ABSTRACT
Arbuscular mycorrhizal fungi (AMF) provide essential nutrients to crops and are critically impacted by fertilization in agricultural ecosystems. Understanding shifts in AMF communities in and around crop roots under different fertilization regimes can provide important lessons for improving agricultural production and sustainability. Here, we compared the responses of AMF communities in the rhizosphere (RS) and root endosphere (ES) of wheat (Triticum aestivum) to different fertilization treatments, nonfertilization (control), mineral fertilization only (NPK), mineral fertilization plus wheat straw (NPKS), and mineral fertilization plus cow manure (NPKM). We employed high-throughput amplicon sequencing and investigated the diversity, community composition, and network structure of AMF communities to assess their responses to fertilization. Our results elucidated that AMF communities in the RS and ES respond differently to fertilization schemes. Long-term NPK application decreased the RS AMF alpha diversity significantly, whereas additional organic amendments (straw or manure) had no effect. In contrast, NPK fertilization increased the ES AMF alpha diversity significantly, while additional organic amendments decreased it significantly. The effect of different fertilization schemes on AMF network complexity in the RS and ES were similar to their effects on alpha diversity. Changes to AMF communities in the RS and ES correlated mainly with the pH and phosphorus level of the rhizosphere soil under long-term inorganic and organic fertilization regimes. We suggest that the AMF community in the roots should be given more consideration when studying the effects of fertilization regimes on AMF in agroecosystems.
IMPORTANCE Arbuscular mycorrhizal fungi are an integral component of rhizospheres, bridging the soil and plant systems and are highly sensitive to fertilization. However, surprisingly little is known about how the response differs between the roots and the surrounding soil. Decreasing arbuscular mycorrhizal fungal diversity under fertilization has been reported, implying a potential reduction in the mutualism between plants and arbuscular mycorrhizal fungi. However, we found opposing responses to long-term fertilization managements of arbuscular mycorrhizal fungi in the wheat roots and rhizosphere soil. These results suggested that changes in the arbuscular mycorrhizal fungal community in soils do not reflect those in the roots, highlighting that the root arbuscular mycorrhizal fungal community is pertinent to understand arbuscular mycorrhizal fungi and their crop hosts’ responses to anthropogenic influences.
KEYWORDS: arbuscular mycorrhizal fungi, co-occurrence network, long-term fertilization, organic matter, wheat
INTRODUCTION
Arbuscular mycorrhizal fungi (AMF) form one of the most ancient obligate symbioses with the majority of land plants and almost all crops, including wheat (1, 2). They have a pivotal role in the productivity and sustainability of ecosystems because of their contribution to plant performance and soil function (3). Arbuscular mycorrhizal symbiosis provides plants with many benefits, including improved access to immobile nutrients (4), tolerance to abiotic stresses (5), and protection against pathogens (6). Arbuscular mycorrhizal symbiosis can also influence a range of soil properties, such as soil aggregation (7), nutrient cycling (8), and soil stability (9), and it holds great potential to improve the sustainability of agriculture (10–12).
AMF represent an intricate belowground ecosystem and are highly sensitive to fertilization. Many studies have investigated the impact of fertilization practices on AMF communities. Fertilization events lead to increased nutrient availability in the soil; therefore, it is not surprising that AMF communities are highly susceptible to such practices (13). Increasing nutrient availability by applying mineral fertilizer has been shown to suppress AMF colonization, biomass, and diversity (14–16). Moreover, long-term application of mineral fertilizer has adverse effects on soil life (11, 17). Thus, various organic materials, including straw and manure, have been recommended for combining with inorganic fertilizer to improve agricultural soil systems (18, 19). AMF have a crucial role in the nutrient cycling of organic sources (20), and the hyphae have been found to preferentially proliferate (21) and capture nitrogen from organic substrates (22). Field studies have shown that the addition of organic materials stimulates mycorrhiza formation (23, 24) and increases AMF diversity (25). However, several studies found that the application of organic amendments also inhibited AMF diversity due to nutrient surpluses (26, 27). So far, studies mainly focused on the influence of agricultural management on AMF communities in soil compartments. However, the exchange of essential nutrients between plants and fungi takes place in the roots—intraradically. Thus, shifts of AMF communities due to management in the root endosphere are of higher importance for crop performance. Until now, few studies investigated how different fertilization regimes affect AMF communities in the root endosphere, and it remains unclear if responses of AMF communities to changes in agricultural management differ between the rhizosphere and root endosphere. Insight into both mycorrhizal compartments, the intraradical and extraradical, is foundational to comprehend the response of AMF to management in agroecosystems.
Although the impact of fertilization on AMF abundance and diversity is well-known, to date, we lack a detailed assessment of the response of the AMF community composition and network structure. Network analyses are powerful tools to describe microbial community responses (28) and thus can be used to assess the impact of fertilizer treatments of AMF communities in different plant root-associated compartments. This would improve our understanding of the consequences of fertilization strategies on beneficial soil microbes. This increased understanding will allow us to determine appropriate fertilization practices to ensure the stability of AMF communities and thereby support the sustainability of agroecosystems. In this study, we explored the responses of AMF diversity, community structure, and co-occurrence network in the wheat rhizosphere and root endosphere to different long-term (35 years) fertilization regimes, using high-throughput amplicon sequencing. This long-term experiment studied the effects of the common fertilization strategies on lime concretion black soils in China. These strategies included a balanced mineral fertilizer comprising nitrogen, phosphorus, and potassium (NPK), NPK combined with straw (NPKS), and NPK combined with manure (NPKM). We aimed to address the following main questions. (i) How do fertilization treatments with different organic amendments affect the diversity and structure of crop root-associated AMF communities? (ii) Does fertilization management significantly affect AMF co-occurrence networks in the rhizosphere and root endosphere? (iii) Do the AMF communities in the rhizosphere (RS) and root endosphere (ES) show consistent responses to long-term fertilization?
RESULTS
Rhizosphere soil physicochemical properties and root nutritional status under different fertilization treatments.
We investigated 21 physicochemical properties of rhizosphere soil and 13 nutritional statuses of wheat roots under different fertilizer treatments (Table S1 in the supplemental material). Among the measured rhizosphere soil characteristics, the pH and carbon-to-phosphorus ratio (C/P ratio) were significantly lower, while the dissolved organic carbon (DOC), dissolved organic nitrogen (DON), total nitrogen (TN), total phosphorus (TP), available phosphorus (AP), and the nitrogen-to-phosphorus ratio (N/P ratio) were significantly higher under long-term NPK fertilization than under the nonfertilizer treatment (Table S1). Compared with NPK treatment, additional manure amendment increased the pH, total nutrients, available nutrients, and Zn in the RS significantly (Table S1).
Among all the measured characteristics of the roots, long-term NPK treatment increased the TP and Mn contents significantly but decreased total carbon (TC) content, the C/P ratio, the N/P ratio, and the Zn content (Table S1). Additional manure amendment (NPKM) increased the TP, total potassium (TK), and Ca contents significantly and decreased the C/P ratio and Mn content in the roots compared with those under NPK treatment (Table S1). However, compared with NPK treatment, straw amendment (NPKS) did not have a marked influence on any of the measured variables of the rhizosphere soil and roots.
AMF diversity and community structure in the RS and ES are influenced by fertilization treatments.
