiotics should be included in the empiric regimen for any patient with severe pneumonia. Outcomes of ECMO therapy in this population are encouraging.
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
REFERENCES
- 1.Cunha BA, Burillo A, Bouza E. Legionnaires’ disease. Lancet. 2016; 387:376–385 [DOI] [PubMed] [Google Scholar]
- 2.Musher DM, Abers MS, Bartlett JG. Evolving understanding of the causes of pneumonia in adults, with special attention to the role of pneumococcus. Clin Infect Dis. 2017; 65:1736–1744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fraser DW, Tsai TR, Orenstein W, et al. Legionnaires’ disease: Description of an epidemic of pneumonia. N Engl J Med. 1977; 297:1189–1197 [DOI] [PubMed] [Google Scholar]
- 4.Burillo A, Pedro-Botet ML, Bouza E. Microbiology and epidemiology of Legionnaire’s disease. Infect Dis Clin North Am. 2017; 31:7–27 [DOI] [PubMed] [Google Scholar]
- 5.Chahin A, Opal SM. Severe pneumonia caused by Legionella pneumophila: Differential diagnosis and therapeutic considerations. Infect Dis Clin North Am. 2017; 31:111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sopena N, Sabrià-Leal M, Pedro-Botet ML, et al. Comparative study of the clinical presentation of Legionella pneumonia and other community-acquired pneumonias. Chest. 1998; 113:1195–1200 [DOI] [PubMed] [Google Scholar]
- 7.Legionnaires’ Disease Surveillance Summary. Report, United States 2016–2017, 2020, pp 1–50 [Google Scholar]
- 8.Irons JF, Dunn MJ, Kefala K, et al. The effect of a large Legionnaires’ disease outbreak in Southwest Edinburgh on acute and critical care services. QJM. 2013; 106:1087–1094 [DOI] [PubMed] [Google Scholar]
- 9.Falcone M, Russo A, Tiseo G, et al. Predictors of intensive care unit admission in patients with Legionella pneumonia: Role of the time to appropriate antibiotic therapy. Infection. 2021; 49:321–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He CW, Tang X, Sun B, et al. [Severe community-acquired pneumonia caused by Legionella pneumophila with acute respiratory failure: Clinical characteristics and prognosis of 34 cases]. Zhonghua Jie He He Hu Xi Za Zhi. 2020; 43:557–563 [DOI] [PubMed] [Google Scholar]
- 11.el-Ebiary M, Sarmiento X, Torres A, et al. Prognostic factors of severe Legionella pneumonia requiring admission to ICU. Am J Respir Crit Care Med. 1997; 156:1467–1472 [DOI] [PubMed] [Google Scholar]
- 12.van Riemsdijk-van Overbeeke IC, van den Berg B. Severe Legionnaire’s disease requiring intensive care treatment. Neth J Med. 1996; 49:196–201 [DOI] [PubMed] [Google Scholar]
- 13.Rello J, Gattarello S, Souto J, et al. ; Community-Acquired Pneumonia in Unidad de Cuidados Intensivos 2 CAPUCI 2 Study Investigators. Community-acquired Legionella pneumonia in the intensive care unit: Impact on survival of combined antibiotic therapy. Med Intensiva. 2013; 37:320–326 [DOI] [PubMed] [Google Scholar]
- 14.Harris NJ, Harris AC, Spiro M. Management of Legionella in the intensive care setting. BMJ Case Rep. 2011; 2011:bcr1220103587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kojicic M, Li G, Schramm GE. Acute respiratory distress syndrome in patients with Legionella pneumonia. Acta Medica Academica. 2011; 40:39–44. [Google Scholar]
- 16.Gacouin A, Le Tulzo Y, Lavoue S, et al. Severe pneumonia due to Legionella pneumophila: Prognostic factors, impact of delayed appropriate antimicrobial therapy. Intensive Care Med. 2002; 28:686–691 [DOI] [PubMed] [Google Scholar]
