Abstract
We identified an extremely rare congenital porcine type 0 lateral bicuspid aortic valve from a fresh porcine heart. Using a 3-dimensionally printed ex vivo left heart simulator, we analyzed valvular hemodynamics at baseline, in an aortic aneurysm disease model, and after valve-sparing root replacement. We showed that bicuspid aortic valve regurgitation due to aortic aneurysm can be successfully repaired without significant hemodynamic impairment using the valve-sparing root replacement technique in an individualized approach. Our results provide direct hemodynamic evidence supporting the use of valve-sparing root replacement for patients with bicuspid aortic valve regurgitation.
INTRODUCTION
Bicuspid aortic valve (BAV) is one of the most prevalent congenital heart defects and is also commonly associated with aortic aneurysm and aortic regurgitation (AR)1,2. Valve-sparing root replacement (VSRR) has been adopted in an effort to restore functional root geometry without replacing the aortic valve3. Although satisfactory results have been reported3,4, it is impossible to directly visualize and compare a competent BAV and a diseased BAV in an aneurysm versus after VSRR repair in human. Additionally, explanted human BAV specimens rarely contain intact aortic root apparatus to allow ex vivo evaluation, and the cusps are typically diseased with calcification.
DESCRIPTION
A fresh porcine heart was obtained from a meat abattoir, and the explanted aortic valve was found to be a rare type 0 lateral BAV. The annulus, sinus of Valsalva, and ascending aorta were measured to be 29 mm, 42 mm, and 34 mm in diameter, respectively. The distal left ventricular outflow tract was attached to a 3-dimensionally printed mount. Baseline measurements of the valve in its native geometry were taken.
To generate an aortic aneurysm model, the valve was prepared in the same fashion as for VSRR. Two 24-mm straight Dacron grafts (Gelweave; Vascutek Terumo, Renfrewshire, Scotland) were divided longitudinally and sewn together with running 4–0 polypropylene sutures to create a 46-mm graft. The aortic valve was reimplanted with commissural posts positioned in its natural configuration at a 180° orientation (Figure 1A).
Figure 1.

En face views of the same porcine bicuspid aortic valve after reimplantation in (A) a combined graft of 46-mm in diameter, (B) a 28-mm straight graft with free margin plication, and (C) a 34-mm straight graft.
Straight Dacron grafts of 28mm (undersized VSRR) and 34mm (proper-sized VSRR) were used for VSRR repair (Figure 1b,c). Commissural posts were positioned at a 180° orientation. The valve implanted in the 28mm graft were further repaired with free margin plication using CV-6 polytetrafluoroethylene sutures (Gore-Tex® Suture, WL Gore & Associates Inc., Flagstaff, Arizona).
Hemodynamics data were collected using pressure transducers (Utah Medical Products Inc., Midvale, UT) and electromagnetic flow probes (Carolina Medical Electronics, East Bend, NC).5 Mean aortic flow tracing from the aneurysm model demonstrated decreased forward flow during systole and flow reversal during diastole (Figure 2). The regurgitant fractions for the baseline, aortic aneurysm model, undersized VSRR, and proper-sized VSRR were 12.9%, 33.8%, 11.2%, and 13.3%, respectively. The detailed hemodynamics data are shown in Supplemental Table 1.
Figure 2.