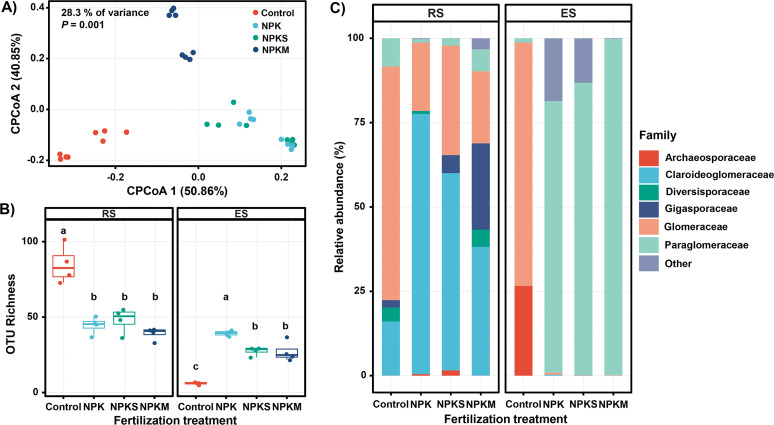
We analyzed the AMF communities in the extraradical and intraradical compartments (RS and ES) of the mycorrhizal symbiosis under different fertilization treatments. Permutational multivariate analysis of variance (PERMANOVA) based on distance matrices (adonis) revealed substantial contributions of compartment and fertilization treatments of 30% and 32%, respectively, to the explained variance (Fig. 1). To highlight the fertilization effects, we used partial canonical analysis of principal coordinates (CAP) to control for the strong compartment effect (Fig. 2A). Using this constrained method, we found that the AMF community structure varied significantly between fertilization treatments, which was in agreement with the PERMANOVA results (Fig. 2A; Table S2).
FIG 1.
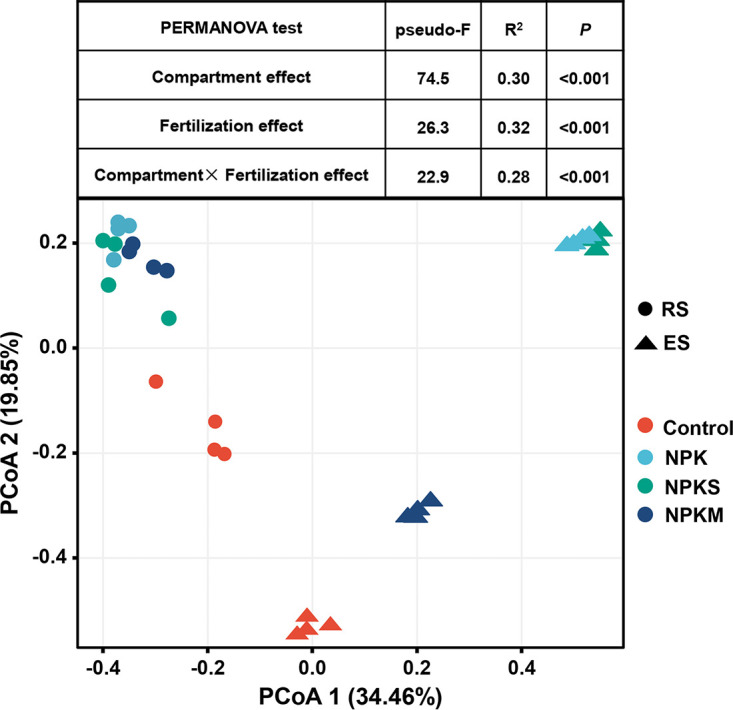
Principal coordinate analysis (PCoA) analysis on the arbuscular mycorrhizal fungal (AMF) community based on Bray-Curtis distance and results of PERMANOVA testing the effects of compartment and fertilization on AMF communities. Control, no fertilization; NPK, mineral fertilizer only; NPKS, mineral fertilizer plus wheat straw; NPKM, mineral fertilizer plus cow manure.
FIG 2.
Fertilization treatment significantly affects the arbuscular mycorrhizal fungal (AMF) communities in the rhizosphere soil (RS) and root endosphere (ES). (A) Canonical analysis of principal coordinates (CAP) based on Bray-Curtis distances constrained to fertilization treatment. (B) AMF operational taxonomic unit (OTU) richness at a sequencing depth of 10,000 reads per sample among different fertilization treatments in the RS and ES. Significant effects are indicated with different lowercase letters, as detected using Wilcoxon rank-sum tests. (C) Relative abundance of the main AMF families in the RS and ES across four treatments. For abbreviations, see the legend to Fig. 1.
Alpha diversity measurements in the RS and ES revealed that the fertilization treatments had significant but contrasting effects on AMF diversity (Fig. 2B). Long-term NPK application had a negative effect on the AMF operational taxonomic unit (OTU) richness in the RS; however, it increased the OTU richness in the ES significantly relative to the control (Fig. 2B). Compared with NPK fertilization, NPKS and NPKM treatments decreased the AMF OTU richness in the ES significantly but did not affect the AMF OTU richness in the RS (Fig. 2B).
Variation of the AMF community composition in the RS and ES under different fertilization treatments.
Overall, 241 OTUs obtained from all samples were assigned to Archaeosporaceae, Claroideoglomeraceae, Diversisporaceae, Gigasporaceae, Glomeraceae, and Paraglomeraceae (Fig. 2C). In each treatment, the AMF taxonomic composition in the ES differed remarkably from that in the RS (Fig. 2C). The relative abundances of the Glomeraceae and Archaeosporaceae were significantly higher in the ES under nonfertilization treatment. Under long-term treatments with NPK, NPKS, and NPKM, the relative abundance of Paraglomeraceae increased significantly in the ES relative to that in the RS (Fig. 2C).
The Glomeraceae and Claroideoglomeraceae were the most abundant taxa in the RS, accounting for 35.8% and 47.5% of the total reads, respectively (Fig. 2C; Table S3). Long-term NPK fertilization increased the relative abundance of the Claroideoglomeraceae and decreased those of the Glomeraceae and Paraglomeraceae significantly relative to the no-fertilizer control (Table S3). Compared with NPK treatment, NPKS treatment did not affect the abundance of these AMF families. However, NPKM treatment reduced the relative abundance of the Claroideoglomeraceae significantly (Table S3).
In contrast to the prevailing family in the RS, the predominant family in the ES was identified as the Paraglomeraceae (67.0% of all AMF sequences detected in the wheat roots), followed by the Glomeraceae (18.2% of all AMF sequences detected in the roots) (Fig. 2C). Long-term NPK treatment increased the relative abundance of the Paraglomeraceae and reduced the relative abundance of the Glomeraceae significantly compared with those under control treatment (Table S3). Treatment with straw (NPKS) or manure (NPKM) did not influence the relative abundance of these AMF families significantly compared with that of NPK fertilization (Table S3).
Effects of fertilization on the AMF co-occurrence network in the RS and ES.