- 17.Bartlett JG, Dowell SF, Mandell LA, et al. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis. 2000; 31:347–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kalil AC, Metersky ML, Klompas M, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016; 63:e61–e111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naqvi A, Kapoor S, Pradhan M, et al. Outcomes of severe Legionella pneumonia requiring extracorporeal membrane oxygenation (ECMO). J Crit Care. 2021; 61:103–106 [DOI] [PubMed] [Google Scholar]
- 20.Posner MR, Caudill MA, Brass R, et al. Legionnaires’ disease associated with rhabdomyolysis and myoglobinuria. Arch Intern Med. 1980; 140:848–850 [PubMed] [Google Scholar]
- 21.Soni AJ, Peter A. Established association of Legionella with rhabdomyolysis and renal failure: A review of the literature. Respir Med Case Rep. 2019; 28:100962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sposato B, Mariotta S, Ricci A, et al. [Legionnaire’s pneumonia with rhabdomyolysis and acute renal failure. A case report]. Recenti Prog Med. 2003; 94:391–394 [PubMed] [Google Scholar]
- 23.Shah A, Check F, Baskin S, et al. Legionnaires’ disease and acute renal failure: case report and review. Clin Infect Dis. 1992;14:204–207. [DOI] [PubMed] [Google Scholar]
- 24.Nishitarumizu K, Tokuda Y, Uehara H, et al. Tubulointerstitial nephritis associated with Legionnaires’ disease. Intern Med. 2000; 39:150–153 [DOI] [PubMed] [Google Scholar]
- 25.Cargnelli S, Powis J, Tsang JL. Legionella pneumonia in the Niagara Region, Ontario, Canada: A case series. J Med Case Rep. 2016; 10:336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viasus D, Di Yacovo S, Garcia-Vidal C, et al. Community-acquired Legionella pneumophila pneumonia: A single-center experience with 214 hospitalized sporadic cases over 15 years. Medicine (Baltimore). 2013; 92:51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heath CH, Grove DI, Looke DF. Delay in appropriate therapy of Legionella pneumonia associated with increased mortality. Eur J Clin Microbiol Infect Dis. 1996; 15:286–290 [DOI] [PubMed] [Google Scholar]
- 28.Levcovich A, Lazarovitch T, Moran-Gilad J, et al. Complex clinical and microbiological effects on Legionnaires’ disease outcone; a retrospective cohort study. BMC Infect Dis. 2016; 16:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Metlay JP, Waterer GW, Long AC, et al. Diagnosis and treatment of adults with community-acquired pneumonia. An official clinical practice guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. 2019; 200:e45–e67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allgaier J, Lagu T, Haessler S, et al. Risk factors, management, and outcomes of Legionella pneumonia in a large, nationally representative sample. Chest. 2021; 159:1782–1792 [DOI] [PubMed] [Google Scholar]
- 31.Gershengorn HB, Keene A, Dzierba AL, et al. The association of antibiotic treatment regimen and hospital mortality in patients hospitalized with Legionella pneumonia. Clin Infect Dis. 2015; 60:e66–e79 [DOI] [PubMed] [Google Scholar]
- 32.Garcia-Vidal C, Sanchez-Rodriguez I, Simonetti AF, et al. Levofloxacin versus azithromycin for treating Legionella pneumonia: A propensity score analysis. Clin Microbiol Infect. 2017; 23:653–658 [DOI] [PubMed] [Google Scholar]
- 33.Cecchini J, Tuffet S, Sonneville R, et al. Antimicrobial strategy for severe community-acquired Legionnaires’ disease: A multicentre retrospective observational study. J Antimicrob Chemother. 2017; 72:1502–1509 [DOI] [PubMed] [Google Scholar]
- 34.Peci A, Winter AL, Gubbay JB. Evaluation and comparison of multiple test methods, including real-time PCR, for Legionella detection in clinical specimens. Front Public Health. 2016; 4:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Diederen BM. Legionella spp. and Legionnaires’ disease. J Infect. 2008; 56:1–12 [DOI] [PubMed] [Google Scholar]