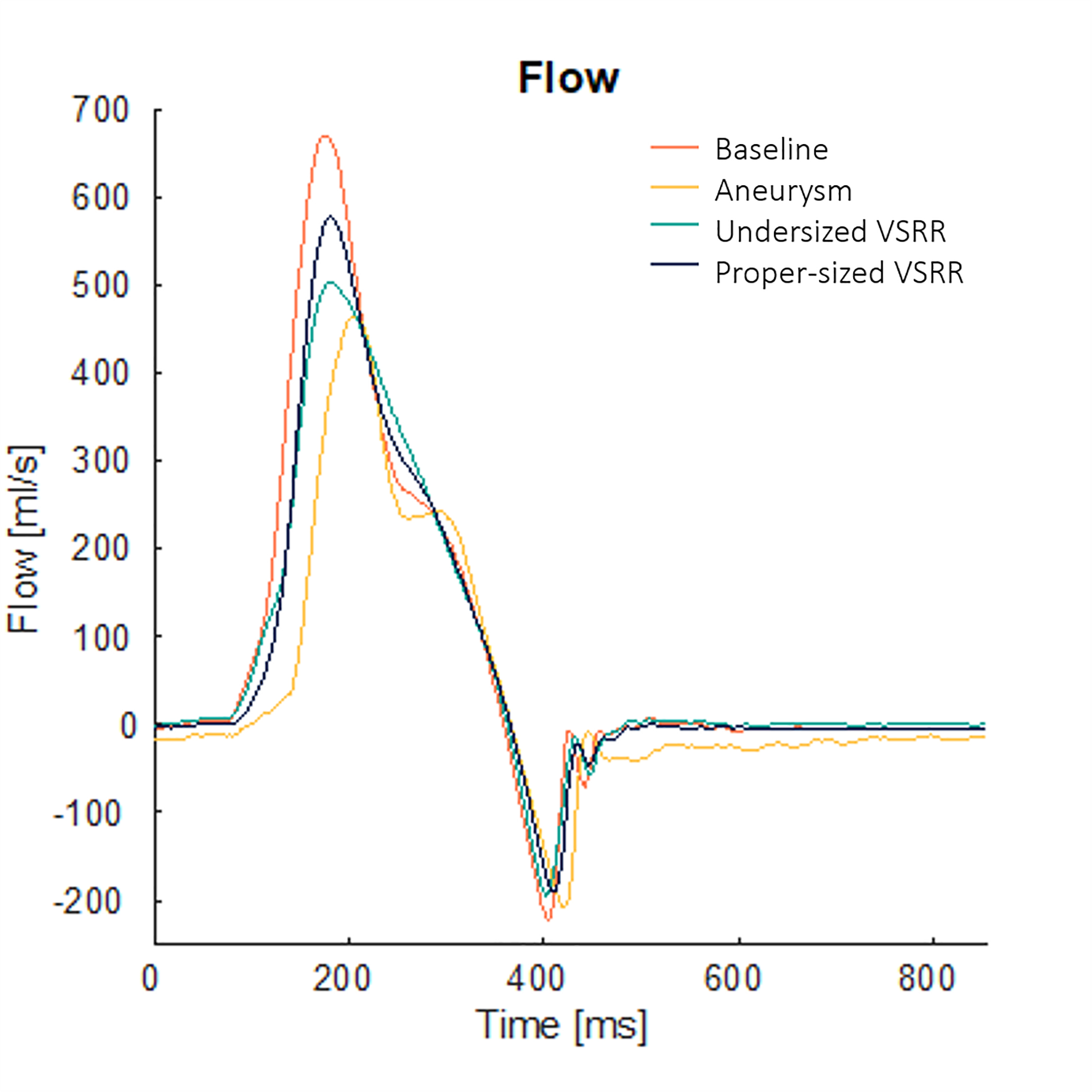
Mean aortic flow tracings from for the baseline, aortic aneurysm model, undersized valve-sparing root replacement (VSRR), and proper-sized VSRR. The aneurysm model flow confirmed the presence of aortic regurgitation as evidenced by the decreased forward flow during systole and flow reversal during diastole.
High-speed videography was obtained (Chronos 1.4, Kron Technologies, Burnaby, British Columbia, Canada) to evaluate for leaflet morphology and motion. The baseline valve demonstrated adequate leaflet coaptation without any restriction in leaflet motion (Figure 3A, Video 1). The aortic aneurysm model showed inadequate leaflet coaptation (Figure 3B, Video 2). The valve repaired with undersized VSRR demonstrated considerable leaflet motion restriction (Figure 3C, Video 3), and the valve repaired with proper-sized VSRR showed competent cusps without considerable leaflet motion limitations (Figure 3D, Video 4).
Figure 3.