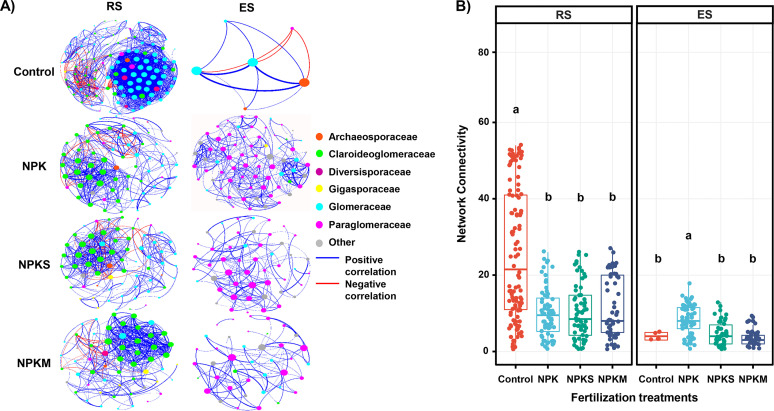
The AMF co-occurrence networks in the RS and ES revealed distinct co-occurrence patterns (Fig. S1). The dominant nodes of the AMF co-occurrence network in the RS were formed by the Glomeraceae (47.6% of nodes), whereas the dominant nodes of the network in the ES belonged to the Paraglomeraceae (54.0% of nodes; Fig. S1). The AMF co-occurrence network in the RS had more negative correlations than that in the ES (7.8% versus 0.8%; Fig. S1), suggesting a more competitive relationship among AMF members in the rhizosphere. The co-occurrence network in the ES had the simplest pattern, with the lowest numbers of nodes (100), edges (364), average degree (7.3), and transitivity (0.6; Fig. S1). In addition, we found that the natural connectivity of the network in the RS was greater than that in the ES, suggesting that the rhizosphere AMF network was more robust (Fig. S2).
Subnetworks for each treatment were generated to explore the impact of fertilization regimes on the network patterns of the AMF communities in the RS and ES. The networks displayed observable differences in their structures and topological features across different fertilization treatments (Fig. 3A; Table S4). The AMF co-occurrence network of the nonfertilization treatment in the RS had the most nodes (114), edges (1,468), and average degree (25.8) and the lowest proportion of negative correlations (5.9%; Table S4). In contrast, the network of nonfertilization treatment in the ES had the lowest number of nodes (6), edges (12), and average degree (4.0) and the highest proportion of negative correlations (25.0%; Table S4). Moreover, the Wilcoxon rank-sum test showed that NPK fertilization significantly reduced the network connectivity of the RS AMF network, whereas it increased the network connectivity of the ES AMF community relative to the control (Fig. 3B). Compared with NPK treatment, amendments with straw and manure only led to a significant reduction of the network connectivity in the ES (Fig. 3B).
FIG 3.
Effects of fertilization on arbuscular mycorrhizal fungi (AMF) co-occurrence networks on the rhizosphere soil (RS) and root endosphere (ES). (A) Co-occurrence subnetworks of different fertilization treatments in the RS and ES, respectively. The subnetworks of four fertilization treatments were visualized to show the significant associations (r > 0.60, P < 0.05) between AMF operational taxonomic units (OTUs) filtered from the overall co-occurrence networks of each compartment. Each dot represents an OTU, different colors represent different families, the node size represents the degree of the node, and edges denote significant correlations between OTUs. (B) Network connectivity as represented by node degrees for different fertilization treatments in the RS and ES, respectively. Different lowercase letters indicate statistically significant (P < 0.05) differences as detected using Wilcoxon rank-sum tests. For abbreviations, see the legend to Fig. 1.
Relationships between AMF communities and environmental variables.
The best multivariate model (distance-based linear modeling [DISTLM]) revealed that the variation in AMF community composition could be attributed to the mixed effects of rhizosphere soil physicochemical variables and the root nutritional status. Taken together, the predictor variables could explain 99.7% and 97.4% of AMF community composition variation in the RS and ES, respectively (Table 1). In particular, the soil pH was the primary driver of the AMF community composition, which individually explained 33.0% and 51.8% of the variation in the RS and ES, respectively (Table 1). In addition, the TP and AP of the rhizosphere soil individually explained 30.9% and 39.1% of the variation in AMF community composition in the RS and ES, respectively (Table 1). Therefore, the relative abundances of the Glomeraceae in the RS and ES correlated negatively with the TP and AP of the rhizosphere soil and correlated positively with the root C/P ratio (Table S5). The relative abundances of the Paraglomeraceae in the RS exhibited strong relationships with the pH, but in the ES, this family correlated significantly with any of the nutrients of the rhizosphere soil (Table S5). In terms of alpha diversity, according to the Spearman correlation test, the AMF OTU richness in the RS correlated most negatively with the rhizosphere soil DOC (r = 0.78, P < 0.001) and AP (r = 0.77, P < 0.001) and correlated positively with the root C/P ratio (r = 0.77, P < 0.001; Table S6). In contrast to the RS, the AMF OTU richness in the ES correlated most positively with the NH4+-N of the rhizosphere soil (r = 0.68, P < 0.010) and correlated negatively with the root Zn content (r = 0.63, P < 0.010; Table S6).
TABLE 1.
Best multivariate model (DISTLM) for community composition of AMF in the RS and root ES, respectivelya
| Compartment | Variable | Pseudo-F | P | R2 (conditional) | R2 (cumulative) |
|---|---|---|---|---|---|
| RS | RS_pH | 6.88 | 0.001 | 0.330 | 0.330 |
| RS_TP | 11.10 | 0.001 | 0.309 | 0.638 | |
| RS_NH4+-N | 2.75 | 0.029 | 0.067 | 0.706 | |
| RS_DON | 2.35 | 0.011 | 0.052 | 0.758 | |
| Root_TP | 1.85 | 0.045 | 0.038 | 0.796 | |
| Root_TK | 9.93 | 0.036 | 0.015 | 0.997 | |
| ES | RS_pH | 15.07 | 0.001 | 0.518 | 0.518 |
| RS_AP | 55.89 | 0.001 | 0.391 | 0.909 | |
| RS_AK | 5.87 | 0.001 | 0.030 | 0.939 | |
| Root_TP | 4.23 | 0.003 | 0.017 | 0.956 | |
| RS_NO3−-N | 2.41 | 0.040 | 0.009 | 0.965 | |
| RS_Ca | 3.34 | 0.013 | 0.010 | 0.974 |
Only the significant explanatory variables are shown. DON, dissolved organic nitrogen; NO3−-N, nitrate nitrogen; NH4+-N, ammonium nitrogen; TP, total phosphorus; TK, total potassium; AP, available phosphorous; AK, available potassium; Ca, calcium.
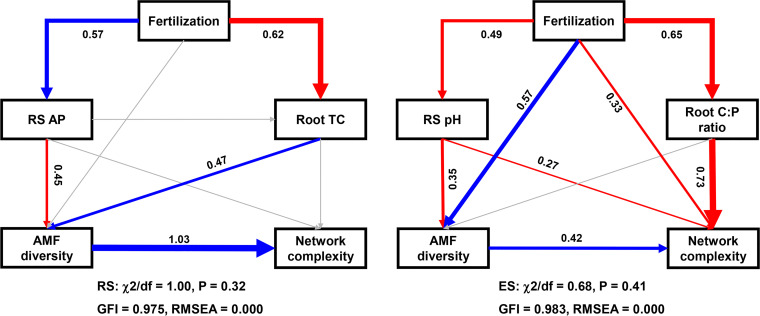
Furthermore, we used structural equation models (SEMs) to investigate the multiple direct and indirect associations between fertilization treatment, rhizosphere soil properties, root nutritional status, AMF richness, and AMF network complexity in the RS and ES, respectively. We observed that the rhizosphere AP and the root TC, which were affected by long-term fertilization, could impact the AMF network complexity by directly influencing the diversity in the RS (Fig. 4). In contrast, the rhizosphere soil pH and the root C/P ratio were associated directly with the AMF network complexity in the ES (Fig. 4). The SEM analysis showed that AMF richness correlated positively and significantly with the complexity of the AMF network in both the RS and ES (Fig. 4).
FIG 4.
Structural equation model (SEM) showing the relationships among fertilization treatments, rhizosphere soil chemical properties, root nutrients, arbuscular mycorrhizal fungal (AMF) diversity, and the network complexity of AMF communities in the rhizosphere soil (RS) and root endosphere (ES), respectively. Each box represents a variable in the model, whereas the number above each arrow represents the value of the standardized path coefficient. Blue lines indicate positive effects, red lines indicate negative effects, and gray lines indicate no significant correlation. The final models fit the data well, as assessed using the maximum-likelihood method.
DISCUSSION
AMF display one of the most ancient and obligate plant-root symbiotic relationships, which has critical benefits for hosts and agricultural ecosystems (4, 29). As a common agricultural practice, mineral fertilizer, eventually combined with organic materials, has profound effects on AMF communities in agricultural soils. However, the responses of the AMF community in the intraradical compartment, the root endosphere, to long-term fertilization management are largely unknown. Understanding the intraradical AMF community changes might provide important information about the influence of agricultural management on the intimate relationship between AMF and their hosts. In the present study, we provided the first insight into how AMF communities in the rhizosphere and root endosphere respond differently to long-term fertilization treatments.
Plant hosts provide specific niches for AMF in their root endosphere. For example, different plant species have often been shown to recruit different AMF communities from the same soil in their root endosphere (30, 31). In the present study, we extend knowledge of endosphere-specific responses of AMF communities by their differential response to agricultural management. For instance, we found a significant decrease in AMF richness in the ES, but not the RS, under nonfertilization conditions. Consistent with previous reports (16, 32), we observed that different long-term fertilizer inputs decreased the AMF richness in the RS compared with that in the nonfertilized control (Fig. 2B). Such a decrease in diversity is probably related to the unfavorable conditions for mycorrhizal symbiosis under high nutrient availability (33). However, in the present study, we found, remarkably, that the AMF richness in the ES increased under long-term fertilization treatments (Fig. 2B). One possible explanation is that the more developed host roots under long-term fertilization might provide more ecological niches for AMF. In addition, AMF richness in the ES correlated positively with mineral nitrogen in the rhizosphere soil (Table S6), which may indicate that under nitrogen fertilization, competition between host (crop) and AMF diminished, promoting mycorrhizal symbiosis (13). Moreover, we also found significant correlations between the AMF richness in the roots and the pH, root manganese, and root zinc contents (Table S6). Long-term fertilization with high nitrogen inputs can facilitate soil acidification (34) and might alter the availabilities of many mineral nutrients (35) and toxic heavy metal ions (36). In this case, plants might need more AMF to adapt to specific certain stresses caused by long-term fertilization treatments with the shift of AMF functional traits.
The AMF community composition established in the rhizosphere and roots showed clear differences, similar to previous findings in various host plants (37, 38). The Glomeraceae are reported to represent the predominating family in soils and plant roots (25, 33, 38, 39). In the present study, we also found that the Glomeraceae dominated in both the RS and ES under nonfertilization conditions; however, under long-term fertilization treatment, the Claroideoglomeraceae and Paraglomeraceae dominated in the RS and ES, respectively (Fig. 2C). This suggested the importance of host selection in shaping the AMF community composition when crops are faced with different fertilization regimes in the agroecosystem. These AMF taxa are defined by functional characteristics that are likely to influence the ecology of AMF, i.e., the changes in the community composition of AMF under different fertilization regimes might have functional implications (40). Therefore, more studies are needed to examine possible capabilities of AMF members under sufficient soil nutritional conditions. This would facilitate research into specific strategies used by AMF families to assist their hosts in adapting to specific certain stresses caused by fertilization.
In the present study, long-term fertilization treatments significantly affected the AMF community composition in both the RS and ES (Fig. 1; Fig. 2A). This could result from the profound alteration of soil properties caused by long-term fertilization (Table 1). We found that the rhizosphere soil pH and phosphorus (total phosphorus or available phosphorus) were the major influencing factors in the RS and ES, similar to many earlier studies of AMF (33, 39, 41, 42). Dumbrell et al. (43) reported pH as the major driver in structuring communities. Different AMF species respond differently to soil pH; for example, Glomus mosseae is not tolerant of a pH below about 5, whereas other members of the Glomeraceae are acid tolerant (44). Meanwhile, changes in pH might also regulate soil phosphorus availability, with fundamental consequences for AMF roles in plant phosphorus uptake (45). Thus, the level of phosphorus in the rhizosphere was strongly associated with root-associated AMF community compositions. The relative abundances of the Glomeraceae in both the RS and ES correlated negatively with the rhizosphere soil total phosphorus and available phosphorus and correlated positively with the root carbon-to-phosphorus ratio (Table S5). Phosphorus transporters were identified in many members of the Glomeraceae; these transporters are not only expressed in the extraradical mycelium, where phosphorus is absorbed for plants from the soil, but also in the arbuscules, where the phosphorus flux is directed toward the plant cell (46–48). Moreover, studies have shown that the knockout of these phosphate transporters stunts the symbiosis with the Glomeraceae AMF, suggesting the essential role of phosphorus acquisition for the Glomeraceae fungi (49). In contrast, little information about the Paraglomeraceae is available because traditional approaches usually fail to study members of this family (http://fungi.invam.wvu.edu/the-fungi/classification/paraglomaceae.html). However, we found that the Paraglomeraceae in the wheat roots accumulated largely under long-term fertilization regimes (Fig. 2C), and the relative abundance of this family in the ES correlated strongly with the rhizosphere soil nutrient conditions (Table S5). Thus, the Paraglomeraceae might play special roles in nutrient uptake of the crop roots in fertilized and productive agricultural systems. AMF live in both soil and roots; therefore, their changes depend on both the edaphic conditions and the status of the host plants (50, 51). We found that the root phosphorus content had significant influences on the rhizosphere and root AMF community compositions (Table 1). This indicates that the host plant might adjust its signaling compounds according to the root phosphorus content, allowing it to control the initiation and degree of AMF colonization (52). Meanwhile, the root phosphorus content has strongly correlation with the pH and available phosphorus of the rhizosphere soil. Therefore, we suggest that precision fertilization in the root zone (e.g., rhizosphere) may be a green and efficient fertilization strategy that will rapidly assemble the environmentally friendly AMF community in the crop roots and thus enhance plant growth.
Previous studies mainly focused on AMF alpha and beta diversity patterns. However, microorganisms usually interact with each other and form co-occurrence networks rather than thrive in isolation. Such networks are critical to gain insights into the microbiome structure and its response to changing environments (53). The impact of different fertilization strategies on the AMF network structure has not been assessed to date. In the present study, co-occurrence networks were constructed to examine the relationships among the AMF in the RS and ES, which showed distinct patterns (Fig. S1 in the supplemental material). Concerning the network features, the AMF co-occurrence network showed a lower number of nodes, edges, and average degree in the ES (Fig. S1). These network features suggested a simple co-occurrence pattern of AMF in the roots compared with that in the rhizosphere, consistent with their less complex community composition. In addition, the higher number of negative correlations in the rhizosphere AMF network could be regarded as indicating a more stable network under fertilizer-induced disturbances (Fig. S1). The natural connectivity of the different networks also supported the view that the network structure was more stable in the rhizosphere (Fig. S2) (54).