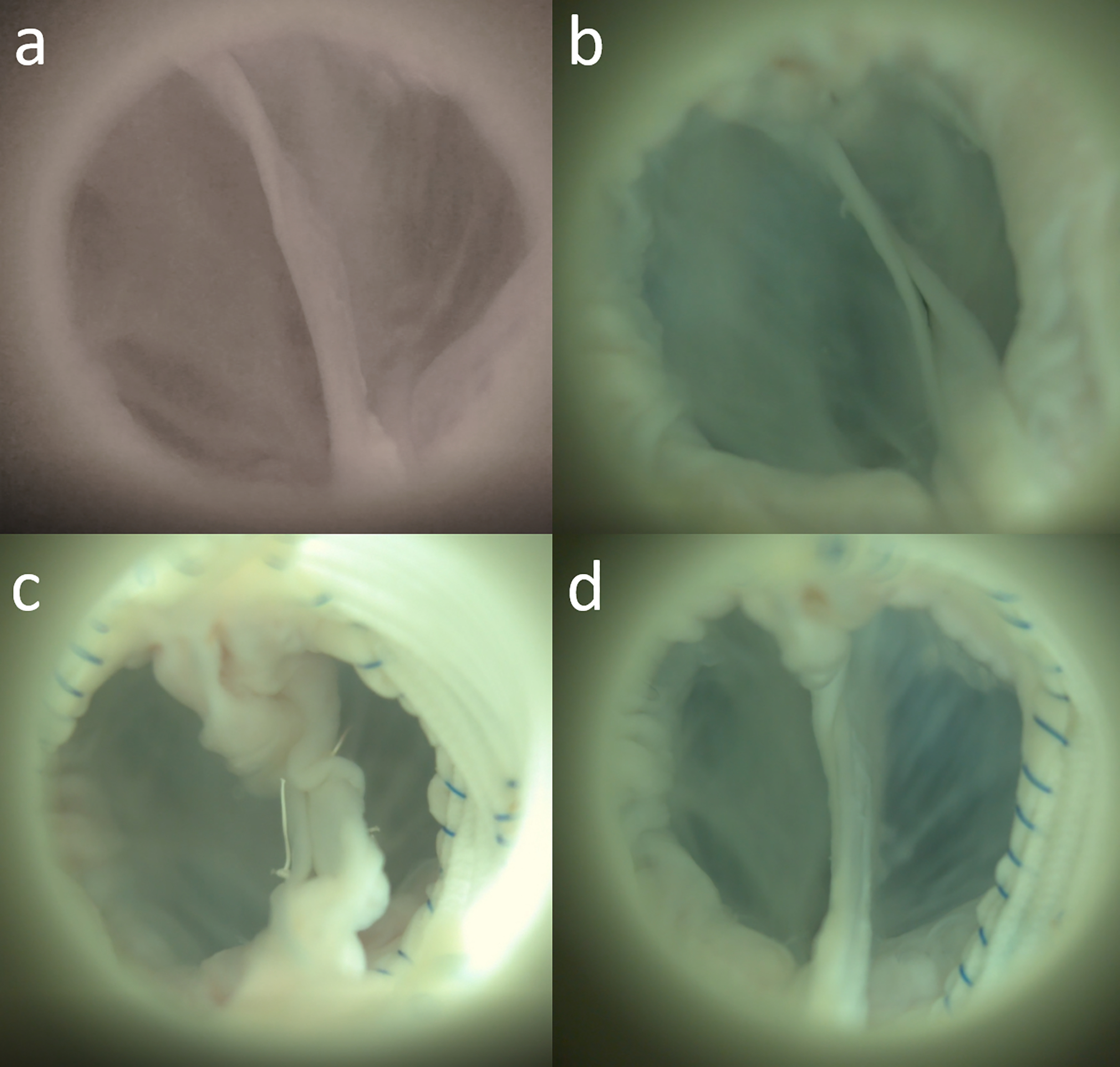
En face views during diastole of the same valve in the ex vivo left heart simulator: (a) baseline, (b) aortic aneurysm model with inadequate leaflet coaptation, (c) undersized valve-sparing root replacement (VSRR) with redundant cusp tissues, and (d) proper-sized VSRR.
Echocardiographic data was obtained using a Phillips iE33 system with a S5–1 transthoracic probe (Koninklijke Philips NV, Amsterdam, The Netherlands). Continuous-wave Doppler images showed that the peak aortic jet velocity, peak instantaneous systolic gradient, and mean gradient across the valve for the baseline, aneurysm model, undersized VSRR, and proper-sized VSRR were 190 cm/s, 14 mm Hg, and 5 mm Hg vs 278 cm/s, 31 mm Hg, and 9 mm Hg vs 474 cm/s, 90 mm Hg, and 15 mm Hg vs 248 cm/s, 24 mm Hg, and 4 mm Hg, respectively.
COMMENT
We describe a rare congenital porcine type 0 lateral BAV. We directly visualized the valve and investigated its valvular hemodynamics in an aneurysm model and after VSRR. We generated an aneurysm model and confirmed AR using the left heart simulator. With an undersized graft, we observed substantial hemodynamic impairments with aortic stenosis. By choosing a properly sized graft as the conduit for VSRR, however, AR due to distorted root geometry was eliminated without affecting valvular hemodynamics.