Similarly, the fertilization-specific co-occurrence networks had distinct structures in the RS and ES (Fig. 3A; Table S4). The AMF network in the ES under fertilization regimes showed markedly higher modularity (Fig. 3A; Table S4). This might suggest more niche overlap or common resource requirements among co-occurring AMF taxa when the roots developed better (55). Notably, long-term fertilization strategies significantly reduced network connectivity in the RS (Fig. 3B), demonstrating a loss of network complexity when AMF was grown under ample nutrient availability conditions. In contrast, long-term NPK treatment increased the network connectivity of AMF significantly in the ES (Fig. 3B). Compared with NPK treatment, organic amendments decreased the AMF network connectivity significantly in the ES (Fig. 3B). This might be explained by the change of AMF richness influenced by different fertilization strategies, as assessed using SEMs (Fig. 4). Theoretically, the presence of more taxa within a community would imply more potential interactions (56). Thus, it could be that the higher the diversity, the greater the complexity of a community. We also found that the extraradical AMF community might be predominantly and indirectly determined by the soil phosphorus availability and the root carbon content (indicating the amount of root exudates). However, the intraradical community might be determined more directly by the plant roots' carbon and phosphorus status. A cornerstone of the mutualism between plants and AMF is the reciprocal exchange of carbon and phosphorus (45). Therefore, the plant root carbon-phosphorus status might influence the AMF network complexity by directly affecting the mycorrhizal carbon and phosphorus exchange. However, further studies are required to explore the relationship between the richness and network complexity, while simultaneously considering the physiological regulation by the host plant.
Conclusions.
This study revealed that different long-term fertilization regimes affected the root-associated AMF diversity, community structure, and co-occurrence network. The responses of AMF communities were shown to differ between the rhizosphere and root endosphere markedly. However, we only revealed the responses of AMF communities associated with wheat roots to long-term fertilization at the booting stage. The next step to assess the temporal consistency and predictability of these findings will be to perform repeated sampling over the growing season of the crops or over several years. Furthermore, a traditional methodology, such as the determination of AMF by root colonization and spore diversity, could complement the results of molecular approaches used in the current study and help better understand the root-associated AMF ecology. In summary, this study provides insight into the responses of wheat root-associated AMF communities to different long-term fertilization strategies, which has important implications to understand the role of the AMF in long-term fertilized and productive agricultural ecosystems.
MATERIALS AND METHODS
Experimental design and sample collection.
The study was superimposed on the ongoing long-term (35 years) field experiment at the Madian Agro Ecological Station in Mengcheng County, Anhui Province, China (33°13′N, 116°35′E). This region possesses a typical lime concretion black soil and has been in wheat-soybean crop rotation since 1982. The field experiment was established using a complete randomized block design. Four treatments with four replicates (four blocks) were chosen for this study, including no fertilization (control), NPK, NPKS, and NPKM (Table S7 in the supplemental material). All fertilizers were applied in October, once a year, before the sowing of wheat. Root and soil samples were collected in April 2017 at the booting stage of the wheat crop.
In each trial plot, 30 healthy wheat (Triticum aestivum L.) roots with adhering soil were collected randomly. Soil attached to the roots was defined as the RS (57). All the rhizosphere or root samples from each plot were well mixed and pooled into sealed polyethylene bags. In total, 32 samples were collected. All the samples were transported on ice in a cooler box to the laboratory within 8 h and divided into two parts: one part was stored at 4°C to determine the soil properties, nutrient concentrations, and root nutrient elements, and the other was frozen at −40°C for DNA extraction within 2 weeks. In detail, each rhizosphere soil sample was passed through a 2-mm sieve to remove impurities, and the root samples were surface sterilized using a modification of the ethanol-sodium hypochlorite method to remove the microorganisms from the root surface. These root samples were then ground with liquid nitrogen using sterile pestles and mortars in a sterile room and finally stored at −40°C in airtight aseptic tubes until further processing. These samples were used to characterize the AMF in the ES.
Nutrient element analyses.
The rhizosphere soil properties and the root nutritional status were measured. For the soil samples, soil moisture was measured using a QS-SFY soil moisture meter 12 times for each sample. The soil pH was measured using a pH meter (Orion-868; Thermo Fisher Scientific, Waltham, MA, USA) (the soil/water ratio was 1:5). The TC and TN were determined using a carbon-hydrogen-nitrogen (CHN) elemental analyzer (CNS-2000; Leco, St. Joseph, MI, USA). The TP and TK were determined using the molybdenum blue colorimetric method and flame spectrophotometry after HNO3-HF-HClO4 digestion, respectively. The AP was measured using the molybdenum blue method after extraction with 0.5 M NaHCO3. The AK was extracted using 1 M ammonium acetate and determined using flame photometry (FP640; Inasa, Shanghai, China). The DOC was measured using a total organic carbon analyzer (Multi N/C 3000; Analytik, Jena, Germany) after extraction with distilled water and vacuum filtration. Nitrate nitrogen (NO3−-N), ammonium nitrogen (NH4+-N), and the dissolved total nitrogen (DTN) were measured using a continuous flow analyzer (San++ system; Skalar, Holland) after extraction with 2 M KCl. The DON was calculated according to the formula DON = DTN − NH4+-N − NO3−-N. The other soil elements, including calcium (Ca), magnesium (Mg), sodium (Na), iron (Fe), manganese (Mn), and zinc (Zn), were measured using an Optima 8000 inductively coupled plasma optical emission spectrometry (ICP-OES; PerkinElmer, Waltham, MA, USA) after digestion with hydrogen nitrate, hydrofluoric acid, and perchloric acid.
For the root samples, all roots of each subsample were ground into a powder. Root TC and TN were measured using a C/N elemental analyzer, Vario Max (Elementar, Langenselbold, Germany). Root TP, TK, Ca, Mg, and the other microelements, including Na, Fe, Mn, and Zn, were extracted using concentrated HNO3 and HClO4 solution (4:1, vol/vol) and measured using an Optima 8000 ICP-OES (PerkinElmer). Rhizosphere soil properties and the nutritional status of wheat crop roots under the different fertilization management strategies are shown in Table S1.
DNA extraction and amplicon sequencing.
The total DNAs of rhizosphere soil samples and roots (ES) were extracted according to the manufacturer’s instruction using a FastDNA spin kit (Bio 101, Carlsbad, CA, USA) and DNeasy plant minikit (Qiagen, Hilden, Germany) using a modified standard extraction protocol as described by Zimmerman and Vitousek (58), respectively. The primers AML1F and AML2R were used to amplify the V4-V5 hypervariable regions of the fungal 18S rRNA gene in a thermocycler PCR system (GeneAmp 9700; ABI, Foster City, CA, USA) (59). The second amplification used the AMF-specific primer set AMV4.5NF and AMDGR (59). The reactions were carried out in a volume of 20 μl, which comprised of 0.4 μl of TransStart FastPfu DNA polymerase (catalog no. AP221-02; TransGen Biotech, Beijing, China), 4 μl of 5 × FastPfu buffer, 2 μl of 2.5 mM deoxynucleoside triphosphates (dNTPs), 0.8 μl each of 5 μM forward and reverse primers, 0.2 μl of bovine serum albumin (BSA), 1 μl of DNA template (10 ng μl−1), and distilled water. The PCR amplification was performed at 95°C for 3 min, followed by 32 cycles of 30 s at 95°C, 30 s at 55°C, and 45 s at 72°C, with a final extension at 72°C for 10 min. Sequences were determined on the Illumina MiSeq PE250 platform (Illumina, Inc. San Diego, CA, USA).
Bioinformatics and statistical analyses.
Raw sequencing data were processed using QIIME 1.9.0 (60) and Cutadapt 1.9.1 (61). Sequences were quality filtered (quality threshold, 25) and trimmed (minimum length, 220 bp), and chimeras were removed. Then, the remaining sequences were clustered into OTUs at 97% similarity using UCLUST (62). Singletons and low-abundance OTUs (sequence count < 10) were discarded. The taxonomic identities of the representative sequences were checked against the MaarjAM AMF database online (http://maarjam.botany.ut.ee/). In total, 435,606 high-quality sequences covering 241 OTUs were obtained, with a mean number of sequences per sample of 13,612 (minimum, 10,029; maximum, 18,346). The command alpha_diversity.py was used on the OTU table after rarefaction to 10,000 reads per sample to determine the Chao1 index (OTU richness).
Partial CAP on Bray-Curtis distance was performed using the function capscale from the package vegan (https://CRAN.R-project.org/package=vegan). This analysis can be constrained to fertilization treatment to better understand the quantitative impact of the factor on the AMF composition. PERMANOVA was performed to test the significance of the explanatory power of the compartment and of the fertilization treatment on AMF dissimilarity using the vegan package. DISTLM was applied to examine the relative effects of the rhizosphere and root properties on the AMF community composition. Figures were prepared using the package ggplot2 in the R software (https://CRAN.R-project.org/package=ggplot2).
Cooccurrence networks were constructed to better comprehend the interrelationships within the AMF community in each compartment based on Spearman correlation coefficients using the WGCNA package (63). Networks of significant correlations (r > 0.6, P < 0.05) were visualized using the Fruchterman-Reingold layout in Gephi (http://gephi.github.io/). Subnetworks were further generated for each treatment from the whole-community network in each compartment by preserving OTUs present in each treatment using the subgraph functions in igraph (http://igraph.org). Three main topological features of the networks were also calculated in igraph. These features included average degree (reflecting network complexity), transitivity (clustering coefficient, reflecting the network cohesion), and modularity (reflecting the extent of smaller components in the network). Degrees of nodes associated with individual fertilization were calculated to represent the network connectivity (28).
Rhizosphere soil mineral properties, root nutritional status, and the AMF community abundance among fertilization treatments and compartments were analyzed using one- and two-way ANOVA in IBM SPSS statistical software package version 20 (IBM Corp., Armonk, NY, USA). The Wilcoxon rank-sum test was used to test for differences in AMF OTU richness and network connectivity between fertilization treatments in the R software. Spearman correlation tests were used to examine the correlations between the diversity and edaphic and root properties. SEMs were conducted using IBM SPSS Amos 24 to evaluate the direct and indirect effects of prominent factors, including fertilization treatments, rhizosphere variables (soil pH, available phosphorus), root nutritional status (total carbon, carbon/phosphorus ratio), AMF diversity, and the complexity of the AMF co-occurrence network. The SEM fitness was examined on the basis of a nonsignificant chi-squared test (P > 0.05), the goodness-of-fit index (GFI), and the root mean square error of approximation (RMSEA).
Data availability.
All raw sequences were deposited at the National Center for Biotechnology Information (NCBI) under the Sequence Read Archive (SRA) accession number PRJNA611471 (BioSample accession numbers SAMN14331863 to SAMN14331894), which are publicly available at https://www.ncbi.nlm.nih.gov/sra/.
ACKNOWLEDGMENTS
We thank Zhibin Guo, Keke Hua, Yingying Ni, Kunkun Fan, and Hongfei Wang for their assistance in field management and soil sampling. We appreciate the constructive comments from the two anonymous reviewers, which strengthened the manuscript.
Funding for this work was supported by the National Natural Science Foundation of China (grant number 31870480) and the Strategic Priority Research Program of the Chinese Academy of Sciences (grant number XDB15010101).
Footnotes
Supplemental material is available online only.
Contributor Information
Haiyan Chu, Email: hychu@issas.ac.cn.
Isaac Cann, University of Illinois at Urbana-Champaign.
REFERENCES
- 1.Ellouze W, Hamel C, Singh AK, Mishra V, DePauw RM, Knox RE. 2018. Abundance of the arbuscular mycorrhizal fungal taxa associated with the roots and rhizosphere soil of different durum wheat cultivars in the Canadian prairies. Can J Microbiol 64:527–536. 10.1139/cjm-2017-0637. [DOI] [PubMed] [Google Scholar]
- 2.Martin FM, Uroz S, Barker DG. 2017. Ancestral alliances: plant mutualistic symbioses with fungi and bacteria. Science 356:eaad4501. 10.1126/science.aad4501. [DOI] [PubMed] [Google Scholar]
- 3.Castillo CG, Borie F, Oehl F, Sieverding E. 2016. Arbuscular mycorrhizal fungi biodiversity: prospecting in southern-central zone of Chile. A review. J Soil Sci Plant Nutr 16:0–422. 10.4067/S0718-95162016005000036. [DOI] [Google Scholar]
- 4.Basu S, Rabara RC, Negi S. 2018. AMF: the future prospect for sustainable agriculture. Physiol Mol Plant Pathol 102:36–45. 10.1016/j.pmpp.2017.11.007. [DOI] [Google Scholar]
- 5.Wu S, Zhang X, Huang L, Chen B. 2019. Arbuscular mycorrhiza and plant chromium tolerance. Soil Ecol Lett 1:94–104. 10.1007/s42832-019-0015-9. [DOI] [Google Scholar]
- 6.Campo S, Martin-Cardoso H, Olive M, Pla E, Catala-Forner M, Martinez-Eixarch M, San Segundo B. 2020. Effect of root colonization by arbuscular mycorrhizal fungi on growth, productivity and blast resistance in rice. Rice (N Y) 13:42. 10.1186/s12284-020-00402-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rillig MC, Mummey DL. 2006. Mycorrhizas and soil structure. New Phytol 171:41–53. 10.1111/j.1469-8137.2006.01750.x. [DOI] [PubMed] [Google Scholar]
- 8.Lanfranco L, Fiorilli V, Gutjahr C. 2018. Partner communication and role of nutrients in the arbuscular mycorrhizal symbiosis. New Phytol 220:1031–1046. 10.1111/nph.15230. [DOI] [PubMed] [Google Scholar]
- 9.Mueller RC, Bohannan BJM. 2015. Shifts in the phylogenetic structure of arbuscular mycorrhizal fungi in response to experimental nitrogen and carbon dioxide additions. Oecologia 179:175–185. 10.1007/s00442-015-3337-z. [DOI] [PubMed] [Google Scholar]
- 10.Rillig MC. 2004. Arbuscular mycorrhizae and terrestrial ecosystem processes. Ecol Lett 7:740–754. 10.1111/j.1461-0248.2004.00620.x. [DOI] [Google Scholar]