The BAV phenotype follows a continuous spectrum1. Type 0 lateral BAV is a true BAV with a prevalence of 2 to 8 in 10,000 individuals1. Although little is known regarding congenital porcine BAVs, aneurysm observed in human appears to be conserved in this porcine specimen6. BAV associated aneurysm has a complex pathophysiology, which is not fully understood7. Explanted human specimens rarely contain intact root structures to allow for meaningful ex vivo evaluations. Animal studies using porcine specimens thus may be another avenue to investigate the pathophysiology of BAV. Furthermore, the aneurysm model generated in this study can potentially be generalized to other ex vivo models for future AR evaluations.
VSRR has gained significant popularity in the contemporary era for repairing aortic valves with regurgitation due to altered root geometry3. The excellent results of VSRR have encouraged the application of this technique for the BAV population, although the implantation technique details remain controversial3,8. We reimplanted this porcine aortic valve with the commissural posts positioned in its natural orientation, given that the valve was competent at baseline and has roughly equal cusp size and free margin length. This reimplantation orientation, however, should be evaluated in an individualized fashion. Additionally, the appropriate graft size must be chosen to avoid accelerated jet velocity across the valve, as excessive stress imposed on the cusps that may affect the long-term durability of the repair.
In conclusion, we identified a rare congenital porcine BAV with a type 0 lateral morphology and studied the valvular hemodynamics using an ex vivo left heart simulator. We created an aneurysm model with AR and demonstrated excellent VSRR repair without significant hemodynamic impairment in an individualized approach. Our results provide direct hemodynamic evidence in support of VSRR for the BAV population.
Supplementary Material
ACKNOWLEDGMENT
This work was supported by the National Institutes of Health (NIH R01 HL089315–01, YJW), the Thoracic Surgery Foundation Resident Research Fellowship (YZ), the National Science Foundation Graduate Research Fellowship Program (AMI), and a generous donation from the Rittenberg Family Foundation (Andy, Amy, Leon and Cindy). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders.
Funding:
This work was supported by the National Institutes of Health (NIH R01 HL089315–01, YJW), the Thoracic Surgery Foundation Resident Research Fellowship (YZ), and the National Science Foundation Graduate Research Fellowship Program (AMI).
Footnotes
DISCLOSURES
None.
REFERENCES
- 1.Sievers HH, Schmidtke C. A classification system for the bicuspid aortic valve from 304 surgical specimens. J Thorac Cardiovasc Surg. 2007;133:1226–1233. [DOI] [PubMed] [Google Scholar]
- 2.Braverman AC, Güven H, Beardslee MA, et al. The bicuspid aortic valve. Curr Probl Cardiol. 2005;30:470–522. [DOI] [PubMed] [Google Scholar]
- 3.Ouzounian M, Feindel CM, Manlhiot C, et al. Valve-sparing root replacement in patients with bicuspid versus tricuspid aortic valves. J Thorac Cardiovasc Surg. 2019;158:1–9. [DOI] [PubMed] [Google Scholar]
- 4.Schneider U, Feldner SK, Hofmann C, et al. Two decades of experience with root remodeling and valve repair for bicuspid aortic valves. J Thorac Cardiovasc Surg. 2017;153:65–71. [DOI] [PubMed] [Google Scholar]
- 5.Paulsen MJ, Kasinpila P, Imbrie-Moore AM, et al. Modeling conduit choice for valve-sparing aortic root replacement on biomechanics with a 3-dimensional-printed heart simulator. J Thorac Cardiovasc Surg. 2019;158:392–403. [DOI] [PubMed] [Google Scholar]
- 6.Wang C, Lachat M, Regar E, et al. Suitability of the porcine aortic model for transcatheter aortic root repair. Interact Cardiovasc Thorac Surg. 2018;26:1002–1008. [DOI] [PubMed] [Google Scholar]
- 7.Borger MA, Fedak PWM, Stephens EH, et al. The American Association for Thoracic Surgery consensus guidelines on bicuspid aortic valve-related aortopathy: Full online-only version. J Thorac Cardiovasc Surg. 2018;156:41–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyahara S, Abe N, Matsueda T, et al. Impact of positional relationship of commissures on cusp function after valve-sparing root replacement for regurgitant bicuspid aortic valve. Eur J Cardiothorac Surg. 2016;50:75–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