- 11.Bender SF, Wagg C, van der Heijden MGA. 2016. An underground revolution: biodiversity and soil ecological engineering for agricultural sustainability. Trends Ecol Evol 31:440–452. 10.1016/j.tree.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 12.Lehmann J, Bossio DA, Kögel-Knabner I, Rillig MC. 2020. The concept and future prospects of soil health. Nat Rev Earth Environ 1:544–553. 10.1038/s43017-020-0080-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson NC. 2010. Resource stoichiometry elucidates the structure and function of arbuscular mycorrhizas across scales. New Phytol 185:631–647. 10.1111/j.1469-8137.2009.03110.x. [DOI] [PubMed] [Google Scholar]
- 14.Williams A, Manoharan L, Rosenstock NP, Olsson PA, Hedlund K. 2017. Long-term agricultural fertilization alters arbuscular mycorrhizal fungal community composition and barley (Hordeum vulgare) mycorrhizal carbon and phosphorus exchange. New Phytol 213:874–885. 10.1111/nph.14196. [DOI] [PubMed] [Google Scholar]
- 15.Cheng Y, Ishimoto K, Kuriyama Y, Osaki M, Ezawa T. 2013. Ninety-year-, but not single, application of phosphorus fertilizer has a major impact on arbuscular mycorrhizal fungal communities. Plant Soil 365:397–407. 10.1007/s11104-012-1398-x. [DOI] [Google Scholar]
- 16.Sheng M, Lalande R, Hamel C, Ziadi N. 2013. Effect of long-term tillage and mineral phosphorus fertilization on arbuscular mycorrhizal fungi in a humid continental zone of Eastern Canada. Plant Soil 369:599–613. 10.1007/s11104-013-1585-4. [DOI] [Google Scholar]
- 17.Bünemann EK, Schwenke GD, Van Zwieten L. 2006. Impact of agricultural inputs on soil organisms - a review. Soil Res 44:379–406. 10.1071/SR05125. [DOI] [Google Scholar]
- 18.Insam H, Gómez-Brandón M, Ascher J. 2015. Manure-based biogas fermentation residues - friend or foe of soil fertility? Soil Biol Biochem 84:1–14. 10.1016/j.soilbio.2015.02.006. [DOI] [Google Scholar]
- 19.Sun RB, Zhang XX, Guo XS, Wang DZ, Chu HY. 2015. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol Biochem 88:9–18. 10.1016/j.soilbio.2015.05.007. [DOI] [Google Scholar]
- 20.Sheldrake M, Rosenstock NP, Revillini D, Olsson PA, Mangan S, Sayer EJ, Wallander H, Turner BL, Tanner EV. 2017. Arbuscular mycorrhizal fungal community composition is altered by long-term litter removal but not litter addition in a lowland tropical forest. New Phytol 214:455–467. 10.1111/nph.14384. [DOI] [PubMed] [Google Scholar]
- 21.Hodge A, Fitter AH. 2010. Substantial nitrogen acquisition by arbuscular mycorrhizal fungi from organic material has implications for N cycling. Proc Natl Acad Sci U S A 107:13754–13759. 10.1073/pnas.1005874107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leigh J, Hodge A, Fitter AH. 2009. Arbuscular mycorrhizal fungi can transfer substantial amounts of nitrogen to their host plant from organic material. New Phytol 181:199–207. 10.1111/j.1469-8137.2008.02630.x. [DOI] [PubMed] [Google Scholar]
- 23.Aguilar R, Carreón-Abud Y, López-Carmona D, Larsen J. 2017. Organic fertilizers alter the composition of pathogens and arbuscular mycorrhizal fungi in maize roots. J Phytopathol 165:448–454. 10.1111/jph.12579. [DOI] [Google Scholar]
- 24.Gosling P, Ozaki A, Jones J, Turner M, Rayns F, Bending GD. 2010. Organic management of tilled agricultural soils results in a rapid increase in colonisation potential and spore populations of arbuscular mycorrhizal fungi. Agr Ecosyst Environ 139:273–279. 10.1016/j.agee.2010.08.013. [DOI] [Google Scholar]
- 25.Verbruggen E, Kiers ET. 2010. Evolutionary ecology of mycorrhizal functional diversity in agricultural systems. Evol Appl 3:547–560. 10.1111/j.1752-4571.2010.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svanbäck A, McCrackin ML, Swaney DP, Linefur H, Gustafsson BG, Howarth RW, Humborg C. 2019. Reducing agricultural nutrient surpluses in a large catchment - links to livestock density. Sci Total Environ 648:1549–1559. 10.1016/j.scitotenv.2018.08.194. [DOI] [PubMed] [Google Scholar]
- 27.Zheng L, Zhang QW, Zhang AP, Hussain HA, Liu XR, Yang ZL. 2019. Spatiotemporal characteristics of the bearing capacity of cropland based on manure nitrogen and phosphorus load in mainland China. J Clean Prod 233:601–610. 10.1016/j.jclepro.2019.06.049. [DOI] [Google Scholar]
- 28.Banerjee S, Schlaeppi K, van der Heijden MGA. 2018. Keystone taxa as drivers of microbiome structure and functioning. Nat Rev Microbiol 16:567–576. 10.1038/s41579-018-0024-1. [DOI] [PubMed] [Google Scholar]
- 29.Shao YD, Zhang DJ, Hu XC, Wu QS, Jiang CJ, Xia TJ, Gao XB, Kuca K. 2018. Mycorrhiza-induced changes in root growth and nutrient absorption of tea plants. Plant Soil Environ 64:283–289. [Google Scholar]
- 30.Gottel NR, Castro HF, Kerley M, Yang ZM, Pelletier DA, Podar M, Karpinets T, Uberbacher E, Tuskan GA, Vilgalys R, Doktycz MJ, Schadt CW. 2011. Distinct microbial communities within the endosphere and rhizosphere of Populus deltoides roots across contrasting soil types. Appl Environ Microbiol 77:5934–5944. 10.1128/AEM.05255-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldmann K, Schröter K, Pena R, Schöning I, Schrumpf M, Buscot F, Polle A, Wubet T. 2016. Divergent habitat filtering of root and soil fungal communities in temperate beech forests. Sci Rep 6:31439. 10.1038/srep31439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang R, Mu Y, Li X, Li S, Sang P, Wang X, Wu H, Xu N. 2020. Response of the arbuscular mycorrhizal fungi diversity and community in maize and soybean rhizosphere soil and roots to intercropping systems with different nitrogen application rates. Sci Total Environ 740:139810–139810. 10.1016/j.scitotenv.2020.139810. [DOI] [PubMed] [Google Scholar]
- 33.Ma M, Ongena M, Wang Q, Guan D, Cao F, Jiang X, Li J. 2018. Chronic fertilization of 37 years alters the phylogenetic structure of soil arbuscular mycorrhizal fungi in Chinese Mollisols. AMB Express 8:57. 10.1186/s13568-018-0587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS. 2010. Significant acidification in major Chinese croplands. Science 327:1008–1010. 10.1126/science.1182570. [DOI] [PubMed] [Google Scholar]
- 35.Ye CL, Chen DM, Hall SJ, Pan S, Yan XB, Bai TS, Guo H, Zhang Y, Bai YF, Hu SJ. 2018. Reconciling multiple impacts of nitrogen enrichment on soil carbon: plant, microbial and geochemical controls. Ecol Lett 21:1162–1173. 10.1111/ele.13083. [DOI] [PubMed] [Google Scholar]
- 36.Shi W, Zhang Y, Chen S, Polle A, Rennenberg H, Luo Z-B. 2019. Physiological and molecular mechanisms of heavy metal accumulation in nonmycorrhizal versus mycorrhizal plants. Plant Cell Environ 42:1087–1103. 10.1111/pce.13471. [DOI] [PubMed] [Google Scholar]
- 37.Qin Z, Zhang H, Feng G, Christie P, Zhang J, Li X, Gai J. 2020. Soil phosphorus availability modifies the relationship between AM fungal diversity and mycorrhizal benefits to maize in an agricultural soil. Soil Biol Biochem 144:107790. 10.1016/j.soilbio.2020.107790. [DOI] [Google Scholar]
- 38.Zhu X, Yang W, Song F, Li X. 2020. Diversity and composition of arbuscular mycorrhizal fungal communities in the cropland black soils of China. Glob Ecol Conserv 22:e00964. 10.1016/j.gecco.2020.e00964. [DOI] [Google Scholar]
- 39.Qin H, Lu KP, Strong PJ, Xu QF, Wu QF, Xu ZX, Xu J, Wang HL. 2015. Long-term fertilizer application effects on the soil, root arbuscular mycorrhizal fungi and community composition in rotation agriculture. Appl Soil Ecol 89:35–43. 10.1016/j.apsoil.2015.01.008. [DOI] [Google Scholar]
- 40.Weber SE, Diez JM, Andrews LV, Goulden ML, Aronson EL, Allen MF. 2019. Responses of arbuscular mycorrhizal fungi to multiple coinciding global change drivers. Fungal Ecol 40:62–71. 10.1016/j.funeco.2018.11.008. [DOI] [Google Scholar]
- 41.Xu XH, Chen C, Zhang Z, Sun ZH, Chen YH, Jiang JD, Shen ZG. 2017. The influence of environmental factors on communities of arbuscular mycorrhizal fungi associated with Chenopodium ambrosioides revealed by MiSeq sequencing investigation. Sci Rep 7:45134. 10.1038/srep45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding JL, Jiang X, Guan DW, Zhao BS, Ma MC, Zhou BK, Cao FM, Yang XH, Li L, Li J. 2017. Influence of inorganic fertilizer and organic manure application on fungal communities in a long-term field experiment of Chinese Mollisols. Appl Soil Ecol 111:114–122. 10.1016/j.apsoil.2016.12.003. [DOI] [Google Scholar]
- 43.Dumbrell AJ, Nelson M, Helgason T, Dytham C, Fitter AH. 2010. Relative roles of niche and neutral processes in structuring a soil microbial community. ISME J 4:337–345. 10.1038/ismej.2009.122. [DOI] [PubMed] [Google Scholar]
- 44.Wang GM, Stribley DP, Tinker PB, Walker C. 1993. Effects of pH on arbuscular mycorrhiza I. Field observations on the long-term liming experiments at Rothamsted and Woburn. New Phytol 124:465–472. 10.1111/j.1469-8137.1993.tb03837.x. [DOI] [Google Scholar]
- 45.Ferrol N, Azcon-Aguilar C, Perez-Tienda J. 2019. Review: arbuscular mycorrhizas as key players in sustainable plant phosphorus acquisition: an overview on the mechanisms involved. Plant Sci 280:441–447. 10.1016/j.plantsci.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 46.Maldonado-Mendoza IE, Dewbre GR, Harrison MJ. 2001. A phosphate transporter gene from the extra-radical mycelium of an arbuscular mycorrhizal fungus Glomus intraradices is regulated in response to phosphate in the environment. Mol Plant Microbe Interact 14:1140–1148. 10.1094/MPMI.2001.14.10.1140. [DOI] [PubMed] [Google Scholar]
- 47.Benedetto A, Magurno F, Bonfante P, Lanfranco L. 2005. Expression profiles of a phosphate transporter gene (GmosPT) from the endomycorrhizal fungus Glomus mosseae. Mycorrhiza 15:620–627. 10.1007/s00572-005-0006-9. [DOI] [PubMed] [Google Scholar]
- 48.Balestrini R, Gomez-Ariza J, Lanfranco L, Bonfante P. 2007. Laser microdissection reveals that transcripts for five plant and one fungal phosphate transporter genes are contemporaneously present in arbusculated cells. Mol Plant Microbe Interact 20:1055–1062. 10.1094/MPMI-20-9-1055. [DOI] [PubMed] [Google Scholar]
- 49.Javot H, Penmetsa RV, Terzaghi N, Cook DR, Harrison MJ. 2007. A Medicago truncatula phosphate transporter indispensable for the arbuscular mycorrhizal symbiosis. Proc Natl Acad Sci U S A 104:1720–1725. 10.1073/pnas.0608136104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vályi K, Mardhiah U, Rillig MC, Hempel S. 2016. Community assembly and coexistence in communities of arbuscular mycorrhizal fungi. ISME J 10:2341–2351. 10.1038/ismej.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vályi K, Rillig MC, Hempel S. 2015. Land-use intensity and host plant identity interactively shape communities of arbuscular mycorrhizal fungi in roots of grassland plants. New Phytol 205:1577–1586. 10.1111/nph.13236. [DOI] [PubMed] [Google Scholar]
- 52.Schmitz AM, Harrison MJ. 2014. Signaling events during initiation of arbuscular mycorrhizal symbiosis. J Integr Plant Biol 56:250–261. 10.1111/jipb.12155. [DOI] [PubMed] [Google Scholar]
- 53.Ramirez KS, Geisen S, Morrien E, Snoek BL, van der Putten WH. 2018. Network analyses can advance above-belowground ecology. Trends Plant Sci 23:759–768. 10.1016/j.tplants.2018.06.009. [DOI] [PubMed] [Google Scholar]
- 54.Peng GS, Wu J. 2016. Optimal network topology for structural robustness based on natural connectivity. Physica A 443:212–220. 10.1016/j.physa.2015.09.023. [DOI] [Google Scholar]
- 55.Poudel R, Jumpponen A, Schlatter DC, Paulitz TC, Gardener BBM, Kinkel LL, Garrett KA. 2016. Microbiome networks: a systems framework for identifying candidate microbial assemblages for disease management. Phytopathology 106:1083–1096. 10.1094/PHYTO-02-16-0058-FI. [DOI] [PubMed] [Google Scholar]
- 56.Lupatini M, Suleiman AKA, Jacques RJS, Antoniolli ZI, de Siqueira Ferreira A, Kuramae EE, Roesch LFW. 2014. Network topology reveals high connectance levels and few key microbial genera within soils. Front Environ Sci 2:10. 10.3389/fenvs.2014.00010. [DOI] [Google Scholar]
- 57.Philippot L, Spor A, Henault C, Bru D, Bizouard F, Jones CM, Sarr A, Maron PA. 2013. Loss in microbial diversity affects nitrogen cycling in soil. ISME J 7:1609–1619. 10.1038/ismej.2013.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zimmerman NB, Vitousek PM. 2012. Fungal endophyte communities reflect environmental structuring across a Hawaiian landscape. Proc Natl Acad Sci U S A 109:13022–13027. 10.1073/pnas.1209872109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lumini E, Orgiazzi A, Borriello R, Bonfante P, Bianciotto V. 2010. Disclosing arbuscular mycorrhizal fungal biodiversity in soil through a land-use gradient using a pyrosequencing approach. Environ Microbiol 12:2165–2179. 10.1111/j.1462-2920.2009.02099.x. [DOI] [PubMed] [Google Scholar]
- 60.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. 2010. QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336. 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17:10–12. 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 62.Edgar RC. 2010. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461. 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- 63.Langfelder P, Horvath S. 2012. Fast R functions for robust correlations and hierarchical clustering. J Stat Softw 46:i11. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1 to S7, Fig. S1 and S2. Download AEM.00349-21-s0001.pdf, PDF file, 1.1 MB (1.1MB, pdf)
Data Availability Statement
All raw sequences were deposited at the National Center for Biotechnology Information (NCBI) under the Sequence Read Archive (SRA) accession number PRJNA611471 (BioSample accession numbers SAMN14331863 to SAMN14331894), which are publicly available at https://www.ncbi.nlm.nih.gov/sra/.